ABSTRACT
Rilpivirine (RPV), dapivirine (DPV), and MIV-150 are in development as microbicides. It is not known whether they will block infection of circulating nonnucleoside reverse transcriptase inhibitor (NNRTI)-resistant human immunodeficiency virus type 1 (HIV-1) variants. Here, we demonstrate that the activity of DPV and MIV-150 is compromised by many resistant viruses containing single or double substitutions. High DPV genital tract concentrations from DPV ring use may block replication of resistant viruses. However, MIV-150 genital tract concentrations may be insufficient to inhibit many resistant viruses, including those harboring K103N or Y181C.
KEYWORDS: HIV-1, MIV-150, NNRTI, antiretroviral resistance, dapivirine, prevention
TEXT
Nonnucleoside reverse transcriptase (RT) inhibitors (NNRTIs) comprise a group of small amphiphilic compounds with diverse chemical structures that inhibit human immunodeficiency virus type 1 (HIV-1) (but not HIV-2) replication. They interact with HIV-1 RT by binding to a single site, termed the NNRTI-binding pocket, on the p66 subunit of the p66/p51 heterodimeric enzyme (1). In many low- and middle-income countries (LMIC), particularly in sub-Saharan Africa, NNRTIs are used in both HIV-1 treatment and prevention strategies (2). Specifically, the NNRTIs nevirapine, efavirenz, and rilpivirine (RPV) are used in first-line antiretroviral therapies, whereas etravirine is reserved for salvage therapy. For prevention of HIV-1 infection, the following drugs are used or being tested. Nevirapine is used to block mother-to-child transmission. A dapivirine (DPV)-containing ring provided moderate efficacy in HIV-1-negative female participants, particularly in compliant women over 25 years of age (3, 4). A microbicide gel formulation (PC-1005) containing the phenylethylthiazolylthiourea derivative MIV-150 is in phase I clinical studies (5), and an injectable long-acting RPV formulation was evaluated in the clinical study HPTN 076 for preexposure prophylaxis (6, 7).
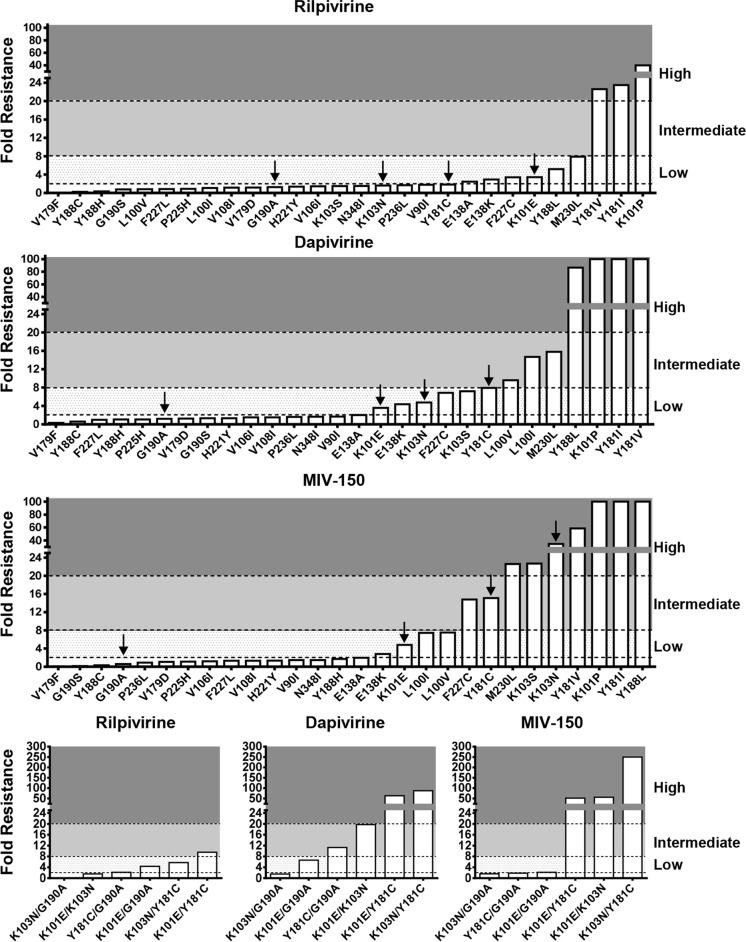
Due to their extensive use in LMIC, there have been significant increases in acquired NNRTI drug resistance, and consequently, the proportion of newly infected patients with transmitted drug resistance has also increased (8, 9). In this regard, four NNRTI resistance mutations—K101E, K103N, Y181C, and G190A—account for >80% of NNRTI-associated transmitted drug resistance in all regions and subtypes (10). Currently, it is unknown whether the NNRTIs used in prevention strategies (e.g., DPV, RPV, or MIV-150) will prevent infection of circulating NNRTI-resistant HIV-1 variants. Importantly, and relevant to this study, there is also a paucity of information in regard to the resistance and cross-resistance profiles of DPV and MIV-150. To address these important knowledge gaps, we constructed by site-directed mutagenesis 28 subtype B HIV-1LAI infectious viruses containing single NNRTI resistance mutations spanning 17 different codons (V90I, L100I/V, K101E/P, K103N/S, V106I, V108I, E138A/K, V179D/F, G190A/S, I181C/I/V, Y188C/H/L, H221Y, P225H, F227C/L, M230L, P236L, and N348I). We also constructed six subtype B HIV-1LAI infectious viruses containing two NNRTI resistance mutations (K101E and G190A [K101E/G190A], K101E/K103N, K101E/Y181C, K103N/G190A, K103N/Y181C, and Y181C/G190A). Drug susceptibility in a single cycle assay using TZM-bl cells was determined for RPV (Selleckchem, TX, USA), DPV (Selleckchem, TX, USA), and MIV-150 (Cayman Chemical Company, MI, USA) as described previously (11, 12). Low-, intermediate-, and high-level resistance was defined as 2- to 8-fold, 8- to 20-fold, and >20-fold changes in drug susceptibility, respectively, compared to the wild-type (WT) virus. Of the three NNRTIs studied, RPV exhibited the best antiviral activity across the panel of mutant viruses tested and retained full sensitivity against 19 of 28 variants containing a single substitution and 2 of 6 variants containing double substitutions (Table 1 and Fig. 1). The E138A/K, F227C, K101E, Y188L, M230L, K101E/G190A, and K103N/Y181C substitutions conferred low-level RPV resistance, while the Y181I/V and K101P substitutions conferred high-level resistance. The RPV resistance profile reported in this study is consistent with those previously published (13, 14). In contrast to RPV, DPV retained activity against only 15 of the 28 viruses containing a single substitution and 1 of 6 viruses containing double substitutions (Table 1 and Fig. 1). The K101E, E138K, K103N/S, F227C, Y181C, and K101E/G190A substitutions conferred low-level resistance to DPV, whereas the L100I/V, M230L, K101E/K103N, and Y181C/G190A substitutions and the Y188L, K101P, Y181I/V, K101E/Y181C, and K103N/Y181C substitutions were found to confer intermediate and high-level resistance, respectively. The DPV cross-resistance profile reported in our study is consistent with prior in vitro studies of DPV resistance selection and cross-resistance profiling (15–17). Additionally, Penrose et al. recently reported that there was frequent cross-resistance to DPV in subtype C-infected individuals after first-line therapy failure and reported that the L100I and K103N substitutions were significantly more frequent in samples with >500-fold resistance to DPV compared to samples with ≤500-fold resistance (18). However, the limitation of this study (18) is that each clinical isolate contained on average three NNRTI resistance mutations, making it difficult to identify the genetic determinants for resistance. Similar to DPV, MIV-150 was also found to be active against only 15 of the HIV-1 variants containing single NNRTI substitutions tested and 2 of 6 variants containing two substitutions tested. However, high-level resistance was more frequently observed for MIV-150 than for either DPV or RPV (Table 1 and Fig. 1). Notably, the M230L, K103S, K103N, Y181V, K101P, Y181I, Y188L, K101E/K103N, K101E/Y181C, and K103N/Y181C substitutions all conferred high-level resistance. The F227C and Y181C substitutions and the L100L/V, K101E, and K101E/G190A substitutions were found to confer intermediate- and low-level MIV-150 resistance, respectively (Table 1 and Fig. 1). To our knowledge, this is the first study to define in detail the cross-resistance profile for MIV-150, although one prior study identified different combinations of E138K, Y181I, Y181C, K103N, L100I, or K101E in simian immunodeficiency viruses expressing HIV reverse transcriptase (SHIV-RT viruses) exposed to MIV-150 in rhesus macaques, although no phenotypic data were provided (19). Additionally, prior studies have reported on the resistance profiles of the MIV-150 analogs, namely, MIV-160 and MIV-170 (16, 17).
TABLE 1.
Susceptibility of HIV-1 containing single or double NNRTI resistance mutations to RPV, DPV, and MIV-150
| Virus | RPV |
DPV |
MIV-150 |
|||
|---|---|---|---|---|---|---|
| EC50 (nM)a | Fold Rb (P value) | EC50 (nM) | Fold R (P value) | EC50 (nM) | Fold R (P value) | |
| WT | 0.33 ± 0.13 | 0.64 ± 0.13 | 0.68 ± 0.09 | |||
| V90I | 0.59 ± 0.13 | 1.8 | 1.09 ± 0.47 | 1.7 | 0.98 ± 0.14 | 1.4 |
| L100I | 0.36 ± 0.13 | 1.1 | 9.36 ± 1.37 | 14.7 (<0.01) | 5.03 ± 1.35 | 7.4 (<0.01) |
| L100V | 0.27 ± 0.13 | 0.8 | 6.15 ± 1.79 | 9.6 (<0.01) | 5.06 ± 2.42 | 7.5 (0.02) |
| K101E | 1.12 ± 0.24 | 3.5 (<0.01) | 2.29 ± 0.68 | 3.6 (<0.01) | 3.25 ± 0.75 | 4.8 (<0.01) |
| K101P | 13.10 ± 1.70 | 40.1 (<0.01) | ≥62.5 | ≥100 (<0.01) | ≥62.5 | ≥100 (<0.01) |
| K103N | 0.53 ± 0.11 | 1.6 | 3.03 ± 0.47 | 4.8 (<0.01) | 23.40 ± 2.81 | 34.6 (<0.01) |
| K103S | 0.49 ± 0.11 | 1.5 | 4.61 ± 0.43 | 7.2 (<0.01) | 15.30 ± 2.03 | 22.7 (<0.01) |
| V106I | 0.48 ± 0.12 | 1.5 | 0.94 ± 0.14 | 1.5 | 0.78 ± 0.11 | 1.20 |
| V108I | 0.38 ± 0.01 | 1.2 | 0.96 ± 0.20 | 1.5 | 0.89 ± 0.15 | 1.3 |
| E138A | 0.80 ± 0.41 | 2.5 (0.06) | 1.29 ± 0.33 | 2.0 (0.02) | 1.32 ± 0.23 | 2.0 (0.01) |
| E138K | 0.96 ± 0.18 | 2.9 (<0.01) | 2.79 ± 0.70 | 4.4 (<0.01) | 1.89 ± 0.17 | 2.8 (<0.01) |
| V179D | 0.39 ± 0.15 | 1.2 | 0.81 ± 0.33 | 1.3 | 0.73 ± 0.35 | 1.1 |
| V179F | 0.003 ± 0.002 | 0.01 (>0.05) | 0.20 ± 0.01 | 0.3 (<0.01) | 0.03 ± 0.01 | 0.1 |
| G190A | 0.42 ± 0.03 | 1.3 | 0.77 ± 0.15 | 1.2 | 0.40 ± 0.09 | 0.6 |
| G190S | 0.25 ± 0.17 | 0.8 | 0.84 ± 0.01 | 1.3 | 0.09 ± 0.01 | 0.1 |
| Y181C | 0.60 ± 0.25 | 1.8 | 5.06 ± 1.50 | 7.9 (<0.01) | 10.20 ± 1.60 | 15.1 (<0.01) |
| Y181I | 7.68 ± 0.78 | 23.5 (<0.01) | ≥62.5 | ≥100 (<0.01) | ≥62.5 | ≥100 (<0.01) |
| Y181V | 7.36 ± 1.20 | 22.6 (<0.01) | ≥62.5 | ≥100 (<0.01) | 39.60 ± 4.10 | 58.6 (<0.01) |
| Y188C | 0.08 ± 0.04 | 0.3 (>0.05) | 0.39 ± 0.20 | 0.6 | 0.21 ± 0.05 | 0.3 (>0.05) |
| Y188H | 0.11 ± 0.04 | 0.3 (>0.05) | 0.69 ± 0.33 | 1.1 | 1.16 ± 0.48 | 1.7 |
| Y188L | 1.69 ± 0.23 | 5.2 (<0.01) | 55.30 ± 6.53 | 86.7 (<0.01) | ≥62.5 | ≥100 (<0.01) |
| H221Y | 0.45 ± 0.20 | 1.37 | 0.85 ± 0.20 | 1.3 | 0.91 ± 0.19 | 1.3 |
| P225H | 0.29 ± 0.17 | 0.9 | 0.69 ± 0.25 | 1.1 | 0.76 ± 0.32 | 1.1 |
| F227C | 1.11 ± 0.27 | 3.40 (0.01) | 4.37 ± 0.78 | 6.9 (<0.01) | 10.01 ± 3.16 | 14.8 (<0.01) |
| F227L | 0.28 ± 0.26 | 0.9 | 0.61 ± 0.19 | 1.0 | 0.88 ± 0.31 | 1.3 |
| M230L | 2.58 ± 1.13 | 7.9 (0.01) | 10.10 ± 0.38 | 15.8 (<0.01) | 15.30 ± 2.87 | 22.6 (<0.01) |
| P236L | 0.55 ± 0.10 | 1.7 | 1.00 ± 0.18 | 1.6 | 0.63 ± 0.07 | 0.9 |
| N348I | 0.50 ± 0.13 | 1.5 | 1.07 ± 0.47 | 1.7 | 0.99 ± 0.34 | 1.5 |
| K101E/G190A | 1.42 ± 0.22 | 4.4 (<0.01) | 4.22 ± 0.31 | 6.6 (<0.01) | 1.45 ± 0.21 | 2.2 (<0.01) |
| K101E/K103N | 0.50 ± 0.07 | 1.5 | 12.60 ± 1.84 | 19.7 (<0.01) | 38.10 ± 7.38 | 56.3 (<0.01) |
| K101E/Y181C | 3.12 ± 0.48 | 9.5 (<0.01) | 40.70 ± 9.86 | 63.8 (<0.01) | 35.50 ± 13.80 | 52.5 (<0.01) |
| K103N/G190A | 0.02 ± 0.01 | 0.06 (<0.01) | 0.98 ± 0.17 | 1.5 | 1.07 ± 0.25 | 1.6 |
| K103N/Y181C | 1.87 ± 0.34 | 5.7 (<0.01) | 56.20 ± 3.06 | 88.0 (<0.01) | 182.00 ± 51.20 | ≥250 (<0.01) |
| Y181C/G190A | 0.70 ± 0.10 | 2.1 (<0.01) | 7.22 ± 2.27 | 11.3 (<0.01) | 1.26 ± 0.39 | 1.9 |
The concentrations of drug required to inhibit viral replication by 50% (EC50s) from three independent experiments. Data reported as means ± standard deviations from at least three independent experiments.
Mean fold change in the EC50 of mutant virus versus WT virus. EC50s were compared for statistically significant differences (P value of <0.05) using a nonpaired, two-sample equal-variance (homoscedastic) test.
FIG 1.
NNRTI cross-resistance profiles for RPV, DPV, and MIV-150. Low-, intermediate- and high-level resistance was defined as 2- to 8-fold, 8- to 20-fold, and >20-fold changes in drug susceptibility compared to the WT virus. The black arrows indicate the four most commonly transmitted drug resistance mutations, G190A, K101E, Y181C, and K103N.
Recently, we reported that an E138A substitution occurs more frequently in subtype C sequences (range, 5.9 to 7.5%) than subtype B sequences (range, 0 to 2.3%) from treatment-naive individuals (P < 0.01) (11). Because E138A in subtype C HIV-1 decreases RPV susceptibility, we previously proposed that this polymorphism may impact prevention (and treatment) strategies that include RPV in geographic areas where subtype C infection is prevalent (11). Accordingly, in this study, we synthesized (GenScript, NJ, USA) and cloned into our HIV-1LAI viral vector (as described previously [12]) full-length subtype C RT sequences from two antiretroviral-naive individuals that did not harbor E138A and from six antiretroviral-naive individuals that contained E138A. Phenotypic analyses revealed that 2 of the recombinant viruses that contained E138A conferred low-level resistance (2.4- and 2.0-fold, respectively) to RPV (Table 2). In contrast, four of the six recombinant viruses that contained E138A conferred decreased susceptibility to DPV (range, 2.1- to 4.7-fold) and MIV-150 (range, 1.9- to 3.4-fold) (Table 2). These data highlight that the RT genetic backbone influences, at least to some extent, the ability of E138A to decrease NNRTI susceptibility and suggest that the low-level resistance conferred by E138A is unlikely to impact RPV, DPV, or MIV-150 activity.
TABLE 2.
Susceptibility of recombinant viruses containing full-length patient-derived WT subtype C RT sequences with and without E138A to RPV, DPV, and MIV-150
| Virus accession no.a | E138A present | RPV |
DPV |
MIV-150 |
|||
|---|---|---|---|---|---|---|---|
| EC50 (μM)b | Fold Rc (P value) | EC50 (μM) | Fold R (P value) | EC50 (μM) | Fold R (P value) | ||
| AF361879 | No | 0.30 ± 0.03 | 0.68 ± 0.10 | 0.56 ± 0.10 | |||
| AY043176 | No | 0.24 ± 0.04 | 0.49 ± 0.02 | 0.36 ± 0.05 | |||
| Avgd | No | 0.27 ± 0.04 | 0.58 ± 0.13 | 0.46 ± 0.13 | |||
| DQ351238 | Yes | 0.40 ± 0.12 | 1.5 | 1.20 ± 0.33 | 2.1 (<0.01) | 1.40 ± 0.59 | 3.1 (<0.01) |
| AY901981 | Yes | 0.64 ± 0.14 | 2.4 (<0.01) | 1.80 ± 0.51 | 3.0 (<0.01) | 1.50 ± 0.23 | 3.2 (<0.01) |
| AF443097 | Yes | 0.41 ± 0.12 | 1.5 | 1.30 ± 0.50 | 2.3 (<0.01) | 0.86 ± 0.16 | 1.9 (<0.01) |
| AY253303 | Yes | 0.23 ± 0.04 | 0.9 | 0.83 ± 0.13 | 1.4 | 0.60 ± 0.05 | 1.3 |
| AY734559 | Yes | 0.36 ± 0.14 | 1.3 | 0.77 ± 0.26 | 1.3 | 0.63 ± 0.15 | 1.4 |
| FJ199637 | Yes | 0.54 ± 0.07 | 2.0 (<0.01) | 2.70 ± 0.19 | 4.7 (<0.01) | 1.60 ± 0.31 | 3.4 (<0.01) |
The GenBank accession number or sequence identifier for the full-length subtype C RT gene for the virus.
The concentrations of drug required to inhibit viral replication by 50% (EC50s) are reported as means ± standard deviations from at least three independent experiments.
Mean fold change in the EC50 of WT virus with the E138A substitution vs WT virus. EC50s from three independent experiments were compared for statistically significant differences (P value of <0.05) using a nonpaired, two-sample equal-variance (homoscedastic) test.
In summary, this study provides the first detailed insights into the antiviral activity of RPV, DPV, and MIV-150 against a broad panel of recombinant viruses containing substitutions that are known to decrease NNRTI susceptibility. We also evaluated their activity against WT subtype C RTs that contained E138A. The pharmacokinetics of the long-acting RPV formulation has been investigated in healthy individuals in two different studies (20, 21). In cervicovaginal fluid (CVL), RPV concentrations at day 28 postadministration were 12, 15, and 98 ng/ml (68, 107, and 232 nM, respectively) following injected doses of 300, 600, and 1,200 mg, respectively. In the rectal fluid (RF), RPV concentrations at day 28 postadministration were 11.9 ng/ml (32 nM), following a 600- mg injection. The RPV concentrations in the CVL and RF exceed the concentrations of drug required to inhibit viral replication by 50% (EC50s) for all of the NNRTI-resistant variants listed in Table 1, suggesting that RPV may prevent infection from transmitted NNRTI-resistant viruses. With regard to DPV, pharmacokinetic studies have shown that the vaginal fluid concentration on day 28 of DPV ring use ranged from 14.9 to 65 μg/ml (45 to 198 μM) (22, 23). These concentrations far exceed the reported EC50s for the WT and mutant HIV-1 in Tables 1 and 2, suggesting that the ring would effectively inhibit replication of all the resistant viruses tested. (Note that exact EC50s for DPV for the K101P and Y181I/V HIV-1 viruses could not be determined, as they exceeded the highest concentration of drug used in the assay.) In contrast, pharmacokinetic studies of PC-1005 (MIV-150 and zinc acetate in a carrageenan gel) yielded concentrations of MIV-150 in cervicovaginal lavage fluid samples ranging from ∼100 to 170 nM (5). In this regard, it is questionable whether these concentrations will effectively block the mutant viruses which exhibited high-level MIV-150 resistance (K101P, K103N/S, Y181C/I/V, F227C, M230L, K101E/K103N, K101E/Y181C, and K103N/Y181C) and for which EC50s range from 10 to 100 nM. Importantly, both K103N and Y181C, which are frequently associated with transmitted NNRTI resistance, fall into this category.
ACKNOWLEDGMENTS
This work was supported by a National Institutes of Health award (R01AI081571 and R01GM068406) to N.S.-C.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. 1992. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 2.Sluis-Cremer N. 2014. The emerging profile of cross-resistance among the nonnucleoside HIV-1 reverse transcriptase inhibitors. Viruses 6:2960–2973. doi: 10.3390/v6082960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, Siva S, Bekker LG, Jeenarain N, Gaffoor Z, Martinson F, Makanani B, Pather A, Naidoo L, Husnik M, Richardson BA, Parikh UM, Mellors JW, Marzinke MA, Hendrix CW, van der Straten A, Ramjee G, Chirenje ZM, Nakabiito C, Taha TE, Jones J, Mayo A, Scheckter R, Berthiaume J, Livant E, Jacobson C, Ndase P, White R, Patterson K, Germuga D, Galaska B, Bunge K, Singh D, Szydlo DW, Montgomery ET, Mensch BS, Torjesen K, Grossman CI, Chakhtoura N, Nel A, Rosenberg Z, McGowan I, Hillier S, MTN-020–ASPIRE Study Team. 2016. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 375:2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, Van Roey J, Parys W, Vangeneugden T, Van Baelen B, Rosenberg Z, Ring Study Team. 2016. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 375:2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 5.Friedland BA, Hoesley CJ, Plagianos M, Hoskin E, Zhang S, Teleshova N, Alami M, Novak L, Kleinbeck KR, Katzen LL, Zydowsky TM, Fernández-Romero JA, Creasy GW. 2016. First-in-human trial of MIV-150 and zinc acetate coformulated in a carrageenan gel: safety, pharmacokinetics, acceptability, adherence, and pharmacodynamics. J Acquir Immune Defic Syndr 73:489–496. doi: 10.1097/QAI.0000000000001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van't Klooster G, Hoeben E, Borghys H, Looszova A, Bouche MP, van Velsen F, Baert L. 2010. Pharmacokinetics and disposition of rilpivirine (TMC278) nanosuspension as a long-acting injectable antiretroviral formulation. Antimicrob Agents Chemother 54:2042–2050. doi: 10.1128/AAC.01529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spreen WR, Margolis DA, Pottage JC Jr. 2013. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frentz D, Boucher CA, van de Vijver DA. 2012. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev 14:17–27. [PubMed] [Google Scholar]
- 9.Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DH, Gregson J, Sawyer AW, Hamers RL, Ndembi N, Pillay D, Bertagnolio S. 2012. Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: a global collaborative study and meta-regression analysis. Lancet 380:1250–1258. doi: 10.1016/S0140-6736(12)61038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhee SY, Blanco JL, Jordan MR, Taylor J, Lemey P, Varghese V, Hamers RL, Bertagnolio S, Rinke de Wit TF, Aghokeng AF, Albert J, Avi R, Avila-Rios S, Bessong PO, Brooks JI, Boucher CA, Brumme ZL, Busch MP, Bussmann H, Chaix ML, Chin BS, D'Aquin TT, De Gascun CF, Derache A, Descamps D, Deshpande AK, Djoko CF, Eshleman SH, Fleury H, Frange P, Fujisaki S, Harrigan PR, Hattori J, Holguin A, Hunt GM, Ichimura H, Kaleebu P, Katzenstein D, Kiertiburanakul S, Kim JH, Kim SS, Li Y, Lutsar I, Morris L, Ndembi N, Ng KP, Paranjape RS, Peeters M, Poljak M, Price MA, Ragonnet-Cronin ML, et al. 2015. Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted HIV-1 drug resistance: an individual-patient- and sequence-level meta-analysis. PLoS Med 12:e1001810. doi: 10.1371/journal.pmed.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sluis-Cremer N, Jordan MR, Huber K, Wallis CL, Bertagnolio S, Mellors JW, Parkin NT, Harrigan PR. 2014. E138A in HIV-1 reverse transcriptase is more common in subtype C than B: implications for rilpivirine use in resource-limited settings. Antiviral Res 107:31–34. doi: 10.1016/j.antiviral.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brehm JH, Koontz DL, Wallis CL, Shutt KA, Sanne I, Wood R, McIntyre JA, Stevens WS, Sluis-Cremer N, Mellors JW, CIPRA-SA Project 1 Study Team. 2012. Frequent emergence of N348I in HIV-1 subtype C reverse transcriptase with failure of initial therapy reduces susceptibility to reverse-transcriptase inhibitors. Clin Infect Dis 55:737–745. doi: 10.1093/cid/cis501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azijn H, Tirry I, Vingerhoets J, de Béthune MP, Kraus G, Boven K, Jochmans D, Van Craenenbroeck E, Picchio G, Rimsky LT. 2010. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basson AE, Rhee SY, Parry CM, El-Khatib Z, Charalambous S, De Oliveira T, Pillay D, Hoffmann C, Katzenstein D, Shafer RW, Morris L. 2015. Impact of drug resistance-associated amino acid changes in HIV-1 subtype C on susceptibility to newer nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 59:960–971. doi: 10.1128/AAC.04215-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schader SM, Oliveira M, Ibanescu RI, Moisi D, Colby-Germinario SP, Wainberg MA. 2012. In vitro resistance profile of the candidate HIV-1 microbicide drug dapivirine. Antimicrob Agents Chemother 56:751–756. doi: 10.1128/AAC.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selhorst P, Vazquez AC, Terrazas-Aranda K, Michiels J, Vereecken K, Heyndrickx L, Weber J, Quiñones-Mateu ME, Ariën KK, Vanham G. 2011. Human immunodeficiency virus type 1 resistance or cross-resistance to nonnucleoside reverse transcriptase inhibitors currently under development as microbicides. Antimicrob Agents Chemother 55:1403–1413. doi: 10.1128/AAC.01426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ariën KK, Venkatraj M, Michiels J, Joossens J, Vereecken K, Van der Veken P, Heeres J, De Winter H, Heyndrickx L, Augustyns K, Vanham G. 2016. Resistance and cross-resistance profile of the diaryltriazine NNRTI and candidate microbicide UAMC01398. J Antimicrob Chemother 71:1159–1168. doi: 10.1093/jac/dkv501. [DOI] [PubMed] [Google Scholar]
- 18.Penrose KJ, Wallis CL, Brumme CJ, Hamanishi KA, Gordon KC, Viana RV, Harrigan PR, Mellors JW, Parikh UM. 2017. Frequent cross-resistance to dapivirine in HIV-1 subtype C-infected individuals after first-line antiretroviral therapy failure in South Africa. Antimicrob Agents Chemother 61:e01805-16. doi: 10.1128/AAC.01805-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu M, Keele BF, Aravantinou M, Krawczyk N, Seidor S, Abraham CJ, Zhang S, Rodriguez A, Kizima L, Derby N, Jean-Pierre N, Mizenina O, Gettie A, Grasperge B, Blanchard J, Piatak MJ Jr, Lifson JD, Fernández-Romero JA, Zydowsky TM, Robbiani M. 2014. Exposure to MIV-150 from a high-dose intravaginal ring results in limited emergence of drug resistance mutations in SHIV-RT infected rhesus macaques. PLoS One 9:e89300. doi: 10.1371/journal.pone.0089300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson AG, Else LJ, Mesquita PM, Egan D, Back DJ, Karolia Z, Ringner-Nackter L, Higgs CJ, Herold BC, Gazzard BG, Boffito M. 2014. A compartmental pharmacokinetic evaluation of long-acting rilpivirine in HIV-negative volunteers for pre-exposure prophylaxis. Clin Pharmacol Ther 96:314–323. doi: 10.1038/clpt.2014.118. [DOI] [PubMed] [Google Scholar]
- 21.McGowan I, Dezzutti CS, Siegel A, Engstrom J, Nikiforov A, Duffill K, Shetler C, Richardson-Harman N, Abebe K, Back D, Else L, Egan D, Khoo S, Egan JE, Stall R, Williams PE, Rehman KK, Adler A, Brand RM, Chen B, Achilles S, Cranston RD. 2016. Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): an open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 3:e569–e578. doi: 10.1016/S2352-3018(16)30113-8. [DOI] [PubMed] [Google Scholar]
- 22.Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N. 2014. A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS 28:1479–1487. doi: 10.1097/QAD.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 23.Chen BA, Panther L, Marzinke MA, Hendrix CW, Hoesley CJ, van der Straten A, Husnik MJ, Soto-Torres L, Nel A, Johnson S, Richardson-Harman N, Rabe LK, Dezzutti CS. 2015. Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: a double-blind randomized trial. J Acquir Immune Defic Syndr 70:242–249. doi: 10.1097/QAI.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]