ABSTRACT
Amoxicillin-clavulanate (A/C) is currently the most effective oral antimicrobial in treating children with acute otitis media (AOM), but the standard dosage of 90 mg amoxicillin/6.4 mg clavulanate/kg of body weight/day commonly causes diarrhea. We examined whether an A/C formulation containing lower concentrations of clavulanate would result in less diarrhea while maintaining plasma levels of amoxicillin and clavulanate adequate to eradicate middle-ear pathogens and to achieve clinical success. We conducted an open-label study in children with AOM who were 6 to 23 months of age. In phase 1, we treated 40 children with a reduced-clavulanate A/C formulation providing 90 mg amoxicillin/3.2 mg clavulanate/kg/day for 10 days. In phase 2, we treated 72 children with the same formulation at a dosage of 80 mg amoxicillin/2.85 mg clavulanate/kg/day for 10 days. We compared the rates of protocol-defined diarrhea (PDD), diaper dermatitis, and AOM clinical response in these children with rates we had reported in children who received the standard A/C regimen, and we obtained plasma levels of amoxicillin and clavulanate at various time points. Outcomes in phase 1 children and in children who had received the standard regimen did not differ significantly. Rates of PDD in children receiving phase 2 and standard regimens were 17% and 26%, respectively (P = 0.10). The corresponding rates of diaper dermatitis were 21% and 33% (P = 0.04) and of AOM treatment failure were 12% and 16% (P = 0.44). Symptomatic responses did not differ significantly between regimens; both gave clavulanate levels sufficient to inhibit β-lactamase activity. In young children with AOM, clavulanate dosages lower than those currently used may be associated with fewer side effects without reducing clinical efficacy. (This study has been registered at ClinicalTrials.gov under registration no. NCT02630992.)
KEYWORDS: acute otitis media, amoxicillin-clavulanate, antimicrobial treatment
INTRODUCTION
Next to the common cold, acute otitis media (AOM) is the most frequently diagnosed illness in children in the United States (1) and the most commonly cited indication for antimicrobial treatment (2). Findings of two recent placebo-controlled trials support routine antimicrobial treatment of AOM in very young children. In those trials, affected children under 3 years of age who received antimicrobial treatment for 7 or 10 days experienced more-favorable outcomes than those who received placebo (3, 4). In both studies, amoxicillin-clavulanate was used as the active treatment—albeit in different dosing regimens—because it had been previously shown to have the highest rates of eradication of AOM pathogens, with high resultant rates of clinical cure. The efficacy of A/C is attributable in part to inactivation, by the clavulanate component, of β-lactamases produced by Haemophilus influenzae and Moraxella catarrhalis, which, together with Streptococcus pneumoniae, comprise the main causative bacteria in AOM.
Formulations of A/C have used various ratios of the two components, ranging from 4:1 to 14:1 (5, 6). Over time, the trend has been to reduce the total dose of clavulanate in order to lower the incidence of diarrhea and, in the latest formulation, to increase the dose of amoxicillin to improve efficacy against nonsusceptible S. pneumoniae, while maintaining the dose of clavulanate to preserve efficacy against H. influenzae and M. catarrhalis (7). Although the lowest dose of clavulanate required to inactivate β-lactamases has not been determined, the currently available formulation (600 mg amoxicillin/42.9 mg clavulanate/5 ml) at the recommended dosing regimen for young children (90 mg amoxicillin/6.4 mg clavulanate/kg of body weight/day administered in two divided doses for 10 days) (8) provides children with almost twice the clavulanate dose (in milligrams per kilogram per day) currently in use for adults.
The relatively high incidence of diarrhea associated with A/C treatment constitutes a troublesome side effect and may occasion delays in the return of children to day care and the return of parents to work. Rates of diarrhea reported in the aforementioned trials ranged between 25% and 48% (3, 4). Most recently, in children under age 2 years with initially observed episodes of AOM who were randomized to receive A/C for either 10 days or 5 days, protocol-defined diarrhea (PDD) occurred in 70 (27%) of 257 children assigned to the 10-day treatment group (9). Although diarrhea may be caused also by the amoxicillin component (10, 11), evidence points to the clavulanate component as mainly responsible. A reduction in the total effective dose of clavulanate and in the number of daily doses has been associated with reduced rates of diarrhea (5). Accordingly, we examined, in children aged 6 to 23 months with AOM, whether a pediatric formulation of A/C containing a reduced concentration of clavulanate would result in lower rates of diarrhea while maintaining plasma levels of A/C sufficient to eradicate H. influenzae and M. catarrhalis from the middle ear.
RESULTS
We enrolled 40 children in phase 1 and 72 children in phase 2. Demographic and clinical characteristics of these children, compared with those of historical controls, are shown in Table 1; no differences, except as noted, were apparent.
TABLE 1.
Selected demographic and clinical characteristics of children according to amoxicillin-clavulanate treatment regimen
| Characteristic | Values for indicated treatment group and A/C treatment regimenh |
||
|---|---|---|---|
| Historical controls (90 mg amoxicillin/6.4 mg clavulanate/kg/day × 10 days) (n = 401) | Phase 1 children (90 mg amoxicillin/3.2 mg clavulanate/kg/day × 10 days) (n = 40) | Phase 2 children(80 mg amoxicillin/2.85 mg clavulanate/kg/day × 10 days) (n = 72) | |
| Age at entry—no. (%) of children | |||
| 6–11 mo | 212 (53) | 23 (58) | 36 (50) |
| 12–23 mo | 189 (47) | 17 (42) | 36 (50) |
| Sex—no. (%) of children | |||
| Female | 184 (46) | 15 (38) | 30 (42) |
| Male | 217 (54) | 25 (62) | 42 (58) |
| Race—no. (%) of childrena | |||
| White | 182 (45)b | 10 (25) | 7 (10) |
| Black/African-American | 172 (43) | 24 (60) | 50 (69) |
| Other | 47 (12) | 6 (15) | 15 (21) |
| Ethnicity—no. (%) of childrena | |||
| Not Hispanic or Latino | 356 (89) | 33 (82) | 66 (92) |
| Hispanic or Latino | 45 (11) | 6 (15) | 6 (8) |
| Unknown | 1 (2) | ||
| Maternal level of education—no. (%) of childrenc | |||
| Below high school graduate | 55 (14) | 4 (10) | 3 (4) |
| High school graduate or equivalent | 240 (60) | 24 (60) | 56 (78) |
| College graduate | 106 (26) | 12 (30) | 12 (17) |
| Unknown | 1 (1) | ||
| Type of health insurance—no. (%) of children | |||
| Private | 127 (32) | 14 (35) | 20 (28) |
| Public | 269 (67) | 26 (65) | 52 (72) |
| None | 5 (1) | ||
| Exposure to other children—no. (%) of childrend | |||
| No | 184 (46) | 16 (40) | 31 (43) |
| Yes | 217 (54) | 24 (60) | 41 (57) |
| AOM-SOS score at entry—no. (%) of childrene | |||
| 0–2 | 11 (3) | ||
| 3–5 | 128 (32) | 19 (48) | 21 (29) |
| 6–8 | 196 (49) | 16 (40) | 34 (47) |
| 9–10 | 66 (16) | 5 (12) | 17 (24) |
| AOM-SOS score at entry—mean (SD) | 6.5 (2) | 6.1 (2) | 6.6 (2) |
| Laterality of acute otitis media—no. (%) of childrenf | |||
| Unilateral | 205 (51) | 19 (48) | 46 (64) |
| Bilateral | 196 (49) | 21 (52) | 26 (36) |
| Degree of tympanic membrane bulging in worse ear—no. (%) of childreng | |||
| Slight | 77 (19) | 2 (5) | 7 (10) |
| Moderate or marked | 324 (81) | 38 (95) | 65 (90) |
Race and ethnicity were reported by the children's parents.
For comparisons of phase 1 children to historical controls, white to nonwhite, P = 0.02. For comparisons of phase 2 children to historical controls, white to nonwhite, P = <0.001.
For comparisons of phase 2 children to historical controls, P = 0.006.
Exposure to other children was defined as exposure to at least three children for at least 10 h per week.
The Acute Otitis Media Severity of Symptoms (AOM-SOS) scale consists of five discrete items—tugging of ears, crying, irritability, difficulty sleeping, and fever. Parents are asked to rate these symptoms, in comparison with the child's usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2. Thus, total scores range from 0 to 10, with higher scores indicating greater severity of symptoms.
For comparisons of phase 2 children to historical controls, P = 0.06.
For comparisons of phase 2 children to historical controls, P = 0.08.
A/C, amoxicillin-clavulanate.
Outcomes.
The data are summarized in Table 2 and Fig. 1.
TABLE 2.
Adverse side effects, clinical efficacies, and symptomatic responses according to amoxicillin-clavulanate treatment regimena
| Outcome measure | Values for indicated treatment group and A/C treatment regimen |
|||
|---|---|---|---|---|
| Historical controls (90 amoxicillin/6.4 mg clavulanate/kg/day × 10 days) (n = 401) | Phase 1 children (90 amoxicillin/3.2 mg clavulanate/kg/day × 10 days) (n = 40) | Phase 2 children (80 amoxicillin/2.85 mg clavulanate/kg/day × 10 days) (n = 72) | P value | |
| Adverse effects of study medication | ||||
| Occurrence of PDDb—no. of children (%) | 104 (26) | 10 (25) | 12 (17) | 0.10c |
| No. days until 15% of parents reported PDD | 4 days | 4 days | 6 days | 0.16d |
| Peak time with PDD; no. of children with PDD/total no. of children (%) | Day 6; 64/394 (16) | Days 5 & 6; 8/39 (20) | Day 6; 12/68 (18) | N/A |
| No. of days with PDD—mean ± SD (no. of children with data) | 1.1 ± 2.2 (401) | 1.3 ± 2.6 (39) | 1.1 ± 2.7 (68) | 0.88e |
| Occurrence of diaper dermatitis—no. of children (%) | 134 (33) | 10 (25) | 15 (21) | 0.04c |
| Discontinued study medication because of diarrhea and/or diaper dermatitisf—no. of children (%) | 39 (10) | 2 (5) | 1 (1) | <0.05c |
| Discontinued temporarily | 32 (8) | 2 (5) | 1 (1) | |
| Discontinued permanently | 7 (2) | 0 | 0 | |
| Clinical failure—proportion of children (%) (95% CI) | 62/380 (16) (13 to 20) | 4/35 (11) (1 to 22) | 8/64 (12) (4 to 21) | 0.44c |
| AOM-SOS score from day 1 to day 7—mean ± SD (no. of children) | ||||
| All children | 2.4 ± 1.6 (367) | 2.7 ± 1.4 (36) | 2.2 ± 1.7 (59) | 0.32g |
| Children who experienced clinical success | 2.4 ± 1.6 (304) | 2.5 ± 1.2 (29) | 2.3 ± 1.7 (49) | 0.52g |
| Children who experienced clinical failure | 2.7 ± 1.7 (56) | 4.3 ± 1.8 (4) | 1.7 ± 1.8 (7) | 0.58g |
| Decrease of >50% in AOM-SOS score from baseline to the end-of-treatment assessment—no. of children with decrease/total no. of children (%)h | ||||
| All children | 320/362 (88) | 30/35 (86) | 57/64 (89) | 0.88c |
| Children who experienced clinical success | 280/308 (91) | 28/31 (90) | 52/56 (93) | 0.66c |
| Children who experienced clinical failure | 40/54 (74) | 2/4 (50) | 5/8 (62) | 0.66c |
| Parents' stated level of satisfaction with child's therapy at end-of-treatment visit—mean ± SD (no. of children)i | 4.47 ± 0.88 (371) | 4.69 ± 0.72 (35) | 4.75 ± 0.76 (4) | 0.02e |
Data were missing for some children for some analyses. CI, confidence interval. N/A, not applicable. SD, standard deviation.
PDD, protocol-defined diarrhea.
Comparison of historical controls to phase 2 children, logistic regression.
Comparison of historical controls to phase 2 children, log rank test.
Comparison of historical controls to phase 2 children, t test.
Study medication was discontinued only because of diaper dermatitis in 2 children.
Comparison of historical controls to phase 2 children, generalized estimated equations.
The AOM-SOS scale consists of five discrete items—tugging of ears, crying, irritability, difficulty sleeping, and fever. Parents are asked to rate these symptoms, in comparison with the child's usual state, as “none,” “a little,” or “a lot,” with corresponding scores of 0, 1, and 2. Thus, total scores range from 0 to 10, with higher scores indicating greater severity of symptoms. Data are restricted to children with an AOM-SOS score of ≥3 at enrollment. The cutoff between ≤50% and >50% was based on data from a study of minimal important difference in AOM-SOS scores (12).
Levels of satisfaction were scored on the following scale: 1, very dissatisfied; 2, somewhat dissatisfied; 3, neutral; 4, somewhat satisfied; 5, very satisfied.
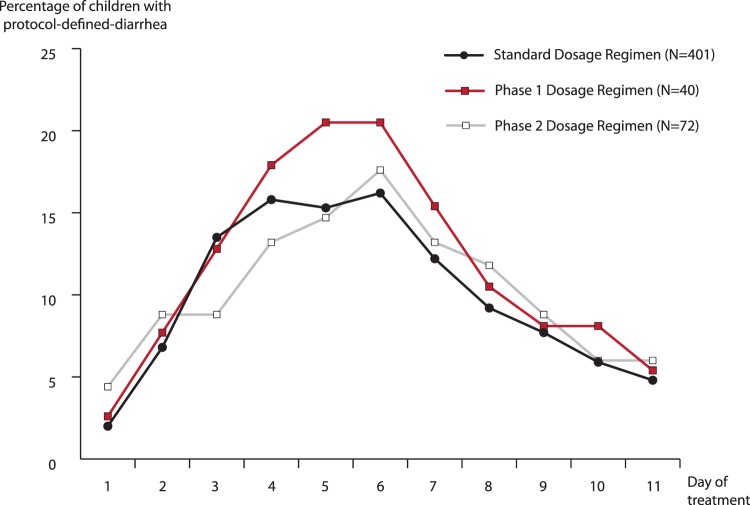
FIG 1.
Percentages of children with protocol-defined diarrhea according to day of treatment and amoxicillin-clavulanate dosage regimen.
Diarrhea and diaper dermatitis.
Compared with the proportion of children who had received the standard A/C formulation and dosing regimen (i.e., the historical controls) who experienced PDD at any time (104/401 [26%]), the proportion of phase 1 children who did so was 10/40 (25%) (P = 0.95) and that of phase 2 children was 12/72 (17%) (P = 0.10). Time-to-event analysis showed no between-group differences in the number of days until 15% of parents reported PDD in their children or in the mean number of days that children experienced PDD. The proportion of children experiencing PDD peaked on or about day 6 in each of the three study groups (Fig. 1). The proportion of phase 2 children who had developed diaper dermatitis that occasioned prescription of an antifungal cream (15/72 [22%]) was lower than the proportion of historical controls who did so (134/401 [33%]) (P = 0.04). PDD and/or diaper dermatitis resulted in discontinuation of study medication in 10%, 5%, and 1% of children who received standard A/C, phase 1, and phase 2 formulation and dosing regimens, respectively.
Clinical failure and symptomatic response.
We found no significant differences between the historical controls, the phase 1 children, and the phase 2 children in the proportions categorized as experiencing clinical failure (16%, 11%, and 12%, respectively). The two-sided 95% confidence interval of the difference between the values from the historical controls and those from the phase 2 children (i.e., 4%) is expressed as −0.06 to 0.14. Key measures of symptomatic response based on parent-recorded acute otitis media severity of symptoms (AOM-SOS) scores did not differ significantly between treatment groups. Within each treatment group, the proportion of children showing a decrease of more than 50% from baseline to the end of treatment was greater in children categorized on the basis of otoscopic findings as having experienced clinical success than in children categorized as having experienced clinical failure. At the end-of-treatment visit, parental satisfaction with their children's treatment was higher among phase 2 children than among historical controls (on a scale of 1 to 5, mean, 4.75 versus 4.47, P = 0.02).
Overall day 7 assessment.
At the day 7 visit, on the basis of AOM-attributable symptoms and otoscopic signs, study clinicians considered discontinuing antimicrobial therapy unsuitable in 11 of 34 children (32%) in phase 1 and 31 of 62 children (50%) in phase 2 (P = 0.15). Irrespective of those judgments, parents were asked to complete the 10-day treatment course. No comparable information was available regarding historical controls.
Pharmacokinetic characteristics.
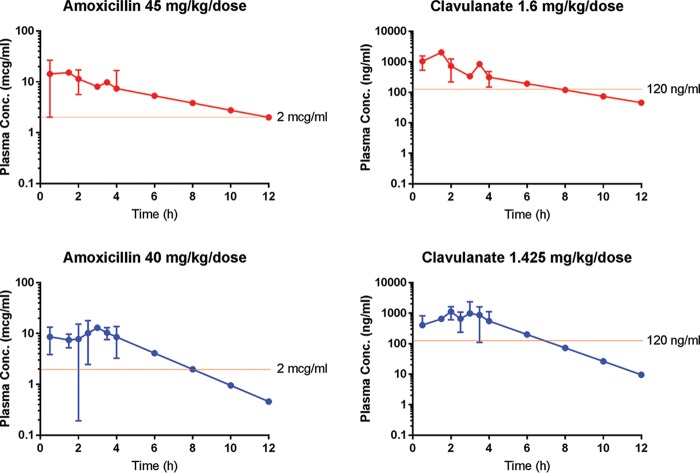
Table 3 shows selected pharmacokinetic findings regarding the standard, phase 1, and phase 2 amoxicillin-clavulanate regimens, and Fig. 2 shows population plasma concentrations of amoxicillin and of clavulanate in relation to time, for children receiving the reduced-clavulanate formulation of A/C at the respective dosing regimens of phase 1 (90 mg amoxicillin/3.2 mg clavulanate/kg of body weight/day) and phase 2 (80 mg amoxicillin/2.85 mg clavulanate/kg/day). At all time points measured, the mean plasma concentration of amoxicillin was substantially higher than 1 μg/ml and, extrapolating the slope of the plasma level time profile beyond 4 h, would have provided MIC levels for S. pneumoniae above 2 μg/ml for 100% of the dosing interval in the case of the phase 1 regimen and for 66% of the dosing interval in the case of the phase 2 regimen—both being above the 40% needed to achieve optimal efficacy against S. pneumoniae.
TABLE 3.
Pharmacokinetic profile following administration of a single dose of standard, phase 1, or phase 2 amoxicillin-clavulanate formulationa
| Parameter | A/C formulation and single-dose size of each A/C component |
|||||
|---|---|---|---|---|---|---|
| Standard formulationb (600 mg amoxicillin/42.9 mg clavulanate/5 ml); amoxicillin, 45 mg/kg/dose | Phase 1 formulationc (600 mg amoxicillin/21.5 mg clavulanate/5 ml); amoxicillin, 45 mg/kg/dose | Phase 2 formulationc (600 mg amoxicillin/21.5 mg clavulanate/5 ml); amoxicillin, 40 mg/kg/dose | Standard formulationb (600 mg amoxicillin/42.9 mg clavulanate/5 ml); clavulanate, 3.2 mg/kg/dose | Phase 1 formulationc (600 mg amoxicillin/21.5 mg clavulanate/5 ml); clavulanate, 1.6 mg/kg/dose | Phase 2 formulationc (600 mg amoxicillin/21.5 mg clavulanate/5 ml); clavulanate, 1.425 mg/kg/dose | |
| Cmax (μg/ml)—mean ± SD | 15.7 ± 7.7 | 15.3 | 13.0 ± 0.5 | 1.7 ± 0.9 | 2.1 | 1.13 ± 0.4 |
| Tmax (h)—mean (range) | 2.0 (1.0–4.0) | 1.5 | 2.9 (2.8–2.9) | 1.1 (1.0–4.0) | 1.5 | 2.0 (1.9–2.1) |
| AUC0–t (μg · h/ml)—mean ± SD | 59.8 ± 20.0 | 77.4 | 55.3 | 4.0 ± 1.9 | 4.9 | 3.8 |
| t1/2 (h)—mean ± SD | 1.4 ± 0.3 | 4.2 | 1.9 | 1.1 ± 0.3 | 2.9 | 1.4 |
| CL/F (liters/h/kg)—mean ± SD | 0.9 ± 0.4 | 0.5 | 0.69 | 1.1 ± 1.1 | 0.31 | 0.37 |
A/C, amoxicillin-clavulanate. Cmax, maximum serum concentration. Tmax, time after administration when the maximum serum concentration was reached. AUC0–t, area under the plasma concentration-time curve from the beginning to the end of the dosing interval (in the present study, 12 h). In the present study, the last actual sample was obtained 4 h after administration of medication, and the AUC from 4 to 12 h after administration was projected using the estimated terminal half-life. t1/2, half-life. CL/F, apparent total clearance of the drug from plasma after oral administration.
Data taken from the Augmentin prescribing information.
Where SD and range are not provided, data were obtained from a single sample or a composite analysis using multiple data points (t1/2; AUC; CL/F).
FIG 2.
Population plasma concentration versus time curves for children receiving the reduced-clavulanate formulation of amoxicillin-clavulanate using various dosage regimens during phase 1 (90/3.2 mg/kg/day) and phase 2 (80/2.85 mg/kg/day). Plasma concentrations (Conc.) for time points 6, 8, 10, and 12 h were extrapolated on the basis of the elimination rate constant calculated from data collected up to 4 h. Data points up to 4 h without a standard deviation bar indicate that the assessment was available for only one child.
A 55% reduction in the clavulanate concentration from the standard formulation to the phase 2 formulation resulted in a reduction of only 33% in the maximum serum concentration (Cmax). The mean plasma clavulanate concentration in phase 2 children exceeded 0.3 μg/ml at all time points beyond 30 min. Both reduced-clavulanate dosing regimens resulted in plasma clavulanate levels that would provide middle-ear fluid levels (25% to 41% as high as plasma levels) sufficient to adequately inhibit (defined as at least 50% inhibition) β-lactamases produced by H. influenzae and M. catarrhalis (0.002 to 0.06 μg/ml) (13). Further, plasma clavulanate levels greater than 0.12 μg/ml were maintained for at least 6 h and would be expected, conservatively, to result in middle-ear fluid levels sufficient to inhibit 93% of β-lactamases produced by H. influenzae in an environment of 2 μg/ml of amoxicillin (14).
DISCUSSION
In this proof-of-concept study of children aged 6 to 23 months with AOM, we compared findings using a novel formulation of A/C containing a reduced concentration of clavulanate, namely, 21.5 mg/5 ml, against findings using the standard formulation containing 42.9 mg/5 ml, with the goal of determining whether use of the reduced-clavulanate formulation would result in less diarrhea and diaper dermatitis, the most common adverse side effects of A/C use, while maintaining maximal clinical effectiveness against S. pneumoniae, H. influenzae, and M. catarrhalis. Current recommendations call for using the standard A/C formulation at a dosage of 90 mg of amoxicillin and 6.4 mg of clavulanate (90/6.4) per kg per day for 10 days. In children receiving the reduced-clavulanate formulation at a dosage of 90 mg amoxicillin/3.2 mg clavulanate/kg of body weight/day for 10 days (phase 1), we found no appreciable differences in outcomes compared with outcomes in historical control children who had received the standard formulation and dosing regimen. Children receiving the same reduced-clavulanate formulation at a dosage of 80 mg amoxicillin/2.85 mg clavulanate/kg/day for 10 days (phase 2) had a lower rate of PDD than children who had received the standard formulation (although the difference did not reach significance [P = 0.10]), a significantly lower rate of diaper dermatitis (P = 0.04), and a significantly lower rate of temporary or permanent discontinuation of study medication because of PDD and/or diaper dermatitis (P < 0.05). There was no appreciable difference in clinical outcomes. Parental satisfaction with the therapy given to their children was marginally although significantly higher among the phase 2 children than among the historical controls. Satisfactory plasma levels of both amoxicillin and clavulanate were maintained in both phase 1 and phase 2 children.
Strengths of our study included limiting enrollment to children aged 6 to 23 months, the age group most prone to development of diarrhea and diaper dermatitis with A/C treatment; reliance on validated otoscopists who applied stringent diagnostic criteria; and our use of a validated scale for rating the severity of symptoms. These same attributes may also be considered limitations since they limit the generalizability of our findings. Other limitations include the study's open-label, nonrandomized design; its small sample sizes (with accordingly low statistical power); and its use of historical controls. Regarding the latter detail, one should note that rates of PDD have been very consistent across our two recent clinical trials, which enrolled children who had the same demographic and clinical characteristics and who were diagnosed, followed, and managed by mainly the same study personnel (3, 9).
Previous studies of children aged 6 to 30 months with AOM who were treated with amoxicillin (but not clavulanate) have described rates of diarrhea ranging from 10% to 17.5%, depending on the duration of treatment at the time of the report (10, 11). In one of the studies, the rate in children receiving only placebo ranged from 8% to 10% (10). In studies performed over the years, the differences in the rates of diarrhea between children receiving various formulations of A/C and children receiving placebo have for the most part been greater than the differences between children receiving amoxicillin alone and children receiving placebo. In two previous studies in children with AOM treated with A/C, reductions in the total daily dose of clavulanate and in the frequency of dosing resulted in reductions in the rate of diarrhea (5, 15). In a review and meta-analysis of 25 randomized placebo-controlled trials involving adults and children of various ages and with various indications, some of whom were treated with amoxicillin alone and others with A/C, diarrhea was attributable mainly to administration of amoxicillin-clavulanate, whereas candidiasis was attributable both to administration of amoxicillin alone and to administration of A/C. However, the numbers of subjects considered were small, and the reviewers concluded that underreporting of both diarrhea and candidiasis was widespread (16).
We conclude that a novel reduced-clavulanate formulation of A/C (600 mg amoxicillin/21.5 mg clavulanate/5 ml) with a dosing regimen of 80 mg amoxicillin/2.85 mg clavulanate/kg/day for 10 days may afford an improved safety profile, with rates of PDD that may approximate those seen with amoxicillin alone, while maintaining plasma levels likely to be maximally efficacious. We hope to test this regimen in a larger clinical trial to further assess its safety and efficacy in very young children with AOM.
MATERIALS AND METHODS
Eligibility and enrollment.
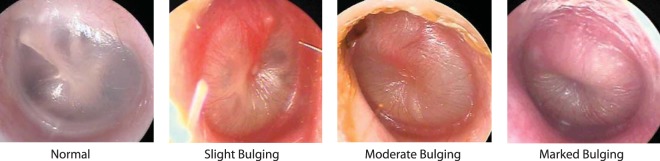
We conducted an open-label study between December 2015 and June 2016 at Children's Hospital of Pittsburgh of the University of Pittsburgh Medical Center (UPMC). The protocol was approved by the institutional review board; written informed consent was obtained from a parent of each enrolled child. To be eligible for enrollment, children were required to have AOM diagnosed on the basis of three criteria: onset of symptoms within the preceding 48 h, with a total score of 2 or more on the AOM-SOS scale (17, 18); middle-ear effusion; and moderate or marked bulging of the tympanic membrane (TM) or slight bulging accompanied by apparent otalgia or marked TM erythema (Fig. 3). Children were excluded who had TM perforation or another illness, were allergic to amoxicillin, or had received an antimicrobial within 96 h. All study clinicians had successfully completed an otoscopic validation program (19, 20).
FIG 3.
Images of tympanic membranes.
Treatment.
In phase 1, parents of 40 children were provided with a novel formulation of A/C containing a reduced concentration of clavulanate (600 mg amoxicillin/21.5 mg clavulanate/5 ml) that had been prepared at the UPMC Investigational Drug Service. The children were treated with 90 mg/kg of body weight/day and 3.2 mg/kg per day of the amoxicillin and clavulanate components, respectively, in two divided doses for 10 days. Because of the negative findings in phase 1, 72 additional children were treated with the same reduced-clavulanate formulation (600 mg amoxicillin/21.5 mg clavulanate/5 ml) but were dosed at 80 mg amoxicillin/2.85 mg clavulanate/kg/day in two divided doses for 10 days (phase 2). For children reporting or exhibiting discomfort, we suggested administration of acetaminophen.
Follow-up, examination, assessment, and management.
We assessed children on a day 7 visit and on an end-of-treatment visit, usually on day 12, 13, or 14. We also asked parents to record daily their children's AOM-SOS scores and the number and consistency of their bowel movements. At either the enrollment day visit or the day 7 visit, we obtained, from each child whose parents gave consent, a single convenience sample of blood—at time points ranging from 30 min to 4 h after administration of study medication—for determination of amoxicillin and clavulanate plasma levels. At the end-of-treatment visit, we categorized children as having experienced either clinical success or clinical failure, which we defined as worsening of symptoms or of otoscopic signs of infection (mainly TM bulging) or as failure to achieve, by the end of treatment, complete or nearly complete resolution of AOM-attributable symptoms and signs—without regard to persistence or resolution of middle-ear effusion. We treated children experiencing clinical failure with a rescue regimen, consisting preferentially of 90 mg amoxicillin/6.4 mg clavulanate/kg/day for 10 days, or, in children considered allergic to amoxicillin or whose response to amoxicillin-clavulanate was unsatisfactory, with ceftriaxone, usually in 2 doses of 75 mg/kg each, administered intramuscularly 2 days apart.
Outcome measures.
All outcome measures were prespecified. The primary outcome was the proportion of children developing PDD, which we defined as the occurrence of three or more watery stools on 1 day or two or more watery stools on 2 consecutive days. Secondary measures included (i) a comparison of the aforementioned standard dosing regimen of amoxicillin-clavulanate to the reduced-clavulanate regimen used in phase 2, regarding the proportion of children who experienced diaper dermatitis occasioning prescription of an antifungal cream; (ii) clinical failure at the day 12 to 14 visit; and (iii) the symptom burden of the children over time and the proportions of children whose symptomatic response we considered satisfactory, defined as a greater than 50% decrease in AOM-SOS scores from baseline (12, 21). We also asked study clinicians to record at the day 7 visit, on the basis of each child's symptoms and otoscopic signs, their judgment regarding the suitability of discontinuing the child's antimicrobial treatment. To obtain a historical control group, we combined the subgroups of children enrolled in two recent clinical trials (which had the same inclusion and exclusion criteria as the present study) who were treated for 10 days with the standard A/C formulation and dosing regimen (3, 9). At the end-of-treatment visit, we asked parents to rate their satisfaction with their children's therapy.
Statistical analysis.
We designed this study as proof of concept in nature. We expected the proportion of children receiving a reduced-clavulanate formulation who developed PDD to be in the range of 10% to 20%. With a population of 75 children and applying a 90% confidence interval, we expected the length of the confidence interval regarding the proportion of children developing PDD to be less than 0.12; applying an 80% confidence interval, we expected the length to be less than 0.08. For all analyses, we compared present findings with previously reported findings from use of the standard A/C formulation and dosing regimen (3, 9). We used logistic regression to compare the proportions of children who experienced adverse effects such as PDD and diaper dermatitis, the proportions experiencing clinical failure, and the proportions whose symptomatic response to treatment we considered satisfactory as defined above. We also used time-to-event analysis to estimate the number of days until 15% of parents reported PDD in their children, and we compared average AOM-SOS scores over specified periods using generalized estimated equations.
ACKNOWLEDGMENTS
We are grateful to the many Children's Hospital of Pittsburgh house officers, to the General Academic Pediatrics faculty and nurses, and to Katherine Prochownik, Nicholas Celender, Robert Parieser, and Brian Kiesel for their technical assistance. We are particularly indebted to the children and their families for their generosity and cooperation in participating in this trial.
REFERENCES
- 1.Schappert SM, Rechtsteiner EA. 2011. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13 2011:1–38. [PubMed] [Google Scholar]
- 2.Finkelstein JA, Metlay JP, Davis RL, Rifas-Shiman SL, Dowell SF, Platt R. 2000. Antimicrobial use in defined populations of infants and young children. Arch Pediatr Adolesc Med 154:395–400. doi: 10.1001/archpedi.154.4.395. [DOI] [PubMed] [Google Scholar]
- 3.Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, Colborn DK, Kurs-Lasky M, Bhatnagar S, Haralam MA, Zoffel LM, Jenkins C, Pope MA, Balentine TL, Barbadora KA. 2011. Treatment of acute otitis media in children under 2 years of age. N Engl J Med 364:105–115. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tähtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. 2011. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med 364:116–126. doi: 10.1056/NEJMoa1007174. [DOI] [PubMed] [Google Scholar]
- 5.Hoberman A, Paradise JL, Burch DJ, Valinski WA, Hedrick JA, Aronovitz GH, Drehobl MA, Rogers JM. 1997. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin) for treatment of acute otitis media in children. Pediatr Infect Dis J 16:463–470. doi: 10.1097/00006454-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hoberman A, Dagan R, Leibovitz E, Rosenblut A, Johnson CE, Huff A, Bandekar R, Wynne B. 2005. Large dosage amoxicillin/clavulanate, compared with azithromycin, for the treatment of bacterial acute otitis media in children. Pediatr Infect Dis J 24:525–532. doi: 10.1097/01.inf.0000164794.50281.1a. [DOI] [PubMed] [Google Scholar]
- 7.Dagan R, Hoberman A, Johnson C, Leibovitz EL, Arguedas A, Rose FV, Wynne BR, Jacobs MR. 2001. Bacteriologic and clinical efficacy of high dose amoxicillin/clavulanate in children with acute otitis media. Pediatr Infect Dis J 20:829–837. doi: 10.1097/00006454-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, Joffe MD, Miller DT, Rosenfeld RM, Sevilla XD, Schwartz RH, Thomas PA, Tunkel DE. 2013. The diagnosis and management of acute otitis media. Pediatrics 131:e964–e999. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 9.Hoberman A, Paradise JL, Rockette HE, Kearney DH, Bhatnagar S, Shope TR, Martin JM, Kurs-Lasky M, Copelli SJ, Colborn DK, Block SL, Labella JJ, Lynch TG, Cohen NL, Haralam M, Pope MA, Nagg JP, Green MD, Shaikh N. 2016. Shortened antimicrobial treatment for acute otitis media in young children. N Engl J Med 375:2446–2456. doi: 10.1056/NEJMoa1606043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damoiseaux RA, van Balen FA, Hoes AW, Verheij TJ, de Melker RA. 2000. Primary care based randomised, double blind trial of amoxicillin versus placebo for acute otitis media in children aged under 2 years. Br Med J 320:350–354. doi: 10.1136/bmj.320.7231.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arguedas A, Emparanza P, Schwartz RH, Soley C, Guevara S, de Caprariis PJ, Espinoza G. 2005. A randomized, multicenter, double blind, double dummy trial of single dose azithromycin versus high dose amoxicillin for treatment of uncomplicated acute otitis media. Pediatr Infect Dis J 24:153–161. doi: 10.1097/01.inf.0000151024.11703.4a. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Rockette HE, Hoberman A, Kurs-Lasky M, Paradise JL. 2015. Determination of the minimal important difference for the acute otitis media severity of symptom scale. Pediatr Infect Dis J 34:e41–e43. doi: 10.1097/INF.0000000000000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Payne DJ, Cramp R, Winstanley DJ, Knowles DJ. 1994. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important beta-lactamases. Antimicrob Agents Chemother 38:767–772. doi: 10.1128/AAC.38.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper CE, Slocombe B, White AR. 1990. Effect of low concentrations of clavulanic acid on the in-vitro activity of amoxycillin against beta-lactamase-producing Branhamella catarrhalis and Haemophilus influenzae. J Antimicrob Chemother 26:371–380. doi: 10.1093/jac/26.3.371. [DOI] [PubMed] [Google Scholar]
- 15.Bottenfield GW, Burch DJ, Hedrick JA, Schaten R, Rowinski CA, Davies JT. 1998. Safety and tolerability of a new formulation (90 mg/kg/day divided every 12 h) of amoxicillin/clavulanate (Augmentin) in the empiric treatment of pediatric acute otitis media caused by drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J 17:963–968. doi: 10.1097/00006454-199810000-00041. [DOI] [PubMed] [Google Scholar]
- 16.Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, Del Mar C. 2015. Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Can Med Assoc J 187:E21–E31. doi: 10.1503/cmaj.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaikh N, Hoberman A, Paradise JL, Rockette HE, Kurs-Lasky M, Colborn DK, Kearney DH, Zoffel LM. 2009. Responsiveness and construct validity of a symptom scale for acute otitis media. Pediatr Infect Dis J 28:9–12. doi: 10.1097/INF.0b013e318185a3a0. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh N, Hoberman A, Paradise JL, Wald ER, Switze GE, Kurs-Lasky M, Colborn DK, Kearney DH, Zoffel LM. 2009. Development and preliminary evaluation of a parent-reported outcome instrument for clinical trials in acute otitis media. Pediatr Infect Dis J 28:5–8. doi: 10.1097/INF.0b013e318185a387. [DOI] [PubMed] [Google Scholar]
- 19.Kaleida PH, Stool SE. 1992. Assessment of otoscopists' accuracy regarding middle-ear effusion. Otoscopic validation. Am J Dis Child 146:433–435. doi: 10.1001/archpedi.1992.02160160053013. [DOI] [PubMed] [Google Scholar]
- 20.Kaleida PH, Ploof DL, Kurs-Lasky M, Shaikh N, Colborn DK, Haralam MA, Ray S, Kearney D, Paradise JL, Hoberman A. 2009. Mastering diagnostic skills: Enhancing Proficiency in Otitis Media, a model for diagnostic skills training. Pediatrics 124:e714–e720. doi: 10.1542/peds.2008-2838. [DOI] [PubMed] [Google Scholar]
- 21.Shaikh N, Hoberman A, Rockette HE, Kurs-Lasky M, Paradise JL. 2015. Toward an improved scale for assessing symptom severity in children with acute otitis media. J Pediatric Infect Dis Soc 4:367–369. doi: 10.1093/jpids/piu062. [DOI] [PubMed] [Google Scholar]