ABSTRACT
In Suriname, an artesunate monotherapy therapeutic efficacy trial was recently conducted to evaluate partial artemisinin resistance emerging in Plasmodium falciparum. We genotyped the PfK13 propeller domain of P. falciparum in 40 samples as well as other mutations proposed to be associated with artemisinin-resistant mutants. We did not find any mutations previously associated with artemisinin resistance in Southeast Asia, but we found fixed resistance mutations for chloroquine (CQ) and sulfadoxine-pyrimethamine. Additionally, the PfCRT C350R mutation, associated with reversal of CQ resistance and piperaquine-selective pressure, was present in 62% of the samples. Our results from neutral microsatellite data also confirmed a high parasite gene flow in the Guiana Shield. Although recruiting participants for therapeutic efficacy studies is challenging in areas where malaria endemicity is very low due to the low number of malaria cases reported, conducting these studies along with molecular surveillance remains essential for the monitoring of artemisinin-resistant alleles and for the characterization of the population structure of P. falciparum in areas targeted for malaria elimination.
KEYWORDS: artemisinin, K13, malaria, Plasmodium falciparum, South America, Suriname, therapeutic efficacy trial, crt, drug resistance, multidrug resistance
INTRODUCTION
In 2004, artemisinin combination therapy (ACT) was adopted in Suriname as part of its malaria control program. Currently, Suriname uses artemether-lumefantrine (AL) plus primaquine (PQ) as the first-line regimen for treatment of Plasmodium falciparum malaria and mefloquine (MQ) as prophylaxis for travelers and treatment for pregnant women. Suriname successfully reduced the number of malaria cases from 11,361 in 2000 to 374 in 2014. In 2014, only 160 P. falciparum cases were reported (1). Most of the malaria patients were gold miners or their relatives working in bordering countries, who then sought treatment in Suriname. Since most of the P. falciparum cases in Suriname are imported, continuous monitoring of the treatment efficacy is necessary to guide treatment recommendations.
The World Health Organization (WHO) currently recommends monitoring the efficacy of ACT every 2 years in countries where falciparum malaria is endemic. The therapeutic efficacy study (TES) conducted in Suriname in 2005 to 2006 revealed a 98% efficacy (2). Subsequently, another TES conducted in 2011 showed an adequate clinical and parasitological response but a day 3 positivity rate of 31% (3). The proportion of patients who are parasitemic on day 3 is a key indicator for routine monitoring to identify suspected artemisinin resistance in P. falciparum. According to the WHO, if ≥10% of patients show persistent parasitemia by microscopy on day 3 after treatment with ACT or artesunate monotherapy, then partial artemisinin resistance is suspected (2). To further investigate this possibility, from July 2013 until July 2014, a TES consisting of 3 days of artesunate monotherapy followed by mefloquine and primaquine was conducted in Suriname to determine the efficacy of artemisinin, without confounding partner drugs. In that study, the day 3 positivity rate for P. falciparum was 10%, and at least 17.9% of the samples exhibited a parasite half-life of ≥5 h, suggesting suspected partial artemisinin resistance (24).
Additionally, WHO recommends conducting molecular surveillance to detect mutations in the K13 propeller domain as a complementary tool in assessing the presence of artemisinin resistance in countries of endemicity. Currently, eight K13 mutations, P441L, F446I, S449A, N458Y, P553L, V568G, P574L, and L675V, have been associated with delayed parasite clearance. In addition, five K13 mutations, Y493H, R539T, I543T, R561H, and C580Y, have been confirmed as K13 resistance mutations by in vivo and in vitro data (2). Studies conducted in Southeast Asia using whole-genome analysis identified polymorphisms in other genes, such as fd (ferredoxin), mdr2 (multidrug resistance protein 2), and crt (chloroquine resistance transporter), associated with the resistance-causing K13 propeller mutations (4). It remains to be further validated if these proposed molecular markers are relevant for monitoring artemisinin resistance in other geographical regions, including South America.
Recently, Pelleau et al. described a new C-to-R mutation at codon 350 in the Pfcrt gene that was found to cause CQ-resistant, SVMNT phenotype parasites to revert to a CQ-sensitive phenotype. Further, it was proposed that this genetic change also impaired susceptibility to piperaquine (PPQ), a drug commonly used by migrant workers in the Guiana Shield (5). Although AL combination therapy was officially introduced in 2008, illegal gold miners are known to self-medicate, creating a drug-selective pressure on the parasite. We recently showed that 5.1% of the blood samples collected in Guyana in 2010 had the C580Y mutation. These mutant parasites shared a common haplotype based on K13 flanking microsatellites, which were different from those reported in Southeast Asia. On the basis of this finding, it was proposed that the C580Y allele found in Guyana had emerged independently in this region (6). In the current study, the 41 samples collected in Suriname from the most recent therapeutic efficacy study were used to determine the presence of any mutations associated with resistance to artemisinin and other antimalarial drugs. In addition, we characterized the population structures of these isolates by using neutral microsatellite markers and compared them with those of isolates found in other countries within the Guiana Shield.
RESULTS
A total of 40 out of 41 samples were positive for P. falciparum according to our photo-induced electron transfer (PET)-PCR results; all genes were successfully amplified for 38 of these samples. Of these samples, 7 displayed a mixed P. falciparum infection as represented by different P. falciparum neutral microsatellite haplotypes in the sample. No mutations in the K13 propeller domain were found. In addition, the recently reported polymorphisms in PfFD (N193Y), PfMDR2 (T484I), and PfCRT (I356T) associated with artemisinin resistance alleles in Southeast Asia (4) were not found in these samples (Fig. 1) but only in laboratory controls W2 and Dd2. Instead, we found nonsynonymous mutations encoded at position 105 (A/T) of Pffd and at codons 423 (F/Y) and 429 (I/V) of Pfmdr2 in all samples. These mutations were also found in the South American 7G8 laboratory strain (Table 1).
FIG 1.
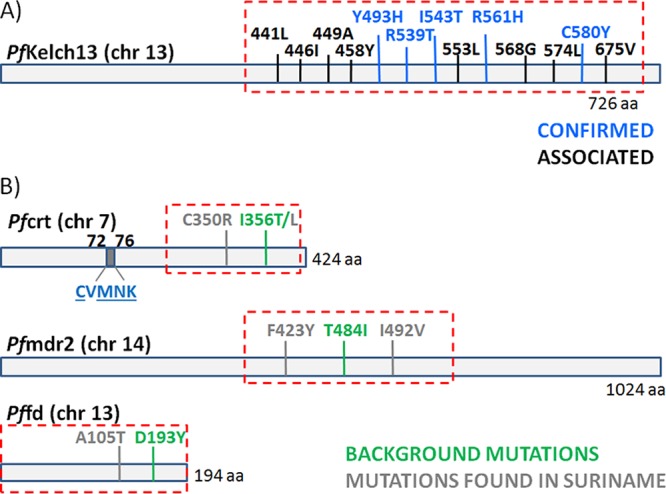
Gene products related to artemisinin resistance and mutations found within them. aa, amino acids.
TABLE 1.
Amino acid mutations encoded in genomic markers associated with K13 resistance mutationsa
| Sample (country) | PfCRT |
PfMDR2 |
PfFD |
||||
|---|---|---|---|---|---|---|---|
| C350R | I356T/L | F423Y | T484I | I492V | A105T | D193Y | |
| Suriname | C/R | L | Y | T | V | A | D |
| 3D7 | C | I | F | T | V | T | D |
| 7G8 (Brazil) | C | L | Y | T | V | A | D |
| W2 (Indochine) | C | T | Y | I | I | T | Y |
| HB3 (Honduras) | C | I | Y | T | I | T | D |
| Dd2 (Indochine) | C | T | Y | I | I | T | Y |
Genetic background-encoded mutations associated with artemisinin resistance previously found in Southeast Asia are in bold.
Also, the Pfcrt mutation leading to I356L was found in all isolates from Suriname, as well as an insertion of four AT repetitive motifs in positions 2477 to 2485. These samples also exhibited the PfCRT C72S K76T double mutation, the PfDHPS A437G K540E A581G triple mutation, and the PfDHFR C50R N51I S108N triple mutation (Table 2). More importantly, the PfCRT C350R mutation, recently described as a reverse phenotypic mutation associated with CQ sensitivity and piperaquine resistance, was found in 62% of the samples. We also found two Pfmdr1 mutant genotypes, those resulting in Y184F N1042D D1246Y (triple mutant) and Y184F S1034C N1042D D1246Y (quadruple mutant). The Pfmdr1 copy number determination indicated that only a single sample had 2 gene copies. The frequency of mutations and the copy number results are shown in Fig. 2.
TABLE 2.
Molecular profile of drug resistance genes for Suriname
| Locus | Phenotypea | % of samples with indicated phenotype | |||||
|---|---|---|---|---|---|---|---|
| Pfcrt | C72S | 73V | M74I | N75E | K76T | C350R | |
| S | V | M | N | T | C | 38 | |
| S | V | M | N | T | R | 62 | |
| Pfmdr1 | N86Y | Y184F | S1034C | N1042D | D1246Y | ||
| N | F | S | D | Y | 61 | ||
| N | F | C | D | Y | 39 | ||
| Pfdhfr | C50R | N51I | C59R | S108N | I164L | ||
| R | I | C | N | I | 100 | ||
| Pfdhps | S436A | A437G | K540E | A581G | |||
| S | G | E | G | 100 |
Mutations associated with drug resistance are in bold.
FIG 2.
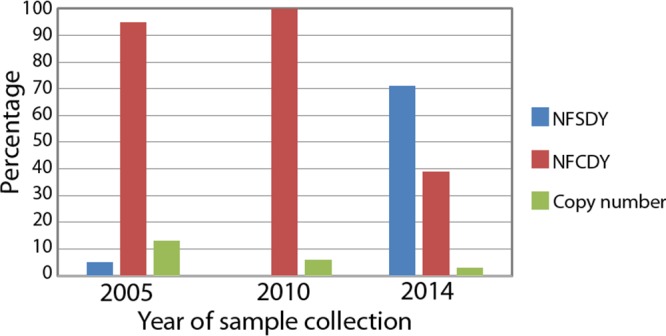
Percentages of Pfmdr1 mutants (leading to NFSDY and NFCDY phenotypes) and gene copy numbers found in Suriname samples. Previously reported data collected in 2005 and 2010 by Adhin et al. (25) were also included.
Neutral microsatellite analysis.
We found at least 12 different neutral microsatellite haplotypes from Suriname and its bordering regions in these Surinamese samples (Table 3). By doing a comparative analysis of our data with previously reported data from Suriname (7) and Guyana (6), we found at least two Plasmodium populations represented by the red and green clusters (Fig. 3A). Each cluster is composed of highly similar P. falciparum haplotypes. Moreover, using tests of genetic differentiation between sampling populations (fixation index [FST]), we found that there was little genetic differentiation between samples from Venezuela and Guyana, Venezuela and Suriname, Venezuela and Brazil, Guyana and Brazil, and Brazil and Suriname (Fig. 3B; Tables 4 and 5), as represented by similar Plasmodium haplotypes circulating in all of these countries (clusters represented by blue, red, and green were found in all sampling populations). No significant genetic difference was observed between samples from Guyana and Suriname given that similar P. falciparum haplotypes (clusters represented by red and green) were shared by both countries.
TABLE 3.
P. falciparum haplotypes found in Suriname using neutral microsatellites
| Haplotype | TA1 | Polyα | PfPK2 | TA109 | 2490 | C2M34 | C3M69 | Frequency |
|---|---|---|---|---|---|---|---|---|
| Haplo-1 | 172 | 185 | 172 | 176 | 84 | 238 | 134 | 0.18 |
| Haplo-2 | 172 | 185 | 172 | 176 | 84 | 226 | 134 | 0.15 |
| Haplo-3 | 172 | 183 | 172 | 176 | 84 | 226 | 134 | 0.15 |
| Haplo-4 | 172 | 185 | 172 | 176 | 84 | 238 | 151 | 0.15 |
| Haplo-5 | 172 | 183 | 172 | 176 | 84 | 238 | 134 | 0.15 |
| Haplo-6 | 172 | 183 | 172 | 176 | 84 | 238 | 151 | 0.06 |
| Haplo-7 | 140 | 183 | 172 | 176 | 84 | 238 | 151 | 0.03 |
| Haplo-8 | 172 | 150 | 172 | 176 | 80 | 238 | 134 | 0.03 |
| Haplo-9 | 172 | 185 | 172 | 176 | 84 | 226 | 151 | 0.03 |
| Haplo-10 | 172 | 183 | 172 | 176 | 84 | 226 | 151 | 0.03 |
| Haplo-11 | 172 | 150 | 172 | 176 | 84 | 238 | 134 | 0.03 |
| Haplo-12 | 172 | 183 | 172 | 176 | 84 | 238 | 138 | 0.03 |
FIG 3.
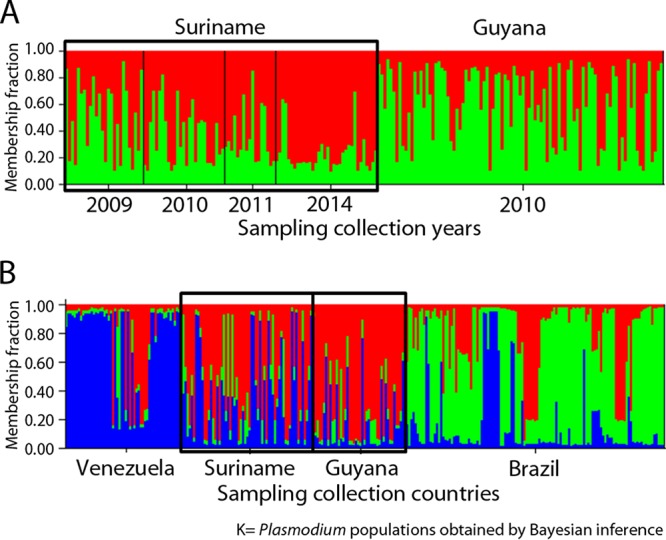
Population structure determined using neutral microsatellite loci. Each sample is represented by a vertical line which is partitioned into K color segments that represent the individual's estimated membership fraction in each of the K clusters. (A) Clustering (K = 2; colors red and green) per year using Structure v2.3; (B) clustering (K = 3; colors blue, red, and green) including other countries.
TABLE 4.
Population genetic differentiation using six neutral microsatellite loci: genetic diversity between sampling populationsa
| Population | n | Collection yrs | No. of haplotypes | No. of unique haplotypes | He (mean) | He (SD) |
|---|---|---|---|---|---|---|
| Venezuela | 54 | 2003-2004 | 22 | 15 | 0.4946 | 0.0624 |
| Guyana | 65 | 2010 | 32 | 21 | 0.4481 | 0.0618 |
| Suriname | 43 | 2009-2011 | 24 | 13 | 0.3640 | 0.0946 |
| Brazil | 122 | 1983-1999 | 61 | 52 | 0.5115 | 0.0472 |
Microsatellite results for at least 80% of the population with a clear pattern were included. He, heterozygosity; SD, standard deviation.
TABLE 5.
Population genetic differentiation using six neutral microsatellite loci: FST comparisons between sampling populationsa
| Population |
FST comparisonb |
||
|---|---|---|---|
| Venezuela | Guyana | Suriname | |
| Guyana | 0.044* | ||
| Suriname | 0.043* | 0.009 | |
| Brazil | 0.044* | 0.027* | 0.019* |
Microsatellite results for at least 80% of the population with a clear pattern were included.
*, significant at a P of <0.05.
DISCUSSION
Historically, resistance to widely used first-line antimalarial drugs such as CQ and sulfadoxine-pyrimethamine (SP) evolved at about the same time in South America and Southeast Asia and spread to other regions. Artemisinin resistance as defined by delayed parasite clearance has been well documented in Southeast Asia, but it has not been definitively confirmed in South America. Although the overall efficacy of ACT remains high in Suriname, the day 3 positivity rate for the in vivo study conducted in 2011 and the parasite half-life results from 2014 showed evidence of suspected artemisinin resistance in this population (3). Due to previous evidence for the independent emergence of the K13 C580Y allele in Guyana (6), it was important to assess the molecular profile of the 2014 samples from Suriname to further determine if artemisinin resistance-associated alleles were present in these samples. The limited number of samples available from the 2014 TES study showed no evidence of K13 resistance alleles, including those samples with low parasite clearance rates. Although several mutations in K13 have been shown to be strongly associated with artemisinin resistance, some parasites with K13 wild-type alleles in Southeast Asia also exhibited a delayed parasite clearance phenotype (8, 9). The lack of association between resistance phenotypes and K13 polymorphisms in some field isolates suggests that additional genes may be involved in the development of artemisinin resistance in P. falciparum. Another explanation may be that the limited sample size for this study may have failed to detect low-prevalence K13 resistance alleles. The high level of sequence conservation of the Kelch propeller domain in Plasmodium and the limited spread of artemisinin resistance-causing K13 mutations imply that there is a substantial fitness cost in the absence of sustained drug pressure. These fitness costs may be compensated by other genetic variants, either in kelch13 or elsewhere in the Plasmodium genome (10).
We further investigated mutations in gene markers such as Pfcrt, Pffd, and Pfmdr2 that are reported to be associated with the artemisinin resistance phenotype in Southeast Asia (4). The lack of these mutations in Suriname samples and the presence of different nonsynonymous mutations suggest that parasite isolates in Suriname are evolving independently. Moreover, K13 wild-type laboratory strains of P. falciparum from Southeast Asia also presented the mutations previously associated with artemisinin resistance, which suggests a very particular P. falciparum genetic profile present in that part of the world. It is possible that K13 resistance mutants in South America arise with a different background and increase in frequency due to a greater selective pressure. However, the potential role of background mutations and their association with artemisinin resistance needs further validation in South America.
Additionally, most of the Suriname samples (62%) had the PfCRT C350R mutation, which has been associated with a CQ phenotypic reversion and a strong piperaquine drug-selective pressure (5). Given that gold miners living in the forest are known to self-medicate with dihydroartemisinin-piperaquine-trimethoprim tablets, it is not surprising that this PfCRT mutation is found in high frequency in these study samples, especially if we consider that 87% of the patients came from artisanal gold mining areas in French Guiana. In addition, dhfr and dhps mutations associated with SP resistance were fixed in these samples.
Pfmdr1 alleles leading to N86Y, Y184F, and D1246Y are common in P. falciparum populations in Africa; however, reduced susceptibility to lumefantrine has been linked to haplotypes encoding the N86, 184F, and D1246 residues and to the K76 residue in PfCRT (8). In Suriname, this particular profile was not found. The majority of the isolates displayed the Y184F N1042D D1246Y mutations while Y184F S1034C N1042D D1246Y mutations were found with less frequency. These Pfmdr1 mutants have also been found in other countries of the Guiana Shield, such as Guyana (6), Venezuela (11), and Brazil (12). The different results in genotype prevalence and gene copy number might be attributed to a different target population. Most of the recent samples from 2014 originated from small-scale gold miners coming from French Guiana, while the previous samples collected in 2010 corresponded mainly to people working or living in the villages in the interior, so most of the drug resistance profile results in this study corresponded to imported malaria cases. Changes in prevalence of these alleles might also indicate selection by a partner drug. A decrease in the partner drug's efficacy might facilitate the emergence of new foci of resistance to artemisinin, as observed in the Mekong region (13).
Furthermore, in the illegal gold mining areas of the Guiana Shield, nonrecommended treatments, including artemisinin monotherapy and nonregistered artemisinin derivatives, are available through the informal sector (14). Indeed, infected gold miners could reintroduce malaria in areas where competent vectors exist, possibly resulting in the spread of artemisinin-resistant parasites. Moreover, our results from cluster analyses, which included data from Venezuela, Suriname, Brazil, and Guyana, reflect the high parasite gene flow in the Guiana Shield. In particular, P. falciparum isolates from Guyana and Suriname are highly genetically related and behaved as a single parasite population.
Although K13 genotypes associated with artemisinin resistance were not detected, our findings highlight the presence of multidrug resistance genotypes in Suriname. Given that the prevalence of P. falciparum in Suriname has been dramatically reduced in recent years, it has become more challenging to conduct in vivo studies to assess the therapeutic efficacy of artemisinin. Therefore, molecular surveillance continues to be an important method for monitoring changes in prevalence of drug resistance genotypes.
MATERIALS AND METHODS
Study site: Plasmodium isolates.
We tested 41 Plasmodium falciparum blood samples collected from July 2013 to June 2014 for an artesunate-based monotherapy efficacy trial. The study was approved by the national ethics committee (CMWO) of the Ministry of Health in Suriname. Patients and/or their legal guardians provided written consent to participate in the study. Patients with uncomplicated falciparum malaria, who met the study inclusion criteria, were enrolled at the Tourtonne laboratory, a malaria diagnostic and treatment facility in the north of Paramaribo. Most gold miners dwell in this neighborhood while in the capital. The participants were febrile persons aged ≥2 years with microscopically confirmed uncomplicated P. falciparum infection presenting at the clinic.
DNA isolation and genotyping methods.
Genomic DNA was isolated from blood spots taken at enrollment (day 0) using a QIAamp DNA minikit (Qiagen, Valencia, CA). Samples were screened using the multiplex photo-induced electron transfer (PET)-PCR (15). For each sample, duplicate PET-PCRs were run with 5 μl of DNA template used in the PCR. All assays were performed using Agilent Mx3005pro thermocyclers (Agilent Technologies, Santa Clara, CA, USA). As previously established, a CT value of 40 was considered the cutoff to score a reaction as positive; samples with CT values above 40 were considered to be negative. The confirmed P. falciparum samples were used to amplify the K13 propeller domain using previously described methods (6). PCR amplifications of specific codons in Pfcrt (codons 350 and 356), Pffd (codon 139), and Pfmdr2 (codon 484) were carried out in 20-μl reaction volumes using 20 ng of total genomic DNA, 1× PCR buffer with MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), 0.75 μM each forward and reverse primers (PF535 [5′-CCATATAATTTTTCATTTTC-3′] and PF536 [5′-GTTCTCTTACAACATCAC-3′] for Pfcrt; PF11723 [5′-TTGTTAGAATCATGAATATTG-3′] and PF11724 [5′-GATTGAGGACAAATTACATG-3′] for Pffd; PF10283 [5′-GCAAAAGGATAGATATGAAAG-3′] and PF10284 [5′-CCTATAAATAATACACTACC-3′] for Pfmdr2), and 0.6 U/μl of high-fidelity Taq polymerase (Expand high-fidelity PCR system; Roche). The cycling conditions were as follows: an initial denaturation step at 94°C for 5 min; 35 cycles of denaturation at 94°C for 30 s; annealing at 52°C for Pfcrt, 46°C for Pffd, and 50°C for Pfmdr2 for 30 s; and extension at 68°C for 1 min, followed by a final extension step at 68°C for 10 min. PCR products were confirmed after ExoSAP cleanup using 1.8% agarose gel electrophoresis and GelRed (Biotium, Hayward, CA, USA). We also included the artemisin-resistant laboratory control samples 3D7, 7G8, W2, HB3, and Dd2 for comparison.
The samples were also genotyped by direct sequencing for Pfcrt (codons 72 to 76), Pfdhfr (codons 50, 51, 59, 108, and 164), and Pfdhps (codons 436, 437, 540, 581, and 613) using an Applied Biosystems 3130 capillary sequencer. In addition, Pfmdr1 copy number and codon mutations (positions 86, 184, 1034, 1042, and 1246) were evaluated. The PCR primers and conditions for these Pfcrt, Pfdhps, Pfdhfr, and Pfmdr1 codons have been previously described (11, 16). Pfmdr1 copy number was determined with a TaqMan real-time PCR (Stratagene MX3005P; Agilent Technologies, La Jolla, CA) using a previously described protocol (16).
Microsatellite analysis.
Seven neutral microsatellites (TA1, Polyα, PfPK2, TA109, C2M34, C3M69, and 2490) located in chromosomes 2, 3, 4, 6, and 12 were PCR amplified using previously published methods for analyzing Plasmodium population structure (17, 18). Fluorescently labeled PCR products were separated on an Applied Biosystems 3130 capillary sequencer and scored using Gene Marker v1.95 (SoftGenetics LLC). The discovery of one or more additional alleles in a single locus was interpreted as a coinfection with two or more genetically distinct clones in the same isolate. Missing data (no amplifications) were observed for some loci but not considered for defining haplotypes. Neutral microsatellite data from previously published data in Suriname (7) were also included to evaluate any changes in the haplotypes circulating in the country. Moreover, results from samples collected from 2003 to 2004 in Venezuela (19), 2010 in Guyana (6), and 1983 to 1999 in Brazil (20) were included to compare historical haplotypes circulating in this region.
We used Structure v2.1 (21) to test whether P. falciparum samples from different countries clustered as a single population. This Bayesian clustering approach assigns isolates to K (number of genetically related) populations or clusters characterized by the allele frequencies at each locus. The sample assignment was evaluated at different K values (K = 2 to 10). Given that this algorithm relies on stochastic simulations, each K value was run independently 10 times with a burn-in period of 10,000 iterations, followed by 50,000 iterations. The admixture model was used to allow for the presence of individuals with ancestry in two or more of the K populations. Heterozygosity (He) and the fixation index (FST) were calculated using Arlequin 3.5 (22). FST values were classified as follows: <0.05, little genetic differentiation; 0.05 to 0.15, moderate genetic differentiation; 0.15 to 0.25, great genetic differentiation; and >0.25, very great genetic differentiation (23).
ACKNOWLEDGMENTS
We thank Ira Goldman for reading and commenting on the manuscript.
This work was supported by the Centers for Disease Control and Prevention Antimicrobial Resistance Working Group and the Amazon Malaria Initiative funded by the U.S. Agency for International Development. Stella M. Chenet was supported by the American Society of Microbiology/CDC Postdoctoral Research Fellowship. This study was also partially supported by The Atlanta Research and Education Foundation, Atlanta VA Medical Center.
REFERENCES
- 1.World Health Organization. 2014. World Malaria Report 2014. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2015. Status report on artemisinin and ACT resistance. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Vreden SG, Jitan JK, Bansie RD, Adhin MR. 2013. Evidence of an increased incidence of day 3 parasitaemia in Suriname: an indicator of the emerging resistance of Plasmodium falciparum to artemether. Mem Inst Oswaldo Cruz 108:968–973. doi: 10.1590/0074-0276130167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miotto O, Amato R, Ashley EA, MacInnis B, Almagro-Garcia J, Amaratunga C, Lim P, Mead D, Oyola SO, Dhorda M, Imwong M, Woodrow C, Manske M, Stalker J, Drury E, Campino S, Amenga-Etego L, Thanh TN, Tran HT, Ringwald P, Bethell D, Nosten F, Phyo AP, Pukrittayakamee S, Chotivanich K, Chuor CM, Nguon C, Suon S, Sreng S, Newton PN, Mayxay M, Khanthavong M, Hongvanthong B, Htut Y, Han KT, Kyaw MP, Faiz MA, Fanello CI, Onyamboko M, Mokuolu OA, Jacob CG, Takala-Harrison S, Plowe CV, Day NP, Dondorp AM, Spencer CC, McVean G, Fairhurst RM, White NJ, Kwiatkowski DP. 2015. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet 47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelleau S, Moss EL, Dhingra SK, Volney B, Casteras J, Gabryszewski SJ, Volkman SK, Wirth DF, Legrand E, Fidock DA, Neafsey DE, Musset L. 2015. Adaptive evolution of malaria parasites in French Guiana: reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc Natl Acad Sci U S A 112:11672–11677. doi: 10.1073/pnas.1507142112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenet SM, Akinyi Okoth S, Huber CS, Chandrabose J, Lucchi NW, Talundzic E, Krishnalall K, Ceron N, Musset L, Macedo de Oliveira A, Venkatesan M, Rahman R, Barnwell JW, Udhayakumar V. 2016. Independent emergence of the Plasmodium falciparum Kelch propeller domain mutant allele C580Y in Guyana. J Infect Dis 213:1472–1475. doi: 10.1093/infdis/jiv752. [DOI] [PubMed] [Google Scholar]
- 7.Akinyi Okoth S, Abdallah JF, Ceron N, Adhin MR, Chandrabose J, Krishnalall K, Huber CS, Goldman IF, Macedo de Oliveira A, Barnwell JW, Udhayakumar V. 2015. Variation in Plasmodium falciparum histidine-rich protein 2 (Pfhrp2) and Plasmodium falciparum histidine-rich protein 3 (Pfhrp3) gene deletions in Guyana and Suriname. PLoS One 10:e0126805. doi: 10.1371/journal.pone.0126805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, et al. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye R, Hu D, Zhang Y, Huang Y, Sun X, Wang J, Chen X, Zhou H, Zhang D, Mungthin M, Pan W. 2016. Distinctive origin of artemisinin-resistant Plasmodium falciparum on the China-Myanmar border. Sci Rep 6:20100. doi: 10.1038/srep20100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MalariaGEN Plasmodium falciparum Community Project. 2016. Genomic epidemiology of artemisinin resistant malaria. eLife 5:e08714. doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T, Barnwell JW, Escalante AA, Wongsrichanalai C, Ariey F, Meshnick SR, Udhayakumar V. 2010. Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLoS Pathog 6:e1000830. doi: 10.1371/journal.ppat.1000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalis MG, Pang L, Silveira MS, Milhous WK, Wirth DF. 1998. Characterization of Plasmodium falciparum isolated from the Amazon region of Brazil: evidence for quinine resistance. Am J Trop Med Hyg 58:630–637. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesan M, Gadalla NB, Stepniewska K, Dahal P, Nsanzabana C, Moriera C, Price RN, Martensson A, Rosenthal PJ, Dorsey G, Sutherland CJ, Guerin P, Davis TM, Menard D, Adam I, Ademowo G, Arze C, Baliraine FN, Berens-Riha N, Bjorkman A, Borrmann S, Checchi F, Desai M, Dhorda M, Djimde AA, El-Sayed BB, Eshetu T, Eyase F, Falade C, Faucher JF, Froberg G, Grivoyannis A, Hamour S, Houze S, Johnson J, Kamugisha E, Kariuki S, Kiechel JR, Kironde F, Kofoed PE, LeBras J, Malmberg M, Mwai L, Ngasala B, Nosten F, Nsobya SL, Nzila A, Oguike M, Otienoburu SD, Ogutu B, et al. 2014. Polymorphisms in Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes: parasite risk factors that affect treatment outcomes for P. falciparum malaria after artemether-lumefantrine and artesunate-amodiaquine. Am J Trop Med Hyg 91:833–843. doi: 10.4269/ajtmh.14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans L III, Coignez V, Barojas A, Bempong D, Bradby S, Dijiba Y, James M, Bretas G, Adhin M, Ceron N, Hinds-Semple A, Chibwe K, Lukulay P, Pribluda V. 2012. Quality of anti-malarials collected in the private and informal sectors in Guyana and Suriname. Malar J 11:203. doi: 10.1186/1475-2875-11-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, Hill V, Udhayakumar V. 2013. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 8:e56677. doi: 10.1371/journal.pone.0056677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffing S, Syphard L, Sridaran S, McCollum AM, Mixson-Hayden T, Vinayak S, Villegas L, Barnwell JW, Escalante AA, Udhayakumar V. 2010. pfmdr1 amplification and fixation of pfcrt chloroquine resistance alleles in Plasmodium falciparum in Venezuela. Antimicrob Agents Chemother 54:1572–1579. doi: 10.1128/AAC.01243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCollum AM, Schneider KA, Griffing SM, Zhou Z, Kariuki S, Ter-Kuile F, Shi YP, Slutsker L, Lal AA, Udhayakumar V, Escalante AA. 2012. Differences in selective pressure on dhps and dhfr drug resistant mutations in western Kenya. Malar J 11:77. doi: 10.1186/1475-2875-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. 1999. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology 119:113–125. doi: 10.1017/S0031182099004552. [DOI] [PubMed] [Google Scholar]
- 19.Chenet SM, Schneider KA, Villegas L, Escalante AA. 2012. Local population structure of Plasmodium: impact on malaria control and elimination. Malar J 11:412. doi: 10.1186/1475-2875-11-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffing SM, Viana GM, Mixson-Hayden T, Sridaran S, Alam MT, Macedo de Oliveira A, Barnwell JW, Escalante AA, Povoa MM, Udhayakumar V. 2013. Historical shifts in Brazilian P. falciparum population structure and drug resistance alleles. PLoS One 8:e58984. doi: 10.1371/journal.pone.0058984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155:945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resources 10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 23.Hartl DL, Clark AG. 1997, Principles of population genetics, 2nd ed Sinauer Associates, Inc., Sunderland, MA. [Google Scholar]
- 24.Vreden SGS, Bansie RD, Jitan JK, Adhin MR. 2016. Assessing parasite clearance during uncomplicated Plasmodium falciparum infection treated with artesunate monotherapy in Suriname. Infect Drug Resist 9:261–267. doi: 10.2147/IDR.S113861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adhin MR, Labadie-Bracho M, Bretas G. 2013. Molecular surveillance as monitoring tool for drug-resistant Plasmodium falciparum in Suriname. Am J Trop Med Hyg 89:311–316. doi: 10.4269/ajtmh.12-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]


