ABSTRACT
Certain Staphylococcus aureus strains exhibit an inoculum effect (InE) with cefazolin (CFZ) that has been associated with therapeutic failures in high-inoculum infections. We assessed the in vitro activities of ceftaroline (CPT), CFZ, and nafcillin (NAF) against 17 type A β-lactamase (βla)-producing, methicillin-susceptible S. aureus (MSSA) strains, including the previously reported TX0117, which exhibits the CFZ InE, and its βla-cured derivative, TX0117c. Additionally, we determined the pharmacokinetics of CPT in rats after single intramuscular doses of 20 and 40 mg/kg of body weight and evaluated the activities of CPT (40 mg/kg every 8 h [q8h]), CFZ, and NAF against TX0117 and TX0117c in a rat model of infective endocarditis. No InE was observed for CPT or NAF, whereas a marked InE was detected for CFZ (MIC, 8 to ≥128 μg/ml). CPT and NAF treatment against TX0117 resulted in mean bacterial counts of 2.3 and 2.1 log10 CFU/g in vegetations, respectively, compared to a mean of 5.9 log10 CFU/g in the CFZ-treated group (CPT and NAF versus CFZ, P = 0.001; CPT versus NAF, P = 0.9830). Both CFZ and CPT were efficacious against the βla-cured derivative, TX0117c, compared to time zero (t0) (P = <0.0001 and 0.0015, respectively). Our data reiterate the in vivo consequences of the CFZ InE and show that CPT is not affected by this phenomenon. CPT might be considered for high-inoculum infections caused by MSSA exhibiting the CFZ InE.
KEYWORDS: β-lactamase, Staphylococcus aureus, ceftaroline, endocarditis
INTRODUCTION
Staphylococcus aureus continues to be a leading cause of bacterial infections worldwide, including skin and soft tissue infections, bacteremia, pneumonia, endocarditis, septic arthritis, and osteomyelitis (1–3). The prevalence of methicillin-susceptible S. aureus (MSSA) isolates exhibiting the cefazolin (CFZ) inoculum effect (InE) in the United States has been reported to range from 19% to 27% (4, 5). Besides the United States, the overall prevalence of the cefazolin InE was reported to be 36% in South America (Colombia, Ecuador, Peru, and Venezuela), where MSSA β-lactamase (βla) type A and type C were 66% and 31%, respectively (6). In South Korea, the blaZ gene was detected in 92% of 220 MSSA isolates studied, and a pronounced cefazolin InE was observed in 13%, most of which (79%) expressed type A β-lactamase (7). More recently, a study using a PubMed database search (January 1996 to June 2016) stated that most of the reports of clinical failure with cefazolin are case reports or case series and that the clinical relevance of the cefazolin InE is not entirely clear, especially as susceptibility testing in clinical microbiology laboratories uses a standardized inoculum (8). This review states that, in addition, limited by small sample size and possible selection bias, the only comparative study to date examining the clinical impact of the cefazolin InE (i.e., the study referred to above from Asia [South Korea] [7]) did not show any significant differences in outcomes when comparing isolates with and without cefazolin InE. However, it is not clear what percentage of the patients had endocarditis or deep-seated and/or undrained infections, which would be more likely to have a large inoculum present.
Isolates of MSSA often harbor one of four different variants of βla (types A, B, C, and D) capable of hydrolyzing penicillins, except isoxazolyl-penicillins (5, 6, 9–13). Among the β-lactamase types, βla type A has been associated with clinical failures in patients with endocarditis treated with CFZ (5, 14–16). When available, isolates recovered from these infections often exhibit the cefazolin InE, which can be detected by a marked increase (≥4-fold) in the MIC of CFZ when a large inoculum is used (107 CFU/ml compared to the standard 105 CFU/ml) (5, 6, 16). The inoculum effect is of potential concern, since CFZ is recommended as an option for the treatment of MSSA endocarditis in patients with non-immediate-type hypersensitivity to penicillin (15). Additionally, although nafcillin (NAF) and its derivatives are the drugs of choice for deep-seated infections caused by MSSA, the need for frequent administration (i.e., every 4 h) precludes its use for outpatient therapy in patients who cannot afford an infusion pump. Thus, CFZ is frequently used for outpatient administration in the setting of endovascular or other severe infections caused by MSSA (17, 18); however, the InE may jeopardize the successful treatment of some patients with MSSA infections treated with CFZ.
Among the newer agents, ceftaroline (CPT) (the active metabolite of the prodrug ceftaroline fosamil) is a broad-spectrum cephalosporin agent with bactericidal activity against Gram-positive pathogens (including S. aureus). CPT is currently approved by the FDA for the treatment of acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia in adults. CPT at the standard inoculum has been shown to have 2- to 4-fold-greater activity (MIC50/90, 0.25 μg/ml; 100% susceptible) than against methicillin-resistant S. aureus (MRSA) (MIC50/90, 0.5 and 1 μg/ml; 96.2% susceptible) (19, 20); however, its activity against MSSA producing type A β-lactamase at a high inoculum is unknown.
The goal of the present study was to assess the in vivo efficacy of CPT against a previously characterized MSSA strain that exhibits the CFZ InE (TX0117) and its βla-cured derivative (TX0117c) (21, 22) using a rat model of infective endocarditis (IE). Moreover, using the same model, we compared the in vivo efficacy of CPT with those of CFZ and NAF. Additionally, we determined the in vitro activity of CPT at standard and high inocula against other MSSA strains harboring βla type A.
RESULTS
MICs.
All 17 MSSA strains, including TX0117 and TX0117c, were susceptible to CPT, CFZ, and NAF at the standard inoculum (105 CFU/ml) (MIC, 0.125 to 1 μg/ml) (Table 1). No InE was observed at the high (107) inoculum for CPT or NAF (MIC, 0.25 to 2 μg/ml) compared with a marked inoculum effect when CFZ was tested at high inoculum (107 CFU/ml) against all the strains (MICs, 8 to ≥128 μg/ml). The InE was abolished in the βla-cured strain TX0117c, as previously described (21).
TABLE 1.
MICs of ceftaroline, naficillin, and cefazolin against S. aureus (MSSA; βla type A)
| Strain | MIC (μg/ml)a |
|||||
|---|---|---|---|---|---|---|
| Standard inoculum (105 CFU/ml) |
High inoculum (107 CFU/ml) |
|||||
| CFZ | NAF | CPT | CFZ | NAF | CPT | |
| S. aureus ATCC 29213b | 0.5 | 0.5 | 0.25 | 8 | 1 | 0.25 |
| S. aureus ATCC 25923c | 0.5 | 0.5 | 0.125 | 0.5 | 1 | 0.125 |
| TX0117 (MSSA; βla type A) | 1 | 0.5 | 0.25 | 32 | 2 | 1 |
| TX0117c (βla cured) | 0.5 | 0.5 | 0.25 | 0.5 | 2 | 0.5 |
| Other MSSA (βla type A strains) (n = 15) | ||||||
| MIC range | 0.5 to 1 | 0.25 to 1 | 0.12 to 0.5 | 8 to >128 | 0.5 to 2 | 0.5 to 1 |
| MIC50 | 0.5 | 0.5 | 0.25 | 32 | 1 | 1 |
| MIC90 | 1 | 0.5 | 0.25 | 64 | 2 | 1 |
MIC determined by broth microdilution.
Type A βla producer.
βla negative.
PK analysis.
The pharmacokinetic (PK) parameters obtained after a single intramuscular (i.m.) injection of CPT (20 mg/kg of body weight) are summarized in Table 2. Andes and Craig determined that the percentage of time that the concentration remains above the MIC (fT>MIC) was the pharmacokinetic/pharmacodynamic index that best correlated with efficacy (23). Based on our PK analysis, dosing of CPT at 40 mg/kg i.m. q8h has a predicted CPT fT>MIC of ∼35% (23, 24).
TABLE 2.
Pharmacokinetic parameters following a single intramuscular administration of ceftaroline in rats
| Parametera | Value (±SD) |
|
|---|---|---|
| 20 mg/kg | 40 mg/kg | |
| ke (h−1) | 2.42 ± 0.17 | 1.96 ± 0.18 |
| ka (h−1) | 2.52 ± 0.202 | 2.18 ± 0.22 |
| Cmax (mg/liter) | 28.6 ± 4.02 | 48.50 ± 4.06 |
| t1/2 (h) | 0.287 ± 0.211 | 0.356 ± 0.035 |
| V (liter/kg) | 0.269 ± 0.039 | 0.321 ± 0.024 |
| AUC0–∞ (mg · h/liter) | 30.89 ± 2.23 | 63.91 ± 2.13 |
ke, elimination rate constant; ka, absorption rate constant; t1/2, elimination half-life; V, volume of distribution; AUC0–∞, area under the concentration-time curve from 0 h to infinity.
Experimental endocarditis model. (i) ID90 determination.
The 90% infective doses (ID90s) of TX0117 and TX0117c were 2.3 × 105 CFU/g and 1.2 × 105 CFU/g, respectively, indicating that the two strains possess very similar infectivities in the rat IE model. As mentioned above, we used ∼10 times the ID90 to infect cardiac valves for both TX0117 and TX0117c strains.
(ii) Antibiotic efficacy.
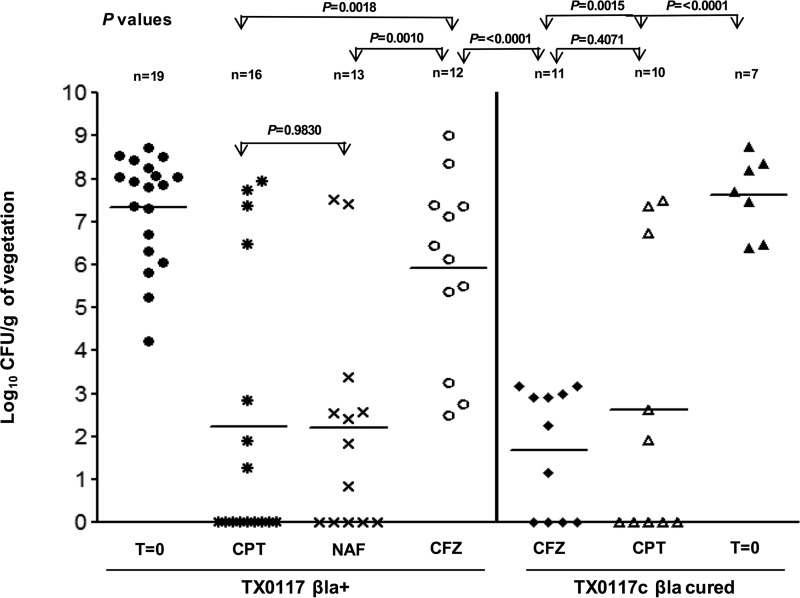
The therapy results for CPT, CFZ, and NAF against TX0117-infected (left) and TX0117c-infected (right) rats are shown in Fig. 1. A total of 19 animals infected with TX0117 served as the time zero (t0) baseline control (no antibiotics). These animals were sacrificed at the time of therapy initiation (36 h after inoculation) and showed a mean of 7.3 ± 1 log10 CFU/g in vegetations. In animals infected with TX0117, the means in vegetations 24 h after the last dose were 2.3 ± 3, 2.1 ± 2, and 5.9 ± 2 log10 CFU/g (±standard deviation [SD]) for the CPT, NAF, and CFZ treatment groups, respectively (CPT versus CFZ, P = 0.0018; NAF versus CFZ, P = 0.0010; CPT versus NAF, P = 0.9830) (Fig. 1, left). Of note, 9 out of 16 rats in the CPT group had sterile vegetations. In contrast, no animal in the CFZ-treated group exhibited sterile vegetations (Fig. 1, left). Animals that survived >24 h (and thus were included in the final analysis) but died before receiving the full 3 days of antibiotic therapy showed high bacterial counts in aortic valves/vegetations, ranging from 107 to 109 CFU/g. They included 3 rats in the TX0117-infected and CPT-treated group (1 rat with 4 out of 9 doses and 2 rats with 7 out of 9 doses) and 2 rats in the CFZ group (1 rat each with 3 out of 9 and 6 out of 9 doses). Autopsy of the dead animals revealed infarcted hearts with punctured cardiac tissue as the likely cause of death.
FIG 1.
Efficacy of antibiotic therapy in the rat IE model of infection with S. aureus TX0117 (MSSA; βla+) and TX0117c (MSSA; βla cured). The results of therapy with CPT, CFZ, and NAF for TX0117-infected (left) and TX0117c-infected (right) rats are shown. The rats were treated for 3 days, starting 36 h after inoculation (t0), with CPT, NAF, and CFZ and sacrificed 24 h after the last dose. The P values shown were between CPT and NAF versus CFZ in TX0117-infected rats and CPT versus CFZ and versus t0 in TX0117c-infected rats. The data were log transformed, and an unpaired t test was performed to obtain the P values. Horizontal bars represent the geometric mean CFU titers.
Seven animals infected with TX0117c (t0) showed a mean of 7.6 ± 0.9 log10 CFU/g (±SD) in vegetations (Fig. 1, right). In animals infected with TX0117c, the mean in vegetations was 2.6 ± 3 log10 CFU/g (±SD), and it was 1.6 ± 1 for the CPT, and CFZ treatment groups, respectively (CPT versus CFZ, P = 0.4071; CPT and CFZ versus t0, P = 0.0015 and <0.0001, respectively) (Fig. 1, right), indicating that CPT and CFZ were equally efficacious in animals when TX0117 was cured of βla production. In addition, there was no significant difference for results with CPT therapy for TX0117 (βla+) or TX0117c (P = 0.7661), indicating that the enzyme production did not have an in vivo effect against CPT. One rat infected with TX0117c and treated with CPT received only 5 out of 9 doses and showed only 101 CFU/g, equivalent to the minimum detection limit. This animal was included in the final analysis. Autopsy of the dead animal also revealed infarcted heart with punctured tissue as the likely cause of death.
DISCUSSION
CPT is a member of a newer broad-spectrum cephalosporin class with the added characteristic of exhibiting potent activity against MRSA. CPT is currently approved for acute skin and soft tissue infections and community-acquired bacterial pneumonia (25–27). The in vivo efficacy of CPT against S. aureus infections at a high inoculum (109 CFU/infection site) has been reported previously in a murine thigh infection model (28), where the use of high inocula did not affect CPT efficacy against three staphylococcal strains tested, namely, MRSA, vancomycin-intermediate S. aureus (VISA), and heterogeneous vancomycin-intermediate S. aureus (hVISA) strains. Moreover, Zhanel et al. (29) evaluated the in vitro pharmacodynamics of a humanized regimen of CPT at 600 mg q12h for 96 h against MRSA, hVISA, and VISA isolates using an inoculum of 106 to 108 CFU/ml (30) and reported no inoculum effect. In this simulated pharmacodynamic model, which has been described in the literature as simulating the treatment of bacteremic infections over 48 h (31, 32), CPT showed a greater reduction in CFU between 24 and 96 h (>5 log10 CFU) than the comparators (vancomycin and daptomycin). CPT has also been tested in vivo in a rabbit model of endocarditis using a regimen that simulates a human dose using 10 mg/kg every 12 h. After 4 days of therapy in this model, CPT exhibited a potent bactericidal effect against two MRSA strains, achieving sterilization of 90% and 60% of the vegetations infected with a fully vancomycin-susceptible MRSA strain and an hVISA strain, respectively (33). However, more recently, CPT resistance in MRSA has been observed both in vitro (34) and in vivo (35).
The in vivo and in vitro efficacies of CPT against MSSA isolates that produce type A β-lactamase and that exhibit the inoculum effect have not been systematically evaluated. Our results provide evidence that CPT, similar to NAF (21, 36), is not affected by the InE in vitro or in vivo. Moreover, using a rat model of endocarditis, our results clearly showed that CPT- and NAF-treated animals demonstrated comparable in vivo efficacies against the type A βla-producing strain TX0117 and that the reduction in bacterial counts in vegetations obtained from animals treated with NAF and CPT was significantly lower than in animals treated with CFZ. These results provide compelling evidence of the in vivo bactericidal efficacy of CPT against βla type A-producing MSSA exhibiting the CFZ InE using a stringent model to assess antibiotic activity in infections with high bacterial burdens. We note that, for unknown reasons, infected animals in the CPT (1 animal) and NAF (2 animals) therapy groups that completed the antibiotic course (3 days) still showed high numbers of CFU per gram. We frequently observe outliers in our endocarditis experiments, again, for unknown reasons. However, in the case of CPT, the development of drug-tolerant/resistant mutants in TX0117-infected rats is a possibility.
There are published reports (including from our group) that correlate the CFZ inoculum effect with clinical failure (4, 5, 7, 14, 16). These reports have shown that, in the majority of the strains, an increase in the CFZ MICs (tested at high inoculum) was associated with the presence of type A β-lactamase (4, 5, 7). Two different studies that included MSSA isolates collected from multicenter surveillance studies (2001-02 and 2006-08) also showed a high prevalence of the CFZ InE in MSSA isolates recovered from bloodstream and bone infections, and most isolates with this phenotype harbored βla type A (6). Nonetheless, clinical studies have not consistently shown clinical relevance of the CFZ InE, although some have suggested that CFZ failure is associated with the site of MSSA infection and that this is especially important for endocarditis and pneumonia (which are high-inoculum infections). However, these studies have limitations in terms of the numbers of patients and the retrospective nature of the data (37, 38). Conflicting results have also been reported in animal models of rat endocarditis. In one study, the in vivo effect of the CFZ InE was observed (39) for an MSSA strain, but in a second study using the same strain and animal model, the previous results could not be reproduced (40). High serum CFZ concentrations achieved in the first study and the use of different rabbit strains in the studies were cited as possible causes for these discrepancies (39, 40).
In a murine model of intraperitoneal infection using an MSSA strain that exhibited the CFZ InE, CFZ-treated animals exhibited higher mortality than the strain that did not show an InE (36). In our previously published study using a rat IE model, we showed that the CFZ InE was evident in vivo against MSSA TX0117. Indeed, a significant difference in bacterial counts in vegetations was observed in CFZ-treated animals compared with daptomycin- and NAF-treated animals. The efficacy of CFZ in vivo was restored when the strain was cured of β-lactamase (TX0117c) (21). The results of the current work reiterate that the CFZ InE can influence the efficacy of CFZ in vivo (4, 5). While the percentage of MSSA infections in which the InE may have a clinical effect appears to be quite small, it nonetheless is of concern when the inoculum is large, such as a large vegetation or an undrained abscess. At the time of therapy initiation in humans with staphylococcal infections, the rapid identification and differentiation of MSSA versus MRSA strains (43), in combination with high-inoculum MIC testing and/or DNA sequencing of the beta-lactamase gene (5), may help in clinical settings to place patients on targeted therapy more quickly.
This study has several limitations which should be noted. We utilized doses of NAF and CFZ that, although shown to be effective in previous experimental IE models, were chosen without guidance from PK evaluation or serum concentrations (21, 44, 45). However, the efficacy of these agents (NAF and CFZ) was confirmed in our experiments despite the limitation. It is also important to note that the fT>MIC of CPT employed in our study is readily achievable in humans, where the fT>MIC of CPT with the FDA-approved dosing is ≥60% (46–49). Although our fT>MIC goal was guided by results from animal models, the application of the results in this study to clinical settings may be difficult until further studies can be done to validate all regimens using human simulated PK parameters. However, our results suggest that CPT is potentially applicable in humans where dosing of CPT would yield a higher fT>MIC.
In summary, our results show that CPT is as efficacious as NAF against an MSSA strain exhibiting the CFZ InE. For serious infections in humans caused by βla type A MSSA with a high bacterial burden, CPT or a CPT-containing antibiotic regimen may be an attractive consideration, especially for patients who fail CFZ therapy.
MATERIALS AND METHODS
Bacterial strains used in in vitro and in vivo experiments.
Fifteen MSSA (βla type A) strains from a previously published study (5) obtained from patients included in clinical studies of acute bacterial skin/soft tissue infections (ATLAS phase III trials), hospital-associated pneumonia (ATTAIN phase III trials), and endocarditis (ICE cohort) (5, 50–53) were included to determine CPT MICs. The previously described strains S. aureus TX0117 (harboring βla type A and exhibiting the CFZ InE) and its βla-cured derivative, TX0117c (21, 22), were used in the rat IE model. The sequences of blaZ genes from all MSSA strains were already available from a previous study (5), confirming that all the strains harbored βla type A exhibiting Thr128 and Ser216 substitutions, which distinguishes it from other βla types (12).
Antibiotics and MIC determination.
CFZ and NAF were acquired from Santa Cruz Biotechnology (Santa Cruz, CA) and MP Biomedicals, LLC (Solon, OH), respectively. Ceftaroline fosamil (batch number 0019D2B) was provided by Allergan (formerly Forest Research Institute and Actavis) (Parsippany, NJ). These antibiotics were reconstituted as recommended by the manufacturers. Nitrocefin was purchased from Calbiochem (Billerica, MA). CFZ, NAF, and CPT MICs against 17 MSSA strains, including TX0117 and TX0117c, were determined by the broth microdilution method using cation-adjusted BBL Mueller-Hinton II broth (BD, Sparks, MD), following Clinical and Laboratory Standards Institute (CLSI) guidelines (54) at the standard inoculum (105 CFU/ml). For the high inoculum (107 CFU/ml), MICs were determined by broth microdilution following our previously published method (5). MIC results for CPT were evaluated at 18 h, following the drug manufacturer's package insert, and for other drugs after 18 to 24 h of incubation at 37°C. S. aureus ATCC 29213 (known to produce small amounts of βla type A) and βla-negative S. aureus ATCC 25923 were used as controls.
Rat PK analysis.
A total of three independent male Sprague-Dawley rats (weight, ∼200 g) with cannulated jugular veins (JVC) (Harlan Laboratories, Houston, TX) were used to facilitate blood sampling. Each animal was given a single i.m. dose of CPT at 20 mg/kg or 40 mg/kg. Serial blood samples were collected at 0 h (prior to CPT dosing); at 5, 15, and 30 min; and at 1, 1.5, 2, 3, 4, 6, and 8 h. The animals were not anesthetized during the blood collection process and were sacrificed after the last blood sample was collected following the approved protocol. Blood samples (0.2 ml each) were collected via the jugular vein cannula and were placed in tubes prealiquoted with 2 μl of Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich). The samples were centrifuged immediately at ∼1,500 × g for 10 min in a refrigerated centrifuge, and plasma samples were stored immediately at −80°C. The plasma samples were analyzed at Keystone Bioanalytical, Inc. (North Wales, PA, USA) using a validated liquid chromatography-tandem mass spectrometry (LC–MS-MS) assay (lower limit of detection, 50 ng/ml). Data obtained from plasma samples were analyzed by a noncompartmental model using PKSolver 2.0 software. Drug exposures were expressed as the area under the concentration-time curve from 0 h to infinity (AUC0–∞). The terminal half-life (t1/2), highest plasma concentration observed (Cmax), and time to Cmax (Tmax) for CPT were determined. The fT>MIC was estimated based on the terminal elimination constant (ke) derived from the PK study. Assuming 20% protein binding for CPT (55), the fT>MIC was estimated for TX0117 based on its MIC of 0.25 μg/ml. The CPT dosing regimen for actual treatment of TX0117- and TX0117c-inoculated rats was guided by the results of the PK analysis (see below). The study was approved by the University of Texas Health Science Center at Houston Animal Welfare Committee (UTHSC-AWC-14-036).
Rat endocarditis model.
Aortic valve endocarditis was produced in male Sprague-Dawley rats weighing ∼200 g following our previously published methods (21, 56). In brief, the animals were anesthetized with isoflurane for intravascular catheter placement. The right carotid artery was accessed, and a sterile polyethylene catheter (Intramedic PE 10; Clay Adams, Parsippany, NJ) was inserted and advanced into the left ventricle across the aortic valve, where it was ligated and left in place for the whole duration of the experiment (21, 56). Bacteria for inoculum preparation were grown in BD Tryptic Soy Broth (BD, Sparks, MD) overnight with gentle shaking. Cells were harvested at 10,000 rpm for 10 min, and the bacterial pellets were resuspended in saline solution.
The inoculum that infected 90% of the rats (ID90) for TX0117 and TX0117c (in saline suspension) was determined by injecting various inocula (ranging from 101 to 107 CFU/rat) intravenously (i.v.) via the tail vein ∼24 h after catheter placement. The ID90 was determined by the method of Reed and Muench (57) by scoring infected versus noninfected vegetations. We estimated an inoculum of at least 10 times the ID90 (by A600). The actual inocula, determined by CFU enumeration, were confirmed as ∼10 times the ID90 of the infecting organism. Bacterial inocula were administered i.v. via the tail vein ∼24 h after vascular catheterization (21).
Antimicrobial therapy.
Antibiotic doses administered to the rats were based on our own PK/PD data for CPT. The CPT dose was selected to achieve an fT>MIC that has been shown to have efficacy against S. aureus in murine thigh and lung infection and rabbit endocarditis models (24, 58, 59). Doses of NAF and CFZ were selected based on previously published studies demonstrating in vivo efficacy in experimental endocarditis (21, 44, 45). Antibiotic treatment was initiated ∼36 h after the bacterial challenge. Baseline (t0) numbers of CFU per gram of bacteria in vegetations at the time of therapy initiation were determined by sacrificing 2 or 3 animals in each experiment and then plating serial dilutions of homogenized aortic valves containing vegetations onto BD Brain Heart Infusion Agar (BHIA) (BD, Sparks, MD).
Antibiotic regimens were administered for 3 days and included (i) CPT at 40 mg/kg q8h i.m., (ii) CFZ at 50 mg/kg q8h i.m. (45), and (iii) NAF at 400 mg/kg q8h subcutaneously (s.c.) (44). The animals were sacrificed ∼15 h after the last antibiotic dose, and vegetations formed on the aortic valve and surrounding tissues were aseptically removed, weighed, and homogenized in 1 ml of 0.9% saline solution. Sequential dilutions of the homogenized tissues were carried out, and subsequently, the entire volume of each dilution (including the undiluted sample) was plated onto BHIA. The geometric mean log10 CFU per gram ± standard deviations were calculated from colonies recovered from vegetations and then compared with t0 controls and among treatment groups. Animals were included in the final analysis only if the catheters were found across the aortic valve in the left ventricle, and only rats that survived beyond the first 24 h of therapy were included in the treatment group (21, 60). The minimum detection limit of bacteria by this method was 101 CFU/g of tissue. The production of β-lactamase in bacteria recovered from tissues was confirmed by the nitrocefin liquid test as previously described (21).
Data analysis.
The numbers of bacterial CFU per gram were log transformed to negate the effect of large positive skewing of recovery values prior to performing unpaired t tests to obtain P values (21, 56, 61–64). Cultures yielding no growth were scored as sterile and were assigned a value of 1 CFU for statistical analysis or to obtain geometric means of CFU per gram of vegetation. In animals that had only 1 colony recovered from the entire undiluted tissue homogenate, this value was converted to the number per gram of tissue (as was done with other recovered CFU) to determine the minimum detection limit (MDL) CFU per gram (21, 56, 61–64). Data and graphs were generated using Prism for Windows (version 4.00; GraphPad Software). Overall, differences were considered significant at a P level of <0.05.
ACKNOWLEDGMENTS
This work was supported by Allergan (formerly Forest Research Institute and Actavis, Inc.) in the form of a grant to the University of Texas Health Science Center, McGovern Medical School (K.V.S., principal investigator). T.T.T. is supported by NIH/NIAID grant K08-AI113317. C.A.A. is supported by NIH/NIAID grants R01-AI093749 and K24-AI114818. B.E.M. and K.V.S. were supported in part by NIH/NIAID R01 AI047923.
We thank Isabel Reyes and Karen Jacques-Palaz for technical assistance.
REFERENCES
- 1.Boucher H, Miller LG, Razonable RR. 2010. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 51(Suppl 2):S183–S197. doi: 10.1086/653519. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother 40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 3.Moellering RC., Jr 2012. MRSA: the first half century. J Antimicrob Chemother 67:4–11. doi: 10.1093/jac/dkr437. [DOI] [PubMed] [Google Scholar]
- 4.Livorsi DJ, Crispell E, Satola SW, Burd EM, Jerris R, Wang YF, Farley MM. 2012. Prevalence of blaZ gene types and the inoculum effect with cefazolin among bloodstream isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 56:4474–4477. doi: 10.1128/AAC.00052-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2009. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 53:3437–3441. doi: 10.1128/AAC.00317-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rincon S, Reyes J, Carvajal LP, Rojas N, Cortes F, Panesso D, Guzman M, Zurita J, Adachi JA, Murray BE, Nannini EC, Arias CA. 2013. Cefazolin high-inoculum effect in methicillin-susceptible Staphylococcus aureus from South American hospitals. J Antimicrob Chemother 68:2773–2778. doi: 10.1093/jac/dkt254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong YP, Park SJ, Kim ES, Bang KM, Kim MN, Kim SH, Lee SO, Choi SH, Jeong JY, Woo JH, Kim YS. 2015. Prevalence of blaZ gene types and the cefazolin inoculum effect among methicillin-susceptible Staphylococcus aureus blood isolates and their association with multilocus sequence types and clinical outcome. Eur J Clin Microbiol Infect Dis 34:349–355. doi: 10.1007/s10096-014-2241-5. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Echevarria KL, Traugott KA. 2017. Beta-lactam therapy for methicillin-susceptible Staphylococcus aureus bacteremia: a comparative review of cefazolin versus antistaphylococcal penicillins. Pharmacotherapy 37:346–360. doi: 10.1002/phar.1892. [DOI] [PubMed] [Google Scholar]
- 9.Kernodle DS, McGraw PA, Stratton CW, Kaiser AB. 1990. Use of extracts versus whole-cell bacterial suspensions in the identification of Staphylococcus aureus beta-lactamase variants. Antimicrob Agents Chemother 34:420–425. doi: 10.1128/AAC.34.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kernodle DS, Stratton CW, McMurray LW, Chipley JR, McGraw PA. 1989. Differentiation of beta-lactamase variants of Staphylococcus aureus by substrate hydrolysis profiles. J Infect Dis 159:103–108. doi: 10.1093/infdis/159.1.103. [DOI] [PubMed] [Google Scholar]
- 11.Richmond MH. 1975. Immunological techniques for studying beta-lactamases. Methods Enzymol 43:86–100. doi: 10.1016/0076-6879(75)43082-8. [DOI] [PubMed] [Google Scholar]
- 12.Voladri RK, Kernodle DS. 1998. Characterization of a chromosomal gene encoding type B beta-lactamase in phage group II isolates of Staphylococcus aureus. Antimicrob Agents Chemother 42:3163–3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nannini EC, Stryjewski ME, Singh KV, Rude TH, Corey GR, Fowler VG Jr, Murray BE. 2010. Determination of an inoculum effect with various cephalosporins among clinical isolates of methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother 54:2206–2208. doi: 10.1128/AAC.01325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant RE, Alford RH. 1977. Unsuccessful treatment of staphylococcal endocarditis with cefazolin. JAMA 237:569–570. [PubMed] [Google Scholar]
- 15.Fernandez-Guerrero ML, de Gorgolas M. 2005. Cefazolin therapy for Staphylococcus aureus bacteremia. Clin Infect Dis 41:127. doi: 10.1086/430833. [DOI] [PubMed] [Google Scholar]
- 16.Quinn EL, Pohlod D, Madhavan T, Burch K, Fisher E, Cox F. 1973. Clinical experiences with cefazolin and other cephalosporins in bacterial endocarditis. J Infect Dis 128(Suppl):S386–S389. doi: 10.1093/infdis/128.Supplement_2.S386. [DOI] [PubMed] [Google Scholar]
- 17.Forsblom E, Ruotsalainen E, Jarvinen A. 2016. Comparable effectiveness of first week treatment with anti-staphylococcal penicillin versus cephalosporin in methicillin-sensitive Staphylococcus aureus bacteremia: a propensity-score adjusted retrospective study. PLoS One 11:e0167112. doi: 10.1371/journal.pone.0167112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vardakas KZ, Apiranthiti KN, Falagas ME. 2014. Antistaphylococcal penicillins versus cephalosporins for definitive treatment of meticillin-susceptible Staphylococcus aureus bacteraemia: a systematic review and meta-analysis. Int J Antimicrob Agents 44:486–492. doi: 10.1016/j.ijantimicag.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Flamm RK, Sader HS, Jones RN. 2014. Ceftaroline activity against organisms isolated from respiratory tract infections in U S A hospitals: results from the AWARE Program, 2009-2011. Diagn Microbiol Infect Dis 78:437–442. doi: 10.1016/j.diagmicrobio.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2015. Activity of ceftaroline and comparator agents tested against Staphylococcus aureus from patients with bloodstream infections in US medical centres (2009-13). J Antimicrob Chemother 70:2053–2056. doi: 10.1093/jac/dkv076. [DOI] [PubMed] [Google Scholar]
- 21.Nannini EC, Singh KV, Arias CA, Murray BE. 2013. In vivo effect of cefazolin, daptomycin, and nafcillin in experimental endocarditis with a methicillin-susceptible Staphylococcus aureus strain showing an inoculum effect against cefazolin. Antimicrob Agents Chemother 57:4276–4281. doi: 10.1128/AAC.00856-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nannini EC, Singh KV, Murray BE. 2003. Relapse of type A beta-lactamase-producing Staphylococcus aureus native valve endocarditis during cefazolin therapy: revisiting the issue. Clin Infect Dis 37:1194–1198. doi: 10.1086/379021. [DOI] [PubMed] [Google Scholar]
- 23.Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 50:1376–1383. doi: 10.1128/AAC.50.4.1376-1383.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keel RA, Crandon JL, Nicolau DP. 2011. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob Agents Chemother 55:4028–4032. doi: 10.1128/AAC.00372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frampton JE. 2013. Ceftaroline fosamil: a review of its use in the treatment of complicated skin and soft tissue infections and community-acquired pneumonia. Drugs 73:1067–1094. doi: 10.1007/s40265-013-0075-6. [DOI] [PubMed] [Google Scholar]
- 26.Lodise TP, Low DE. 2012. Ceftaroline fosamil in the treatment of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections. Drugs 72:1473–1493. doi: 10.2165/11635660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Mpenge MA, MacGowan AP. 2015. Ceftaroline in the management of complicated skin and soft tissue infections and community acquired pneumonia. Ther Clin Risk Manag 11:565–579. doi: 10.2147/TCRM.S75412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.So W, Crandon JL, Zhanel GG, Nicolau DP. 2014. Comparison of in vivo and in vitro pharmacodynamics of a humanized regimen of 600 milligrams of ceftaroline fosamil every 12 hours against Staphylococcus aureus at initial inocula of 106 and 108 CFU per milliliter. Antimicrob Agents Chemother 58:6931–6933. doi: 10.1128/AAC.03652-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhanel GG, Rossnagel E, Nichol K, Cox L, Karlowsky JA, Zelenitsky S, Noreddin AM, Hoban DJ. 2011. Ceftaroline pharmacodynamic activity versus community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus, heteroresistant vancomycin-intermediate S. aureus, vancomycin-intermediate S. aureus and vancomycin-resistant S. aureus using an in vitro model. J Antimicrob Chemother 66:1301–1305. doi: 10.1093/jac/dkr110. [DOI] [PubMed] [Google Scholar]
- 30.Bhalodi AA, Hagihara M, Nicolau DP, Kuti JL. 2014. In vitro pharmacodynamics of human simulated exposures of ceftaroline and daptomycin against MRSA, hVISA, and VISA with and without prior vancomycin exposure. Antimicrob Agents Chemother 58:672–677. doi: 10.1128/AAC.01516-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrison MW, Vance-Bryan K, Larson TA, Toscano JP, Rotschafer JC. 1990. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother 34:1925–1931. doi: 10.1128/AAC.34.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelenitsky SA, Booker B, Laing N, Karlowsky JA, Hoban DJ, Zhanel GG. 1999. Synergy of an investigational glycopeptide, LY333328, with once-daily gentamicin against vancomycin-resistant Enterococcus faecium in a multiple-dose, in vitro pharmacodynamic model. Antimicrob Agents Chemother 43:592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacqueline C, Caillon J, Le Mabecque V, Miegeville AF, Hamel A, Bugnon D, Ge JY, Potel G. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob Agents Chemother 51:3397–3400. doi: 10.1128/AAC.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan LC, Basuino L, Diep B, Hamilton S, Chatterjee SS, Chambers HF. 2015. Ceftobiprole- and ceftaroline-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 59:2960–2963. doi: 10.1128/AAC.05004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, Perez KK, Musick WL, Rosato AE, Musser JM. 2014. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapman SW, Steigbigel RT. 1983. Staphylococcal beta-lactamase and efficacy of beta-lactam antibiotics: in vitro and in vivo evaluation. J Infect Dis 147:1078–1089. doi: 10.1093/infdis/147.6.1078. [DOI] [PubMed] [Google Scholar]
- 37.Lee S, Choe PG, Song KH, Park SW, Kim HB, Kim NJ, Kim EC, Park WB, Oh MD. 2011. Is cefazolin inferior to nafcillin for treatment of methicillin-susceptible Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 55:5122–5126. doi: 10.1128/AAC.00485-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul M, Zemer-Wassercug N, Talker O, Lishtzinsky Y, Lev B, Samra Z, Leibovici L, Bishara J. 2011. Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect 17:1581–1586. doi: 10.1111/j.1469-0691.2010.03425.x. [DOI] [PubMed] [Google Scholar]
- 39.Goldman PL, Petersdorf RG. 1980. Importance of beta-lactamase inactivation in treatment of experimental endocarditis caused by Staphylococcus aureus. J Infect Dis 141:331–337. doi: 10.1093/infdis/141.3.331. [DOI] [PubMed] [Google Scholar]
- 40.Carrizosa J, Kobasa WD, Snepar R, Kaye KM, Kaye D. 1982. Cefazolin versus cephalothin in beta-lactamase-producing Staphylococcus aureus endocarditis in a rabbit experimental model. J Antimicrob Chemother 9:387–393. doi: 10.1093/jac/9.5.387. [DOI] [PubMed] [Google Scholar]
- 41.Reference deleted.
- 42.Reference deleted.
- 43.Nicolsen NC, LeCroy N, Alby K, Martin KE, Laux J, Lin FC, Daniels L, Weber DJ, Miller MB. 2013. Clinical outcomes with rapid detection of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from routine blood cultures. J Clin Microbiol 51:4126–4129. doi: 10.1128/JCM.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catherall EJ, Gillon V, Hurn S, Irwin R, Mizen L. 1992. Efficacy of ticarcillin-clavulanic acid for treatment of experimental Staphylococcus aureus endocarditis in rats. Antimicrob Agents Chemother 36:458–462. doi: 10.1128/AAC.36.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. 2005. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother 49:380–387. doi: 10.1128/AAC.49.1.380-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhavnani SM, Hammel JP, Van Wart SA, Rubino CM, Reynolds DK, Forrest A, Drusano GL, Khariton T, Friedland HD, Riccobene TA, Ambrose PG. 2015. Pharmacokinetic-pharmacodynamic analysis for efficacy of ceftaroline fosamil in patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 59:372–380. doi: 10.1128/AAC.02531-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhavnani SM, Hammel JP, Van Wart SA, Rubino CM, Reynolds DK, Forrest A, Khariton T, Friedland HD, Riccobene TA, Ambrose PG. 2013. Pharmacokinetic-pharmacodynamic analyses for efficacy of ceftaroline fosamil in patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother 57:6348–6350. doi: 10.1128/AAC.01748-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Justo JA, Mayer SM, Pai MP, Soriano MM, Danziger LH, Novak RM, Rodvold KA. 2015. Pharmacokinetics of ceftaroline in normal body weight and obese (classes I, II, and III) healthy adult subjects. Antimicrob Agents Chemother 59:3956–3965. doi: 10.1128/AAC.00498-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matzneller P, Lackner E, Lagler H, Wulkersdorfer B, Osterreicher Z, Zeitlinger M. 2016. Single- and repeated-dose pharmacokinetics of ceftaroline in plasma and soft tissues of healthy volunteers for two different dosing regimens of ceftaroline fosamil. Antimicrob Agents Chemother 60:3617–3625. doi: 10.1128/AAC.00097-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG Jr, International Collaboration on Endocarditis Merged Database Study Group. 2005. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis 41:507–514. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 51.Rubinstein E, Lalani T, Corey GR, Kanafani ZA, Nannini EC, Rocha MG, Rahav G, Niederman MS, Kollef MH, Shorr AF, Lee PC, Lentnek AL, Luna CM, Fagon JY, Torres A, Kitt MM, Genter FC, Barriere SL, Friedland HD, Stryjewski ME, ATTAIN Study Group . 2011. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis 52:31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stryjewski ME, Chu VH, O'Riordan WD, Warren BL, Dunbar LM, Young DM, Vallee M, Fowler VG Jr, Morganroth J, Barriere SL, Kitt MM, Corey GR, FAST 2 Investigator Group . 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother 50:862–867. doi: 10.1128/AAC.50.3.862-867.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stryjewski ME, Graham DR, Wilson SE, O'Riordan W, Young D, Lentnek A, Ross DP, Fowler VG, Hopkins A, Friedland HD, Barriere SL, Kitt MM, Corey GR, Assessment of Telavancin in Complicated Skin, Skin-Structure Infections Study. 2008. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis 46:1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 54.CLSI. 2016. Performance standard for antimicrobial susceptibility testing; 24th informational supplement. CLSI M100-S27 Clinical and Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 55.Saravolatz LD, Stein GE, Johnson LB. 2011. Ceftaroline: a novel cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Clin Infect Dis 52:1156–1163. doi: 10.1093/cid/cir147. [DOI] [PubMed] [Google Scholar]
- 56.Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. 2010. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog 6:e1000716. doi: 10.1371/journal.ppat.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent end points. Am J Hyg 27:493–497. [Google Scholar]
- 58.Bhalodi AA, Crandon JL, Biek D, Nicolau DP. 2012. Efficacy of ceftaroline fosamil in a staphylococcal murine pneumonia model. Antimicrob Agents Chemother 56:6160–6165. doi: 10.1128/AAC.01078-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacqueline C, Caillon J, Batard E, Le Mabecque V, Amador G, Ge Y, Biek D, Potel G. 2010. Evaluation of the in vivo efficacy of intramuscularly administered ceftaroline fosamil, a novel cephalosporin, against a methicillin-resistant Staphylococcus aureus strain in a rabbit endocarditis model. J Antimicrob Chemother 65:2264–2265. doi: 10.1093/jac/dkq328. [DOI] [PubMed] [Google Scholar]
- 60.Entenza JM, Hohl P, Heinze-Krauss I, Glauser MP, Moreillon P. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob Agents Chemother 46:171–177. doi: 10.1128/AAC.46.1.171-177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Svanborg Eden C. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46:839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayo SA, Song YK, Cruz MR, Phan TM, Singh KV, Garsin DA, Murray BE, Dial EJ, Lichtenberger LM. 2016. Indomethacin injury to the rat small intestine is dependent upon biliary secretion and is associated with overgrowth of enterococci. Physiol Rep 4:e12725. doi: 10.14814/phy2.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh KV, Nallapareddy SR, Murray BE. 2007. Importance of the ebp (endocarditis- and biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infect Dis 195:1671–1677. doi: 10.1086/517524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pinkston KL, Singh KV, Gao P, Wilganowski N, Robinson H, Ghosh S, Azhdarinia A, Sevick-Muraca EM, Murray BE, Harvey BR. 2014. Targeting pili in enterococcal pathogenesis. Infect Immun 82:1540–1547. doi: 10.1128/IAI.01403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]