LETTER
The dissemination of genetic elements encoding VEB-type β-lactamases among Gram-negative bacteria has become a public health concern worldwide (1, 2). Recently, a blaVEB-2 gene was detected in a novel plasmid in Vibrio parahaemolyticus, an important foodborne pathogen causing gastroenteritis in humans (3, 4). Following our previous study (4), we investigated the genetic features of a novel blaVEB-18 variant in V. parahaemolyticus and Vibrio alginolyticus, both of which belong to the Vibrio harveyi clade.
Isolates of V. parahaemolyticus VPS72 and V. alginolyticus VAS24 were obtained from shrimp samples in supermarkets in Shenzhen, China, in 2015. These two isolates were shown to be resistant to cephalosporin antibiotics by determination of the MICs by the broth microdilution method in accordance with the CLSI standard (5). PCR assays performed as previously described identified a blaVEB-type extended-spectrum β-lactamase (ESBL)-encoding gene in these two isolates (6). The full length of blaVEB alleles was amplified with primers VEB-F (ATGAAAATCGTAAAAAGGAT) and VEB-R (TTATTTATTCAAATAGTAAT) and sequenced by the Sanger method. Sequences of the blaVEB PCR products obtained from VPS72 and VAS24 showed that they belong to a new blaVEB variant, the deduced amino acid sequence of which revealed the presence of three substitutions (V19A, T104M, and N294D) compared with VEB-1. We designated this new blaVEB allele blaVEB-18 (accession no. NG_051319). To check for the activity of the novel β-lactamase, VEB-18, the entire coding sequence of VEB-18 was amplified with primers VEB18-F (GAGAAGATCATCACCA) and VEB-R (TTATTTATTCAAATAGTAAT), purified, and cloned into the pCR2.1-TOPO vector to obtain pCR2.1-blaVEB-18. Escherichia coli DH5α carrying pCR2.1-blaVEB-18 and pCR2.1-blaVEB-2 exhibited similar cefotaxime, ceftazidime, and ceftriaxone MICs of 8 to 16 mg/liter, suggesting that this variant exhibits activity similar to that of VEB-2.
A conjugation experiment performed as previously described (7) showed that the blaVEB-18 gene in V. parahaemolyticus VAS24 was transferred to E. coli J53 (Azr), whereas the cephalosporin resistance phenotype encoded by the blaVEB-18 gene in V. alginolyticus VPS72 could not be transferred to E. coli J53. However, the gene could be transferred to E. coli TG1 through electroporation. S1 pulsed-field gel electrophoresis (PFGE) analysis of VPS72, VAS24, and their corresponding transformant/transconjugant strains showed that one of the parental strains, VAS24, contained one plasmid of ∼240 kb, yet no plasmid was observed in the other three strains. A Southern hybridization experiment showed that the blaVEB-18 probe could not be positively hybridized to either the chromosome or plasmids in these strains. These data suggested that the blaVEB-18 allele may be harbored by small plasmids that could not be detected by S1 PFGE and hybridization experiments. Plasmids extracted from the transformant/transconjugant strains of VPS72 and VAS24 with the Qiagen Midi kit could be visualized on regular agarose gel with a size of ∼7 to 9 kb (data not shown).
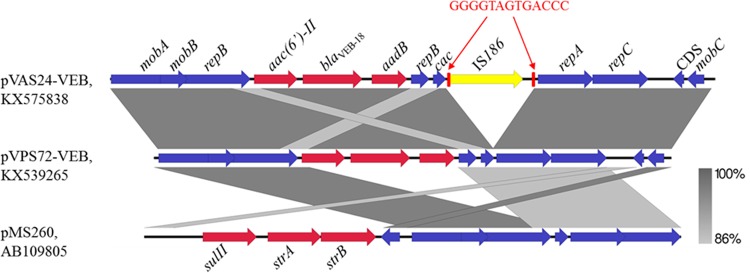
These plasmids were subjected to Illumina sequencing and then de novo assembly by SPAdes 3.5 (8). The blaVEB-bearing contigs were circularized by the primer-walking method. The blaVEB-18-bearing plasmid obtained from strain VPS72, pVPS72-VEB (accession no. KX539265), was found to be 7, 831 bp in length. The backbone of pVPS72-VEB was found to be similar to that of small, broad-host-range, IncQ-like plasmids with sizes of 5.1 to 14.2 kb (9). A BLASTn search of the nr database for the blaVEB-18 gene cassette indicated that it is a novel gene cassette and the blaVEB-aadB gene fragment could be found in different integrons in the NCBI database. Similarly, pVAS24-VEB (accession no. KX575838), which harbors the blaVEB-18 gene, is also an IncQ-like plasmid that exhibits homology with pVPS72-VEB, with IS186 inserted in a region between aadB and repA, resulting in a 13-bp target site duplication (Fig. 1). This event is similar to that mediated by IS186 in E. coli, which prefers to target the palindromic sequence structure 5′-GGGG(N6/N7)CCCC-3′ (10). The most similar backbone structure, with 86% identity to pVPS72-VEB and pVAS24-VEB, was found in an IncQ plasmid, pMS260, in Actinobacillus pleuropneumoniae (Fig. 1) (11). It has been reported that IncQ plasmids are highly mobilizable with the help of large conjugative plasmids and are able to nonselectively capture resistance genes in the environment (9). The pVAS24-VEB plasmid was transferrable in a conjugation assay; however, no transconjugant could be harvested for pVPS72-VEB. The ∼240-kb plasmid in VAS24 may be a conjugative plasmid that enabled the transfer of pVAS24-VEB to other bacteria. Although IncQ-type plasmids have been detected in various environmental niches, in various species of bacteria, and even in plants, this report is the first description of IncQ plasmids harboring blaVEB-18 in V. parahaemolyticus and V. alginolyticus. Because of their broad host range and wide distribution in various bacterial species, the discovery of IncQ plasmids harboring cephalosporinase genes would pose a significant threat to human health since they may facilitate the spread of this ESBL gene among aquatic pathogens or the transmission of such genes from aquatic bacteria to other pathogens.
FIG 1.
Schematic representation of the alignment of plasmids pVAS24-VEB, pVPS72-VEB, and pMS260 with similar IncQ plasmid backbones. The two red rectangles surrounding IS186 denote the left and right inverted repeats. The 13-bp segment is the target site duplication upon the insertion of IS186. Red arrows indicate the resistance genes, and blue arrows stand for maintenance genes in plasmids. The gray scale at the bottom right depicts percentages of sequence homology.
ACKNOWLEDGMENTS
This work was supported by the Chinese National Key Basic Research and Development (973) Program (2013CB127200) and Health and Medical Research Fund from Hong Kong (13121422).
We have no competing interests to declare.
No ethical approval was required for this study.
REFERENCES
- 1.Zong Z, Partridge SR, Iredell JR. 2009. A blaVEB-1 variant, blaVEB-6, associated with repeated elements in a complex genetic structure. Antimicrob Agents Chemother 53:1693–1697. doi: 10.1128/AAC.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naas T, Bogaerts P, Bauraing C, Degheldre Y, Glupczynski Y, Nordmann P. 2006. Emergence of PER and VEB extended-spectrum beta-lactamases in Acinetobacter baumannii in Belgium. J Antimicrob Chemother 58:178–182. doi: 10.1093/jac/dkl178. [DOI] [PubMed] [Google Scholar]
- 3.Letchumanan V, Chan KG, Lee LH. 2014. Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol 5:705. doi: 10.3389/fmicb.2014.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R, Ye L, Zheng Z, Chan EW, Chen S. 2016. Genetic characterization of a blaVEB-2-carrying plasmid in Vibrio parahaemolyticus. Antimicrob Agents Chemother 60:6965–6968. doi: 10.1128/AAC.01749-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CLSI. 2010. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious bacteria; approved guideline, 2nd ed CLSI document M45-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 6.Dallenne C, Da Costa A, Decre D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother 65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 7.Chiou CS, Alam M, Kuo JC, Liu YY, Wang PJ. 2015. Chromosome-mediated multidrug resistance in Salmonella enterica serovar Typhi. Antimicrob Agents Chemother 59:721–723. doi: 10.1128/AAC.04081-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftie-Eaton W, Rawlings DE. 2012. Diversity, biology and evolution of IncQ-family plasmids. Plasmid 67:15–34. doi: 10.1016/j.plasmid.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Doak TG, Popodi E, Foster PL, Tang H. 2016. Insertion sequence-caused large-scale rearrangements in the genome of Escherichia coli. Nucleic Acids Res 44:7109–7119. doi: 10.1093/nar/gkw647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito H, Ishii H, Akiba M. 2004. Analysis of the complete nucleotide sequence of an Actinobacillus pleuropneumoniae streptomycin-sulfonamide resistance plasmid, pMS260. Plasmid 51:41–47. doi: 10.1016/j.plasmid.2003.10.001. [DOI] [PubMed] [Google Scholar]