ABSTRACT
We have shown previously that oral treatment with sodium butyrate or phenylbutyrate in an experimental model of shigellosis improves clinical outcomes and induces the expression of the antimicrobial peptide CAP-18 in the large intestinal epithelia. In a subsequent study, we found that entinostat, an aroylated phenylenediamine compound, has similar therapeutic potential against shigellosis. In this study, we aimed to evaluate entinostat as a potential candidate for host-directed therapy against cholera in an experimental model. Vibrio cholerae-infected rabbits were treated with two different dose regimens of entinostat: either 0.5 mg twice daily for 2 days or 1 mg once daily for 2 days. The effects of treatment on clinical outcomes and V. cholerae shedding (CFU count in stool) were observed. Immunohistochemical analysis was carried out to assess CAP-18 expression in ileal and jejunal mucosae. The serum zonulin level was measured by an enzyme-linked immunosorbent assay (ELISA) to evaluate gut permeability. Infection of rabbits with V. cholerae downregulated CAP-18 expression in the ileal epithelium; the expression was replenished by oral treatment with entinostat at either dose regimen. The level of zonulin, a marker of gut permeability, in serum was upregulated after infection, and this upregulation was counteracted after treatment with entinostat. Entinostat treatment also led to recovery from cholera and a decline in the V. cholerae count in stool. In conclusion, the improved clinical outcome of cholera for rabbits treated with entinostat is associated with the induction of CAP-18 and the reduction of gut epithelial permeability.
KEYWORDS: animal models, antimicrobial peptides, cathelicidin, enteric pathogens, host-directed therapy, infectious diseases, innate immunity
INTRODUCTION
Cholera, an acute secretory diarrhea caused by infection of the small intestine with the bacterium Vibrio cholerae, remains one of the major public health concerns in many parts of the world. According to the World Health Organization, a total of 172,454 cholera cases, with 1,304 deaths, were reported in 42 countries in 2015, resulting in a case fatality rate of 0.8% (1). After entering the host in contaminated food or water, V. cholerae colonizes the small intestine and releases cholera enterotoxin (CT). By activating adenylate cyclase, CT induces the production of cyclic AMP, which, in turn, signals the opening of chloride ion channels and induces a subsequent net loss of salt and water from the intestine (2, 3). Loss of water and electrolytes quickly leads to severe dehydration and death if timely treatment is not given. V. cholerae secretes additional toxins, e.g., zonula occludens toxin, and hemagglutinin/protease, which increase the paracellular permeability of the epithelial barrier and thus add to the CT-mediated fluid secretion (4, 5).
Patients with mild cholera are managed by compensation of fluid loss through oral or intravenous rehydration therapy, but for patients with moderate to severe cholera, additional treatment with an antibiotic is recommended (6). The continued emergence of multidrug-resistant bacteria and the adverse effects of antibiotic treatment (e.g., disruption of the normal flora, allowing secondary infections and the release of toxic microbial components) limit the use of antibiotics for the management of infectious diseases (7, 8). Therefore, the development of novel antimicrobial therapies is warranted.
Antimicrobial peptides (AMPs) are effectors of the innate immune system, limiting the growth and virulence of pathogens at the host-microbe interface (9). Cathelicidins and defensins are two major classes of AMPs in mammals that have a wide range of antimicrobial activity. However, several pathogens have evolved strategies to suppress the expression of these AMPs and thereby constitute an immune escape mechanism. Our group previously demonstrated that expression of the sole human cathelicidin LL-37 and human beta-defensin 1 (HBD-1) is downregulated in the intestinal epithelial cells of patients during acute shigellosis and watery diarrhea (10). Besides, in polarized human intestinal cells, Shigella flexneri suppressed LL-37 and HBD-3 (11). In an experimental model of diarrhea induced by enteropathogenic Escherichia coli (EPEC), we showed the downregulation of CAP-18, a rabbit homologue of LL-37, in intestinal epithelia (12). Vibrio cholerae and enterotoxigenic Escherichia coli (ETEC) have been shown to suppress CAP-18 and the mouse cathelicidin mCRAMP in the ileal mucosae of rabbits and mice, respectively (13). The suppression was mediated by the CT and heat-labile toxin (LT) of V. cholerae and ETEC, respectively (13). Downregulation of LL-37 in duodenal epithelia was confirmed for patients with cholera and ETEC diarrhea (14). A novel therapeutic approach would be to compensate for the loss of AMPs by inducing their production at local sites of infection. Indeed, we have shown that treatment of Shigella- or EPEC-infected rabbits with sodium butyrate and/or its analogue phenylbutyrate induces CAP-18 in the intestinal epithelia, which is associated with improved clinicopathological features (12, 15, 16). We also conducted a clinical trial with Shigella-infected patients, where the use of butyrate simultaneously enhanced LL-37 expression and reduced the local inflammation of the large intestine (17). However, we did not observe a therapeutic effect of butyrate or phenylbutyrate in an experimental model of cholera (R. Raqib, unpublished data).
For large-scale screening of novel inducers of LL-37 expression, a reporter cell line (MN8CampLuc) producing a luciferase–LL-37 fusion protein has recently been constructed (18). Using MN8CampLuc, we have discovered a novel class of compounds, aroylated phenylenediamines (APDs), that are potent inducers of LL-37 (19). Entinostat was the strongest inducer in this class, and its treatment potential was subsequently tested in a rabbit model of shigellosis. We observed that oral administration of entinostat counteracted the downregulation of CAP-18, an effect that correlated with clinical recovery from shigellosis (19).
In the present study, building on these findings, we aimed to evaluate the efficiency of entinostat in treating acute secretory diarrhea caused by V. cholerae infection in a rabbit model. We investigated whether entinostat, given in one of two dose regimens—either 0.5 mg twice a day for 2 days or 1 mg once a day for 2 days—can lead to recovery from cholera symptoms, reduce V. cholerae counts in stool, and upregulate CAP-18 expression in ileal and jejunal epithelia. Additionally, the impact of entinostat treatment on gut epithelial barrier function was explored.
RESULTS
Improvement of the clinical outcome of cholera with oral entinostat treatment.
Infected rabbits (n = 13) developed mild to moderate cholera within 24 h of infection (on day 1), which was apparent by liquid stool mixed with soft stool pellets. One rabbit developed severe watery diarrhea and died within approximately 16 h of infection; this rabbit was considered untreated. Autopsy and stool specimens were collected from this rabbit, but blood could not be collected. One rabbit exhibited diarrheal symptoms on day 2 and was not treated. It is worth mentioning that this rabbit did not defecate on day 1. All rabbits became anorexic and lethargic and lost some body weight; a few rabbits (n = 4) developed transient fevers. Untreated rabbits were sacrificed within 4 h after the development of diarrhea on day 1 (n = 3) or day 2 (n = 1). In previous studies, rabbits challenged with V. cholerae died within 18 to 25 h postchallenge (20, 21). Therefore, in the present study, no effort was made to keep these rabbits alive, because they were weak and might die at night, in which case biopsy specimens could not be collected. Treatment with entinostat for 2 days (0.5 mg twice daily or 1 mg once daily) led all infected rabbits to recover from the disease; formed stool reappeared, and rabbits revived from lethargy and anorexia. However, their body weights remained similar.
Entinostat treatment reduces the Vibrio cholerae count in the intestine.
To investigate if recovery from cholera after entinostat treatment is linked to the number of pathogens in the gut, the shedding of V. cholerae in stool was measured over time. Oral treatment of infected rabbits with either dose regimen of entinostat led to a gradual decrease in the V. cholerae count in stool (Fig. 1). On day 4, the numbers of bacteria were 3 to 4 log units lower than those on day 1, i.e., before the start of the treatment.
FIG 1.
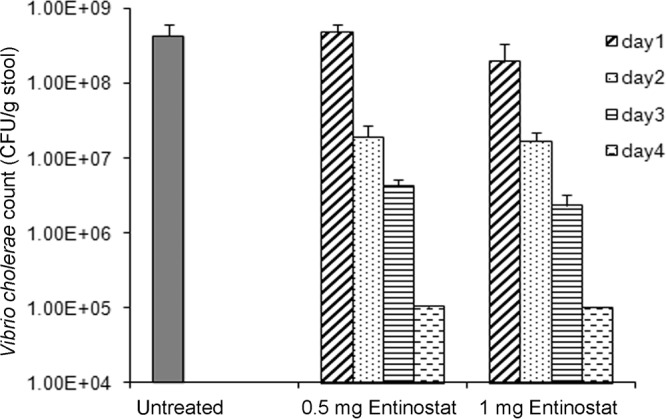
Effect of entinostat treatment on the shedding of Vibrio cholerae in stool. Stool samples were collected from V. cholerae-infected rabbits that were either left untreated (n = 5) or treated with entinostat at a dose of 0.5 mg twice daily for 2 days (n = 5) or 1 mg once a day for 2 days (n = 5). Stool samples were collected from untreated rabbits once before sacrifice. For treated rabbits, stool samples were collected before the start of the treatment (day 1) and during or after the treatment (days 2 to 4). Serially diluted stool suspensions were plated onto TTGA plates, and colonies were counted after overnight incubation at 37°C. The results are expressed as CFU counts per gram of stool. Data are means ± SEM.
Levels of CAP-18, which is downregulated in the ileum by V. cholerae infection, are restored by entinostat.
Immunostaining of ileal and jejunal mucosae of healthy rabbits with a CAP-18-specific antibody revealed the localization of CAP-18 peptide/protein in the surface epithelia (SE) of the villi (Fig. 2A, top left). Upon semiquantitative image analysis, significant downregulation of CAP-18 expression was observed in the SE of the ileum villi after infection with V. cholerae (P < 0.001) (Fig. 2B). The downregulation of CAP-18 in the ileal SE of the infected rabbits was accompanied by partial shedding of the epithelial cells (Fig. 2A, top right). After the treatment of infected rabbits with either dose regimen of entinostat, CAP-18 expression was enhanced significantly (P, 0.001 for the 0.5-mg dose regimen and 0.02 for the 1-mg dose regimen) (Fig. 2B); eroded epithelium also healed after treatment (Fig. 2A, bottom). In jejunal epithelia, after infection and treatment, CAP-18 expression did not differ significantly from that in healthy rabbits.
FIG 2.
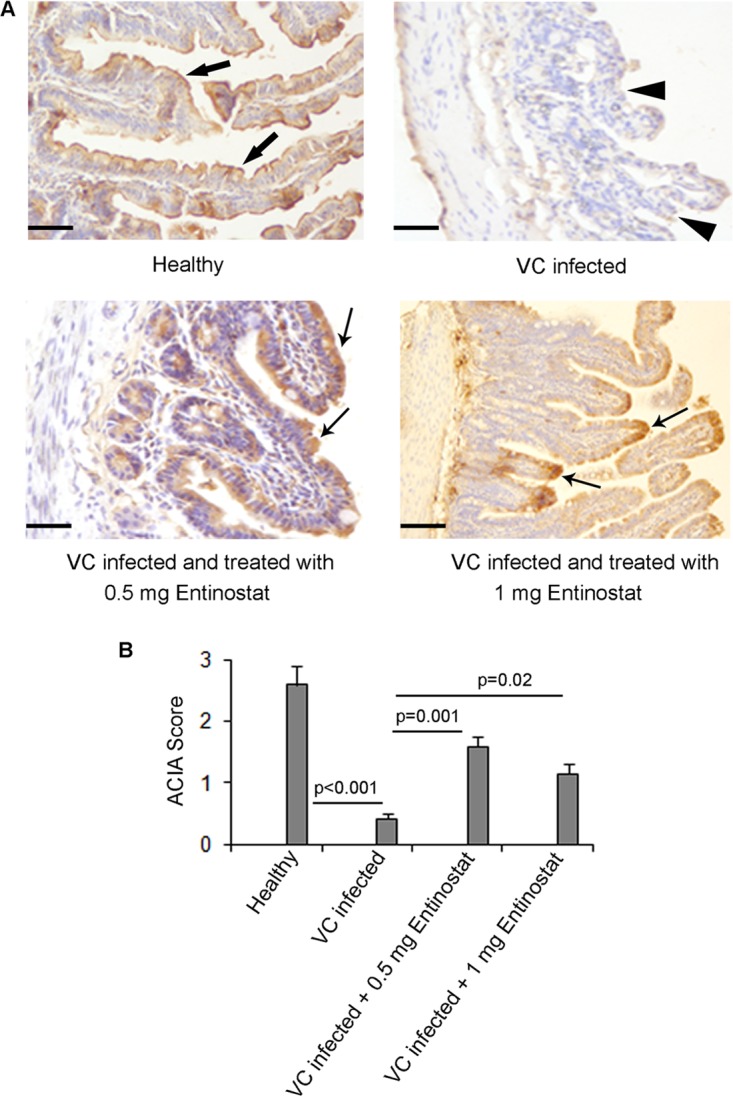
CAP-18 peptide/protein expression in the ileal mucosae of healthy rabbits, Vibrio cholerae-infected rabbits, and rabbits that were infected and treated with entinostat. Mucosal sections of ileum from healthy rabbits (n = 5), V. cholerae-infected rabbits (n = 5), and infected rabbits treated with 0.5 mg entinostat twice a day for 2 days (n = 5) or with 1 mg entinostat once a day for 2 days (n = 5) were stained with a CAP-18-specific antibody. (A) Representative photomicrographs. CAP-18-positive staining is brown; cell nuclei are stained blue with hematoxylin. (Top left) Prominent expression of CAP-18 (thick arrows) in the SE of healthy ileal mucosae. (Top right) Downregulation of CAP-18 expression in the SE after V. cholerae infection. Partial erosion of SE (arrowheads) was also noted. (Bottom) Recovery of epithelial erosion and restoration of CAP-18 expression (arrows) in the SE after treatment with 0.5 mg entinostat twice a day for 2 days (left) or 1 mg entinostat once a day for 2 days (right). Bars, 50 μm (top and bottom left); 100 μm (bottom right). (B) Semiquantitative expression of CAP-18 in SE, given as ACIA scores (see Materials and Methods). Data are means ± SEM. Differences in outcomes between different groups were estimated by mixed-model ANOVA. Differences are significant at a P value of <0.05.
Entinostat restores epithelial barrier integrity.
Increased paracellular permeability of intestinal epithelia may contribute to fluid loss in cholera. Therefore, the effects of V. cholerae infection and of entinostat treatment on gut permeability were studied in the current experimental model. For this purpose, the levels of circulatory zonulin (ZON), which has been identified as a marker of gut permeability in several diseases (22, 23), were measured. In infected, untreated rabbits, the concentration of zonulin in serum was significantly higher than that for healthy controls (P = 0.024). Treatment of infected rabbits with entinostat at either dose regimen resulted in a significant decrease in the serum zonulin level (P, 0.005 for the 0.5-mg dose regimen and 0.002 for the 1-mg dose regimen) (Fig. 3).
FIG 3.
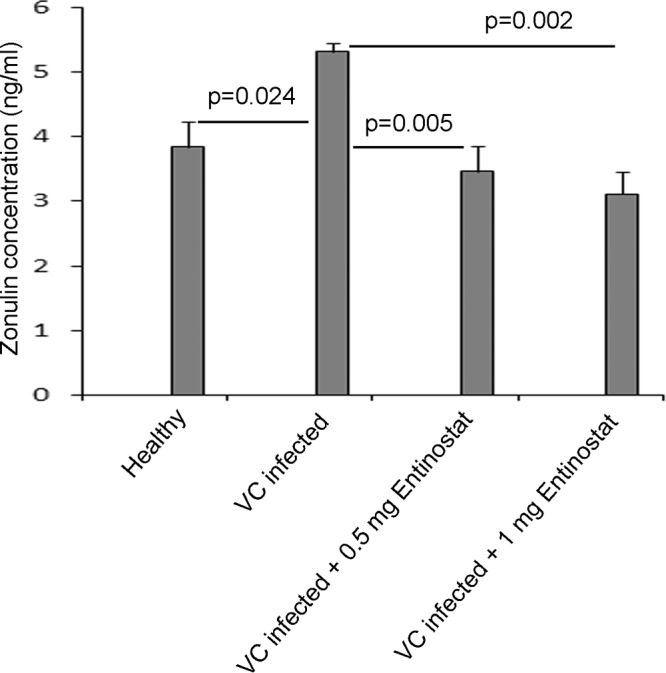
Serum zonulin levels in healthy, Vibrio cholerae-infected, and infected and entinostat-treated rabbits. Serum was collected from healthy rabbits (n = 5), untreated V. cholerae-infected rabbits (n = 4), and infected rabbits treated with 0.5 mg entinostat twice daily for 2 days (n = 5) or with 1 mg entinostat once a day for 2 days (n = 5). Levels of zonulin, a marker of gut permeability, in serum were quantified by using a commercially available rabbit zonulin ELISA kit. Data are means ± SEM. Differences in outcomes between different groups were estimated by mixed-model ANOVA. Differences are significant at a P value of <0.05.
In vitro antibacterial activities of entinostat and CAP-18.
To determine whether the reduction in the V. cholerae count in stool after treatment is due to the direct inhibitory effect of entinostat or to increased CAP-18 levels in the ileal epithelium, the antibacterial activity of entinostat or a synthetic CAP-18 peptide against V. cholerae was assessed in an in vitro experiment. Entinostat at a concentration range of 0.25 to 1.0 mg/ml did not reduce the bacterial count when incubated for 2 h (Fig. 4A) or overnight. After 2 h of incubation, CAP-18 at concentrations of 3.0, 3.5, 4.0, 4.5, and 5.0 μg/ml exhibited a dose-dependent vibriocidal effect, and complete clearance of V. cholerae was observed with a 5-μg/ml concentration (Fig. 4B). After overnight incubation, the killing of V. cholerae by CAP-18 was not dose specific. In some experiments, 4 μg/ml of CAP-18 killed all bacteria; in others, there was no significant reduction in the bacterial count with CAP-18 concentrations up to 4.5 μg/ml, but complete killing occurred with 5 μg/ml CAP-18. It is possible that after the initial dose-dependent reduction, the remaining viable bacteria grew overnight to a saturation level in the growth medium, leading to similar counts at different doses of CAP-18. When CAP-18 and entinostat were used in combination for 2 h, entinostat (1.0 mg/ml) did not display any additive/synergistic effect on CAP-18-mediated killing.
FIG 4.
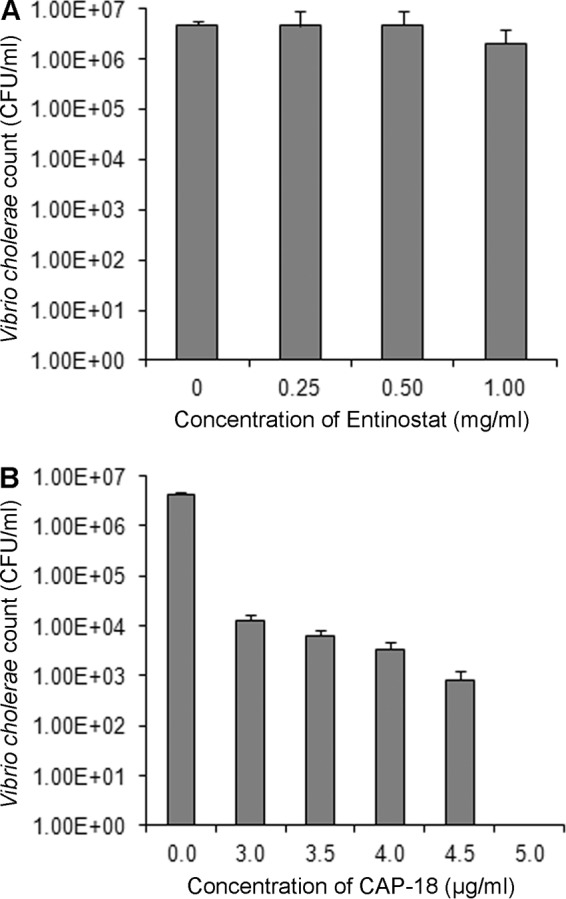
In vitro inhibitory effect of entinostat or CAP-18 on the growth of Vibrio cholerae. A suspension of V. cholerae Ogawa was incubated with different concentrations of entinostat (A) or synthetic CAP-18 (B) in MHB in wells of a microtiter plate at 37°C for 2 h. Bacterial suspensions from individual wells were plated on TTGA, and CFU were counted after overnight incubation. Data are means ± SEM from 2 and 4 individual experiments for entinostat and CAP-18, respectively.
Entinostat has no harmful effect on the renal and hepatic functions of rabbits.
To exclude any toxic effect of entinostat treatment on renal and hepatic functions, concentrations of renal biomarkers (creatinine and blood urea nitrogen [BUN]) and hepatic enzymes (alanine transaminase [ALT], aspartate transaminase [AST], and gamma-glutamyl transferase [GGT]) were measured in serum. No significant differences were observed in the levels of these biomarkers in serum between untreated healthy rabbits, untreated infected rabbits, and infected rabbits treated with entinostat (Table 1), indicating that entinostat was safe for rabbits at the doses given.
TABLE 1.
Levels of hepatic and renal biomarkers in serum samples from healthy rabbits, Vibrio cholerae-infected rabbits, and infected rabbits treated with entinostata
| Rabbit group (n) | Levels of hepatic enzymes (U/liter) |
Levels of renal markers |
|||
|---|---|---|---|---|---|
| ALT | AST | GGT | Creatinine (μmol/liter) | BUN (mmol/liter) | |
| Healthy (5) | 55.9 ± 6.2 | 66.8 ± 14.4 | 10.6 ± 1.0 | 105.6 ± 11.9 | 4.08 ± 1.0 |
| Infected and not treated (4) | 62.02 ± 16.2 | 30.69 ± 6.1 | 10.1 ± 3.2 | 70.83 ± 16.3 | 17.05 ± 5.8 |
| Infected and treated with entinostat | |||||
| 0.5 mg b.d.s. for 2 days (5) | 39.91 ± 11.4 | 30.18 ± 6.6 | 11.25 ± 2.8 | 63.42 ± 12.5 | 19.3 ± 6.9 |
| 1 mg q.d. for 2 days (5) | 49.78 ± 8.2 | 41.6 ± 14.8 | 6.4 ± 1.2 | 77.82 ± 2.3 | 8.2 ± 1.4 |
Data are expressed as means ± SEM. ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transferase; BUN, blood urea nitrogen; b.d.s., twice daily; q.d., once a day.
DISCUSSION
In an experimental rabbit model, we have demonstrated that oral treatment with entinostat led to clinical recovery from cholera. The improved clinical outcome was accompanied by a marked reduction in the V. cholerae count in the stool and by the restoration of epithelial barrier integrity. V. cholerae infection resulted in significant downregulation of the cathelicidin CAP-18 in the ileal epithelium, an effect that was counteracted by entinostat therapy.
The downregulation of the cathelicidin family of AMPs in intestinal epithelial cells by V. cholerae, as shown previously in rabbit and mouse ilea (13) and in patients' duodena (14), has been reproduced here in a reversible intestinal tie-adult rabbit diarrhea (RITARD) model. The experimental setup has been established in order to test the treatment approach of compensating for the loss of AMPs at the local sites of infection.
In the present study, the downregulated CAP-18 in the ileal epithelium during V. cholerae infection was restored after treatment with either of the two different dose regimens of entinostat (0.5 mg twice daily, or 1 mg once daily, for 2 days). Importantly, infected rabbits recovered from disease symptoms after the treatment; treated rabbits were no longer lethargic or anorexic and passed normal stool. Recovery from disease symptoms was accompanied by reduced numbers of V. cholerae bacteria in the gut lumen. Epithelial cells that underwent hydropic degeneration, death, and subsequent shedding due to cholerogenic V. cholerae (24) were replaced with new cells after the treatment. Induced CAP-18 could be involved, at least in part, in the reepithelialization of intestinal mucosae, as shown previously for LL-37 in human skin wounds (25).
In an in vitro antibacterial assay, a synthetic CAP-18 peptide killed, in a dose-dependent manner, the V. cholerae Ogawa isolate that was used to infect the rabbits. Entinostat neither exhibited a vibriocidal effect nor enhanced the killing capacity of the CAP-18 peptide. These findings indicate that entinostat has no direct involvement in eliminating V. cholerae from the gut lumen but contributes indirectly by inducing CAP-18, and possibly additional AMPs, in intestinal epithelia.
Enhanced acetylation of histones by histone deacetylase inhibitors (HDACi), e.g., butyrate, phenylbutyrate, and trichostatin A (TSA), has been proposed to mediate the transcriptional induction of LL-37 (26–28). A panel of HDACi that are analogues of butyrate or phenylbutyrate also induced LL-37 in the MN8CampLuc reporter cell line (18). Entinostat is a benzamide histone deacetylase inhibitor; hence, this activity could be included in the induction of LL-37. However, the much higher induction by entinostat and other APDs than by more-potent HDAC inhibitors (e.g., vorinostat and trichostatin A) suggests that HDAC inhibition cannot be the major route (19). Other mechanisms are likely to be responsible for the efficient induction of LL-37 by APDs. One major route seems to be activation of STAT3, which, in turn, induces HIF-1; HIF-1 subsequently increases the expression of LL-37 and HBD-1 (29).
The intestinal epithelium forms a physical barrier between the gut lumen and underlying tissue compartments, allowing the absorption of nutrients and the selective passage of molecules while preventing the entry of noxious luminal contents. The paracellular permeability of this barrier is tightly regulated by the apical junction complex, consisting of the tight junction, adherens junction, and desmosome (30). Zonula occludens toxin and hemagglutinin/protease produced by V. cholerae have been shown to modify tight junctions and thus increase the paracellular permeability of the epithelial barrier (4, 5, 31, 32). Interestingly, the production of zonula occludens toxin correlated with the diarrheagenicity of V. cholerae strains in volunteers (4). Zonulin, a mammalian protein, was identified as the analogue of zonula occludens toxin and to date is the only known physiologic modulator of intercellular tight junctions (22, 33). The circulating zonulin level has been established as the marker of gut permeability in several diseases (22, 23). In this study, we demonstrated increased concentrations of zonulin in the sera of rabbits infected with V. cholerae. After treatment with either dose regimen of entinostat, zonulin levels decreased to the levels in healthy controls, indicating the reversal of gut permeability. However, due to the lack of a rabbit-specific antibody, we could not detect the expression of tight-junction proteins in the intestinal tissue and hence could not identify the tight-junction modification after infection and treatment. Interestingly, butyrate and other HDACi have been shown to maintain epithelial barrier integrity by altering tight-junction permeability (34–36). It is plausible that entinostat, through HDAC inhibition, can reverse V. cholerae-induced disruption of intestinal tight junctions and consequently decrease gut permeability. On the other hand, recent studies have reported that host defense peptides play roles in intestinal barrier function by directly regulating mucin and tight-junction protein expression (37). Cathelicidin-BF, a peptide purified from the venom of the Bungarus fasciatus snake, has been shown to ameliorate lipopolysaccharide (LPS)-induced disruption of the epithelial barrier in rats via prevention of the downregulation of tight-junction proteins (38). Therefore, it is quite possible that similar modification of tight-junction proteins by entinostat-induced CAP-18 may contribute to the reestablishment of epithelial barrier integrity.
Entinostat at a concentration of 6 to 8 mg/m2 has been reported to be safe and well tolerated by patients with myeloid leukemia, solid tumors, and lymphomas in phase I clinical trials (39–41). Entinostat in combination with other drugs has also been found safe and efficacious in phase II trials (42, 43). In rat liver, no significant histopathological changes were shown after the administration of as much as 49 mg entinostat/kg of body weight for 7 days (44). In the current study, we used a much lower concentration of entinostat to treat infected rabbits based on the strong in vitro LL-37-inducing capacity of entinostat. As expected, no toxic effect was observed in rabbits treated with entinostat.
In conclusion, we have demonstrated that entinostat can be used in treating watery diarrhea caused by V. cholerae through its ability to induce epithelial barrier integrity and innate defenses. The induction of AMPs suggests that entinostat and other APDs are possible candidates against infectious diseases in general, where pathogens downregulate the mucosal expression of AMPs as a means of evading host immunity, as shown, for example, in shigellosis and cholera.
MATERIALS AND METHODS
Ethics statement.
This study was approved by the Animal Experimentation Ethics Committee of the icddr,b. All experiments conformed to the rules and guidelines of the icddr,b, which were developed based on the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) (45).
Bacteria.
Vibrio cholerae 01 Ogawa was isolated from the stool of a cholera patient; the isolate was a kind gift from the Clinical Microbiology and Immunology Laboratory of icddr,b. The virulence of the clinical isolate was confirmed by a rabbit ileal loop assay; ileal segments infected with this isolate resulted in pronounced accumulation of fluid.
Bacteria were maintained on nutritive soft agar (T1N1; 0.75% agar, mineral oil) at room temperature until further use.
Preparation of infectious inocula.
Vibrio cholerae 01 Ogawa from stock culture was streaked onto taurocholate-tellurite-gelatin agar (TTGA) plates (icddr,b media facility) to obtain individual colonies. Two to three isolated colonies were taken from a plate in a culture flask containing ∼20 ml tryptic soya broth (TSB; Becton Dickinson, NJ, USA) and were grown to exponential phase by incubating at 37°C for 2 h in a shaker incubator. Bacterial cells were then pelleted by centrifugation at 7,000 rpm for 10 min. After two washes with sterile normal saline, the concentration of bacteria was adjusted to an optical density at 595 nm (OD595) of 0.2, which corresponds to an infectious dose of ∼1 × 108 CFU/ml. A 2-fold dilution was made to prepare the infectious dose of ∼5 × 107 CFU/ml for each rabbit.
RITARD model of infection and treatment procedure.
The previously established reversible intestinal tie-adult rabbit diarrhea (RITARD) model for V. cholerae and ETEC was adopted with slight modifications (20). Briefly, inbred New Zealand White rabbits (Charles River Laboratories, Wilmington, MA, USA) of either sex, aged 2.5 to 3 months and weighing 1.7 to 1.9 kg, were maintained in the animal resource facilities of icddr,b. Healthy rabbits free of enteric pathogens (e.g., Salmonella, Shigella, V. cholerae, and coccidia) were starved for 24 h before inoculation with V. cholerae. On the day of inoculation (day 0), rabbits were anesthetized intravenously with 33 mg of sodium pentobarbital (Sigma-Aldrich, St. Louis, MO, USA) per kg of body weight. The cecum was brought out through a midline incision and was ligated close to the ileocecal junction. The small intestine was brought out, and a slip knot was tied around it close to the mesoappendix. Thereafter, 1 ml of a V. cholerae Ogawa suspension containing ∼5 × 107 CFU in sterile normal saline was injected into the lumen of the anterior jejunum. The intestine and cecum were returned to the peritoneal cavity, and the incision was closed. The rabbit was kept in a special holding box, and the temporary tie was removed 2 h after bacterial challenge. The unclosed portion of the skin incision was sutured, and the animal was returned to its cage and was provided with food and water ad libitum.
After the development of diarrheal symptoms (usually within 24 h of inoculation, i.e., on day 1), infected rabbits were either left untreated (n = 5) or treated with 0.5 mg entinostat (LC Laboratories, Woburn, MA, USA) twice a day for 2 days (n = 5) or 1 mg entinostat once a day for 2 days (n = 5). Entinostat was given in 7 ml of normal saline via a sterile orogastric feeding tube. The treatment regimen was started on the afternoon of day 1 and was completed on day 3. All treated rabbits were sacrificed on day 4 with an overdose of intravenous sodium pentobarbital (66 mg/kg of body weight). Infected but untreated rabbits were sacrificed within 4 h after the development of diarrhea in order to relieve their suffering and to collect biopsy specimens before their death. Figure 5 outlines the course of inoculation and treatment and shows when the rabbits were sacrificed. An additional five rabbits were used as healthy controls.
FIG 5.
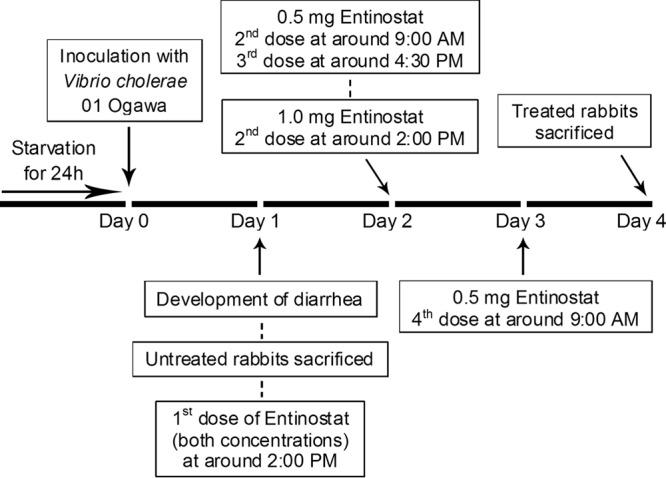
Schematic diagram showing the infection and treatment schedules of the rabbits and the times of sacrifice.
Specimen collection.
Stool specimens from untreated infected rabbits were collected once after the development of diarrhea. For rabbits in the treatment group, stool samples were collected once on day 1 before the start of treatment and once or twice daily during and after treatment (days 2 to 4), based on availability. Blood was collected by puncturing the heart immediately after the rabbits were sacrificed. The abdomen of each sacrificed rabbit was opened, and tissue samples from the ileum and jejunum were collected in 10% buffered formalin for immunohistochemical evaluations. Stool, blood, and autopsy samples were also collected from healthy rabbits.
Vibrio cholerae count in stool.
V. cholerae bacteria were counted in stool specimens collected at the time points specified in the preceding section (days 1 to 4). The stool specimen (100 mg) was mixed well with normal saline to prepare a 5-ml suspension, followed by 10-fold serial dilutions up to a 10−4 dilution. One hundred microliters of the 10−2, 10−3, or 10−4 dilution was cultured (by the spread plate method) onto TTGA plates; plating was done in duplicate. Colonies were counted after overnight incubation at 37°C. Only plates with 30 to 300 colonies were counted, as a rule of thumb. Counts of duplicate plates were averaged, and the CFU count per gram of stool was calculated as follows. The CFU count per 100 μl of the diluted suspension (the colonies on the plate) was multiplied by 10 to give the CFU count per milliliter, which was then multiplied by the dilution factor to yield the CFU count per milliliter of the original suspension. The CFU count per milliliter of the original suspension was multiplied by 5 to yield the CFU count per 100 mg of stool (equivalent to 5 ml of the original suspension), which was multiplied by 10. When stool was collected twice a day, the average count for the two time points was used.
The antimicrobial peptide CAP-18 and the corresponding specific antibody.
The synthetic CAP-18 peptide GLRKRLRKFRNKIKEKLKKIGQKIQGLLPKLAPRTDY (Innovagen, Lund, Sweden) is the rabbit homologue of the human cathelicidin LL-37. The affinity-purified polyclonal chicken antiserum against CAP-18 (Innovagen) recognizes both the proform and the mature CAP-18 peptide. The proform/mature CAP-18 peptide has been designated the CAP-18 protein/peptide in this study.
In situ immunohistochemical staining for CAP-18 detection.
Formalin-fixed pieces of ileum and jejunum tissues were embedded in paraffin and were cut into 3-μm-thick sections by utilizing a microtome (Thermo Scientific, Walldorf, Germany). Tissue sections were deparaffinized and were microwave treated in a retrieval buffer (Dako, Glostrup, Denmark) for antigen retrieval, followed by quenching of endogenous peroxidase activity by hydrogen peroxide. Sections were then incubated sequentially with the CAP-18 antibody (0.5 μg/ml), biotinylated goat anti-chicken IgG(H+L) (Vector Laboratories Inc., CA, USA), and an avidin-biotin complex conjugated with peroxidase enzyme (Vector Laboratories Inc.). A color reaction was developed by adding hydrogen peroxide as the substrate and 3,3′-diaminobenzidine (DAB; Sigma-Aldrich) as the chromogen; a brown color represents positive staining of CAP-18. Sections were counterstained with hematoxylin (Fisher Scientific, NJ, USA), resulting in a blue nuclear stain; dehydrated; and mounted with a permanent mounting medium (Vector Laboratories Inc.).
Quantification of CAP-18 peptide/protein expression in ileal/jejunal mucosae of rabbits.
CAP-18 expression in ileal and jejunal tissues was analyzed by using a microscope (Leica Microsystems GmbH, Wetzlar, Germany) and the Quantimate Q550 image analysis system (Leica). CAP-18 staining was quantified in the epithelial area of the whole-tissue section. The results were given as ACIA (acquired computerized image analysis) scores, calculated as (total positively stained area × total mean intensity [1 to 256 levels per pixel] of the positive area)/total cell area (16).
Quantitative determination of zonulin levels in serum by ELISA.
Serum was separated from blood, aliquoted, and stored at −80°C. Zonulin, a marker of gut permeability (22), was quantified in stored serum by using a commercially available rabbit zonulin (ZON) enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, CA, USA). The kit applies the competitive enzyme immunoassay technique utilizing a monoclonal anti-ZON antibody and a ZON–horseradish peroxidase (HRP) conjugate.
In vitro bacterial killing.
The Vibrio cholerae 01 Ogawa isolate was grown to exponential phase as described in “Preparation of infectious inocula” above. Different concentrations of the synthetic CAP-18 peptide (3.0, 3.5, 4.0, 4.5, and 5.0 μg/ml) or entinostat (0.25, 0.5, and 1.0 mg/ml), or a combination of both, were added to the bacterial suspension (∼2 × 104 CFU/ml) in Mueller-Hinton broth (MHB; Becton Dickinson, NJ, USA) in the wells of a microtiter plate (Thermo Fisher Scientific, Roskilde, Denmark) in a final volume of 200 μl. Control wells contained MHB alone (negative control) or bacteria in MHB without CAP-18 or entinostat (positive control). The plate was incubated at 37°C in a shaker incubator for 2 h or overnight. Bacterial suspensions or media from individual wells were plated on TTGA, and CFU were counted after overnight incubation at 37°C.
Evaluation of the toxic effect of entinostat treatment.
For safety evaluation, levels of hepatic enzymes (e.g., alanine transaminase [ALT], aspartate transaminase [AST], and gamma-glutamyl transferase [GGT]) and renal markers (e.g., creatinine and blood urea nitrogen [BUN]) in the sera of infected and entinostat-treated rabbits were assessed in the Cobas C311 Clinical Chemistry Analyzer (Roche Diagnostics GmbH, Mannheim, Germany) and were compared to those in untreated healthy and infected rabbits.
Statistical analyses.
Statistical analyses were performed by using IBM SPSS Statistics, version 20.0. Data are expressed as means ± standard errors of the means (SEM). Data that were not normally distributed and/or that failed the equal variance test were log transformed. Differences in outcomes between different groups were estimated by mixed-model analysis of variance (ANOVA). The least significant difference (LSD) test was used for multiple comparisons of the means of outcomes across the different groups. Probabilities were regarded as significant at a P value of <0.05.
ACKNOWLEDGMENTS
We acknowledge K. M. Nasirul Islam, Suman Kumer Paul, and other members of the Animal Facility staff of icddr,b for support in carrying out animal experimentation. We thank Nazrul Islam of the Clinical Microbiology and Immunology Laboratory of icddr,b for providing the clinical isolate of V. cholerae. We also thank Ahsanul Haq for assisting with the statistical analysis.
icddr,b acknowledges with gratitude the commitment of the Swedish International Development Cooperation Agency (Sida), the Swedish Foundation for Strategic Research (SSF), the Swedish Research Council (VR), and the Karolinska Institutet (KI) to its research efforts. icddr,b is also grateful to the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
R.S., G.H.G., and B.A. are cofounders of a company named Akthelia that applied for a patent for the use of aroylated phenylenediamines (including entinostat) in the treatment of infections. P.S., A.B., and R.R. declare no conflict of interest.
REFERENCES
- 1.World Health Organization. 2016. Cholera, 2015. Wkly Epidemiol Rec 91:433–440. [PubMed] [Google Scholar]
- 2.Field M, Fromm D, al-Awqati Q, Greenough WB III. 1972. Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest 51:796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sears CL, Kaper JB. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev 60:167–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, Kaper JB. 1991. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A 88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, Milton D, Nybom P, Sjo A, Magnusson KE. 1996. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog 21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- 6.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett JG. 2002. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 8.Nau R, Eiffert H. 2002. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev 15:95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7:180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 11.Sperandio B, Regnault B, Guo J, Zhang Z, Stanley SL Jr, Sansonetti PJ, Pedron T. 2008. Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J Exp Med 205:1121–1132. doi: 10.1084/jem.20071698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Mamun A, Mily A, Sarker P, Tiash S, Navarro A, Akter M, Talukder KA, Islam MF, Agerberth B, Gudmundsson GH, Cravioto A, Raqib R. 2013. Treatment with phenylbutyrate in a pre-clinical trial reduces diarrhea due to enteropathogenic Escherichia coli: link to cathelicidin induction. Microbes Infect 15:939–950. doi: 10.1016/j.micinf.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty K, Ghosh S, Koley H, Mukhopadhyay AK, Ramamurthy T, Saha DR, Mukhopadhyay D, Roychowdhury S, Hamabata T, Takeda Y, Das S. 2008. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol 10:2520–2537. doi: 10.1111/j.1462-5822.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirin T, Rahman A, Danielsson A, Uddin T, Bhuyian TR, Sheikh A, Qadri SS, Qadri F, Hammarstrom ML. 2011. Antimicrobial peptides in the duodenum at the acute and convalescent stages in patients with diarrhea due to Vibrio cholerae O1 or enterotoxigenic Escherichia coli infection. Microbes Infect 13:1111–1120. doi: 10.1016/j.micinf.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Raqib R, Sarker P, Bergman P, Ara G, Lindh M, Sack DA, Nasirul Islam KM, Gudmundsson GH, Andersson J, Agerberth B. 2006. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc Natl Acad Sci U S A 103:9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarker P, Ahmed S, Tiash S, Rekha RS, Stromberg R, Andersson J, Bergman P, Gudmundsson GH, Agerberth B, Raqib R. 2011. Phenylbutyrate counteracts Shigella mediated downregulation of cathelicidin in rabbit lung and intestinal epithelia: a potential therapeutic strategy. PLoS One 6:e20637. doi: 10.1371/journal.pone.0020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raqib R, Sarker P, Mily A, Alam NH, Arifuzzaman AS, Rekha RS, Andersson J, Gudmundsson GH, Cravioto A, Agerberth B. 2012. Efficacy of sodium butyrate adjunct therapy in shigellosis: a randomized, double-blind, placebo-controlled clinical trial. BMC Infect Dis 12:111. doi: 10.1186/1471-2334-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nylén F, Miraglia E, Cederlund A, Ottosson H, Stromberg R, Gudmundsson GH, Agerberth B. 2014. Boosting innate immunity: development and validation of a cell-based screening assay to identify LL-37 inducers. Innate Immun 20:364–376. doi: 10.1177/1753425913493338. [DOI] [PubMed] [Google Scholar]
- 19.Ottosson H, Nylen F, Sarker P, Miraglia E, Bergman P, Gudmundsson GH, Raqib R, Agerberth B, Stromberg R. 2016. Potent inducers of endogenous antimicrobial peptides for host directed therapy of infections. Sci Rep 6:36692. doi: 10.1038/srep36692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spira WM, Sack RB, Froehlich JL. 1981. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun 32:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson K. 1991. Roles of motility and flagellar structure in pathogenicity of Vibrio cholerae: analysis of motility mutants in three animal models. Infect Immun 59:2727–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fasano A. 2011. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 23.Fasano A. 2012. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol 10:1096–1100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monakhova EV, Fedorenko GM, Mazrukho AB, Bardakhch'yan EA. 2015. Ultrastructural changes in the intestine of suckling rabbits infected with cholerogenic and non-cholerogenic nonO1/nonO139 Vibrio cholerae strains. Bull Exp Biol Med 159:675–679. doi: 10.1007/s10517-015-3045-z. [DOI] [PubMed] [Google Scholar]
- 25.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. 2003. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol 120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 26.Kida Y, Shimizu T, Kuwano K. 2006. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human lung epithelial cell line, EBC-1. Mol Immunol 43:1972–1981. doi: 10.1016/j.molimm.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, Menzel T, Gostner A, Luhrs H, Scheppach W. 2004. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol 41:847–854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Steinmann J, Halldorsson S, Agerberth B, Gudmundsson GH. 2009. Phenylbutyrate induces antimicrobial peptide expression. Antimicrob Agents Chemother 53:5127–5133. doi: 10.1128/AAC.00818-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miraglia E, Nylen F, Johansson K, Arner E, Cebula M, Farmand S, Ottosson H, Stromberg R, Gudmundsson GH, Agerberth B, Bergman P. 2016. Entinostat up-regulates the CAMP gene encoding LL-37 via activation of STAT3 and HIF-1α transcription factors. Sci Rep 6:33274. doi: 10.1038/srep33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttman JA, Finlay BB. 2009. Tight junctions as targets of infectious agents. Biochim Biophys Acta 1788:832–841. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Goldblum SE, Rai U, Tripathi A, Thakar M, De Leo L, Di Toro N, Not T, Ramachandran R, Puche AC, Hollenberg MD, Fasano A. 2011. The active Zot domain (aa 288-293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J 25:144–158. doi: 10.1096/fj.10-158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z, Nybom P, Magnusson KE. 2000. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol 2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- 33.Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. 2000. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 355:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 34.Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF. 2012. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci 90(Suppl 4):S266–S268. doi: 10.2527/jas.50965. [DOI] [PubMed] [Google Scholar]
- 35.Ohata A, Usami M, Miyoshi M. 2005. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 21:838–847. doi: 10.1016/j.nut.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. 2011. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson K, Deng Z, Hou Y, Zhang G. 2015. Regulation of the intestinal barrier function by host defense peptides. Front Vet Sci 2:57. doi: 10.3389/fvets.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han F, Lu Z, Liu Y, Xia X, Zhang H, Wang X, Wang Y. 2016. Cathelicidin-BF ameliorates lipopolysaccharide-induced intestinal epithelial barrier disruption in rat. Life Sci 152:199–209. doi: 10.1016/j.lfs.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 39.Gojo I, Jiemjit A, Trepel JB, Sparreboom A, Figg WD, Rollins S, Tidwell ML, Greer J, Chung EJ, Lee MJ, Gore SD, Sausville EA, Zwiebel J, Karp JE. 2007. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood 109:2781–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gore L, Rothenberg ML, O'Bryant CL, Schultz MK, Sandler AB, Coffin D, McCoy C, Schott A, Scholz C, Eckhardt SG. 2008. A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin Cancer Res 14:4517–4525. doi: 10.1158/1078-0432.CCR-07-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pili R, Salumbides B, Zhao M, Altiok S, Qian D, Zwiebel J, Carducci MA, Rudek MA. 2012. Phase I study of the histone deacetylase inhibitor entinostat in combination with 13-cis retinoic acid in patients with solid tumours. Br J Cancer 106:77–84. doi: 10.1038/bjc.2011.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witta SE, Jotte RM, Konduri K, Neubauer MA, Spira AI, Ruxer RL, Varella-Garcia M, Bunn PA Jr, Hirsch FR. 2012. Randomized phase II trial of erlotinib with and without entinostat in patients with advanced non-small-cell lung cancer who progressed on prior chemotherapy. J Clin Oncol 30:2248–2255. doi: 10.1200/JCO.2011.38.9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yardley DA, Ismail-Khan RR, Melichar B, Lichinitser M, Munster PN, Klein PM, Cruickshank S, Miller KD, Lee MJ, Trepel JB. 2013. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol 31:2128–2135. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q, Zhang Q, Wen C, Hu L, Wang X, Lin G. 2015. The effect of MS-275 on CYP450 isoforms activity in rats by cocktail method. Int J Clin Exp Pathol 8:9360–9367. [PMC free article] [PubMed] [Google Scholar]
- 45.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]


