ABSTRACT
The plasmid-mediated mcr-1 gene encodes a phosphoethanolamine transferase that confers resistance to polymyxins. The mcr-1 gene is associated with insertion sequence ISApl1 (IS30 family). In vitro mobilization assays demonstrated the functionality of the composite transposon structure ISApl1-mcr-1-ISApl1. Transposition generated a 2-bp duplication and occurred in AT-rich DNA regions. This is the first report demonstrating the mobility of the mcr-1 gene by transposition.
KEYWORDS: ISApl1, composite transposon, mcr-1, plasmid, polymyxins, transposition
TEXT
Since its discovery by the end of 2015 (1), the occurrence of the plasmid-mediated colistin resistance gene mcr-1 has been reported worldwide. This gene has now been reported in a variety of enterobacterial species, mostly in Escherichia coli, from human, environmental, and animal samples (2), and also from retail food (3). Retrospective studies reported MCR-1-producing colistin-resistant isolates as early as in the late 1980s (4), but several studies suggest that spread of the mcr-1 gene is on a rising trend (5).
Various plasmids may carry the mcr-1 gene, including those belonging to the incompatibility groups IncX4, IncI2, InHI2, IncF, IncY, and IncP (6–10). This gene is often identified in association with the insertion sequence ISApl1, which may play a major role in its mobilization (11–13).
ISApl1 belongs to the IS30 family and was first identified in Actinobacillus pleuropneumoniae (14), a Gram-negative rod of the Pasteurellaceae family that is a causative agent of porcine necrotic pleuropneumonia. It is a 1,070-bp-long mobile element possessing a 924-bp open reading frame (ORF) encoding a 307-amino-acid transposase that contains a DDE domain containing the carboxylate residues believed to be responsible for coordinating metal ions needed for catalysis. ISApl1 is flanked by two imperfect 27-bp inverted repeats (IRs) exhibiting 6 base pair mismatches. In a recent study (11), an intermediate circular form of ISApl1 associated with mcr-1 was detected, suggesting that ISApl1 might be involved in the mobilization of this resistance gene. Moreover, a ca. 790-bp open reading frame has been identified downstream of the mcr-1 gene in most of the MCR-1 producers. This sequence is not believed to play any role in colistin resistance (15); nevertheless, its putative role in the mobilization of the mcr-1 gene remains to be determined. Recent works (6, 16) showed that mcr-1 is part of a 2,600-bp cassette containing promoter sequences for mcr-1 expression and is bracketed in most cases by two direct copies of ISApl1, suggesting that it may constitute a composite transposon element. Therefore, the aim of our study was to determine experimentally whether ISApl1 could actually mobilize the mcr-1 gene.
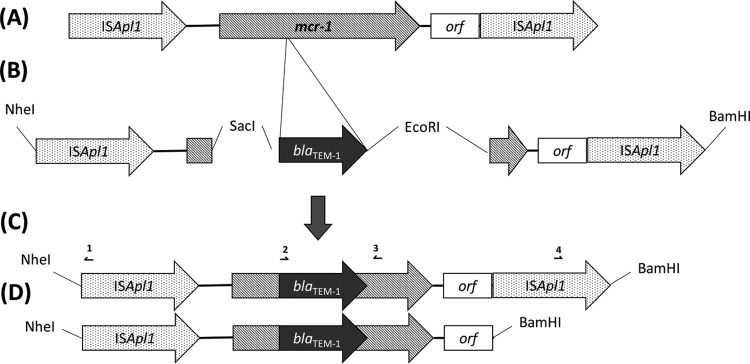
Since preliminary experiments showed that cloning the mcr-1 gene in regular recombinant vectors is difficult, likely due to a toxic effect of MCR-1 once overproduced in E. coli (data not shown), a truncated form of mcr-1 was created by inserting the blaTEM-1 gene into the coding sequence of mcr-1, as shown in Fig. 1. Then, two structures were analyzed, the entire composite transposon bracketed by two copies of ISApl1 and encompassing the mcr-1–blaTEM-1 gene, and the same structure deleted from the right-hand copy of ISApl1. Those two different genetic structures, namely, ISApal1-mcr-1–blaTEM-1-orf-ISApl1 and ISApl1-mcr-1–blaTEM-1-orf, respectively, were obtained by PCR and then ligated and cloned into plasmid pNK1 (p15A-pTOPO-ΔlacP-Kanr), giving rise to recombinant plasmids pNK31 and pNK45, respectively. Those plasmids were transformed into E. coli TOP10 (InvitroGen, Thermo Fisher Scientific, Ecublens, Switzerland) and selected onto Luria-Bertani (LB) agar plates supplemented with 100 μg/ml of ampicillin and 25 μg/ml of kanamycin. Plasmids pNK31 and pNK45 were then transformed into the E. coli strain RZ211 containing the transfer-proficient pOX38 F plasmid carrying a gentamicin resistance gene (17). Plasmid pOX38 is a self-conjugative and IS-free plasmid encoding resistance to gentamicin that serves as a target for transposition events that may be searched after 24 h of growth, as described previously (18). By conjugating the pOX38 plasmid into another E. coli recipient strain using gentamicin as selective marker, it is therefore possible to isolate and identify putative transposition events. The strains used in this study are listed in Table 1.
FIG 1.
Schematic map of the different constructs performed for the transposition study. (A) corresponds to the original transposon ISApl1-mcr-1-orf-ISApl1 identified in clinical isolates; (B) shows the different fragments generated by PCR (with corresponding restriction sites indicated) and used as templates for ligation and subsequent genesis of (C) ISApl1-mcr-1–blaTEM-1-orf-ISApl1 or (D) ISApl1-mcr-1–blaTEM-1-orf genetic structures. Locations of primers used for the inverse PCR strategy (as listed in Table 2) are indicated by small half arrows. Restriction sites of endonucleases used for cloning are indicated (NheI, SacI, EcoRI, and BamHI).
TABLE 1.
E. coli strains used in this study
| Strain | Feature |
|---|---|
| Af31 | MCR-1-producing clinical isolate carrying two copies of ISApl1 |
| Af45 | MCR-1-producing clinical isolate carrying only one copy of ISApl1 |
| RZ211 | Isolate carrying the pOX38-Gen plasmid |
| J53 | Azide-resistant isolate used for mating-out experiments |
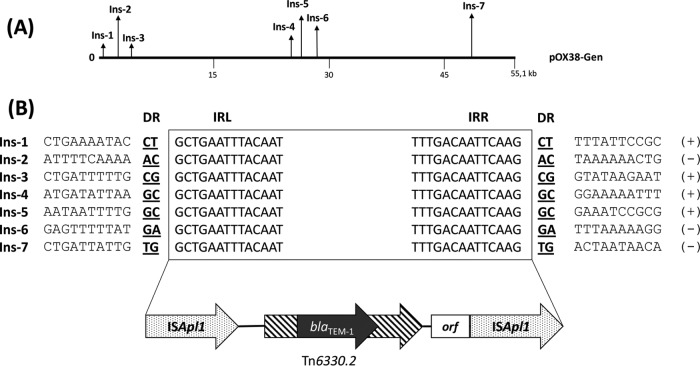
Clones were selected onto LB agar plates supplemented with ampicillin (100 μg/ml), kanamycin (25 μg/ml), and gentamicin (10 μg/ml). E. coli RZ211-harboring recombinant plasmid pNK31 or pNK45 was used as a donor for conjugation experiments with azide-resistant E. coli strain J53. Briefly, the donor and recipient strains were separately cultured overnight and then subcultured for 5 h in order to reach the exponential phase. Mating-out assays were performed on solid medium using filters with a 1:10 donor to recipient ratio. After 5 h of incubation, filters were resuspended in NaCl 0.85% and bacterial mixtures were plated onto agar plates supplemented with gentamicin (10 μg/ml) and sodium azide (100 μg/ml) or onto agar plates supplemented with gentamicin, sodium azide, and ampicillin (100 μg/ml). All GenrAziderAmpr colonies were screened for kanamycin susceptibility to exclude the spontaneous E. coli RZ211 azide-resistant mutants or possible cointegration events that might not correspond to transposition events. The transposition frequency was obtained by dividing the number of Genr Azider Ampr Kans colonies by the number of GenrAzider transconjugants. In total, we randomly selected 100 Genr Azider Ampr Kans transposants recovered from the conjugation experiment, using RZ211-pNK31 as donor, and identified seven distinct transposition events (Fig. 2). No transposant was found with the donor strain RZ211-pNK45 (only a single copy of ISApl1). The transposition frequency determined in E. coli J53 with pNK-31 as donor plasmid was estimated to be 2.2 × 10−8, which is relatively low. The insertion sites of the ISApl1-mcr-1–blaTEM-1-orf-ISApl1 cassette were determined by using an inverse PCR strategy. Briefly, DNA from the transposants was extracted using the GenElute bacterial genomic DNA kit (Sigma-Aldrich) and digested by the PstI restriction enzyme (InVitrogen). Digested fragments were self-circularized by ligation and used as templates for reverse PCR using outward primers, as listed in Table 2 and indicated on the figures. Since two PstI restriction sites were located into the blaTEM-1 and mcr-1 genes, respectively, two PCR amplifications per transposant were performed. The first PCR amplification was performed using primers ISApl1-SP3 and TEM-Fw, and a second PCR was performed using primers mcr-south-Rv and ISApl1-3′-Fw, in order to characterize the 5′ and 3′ genetic contexts of the insertions, respectively. Sequencing of the corresponding amplicons revealed that transposition events occurred in seven different sites, namely, Ins-1 to Ins-7 (Fig. 2A), the whole mobilized transposon being always 5,699-bp in size. Each transposition event generated 2-bp direct repeats at the insertion site (Fig. 2B). High AT-rich DNA sequences were identified on the two flanking regions of all insertion sites (Fig. 2). Our data are in accordance with previous studies showing that ISApl1 like other IS30-like elements targets preferentially AT-rich sequences (14). Noteworthy, in silico analysis showed that in most of the sequenced plasmids, the mcr-1 gene is flanked by AT-rich regions.
FIG 2.
Target sites of the ISApl1-mcr-1–blaTEM-1-orf-ISApl1 composite transposon. (A) Map positions of ISApl1-mcr-1–blaTEM-1-orf-ISApl1 composite transposon in plasmid pOX38-Gen. Insertions of the tagged insertion sequence (Ins-1 to −4) are indicated by a vertical arrow. (B) Nucleotide sequence alignment of the three ISApl1-mcr-1–blaTEM-1-orf-ISApl1 transposon events identified into pOX38-Gen. Nucleotide sequences of the end regions of transposon are boxed. Boldfaced letters indicate target site sequences duplicated upon transposition. Orientations of the insertion sequences are indicated by (+) and (−).
TABLE 2.
Sequences of primers used in this study
| Primer | Sequence (5′ → 3′)a | Position in Fig. 1 |
|---|---|---|
| ISAplSP3 | CAGGCTGCTCTAATTTGCGC | 1 |
| ISApl1-3′-Fw | AGACATCAATCAGTGGAGCG | 4 |
| mcr-south-Rv | GATAGACACCGTTCTCACCC | 3 |
| Nhe-ISApl1 | GATGATGCTAGCGCTGAATTTACAATCCAAGT | |
| SacI-Δmcr-1 | GATGATGAGCTCGTAGGGCATTTTGGAGCATG | |
| Sac-I-TEM-1 | GATGATGAGCTCGTATCCGCTCATGAGACAATA | 2 |
| EcoRI-TEM-1 | GATGATGAATTCTCTAAAGTATATATGAGTAAACTTGGTCTG | |
| EcoRI-Δmcr-1 | GATGATGAATTCCCGAGACCAAGGATCTATTA | |
| BamHI-Cass | GATGATGGATCCGTTATTTCTGTTTGGGGTTG | |
| BamHI-ISApl1 | GATGATGGATCCCATTGCGCAATCCCATACTG |
Underlining indicates the restriction sites.
Our results therefore support the hypothesis made by Snesrud et al. suggesting that the mobilization of mcr-1 is mediated by a composite transposon (12). The fact that the mcr-1 gene was associated with only a single copy of ISApl1 at its 5′ extremity in some studies might be explained by the characteristic of IS30 family members to excise one copy of the IS element by transposition or by illegitimate recombination events after transposition of the original composite transposon, in order to stabilize the genetic structure once integrated (19, 20). This hypothesis agrees with the lack of transposition events observed using the pNK45 construct.
Here, we demonstrate the effective mobilization of the mcr-1 gene located into a composite transposon named Tn6330.2, based on in silico comparison with Tn6330 (11). This work confirms previous hypotheses (12) that the mcr-1 gene had been initially mobilized by two copies of ISApl1 from an unknown progenitor, targeting broad-host range plasmid(s) that subsequently transferred this resistance gene into Enterobacteriaceae. Interestingly, our very recent work showed that Moraxella species are natural sources of mcr-like genes, and may harbor ISApl1 elements (21). Therefore, mobilization of the mcr-1 gene might have occurred into a Moraxella species (still to be precisely identified) through an ISApl1-mediated transposition process. Further studies are being conducted to reproduce mobilization of mcr-like genes from such bacterial sources by ISApl1-mediated transposition.
ACKNOWLEDGMENTS
This work has been funded by the University of Fribourg, by grants from the ANIHWA ERA-NET project, Switzerland, by the OFSP, Bern, Switzerland (grant 16009294), and by the Novartis Foundation for Medical-Biological Research.
REFERENCES
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz S, Johnson AP. 2016. Transferable resistance to colistin: a new but old threat. J Antimicrob Chemother 71:2066–2070. doi: 10.1093/jac/dkw274. [DOI] [PubMed] [Google Scholar]
- 3.Zurfluh K, Poirel L, Nordmann P, Nuesch-Inderbinen M, Hächler H, Stephan R. 2016. Occurrence of the plasmid-borne mcr-1 colistin resistance gene in extended-spectrum-β-lactamase-producing Enterobacteriaceae in river water and imported vegetable samples in Switzerland. Antimicrob Agents Chemother 60:2594–2595. doi: 10.1128/AAC.00066-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen Z, Wang Y, Shen Y, Shen J, Wu C. 2016. Early emergence of mcr-1 in Escherichia coli from food-producing animals. Lancet Infect Dis 16:293. doi: 10.1016/S1473-3099(16)00061-X. [DOI] [PubMed] [Google Scholar]
- 5.Poirel L, Jayol A, Nordmann P. 2017. Polymyxins; antibacterial activity, susceptibility testing, plasmid- and chromosomally-encoded resistance mechanisms. Clin Microbiol Rev 30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poirel L, Kieffer N, Brink A, Coetze J, Jayol A, Nordmann P. 2016. Genetic features of MCR-1-producing colistin-resistant Escherichia coli isolates in South Africa. Antimicrob Agents Chemother 60:4394–4397. doi: 10.1128/AAC.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zurflüh K, Klumpp J, Nuesch-Inderbinen M, Stephan R. 2016. Full-length nucleotide sequences of mcr-1-harboring plasmids isolated from extended-spectrum-β-lactamase-producing Escherichia coli isolates of different origins. Antimicrob Agents Chemother 60:5589–5591. doi: 10.1128/AAC.00935-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao F, Feng Y, Lu X, McNally A, Zong Z. 2017. IncP plasmid carrying colistin resistance gene mcr-1 in Klebsiella pneumoniae from hospital sewage. Antimicrob Agents Chemother 61:e02229-16. doi: 10.1128/AAC.02229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Feng Y, Liu F, Jiang H, Qu Z, Lei M, Wang J, Zhang B, Hu Y, Ding J, Zhu B. 2017. A phage-like IncY plasmid carrying the mcr-1 gene in Escherichia coli from a pig farm in China. Antimicrob Agents Chemother 61:e02035-16. doi: 10.1128/AAC.02035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordmann P, Lienhard R, Kieffer N, Clerc O, Poirel L. 2016. Plasmid-mediated colistin-resistant Escherichia coli in bacteremia in Switzerland. Clin Infect Dis 62:1322–1323. doi: 10.1093/cid/ciw124. [DOI] [PubMed] [Google Scholar]
- 11.Li R, Xie M, Lv J, Wai-Chi Chan E, Chen S. 2017. Complete genetic analysis of plasmids carrying mcr-1 and other resistance genes in an Escherichia coli isolate of animal origin. J Antimicrob Chemother 72:696–699. doi: 10.1093/jac/dkw411. [DOI] [PubMed] [Google Scholar]
- 12.Snesrud E, He S, Chandler M, Dekker JP, Hickman AB, McGann P, Dyda F. 2016. A model for transposition of the colistin resistance gene mcr-1 by ISApl1. Antimicrob Agents Chemother 60:6973–6976. doi: 10.1128/AAC.01457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Li XP, Yang RS, Fang LX, Huo W, Li SM, Jiang P, Liao XP, Liu YH. 2016. Complete nucleotide sequence of an IncI2 plasmid coharboring blaCTX-M-55 and mcr-1. Antimicrob Agents Chemother 60:5014–5017. doi: 10.1128/AAC.00774-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tegetmeyer HE, Fricke K, Baltes N. 2009. An isogenic Actinobacillus pleuropneumoniae AasP mutant exhibits altered biofilm formation but retains virulence. Vet Microbiol 137:392–396. doi: 10.1016/j.vetmic.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Zurfluh K, Kieffer N, Poirel L, Nordmann P, Stephan R. 2016. Features of the mcr-1 cassette related to colistin resistance. Antimicrob Agents Chemother 60:6438–6439. doi: 10.1128/AAC.01519-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, Giani T, Menichetti F, Rossolini GM. 2016. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother 60:5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derbyshire KM, Hwang L, Grindley ND. 1987. Genetic analysis of the interaction of the insertion sequence IS903 transposase with its terminal inverted repeats. Proc Natl Acad Sci U S A 84:8049–8053. doi: 10.1073/pnas.84.22.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poirel L, Lartigue MF, Decousser JW, Nordmann P. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob Agents Chemother 49:447–450. doi: 10.1128/AAC.49.1.447-450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo M, Kiss J, Kotany G, Olasz F. 1999. Importance of illegitimate recombination and transposition in IS30-associated excision events. Plasmid 42:192–209. doi: 10.1006/plas.1999.1425. [DOI] [PubMed] [Google Scholar]
- 20.Szabo M, Kiss J, Nagy Z, Chandler M, Olasz F. 2008. Sub-terminal sequences modulating IS30 transposition in vivo and in vitro. J Mol Biol 375:337–352. doi: 10.1016/j.jmb.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Kieffer N, Nordmann P, Poirel L. 2017. Moraxella species as potential sources of MCR-like polymyxin resistance determinants. Antimicrob Agents Chemother 61:e00129-17. doi: 10.1128/AAC.00129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]