Abstract
A striped dolphin (Stenella coeruleoalba) calf stranded alive because of a Salter-Harris fracture type 1 of a caudal vertebra and remained in a provisional rehabilitation facility for 3 days where the fracture stabilization was attempted, but he died the day after bandaging. Serum and urine samples were collected during hospitalization (days 1, 2 and 3 serum and day 2 urine). Serum analysis showed increased urea, alanine transaminase, aspartate transaminase, and serum amyloid A values, while creatinine was below the lower limit. Urine analysis showed urinary protein-to-creatinine ratio of 5.3 with glomerular proteinuria. Postmortem analyses demonstrated a severe rhabdomyolysis and myoglobinuric nephrosis, suggestive of capture myopathy syndrome. We report, for the first time, the clinico-pathological changes during this condition in a striped dolphin.
Keywords: biochemical analysis, capture myopathy, dolphin, myoglobinuric nephrosis
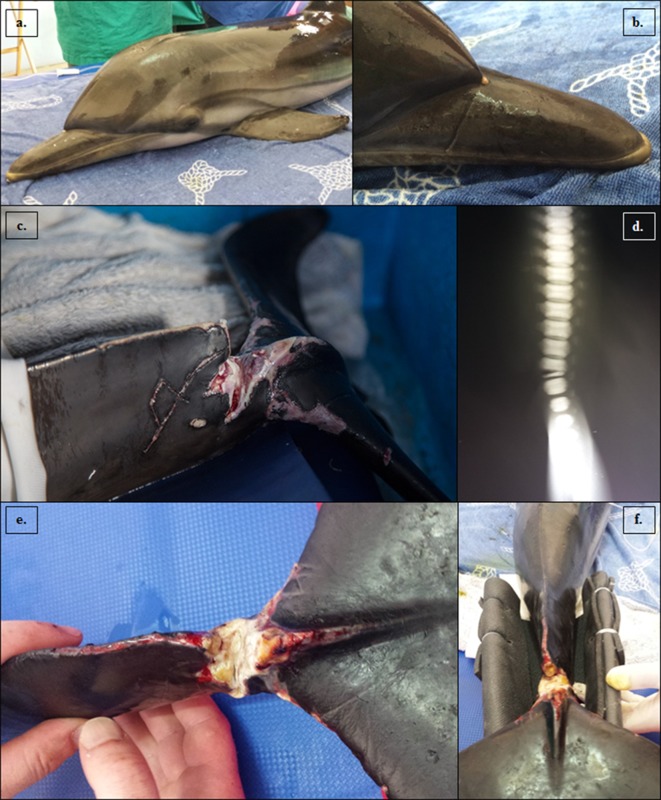
In August 2014, a calf male striped dolphin (Stenella coeruleoalba) was found stranded alive in Bovalino beach (Reggio Calabria, Italy, 38°9ʹ33˝N, 16°11ʹ38˝E), because of a severe wound close (Fig. 1C and 1D) to the caudal peduncle likely caused by entanglement in fishing gear. The animal was 133 cm long and 22 kg of weight; based on these morphometric data, we estimated that the animal was about 1 year old [4]. Despite the severe injury impairing swimming and diving, a good health condition was hypothesized based on respiratory rate, heart rate, physical examination (Fig. 1A and 1B) and complete blood cells count results, and the dolphin was recovered in a provisional pool for 3 days. To correctly characterize the severity of the wound, we did a radiographic examination of the injured area. The radiographic exam showed a physeal fracture, classified as Salter-Harris fracture type 1, of a caudal vertebra (Fig. 1D). In the Salter-Harris type 1, the fracture runs through the physis, but metaphysis is not involved [7]. Because of the prolonged survival and in view of young age, fracture stabilization was attempted using an external splint (Fig. 1F). The animal was sedated using diazepam during manipulation and bandaging procedures. During the hospitalization, the animal was hydrated using 40 ml/kg/day (maintenance fluid requirement) of 0.9% saline. After stabilization, we decided to treat the animal using a long acting antibiotic (Cefovecin, 8 mg/kg, intramuscular administration) and analgesic (tramadol, 0.1 mg/kg twice a day, subcutaneous administration) to control animal’s pain.
Fig. 1.
Striped dolphin (Stenella coeruleoalba), male, 133 cm long, (a) left side of the whole body; (b) the fishing gear marks involving the rostrum; (c, e) severe wound close to the caudal peduncle, before (c) and after (e) chirurgical curettage; (d) radiographic examination of the fracture showed a Salter-Harris fracture type 1; (f) rigid structure of the external splint.
Blood samples were collected from the dorsal fin vein: a complete blood cell count (CBC) was performed the day before bandaging, while biochemical analyses using an automated chemistry analyzer (Cobas Mira, Roche Diagnostics, Basel, Switzerland) and serum electrophoresis (Sebia Italia Srl, Bagno a Ripoli, Firenze, Italy) were performed on serum samples collected before, during and after the application of the external splint. A urine sample collected by free catch during the bandaging procedure was used for urinalysis and sodium dodecyl sulphate-agarose gel electrophoresis (SDS-AGE). Both blood and urine samples were collected in the morning.
Unfortunately, one day after the bandaging, the animal died unexpectedly without specific clinical signs. A detailed necropsy, according to European Cetaceans Society [8] standard necropsy protocol, was carried out 8 hr after death (conservation code 2 according to [4]), and tissues were routinely sampled, formalin fixed, paraffin-embedded and processed for microscopic examination. Skeletal muscles sampled from the longissimus dorsi at the level of the dorsal fin were obtained during necropsy [5]. Transverse and longitudinal sections were trimmed, fixed in 10% buffered formalin ensuring them on a solid surface to prevent contraction and paraffin embedded, and serial sections were obtained for microscopic examination. These slides as well as those obtained by cardiac samples were used for immunohistochemical (IHC) analyses in order to assess any injures related to stressful conditions occurred during stranding and/or rehabilitation efforts. The IHC analysis was performed using a polyclonal rabbit anti-human fibrinogen (Agilent Technologies, Santa Clara, CA, U.S.A., Dako, Agilent technology; dilution 1:200; incubated for 2 hr at 37°C) and polyclonal rabbit anti-human myoglobin (Agilent Technologies, Dako; dilution 1:800, incubated for 16 min at 42°C). The original IHC manual protocol [5] was modified to be performed using an automatic immunostainer (Ventana Benchmark XT, Ventana Medical System Inc., Tucson, AZ, U.S.A.). Finally, virological, microbiological and parasitological analyses were carried out to exclude any possible biological agent.
The CBC showed mild eosinopenia, while all other blood parameters were within the reference intervals (RI) (Table S1). Biochemical abnormalities included increased aspartate transaminase (AST) and alanine transaminase (ALT) activities. Urea concentration was above the upper limit of the RI, and creatinine concentration was under the lower limit in all 3 samples took before, during and after bandaging. Total serum protein (TP) was within the RI before bandaging and increased above the upper limit the day after bandaging with serum electrophoresis showing an increase in the beta-globulins concentrations. In addition, serum amyloid-A (SAA) was above the upper limit of RI in all 3 samples (Table 1). Urine was macroscopically clear and yellow. The urinary protein to creatinine ratio (UPC) revealed a severe glomerular proteinuria (Table 1) as confirmed by SDS-AGE (Fig. 2). Muscular, glomerular injures and an acute phase reaction were supposed on the biochemical profile.
Table 1. Biochemical variables and urine analysis in the striped dolphin. Serum samples were collected the day before, during and the day after the bandaging, while urine sample was collected during bandaging.
| SI units | Pre-bandaging | During bandaging | Post-bandaging | Reference intervals | |
|---|---|---|---|---|---|
| Urea | mmol L-1 | 18.32 | 19.15 | 16.82 | 7.83–13.32 |
| Creatinine | µmol L-1 | 57.46 | 41.55 | 50.39 | 61.88–132.6 |
| ALT | U L-1 | 202 | 151 | 184 | 12.0–70.0 |
| ALP | U L-1 | 231 | 272 | 325 | 96.0–702.0 |
| AST | U L-1 | 1,025 | 984 | 1,024 | 165.0–371.0 |
| CK | U L-1 | 307 | 217 | 342 | 104.0–358.0 |
| Total protein | g L-1 | 80.7 | 72.9 | 88.1 | 64.0–83.0 |
| Albumin | % | nd | nd | 38.3 | 64.00–70.99 |
| Albumin | g L-1 | nd | nd | 33.7 | 31.0–43.0 |
| α-globulin | % | nd | nd | 18.4 | 10.2–14.3 |
| α-globulin | g L-1 | nd | nd | 16.2 | 11.0–18.0 |
| β-globulin | % | nd | nd | 14.5 | 5.4–9.6 |
| β-globulin | g L-1 | nd | nd | 12.8 | 4.0–7.0 |
| γ-globulin | % | nd | nd | 28.8 | 7.6–13.9 |
| γ-globulin | g L-1 | nd | nd | 25.4 | 8.0–30.0 |
| SAA | mg L-1 | 68.4 | 84.9 | 128.0 | 17.5–42.9 |
| Urinary proteins | mg dL-1 | nd | 173.6 | nd | nd |
| Urinary creatinine | mg dL-1 | nd | 32.6 | nd | nd |
| Urinary protein to creatinine ratio | nd | 5.3 | nd | <0.5 |
Biochemical values are compared to the reference intervals of free-ranging, age-matched bottlenose dolphins [12], while the urine values are compared to the reference intervals of domestic animals [8]. SI=système international d’unités; ALT=alanine transaminase; ALP=alkaline phosphatase; AST=aspartate transaminase; CK=creatine kinase; SAA=serum amyloid-A; nd=not determined. Bold; the values outside the reference intervals.
Fig. 2.
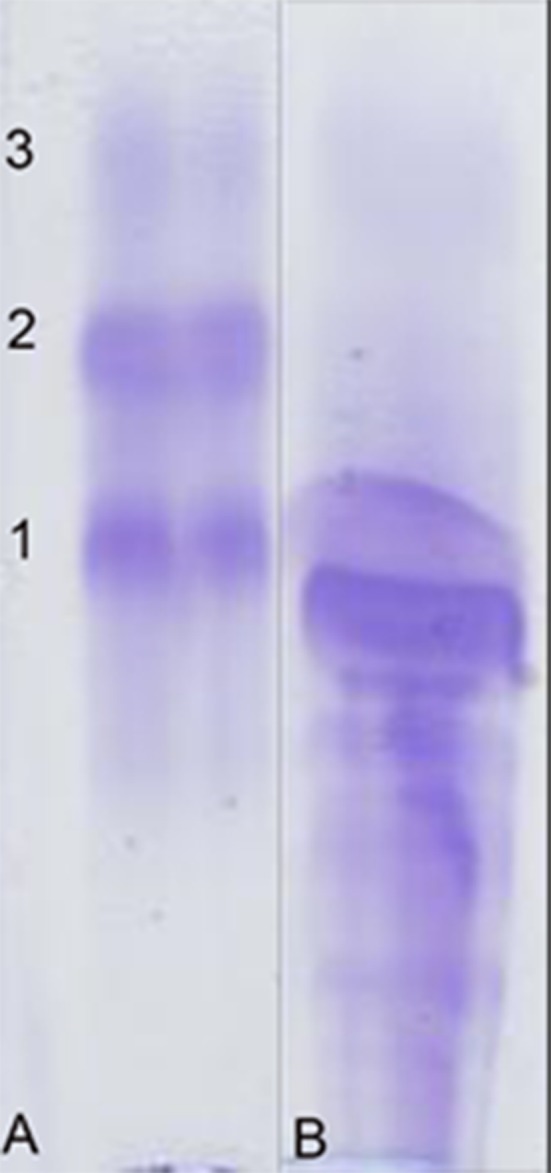
Sodium dodecyl sulphate-agarose gel electrophoresis (SDS-AGE) analysis. Lane A: Molecular weight control (1-Albumin [66 kDa], 2-triose phosphate isomerase [27 kDa] and 3-lysozyme [15 kDa]). Lane B: glomerular pattern of proteinuria seen in striped dolphin. In the lane B, only proteins with a molecular weight equal or higher than albumin are present.
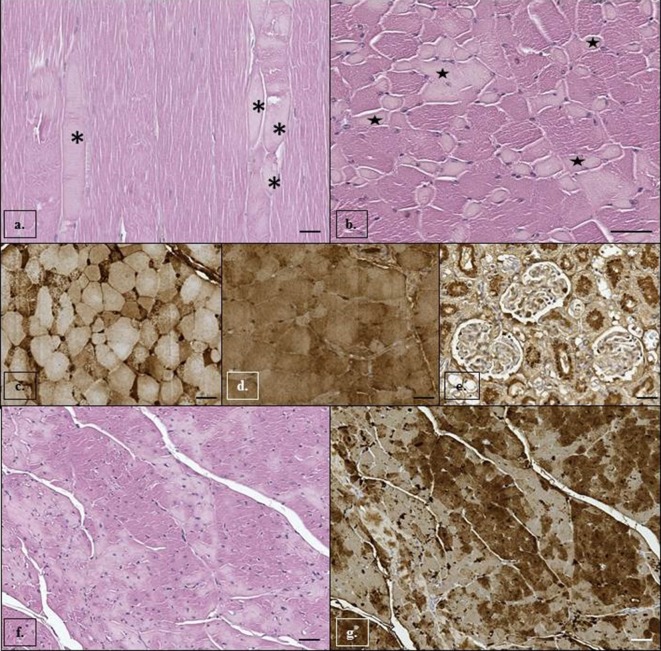
During necropsy, external examination revealed fishing gear marks around the rostrum and the caudal peduncle along with the wound involving the caudal peduncle, the related hemorrhagic changes and the fracture. Grossly, skeletal muscles had multifocal, locally extensive, pale, whitish areas in the muscle fibers, and stomach content analysis showed the presence of scant and digested squid beaks and a mild multifocal granulomatous gastritis associated to Pholeter gastrophylus in the third chamber. Further observed pathological changes were mild diffuse hyperemia of liver and spleen, pale muscle aspect of heart and mild multifocal edema associated with emphysema in lung tissue. Microscopic examination of cardiac muscle, longissimus dorsi and rectus abdominis muscles revealed multiple, multifocal, moderate hypereosinophilic cytoplasm with fragmentation, loss of cross striations, moderate myolysis and multifocal contraction band necrosis (Fig. 3A and 3B), consistent with acute myodegeneration. IHC analysis revealed myoglobin depletion and an intense cytoplasmic immunoreaction with anti-fibrinogen antibody in degenerate myocytes (Fig. 3C and 3D) and cardiomyocytes (Fig. 3F and 3G). Furthermore, renal tubular degeneration and necrosis associated with presence of brown homogeneous pigment, positively stained using the anti-myoglobin antibody, in the tubular lumen, in the apical part of the tubular epithelial cells, and sometimes in Bowman’s spaces were observed (Fig. 3E). Negative results were obtained by molecular analyses looking for dolphin morbillivirus and Toxoplasma gondii and microbiological and microscopic examinations excluded any bacterial agent or related pathological changes.
Fig. 3.
Stenella coeruleoalba. Skeletal muscle (longissimus dorsi): myocytes have (a, b) acute degenerative changes characterized by fibers hyaline degeneration (asterisks) and multifocal loss of cross striations and mild endomisial edema (stars) probably due to an acute ischemic damage. Hematoxylin and eosin staining. Scale bar=50 µm. Immunohistochemical analysis of skeletal muscle (longissimus dorsi): (c) strong intracytoplasmatic positive immunoreaction for fibrinogen in affected myocytes and (d) depletion of myoglobin in degenerated myocytes is demonstrated by decreased immunoreaction for myoglobin. Mayer’s hematoxylin counterstain. In kidney (e), the apical cytoplasm of degenerated tubular cells and granules in the Bowman space and tubular lumen show positive immunoreaction for myoglobin. Mayer’s hematoxylin counterstain. Scale bar=50 µm. Cardiac muscle: (f) cardiomyocytes present multifocal hyaline degeneration and loss of cross striations. Hematoxylin and eosin staining. Scale bar=50 µm. Immunohistochemical analysis of cardiac muscle: (g) decreased immunoreaction for myoglobin in degenerated cardiomyocytes. Mayer’s hematoxylin counterstain. Scale bar=50 µm.
Hematological and biochemical values and pathoanatomical findings suggested that cause of death was consistent with an acute stress syndrome with rhabdomyolysis and myoglobinuric nephrosis, consistent with a capture myopathy (CM) syndrome.
This report describes, for the first time, the changes in serum and urine samples during a CM syndrome in a striped dolphin.
CM is a metabolic muscle disease associated with stress of capture, restraint and transportation [6]. CM has been documented in a broad variety of wild terrestrial and aquatic mammals [1, 5, 6, 10]. Clinical signs of CM are aspecific including depression, hyperthermia, ataxia and torticollis, and the more common laboratories abnormalities increase serum muscle enzymes and urea due to myoglobinuria [5]. The pathogenesis of CM in artiodactyls is complex and related to overstimulation of the sympathetic nervous system in response to stressful stimuli. The overstimulation of the sympathetic nervous system leads to a vasogenic shock caused by systemic vasodilation, resulting in hypotension and systemic hypoperfusion. In animals survived after vasogenic shock, widespread muscle lesions associated with hypoxia and deficient ATP production with severe intracellular acidosis were evident. The myoglobin released from muscle damage is filtered through the glomerulus, and its concentration rises after the reabsorption of water in the tubules, until it precipitates and causes obstructive cast formation [17]. The myoglobin is then degraded in the tubules, and this leads to the release of free iron and free radical production [18]. Thus, severe tubular damage is caused by renal vasoconstriction, intraluminal cast formation and heme-protein-induced cytotoxicity [18].
In terrestrial animals, four different clinical syndromes were described: capture shock syndrome, ataxic myoglobinuric syndrome, ruptured muscle syndrome and delayed-peracute syndrome [14]. Their pathogenesis is the same, but the interval between capture and the onset of clinical signs changes [12].
In cetaceans stranded at the beach ischemic complications secondary to extended muscle compression, acute to subacute myodegeneration affecting both skeletal and cardiac muscle and myoglobinuric nephrosis are also present [6]. Moreover, cetaceans stranded alive are usually debilitated, their condition deteriorates over time, and they often die after a period of captivity [5].
In the case under herein reported, the animal showed a good body condition and no signs of other ongoing diseases suggesting that the cause of the tail fracture and, consequently, of the stranding, was likely related to the interaction with fisheries, while no specific clinical signs suggesting rhabdomyolysis was detected.
The evaluation of clinic-pathological data was challenging because values in striped dolphins are scarce [3] and no RI are reported in literature, so the levels of blood analytes were compared with published values for free-ranging, age-matched bottlenose dolphins (Tursiops truncatus) [2, 13]. Moreover, no data about urinalysis in cetaceans are present in the literature, so the results were compared with those of domestic mammals. In our case, increased ALT and AST values above the upper limits of RI in all the 3 samples were detected and considered markers of muscular or hepatic damage in mammals [16]. Since ALP, more specific for cholestatic disorders, is within RI, muscular damage, was hypothesized, as confirmed also from histologic and IHC analyses. Despite this condition, creatine kinase (CK) was within the RI of bottlenose dolphins. This could be an unexpected finding, since injures to skeletal muscle are usually supported by an increase value of this marker [16] and it was recommended as a sensitive indicator of handling stress in other marine mammals [15]. A possible explanation for the unexpected low levels of CK in our samples could be the short half-life of CK (1.5 hr) [11]. Additionally, the analytical ranges of some commercial assays are too narrow for the animals, and thus sometimes it is necessary to dilute the serum to obtain numeric results when CK activity is markedly increased [16]. Nevertheless, as stated above, the absence of specific RI for this species could affect the evaluation and the analysis of the obtained results, and the increased CK value in the last sample reflects the worsening of the muscular damage. To confirm our hypothesis, the histological and IHC analysis clearly identified the muscle damage. Thus, we think that unexpected findings underline the need of more information on specific assay and RI for wild species.
Pre-renal azotemia was reasonably supported by increased urea’s values in comparison to bottlenose dolphins’ RI, likely related to hypovolemia probably caused by vasodilation. In fact, hypovolemia enhances resorption of sodium and water in proximal tubules, which in turn promotes passive proximal tubular resorption of urea, but not creatinine, because the lower flow rate provides more time for resorption. Hypovolemia also triggers release of anti-diuretic hormone (ADH), which enhances resorption of urea in medullary collecting ducts [16]. The decrease of urea in the third sample is probably related to the fluid administration during recovery. Conversely, creatinine was slightly under the lower RI’s limits. The creatinine concentration is directly proportional to the muscular mass of an animal. The animal in our work was smaller compared to the juvenile bottlenose dolphins (length between 200 and 240 cm) and had less muscle and hypotrophic muscular masses. So, probably, the creatinine in our animal was increased, but within the RI of bottlenose dolphins [13] for this reason. A more accurate age and species-specific RI are needed to confirm this hypothesis.
TP was very close to the upper limit of bottlenose dolphins’ RI in the first and second samples and they overcome the upper limit in the last sample [13]. However, if compared to RI values reported for stranded striped dolphins [3], first and third samples were above the upper limit while the second one is within the range. More in detail, the electrophoretic analysis performed on the post-bandaging sample showed an increase of beta globulins, while albumin, alpha globulin and gamma globulins were within the RI. These values suggest that hyperproteinemia herein reported could be associated to inflammation related to the above described fracture and reported injures. Compared to the bottlenose dolphins’ RI [2], our SAA values were decisively increased, and they rose progressively from the first to the last samples, underlying a worsening in the health condition of the animal. This finding confirms the possible role of SAA as an early marker of inflammation, since leukocytes were within RI.
Finally, urine analysis showed a UPC of 5.3. No data regarding urine analysis in cetaceans have been found for comparison, but considering domestic animals, cut-off values between proteinuric versus non proteinuric animals are <0.5 in dogs and <0.4 in cats, and an UPC value >2.0 is strongly suggestive of glomerular disease [9]. SDS-AGE confirmed the glomerular origin of proteinuria, since these proteins have a greater molecular weight than albumin [19]. Surprisingly, in SDS-AGE analysis, no band at the expected molecular weight of myoglobin (18 kDa), was evident, despite myoglobin’s evidences in the Bowman’s space and in the tubular lumens observed with IHC examination. The absence of visible myoglobin in the urine is probably due to myoglobin precipitates in acidic urine within renal tubules (as seen during microscopic examination). Myoglobin tubular reabsorption has been excluded on the serum features, which appeared to be clear at gross examination [16]. Data herein reported confirm usefulness of SDS-AGE to evaluate proteinuria’s origin (glomerular and/or tubular). Further studies are needed to obtain correct RI in urinalysis in these species, because no data are available in literature.
The present investigation reports for the first time possible clinico-pathological changes in serum and urine samples during a stressful condition in a striped dolphin, underlining the relevant role of SAA in monitoring the health status also in this species. A constant effort in serum and urine sampling on marine mammals could enhance the existing knowledge on health biomarkers RI representing a useful tool for cetaceans’ rehabilitation and release.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Supplementary Material
Acknowledgments
We thank Prof. Antonio Di Bello for the clinical assessment, Centro Studi Cetacei staff for technical and veterinary support, and Centro Recupero Tartarughe Marine in Brancaleone (RC) for the logistical help during the hospitalization period. This study was partially funded by the Italian Ministry of the Environment and by a grant from University of Padua (PRA 2014; Project coordinator: ME. Gelain).
REFERENCES
- 1.Cattet M., Stenhouse G., Bollinger T.2008. Exertional myopathy in a grizzly bear (Ursus arctos) captured by leghold snare. J. Wildl. Dis. 44: 973–978. doi: 10.7589/0090-3558-44.4.973 [DOI] [PubMed] [Google Scholar]
- 2.Cray C., Arheart K. L., Hunt M., Clauss T., Leppert L. L., Roberts K., McCulloch S. D., Goldstein J. D., Gonzalez C., Sweeney J., Stone R., Fair P. A., Bossart G. D.2013. Acute phase protein quantitation in serum samples from healthy Atlantic bottlenose dolphins (Tursiops truncatus). J. Vet. Diagn. Invest. 25: 107–111. doi: 10.1177/1040638712467986 [DOI] [PubMed] [Google Scholar]
- 3.Gales N. J.1992. Mass stranding of striped dolphin, Stenella coeruleoalba, at Augusta, Western Australia: notes on clinical pathology and general observations. J. Wildl. Dis. 28: 651–655. doi: 10.7589/0090-3558-28.4.651 [DOI] [PubMed] [Google Scholar]
- 4.Geraci J. R., Lounsboury V. J.2005. Cetaceans-single strandings. pp. 75–112. In: Marine Mammals Ashore, a Field Guide for Stranding, 2nd ed. (Geraci, J. R. and Lounsboury, V. J. eds.), National Aquarium in Baltimore, Baltimore. [Google Scholar]
- 5.Herráez P., Espinosa de los Monteros A., Fernández A., Edwards J. F., Sacchini S., Sierra E.2013. Capture myopathy in live-stranded cetaceans. Vet. J. 196: 181–188. doi: 10.1016/j.tvjl.2012.09.021 [DOI] [PubMed] [Google Scholar]
- 6.Herráez P., Sierra E., Arbelo M., Jaber J. R., de Los Monteros A. E., Fernández A.2007. Rhabdomyolysis and myoglobinuric nephrosis (capture myopathy) in a striped dolphin. J. Wildl. Dis. 43: 770–774. doi: 10.7589/0090-3558-43.4.770 [DOI] [PubMed] [Google Scholar]
- 7.Johnson A. L., Hulse D. A.2007. Fundamentals of orthopedic surgery and fracture management. pp. 821–900. In: Small Animal Surgery, 3rd ed. (Fossum T. W. ed.), Elsevier, St. Louis. [Google Scholar]
- 8.Kuiken T., García Hartmann M.1991. Cetacean pathology: dissection techniques and tissue sampling. pp. 1–39. In: Proceedings of the First ECS Workshop on Cetacean Pathology, Leiden.
- 9.Lees G. E., Brown S. A., Elliott J., Grauer G. E., Vaden S. L., American College of Veterinary Internal Medicine 2005. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J. Vet. Intern. Med. 19: 377–385. doi: 10.1111/j.1939-1676.2005.tb02713.x [DOI] [PubMed] [Google Scholar]
- 10.Montané J., Marco I., Manteca X., López J., Lavín S.2002. Delayed acute capture myopathy in three roe deer. J. Vet. Med. A Physiol. Pathol. Clin. Med. 49: 93–98. doi: 10.1046/j.1439-0442.2002.jv409.x [DOI] [PubMed] [Google Scholar]
- 11.Poels P. J., Gabreëls F. J.1993. Rhabdomyolysis: a review of the literature. Clin. Neurol. Neurosurg. 95: 175–192. doi: 10.1016/0303-8467(93)90122-W [DOI] [PubMed] [Google Scholar]
- 12.Roe W., Spraker T. R.2012. Capture-related myopathy in marine mammals and exertional rhabdomyolysis in horses: a possible link? Vet. J. 193: 10–11. doi: 10.1016/j.tvjl.2012.03.012 [DOI] [PubMed] [Google Scholar]
- 13.Schwacke L. H., Hall A. J., Townsend F. I., Wells R. S., Hansen L. J., Hohn A. A., Bossart G. D., Fair P. A., Rowles T. K.2009. Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am. J. Vet. Res. 70: 973–985. doi: 10.2460/ajvr.70.8.973 [DOI] [PubMed] [Google Scholar]
- 14.Spraker T. R.1993. Stress and capture myopathy in artiodactylids. pp. 481–488. In: Zoo & Wild Animal Medicine, Current Therapy, 3rd ed. (Fowler, M.E. ed.), W.B. Saunders, Philadelphia. [Google Scholar]
- 15.St Aubin D. J., Austin T. P., Geraci J. R.1979. Effects of handling stress on plasma enzymes in harp seals, Phoca groenlandica. J. Wildl. Dis. 15: 569–572. doi: 10.7589/0090-3558-15.4.569 [DOI] [PubMed] [Google Scholar]
- 16.Stockam S. L., Scott M. A.2008. Enzymes. pp. 639–674. In: Fundamentals of Veterinary Clinical Pathology, 2nd ed. (Stockam, S. L. and Scott, M. A. eds.), Blackwell Publishing, Yowa. [Google Scholar]
- 17.Yen T. H., Lai P. C., Chen C. C., Hsueh S., Huang J. Y.2005. Renal involvement in patients with polymyositis and dermatomyositis. Int. J. Clin. Pract. 59: 188–193. doi: 10.1111/j.1742-1241.2004.00248.x [DOI] [PubMed] [Google Scholar]
- 18.Zager R. A.1996. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 49: 314–326. doi: 10.1038/ki.1996.48 [DOI] [PubMed] [Google Scholar]
- 19.Zini E., Bonfanti U., Zatelli A.2004. Diagnostic relevance of qualitative proteinuria evaluated by use of sodium dodecyl sulfate-agarose gel electrophoresis and comparison with renal histologic findings in dogs. Am. J. Vet. Res. 65: 964–971. doi: 10.2460/ajvr.2004.65.964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.