Abstract
Peroxisome proliferator-activated receptor γ (PPARγ), a member of a nuclear receptor family, has been shown to be implicated in various reproductive processes. Here, we evaluated possible roles of PPARγ in ovulation and luteal development in a gonadotropins-primed immature rat model. Immunoreactive PPARγ was expressed in granulosa cells of eCG-stimulated mature follicles, and its expression level decreased following ovulatory hCG stimulus. Intra-bursal treatment with rosiglitazone (a PPARγ agonist) simultaneously with subcutaneously administered hCG blocked the induction of cyclooxygenase-2 and steroidogenic acute regulatory protein (StAR) in preovulatory follicles. Consistently, tissue levels of their respective products, prostaglandin (PG) E2 and progesterone (P4), were reduced, leading to significantly decreased ovulation rate. GW9662, a PPARγ antagonist, was almost ineffective to alter those values. Local treatment with rosiglitazone 24 hr after hCG administration caused reductions in the size, StAR expression and P4 secretion of corpus luteum 48 hr later. Obtained data are possible functional evidence with rats for granulosa cell PPARγ as a negative regulator of PG and P4 synthesis during follicle rupture and transformation to luteal tissue. LH/hCG-induced decreases in PPARγ expression and its activity would be an early component in the proper induction of following ovulatory cascade and luteal development.
Keywords: luteal formation, ovulatory cascade, PPARγ, rat, rosiglitazone
Granulosa cells in mature follicles are a central player in mammalian ovulation and subsequent formation of corpus luteum (CL) in response to ovulatory stimuli, such as endogenous luteinizing hormone (LH) surge and exogenous human chorionic gonadotropin (hCG) administration [23, 30]. The ovulatory stimulus triggers potential intracellular and intranuclear signaling pathways, which, in large part, represents multi-stages gene expression program. An early stage component is alterations in transcription factors, their regulatory ligands and/or the ligands synthesizing/regulating factors, which are in concert responsible for further transcriptional events. Progesterone (P4)-P4 receptor pathway is one of such pathways acting in an intracrine fashion, and prostaglandin (PG)-PG receptor pathway is the one probably operating in a para/autocrine fashion. The almost essential roles of these two pathways in ovulation and functional CL formation are demonstrated by a number of studies with pharmacologic and genetic inhibition of synthesis or signaling of ligands and receptors [23]. Our group has presented some evidence with a rodent model of induced ovulation supporting for critical roles of a properly regulated endogenous P4 [1] and a cooperated action of group IVA phospholipase A2 (GIVA PLA2) and cyclooxygenase-2 (COX-2) [14].
Peroxisome proliferator-activated receptors (PPARs) are a nuclear receptor and transcription factor superfamily whose activity is supposed to be regulated by their ligands, such as endogenous arachidonic acid and 15-deoxy-delta (12,14)-PGJ2 (15d-PGJ2) and many exogenous chemicals [9, 36]. Three types, PPARα, PPARβ/δ and PPARγ, have all been shown to be expressed in the mammalian ovary and to have some possible roles in fertility [33, 35]. A previous study by Komar et al. has shown that mRNAs of three PPARs are expressed in granulosa cells of equine chorionic gonadotropin (eCG)-stimulated immature rat ovaries and that among PPARs, PPARγ mRNA is most abundantly expressed and down-regulated by human CG (hCG) treatment [12]. PPARγ mRNA expression following hCG treatment was demonstrated to be also down-regulated in macaque granulosa cells in vitro [21], but to be up-regulated in mouse ovary in vivo [8]. Chronic administration of a PPARα/γ dual agonist caused ovarian toxicity and infertility in adult female rats [26], but this drug might have acted on pituitary, exerting impaired gonadotropins secretion [35]. To solve the functional role of ovarian PPARγ signaling in situ, two independent groups have created and characterized mice with conditional gene knockout of PPARγ specific in the ovary [4] and in follicular granulosa cells [8]. Different ovulatory outcomes were found with little alteration in the former mutant mice [4] and severe suppression in the latter mutant mice [8]. Furthermore, another line of studies has been focusing on expression and role(s) of PPARγ in CL formed from a ruptured follicle. A low but notable level of PPARγ mRNA expression was negatively correlated with steroidogenic activity and a steroidogenic enzyme, P450 side chain cleavage (P450scc), mRNA in rat CL [10, 11, 32]. On the other hand, PPARγ protein expression in luteal cells decreased with aging in both non-pregnant and pregnant cow [34] and pseudopregnant rabbit [20] and was down-regulated by luteolytic PGF2α action [34]. The impacts of natural (15d-PGJ2) or synthetic (rosiglitazone) ligands of PPARγ on luteal P4 synthesis have so far been reported to be positive in bovine luteal cells in vitro [18] and in pseudopregnant rabbit CL in vitro [20], none in rat CL in vitro [32] or negative in porcine CL during early pregnancy [17]. Thus, data available are conflicting on the expression and definite functional role(s) of PPARγ in ovulatory follicles and subsequently formed CL.
Here, we address these issues using an immature rat model of gonadotropins-induced ovulation and luteal formation. We first confirm cellular location and temporal changes of PPARγ protein expression following hCG administration with relation to COX-2 and steroidogenic acute regulatory protein (StAR). Second, we evaluate the effects of a bolus dose of PPARγ agonist or antagonist simultaneously with hCG on COX-2 and StAR expression in preovulatory follicles and ovulation rate. Third, we delay the local treatment with PPARγ drugs as late as 24 hr after hCG treatment and evaluate its effect on CL function and structure. The currently obtained data suggest that down-regulation of PPARγ with inhibitory effects on COX-2 and StAR expression is important for inducing normal ovulation and early luteal development in rats.
MATERIALS AND METHODS
Reagents
Equine CG (eCG) and hCG were obtained from Shionogi (Osaka, Japan) and Daiichi-Sankyo (Tokyo, Japan), respectively. Rosiglitazone (a PPARγ agonist) and GW9662 (a PPARγ antagonist) were both obtained from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). Radiolabeled [1, 2, 6, 7-3H]-P4 used in radioimmunoassay (RIA) was obtained from Perkin-Elmer Japan (Yokohama, Japan). An enzyme immunoassay (EIA) kit for PGE2 and antibodies against PPARγ and COX-2 were also from Cayman Chemical. Antibodies against StAR and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). The antibody against rat CD68 was purchased from AbD Serotec (Oxford, U.K.). The antibody against P4 was generated in our laboratory. Vectastain Elite ABC staining kit was purchased from Vector Laboratories (Burlingame, CA, U.S.A.). Protein assay kit was from Bio Rad (Hercules, CA, U.S.A.) or Thermo Scientific (Waltham, MA, U.S.A.). All other reagents including 3, 3′-diaminobenzidine tetrahydrochrolide (DAB) and dimethyl sulfoxide (DMSO) were of analytical grade.
Animals and induction of ovulation and luteinization
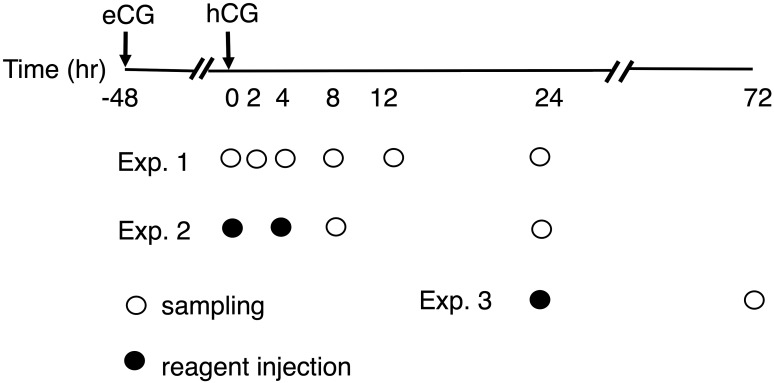
Animal handling and experimental procedures were performed following the guideline and approved by the Committee for Laboratory Animals Care and Use of Kitasato University. Wistar-Imamichi strain female rats of 25–27 day-old were treated with eCG (intraperitoneally, 0.2 IU/g of body weight) followed 48 hr later by hCG (10 IU/rat) to experimentally induce ovulation and luteal formation [1, 15]. Some of these rats were directly used for Experiment 1, and others for Experiments 2 and 3 were subjected to further treatments. The outline of the three experiments is summarized in Fig. 1 and described in detail in the next section. At indicated time points after treatments, rats were sacrificed by cervical dislocation under light anesthesia. In some cases, blood was taken via heart puncture. Ovaries, oviducts and blood plasma were harvested.
Fig. 1.
The outline for treatment and sampling schedules. eCG-primed immature (approximately 25-day old) rats were treated with hCG 48 hr later. They were then subject or not to intra-bursal treatment with vehicle (Veh), rosiglitazone (Ros) or GW9662 (GW) at the indicated time (marked with closed circles). Rats were sacrificed for sampling of ovary, oviduct and blood at the indicated time points (marked with open circles). Details of Exp. 1, 2 and 3 were described in the text.
In vivo experiments (Fig. 1)
Experiment 1: Expression and cellular localization of PPARγ and two ovulation-associated factors (COX-2 and StAR) were examined in the ovary of only eCG/hCG-treated rats. Ovaries were sampled at 0, 2, 4, 8, 12 or 24 hr after hCG administration. In this paper, for example, the time point of 0 hr after hCG was expressed as hCG0h. The organs were strored frozen until Western blot analysis, and some of those harvested at hCG0h, hCG8h and hCG24h were fixed for histology.
Experiment 2: To examine the impacts of PPARγ activity on ovulation, its ligand (agonist or antagonist) was locally treated at hCG0h, and ovulatory mediators (COX-2, StAR, PGE2 and P4) and ovulation rate were evaluated at hCG8h and hCG24h, respectively. Animals under anesthesia were subject to lateral abdominal incisions, and the ovarian bursa was exposed. A 50 µl of vehicle (20% DMSO in physiological saline), rosiglitazone (50 µM) or GW9662 (50 µM) was injected into one ovarian bursa using a syringe and repeated in another side. No visible leakage of the injected solution and swelling of the bursa were ascertained in this procedure. After the injection, ovaries were positioned back to the abdominal cavity, and muscles and skins were sutured separately. To examine the time-dependency of rosiglitazone treatment, another group receiving the agent at hCG4h was also prepared. Ovaries sampled at hCG8h were stored frozen for biochemical analysis of ovulatory mediators and fixed for histology. Oviducts harvested at hCG24h were evaluated for ovulation rate (=the number of released eggs). Eggs present in the ampulla were counted under a light microscope [1, 14].
Experiment 3: To study the impact of PPARγ activity on luteal development, local treatment with its ligands was delayed as late as hCG24h. Forty eight hr later (at hCG72h), ovaries were harvested for Western blot and histological analyses. Blood plasma was also sampled for P4 assay.
Western blot analysis
Western blot analysis of PPARγ, COX-2 and StAR was performed. Whole ovarian tissues were homogenized, sonicated and boiled for 5 min in SDS sample buffer. The samples containing 20 µg of protein were electrophoresed on SDS-PAGE 10% gel (Bio-Rad), and proteins were transferred onto polyvinylidene fluoride membranes (Bio-Rad). Membranes were blocked with 5% blocking buffer (Wako Chemicals, Osaka, Japan) for 1 hr at room temperature and then incubated with primary antibodies: anti-PPARγ (1:500), anti-COX-2 (1:400), anti-StAR (1:2,000) or anti-β-actin (1:5,000) overnight at 4°C. After washing, the membranes were incubated with peroxidase-conjugated goat IgG fraction to mouse IgG or rabbit IgG (1:40,000, GE Healthcare, Buckinghamshire, U.K.) for 2 hr at room temperature. Immunoreactive proteins were detected with ECL Prime Western Blotting Reagent (GE Healthcare). The signal was analyzed with an ImageQuant LAS 4000 digital imaging system (GE Healthcare).
Immunohistochemistry and histology
Localization and expression of PPARγ, COX-2 and StAR by preovulatory follicles were analyzed by immunohistochemistry as reported previously [1, 15]. Detection of macrophages in formed CL was performed by the immunohistochemistry of its marker CD68. Ovaries were fixed in Bouin’s fixative, dehydrated and embedded in paraffin. Samples of more than 3 individual rats in each group were collected and examined. Tissues were serially sectioned (2~4 µm in thickness), deparaffinized and examined. In PPARγ and StAR immunostaining, tissue sections were boiled in 10 mM citrate buffer for antigen retrieval. Endogenous peroxidase was blocked by pretreatment with 0.3% H2O2 in methanol for 30 min. Tissue sections were incubated with anti-PPARγ (used at 1:100), anti-StAR (1:100), anti-COX-2 (1:500) or anti-CD68 (1:400) at 4°C overnight. Antigen/antibody complexes were visualized with the Vectastain ABC staining kit and DAB as peroxidase substrate. Controls were performed with normal (non-immunized) mouse IgG. Most slides were then counterstained with hematoxylin. General cytology of developing CL harvested at hCG72h was examined with hematoxylin and eosin (HE) staining. The size of formed CL was estimated with the areas (mm2) of random tissue sections whose numbers exceeded 170.
Assay of P4 and PGE2
P4 in ovarian homogenate in physiological saline or blood plasma was extracted by n-hexane and was assayed with RIA [16]. PGE2 in ovarian homogenates was determined with an EIA kit as reported previously [1, 14]. Tissue contents of P4 and PGE2 were normalized by wet tissue weight and protein concentrations, respectively.
Statistical analysis
Data were presented as mean and standard error of the mean (SEM) of sample numbers indicated. The means among different groups were analyzed by Tukey-Kramer’s multiple comparison test. A P value less than 0.05 was considered to be significant.
RESULTS
Expression and cellular distribution of PPARγ, COX-2 and StAR during hCG-induced ovulation
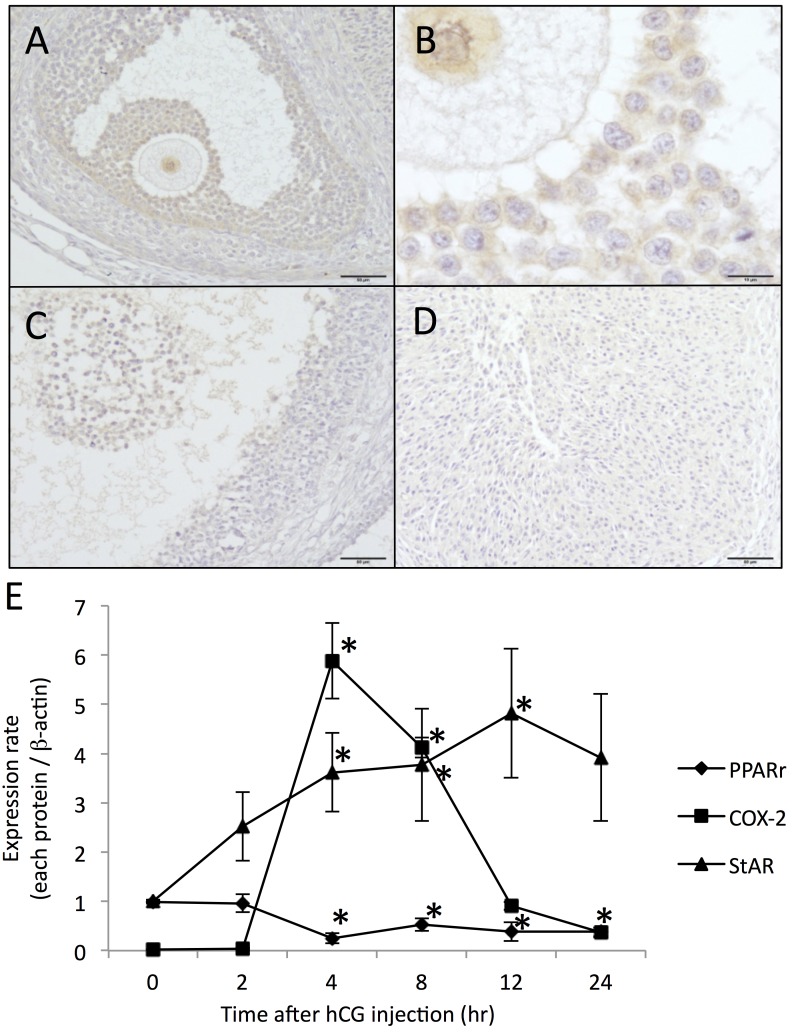
In eCG-stimulated mature follicles, the immunoreactivity for PPARγ was evident in mural and cumulus granulosa cells and oocytes (Fig. 2A and 2B). Its immunoreactivity in granulosa cells decreased in pre-ovulatory follicles at hCG8h (Fig. 2C) and in luteinized tissue (Fig. 2D). The level of PPARγ protein in whole ovarian homogenates was significant at hCG0h, persisted at hCG2h (96% of pre-hCG level) and decreased significantly at hCG4h (29% of pre-hCG level) (Fig. 2E, Supplementary Fig. 1). It remained suppressed until hCG24h (P<0.05, 38~52% of pre-hCG level). Levels of COX-2 protein in eCG-pretreated ovaries showed a temporal and drastic increase following hCG stimulation, while that of StAR protein showed a gradual increase (Fig. 2C, Supplementary Fig. 1). The data on the temporal expressional patterns of these eicosanoidogenic and stroidogenic proteins are consistent with those of the previous reports [1, 14, 25].
Fig. 2.
Ovarian expression of PPARγ, COX-2 and StAR proteins during hCG-triggered ovulation and luteinization. hCG-treated ovaries were sampled at the indicated time points for immunohistochemical (only for PPARγ) and Western blot analyses of PPARγ, COX-2 and StAR. Immunoreactive PPARγ was abundant in granulosa cells of preovulatory follicles at hCG0h (A, B), but markedly reduced in granulosa cells at hCG8h (C) and granulosa-lutein cells at hCG24h (D). Scale bars: 50 µm (A, C, D), 10 µm (B). Expression levels of three proteins were normalized to the internal standard (β-actin) (E). Data are mean with SEM (n=3 per time point). *, P<0.05 versus each value at hCG0h.
Effects of PPARγ agonist and antagonist on eicosanoid and steroid synthesis
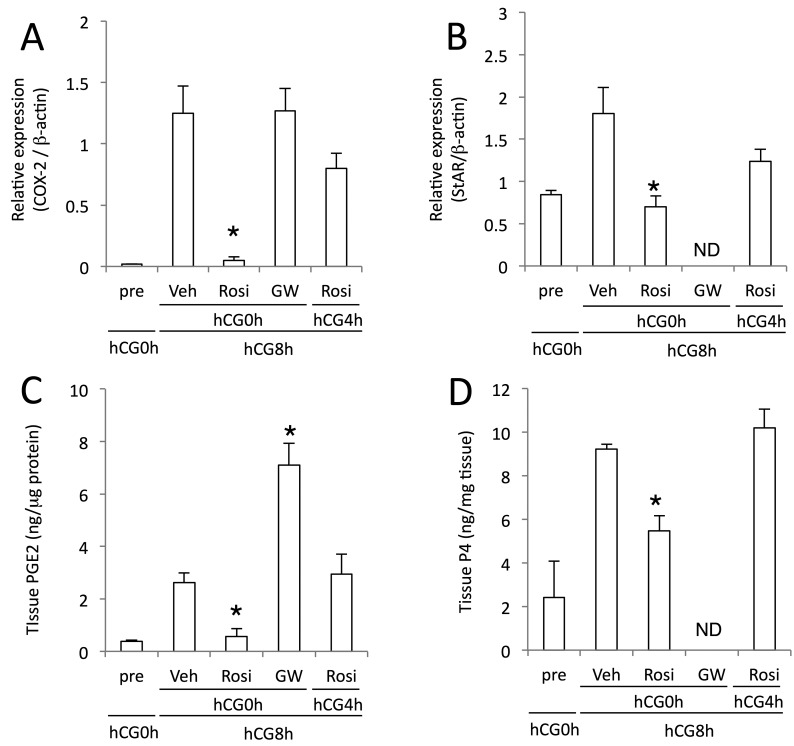
Given hCG-initiated dynamics of PPARγ and other proteins, we next determined the effects of PPARγ agonist or antagonist on COX-2 and StAR expression. hCG induced COX-2 protein in granulosa layer of mature follicles of the vehicle treatment group at hCG8h (Supplementary Fig. 2A). Rosiglitazone administered at hCG0h suppressed COX-2 induction at hCG8h (Fig. 3A, Supplementary Fig. 2C). Its suppressive effect was less potent when administered at hCG4h. GW9662 treatment at hCG0h was without effect. hCG-induced rise in PGE2 level at hCG8h was attenuated by simultaneous treatment with rosiglitazone, but not its treatment with 4 hr delay (Fig. 3C). GW treatment further increased PGE2 level compared to vehicle treated group. Rosiglitazone also blocked hCG-stimulated StAR expression (P<0.05 vs. vehicle treatment group) (Fig. 3B, Supplementary Fig. 2B and 2D), and this effect was decreased when treated at hCG4h. Following hCG stimulation, tissue P4 level in the control group was elevated as StAR was (Fig. 3D). Rosiglitazone inhibited the rise in P4 synthesis when treated at hCG0h, but not at hCG4h.
Fig. 3.
Effects of administration of PPARγ drugs on ovarian levels of COX-2 and StAR proteins and their metabolites. eCG/hCG-treated rats were further treated intrabursally with rosiglitazone (Rosi), GW9662 (GW) or vehicle (Veh) at hCG0h or at hCG4h (only Rosi). Ovaries were sampled at hCG8h and analyzed for levels of COX-2 (A) and StAR (B) proteins, PGE2 (C) and P4 (D) with Western blot, EIA and RIA, respectively. ND, not determined. Data are mean with SEM (n=3 or 4). *, P<0.05 versus Veh treatment group.
Effects of PPARγ agonist and antagonist on ovulation outcome
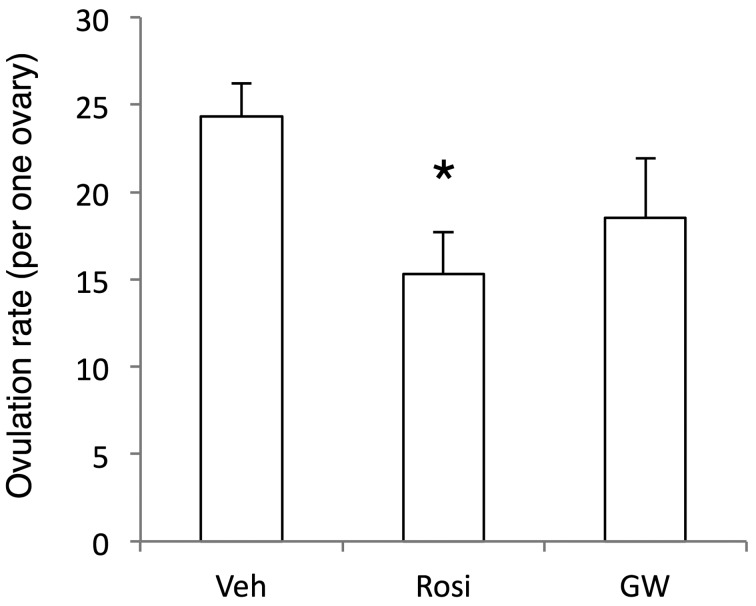
In the present gonadotropins treatment protocol in an immature rat model, 24.3 ± 1.9 (n=40) eggs were ovulated from an ovary and were seen in an ampulla of the vehicle treated group at hCG24h (Fig. 4). In rosiglitazone-treated group, ovulated eggs were decreased to 63% of the control group (P<0.05). GW9662 treatment was with an insignificant effect.
Fig. 4.
Effects of administration of PPARγ drugs on ovulation rates. eCG/hCG-treated rats were further treated intrabursally with rosiglitazone (Rosi), GW9662 (GW) or vehicle (Veh) at hCG0h. Oviducts were sampled at hCG24h and analyzed for counting eggs seen in the ampulla. Data are mean with SEM (n=22~44). *, P<0.05 versus Veh treatment group.
Effects of PPARγ agonist and antagonist on corpus luteum development
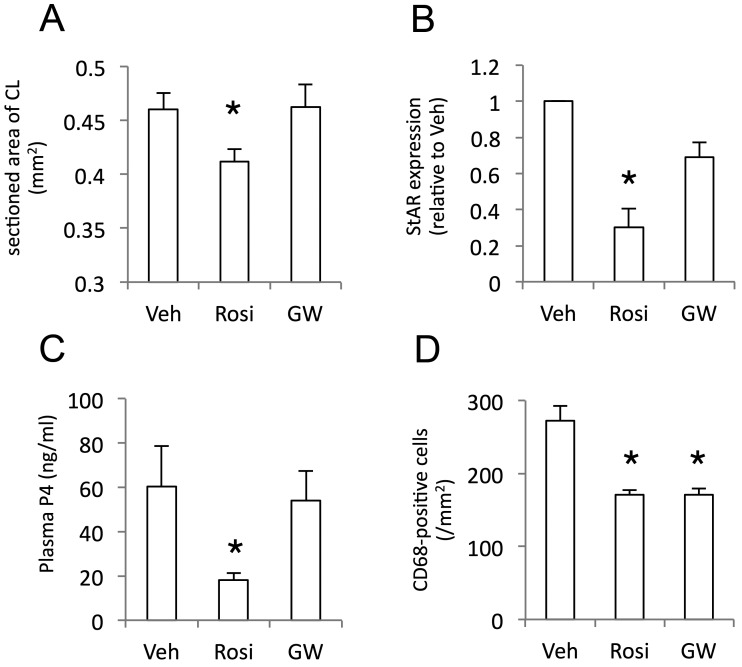
As we found reduced but significant level of PPARγ expression in ovulated and luteinized tissues, we next sought the impact of its activity on luteal development. Rosiglitazone significantly decreased the tissue size harvested at hCG72h (P<0.05 vs. Vehicle group) (Fig. 5A), while GW9662 treatment did not. General histology with HE staining revealed the intact luteal (steroidogenic) cell differentiation with well eosin staining in the control group, but abundant vacuoles and less eosin staining in the cytoplasm of luteal cells in rosiglitazone-treated group (Supplementary Fig. 3A and 3B). StAR expression in formed CL was lower in rosiglitazone-treated group compared to that in the control group (Fig. 5B, Supplementary Fig. 3D). No significant alteration was found in GW9662-treated group. Plasma P4 level, an idex of luteal functional development, was attenuated by a PPARγ agonist, but not its antagonist (Fig. 5C). The number of macrophages infiltrating into a formed CL, assessed by CD68-immunopositive cells in the defined area, was decreased by both PPARγ agonist and antagonist (Fig. 5D).
Fig. 5.
Effects of administration of PPARγ drugs on tissue size, StAR protein expression, and P4 production of formed CL. eCG/hCG-treated rats were further treated intrabursally with rosiglitazone (Rosi), GW9662 (GW) or vehicle (Veh) at hCG24h. Ovaries were sampled at hCG72h and analyzed for CL section size (A), StAR proteins (B) and macrophage number (D) with histological morphometry, Western blotting and immunohistochemistry, respectively. Blood plasma P4 (C) was determined by RIA. Data are mean with SEM (n=174~311 in A,=3 in B,=6 in C,=37~71 in D). *, P<0.05 versus Veh treatment group.
DISCUSSION
The findings of this study using an immature rat model include: 1) The local treatment with a PPARγ agonist suppressed hCG-induced COX-2 and StAR expression in preovulatory follicles, leading to decreased ovulatory rate, 2) hCG-induced up-regulation of COX-2 and StAR expression in granulosa cells of intact preovulatory follicles was associated with or preceded by down-regulation of PPARγ that had been robustly expressed in situ, and 3) A PPARγ agonist treatment also decreased the size and P4 secretory potency of a formed CL.
Experiments of agonist administration in vivo have revealed that PPARγ in the preovulatory follicles could have a negative regulatory effect on the production of critical mediators in ovulatory cascade. Acute activation of PPARγ with rosiglitazone treatment at hCG0h prevented preovulatory ovaries from hCG-induced COX-2 and StAR proteins expression. This repressing effect of rosiglitazone was impaired by a 4 hr delayed treatment. This result is consistent with the finding of temporal changes in PPARγ protein level. Both of COX-2 and StAR are expressed in granulosa layers of preovulatory follicles in a spatio-temporally regulated manner [1, 14, 25]. PPARγ is present in almost all follicles in eCG-treated ovaries [10, 12]. The drug administered to the ovary, however, must have directly acted on and affected, at least, granulosa cells in preovulatory follicles that should have expressed COX-2 and StAR and have underwent luteinization [23]. Consistent reductions in PGE2 and P4 production by rosiglitazone-treated ovaries were also found, and consequently, ovulation rate was decreased.
Many evidences are available supporting for PPARγ repression of COX-2 expression. Indirect evidences are opposite regulation by ovulatory hCG of COX-2 mRNA/protein expression [1, 14, 15, 23] and PPARγ mRNA expression [12] in rodent ovarian follicles. This inverse relationship is confirmed here with the identical sample and has been reported in the human term placenta [5] and human ovarian carcinoma tissue [29]. Direct evidence is that treatment with 15d-PGJ2 or rosiglitazone seemed to inhibit tumor necrosis factor α-induced COX-2 expression in human WISH and amnion cells [2]. Furthermore, molecular analysis reveals that PPARγ agonists repress COX-2 transcription via direct interaction with its gene promoter [7, 31].
Compared to that with COX-2 expression and PG production, the relation of PPARγ with ovarian steroidogenic activity seems more complex. Our data reveal down-regulation by PPARγ of P4 production via impaired StAR expression in both preovulatory follicles and newly formed CL. A previous in situ hybridization study suggested little association of its mRNA level with steroidogenic activity in rat ovarian follicles [12]. In contrast, PPARγ mRNA level in adult rat CL was inversely correlated with functional status and P450scc mRNA level [10, 11, 32]. Data on the effects of PPARγ agonist administration have been further variable. PPARγ activators, 15d-PGJ2 and ciglitazone, stimulated P4 secretion by granulosa cells of eCG-treated mature follicles in rats [12], porcine ovarian follicles [22, 27], bovine CL [18] and rabbit CL of early- and middle-phases of pseudopregnancy [20]. Ciglitazone and rosiglitazone stimulated mRNA and/or protein expression of StAR in KK1 mouse granulosa cell lines [13] and human granulosa cells [28], respectively. The PPARγ up-regulating ligands inhibited P4 production in porcine granulosa cell with inhibited expression of 3β-hydroxy-steroid dehydrogenase [6], human granulosa cells [37] and porcine CL in early pregnancy [17]. It is likely that PPARγ impacts are dependent on cell types, tissues, animal species and their functional states. Our results show, at least, that PPARγ (over) stimulation could inhibit P4 production via abrogated StAR expression in preovulatory follicles.
It is reasonable that the expression of PPARγ protein having an inhibitory potency on ovulatory mediators is instantly down-regulated by ovulatory stimulus. Previous studies showed that PPARγ mRNA highly expressed in granulosa cells of rat preovulatory follicles [9, 10, 12] was down-regulated by hCG [12]. hCG-induced down-regulation of PPARγ mRNA was also found in macaque granulosa cells [21]. Our immunohistochemical analysis confirmed granulosa expression of PPARγ protein, and Western blot analysis revealed its temporal change that was consistent with two previous reports on dynamics in mRNA level [12, 21]. Condsidering that a part of mature follicles in eCG-stimulated ovaries are sensitive to and respond to hCG stimulus, PPARγ protein level in ovulatory follicles and luteinized tissues must be lower than the level detected with Western blot analysis of whole ovarian tissues. Physiologically, PPARγ impact would be masked or diminished by the decrease in its expression level as ovulatory cascade progresses.
PPARγ activity might also affect multiple aspects of luteal development. As described above, PPARγ mRNA expression level in rat mature follicles decreased during luteinization and CL formation [12], and its expression level in CL was further noted to be inversely related with P450scc mRNA level and steroidogenic potency [10, 11]. Our demonstration in vivo of attenuated StAR expression and P4 secretion in rosiglitazone-treated young CL suggests a negative action on luteal steroidogenesis. Inhibition of P4 production by PPARγ activation has very recently been reported in porcine CL of early pregnancy [17]. Impaired functional development was associated with impairments in morphological development. We found decreased size of CL and poor cytoplasmic proteins in luteal cells of rosiglitazone-treated group. Angiogenesis is critical to CL formation from a non-vascularized follicle [30] and was shown to be potently inhibited by PPARγ activation in vivo and in vitro [38]. Furthermore, luteal development in mice is reported to involve accumulation of and promoting action by macrophages [3], whose activation is negatively regulated by PPARγ [24]. Consistent with it, we found the decreased number of CD68-positive cells in rosiglitazone-treated CL. A similar effect of GW9662 on macrophage recruitment is likely due to multiple effects on PPARγ in macrophage and/or less specificity of this inhibitor. Collectively, PPARγ may exert negative actions on CL development directly on luteal steroidogenic cells and indirectly via angiogenesis and macrophage mobilization.
The present experimental approach was a bolus administration of exogenous agonist and antagonist to evaluate PPARγ activity in vivo. A previous study has suggested that PPARγ would be functional in rat granulosa cells [19]. Our results of almost identical responses to GW9662 and control vehicle may suggest little presence and action of endogenous ligand(s) to PPARγ in vivo at least in the time frame (around hCG administration) we focused on. It is generally supposed that 15d-PGJ2, synthesized non-enzymatically from PGD2, and arachidonic acid are the endogenous ligands for PPARγ [36]. As far as we know, the endogenous ligand(s) of PPARγ in ovarian granulosa and luteal cells have remained unidentified. We previously demonstrated that arachidonic acid-selective GIVA PLA2 was up-regulated by hCG, potentiated hCG-induced COX-2 expression and co-localized with the induced COX-2 on nuclear membranes of granulosa cells in rodent preovulatory follicles [14]. Those results have suggested that the synthesized arachidonic acid and/or any PG could interact with unknown transcription factor(s) to up-regulate COX-2 expression by positive feed-forward and/or feedback loop(s). Our current results show the simultaneous occurrence of COX-2 induction and PPARγ repression and PPARγ’s negative impact on COX-2 induction. Taken together, PPARγ is unlikely related to hCG-induced robust COX-2 induction, and it is reasonable to think that decreasing expression of PPARγ is one of mechanisms for optimal induction of PLA2/COX-2 pathway and other and downstream ovulatory cascade.
Chronic and systemic treatment (2–4 weeks) of adult female rats with an overdose of a PPARα/γ dual agonist resulted in infertility including impaired ovulation and luteinization [26]. This toxicological finding has significant implication given the clinical use of PPARγ agonists for lipid/glucose metabolism-related diseases and inflammation [36]. Mice with the ovarian specific deletion of PPARγ gene appeared to have no phenotypes in follicular maturation, ovulation, CL formation or its P4 secretion [4], while mice with granulosa-specific conditional deletion represented drastic ovulation failure [8]. Our study is evidently inconsistent with the latter study claiming that PPARγ is induced and may be actively involved in ovulatory cascade through binding to COX-2-derived metabolites [8]. Further studies are needed to understand definite impacts of PPARγ signaling in terms of cell types and the term of ovarian function.
In conclusion, this study provides some novel information about PPARγ in ovulatory follicles and newly formed CL in rats. This transcription factor highly expressed in granulosa cells has a potency to repress COX-2 and StAR expression, but its possible inhibitory action would be diminished, in some part, by down-regulating its expression level in the early phase post LH/hCG stimulus. PPARγ in CL may affect its structural and functional development at multiple sites, and the regulation of its expression would also be important for normal CL development. These findings help to widen the knowledge on the mechanisms of ovulatory cascade and luteal formation.
Supplementary Material
Acknowledgments
We greatly thank M. Nakata for help in preparation of manuscript.
REFERENCES
- 1.Abe T., Toida D., Satoh H., Yonezawa T., Kawaminami M., Kurusu S.2011. An early single dose of progesterone agonist attenuates endogenous progesterone surge and reduces ovulation rate in immature rat model of induced ovulation. Steroids 76: 1116–1125. doi: 10.1016/j.steroids.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 2.Ackerman W. E., 4th, Zhang X. L., Rovin B. H., Kniss D. A.2005. Modulation of cytokine-induced cyclooxygenase 2 expression by PPARG ligands through NFkappaB signal disruption in human WISH and amnion cells. Biol. Reprod. 73: 527–535. doi: 10.1095/biolreprod.104.039032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Care A. S., Diener K. R., Jasper M. J., Brown H. M., Ingman W. V., Robertson S. A.2013. Macrophages regulate corpus luteum development during embryo implantation in mice. J. Clin. Invest. 123: 3472–3487. doi: 10.1172/JCI60561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui Y., Miyoshi K., Claudio E., Siebenlist U. K., Gonzalez F. J., Flaws J., Wagner K. U., Hennighausen L.2002. Loss of the peroxisome proliferation-activated receptor γ (PPARgamma) does not affect mammary development and propensity for tumor formation but leads to reduced fertility. J. Biol. Chem. 277: 17830–17835. doi: 10.1074/jbc.M200186200 [DOI] [PubMed] [Google Scholar]
- 5.Dunn-Albanese L. R., Ackerman W. E., 4th, Xie Y., Iams J. D., Kniss D. A.2004. Reciprocal expression of peroxisome proliferator-activated receptor-γ and cyclooxygenase-2 in human term parturition. Am. J. Obstet. Gynecol. 190: 809–816. doi: 10.1016/j.ajog.2003.09.052 [DOI] [PubMed] [Google Scholar]
- 6.Gasic S., Bodenburg Y., Nagamani M., Greeen A., Urban R. J.1998. Troglitazone inhibits progesterone production in porcine granulosa cells. Endocrinology 139: 4962–4966. doi: 10.1210/endo.139.12.6385 [DOI] [PubMed] [Google Scholar]
- 7.Inoue H., Tanabe T., Umesono K.2000. Feedback control of cyclooxygenase-2 expression through PPARgamma. J. Biol. Chem. 275: 28028–28032. [DOI] [PubMed] [Google Scholar]
- 8.Kim J., Sato M., Li Q., Lydon J. P., Demayo F. J., Bagchi I. C., Bagchi M. K.2008. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol. Cell. Biol. 28: 1770–1782. doi: 10.1128/MCB.01556-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komar C. M.2005. Peroxisome proliferator-activated receptors (PPARs) and ovarian function-implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod. Biol. Endocrinol. 3: 41. doi: 10.1186/1477-7827-3-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komar C. M., Curry T. E., Jr.2002. Localization and expression of messenger RNAs for the peroxisome proliferator-activated receptors in ovarian tissue from naturally cycling and pseudopregnant rats. Biol. Reprod. 66: 1531–1539. doi: 10.1095/biolreprod66.5.1531 [DOI] [PubMed] [Google Scholar]
- 11.Komar C. M., Curry T. E., Jr.2003. Inverse relationship between the expression of messenger ribonucleic acid for peroxisome proliferator-activated receptor γ and P450 side chain cleavage in the rat ovary. Biol. Reprod. 69: 549–555. doi: 10.1095/biolreprod.102.012831 [DOI] [PubMed] [Google Scholar]
- 12.Komar C. M., Braissant O., Wahli W., Curry T. E., Jr.2001. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology 142: 4831–4838. doi: 10.1210/endo.142.11.8429 [DOI] [PubMed] [Google Scholar]
- 13.Kowalewski M. P., Dyson M. T., Manna P. R., Stocco D. M.2009. Involvement of peroxisome proliferator-activated receptor γ in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod. Fertil. Dev. 21: 909–922. doi: 10.1071/RD09027 [DOI] [PubMed] [Google Scholar]
- 14.Kurusu S., Sapirstein A., Bonventre J. V.2012. Group IVA phospholipase A2 optimizes ovulation and fertilization in rodents through induction of and metabolic coupling with prostaglandin endoperoxide synthase 2. FASEB J. 26: 3800–3810. doi: 10.1096/fj.12-203968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurusu S., Jinno M., Ehara H., Yonezawa T., Kawaminami M.2009. Inhibition of ovulation by a lipoxygenase inhibitor involves reduced cyclooxygenase-2 expression and prostaglandin E2 production in gonadotropin-primed immature rats. Reproduction 137: 59–66. doi: 10.1530/REP-08-0257 [DOI] [PubMed] [Google Scholar]
- 16.Kurusu S., Shingaki S., Munekata Y., Kawaminami M., Hashimoto I.1995. Detection of cytosolic phospholipase A2 in rat corpora lutea: relationship to functional luteolysis. J. Reprod. Dev. 41: 141–147. doi: 10.1262/jrd.41.141 [DOI] [Google Scholar]
- 17.Kurzynska A., Bogacki M., Chojnowska K., Bogacka I.2014. Peroxisome proliferator activated receptor ligands affect progesterone and 17β-estradiol secretion by porcine corpus luteum during early pregnancy. J. Physiol. Pharmacol. 65: 709–717. [PubMed] [Google Scholar]
- 18.Löhrke B., Viergutz T., Shahi S. K., Pöhland R., Wollenhaupt K., Goldammer T., Walzel H., Kanitz W.1998. Detection and functional characterisation of the transcription factor peroxisome proliferator-activated receptor γ in lutein cells. J. Endocrinol. 159: 429–439. doi: 10.1677/joe.0.1590429 [DOI] [PubMed] [Google Scholar]
- 19.Lovekamp-Swan T., Chaffin C. L.2005. The peroxisome proliferator-activated receptor gamma ligand troglitazone induces apoptosis and p53 in rat granulosa cells. Mol. Cell. Endocrinol. 233: 15–24. doi: 10.1016/j.mce.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 20.Parillo F., Maranesi M., Brecchia G., Gobbetti A., Boiti C., Zerani M.2014. In vivo chronic and in vitro acute effects of di(2-ethylhexyl) phthalate on pseudopregnant rabbit corpora lutea: possible involvement of peroxisome proliferator-activated receptor γ. Biol. Reprod. 90: 41. doi: 10.1095/biolreprod.113.109223 [DOI] [PubMed] [Google Scholar]
- 21.Puttabyatappa M., Vandevoort C. A., Chaffin C. L.2010. hCG-induced down-regulation of PPARγ and liver X receptors promotes periovulatory progesterone synthesis by macaque granulosa cells. Endocrinology 151: 5865–5872. doi: 10.1210/en.2010-0698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rak-Mardyła A., Karpeta A.2014. Rosiglitazone stimulates peroxisome proliferator-activated receptor γ expression and directly affects in vitro steroidogenesis in porcine ovarian follicles. Theriogenology 82: 1–9. doi: 10.1016/j.theriogenology.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 23.Richards J. S., Liu Z., Shimada M.2015. Ovulation. pp. 997–1021. In: Physiology of Reproduction, 4th ed. (Plant, T. M. and Zeleznik, A. J. eds.), Elsevier, Amsterdam. [Google Scholar]
- 24.Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K.1998. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391: 79–82. doi: 10.1038/34178 [DOI] [PubMed] [Google Scholar]
- 25.Ronen-Fuhrmann T., Timberg R., King S. R., Hales K. H., Hales D. B., Stocco D. M., Orly J.1998. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology 139: 303–315. doi: 10.1210/endo.139.1.5694 [DOI] [PubMed] [Google Scholar]
- 26.Sato N., Uchida K., Nakajima M., Watanabe A., Kohira T.2009. Collaborative work on evaluation of ovarian toxicity. 13) Two- or four-week repeated dose studies and fertility study of PPAR α/γ dual agonist in female rats. J. Toxicol. Sci. 34Suppl 1: SP137–SP146. [DOI] [PubMed] [Google Scholar]
- 27.Schoppee P. D., Garmey J. C., Veldhuis J. D.2002. Putative activation of the peroxisome proliferator-activated receptor γ impairs androgen and enhances progesterone biosynthesis in primary cultures of porcine theca cells. Biol. Reprod. 66: 190–198. doi: 10.1095/biolreprod66.1.190 [DOI] [PubMed] [Google Scholar]
- 28.Seto-Young D., Avtanski D., Strizhevsky M., Parikh G., Patel P., Kaplun J., Holcomb K., Rosenwaks Z., Poretsky L.2007. Interactions among peroxisome proliferator activated receptor-γ, insulin signaling pathways, and steroidogenic acute regulatory protein in human ovarian cells. J. Clin. Endocrinol. Metab. 92: 2232–2239. doi: 10.1210/jc.2006-1935 [DOI] [PubMed] [Google Scholar]
- 29.Stadlmann S., Gueth U., Wight E., Kunz-Schughart L. A., Hartmann A., Singer G.2007. Expression of peroxisome proliferator activated receptor γ and cyclo-oxygenase 2 in primary and recurrent ovarian carcinoma. J. Clin. Pathol. 60: 307–310. doi: 10.1136/jcp.2005.035717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stouffer R. L., Hennebold J. D.2015. Structure, function, and regulation of the corpus luteum. pp. 1023–1076. In: Physiology of Reproduction, 4th ed. (Plant, T. M. and Zeleznik, A. J. eds.), Elsevier, Amsterdam. [Google Scholar]
- 31.Subbaramaiah K., Lin D. T., Hart J. C., Dannenberg A. J.2001. Peroxisome proliferator-activated receptor gamma ligands suppress the transcriptional activation of cyclooxygenase-2. Evidence for involvement of activator protein-1 and CREB-binding protein/p300. J. Biol. Chem. 276: 12440–12448. doi: 10.1074/jbc.M007237200 [DOI] [PubMed] [Google Scholar]
- 32.Tinfo N., Komar C.2007. Potential role for peroxisome proliferator-activated receptor γ in regulating luteal lifespan in the rat. Reproduction 133: 187–196. doi: 10.1530/REP-06-0134 [DOI] [PubMed] [Google Scholar]
- 33.Vélez L. M., Abruzzese G. A., Motta A. B.2013. The biology of the peroxisome proliferator-activated receptor system in the female reproductive tract. Curr. Pharm. Des. 19: 4641–4646. doi: 10.2174/1381612811319250010 [DOI] [PubMed] [Google Scholar]
- 34.Viergutz T., Loehrke B., Poehland R., Becker F., Kanitz W.2000. Relationship between different stages of the corpus luteum and the expression of the peroxisome proliferator-activated receptor γ protein in bovine large lutein cells. J. Reprod. Fertil. 118: 153–161. doi: 10.1530/reprod/118.1.153 [DOI] [PubMed] [Google Scholar]
- 35.Vitti M., Di Emidio G., Di Carlo M., Carta G., Antonosante A., Artini P. G., Cimini A., Tatone C., Benedetti E.2016. Peroxisome proliferator-activated receptors in female reproduction and fertility. PPAR Res. 2016: 4612306. doi: 10.1155/2016/4612306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wahli W., Michalik L.2012. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol. Metab. 23: 351–363. doi: 10.1016/j.tem.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 37.Willis D. S., White J., Brosens S., Franks S.1999. Effect of 15-deoxy-delta-(12, 14)-prostaglandin J2 (PGJ2) a peroxisome proliferator activating receptor γ (PPARγ) ligand on human ovarian steroidogenesis. Endocrinology 1999: 491. [Google Scholar]
- 38.Xin X., Yang S., Kowalski J., Gerritsen M. E.1999. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J. Biol. Chem. 274: 9116–9121. doi: 10.1074/jbc.274.13.9116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.