Abstract
Ambystoma mexicanum kept as pets are affected by a variety of diseases. However, no reports regarding the incidence of specific diseases are available. This study aimed to identify the diseases that occur frequently in this species by surveying the incidence of conditions in pet A. mexicanum specimens brought to a veterinary hospital. The sample comprised 97 pet A. mexicanum individuals brought to the authors’ hospital during the 82-month period, i.e., from January 2008 to October 2014. In total, 116 diseases were identified. The most common disease was hydrocoelom (32 cases; 27.5% of all cases). Elucidating the pathogenesis of hydrocoelom, which has a high prevalence rate, is vital to maintaining the long-term health of A. mexicanum pets.
Keywords: Ambystoma mexicanum, axolotl, exotic animal, hydrocoelom
Ambystoma mexicanum is a neotenic salamander, which belongs to the family Ambystomatidae [9]. A. mexicanum kept as pets are affected by a variety of diseases that are treated at veterinary hospitals. However, limited information is available regarding these diseases [1, 4, 6,7,8, 10, 11,12,13,14]. The purpose of this study was to identify and survey the incidence of the most common diseases in pet A. mexicanum specimens brought to a veterinary hospital. A total of 97 A. mexicanum specimens were brought to the authors’ hospital for treatment during the 82-month period from January 2008 to October 2014. The clinical diagnosis, sex, body color, tank water temperature and quality, and timing of examination were surveyed from case records. Cases with nonspecific clinical symptoms only were provisionally classified in accordance with the symptom name (e.g., anorexia lethargy). For the disease with the highest incidence, the relationship of the disease to diet, sexual maturity, timing of examination, and use of bedding material were investigated in addition to the other parameters. The body color was classified as white, yellow, black or wild-type. The tap water in Japan is “soft” (i.e., it contains a low concentrations of ions, particularly calcium and magnesium) and hence it is unsuitable for housing A. mexicanum. Accordingly, questions regarding the water quality were asked to investigate its potential relationship with the diseases. The diet was recorded as artificial feed, artificial feed together with live feed, live feed or unknown. Whether the tank floor was covered with material (i.e., “bedding”) such as gravel or pebble was also recorded.
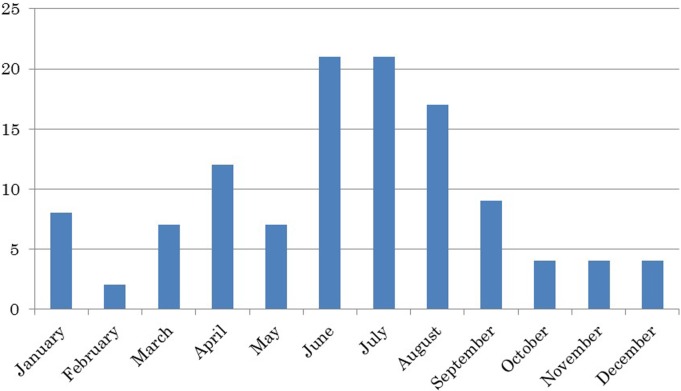
All 97 A. mexicanum specimens had acquired some type of disease. Six were identified as male, 3 as female, and 88 were of an undetermined sex. Eighty-seven individuals were white, 4 were yellow, 1 was black, and 5 were wild-type in coloration. None of the specimens were housed in environments in which the water temperature was controlled using a water cooler or other methods; all were housed at room temperature. With regard to the water quality, none were housed in “hard” (i.e., high mineral content) water. A total of 116 diseases were enumerated in the 97 A. mexicanum specimens. In terms of the examination timing for these 116 diseases, the number of cases recorded in each calendar month is shown in Fig. 1. The incidence of each disease is shown in Fig. 2, and the number of cases in each disease group is shown in Table 1. The most common group was “other,” with 61 cases (52.6%) that included hydrocoelom (Fig. 3) and buoyancy disorders (Fig. 4). Skin disease was the next most prevalent group, with 33 cases (28.4%). Fairly large numbers of cases of gastrointestinal disease (n=9; 7.7%) and urological disease (n=7; 6.0%) were also observed. Of the individual diseases, hydrocoelom was the most common with 32 cases, corresponding to 27.5% of all cases. Individual specimens with hydrocoelom included the following: 3 of the male animals; all 3 of the female animals; 26 of the individuals of undetermined sex; 28 (32.2%) of the 87 white individuals; 2 (50.0%) of the 4 yellow individuals; 0 of 1 black individual; and 2 (40%) of the 5 individuals with wild-type coloration. The animals with hydrocoelom were fed various diets: an artificial diet in 13 animals; artificial and live, 5; live, 1; unknown, 13. Eight individuals (25%) were sexually mature, 19 (59.4%) were sexually immature, and 5 (15.6%) were undetermined. The timing of examination is shown in Fig. 5. Of the 32 individuals with ascites, 84% were examined during the 6-month period beginning in May. Eight individuals (25%) were housed in tanks with bedding material, while 24 (75%) were not. Other fairly common diseases included buoyancy disorders (n=20), bacterial dermatitis (n=18), localized edema (n=5), cephalic subcutaneous abscess (n=5), and gastrointestinal foreign body (n=5).
Fig. 1.
Number of separate diseases among 116 enumerated in total according to month. Y-axis: Number of individuals.
Fig. 2.
Incidence of each disease group in Ambystoma mexicanum. X-axis: Number of individuals.
Table 1. Number of cases in each disease group among the 116 diseases.
| Group | Disease | n |
|---|---|---|
| Skin disease | Bacterial dermatitis | 18 |
| Cephalic subcutaneous abscess | 5 | |
| Fungal dermatitis | 3 | |
| Traumatic dermatitis | 3 | |
| Skin mass | 2 | |
| Burn | 1 | |
| Anchor worm disease | 1 | |
| Gastrointestinal disease | Gastrointestinal foreign body | 5 |
| Constipation | 2 | |
| Duodenal mucosal hyperplasia | 1 | |
| Gastric prolapse | 1 | |
| Urogenital disease | Bladder prolapse | 4 |
| Testicular tumor | 1 | |
| Perivesical abscess | 1 | |
| Retained eggs | 1 | |
| Neurological disease | Malignant olfactory neuroepiyhelioma | 1 |
| Forelimb paralysis | 1 | |
| Torticollis | 1 | |
| Ophthalmologic disease | Corneal injury | 1 |
| Corneal lipidosis | 1 | |
| Musculoskeletal disease | Tail necrosis | 1 |
| Other | Hydrocoelom | 32 |
| Buoyancy disorders | 20 | |
| Localized edema | 5 | |
| Anorexia | 2 | |
| Lethargy | 2 | |
Fig. 3.
Ambystoma mexicanum with hydrocoelom.
Fig. 4.
Ambystoma mexicanum with buoyancy disorder.
Fig. 5.
Number of cases of hydrocoelom in Ambystoma mexicanum according to month. Y-axis: Number of individuals.
The results of this study revealed a high incidence of hydrocoelom (27.5%), which was more prevalent than other diseases. Although the causes of hydrocoelom remain unknown [15], its incidence was highest in June. The examination rate during the 6 month period beginning in May corresponded to an incidence of 84%. A. mexicanum inhabits cold-water regions and has an optimal water temperature of 15 to 18°C [2, 3, 5]. However, none of the owners in this study used water-cooling equipment when housing their A. mexicanum pets. In the Fukuoka prefecture where this study was performed, the atmospheric temperatures begin to rise in May, and the mean temperature exceeds 18°C until October. Thus, an elevated water temperature is believed to be a risk factor for hydrocoelom. With respect to body color, the incidence of hydrocoelom appeared to be high in colored individuals; however, because the number of individuals in the study was small, no definitive conclusion could be reached with regard to a body color bias. With respect to the bedding material, the incidence of hydrocoelom appeared to be high in individuals housed in tanks without bedding material; however, no definitive conclusion could be drawn with regard to the bedding material within the scope of this study. Owing to the very high incidence of hydrocoelom, a further examination of its pathogenesis is needed. This study was performed at only one hospital in the Fukuoka prefecture (Japan), and the number of subjects was relatively small. Furthermore, seasonal factors in the geographical area may have influenced the results. Therefore, future studies should include additional centers and a larger number of subjects.
REFERENCES
- 1.Del-Pozo J., Girling S., Pizzi R., Mancinelli E., Else R. W.2011. Severe necrotizing myocarditis caused by serratia marcescens infection in an axolotl (Ambystoma mexicanum). J. Comp. Pathol. 144: 334–338. doi: 10.1016/j.jcpa.2010.11.001 [DOI] [PubMed] [Google Scholar]
- 2.Duhon S.1988. The I.U. axolotl colony’s short guide to the care and feeding of axolotls: an overview of the methods used at the Indiana University axolotl colony. Axolotl Newslett. 17: 15–18. [Google Scholar]
- 3.Farkas J. E., Monaghan J. R.2015. Housing and maintenance of Ambystoma mexicanum, the Mexican axolotl. Methods Mol. Biol. 1290: 27–46. doi: 10.1007/978-1-4939-2495-0_3 [DOI] [PubMed] [Google Scholar]
- 4.Green D. E., Harshbarger J. C.2001. Spontaneous neoplasia in amphibian. pp. 335–400. In: Amphibian Medicine and Captive Husbandry (Wright, K. M. and Whitaker, B. R. eds.), Krieger Publishing Company, Malabar. [Google Scholar]
- 5.Gresens J.2004. An introduction to the Mexican axolotl (Ambystoma mexicanum). Lab Anim. (NY) 33: 41–47. doi: 10.1038/laban1004-41 [DOI] [PubMed] [Google Scholar]
- 6.Khudoley V. V., Eliseiv V. V.1979. Multiple melanomas in the axolotl Ambystoma mexicanum. J. Natl. Cancer Inst. 63: 101–103. [PubMed] [Google Scholar]
- 7.McMillan M. W., Leece E. A.2011. Immersion and branchial/transcutaneous irrigation anaesthesia with alfaxalone in a Mexican axolotl. Vet. Anaesth. Analg. 38: 619–623. doi: 10.1111/j.1467-2995.2011.00660.x [DOI] [PubMed] [Google Scholar]
- 8.Menger B., Vogt P. M., Jacobsen I. D., Allmeling C., Kuhbier J. W., Mutschmann F., Reimers K.2010. Resection of a large intra-abdominal tumor in the Mexican axolotl: a case report. Vet. Surg. 39: 232–233. doi: 10.1111/j.1532-950X.2009.00609.x [DOI] [PubMed] [Google Scholar]
- 9.Reiß C., Olsson L., Hoßfeld U.2015. The history of the oldest self-sustaining laboratory animal: 150 years of axolotl research. J. Exp. Zoolog. B Mol. Dev. Evol. 324: 393–404. doi: 10.1002/jez.b.22617 [DOI] [PubMed] [Google Scholar]
- 10.Shioda C., Uchida K., Nakayama H.2011. Pathological features of olfactory neuroblastoma in an axolotl (Ambystoma mexicanum). J. Vet. Med. Sci. 73: 1109–1111. doi: 10.1292/jvms.11-0105 [DOI] [PubMed] [Google Scholar]
- 11.Taylor S. K., Green D. E., Wright K. M.2001. Bacterial diseases. pp. 159–180. In: Amphibian Medicine and Captive Husbandry (Wright, K. M. and Whitaker, B. R. eds.), Krieger Publishing Company, Malabar. [Google Scholar]
- 12.Voss S. R., Shaffer H. B.2000. Evolutionary genetics of metamorphic failure using wild-caught vs. laboratory axolotls (Ambystoma mexicanum). Mol. Ecol. 9: 1401–1407. doi: 10.1046/j.1365-294x.2000.01025.x [DOI] [PubMed] [Google Scholar]
- 13.Whited J. L., Tsai S. L., Beier K. T., White J. N., Piekarski N., Hanken J., Cepko C. L., Tabin C. J.2013. Pseudotyped retroviruses for infecting axolotl in vivo and in vitro. Development 140: 1137–1146. doi: 10.1242/dev.087734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams D. L.2002. Amphibians. pp. 257–266. In: BSAVA Manual of Exotic pets, 4th ed. (Meredith, A. and Redrobe, S. eds.), British Small Animal Veterinary Association, Quedgeley. [Google Scholar]
- 15.Wright K. M.2001. Idiopathic syndromes. pp. 239–244. In: Amphibian 263 Medicine and Captive Husbandry (Wright, K. M. and Whitaker, B. R. eds.), Krieger Publishing Company, Malabar. [Google Scholar]