Abstract
An 11-year-old female goat had invasive and metastatic endometrial adenocarcinoma in the uterus. There was a notable proliferation of endometrial epithelial cells in a tubular growth pattern, with a desmoplastic response. The endometrial epithelial tumor cells metastasized to the kidney, liver and lung. In contrast to the primary and metastatic tumor cells, pleomorphic tumor cells with a choriocarcinoma-like growth pattern infiltrated the mesometrium. Cell proliferation activity was high in both types of tumor cells. Both types of tumor cells expressed cytokeratins AE1/AE3, 7 and CAM5.2; choriocarcinomatous cells also had positive immunoreactions to human chorionic gonadotropin, human placental alkaline phosphatase and α-inhibin. The present case was diagnosed as endometrial adenocarcinoma with choriocarcinomatous differentiation.
Keywords: choriocarcinomatous differentiation, endometrial adenocarcinoma, goat, human chorionic gonadotropin, uterus
A choriocarcinomatous component is rarely present in epithelial tumors of several organs, including the human uterus. In the uterus, endometrioid adenocarcinomas with choriocarcinomatous differentiation have been reported in post-menopausal women [5, 12] and unmarried women [1]. A conventional-appearing endometrioid adenocarcinoma was admixed with syncytiotrophoblast-like multinucleated giant cells and highly pleomorphic cytotrophoblast-like mononucleated tumor cells [1]. Transitional areas between adenocarcinomatous and choriocarcinomatous differentiation were observed [1, 5]. Immunohistochemical studies showed the intense reactivity of tumor cells to human chorionic gonadotropin (hCG), confirming the diagnosis of choriocarcinomatous differentiation [1, 5, 12]. To the best of our knowledge, there has been no histological evidence showing choriocarcinomatous differentiation in uterine tumors of animals. We encountered the case of a goat with a metastatic endometrial adenocarcinoma combined with a choriocarcinoma-like component at the infiltrating area in the mesometrium, and here report the pathological characteristics of the tumor cells observed through histopathological and immunohistochemical analyses.
A female goat was raised on the farm at Tokyo University of Agriculture and Technology (Fuchu, Tokyo) for educational purpose. When the goat was 11 years old, an oophorectomy was performed. One month after the operation, the goat exhibited tremor, anorexia with a reduction in body weight from 25 to 21 kg and an intermittent cough, and then died despite intensive care. During the necropsy, approximately 2l of pleural fluid was measured in the thoracic cavity. Multiple gray masses, approximately 5 to 10 mm in diameter, were found scattered in the lung parenchyma and pulmonary pleura. Atelectasis was noted in the lung parenchyma as well. Multiple masses, ranging in color from gray to white and approximately 5 mm in diameter, were also found on the surface and in the parenchyma of the liver and the unilateral side of the kidney, with a wedge-shaped appearance from the cortex to the medulla. Red-tinged intraluminal contents were found in the uterine cavity, and the uterus was adhered to the mesometrium and mesenteric adipose tissues.
Tissues were cut up into small pieces and fixed in 10% neutral-buffered formalin and embedded in paraffin. After embedding the tissues in paraffin, 3-µm sections were prepared and stained with hematoxylin and eosin. For immunohistochemical research, we used the avidin-biotin-peroxidase complex method with the primary antibodies listed in Table 1. The chromogen was 3,3′-diaminobenzidine, and hematoxylin was used for nuclear counterstain. As positive control tissues, the uterus and placenta of cattle, a bovine fetal liver and the kidney of a goat were used. For negative controls, the primary antibody was replaced with non-immunized sera.
Table 1. Antibodies used in immunohistochemistry.
| Antigen | Host species | Clonality | Clone name | Dilution | Antigen retrieval | Manufacture |
|---|---|---|---|---|---|---|
| Cytokeratin (AE1/AE3) | mouse | monoclonal | AE1/AE3 | 1:50 | Microwaving pH6b) | Dako (Glostrup, Denmark) |
| Cytokeratin CAM5.2 | mouse | monoclonal | CAM5.2 | undiluted | Microwaving pH6b) | BD Biosciences (San Jose, CA, U.S.A.) |
| Cytokeratin 7 (CK7) | mouse | monoclonal | OV-TL 12/30 | 1:100 | Proteinase Kd) | Dako (Glostrup, Denmark) |
| Human chorionic gonadotropin (hCG) | rabbit | polyclonal | n.a. | 1:1,000 | Autoclaving pH9a) | Dako (Glostrup, Denmark) |
| α-1-fetoprotein (AFP) | rabbit | polyclonal | n.a. | 1:400 | Microwaving pH6b) | Dako (Glostrup, Denmark) |
| Octamer-binding transcription factor-3/4 (OCT3/4) | mouse | polyclonal | n.a. | 1:100 | None | Santa Cruz Biotechnology, Inc. (Dallas, TX, U.S.A.) |
| Human placental alkaline phosphatase (hPLAP) | mouse | monoclonal | 8A9 | 1:25 | Autoclaving pH9a) | Dako (Glostrup, Denmark) |
| α-inhibin | rabbit | polyclonal | n.a. | 1:100 | Autoclaving pH9a) | Gift from Dr. Gen Watanabe. |
| Vimentin | goat | polyclonal I | n.a. | 1:200 | Microwaving pH6b) | Santa Cruz Biotechnology, Inc. (Dallas, TX, U.S.A.) |
| Ionized calcium-binding adaptor molecule 1 (Iba-1) | rabbit | polyclonal I | n.a. | 1:300 | None | Wako Pure Chemical Industries Ltd. (Osaka, Japan) |
| Proliferating cell nuclear antigen (PCNA) | mouse | monoclonal | PC10 | 1:200 | None | Dako (Glostrup, Denmark) |
| Ki-67 | mouse | monoclonal | MIB1 | 1:200 | Autoclaving pH6c) | Dako (Glostrup, Denmark) |
Abbreviation: n.a, not applicable. a) Autoclaved at 121°C for 10 min in Dako target retrieval solution (pH 9.0). b) Microwaved at 90°C for 10 min in 10 mM citrate buffer (pH 6.0). c) Autoclaved at 121°C for 10 min in 10 mM citrate buffer (pH 6.0). d) Incubated with proteinase K solution (20 µg/ml) for 15 min.
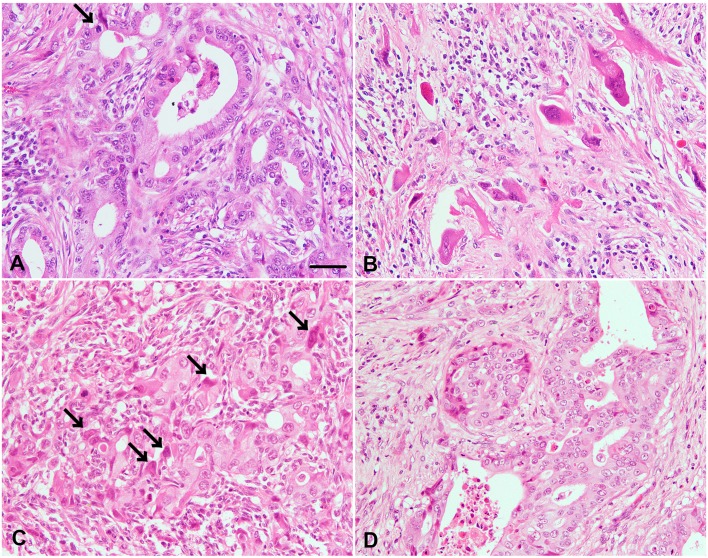
In the histopathological examination, endometrial epithelial tumor cells extensively proliferated in a tubular growth pattern, with a large amount of interstitial tissues in the uterus (Fig. 1A). The tumor cells demonstrated moderate nuclear atypia with prominent nucleoli and poorly demarcated eosinophilic cytoplasm. Mitotic figures and apoptotic bodies were often observed in the tumor cells. Exfoliated epithelial cells were frequently seen in the luminal cavity. In the interstitial connective tissues, desmoplastic reaction with infiltration of inflammatory cells, including lymphocytes and macrophages, was evident, and frequent tumor-cell invasion of the vasculature and myometrium was observed. Hyperplastic smooth muscle layers were also seen. Of note, the mesometrium was found to be heavily infiltrated by solitary (Fig. 1B) or clumped (Fig. 1C) pleomorphic tumor cells in a choriocarcinoma-like growth pattern, mixed with abundant connective tissues and interstitial spindle cells. The tumor cells displayed predominant nuclear atypia, often featuring pleomorphic giant and multinucleated cells, some larger nucleoli, and abundant and poorly demarcated eosinophilic or vacuolated cytoplasm. These pleomorphic cells partially mimicked cytotrophoblasts or syncytiotrophoblasts, and sometimes proliferated in very close contact with ill-defined tubular structures. In the tissues from areas other than the uterus, endometrial tumor cells showed severe infiltration into the kidney, liver and lung, where the tumor cells proliferated mainly in the interstitium with multiple formations of tubular and cribriform structures (Fig. 1D). The choriocarcinoma-like component noted in the mesometrium was obscure in the metastatic lesions.
Fig. 1.
Histopathological findings in the tumors of the uterus and kidney. (A) Epithelial tumor cells exhibit a tubular growth pattern in the endometrium. Mitotic figures are often observed in the tumor cells (arrow). (B) Solitary syncytiotrophoblast-like cells with prominent cellular atypia proliferated in the desmoplastic interstitium of the mesometrium. Mononuclear cell infiltration was observed in the interstitium. (C) A cluster of infiltrated tumor cells includes cytotrophoblast-like cells and syncytiotrophoblast-like cells (arrows) with an ill-defined tubular growth pattern in the mesometrium. (D) Metastatic epithelial tumor cells in the kidney are arranged in a tubular or cribriform growth pattern similar to the growth pattern of tumor cells in the uterus. Desquamative epithelial cells were observed in the luminal cavity (A, D). Scale bar=50 µm (All images have the same magnification).
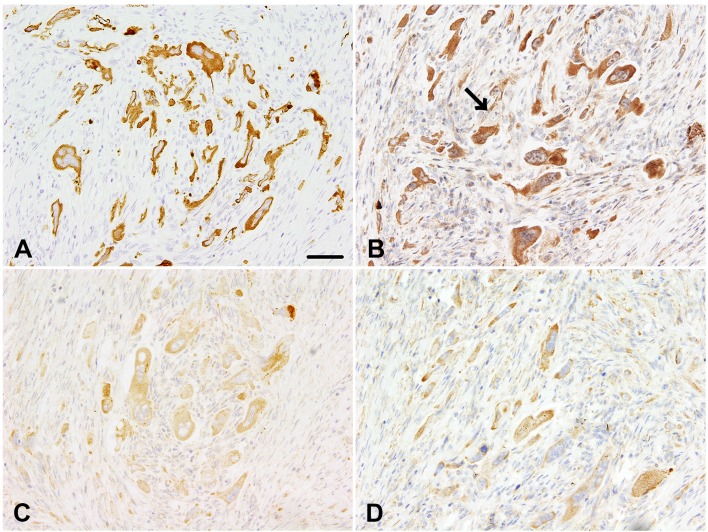
In the immunohistochemical examination, endometrial epithelial tumor cells and choriocarcinoma-like pleomorphic cells tested positive for cytokeratin AE1/AE3 (Fig. 2A), CAM5.2 and cytokeratin 7 (CK7). Interstitial cells tested strongly positive for vimentin. Ionized calcium-binding adaptor molecule 1 (Iba1)-positive macrophages were frequently observed in the desmoplastic interstitium. Some of the choriocarcinoma-like pleomorphic cells in the mesometrium tested strongly positive for hCG (Fig. 2B), but the endometrial epithelial tumor cells in the uterus tested negative for hCG. hCG-expressing pleomorphic cells were scattered throughout the mesometrium we examined. In addition to hCG positivity, some of the pleomorphic cells in the mesometrium showed positive immunoreactivity to α-1-fetoprotein (AFP) and α-inhibin (Fig. 2C). Both types of tumor cells in the uterus and mesometrium also tested immunopositive for human placental alkaline phosphatase (hPLAP) (Fig. 2D), but not for octamer-binding transcription factor-3/4 (OCT3/4). Of note, the positive reactions for AE1/AE3 and CAM5.2 were also detected in the metastatic tumors, although these tested negative for CK7, hCG, PLAP, α-inhibin and AFP. A densely positive reaction to proliferating cell nuclear antigen (PCNA) and Ki-67 was observed in the nuclei of both types of tumor cells, but not in the interstitial cells. In normal uterine and kidney tissues, epithelial cells tested positive for AE1/AE3 and CAM5.2, uterine glands tested positive for CK7, interstitial spindle cells and smooth muscle layers tested positive for vimentin, and interstitial macrophages tested positive for Iba-1. The placental trophoblastic cells tested positive for hCG, α-inhibin and PLAP. An immunopositive reaction to AFP was detected in cells from the bovine fetal liver.
Fig. 2.
Immunohistochemical findings in the tumor cells of the mesometrium. (A) Many pleomorphic tumor cells are positive for the epithelial marker cytokeratin AE1/AE3. (B) Some of the tumor cells are strongly positive for human chorionic gonadotropin (hCG), which is a marker for typical human choriocarcinomas. A tumor cell with a negative reaction to hCG was noted (arrow). (C, D) Most of the tumor cells are weakly positive for α-inhibin (C) and human placental alkaline phosphatase (hPLAP) (D), both reliable markers for choriocarcinomas. Scale bar=50 µm (All images have the same magnification).
The present case was characterized by proliferation of endometrial epithelial tumor cells with irregularly shaped tubular structures in the uterus in association with a remarkable desmoplastic reaction. Based on a typical growth pattern, the tumor is considered to be an adenocarcinoma derived from the endometrium of the uterus as reported in cattle [2, 6, 8, 9] and sheep [10]. In humans, endometrial carcinomas are subdivided into endometrioid, mucinous, serous, clear cell or mixed variants [4]. The present case might be categorized as the endometrioid variant, composed of a confluent glandular pattern with irregular infiltration into a desmoplastic stroma [4]. The metastatic tumor cells in the lung, liver and kidney mimicked epithelial tumor cells that might be derived from primary tumors in the endometrium. The findings on primary and metastatic endometrial tumors were supported by previous studies in cattle [6, 8, 9].
The tumor cells infiltrated the myometrium, which is consistent with findings previously reported regarding uterine adenocarcinoma in cattle [6]. However, the tumor cells infiltrating the mesometrium were highly pleomorphic with a unique choriocarcinoma-like growth pattern. Choriocarcinomas, highly malignant tumors of trophoblastic cells, have been reported in the uterus of animals, such as rabbits [7] and pigs [3]. The tumor cells of those animals were composed of the following two types of pleomorphic cells: (a) cytotrophoblast-like cells, with a round-to-oval nucleus containing prominent nucleoli and granular eosinophilic cytoplasm, and (b) syncytiotrophoblast-like cells, with a single hyperchromatic oval-to-spindle-shaped nucleus or multiple nuclei and dense eosinophilic cytoplasm [3]. The tumor cells in the present case mimic these pleomorphic cells.
The diagnosis of a choriocarcinoma-like component necessitates demonstration of trophoblastic differentiated cells, which generally express chorionic gonadotropin [2, 3, 7, 13]. In this case, the immunohistochemical findings in the mesometrium were similar to findings reported on typical simian choriocarcinoma cells, as shown in the immunohistochemistry for CK7, hCG and α-inhibin [13]. However, the finding that not all the tumor cells in the mesometrium were positive for hCG suggests that a limited number of invasive tumor cells have choricarcinomatous features. Another unexplainable finding is that several pleomorphic tumor cells were also positive for AFP, which is a crucial marker for embryonal carcinomas and yolk sac tumors [11]. This case might have an embryonic feature in part; however, we ruled out embryonal carcinoma as a diagnosis, because there was a lack of sheets, glands and a papillary growth pattern of embryonic (primitive) epithelial cells [11, 13].
Invasive tumor cells with choriocarcinoma-like growth pattern extending to the mesometrium appeared to be similar to the findings in a human case [1]. The mechanism of restricted abnormal differentiation of tumor cells in the mesometrium remains uncertain; however, a population of tumor cells with an embryonic feature might undergo an abnormal transformation to choriocarcinoma-like cells during the invasion process. The interpretation was supported by three hypotheses that have been postulated on choricarcinomatous differentiation in endometrioid adenocarcinomas as follows: (a) dedifferentiation of epithelial cells into the choriocarcinoma, (b) germ cells that fail to complete their migration to the gonads and (c) multidirectional tumor differentiation from a common stem cell [12]. In this case, given that the tumor cells demonstrated presumable dual differentiation to endometrial cells and trophoblastic cells, along with distant metastasis, the most appropriate diagnosis is endometrial adenocarcinoma with choriocarcinomatous differentiation as shown in human cases [5].
Acknowledgments
The authors thank Mrs. Shigeko Suzuki for her technical assistance in preparing the histological specimens.
REFERENCES
- 1.Akbulut M., Tosun H., Soysal M. E., Oztekin O.2008. Endometrioid carcinoma of the endometrium with choriocarcinomatous differentiation: a case report and review of the literature. Arch. Gynecol. Obstet. 278: 79–84. doi: 10.1007/s00404-007-0526-y [DOI] [PubMed] [Google Scholar]
- 2.Giusti A. M. F., Terron A., Belluco S., Scanziani E., Carcangiu M. L.2005. Ovarian epithelioid trophoblastic tumor in a cynomolgus monkey. Vet. Pathol. 42: 223–226. doi: 10.1354/vp.42-2-223 [DOI] [PubMed] [Google Scholar]
- 3.Hirata A., Miyazaki A., Sakai H., Imada N., Kitani R., Nikami H., Yanai T.2014. Choriocarcinoma-like tumor in a potbellied pig (Sus scrofa). J. Vet. Diagn. Invest. 26: 163–166. doi: 10.1177/1040638713515481 [DOI] [PubMed] [Google Scholar]
- 4.Horn L. C., Meinel A., Handzel R., Einenkel J.2007. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann. Diagn. Pathol. 11: 297–311. doi: 10.1016/j.anndiagpath.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Ishida M., Okabe H.2013. Endometrioid adenocarcinoma with choriocarcinomatous differentiation: A case report and review of the literature. Oncol. Lett. 6: 655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kadota K., Watanabe M.1988. Uterine adenocarcinoma with stromal cells containing lipofuscin in a cow. Jpn. J. Vet. Sci. 50: 347–352. doi: 10.1292/jvms1939.50.347 [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann-Bart M., Fischer I.2008. Choriocarcinoma with metastasis in a rabbit (Oryctolagus cuniculi). Vet. Pathol. 45: 77–79. doi: 10.1354/vp.45-1-77 [DOI] [PubMed] [Google Scholar]
- 8.Lingard D. R., Dickinson E. O.1966. Uterine adenocarcinoma with metastasis in the cow. J. Am. Vet. Med. Assoc. 148: 913–915. [PubMed] [Google Scholar]
- 9.Lingard D. R., Dickinson E. O., Jr.1969. Metastatic uterine adenocarcinoma in the cow (a recognizable clinical entity?). Vet. Med. Small Anim. Clin. 64: 234–236. [PubMed] [Google Scholar]
- 10.Terlecki S., Watson W. A.1967. Adenocarcinoma of the uterus of a ewe. Vet. Rec. 80: 516–518. doi: 10.1136/vr.80.17.516 [DOI] [PubMed] [Google Scholar]
- 11.Ulbright T. M.2005. Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod. Pathol. 18Suppl 2: S61–S79. doi: 10.1038/modpathol.3800310 [DOI] [PubMed] [Google Scholar]
- 12.Wakahashi S., Sudo T., Nakagawa E., Ueno S., Muraji M., Kanayama S., Itami H., Kawakami F., Yamada T., Yamaguchi S., Fujiwara K., Nishikawa H., Nishimura R., Ohbayashi C.2012. Endometrioid adenocarcinoma with high-grade transformation with serous and choriocarcinomatous differentiation - a case report. J. Cancer 3: 14–18. doi: 10.7150/jca.3.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokouchi Y., Imaoka M., Sayama A., Sanbuissho A.2011. Mixed germ cell tumor with embryonal carcinoma, choriocarcinoma, and epithelioid trophoblastic tumor in the ovary of a cynomolgus monkey. Toxicol. Pathol. 39: 553–558. doi: 10.1177/0192623311399787 [DOI] [PubMed] [Google Scholar]