Abstract
Hualian No. 4 wild bitter gourd (WBG) is a specific vegetable cultivated by the Hualien District Agricultural Research and Extension Station in Taiwan. WBG is commonly consumed as a vegetable and used as a popular folk medicine. However, few studies have demonstrated the effects of WBG supplementation on exercise performance, physical fatigue and the biochemical profile. The purpose of this study was to evaluate the potential beneficial effects of WBG extract on fatigue and ergogenic functions following physiological challenge. Three groups of male ICR mice (n=8 per group) were orally administered 0, 1 or 2.5 g/kg/day of WBG for 4 weeks. They were respectively designated the vehicle, WBG-1X and WBG-2.5X groups. WBG significantly decreased body weight (BW) and epididymal fat pad (EFP) weight. Concerning physical performance, WBG supplementation dose-dependently increased grip strength and endurance swimming time. Concerning anti-fatigue activity, WBG decreased levels of serum lactate, ammonia, creatine kinase and blood urea nitrogen, and economized glucose metabolism after acute exercise challenge. Glycogen in the liver and gastrocnemius muscle dose-dependently increased with WBG treatment. Concerning the biochemical profile, WBG treatment significantly decreased alanine aminotransferase (ALT), blood urea nitrogen (BUN) and urea acid (UA), and increased total protein (TP). Therefore, 4-week supplementation with WBG may decrease white adipose weight, enhance energy economy, increase glycogen storage to enhance exercise performance and reduce fatigue.
Keywords: anti-fatigue, exercise performance, glycogen storage, wild bitter gourd
Hualian No. 4 wild bitter gourd (Momordica charantia L. var. abbreviata Seringe, WBG) is a wild variety of M. charantia that is cultivated by Hualien District Agricultural Research and Extension Station (HDARES) in Taiwan. WBG, which is normally smaller than domesticated bitter gourd (M. charantia L.), belongs to the family Cucurbitaceae. The fresh fruits of WBG are frequently eaten as a vegetable in soup, fried dishes and other dishes. The inheritance similarity of WBG to bitter gourd is 80–98%. The HDARES, capitalizing on the continuous female flower initiation and high fruit bearing ability of bitter gourd, bred the new cultivars Hualien Nos.1–4, which are high in Vitamin C, anti-oxidants and charantin. Recent publications have reported many pharmacological activities of WBG, such as improving metabolic syndrome [37], countering alcoholic fatty liver [28] and reducing inflammation [18]. The nutritional analysis of bitter gourd fruits shows that it is rich in carbohydrates, proteins, vitamins, fiber and minerals, holds the highest nutritive value among all cucurbits [21]. In general, the main phytoconstituents present in M. charantia are charantin, momordicin, momordin, stigmasta-5, 25-dien-3-β-O-glucoside, β-sitosterol-β-D-glucoside, momordicoside G, momordicoside F1, momordicoside F2, momordicoside I, momordicoside K and momordicoside L [13, 42]. Of these, charantin is the main active constituent of M. charantia fruits, and its potential hypoglycemic activity has been reported [29, 38, 44]. Moreover, several studies have been conducted to establish the therapeutic activities of bitter gourd for various disorders, and it appears to have the ability to target several cancers [19, 27, 31, 39].
WBG is known to increase glycogen storage by potentiating mitochondrial function via activation of the PPARα and PPARγ signaling pathways. However, relatively few studies have directly addressed the possible anti-fatigue function of WBG and its effects on exercise performance. Exercise performance can be defined for both humans and animals as performance in athletic events. Research focusing on exercise performance can provide important insights on oxygen transport, muscle performance, metabolism, cardiovascular control and the operations of various components of the nervous system [23]. Physical fatigue is often accompanied by deterioration in functional performance [15]. The occurrence of physical fatigue can be explained by at least two mechanisms: oxidative stress and energy exhaustion [11]. Exhaustive or intensive exercise can lead to the accumulation of excess reactive free radicals that cause tissue damage. Exhaustion theory suggests that energy source depletion and excess metabolite accumulation can lead to fatigue [43]. A previous article reviewed the physiological effects of WBG on energy metabolism and utilization via the AMPK pathway, peroxisome proliferator-activated α (PPARα) and PPARγ activation [6, 36]. In this study, we evaluated the potential ergogenic, anti-fatigue and health promotion effects of WBG supplementation in mice using our established in vivo platform [9].
MATERIALS AND METHODS
Materials, animals and experiment design
Hualian No. 4 Wild Bitter Gourd (WBG) extract was provided by Aquavan Technology Co., Ltd. (Taipei, Taiwan). Male ICR mice (8 weeks old) grown under specific pathogen-free conditions were purchased from BioLASCO (Yi-Lan, Taiwan). All mice were provided a standard laboratory diet (No. 5001; PMI Nutrition International, Brentwood, MO, U.S.A.) and distilled water ad libitum, and housed in a 12-hr light/12-hr dark cycle at room temperature (22 ± 1°C) and 50–60% humidity. The Institutional Animal Care and Use Committee (IACUC) of National Taiwan Sport University (NTSU) inspected all animal experiments, and this study conformed to the guidelines of protocol IACUC-10501 approved by the IACUC ethics committee. In this study, the dose of WBG designed for humans was 4.8 g per day (WBG extract). The mouse dose (1 g/kg) we used in this study was converted from a human-equivalent dose (HED) based on body surface area by the following formula from the US Food and Drug Administration: Assuming a human weight of 60 kg, the HED for 4.8 (g)/60 (kg)=0.08 × 12.3=1 g/kg; the conversion coefficient 12.3 was used to account for differences in body surface area between mice and humans as we recently described [24]. In total, 24 mice were randomly assigned to 3 groups (8 mice/group) for daily oral WBG treatment for 4 weeks. The three groups were the vehicle group; the WBG-1X group, receiving 1 g/kg; and the WBG-2.5X group, receiving 2.5 g/kg. The vehicle group received the same volume of solution equivalent to individual body weight (BW). The mice were randomly housed in groups of 4 per cage.
Determination of charantin with high performance liquid chromatography (HPLC) equipment and conditions
The WBG extract was dissolved in 25 mg/ml methanol and filtered through a 0.45 µl membrane filter for HPLC analysis. The HPLC system was a Dionex UltiMate 3000 HPLC system, which consisted of an UltiMate 3000 RS pump, an UltiMate 3000 RS autosampler, an UltiMate 3000 RS column compartment, a variable wavelength detector (Dionex, Olten, Switzerland) and a reversed-phase column (Inertsil ODS-3V, 4.6 Â, 150 mm, GL Science, Inc., Tokyo, Japan). Each charantin standard and all samples were prepared in injection volumes of 20 µl with detection at a wavelength of 204 nm and a column flow rate of 1 ml, and the mobile phase was methanol-water 100:2 (V/V). Data were collected and stored in the Chromelion software from Dionex Corporation (Sunnyvale, CA, U.S.A.).
Forelimb grip strength test
A low-force testing system (Model-RX-5, Aikoh Engineering, Nagoya, Japan) was used to measure the forelimb grip strength of treated mice as we previously described [8].
Swimming exercise performance test
The swim-to-exhaustion test involved loads corresponding to 5% of the mouse BW attached to the tail to evaluate endurance time as we previously described [8]. The swimming endurance time of each mouse was recorded from beginning to exhaustion, determined by observing loss of coordinated movements and failure to return to the surface within 7 sec.
Determination of fatigue-associated biochemical variables
The effect of WBG supplementation on fatigue-associated biochemical indices was evaluated after exercise as we previously described [8, 10]. At 1 hr after WBG supplementation, all mice underwent a 15-min swim test without weight loading. Immediately after the swim test, blood samples were collected from the submandibular duct of mice and centrifuged at 1,500 ×g and 4°C for 10 min for serum separation. Serum lactate, ammonia, glucose, creatine kinase (CK) and blood urea nitrogen (BUN) levels were determined with an autoanalyzer (Hitachi 7060, Hitachi, Tokyo, Japan).
Clinical biochemical profiles
Two days after the 15-min swimming test, all mice were sacrificed with 95% CO2 asphyxiation, and blood was immediately collected. Serum was separated by centrifugation and the levels of the clinical biochemical variables, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK), total protein (TP), albumin, blood urea nitrogen (BUN), creatinine, uric acid (UA), total cholesterol (TC), triacylglycerol (TG) and glucose, were measured with an autoanalyzer (Hitachi 7060).
Tissue glycogen determination and visceral organ weight
The stored form of glucose is glycogen, which exists mostly in liver and muscle tissue. Liver and muscle tissues were excised after the mice were sacrificed and weighed for glycogen content analysis as we described previously [20]. The weights of the related visceral organs were recorded.
Histology of tissues
All tissues were carefully removed, minced and fixed in 10% formalin. Samples were embedded in paraffin and cut into 4-µm thick slices for morphological and pathological evaluations. Tissue was stained with hematoxylin and eosin (H&E) and examined under an optical microscope equipped with a CCD camera (BX-51, Olympus, Tokyo, Japan) by a veterinary pathologist.
Statistical analysis
All data are expressed as mean ± SEM, n=8 mice/group. Statistical differences among groups were analyzed by a one-way analysis of variance (ANOVA) and the Cochran-Armitage test for dose-effect trend analysis with SAS 9.0 (SAS Inst., Cary, NC, U.S.A.). P<0.05 was considered statistically significant. Differences between groups were analyzed by one-way analysis of variance (ANOVA) using Duncan’s post-hoc test, and P values <0.05 were considered significant.
RESULTS
Charantin content of WBG extract
We measured the charantin content in Hualian No. 4 Wild Bitter Gourd extract (WBG) by HPLC analysis. The retention time of charantin was approximately 13 min, and the content of charantin in WBG extract was 0.45 mg/g.
Effects of WBG on forelimb grip strength
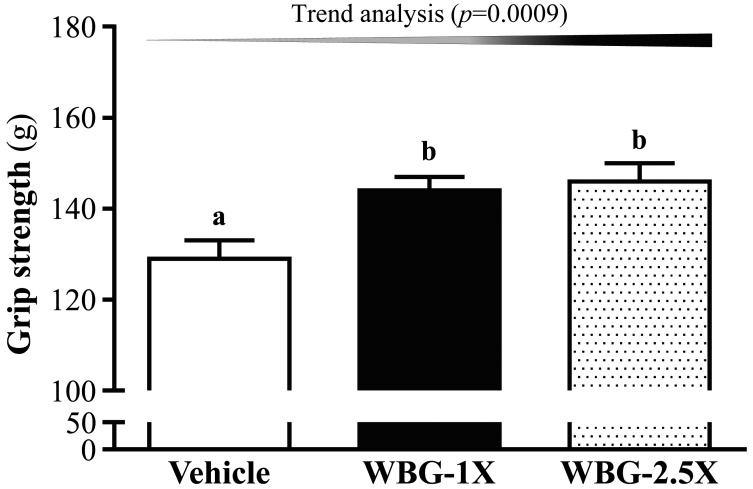
After supplementation with WBG for 4 weeks, the forelimb grip strengths in the vehicle, WBG-1X and WBG-2.5X groups were 129 ± 4, 144 ± 3 and 146 ± 4 g, respectively (Fig. 1). Forelimb grip strength was significantly higher in the WBG-1X and WBG-2.5X groups (by 1.14-fold; P=0.0090 and P=0.0035, respectively) than in the vehicle group. Trend analysis indicated that absolute forelimb grip strength dose-dependently increased with increasing WBG dose (P=0.0009).
Fig. 1.
Effect of WBG (Hualian No. 4 Wild Bitter Gourd) supplementation for 4 weeks on forelimb grip strength. Mice were pretreated with vehicle, WBG-1X or WBG-2.5X for 4 weeks before forelimb grip strength was tested. Data are mean ± SEM, 8 mice/group, by one-way ANOVA. Different letters (a, b) indicate a significant difference at P<0.05.
Effect of WBG on exercise performance in a weight-loaded swimming test
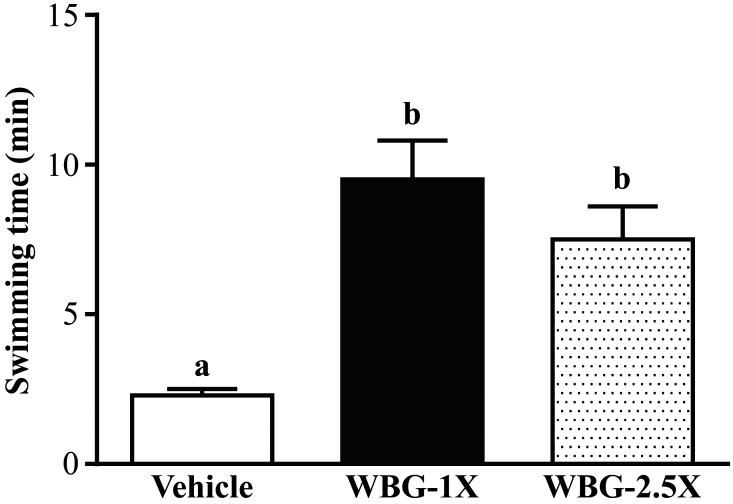
Endurance swimming times were 2.3 ± 0.2, 9.5 ± 1.3 and 7.5 ± 1.1 min in the vehicle, WBG-1X and WBG-2.5X groups, respectively (Fig. 2). The exhaustive swimming times were longer in the WBG-1X and WBG-2.5X groups (by 4.10- and 3.23-fold; P<0.0001 and P=0.0013, respectively) than in the vehicle group.
Fig. 2.
Effect of WBG supplementation on swimming exercise performance. Mice were pretreated with vehicle, WBG-1X or WBG-2.5X for 4 weeks and then 1 hr later performed an exhaustive swimming exercise with a load equivalent to 5% of body weight attached to the tail. Data are mean ± SEM, n=8 mice/group, by one-way ANOVA. Different letters (a, b) indicate a significant difference at P<0.05.
Effect of WBG supplementation on serum lactate, ammonia, glucose, CK and BUN levels after acute exercise challenge
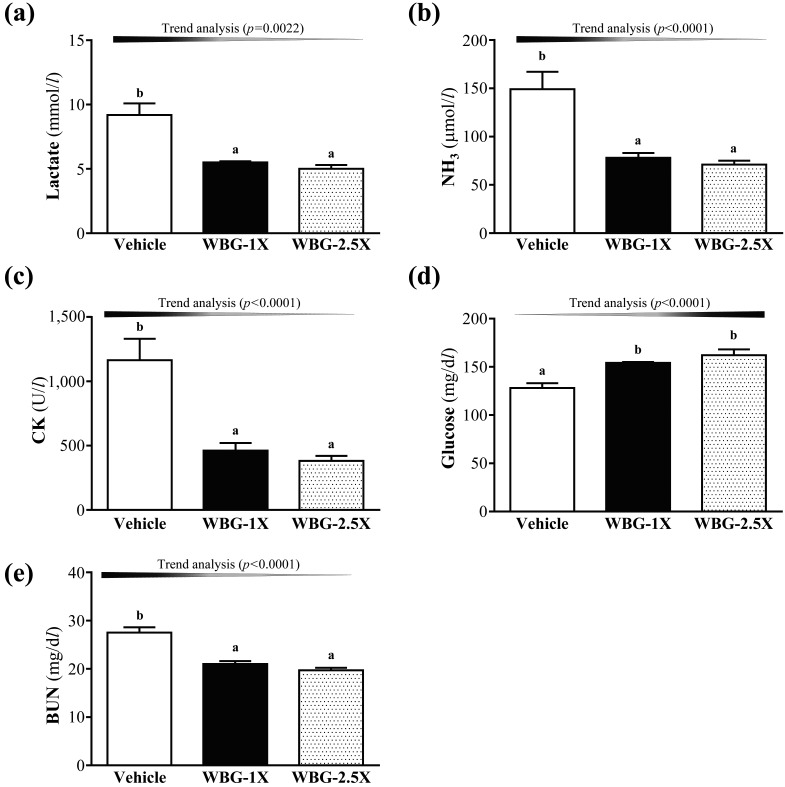
The lactate levels in the vehicle, WBG-1X and WBG-2.5X groups were 9.2 ± 0.9, 5.5 ± 0.1 and 5.0 ± 0.3 mmol/l, respectively (Fig. 3a). The lactate levels were significantly lower in the WBG-1X and WBG-2.5X groups (40.16% and 45.45%; P=0.0005 and P<0.0001, respectively) than in the vehicle treatment group. Trend analysis indicated that serum lactate level dose-dependently decreased with increasing WBG dose (P<0.0001). Therefore, WBG supplementation may have the potential for clearance or utilization of blood lactate during exercise.
Fig. 3.
Effect of WBG supplementation on serum levels of lactate (a); ammonia (b); creatine kinase (CK) (c); glucose (d) and blood urea nitrogen (BUN) (e) after acute exercise challenge. Data are mean ± SEM, n=8 mice/group, by one-way ANOVA. Different letters (a, b) indicate a significant difference at P<0.05.
Ammonia is an important metabolite during energy metabolism for exercise. Serum ammonia is a useful marker of exercise-related metabolic responses in endurance-trained competitive athletes. The serum ammonia levels of the vehicle, WBG-1X and WBG-2.5X groups were 149 ± 18, 78 ± 5 and 71 ± 4 µmol/l, respectively (Fig. 3b). The ammonia levels were significantly lower in the WBG-1X and WBG-2.5X groups (40.16% and 45.45% lower; P=0.0005 and P=0.0002, respectively) than in the vehicle group. Trend analysis indicated that serum ammonia level dose-dependently decreased with increasing WBG dose (P<0.0001). Thus, continuous supplementation with WBG for 4 weeks could decrease ammonia levels during exercise.
Serum CK level is an important clinical biomarker of muscle damage and may represent recovery capacity from exercise-induced muscle damage. The serum CK levels of the vehicle, WBG-1X and WBG-2.5X groups were 1,164 ± 167, 462 ± 58 and 382 ± 39 mg/dl, respectively (Fig. 3c). CK levels were respectively 60.34% (P=0.0004) and 67.22% (P=0.0001) lower in the WBG-1X and WBG-2.5X groups than in the vehicle treatment group. Therefore, WBG supplementation could ameliorate skeletal muscle injury induced by acute exercise challenge. Trend analysis showed that WBG treatment had a significant dose-dependent effect on CK level (P=0.0002).
Blood glucose level is an important index for performance maintenance during exercise. The serum glucose levels in the vehicle, WBG-1X and WBG-2.5X groups were 128 ± 5, 154 ± 1 and 162 ± 6 mg/dl, respectively (Fig. 3d). The glucose levels were significantly higher in the WBG-1X and WBG-2.5X groups (by 1.21- and 1.27-fold; P=0.0011 and P<0.0001, respectively) than in the vehicle group. Trend analysis showed that serum glucose levels dose-dependently increased with WBG supplementation dose (P=0.0022). Therefore, continuous supplementation with WBG for 4 weeks could increase serum glucose levels and glucose uptake capacity to improve anti-fatigue activity.
BUN is an important biochemical parameter related to fatigue. The BUN level is used to measure the amount of nitrogen in blood from the waste product of urea. Urea serves an important role in the metabolism of nitrogen-containing compounds. The serum BUN levels of the vehicle, WBG-1X and WBG-2.5X groups were 27.5 ± 1.1, 21.0 ± 0.6 and 19.7 ± 0.5, respectively (Fig. 3e). The BUN levels in the WBG-1X and WBG-2.5X groups were respectively 23.41% (P<0.0001) and 28.14% (P<0.0001) lower than in the vehicle group. Trend analysis revealed that WBG treatment had a significantly dose-dependent effect on BUN level (P<0.0001). Above all, WBG may have potential as an ergogenic supplement for improving the removal of the metabolic waste during exercise.
Effect of WBG supplementation for 4 weeks on hepatic and muscle glycogen levels
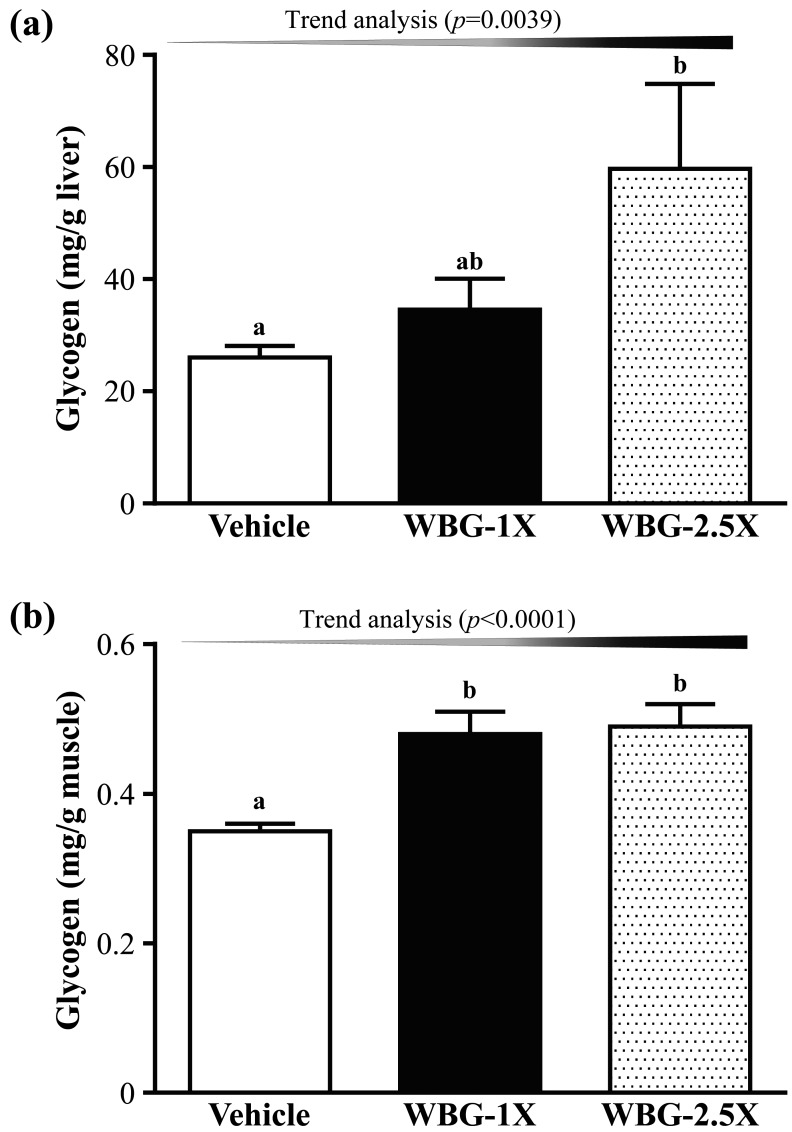
We also measured the glycogen contents of the liver and muscle tissues (Fig. 4). The liver glycogen levels in the vehicle, WBG-1X and WBG-2.5X groups were 26.01 ± 2.11, 34.56 ± 5.55 and 59.68 ± 15.07 mg/g liver, respectively. The liver glycogen level was significantly higher in the WBG-2.5X group (by 2.29-fold; P=0.0122) than in the vehicle group. The muscle glycogen levels in the vehicle, WBG-1X and WBG-2.5X groups were 0.36 ± 0.01, 0.47 ± 0.03 and 0.49 ± 0.03 mg/g muscle. The muscle glycogen content was significantly higher in the WBG-1X and WBG-2.5X groups (by 1.31- and 1.36-fold; P=0.0018 and P=0.0002, respectively) than in the vehicle group. Thus, WBG slightly increased the liver and muscle glycogen storage. Trend analysis indicated that liver glycogen and muscle glycogen increased with WBG dose (P=0.0039 and P<0.0001). After 4 weeks of WBG supplementation, both liver and muscle glycogen increased as exercise fuels to enhance exercise performance.
Fig. 4.
Effect of WBG supplementation on glycogen levels in liver (a) and muscle (b). Data are mean ± SEM, n=8 mice/group, by one-way ANOVA. Different letters (a, b) indicate a significant difference at P<0.05.
General characteristics of mice with WBG supplementation for 4 weeks
Initial BW did not differ among the vehicle, WBG-1X and WBG-2.5X groups (Table 1). After 4-week supplementation with WBG, the final BWs were significantly lower in the WBG-1X and WBG-2.5X groups (9.45% and 9.91%; P=0.0035 and P=0.0024, respectively) than in the vehicle group. In addition, daily intake of diet and water did not differ in the vehicle and WBG supplementation groups. Trend analysis showed that final BW dose-dependently decreased with WBG supplementation (P=0.0019). Thus, the 4-week WBG supplementation reduced the final BW. In addition, the epididymal fat pad (EFP) weight of the WBG-2.5X group was 26.55% (P=0.0164) lower than that of the vehicle treatment group. The brown adipose tissue (BAT) weight was higher in the WBG-1X and WBG-2.5X groups (by 1.19- and 1.17-fold; P=0.0013 and P=0.0051, respectively) than in the vehicle treatment group. Trend analysis indicated that the absolute EFP (P=0.0004) and BAT (P=0.0396) weights varied dose-dependently with WBG treatment. We also measured the effect of WBG on the relative tissue weight (different tissue weights adjusted for individual BW%). The relative EFP weight was 18.72% (P=0.0164) lower in the WBG-2.5X group than in the vehicle treatment group. The relative BAT weights were significantly higher in the WBG-1X and WBG-2.5X groups (by 1.35- and 1.30-fold; P=0.0067 and P=0.0314, respectively) than in the vehicle group. Trend analysis also showed that BAT weight dose-dependently increased with WBG supplementation (P=0.0048).
Table 1. General characteristics of mice with Hualian No. 4 wild bitter gourd (WBG) supplementation.
| Characteristic | Vehicle | WBG-1X | WBG-2.5X | Trend analysis |
|---|---|---|---|---|
| Initial BW (g) | 34.4 ± 0.4 | 34.1 ± 0.4 | 34.8 ± 0.4 | 0.9791 |
| Final BW (g) | 38.2 ± 0.8b) | 34.6 ± 0.6a) | 34.5 ± 0.8a) | 0.0019 |
| Food intake (g/day) | 5.8 ± 0.3 | 5.7 ± 0.1 | 5.5 ± 0.1 | 0.0055 |
| Water intake (ml/day) | 6.9 ± 0.1 | 6.8 ± 0.1 | 6.9 ± 0.1 | 0.4671 |
| Liver (g) | 1.71 ± 0.05 | 1.52 ± 0.03 | 1.59 ± 0.08 | 0.1527 |
| Kidney (g) | 0.51 ± 0.02 | 0.50 ± 0.01 | 0.50 ± 0.01 | 0.8691 |
| Heart (g) | 0.23 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.7113 |
| Lung (g) | 0.22 ± 0.01 | 0.22 ± 0.00 | 0.22 ± 0.01 | 0.9714 |
| Muscle (g) | 0.37 ± 0.01 | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.0590 |
| EFP (g) | 0.34 ± 0.04b) | 0.28 ± 0.02 a,b) | 0.25 ± 0.01a) | 0.0004 (↓) |
| BAT (g) | 0.08 ± 0.00a) | 0.10 ± 0.00 b) | 0.09 ± 0.01b) | 0.0396 (↑) |
| Relative Liver weight (%) | 4.48 ± 0.18 | 4.40 ± 0.12 | 4.63 ± 0.26 | 0.8880 |
| Relative Kidney weight (%) | 1.33 ± 0.07 | 1.42 ± 0.04 | 1.47 ± 0.06 | 0.1493 |
| Relative Heart weight (%) | 0.59 ± 0.03 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.1596 |
| Relative Lung weight (%) | 0.57 ± 0.02 | 0.62 ± 0.04 | 0.63 ± 0.02 | 0.0141 (↑) |
| Relative Muscle weight (%) | 0.98 ± 0.02 | 1.05 ± 0.03 | 1.05 ± 0.05 | 0.1435 |
| Relative EFP weight (%) | 0.91 ± 0.11b) | 0.92 ± 0.07a,b) | 0.74 ± 0.04a) | 0.0835 |
| Relative BAT weight (%) | 0.21 ± 0.01a) | 0.29 ± 0.01b) | 0.28 ± 0.02b) | 0.0048 (↑) |
Data are mean ± SEM, n=8 mice/group. Different letters (a, b) in the same row indicate a significant difference at P<0.05. Food efficiency ratio: body weight (BW) gain (g/day)/food intake (g/day). Muscle mass includes both gastrocnemius and soleus muscles in the back part of the lower legs. BAT: brown adipose tissue; EFP: epididymal fat pad. Mice were pretreated with vehicle, WBG-1X or WBG-2.5X for 4 weeks. The arrows up and down mean dose-dependently increased and decreased by WBG supplementation.
Effect of WBG supplementation on biochemical variables at the end of the experiment
We observed beneficial effects of WBG on grip strength, exhaustive exercise challenge and body composition after 4-week WBG supplementation. We further investigated whether 4-week WBG treatment affected other biochemical markers in healthy mice. We examined tissue- and health status-related biochemical variables and major organs, including skeletal muscle, heart, kidney and lung (Table 2). The levels of the biochemical indices, AST, CK, creatinine, TC, TG and glucose did not differ among groups (P>0.05, Table 2). Serum ALT levels were lower in the WBG-1X and WBG-2.5X groups (20.83 and 21.09%; P=0.0323 and P=0.0304, respectively) than in the vehicle treatment group. Serum BUN levels were significantly lower in the WBG-1X and WBG-2.5X groups (18.54 and 35.44%; P=0.0049 and P<0.0001, respectively) than in the vehicle treatment group. Serum UA levels were significantly lower in the WBG-1X and WBG-2.5X groups (59.05 and 57.76%; P<0.0001 and P<0.0001, respectively) than in the vehicle group. Serum TP was higher in the WBG-1X and WBG-2.5X groups (by 1.08- and 1.09-fold; P=0.0035 and P=0.001, respectively) than in the vehicle treatment group. The trend analysis revealed that WBG treatment had significant dose-dependent effects on ALT (P=0.0483), TP (P<0.0001), BUN (P<0.0001) and UA levels (P<0.0001). In addition, serum albumin was slightly increased by WBG supplementation, but remained within the normal range. Therefore, long-term daily supplementation with WBG may have the potential for tissue protection by enhancing nutrient absorption with increases in serum TP.
Table 2. Biochemical analysis after WBG supplementation, at the end of the experiment.
| Parameter | Vehicle | WBG-1X | WBG-2.5X | Trend analysis |
|---|---|---|---|---|
| AST (U/l) | 90 ± 5 | 83 ± 3 | 80 ± 4 | 0.2419 |
| ALT (U/l) | 48 ± 4b) | 38 ± 2a) | 38 ± 1a) | 0.0483 (↓) |
| CK (U/l) | 255 ± 33 | 253 ± 17 | 242 ± 21 | 0.9089 |
| TP (g/dl) | 4.7 ± 0.1a) | 5.1 ± 0.1b) | 5.2 ± 0.1b) | <0.0001 (↑) |
| Albumin (g/dl) | 2.8 ± 0.0a) | 3.1 ± 0.0b) | 3.0 ± 0.0b) | 0.0222 (↑) |
| BUN (mg/dl) | 25.3 ± 1.4c) | 20.6 ± 0.5b) | 16.3 ± 0.6a) | <0.0001 (↓) |
| Creatinine (mg/dl) | 0.28 ± 0.01 | 0.27 ± 0.02 | 0.27 ± 0.01 | 0.1770 |
| UA (mg/dl) | 2.9 ± 0.3b) | 1.2 ± 0.1a) | 1.2 ± 0.1a) | <0.0001 (↓) |
| TC (mg/dl) | 136 ± 6 | 138 ± 4 | 138 ± 4 | 0.7146 |
| TG (mg/dl) | 153 ± 3 | 148 ± 3 | 141 ± 7 | 0.2868 |
| Glucose (mg/dl) | 142 ± 3 | 151 ± 3 | 140 ± 5 | 1.0000 |
Data are the mean ± SEM for n=8 mice in each group. Values in the same row with different superscript letters (a, b, c) differ significantly, P<0.05, by one-way ANOVA. Muscle mass includes both gastrocnemius and soleus muscles in the back part of the lower legs. The arrows up and down mean dose-dependently increased and decreased by WBG supplementation. AST, aspartate aminotransferase; ALT, alanine aminotransferase; TP, total protein; BUN, blood urea nitrogen; CK, creatine kinase; UA, uric acid; TC, total cholesterol; TG, triacylglycerol.
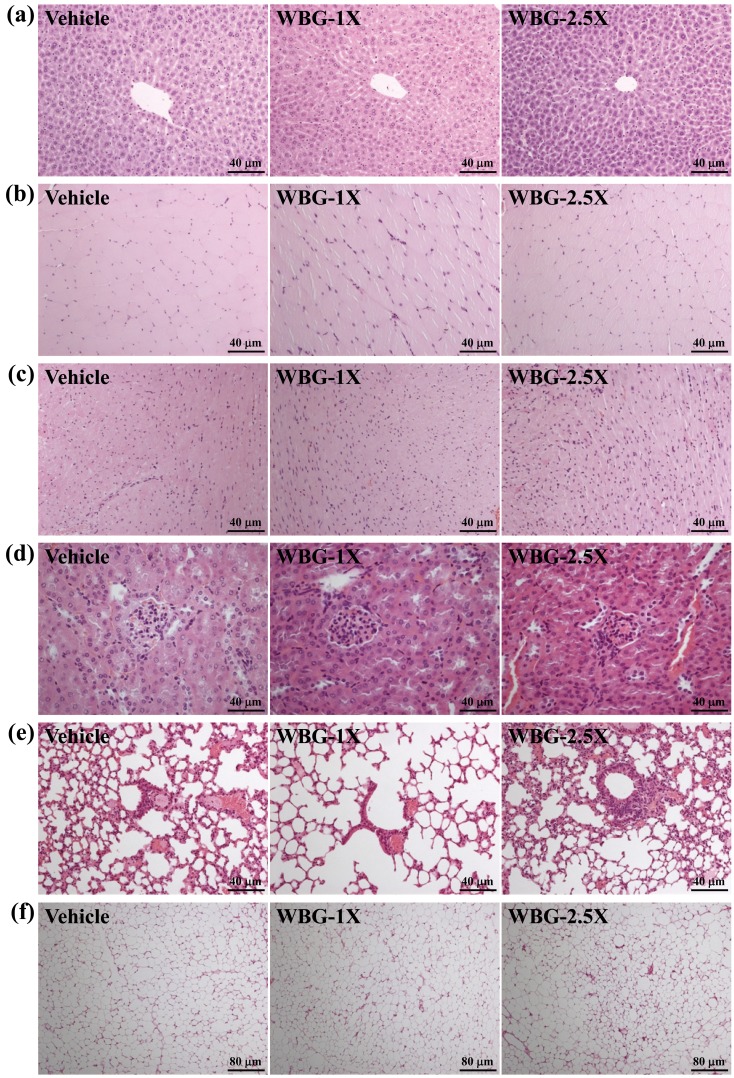
Effect of WBG supplementation on histological examinations at the end of the experiment
We also examined tissue morphology. WBG supplementation for 4 weeks had no adverse effects on the liver, skeletal muscle, heart, kidneys, lungs or EFP. Therefore, the dose of WBG supplementation used in this study was safe (Fig. 5).
Fig. 5.
Effects of WBG supplementation on morphology of liver (a); skeletal muscle (b); heart (c); kidney (d); lungs (e) and EFP (f). Specimens were photographed by light microscopy. H&E stain, magnification: × 200 (a–e) and ×100 (f). EFP: epididymal fat pad.
DISCUSSION
Charantin, the main active constituent of M. charantia, is an insulin-like compound that makes it useful for moderating blood glucose. Previous studies have demonstrated that WBG extract contains charantin and alkaloids for increasing blood glucose uptake [12, 17]. One of the best-known food factors, resveratrol, is under consideration as an additional phytochemical for treatment of metabolic diseases. In a previous study, we found that resveratrol reduces fatigue by increasing utilization of important fuel sources [41]. Thus, WBG could be a potential agent with an anti-fatigue pharmacological effect.
In general, programmed exercise training is required to increase grip strength [7]; however, we found that WBG supplementation increased grip strength, even though the test animals did not undergo a training intervention. Multiple pathways mediate the benefits of exercise in terms of muscle biology, growth and metabolism. Among them, peroxisome proliferator-activated receptor (PPAR) is a coactivator essential to various cellular processes, including mitochondrial biogenesis, oxidative metabolism and energy homeostasis. PPARα and PPARγ, in the PPAR sub-family, may be activated by WBG supplementation [6, 36]. PPARα and PPARγ can reflect athletic performance and activation by exercise training [1, 7]. They are induced in the adipose tissue and skeletal muscle [14, 30] of type 2 diabetes patients following a prescription of exercise [34]. PPARγ is activated in adipose tissue. Thus, WBG supplementation could activate PPAR pathways to improve exercise performance [4].
Exercise-induced muscle fatigue can be evaluated by biochemical indicators including lactate, ammonia, glucose, CK and BUN levels [22, 35]. The clearance of lactate is believed to reduce peripheral neuromuscular fatigue and has positive effects on muscle function [40]. After acute exercise, lactate accumulates in the muscles, and approximately 75% of the total amount of lactate produced is used for oxidative production of energy in the exercising body [2]. A previous study reported that ammonia levels are more intensity-sensitive than blood lactate levels across the whole intensity range during incremental exercise [25]. The reason is that peripheral and central fatigue levels are related to increased ammonia during exercise [5]. As exercise continues, glucose uptake increases and intramuscular glucose concentration decreases as hexokinase inhibition is relieved by a lower glucose 6-phosphate (G-6-P) concentration [26]. This mechanism contributes to the explanation of the temporal relationship between the decrease in muscle glycogen and the progressive increase in glucose uptake during moderate intensity exercise [32]. In our present data, WBG showed a great capacity to remove lactate and decrease levels of ammonia, CK and BUN after exercise and also to economize glucose utilization during exercise.
We focused on glycogen storage in the liver and muscle after 4-week WBG supplementation. WBG supplementation increased energy storage, thereby improving exercise performance. Utilization of carbohydrate in the form of intramuscular glycogen stores and glucose delivered from plasma becomes a crucial energy substrate to the exercise muscle [33]. Charantin and its derivatives may in part be responsible for the observed up-regulatory activities of high glycogen storage, glucose uptake and increased levels of the glucose, transporter Glucose transporter type 4 (GLUT4), in bitter melon extracts [16].
We also evaluated the body compositions of the mice after 4-week WBG supplementation. Data showed that WBG supplementation could increase BAT. Previous research has shown that both aerobic exercise training and AMPK/PPAR agonists accelerate the oxidative metabolism. An expected result would be reduction of the amount of fat in the body [3]. Thus, supplementation with WBG for 4 weeks could increase the BAT weight and decrease the EFP weight via AMPK/PPAR activation, which would stimulate lipid utilization and reduce adipose accumulation.
In summary, WBG is a specific tropical vegetable cultivated in Taiwan. We found that 4-week supplementation with WBG extract significantly improved the forelimb grip strength and swimming time to exhaustion of test mice. WBG reduces fatigue by decreasing plasma lactate, ammonia, CK and BUN levels, and increasing serum glucose levels after acute exercise in mice. Exercise-induced fatigue-related parameters, including lactate, ammonia, CK, glucose and BUN levels, were positively modulated by WBG supplementation in a dose-dependent manner, as confirmed by trend analysis. WBG also had positive effects on liver and kidney protection and enhanced nutrient absorption by decreasing ALT, BUN, albumin and UA levels, and increasing TP levels. Concerning body composition, our data showed that BW decreased after 4-week WBG supplementation; white adipose tissue weight was decreased by WBG-2.5X supplementation. On the other hand, BAT weight and relative weight increased dose-dependently with WBG supplementation. In conclusion, we provide evidence that 4-week WBG supplementation affected biochemical assessments, enhanced exercise performance, had anti-fatigue activity and reduced white adipose tissue. Therefore, we suggest that WBG may be considered for use as a nutrient supplement to improve health, reduce adipose generation and enhance energy storage.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
This study was not supported by any grant, outside sources of funding or industry sponsorship. The authors are grateful to Ms. Sin-Jie Lin for technical assistance in animal experiments. The authors thank Mr. John Ring (National Taiwan University, Taipei City, Taiwan) for his careful editing of the manuscript and Dr. Chien-Chao Chiu for conducting histological examination. The HPLC analysis was performed by Research Center for Industry of Human Ecology and Food Safety Testing Laboratory, College of Human Ecology, Chang Gung University of Science and Technology.
Appendix
Appendix Figure
Figure.
The bioactivities of hualian no. 4 wild bitter gourd (WBG) extract on anti-fatigue in vivo.
REFERENCES
- 1.Ahmetov I. I., Mozhayskaya I. A., Flavell D. M., Astratenkova I. V., Komkova A. I., Lyubaeva E. V., Tarakin P. P., Shenkman B. S., Vdovina A. B., Netreba A. I., Popov D. V., Vinogradova O. L., Montgomery H. E., Rogozkin V. A.2006. PPARalpha gene variation and physical performance in Russian athletes. Eur. J. Appl. Physiol. 97: 103–108. doi: 10.1007/s00421-006-0154-4 [DOI] [PubMed] [Google Scholar]
- 2.Brooks S. P., Storey K. B.1991. A quantitative evaluation of the effect of enzyme complexes on the glycolytic rate in vivo: mathematical modeling of the glycolytic complex. J. Theor. Biol. 149: 361–375. doi: 10.1016/S0022-5193(05)80311-X [DOI] [PubMed] [Google Scholar]
- 3.Bueno Júnior C. R., Pantaleão L. C., Voltarelli V. A., Bozi L. H., Brum P. C., Zatz M.2012. Combined effect of AMPK/PPAR agonists and exercise training in mdx mice functional performance. PLoS ONE 7: e45699. doi: 10.1371/journal.pone.0045699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calvo J. A., Daniels T. G., Wang X., Paul A., Lin J., Spiegelman B. M., Stevenson S. C., Rangwala S. M.2008. Muscle-specific expression of PPARgamma coactivator-1α improves exercise performance and increases peak oxygen uptake. J. Appl. Physiol. 104: 1304–1312. doi: 10.1152/japplphysiol.01231.2007 [DOI] [PubMed] [Google Scholar]
- 5.Carvalho-Peixoto J., Alves R. C., Cameron L. C.2007. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 32: 1186–1190. doi: 10.1139/H07-091 [DOI] [PubMed] [Google Scholar]
- 6.Chao C. Y., Yin M. C., Huang C. J.2011. Wild bitter gourd extract up-regulates mRNA expression of PPARα, PPARγ and their target genes in C57BL/6J mice. J. Ethnopharmacol. 135: 156–161. doi: 10.1016/j.jep.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 7.Chen W. C., Huang W. C., Chiu C. C., Chang Y. K., Huang C. C.2014. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 46: 1517–1524. doi: 10.1249/MSS.0000000000000272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y. M., Lin C. L., Wei L., Hsu Y. J., Chen K. N., Huang C. C., Kao C. H.2016. Sake protein supplementation affects exercise performance and biochemical profiles in power-exercise-trained mice. Nutrients 8: 106. doi: 10.3390/nu8020106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y. M., Tsai Y. H., Tsai T. Y., Chiu Y. S., Wei L., Chen W. C., Huang C. C.2014. Fucoidan supplementation improves exercise performance and exhibits anti-fatigue action in mice. Nutrients 7: 239–252. doi: 10.3390/nu7010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y. M., Wei L., Chiu Y. S., Hsu Y. J., Tsai T. Y., Wang M. F., Huang C. C.2016. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 8: 205. doi: 10.3390/nu8040205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coombes J. S., Rowell B., Dodd S. L., Demirel H. A., Naito H., Shanely R. A., Powers S. K.2002. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 87: 272–277. doi: 10.1007/s00421-002-0631-3 [DOI] [PubMed] [Google Scholar]
- 12.Das D. R., Sachan A. K., Imtiyaz M., Shuaib M.2015. Momordica charantia as a Potential Medicinal Herb: An Overview. J. Med. Plants Stud. 3: 23–26. [Google Scholar]
- 13.Desai S., Tatke P.2015. Charantin: An important lead compound from Momordica charantia for the treatment of diabetes. J. Pharmacogn. Phytochem. 3: 163–166. [Google Scholar]
- 14.Fatone C., Guescini M., Balducci S., Battistoni S., Settequattrini A., Pippi R., Stocchi L., Mantuano M., Stocchi V., De Feo P.2010. Two weekly sessions of combined aerobic and resistance exercise are sufficient to provide beneficial effects in subjects with Type 2 diabetes mellitus and metabolic syndrome. J. Endocrinol. Invest. 33: 489–495. doi: 10.1007/BF03346630 [DOI] [PubMed] [Google Scholar]
- 15.Fitts R. H.1994. Cellular mechanisms of muscle fatigue. Physiol. Rev. 74: 49–94. [DOI] [PubMed] [Google Scholar]
- 16.Hanhineva K., Törrönen R., Bondia-Pons I., Pekkinen J., Kolehmainen M., Mykkänen H., Poutanen K.2010. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 11: 1365–1402. doi: 10.3390/ijms11041365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazarika R., Parida P., Neog B., Yadav R. N.2012. Binding energy calculation of GSK-3 protein of human against some anti-diabetic compounds of Momordica charantia linn (Bitter melon). Bioinformation 8: 251–254. doi: 10.6026/97320630008251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu C., Tsai T. H., Li Y. Y., Wu W. H., Huang C. J., Tsai P. J.2012. Wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) extract and its bioactive components suppress Propionibacterium acnes-induced inflammation. Food Chem. 135: 976–984. doi: 10.1016/j.foodchem.2012.05.045 [DOI] [PubMed] [Google Scholar]
- 19.Hsu H. Y., Lin J. H., Li C. J., Tsang S. F., Tsai C. H., Chyuan J. H., Chuang S. E.2012. Antimigratory effects of the methanol extract from Momordica charantia on human lung adenocarcinoma CL1 cells. Evid-Based Compl. Alt. 2012: 819632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C. C., Hsu M. C., Huang W. C., Yang H. R., Hou C. C.2012. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid-Based Compl. Alt. 2012: 364741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S., Li R., Zhang Z., Li L., Gu X., Fan W., Lucas W. J., Wang X., Xie B., Ni P., Ren Y., Zhu H., Li J., Lin K., Jin W., Fei Z., Li G., Staub J., Kilian A., van der Vossen E. A., Wu Y., Guo J., He J., Jia Z., Ren Y., Tian G., Lu Y., Ruan J., Qian W., Wang M., Huang Q., Li B., Xuan Z., Cao J., Asan, Wu Z., Zhang J., Cai Q., Bai Y., Zhao B., Han Y., Li Y., Li X., Wang S., Shi Q., Liu S., Cho W. K., Kim J. Y., Xu Y., Heller-Uszynska K., Miao H., Cheng Z., Zhang S., Wu J., Yang Y., Kang H., Li M., Liang H., Ren X., Shi Z., Wen M., Jian M., Yang H., Zhang G., Yang Z., Chen R., Liu S., Li J., Ma L., Liu H., Zhou Y., Zhao J., Fang X., Li G., Fang L., Li Y., Liu D., Zheng H., Zhang Y., Qin N., Li Z., Yang G., Yang S., Bolund L., Kristiansen K., Zheng H., Li S., Zhang X., Yang H., Wang J., Sun R., Zhang B., Jiang S., Wang J., Du Y., Li S.2009. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 41: 1275–1281. doi: 10.1038/ng.475 [DOI] [PubMed] [Google Scholar]
- 22.Izquierdo M., González-Izal M., Navarro-Amezqueta I., Calbet J. A., Ibañez J., Malanda A., Mallor F., Häkkinen K., Kraemer W. J., Gorostiaga E. M.2011. Effects of strength training on muscle fatigue mapping from surface EMG and blood metabolites. Med. Sci. Sports Exerc. 43: 303–311. doi: 10.1249/MSS.0b013e3181edfa96 [DOI] [PubMed] [Google Scholar]
- 23.Joyner M. J., Coyle E. F.2008. Endurance exercise performance: the physiology of champions. J. Physiol. 586: 35–44. doi: 10.1113/jphysiol.2007.143834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kan N. W., Ho C. S., Chiu Y. S., Huang W. C., Chen P. Y., Tung Y. T., Huang C. C.2016. Effects of resveratrol supplementation and exercise training on exercise performance in middle-aged mice. Molecules 21: 661. doi: 10.3390/molecules21050661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantanista A., Kusy K., Zarębska E., Włodarczyk M., Ciekot-Sołtysiak M., Zieliński J.2016. Blood ammonia and lactate responses to incremental exercise in highly-trained male sprinters and triathletes. Biomed. Hum. Kinetic. 8: 32–38. doi: 10.1515/bhk-2016-0005 [DOI] [Google Scholar]
- 26.Kawanaka K., Nolte L. A., Han D. H., Hansen P. A., Holloszy J. O.2000. Mechanisms underlying impaired GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. Am. J. Physiol. Endocrinol. Metab. 279: E1311–E1318. [DOI] [PubMed] [Google Scholar]
- 27.Konishi T., Satsu H., Hatsugai Y., Aizawa K., Inakuma T., Nagata S., Sakuda S. H., Nagasawa H., Shimizu M.2004. A bitter melon extract inhibits the P-glycoprotein activity in intestinal Caco-2 cells: monoglyceride as an active compound. Biofactors 22: 71–74. doi: 10.1002/biof.5520220113 [DOI] [PubMed] [Google Scholar]
- 28.Lu K. H., Tseng H. C., Liu C. T., Huang C. J., Chyuan J. H., Sheen L. Y.2014. Wild bitter gourd protects against alcoholic fatty liver in mice by attenuating oxidative stress and inflammatory responses. Food Funct. 5: 1027–1037. doi: 10.1039/c3fo60449g [DOI] [PubMed] [Google Scholar]
- 29.Miura T., Itoh C., Iwamoto N., Kato M., Kawai M., Park S. R., Suzuki I.2001. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J. Nutr. Sci. Vitaminol. (Tokyo) 47: 340–344. doi: 10.3177/jnsv.47.340 [DOI] [PubMed] [Google Scholar]
- 30.Petridou A., Tsalouhidou S., Tsalis G., Schulz T., Michna H., Mougios V.2007. Long-term exercise increases the DNA binding activity of peroxisome proliferator-activated receptor γ in rat adipose tissue. Metabolism 56: 1029–1036. doi: 10.1016/j.metabol.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 31.Rajamoorthi A., Shrivastava S., Steele R., Nerurkar P., Gonzalez J. G., Crawford S., Varvares M., Ray R. B.2013. Bitter melon reduces head and neck squamous cell carcinoma growth by targeting c-Met signaling. PLoS ONE 8: e78006. doi: 10.1371/journal.pone.0078006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter E. A., Hargreaves M.2013. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93: 993–1017. doi: 10.1152/physrev.00038.2012 [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez N. R., Di Marco N. M., Langley S., American Dietetic AssociationDietitians of CanadaAmerican College of Sports Medicine 2009. American College of Sports Medicine position stand. Nutrition and athletic performance. Med. Sci. Sports Exerc. 41: 709–731. [DOI] [PubMed] [Google Scholar]
- 34.Sixt S., Beer S., Blüher M., Korff N., Peschel T., Sonnabend M., Teupser D., Thiery J., Adams V., Schuler G., Niebauer J.2010. Long- but not short-term multifactorial intervention with focus on exercise training improves coronary endothelial dysfunction in diabetes mellitus type 2 and coronary artery disease. Eur. Heart J. 31: 112–119. doi: 10.1093/eurheartj/ehp398 [DOI] [PubMed] [Google Scholar]
- 35.Strojnik V., Komi P. V.2000. Fatigue after submaximal intensive stretch-shortening cycle exercise. Med. Sci. Sports Exerc. 32: 1314–1319. doi: 10.1097/00005768-200007000-00020 [DOI] [PubMed] [Google Scholar]
- 36.Tan M. J., Ye J. M., Turner N., Hohnen-Behrens C., Ke C. Q., Tang C. P., Chen T., Weiss H. C., Gesing E. R., Rowland A., James D. E., Ye Y.2008. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem. Biol. 15: 263–273. doi: 10.1016/j.chembiol.2008.01.013 [DOI] [PubMed] [Google Scholar]
- 37.Tsai C. H., Chen E. C., Tsay H. S., Huang C. J.2012. Wild bitter gourd improves metabolic syndrome: a preliminary dietary supplementation trial. Nutr. J. 11: 4. doi: 10.1186/1475-2891-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang H. Y., Kan W. C., Cheng T. J., Yu S. H., Chang L. H., Chuu J. J.2014. Differential anti-diabetic effects and mechanism of action of charantin-rich extract of Taiwanese Momordica charantia between type 1 and type 2 diabetic mice. Food Chem. Toxicol. 69: 347–356. doi: 10.1016/j.fct.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 39.Weng J. R., Bai L. Y., Chiu C. F., Hu J. L., Chiu S. J., Wu C. Y.2013. Cucurbitane triterpenoid from Momordica charantia induces apoptosis and autophagy in breast cancer cells, in part, through peroxisome proliferator-activated receptor γ activation. Evid-Based Compl. Evid. Based Complement. Alternat. Med. 2013: 935675. doi: 10.1155/2013/935675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White G. E., Wells G. D.2015. The effect of on-hill active recovery performed between runs on blood lactate concentration and fatigue in alpine ski racers. J. Strength Cond. Res. 29: 800–806. doi: 10.1519/JSC.0000000000000677 [DOI] [PubMed] [Google Scholar]
- 41.Wu R. E., Huang W. C., Liao C. C., Chang Y. K., Kan N. W., Huang C. C.2013. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 18: 4689–4702. doi: 10.3390/molecules18044689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu S. J., Ng L. T.2008. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT-Food. Sci. Tech. (Paris) 41: 323–330. [Google Scholar]
- 43.You L., Zhao M., Regenstein J. M., Ren J.2011. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 124: 188–194. doi: 10.1016/j.foodchem.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 44.Yu S. H., Chen S. Y., Li W. S., Dubey N. K., Chen W. H., Chuu J. J., Leu S. J., Deng W. P.2015. Hypoglycemic activity through a novel combination of fruiting body and mycelia of Cordyceps Militaris in high-fat diet-induced type 2 diabetes mellitus mice. J. Diabetes Res. 2015: 723190. doi: 10.1155/2015/723190 [DOI] [PMC free article] [PubMed] [Google Scholar]