Abstract
Obesity and type 2 diabetes mellitus (T2DM) are occurring at epidemic-like rates, and these epidemics appear to have emerged largely from changes in daily diet. In the present study, we compared effects of high-fat diet (HFD) and fructose-rich diet (FRD) in WBN/Kob-Leprfa (WBKDF) rats that spontaneously develop obesity, dyslipidemia and T2DM. After a 4-week feeding of each diet, WBKDF-HFD and WBKDF-FRD rats exhibited aggravated obesity and dyslipidemia compared with WBKDF rats fed standard diet (STD). In contrast, hyperglycemia developed in WBKDF-STD rats was significantly inhibited in WBKDF-FRD rats, but not in WBKDF-HFD rats. The present study demonstrated that the 4-week feeding of HFD and FRD caused diet-induced obesity with a distinct phenotype in the glucose metabolism in WBKDF rats.
Keywords: diet-induced obesity, fructose-rich diet, high-fat diet, type 2 diabetes, WBN/Kob-Leprfa rat
Obesity is rapidly evolving, as one of the major global health issues, as it is frequently associated with type 2 diabetes mellitus (T2DM) and dyslipidemia [2]. This epidemic appears to have emerged largely from a combination of increased food intake and decreased physical activity [3], and is strongly influenced by genetic background [14]. The availability of useful animal models reflecting human obesity is crucial in the search for novel compounds for the pharmacological treatment of obesity.
The Wistar Bonn Kobori (WBN/Kob) diabetic fatty (WBKDF) rat is a new congenic strain developed by introduction of the fa allele of the Zucker fatty rat into the parental WBN/Kob (lean) rat genome [1]. The leptin receptor fatty gene (Leprfa) is a recessive mutation that leads to leptin receptor deficiency, and homozygous animals (fa/fa) exhibit obesity and hyperphagia, in addition to insulin resistance and glucose intolerance [6]. Previous reports demonstrated that WBKDF rats have severe obesity and insulin resistance, both of which lead to developing T2DM [1, 11, 15, 16].
Although several gene mutations causing obesity in humans, such as leptin [13] and leptin receptor [7], have been identified, the increase in worldwide obesity in a short period of time cannot be explained by genetics; there are individual differences in genetic susceptibility to environmental factors, such as inadequate diet. In recent years, concerns have arisen regarding the increased consumption of fat and fructose. Adopting a high-fat diet (HFD) and fructose-rich diet (FRD) has been widely used as a template to induce obesity in laboratory animals including rats [10, 17]. However, animals with similar weight and age at the start of the experimental protocol have different results regarding body weight gain when different types of diets are used [4, 10, 17]. Our aim was to investigate and compare the effects of HFD and FRD on obesity, dyslipidemia and hyperglycemia in WBKDF rats.
Male WBKDF rats and age-matched male Wistar rats were obtained from Japan SLC, Inc. (Hamamatsu, Japan). Prior to dietary manipulation, all rats were fed standard rat chow (STD, catalog number: CE-2, CLEA Japan, Inc., Tokyo, Japan), and were housed in plastic cages with a fixed (12 hr) artificial light period (08:00 to 20:00 hr) at a constant temperature (21 ± 2°C) and humidity (55 ± 5%) throughout the experiment. They were allowed free access to food and fresh tap water from a plastic water bottle.
WBKDF rats (n=24) at 7 weeks of age were divided into three groups: (a) the WBKDF-STD group (n=8), (b) the WBKDF-HFD group (n=8) and (c) the WBKDF-FRD group (n=8), and were fed STD, HFD (45% kcal from fat, catalog number: 58V8, PMI Nutrition International, St. Louis, MO, U.S.A.) or FRD (60% fructose purified diet, catalog number: 5375, PMI Nutrition International) for 4 weeks, respectively. Age-matched Wistar rats fed STD (n=8) were used as normal controls. All rats were allowed free access to food and fresh tap water during the study period. The body weight of the rats was measured weekly between 10:00 and 14:00 hr. Non-fasting plasma glucose and insulin levels were determined weekly using blood samples collected from the tail vein. All experimental protocols were approved by Azabu University Animal Research Ethics Committee.
At the end of the experiment, blood samples (3 ml) for blood chemistry analyses were collected from fasted rats under pentobarbital sodium anesthesia (50 mg/kg IP; Kyoritsu Seiyaku, Tokyo, Japan) and centrifuged at 3,000 × g for 15 min at 4°C, and then, the plasma was removed and flash-frozen for performing blood biomarker analyses later. The rats were then sacrificed using a lethal dose of pentobarbital, and the epididymal fat and mesenteric fat were harvested and weighed.
Plasma levels of total cholesterol (T-Chol), phospholipid (PL), triglycerides (TG) and glucose were measured using an automatic analyzer (JCA-BM 2250; JEOL Ltd., Tokyo, Japan). Plasma insulin levels were quantitated by a rat insulin ELISA kit (Morinaga Institute of Biological Science, Yokohama, Japan).
Values are expressed as the mean ± standard error (SE) unless otherwise stated. Statistical analysis was performed by two-way ANOVA followed by post-hoc Tukey tests. Significance was set at a P-value less than 0.05. All statistical analyses were performed using GraphPad Prism 5 statistical software (GraphPad Software Inc., La Jolla, CA, U.S.A.).
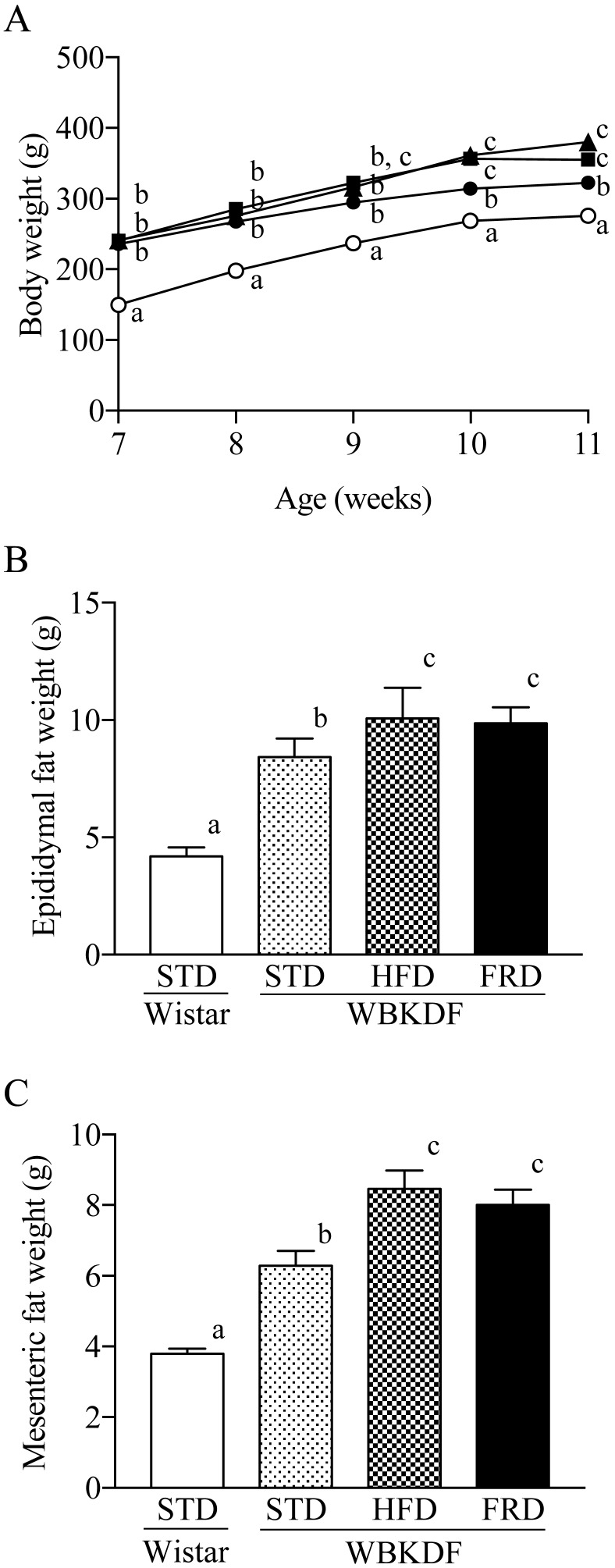
Prior to the start of dietary interventions, there were no significant intergroup differences in body weight of WBKDF rats which were significantly (P<0.01) heavier than Wistar rats (Fig. 1A). After the 4-week feeding, the body weights in WBKDF-HFD and WBKDF-FRD groups were significantly (P<0.01) higher than those in the WBKDF-STD group (Fig. 1A). The epididymal fat weights in the WBKDF-HFD and WBKDF-FRD rats were also significantly (P<0.01) heavier than those in WBKDF-STD rats and Wistar rats (Fig. 1B and 1C).
Fig. 1.
Effects of the 4-week feeding of HFD and FRD on (A) the body weights (open circle, Wistar-STD; closed circle, WBKDF-STD; closed square, WBKDF-HFD; closed triangle, WBKDF-FRD), and (B) epididymal and (C) mesenteric fat weights in WBKDF rats. The fat weights were determined at 11 weeks of age. Data are expressed as mean ± SE (n=8). Different superscript letters (a, b, c) indicate significant differences between groups (P<0.05).
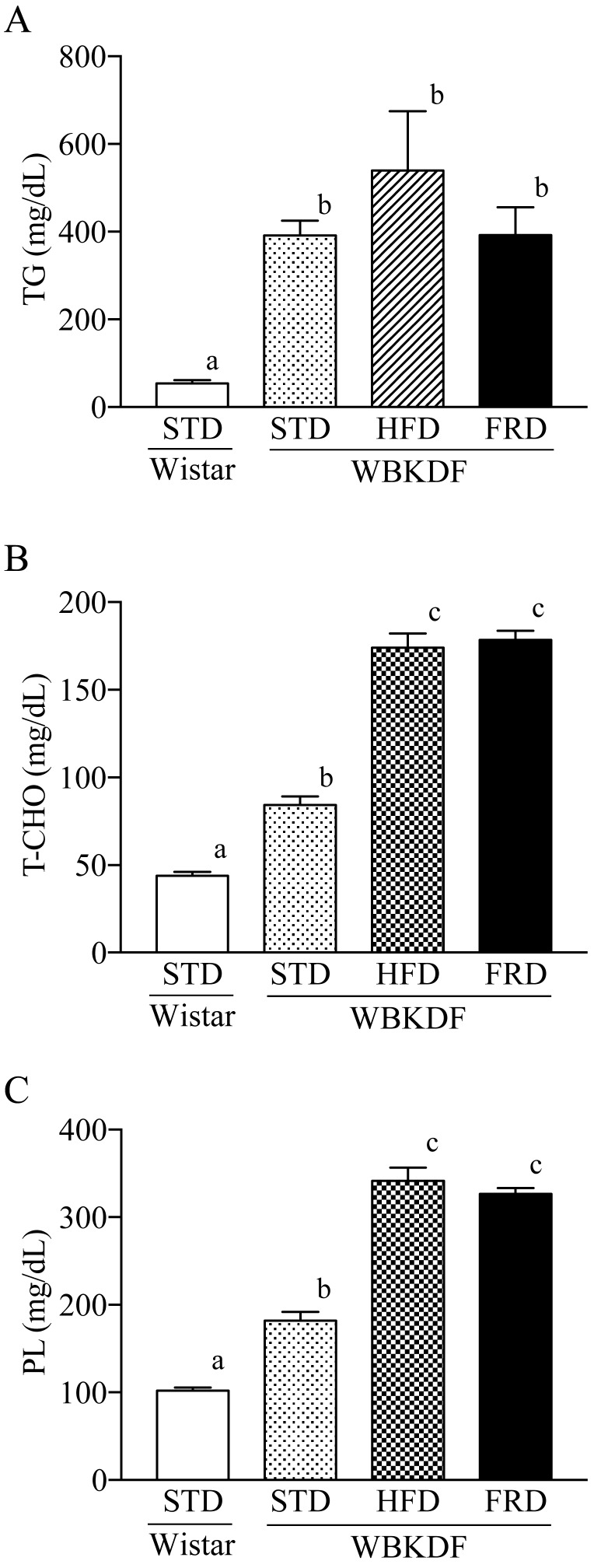
WBKDF-STD developed dyslipidemia: plasma levels of TG, T-Chol and PL were significantly (P<0.01) higher than those in Wistar rats at 11 weeks of age (Fig. 2A–2C). WBKDF-HFD and -FRD rats had significantly (P<0.01) higher levels of T-Chol and PL than those in WBKDF-STD rats (Fig. 2A and 2B).
Fig. 2.
Effects of the 4-week feeding of HFD and FRD on plasma levels of (A) total cholesterol, (B) phospholipid and (C) triglyceride in WBKDF rats. Data are expressed as mean ± SE (n=8). Different superscript letters (a, b, c) indicate significant differences between groups (P<0.05).
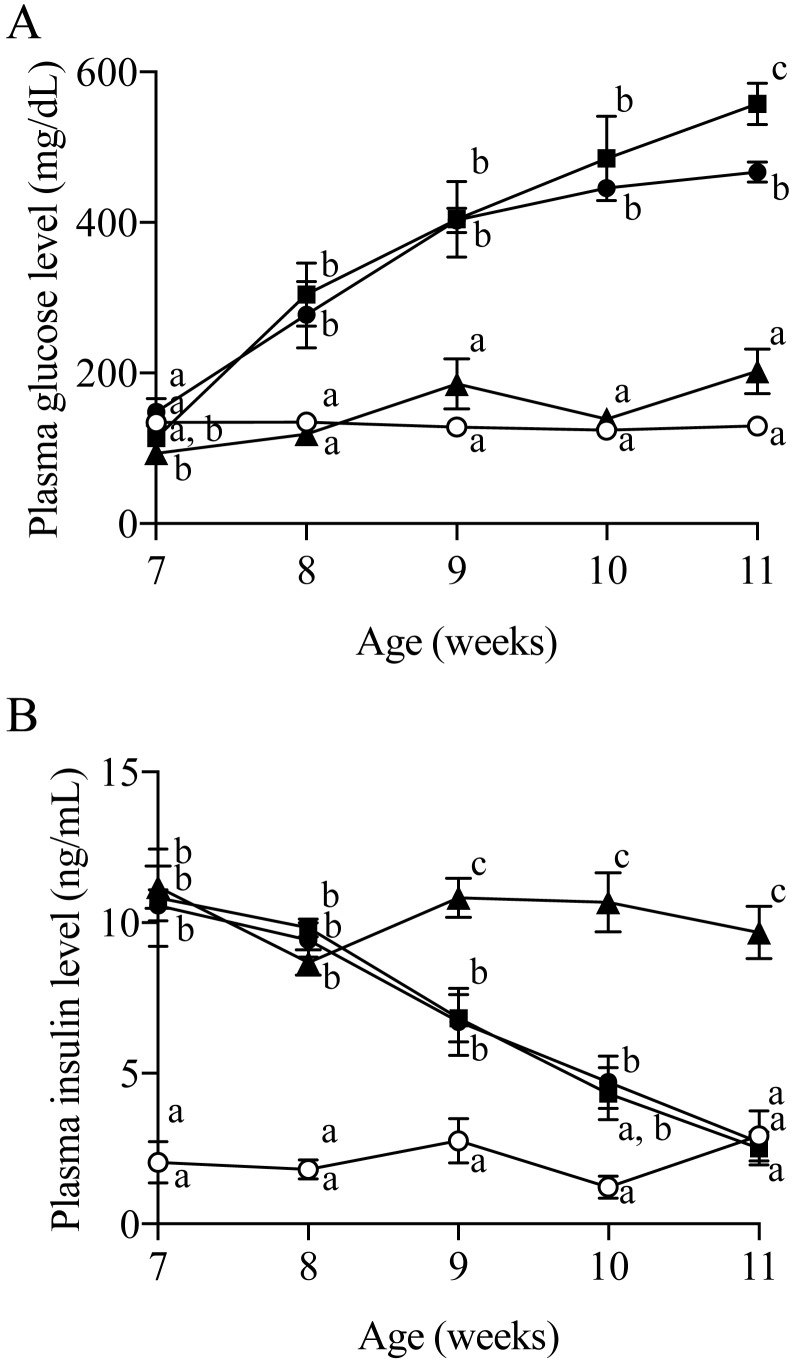
The non-fasting blood glucose level of the WBKDF-STD rats markedly increased from 8 weeks of age and reached a plateau at 10 weeks of age (Fig. 3A). Plasma glucose levels in WBKDF-HFD were higher than that in WBKDF-STD, and significance (P<0.05) was observed at 11 weeks of age. In contrast, the plasma glucose level in the WBKDF-FRD rats did not increase during the study period and was significantly (P<0.01) lower when compared with WBKDF-STD rats (Fig. 3A). There was no significant difference in plasma insulin levels among three groups of WBKDF rats at 7 weeks of age (Fig. 3B), which were significantly higher than those in Wistar rats, indicating that WBKDF rats had hyperinsulinemia. The plasma insulin levels of WBKDF-STD and -HFD rats were comparable and had a tendency to decrease during the study period. On the other hand, the blood insulin level of WBKDF-FRD rats remained steady up to 11 weeks of age (Fig. 3B).
Fig. 3.
Effects of the 4-week feeding of HFD and FRD on (A) plasma glucose concentrations and (B) plasma insulin concentrations in WBKDF rats (open circle, Wistar-STD; closed circle, WBKDF-STD; closed square, WBKDF-HFD; closed triangle, WBKDF-FRD). Data are expressed as mean ± SE (n=8). Different superscript letters (a, b, c) indicate significant differences between groups (P<0.05).
Although it is generally accepted that feeding HFDs or FRDs to rodents reliably induces obesity and dyslipidemia, the metabolic alterations observed in HFD- and FRD-fed rats are highly divergent among studies [5, 18]. Differences between studies include the strain of rats used, the amount of cholesterol and fructose, and the age of animals at the beginning of experiment. In this study, we examined the effects of HFD and FRD on obesity, dyslipidemia and T2DM in WBKDF rats, which spontaneously develop obesity, dyslipidemia and T2DM. The main finding of this study was that a 4-week feeding of HFD and FRD resulted in a significant increase of body weights and visceral fat weights in WBKDF rats, indicating diet-induced visceral obesity. HFD-induced obesity in WBKDF rats was analogous to that in ZDF rats, one of the most well examined T2DM rats [19]. To our knowledge, this is the first study on FRD-induced obesity in rats with T2DM. Although the diet effects depend not only on the diet composition but also considerably on the rat strain, this study clearly demonstrated that the WBKDF rat is susceptible to diet-induced obesity.
In the current study, HFD and FRD aggravated dyslipidemia in WBKDF rats. Our data are analogous to those in previous studies reporting that HFD and FRD cause dyslipidemia in non-diabetic rodents [4, 10, 17]. It is well known that fructose and fat provide excess lipids from different sources; fructose increases the availability of endogenous lipids, whereas fat increases the availability of exogenous lipids. However, as shown in the current study, feeding of the two diets resulted in similar metabolic phenotypes in WBKDF rats, as evidenced by increased plasma T-Chol and PL.
WBKDF-STD rats have been reported to spontaneously develop hyperglycemia and pancreatitis associated with gradual reduction of plasma insulin levels [11, 15, 16]. The present study demonstrated that HFD loading aggravated hyperglycemia in WBKDF rats, which is consistent with the notion that consumption of HFD has a negative influence on the incidence of T2DM [8]. In contrast, FRD loading inhibited hyperglycemia and reduction of the plasma insulin level. The mechanism by which FRD inhibited reduction of plasma insulin levels is not clear. Early studies reported that injury of the pancreatic β-cells precedes occurrence of hyperglycemia in WBKDF rats [1, 16]. Thus, one possible explanation is that FRD attenuated injury of the pancreatic β-cells. Another possibility is that FRD enhanced insulin release from pancreatic β-cells. Indeed, FRD is known to increase plasma insulin levels via enhancing release of glucagon-like peptide-1 (GLP-1), one of the major incretin hormones, from the small intestine [12]. GLP-1 is known to stimulate insulin secretion and regulates glucose homeostasis by inhibiting glucagon secretion, slowing gastric emptying and controlling satiation [9, 20].
Whether high fructose consumption is harmful or not has been a subject of debate [21]. Fructose, a low glycemic sugar compared with glucose, was suggested to be beneficial for the diet of diabetic patients. However, its growing industrial use as a sweetener, especially in soft drinks, has focused attention on its potential harmfulness, as well as being a possible cause of obesity, dyslipidemia and even T2DM. Our present results demonstrating the anti-diabetic effects of FRD in WBKDF rats may provide additional evidence that dietary fructose is beneficial against T2DM, but warrants further studies to prove this possibility.
In summary, feeding of HFD and FRD exacerbated obesity and dyslipidemia spontaneously developed in the WBKDF-STD rats. T2DM spontaneously developed in WBKDF-STD rats was aggravated by HDF, but inversely inhibited by FRD. The present study demonstrated that feeding of HFD and FRD leads to a distinct phenotype in glucose metabolism in WBKDF rats. WBKDF rats with DIO may be a useful model for investigating the causal mechanism and new medical agents against metabolic syndromes.
Acknowledgments
This study was supported in part by a research project grant awarded by Azabu University.
REFERENCES
- 1.Akimoto T., Nakama K., Katsuta Y., Zhang X. J., Ohsuga M., Ishizaki M., Sawai N., Ozawa H.2008. Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-Lepr(fa)) rat. Biochem. Biophys. Res. Commun. 366: 556–562. doi: 10.1016/j.bbrc.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 2.Anderson P. J., Critchley J. A., Chan J. C., Cockram C. S., Lee Z. S., Thomas G. N., Tomlinson B.2001. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int. J. Obes. Relat. Metab. Disord. 25: 1782–1788. doi: 10.1038/sj.ijo.0801837 [DOI] [PubMed] [Google Scholar]
- 3.Bandini L. G., Vu D., Must A., Cyr H., Goldberg A., Dietz W. H.1999. Comparison of high-calorie, low-nutrient-dense food consumption among obese and non-obese adolescents. Obes. Res. 7: 438–443. doi: 10.1002/j.1550-8528.1999.tb00431.x [DOI] [PubMed] [Google Scholar]
- 4.Buettner R., Schölmerich J., Bollheimer L. C.2007. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 15: 798–808. doi: 10.1038/oby.2007.608 [DOI] [PubMed] [Google Scholar]
- 5.Catena C., Giacchetti G., Novello M., Colussi G., Cavarape A., Sechi L. A.2003. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. Am. J. Hypertens. 16: 973–978. doi: 10.1016/S0895-7061(03)01002-1 [DOI] [PubMed] [Google Scholar]
- 6.Chua S. C., Jr, White D. W., Wu-Peng X. S., Liu S. M., Okada N., Kershaw E. E., Chung W. K., Power-Kehoe L., Chua M., Tartaglia L. A., Leibel R. L.1996. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr). Diabetes 45: 1141–1143. doi: 10.2337/diab.45.8.1141 [DOI] [PubMed] [Google Scholar]
- 7.Clément K., Vaisse C., Lahlou N., Cabrol S., Pelloux V., Cassuto D., Gourmelen M., Dina C., Chambaz J., Lacorte J. M., Basdevant A., Bougnères P., Lebouc Y., Froguel P., Guy-Grand B.1998. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401. doi: 10.1038/32911 [DOI] [PubMed] [Google Scholar]
- 8.Drouin-Chartier J. P., Brassard D., Tessier-Grenier M., Côté J. A., Labonté M. È., Desroches S., Couture P., Lamarche B.2016. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv. Nutr. 7: 1026–1040. doi: 10.3945/an.115.011403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drucker D. J.2006. The biology of incretin hormones. Cell Metab. 3: 153–165. doi: 10.1016/j.cmet.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 10.Fellmann L., Nascimento A. R., Tibiriça E., Bousquet P.2013. Murine models for pharmacological studies of the metabolic syndrome. Pharmacol. Ther. 137: 331–340. doi: 10.1016/j.pharmthera.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Kaji N., Okuno A., Ohno-Ichiki K., Oki H., Ishizawa H., Shirai M., Asai F.2012. Plasma profiles of glucose, insulin and lipids in the male WBN/Kob-Lepr(fa) rat, a new model of type 2 diabetes with obesity. J. Vet. Med. Sci. 74: 1185–1189. doi: 10.1292/jvms.12-0045 [DOI] [PubMed] [Google Scholar]
- 12.Kuhre R. E., Gribble F. M., Hartmann B., Reimann F., Windeløv J. A., Rehfeld J. F., Holst J. J.2014. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 306: G622–G630. doi: 10.1152/ajpgi.00372.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montague C. T., Farooqi I. S., Whitehead J. P., Soos M. A., Rau H., Wareham N. J., Sewter C. P., Digby J. E., Mohammed S. N., Hurst J. A., Cheetham C. H., Earley A. R., Barnett A. H., Prins J. B., O’Rahilly S.1997. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387: 903–908. doi: 10.1038/43185 [DOI] [PubMed] [Google Scholar]
- 14.Mutch D. M., Clément K.2006. Unraveling the genetics of human obesity. PLoS Genet. 2: e188. doi: 10.1371/journal.pgen.0020188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagakubo D., Shirai M., Nakamura Y., Kaji N., Arisato C., Watanabe S., Takasugi A., Asai F.2014. Prophylactic effects of the glucagon-like Peptide-1 analog liraglutide on hyperglycemia in a rat model of type 2 diabetes mellitus associated with chronic pancreatitis and obesity. Comp. Med. 64: 121–127. [PMC free article] [PubMed] [Google Scholar]
- 16.Okuno A., Kaji N., Takahashi A., Nagakubo D., Ohno-Ichiki K., Shirai M., Asai F.2013. Role of insulin resistance in the pathogenesis and development of type 2 diabetes in WBN/Kob-Lepr(fa) rats. J. Vet. Med. Sci. 75: 1557–1561. doi: 10.1292/jvms.13-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosini T. C., Silva A. S., Moraes C.2012. Diet-induced obesity: rodent model for the study of obesity-related disorders. Rev. Assoc. Med. Bras. (1992) 58: 383–387. [PubMed] [Google Scholar]
- 18.Shapiro A., Mu W., Roncal C., Cheng K. Y., Johnson R. J., Scarpace P. J.2008. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295: R1370–R1375. doi: 10.1152/ajpregu.00195.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teague J., Gyte A., Peel J. E., Young K. C., Loxham S. J., Mayers R. M., Poucher S. M.2011. Reversibility of hyperglycaemia and islet abnormalities in the high fat-fed female ZDF rat model of type 2 diabetes. J. Pharmacol. Toxicol. Methods 63: 15–23. doi: 10.1016/j.vascn.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 20.Wajchenberg B. L.2007. β-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 28: 187–218. doi: 10.1210/10.1210/er.2006-0038 [DOI] [PubMed] [Google Scholar]
- 21.Wiernsperger N., Geloen A., Rapin J. R.2010. Fructose and cardiometabolic disorders: the controversy will, and must, continue. Clinics (Sao Paulo) 65: 729–738. doi: 10.1590/S1807-59322010000700013 [DOI] [PMC free article] [PubMed] [Google Scholar]