Abstract
Pregnancy is increasingly undertaken in patients with chronic kidney disease (CKD) and, conversely, CKD is increasingly diagnosed in pregnancy: up to 3 % of pregnancies are estimated to be complicated by CKD. The heterogeneity of CKD (accounting for stage, hypertension and proteinuria) and the rarity of several kidney diseases make risk assessment difficult and therapeutic strategies are often based upon scattered experiences and small series. In this setting, the aim of this position statement of the Kidney and Pregnancy Study Group of the Italian Society of Nephrology is to review the literature, and discuss the experience in the clinical management of CKD in pregnancy. CKD is associated with an increased risk for adverse pregnancy-related outcomes since its early stage, also in the absence of hypertension and proteinuria, thus supporting the need for a multidisciplinary follow-up in all CKD patients. CKD stage, hypertension and proteinuria are interrelated, but they are also independent risk factors for adverse pregnancy-related outcomes. Among the different kidney diseases, patients with glomerulonephritis and immunologic diseases are at higher risk of developing or increasing proteinuria and hypertension, a picture often difficult to differentiate from preeclampsia. The risk is higher in active immunologic diseases, and in those cases that are detected or flare up during pregnancy. Referral to tertiary care centres for multidisciplinary follow-up and tailored approaches are warranted. The risk of maternal death is, almost exclusively, reported in systemic lupus erythematosus and vasculitis, which share with diabetic nephropathy an increased risk for perinatal death of the babies. Conversely, patients with kidney malformation, autosomal-dominant polycystic kidney disease, stone disease, and previous upper urinary tract infections are at higher risk for urinary tract infections, in turn associated with prematurity. No risk for malformations other than those related to familiar urinary tract malformations is reported in CKD patients, with the possible exception of diabetic nephropathy. Risks of worsening of the renal function are differently reported, but are higher in advanced CKD. Strict follow-up is needed, also to identify the best balance between maternal and foetal risks. The need for further multicentre studies is underlined.
Keywords: Chronic kidney disease, Evidence based medicine, Pregnancy, Hypertension, Proteinuria, Preeclampsia, Pre-term delivery
Introduction
In 1975, an editorial in the Lancet, entitled Pregnancy and Renal Disease, started with the following sentence: Children of women with renal disease used to be born dangerously or not at all—not at all if their doctors had their way [1]. The paper reviewed the previous grim evidence, with minimal chances of a positive outcome for children whose mothers started pregnancy with blood urea nitrogen (BUN) levels higher than 60 mg/dl and with hypertension. In spite of several negative reports, however, the authors gave space to new more positive data and concluded discussing the advice to be given to a woman with chronic kidney disease (CKD) who wants to undertake a pregnancy. The conclusions are still valid in our era of patient empowerment: the woman should be told that there is a considerable risk to her infant and a small risk to herself, but dogmatic prohibitions do not seem justified today. Instead, obstetrician and physician must batter down the hatches and prepare to ride out the storm together with those determined to set sail [1].
Many of the issues raised in 1975, notably the role of hypertension and proteinuria, the severity of CKD and the presence of specific diseases such as systemic lupus erythematosus (SLE) or autosomal dominant polycystic kidney disease (ADPKD), are still a matter of discussion. However, the context has changed throughout the decades, for several reasons including advances in maternal–foetal care, with a progressive expansion of the foetal viability zone to 22–24 weeks [2–4]. The progressive empowerment of patients with chronic diseases has shifted the attitude from a paternalistic protection of the mother to a shared choice. Meanwhile, the almost unexpected results of pregnancy on dialysis have highlighted the potentials of pregnancy even in the latest stages of CKD [5–13].
The definition of CKD, substituting “renal insufficiency” with the broader concept of “chronic kidney disease”, sets the stage for acknowledging a high prevalence of CKD in pregnancy [14–19].
In this context, the Kidney and Pregnancy Study Group of the Italian Society of Nephrology undertook the present best practice review, aimed at combining the available evidence with an in-depth discussion on shared experiences and open questions.
Evidence-based medicine and pregnancy in CKD: methodological insights
The evidence on pregnancy in CKD shares several methodological problems with pregnancy in dialysis and, more generally, with pregnancy in rare and/or heterogeneous diseases [13, 19–21].
CKD is an etiquette gathering many different diseases that may differently affect pregnancy. Immunologic nephropathies are at risk of flares during and after pregnancy. Pyelonephritis and renal malformations share an increased risk of urinary tract infections. ADPKD presents complex ethical problems as regards prenatal counselling. Diabetic nephropathy is the only kidney disease associated with a higher risk for non-renal malformations [19–30]. The lack of shared definitions partially impairs the pooling of the data for systematic reviews, an issue that has to be considered during a critical analysis of the results [19–21, 28, 30].
Kidney disease, degree of kidney impairment, hypertension and proteinuria are well known factors in the pathogenesis of pregnancy-related adverse outcomes. Their interactions are complex, and so far not completely elucidated [31–33].
A major issue is kidney function assessment in pregnancy, a situation in which no validated formula exists and the physiologic hyperfiltration may alter CKD staging [34–38].
It is difficult to perform randomised controlled trials (RCTs) in pregnancy, and often these are ethically unfeasible [39–41]. Hence, our understanding on pregnancy outcomes in CKD patients mainly relies on observational studies, whose quality is not necessarily inferior as compared to RCTs [42–48]. As a consequence of the “physiological” lack of RCTs and of the paucity of large observational studies, we will deal with low levels of recommendation. We will include several not graded suggestions, whose contribution should however not be underestimated [13].
The research strategy on which the present position statement is based represents an updating, at June 2014, of a previous systematic review on CKD and pregnancy, to which we refer the reader for details on the search strategy and on the paper selection modality [20]. The heterogeneity of the literature and the high number of kidney diseases led us to focus on general features and on the main forms of CKD, highlighting the needs for future research. The Italian Study Group on Kidney and Pregnancy has already covered the issue of pregnancy on dialysis [13]. A further position statement will treat pregnancy in kidney-transplanted patients.
Which CKD patient should be considered at “increased risk” for adverse pregnancy-related outcomes?
All CKD patients should be considered at increased risk for adverse pregnancy-related outcomes, and followed-up accordingly (strong suggestion; evidence form relatively large reports from few settings).
The risks of adverse pregnancy-related outcomes increase in incidence and severity along with the worsening of CKD. Follow-up should be intensified in patients with severe CKD (strong suggestion; evidence from several reports in different settings, CKD stages and/or in different diseases).
Proteinuria, hypertension, type 1 diabetes and immunologic diseases are associated with increased risk. Follow-up should be intensified (strong suggestion; evidence from several reports in different settings).
As already mentioned, the definition of kidney disease has changed over time and the current CKD definition, which includes all patients with signs of kidney disease regardless of the kidney function, dates from the beginning of the new millennium [14–20]. Consequently, most of the older studies regarded advanced CKD and few compared early and late CKD stages in the same settings [14–21, 30, 32, 33, 49–60].
The risks for adverse pregnancy-related outcomes increase from the early CKD stages [20, 21, 28–30, 32, 33, 38, 56–59, 62]. Large studies and meta-analyses demonstrate that the risks further increase along with the worsening of the kidney function, up to dialysis [11, 19, 20, 60–66].
The effect of CKD, even with normal renal function, is confirmed by the significant increase in adverse maternal–foetal outcomes, including preeclampsia (PE), pregnancy induced hypertension and pre-term delivery, in three large cohorts of patients who undertook pregnancy after kidney donation [57, 58, 67]. The reasons why women with single kidney (or kidney scars, glomerulonephritis in remission, etc.) with normal renal function, no proteinuria or hypertension should share a higher risk for adverse pregnancy-related outcomes are not elucidated [32, 38, 57, 58, 67].
In such a context, the working group suggests that all patients with CKD should be considered at increased risk for pregnancy-related adverse events and should be followed, whenever possible, by a multidisciplinary team including nephrology and obstetrics staff. Since the risks increase along with the worsening of the renal function, follow-up should be intensified in severe CKD (see further recommendations). For the management of dialysis patients, please refer to the specific position statement [13].
These conclusions are mostly based upon scattered evidence and underline the need for further studies on large cohorts of CKD patients. Such studies are highly encouraged by the study group.
What are the main adverse pregnancy-related outcomes in CKD patients?
The risks of maternal death are low, and hardly quantified; they are mainly described in active or flared immunologic diseases, above all in lupus erythematosus (strong suggestion; scattered evidence).
The risks of worsening of the kidney function, of developing hypertension and of developing or increasing proteinuria increase along with CKD stage (moderate suggestion; scattered evidence).
The foetal risks are mainly related to prematurity (strong suggestion; scattered evidence).
Perinatal death may be increased in specific diseases (SLE, immunologic systemic diseases, and diabetic nephropathy) (strong suggestion; scattered evidence).
Malformations are not increased in CKD patients, with the possible exception of diabetic nephropathy (strong suggestion, large body of evidence from different sources on non diabetic CKD; one paper only reporting on increase in malformations in diabetic nephropathy).
Risks for adverse materno-foetal outcomes may be increased in multiple pregnancies (moderate suggestion, one large series only).
Pregnancy-related outcomes in CKD may be related to the mother and to the foetus–child. The maternal risks are death, and progression of CKD, including also increase in proteinuria, impaired control or onset of hypertension, eventually persisting after delivery. The risks for the foetus–newborn are perinatal death, malformations, preterm-birth, intellectual deficiencies, diseases in adulthood [18–20].
Maternal death is reported as a very low or non quantifiable risk, at least in Western Countries, where maternal mortality is already low at baseline [18–20, 66]. According to most large series and systematic reviews, maternal deaths are almost exclusively reported in patients with systemic immunologic diseases, first of all SLE, also on account of the fact that SLE represents the most common systemic immunologic disease in pregnancy [18–20, 23, 68–70].
The risk for worsening of kidney function may increase along CKD stages; its entity has been differently calculated, from about 20 to over 80 %; however, a recent systematic review did not find a higher risk of kidney function reduction in CKD patients with versus without pregnancy in stages 1–3 [16–21, 25, 49–53, 60, 62, 71].
An increase in proteinuria is common; it is more frequent in patients already proteinuric at start of pregnancy and may be higher in diabetic nephropathy. However, the definition of a “relevant increase” in proteinuria is quite elusive, and the reported prevalence ranges from 20 to almost 100 % of the cases [25–29, 72–74].
Prematurity (defined as birth before 37 completed gestational weeks), early preterm delivery (before 34 completed gestational weeks) and extremely preterm delivery (before 28 gestational weeks) are increased in CKD pregnancies and, once more, the risk of preterm delivery, as well as the degree of prematurity, rises across CKD stages [18–21, 52–56].
Prematurity brings several short- and long-term risks for the babies: perinatal death, retinopathy, neurological problems (eventually linked to brain haemorrhage), and a possible long-term increase of hypertension, CKD and cardiovascular diseases [75–90]. The last phases of glomerular formation and maturation occur in late gestation; not surprisingly, therefore, a strong correlation has been described between prematurity, low birth weight, low nephron number, and development of kidney disease in adulthood [75–90].
Children from mothers affected by diabetes and systemic-immunologic diseases have a higher risk of perinatal death, also when born at-term or in the late preterm phase. This risk seems more related to the specific disease than to the degree of renal functional impairment [22, 23, 28, 69, 70]. There is no evidence that children from non diabetic CKD mothers have a higher risk of malformations, beside urinary tract malformations, including vescico-ureteral reflux, and related conditions, that are known to have a genetic background, albeit so far partially understood.
One recent study, on a large series, conversely suggests that the presence of nephropathy increases the risk for malformations in children of diabetic mothers. Several different malformations have been reported, with an odds ratio of 2–3 as compared with mothers without nephropathy [91].
According to one case series and a few case reports, multiple pregnancies may have higher risks of adverse pregnancy related outcomes [92–95]. This should be kept in mind in particular in the case of assisted reproduction techniques.
Very few studies have been specifically addressed at the health status of children from CKD mothers; they analysed different aspects of health, from kidney function to psychological well-being. Within the limits of the scattered evidence, it appears that most of the children from CKD mothers are able to attain normal developmental goals, at any level of kidney function impairment [11, 12, 96–98].
According to the Study Group, there is need for further studies, focusing on the clinical and psychological long-term outcome of children from CKD mothers.
Indications and goals for follow-up
The main goals of follow-up are early identification and treatment of complications, including hypertension, anaemia, coagulation disorders, and timely planning of delivery (strong recommendation, indirect evidence).
Follow-up should be intensified in CKD patients with respect to normal pregnancies (strong recommendation, indirect evidence).
Follow-up should be increased along with the increase in CKD stages, and according to the presence of other risk factors, such as hypertension, proteinuria and systemic disease (strong recommendation, indirect evidence).
Follow-up should include at least one nephrological visit with blood and urinary tests every 4–6 weeks in non proteinuric, non hypertensive stage 1 CKD pregnancies and should be increased to weekly in intensely proteinuric, hypertensive patients or in CKD stages 4–5 (strong recommendation, indirect evidence).
No validated formula for glomerular filtration rate (GFR) calculation in CKD pregnancy is available, hence GFR should be assessed by 24-h urine collection (strong recommendation, based on GFR in normal pregnancy and PE).
24-h protein excretion is preferable for quantification of proteinuria (strong recommendation, indirect evidence).
Twice monthly to weekly urinalysis and urinary cultures may be indicated in the prevention of urinary tract infection (UTI) recurrences and for early detection of proteinuria (strong recommendation, evidence limited to one RCT).
Pregnancy is not a disease nor a disease complication; however, it may be complicated by the presence of maternal diseases. Hence, there is no specific “treatment” for pregnancy in CKD, and conversely several therapies that are used in CKD outside of pregnancy are contraindicated in pregnancy (Tables 1, 2, 3, 4). Consequently, the main goals of follow-up are early identification and treatment of potential complications, including hypertension, proteinuria, anaemia, coagulation disorders, and flares of systemic diseases.
Table 1.
Main anti-hypertensive drugs in pregnant patients with CKD
| Drug | Main features | FDA | SOGC |
|---|---|---|---|
| Usually considered FIRST CHOICE drugs [141, 142, 340, 368] | |||
| Alpha-methyl dopa | Widely used in pregnancy, with no reported negative effects on the foetus or on its subsequent development. May not be able to correct severe hypertension in CKD | B | 1-A |
| Niphedipine | The long acting drug most commonly used in hypertension in pregnancy. The increase in peripheral oedema may be a relevant side effect in CKD patients | C | 1-A |
| Labetalole | Usually well tolerated, should be avoided in subjects with asthma. In a RCT it was shown to be comparable to alphamethyldopa [143, 149] | C | 1-A |
| Usually considered SECOND CHOICE drugs [141, 340] | |||
| Beta blockers | The main drawback in older studies was foetal growth restriction, possibly as an effect of overzealous correction [142]. Beta1 selective beta blockers (atenolole) are more often involved. Beta blockers may be more effective than alpha-methyldopa in severe hypertension, alone or in combined therapy. At delivery they may induce hypoglycaemia, hypotension and bradycardia (usually mild and transient) | D atenolole B pindolole C metoprolol |
1-B |
| Clonidine | The effect is similar to alpha-methyldopa; side effects may be more common and hypertensive rebounds at discontinuation are common; slowing foetal growth is occasionally reported [144] | C | |
| Alpha blockers | Other drugs should be preferred as controlled studies are missing | C | |
| Diuretics | They are usually avoided in pregnancy except when there are nephrological or cardiological indications. Thiazides may be continued in patients previously on treatment [145, 158]. In selected cases with Gitelman syndrome, amiloride may be employed | B hydrochloro-thiazide amiloride | |
| To be avoided [141, 340] | |||
| Short acting niphedipine | Contraindicated by the FDA, RCOG and AIPE due to the risk of severe sudden hypotension with detrimental effects on placental flows | D | |
| ACE-i ARB and related drugs | Both drugs are contraindicated in all phases of pregnancy because of the risk of several major malformations, including cardiovascular, central nervous system, renal and bone malformations [153–155] | C 1st D 2nd 3rd trimester |
II 2E |
FDA site of the Food and Drug Administration [340]; FDA rating: A, controlled human studies show no risk; B, no evidence of risk in studies; C, risk cannot be ruled out; D, positive evidence of risk; X, contraindicated in pregnancy; SOGC, Society of Obstetrics and Gynaecology of Canada: guidelines 2014 [102]
Table 2.
Main immunosuppressive drugs in pregnant CKD patients
| Drug | Main features | FDA |
|---|---|---|
| Usually considered as relatively safe, when absolutely needed [341–349] | ||
| Azathioprine | This is the most widely used immunosuppressive drug. It is teratogenic in animal models, but not in humans, possibly because the foetal liver is not able to activate the drug. K-DIGO and European Best Practice Guidelines suggest switching from Mycophenolate to Azathioprine before pregnancy [341–343] | D |
| Cyclosporine A | This Calcineurin inhibitor has not been associated with increased teratogenicity; however, small for gestational age babies and preterm delivery have been reported, possibly due to the maternal disease and not specifically to the drug; levels may vary in pregnancy and the hypertensive, hyperglycaemic and nephrotoxic effects should be mentioned [344] | C |
| Tacrolimus | The drug has similar effects and side effects as Cyclosporine A; since it is a relatively new drug, experience is more limited than with the previous drug [345] | C |
| Steroids | Together with azathioprine these are the most often employed and best known drugs. The most frequently used short-acting corticosteroids include prednisone, methylprednisolone and prednisolone, while betamethasone and dexamethasone are among the long-acting drugs. No major malformations have been reported, and the issue of labio-palatoschisis is debated. A higher risk of premature rupture of membranes has been reported. Other relevant side effects include infectious risk, and the increased risk of gestational diabetes [346] | C |
| Hydroxy-chloroquine | This synthetic anti-malaric agent crosses the placenta but was not found to be associated with foetal toxicity [217–219] | B |
| To be avoided [341 – 349] | ||
| Cyclo-phosphamide | This alkylating agent is contraindicated in pregnancy; a few reports suggest that pregnancy termination is common in the case of inadvertent use or need for life saving therapy. A few positive reports, mainly in women with SLE are also available [68] | D |
| Mycophenolate | Severe foetal malformations are reported, mainly involving cardiovascular and cranial malformations. Discontinuation for at least 6 months, to stabilize kidney function, is usually indicted after kidney transplantation [347, 348] | D |
| Rituximab | There are no data on whether rituximab can cause foetal harm. Rituximab was detected postnatally in the serum of infants exposed in utero: B-cell lymphocytopenia generally lasting less than 6 months can occur in infants. The manufacturer recommends contraception for up to 12 months following therapy [369, 370] | C |
| m-Tor inhibitors | Very few studies have considered their use in pregnancy. They are teratogenic in animals and discontinuation in humans is a matter of debate; KDIGO guidelines suggest discontinuation in anticipation of pregnancy [347, 349] | C |
FDA site of the Food and Drug Administration [340]; FDA rating: A, controlled human studies show no risk; B, no evidence of risk in studies; C, risk cannot be ruled out; D, positive evidence of risk; X, contraindicated in pregnancy
Table 3.
Main antibiotics for pregnant women with urinary tract infections
| Drug | Characteristics | FDA |
|---|---|---|
| Usually considered as safe, when needed [278 – 280, 304, 340, 350 – 354] | ||
| Semi-synthetic penicillin | Ampicillin and Amoxicillin are the first-choice antibiotics | B |
| Clavulanic acid | Bacterial beta-lactamase inhibitor, used in combination with Amoxicillin. The association with beta-lactamase inhibitors is indicated when therapy with only Penicillins and Cephalosporins is not effective | |
| 1st and 2nd generation Cephalosporins | In general, the data available on the use of Cephalosporins, in particular of first and second generation, during pregnancy, does not indicate an increase, over the expectation, of congenital abnormalities on exposed new-borns | |
| 3rd generation Cephalosporins | Indicated for acute pyelonephritis when parenteral administration is necessary. Cefepime and Ceftriaxone: animal studies do not show teratogenic effects. Ceftriaxone should be avoided during the days before delivery because of the possibility of kernicterus (it competes with bilirubin for the binding with albumin) | B |
| Carbapenems | Meropenem should be the first choice in cases of notable severity, according to sensitivity. Animal studies, in fact, showed adverse effects on the foetus with Imipenem-cilastatin | B |
| Aztreonam | Valid alternative in the case of allergy to beta-lactams when parenteral administration is necessary | B |
| Macrolides | Erythromycin represents a valid alternative in the case of allergy to beta-lactams. Clarithromycin and Azithromycin are a second choice but they could be used according to clinical conditions [302] | B |
| Phosphomycin | Indicated for uncomplicated urinary tract-infections [297, 298, 303] | B |
| Nitrofurantoin | Contraindicated in G6PDH-deficient women. Their use during the first trimester should be limited to those situations in which no alternative therapies are available. Contraindicated at the end of the pregnancy (38th–40th week) and during delivery because of the risk of haemolytic anaemia in the new-born [26, 305] | B |
| To be avoided | ||
| Aminoglycosides | They have been associated with ototoxicity. Their use must be avoided | D |
| Fluoroquinolones | Preclinical animal studies demonstrated abnormalities in the development of cartilages. Ciprofloxacin is not a first-choice drug during pregnancy; its administration should be limited to those cases in which the benefits are greater than the risk connected to the therapy [307] | C |
| Tetracycline | Their use must be avoided | D |
| Sulphonamides | Trimethoprim sulfamethoxazole must be avoided during the first trimester (it is a folic acid—antagonist) and at the end of the pregnancy for the risk of kernicterus | D |
FDA Classification [340]: A, controlled human studies show no risk; B, no evidence of risk in studies; C, risk cannot be ruled out; D, positive evidence of risk; X, contraindicated in pregnancy
Table 4.
Other drugs for pregnant CKD women
| Drug | Characteristics | FDA |
|---|---|---|
| Usually considered as safe, when needed | ||
| Acetyl salicylate | Low doses during pregnancy needed for the treatment of diverse medical conditions have not been shown to cause foetal harm; may be protective against pre-eclampsia, favoring placentation (see text); discontinuation before delivery is recommended [161–163, 223, 224] | NC |
| LMWH | Low molecular-weight heparin (LMWH) does not cross the placenta and is safe for the foetus, although bleeding at the utero-placental junction cannot be rued out. Individualized doses of LMWH are well tolerated and safe for prophylaxis and treatment of thromboembolic complications during pregnancy, and post-partum [355]. Twice-daily heparin should be discontinued prior to induction of labour or planned cesarean delivery and can be resumed after delivery [355, 356] | C |
| ESAs | In vitro studies suggest that recombinant erythropoietin does not cross the human placenta; higher doses may be needed in dialysis patients [357, 358] | C |
| Allopurinol | Adverse events were observed in animal studies. Allopurinol crosses the placenta [359]. An increased risk of malformations events has not been observed in humans (limited data) [360] | C |
| Vitamin D | The role for vitamin D supplementation in pregnancy is controversial, but there is no evidence of a reduction in adverse pregnancy outcomes (e.g., preeclampsia, stillbirth, neonatal death) [361] or improvement in bone mineral content in children in studies in which supplementation was given independently from blood levels [362]. Cholecalciferol (vitamin D3) crosses the placenta but the transfer to the foetus from the mother is low. Maternal supplementation has not been shown to affect pregnancy outcomes [363]. Ergocalciferol Adverse events were observed in animal studies. The ergocalciferol (vitamin D2) metabolite, 25(OH) D, crosses the placenta; maternal serum concentrations correlate with foetal concentrations at birth. Calcitriol Teratogenic effects have been observed in animal studies. Adverse effects on foetal development were not observed in women (N = 9) with pseudovitamin D-dependent rickets. Paricalcitol Adverse events were observed in some animal reproduction studies Vitamin D deficiency in a pregnant woman may lead to deficiency in the neonate [364, 365]. Serum 25(OH) D concentrations should be measured in pregnant women at increased risk of deficiency [366]. Current guidelines recommend an intake of 1000–2000 units/day until more safety data is available [366, 367]. Vitamin D and calcium levels should be monitored and kept in the lower normal range |
C |
FDA Classification [340]: A, controlled human studies show no risk; B, no evidence of risk in studies; C, risk cannot be ruled out; D, positive evidence of risk; X, contraindicated in pregnancy; NC, not classified
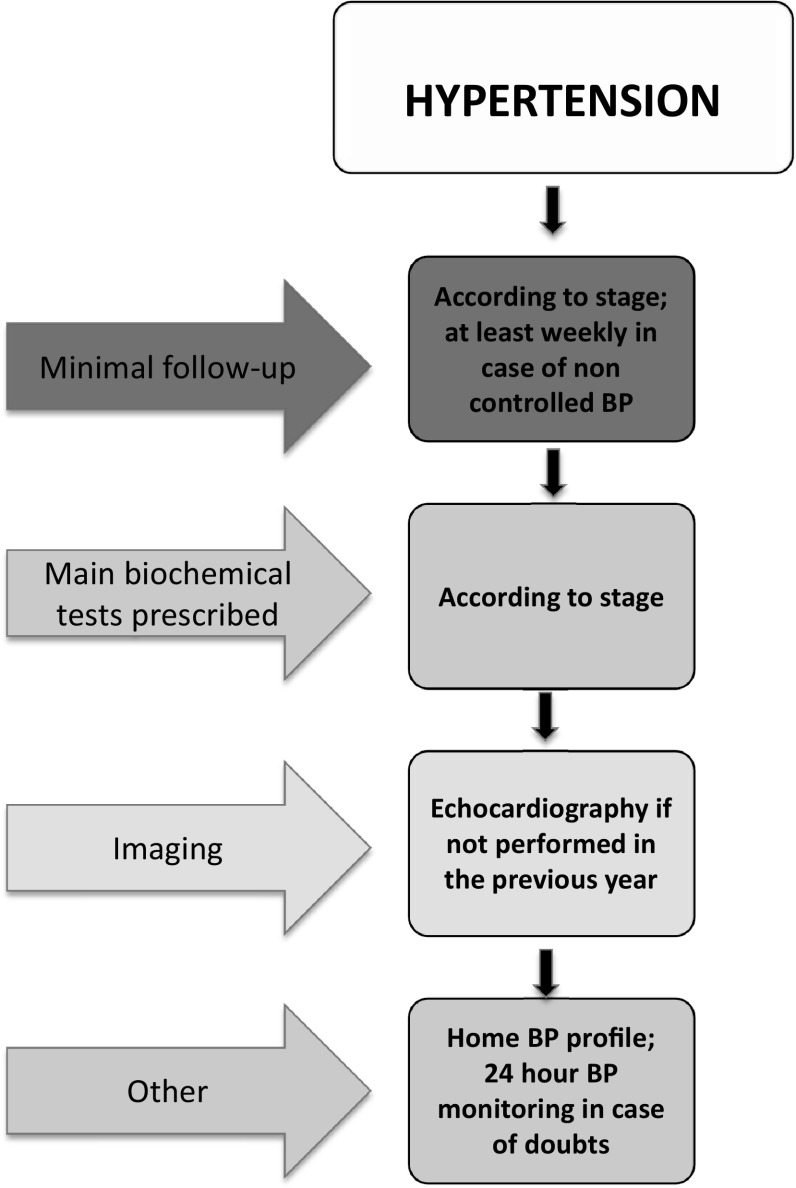
In the absence of RCTs, the Study Group suggests to intensify follow-up in CKD patients as compared with normal pregnancies, increasing the frequency of visits along with the increase in CKD stages, and according to the presence of hypertension, proteinuria and systemic diseases [21, 32, 52]. The flow charts highlight the proposed minimal frequency of controls (Figs. 1, 2, 3).
Fig. 1.
Minimum follow-up for pregnant CKD patients
Fig. 2.
Minimum follow-up for pregnant hypertensive CKD patients
Fig. 3.
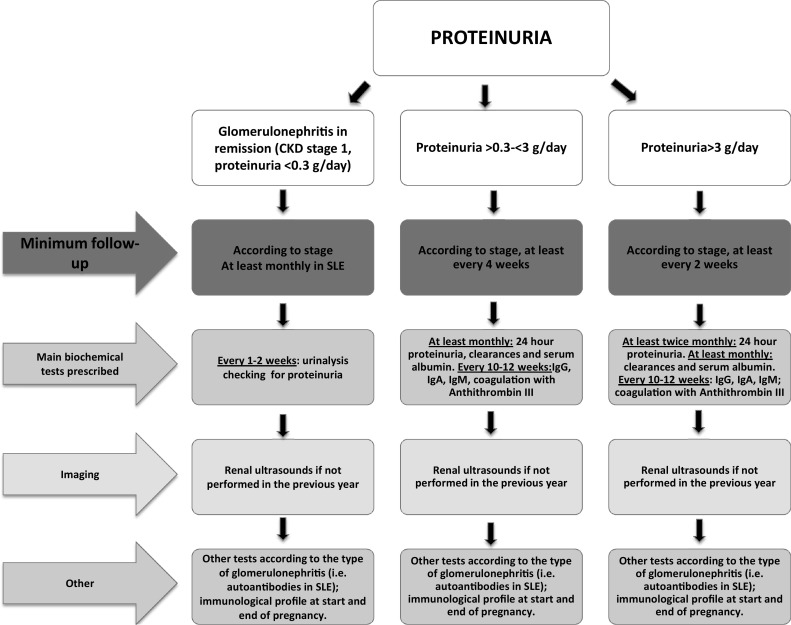
Minimum follow-up for pregnant proteinuric CKD patients
The assessment of kidney function in pregnancy is challenging, due to hyperfiltration, and increase in plasma volume and in volume distribution. To date, no formula is validated for GFR calculation in CKD pregnancy, and it is preferable to assess creatinine clearance on 24-h urine collection, at least in patients trained to follow the indications. This also allows a precise assessment of proteinuria [33–38].
The Study Group suggests a minimum requirement of one nephrology visit with blood and urinary tests every 4–6 weeks in non proteinuric, non hypertensive stage 1 CKD pregnancies, increased up to weekly in patients with various combinations of proteinuria, hypertension or CKD stages 4–5. Patients at risk of recurrent UTIs, of immunologic flares or with glomerulonephritis in remission should control urinalysis for proteinuria and/or urinary cultures every 1–2 weeks, to promptly identify UTIs and/or proteinuria (Figs. 1, 2, 3).
Differential diagnosis between CKD and preeclampsia
The differential diagnosis between PE and CKD, and the definition of superimposed PE on CKD may be impossible on clinical grounds (strong recommendation, limited evidence).
PE of placental origin is usually associated with impaired utero-placental Doppler flows (strong recommendation, scattered evidence).
PE ad CKD may be differentiated by means of the angiogenic-antiangiogenic patterns (soluble Fms-like tyrosine kinase 1 [s-Flt-1], placental growth factor [PIGF]) (medium recommendation, scattered evidence).
Kidney biopsy is not recommended in pregnancy, either for the definition of the kidney disease or for the differential diagnosis between PE and CKD, because of risk of severe complications (strong recommendation, systematic review of scattered evidence).
The broad definition of PE includes both mildly affected subjects, whose situation is compatible with term delivery of “appropriate for gestational age” babies, as well as patients affected by stormy, devastating and even deadly disease, with “small for gestational age” (SGA) babies and risk of long-term complications [99–106]. Several attempts have been made to sub-classify PE, according to severity, gestational age, initiating events and pathophysiological origin; furthermore, PE may be included in a continuum of diseases, from pregnancy-induced hypertension to HELLP syndrome (named for its three features: haemolysis, elevated liver enzymes, and low platelet levels) [107–112].
The classic definition of PE states that it is a reversible condition characterized by the association of hypertension and proteinuria (above 300 mg/day), occurring after the 20th week of pregnancy in previously normotensive, non proteinuric pregnant women [101]. Proteinuria and hypertension should disappear within 1–3 months after delivery. Some authors, however, recently suggested following PE patients for at least 6 months after delivery, to “change public (and clinician) thinking that pregnancy lasts, instead of 9 months, at least 15 months”. This idea is a great way of getting us all to consider the importance of post-partum care particularly for women with PE. In this way we can detect ongoing hypertension or renal issues [102]. The relationship with kidney diseases has been underlined by several authors, and is crucial for the differential diagnosis of PE [113–116].
A paradigm shift occurred when the American College of Obstetricians and Gynaecologists (ACOG) guidelines stated that PE may be diagnosed in the absence of proteinuria, basing diagnosis on new-onset hypertension accompanied by at least one of the following: serum creatinine increase, low platelet count or high liver enzymes, pulmonary edema or central nervous symptoms, once more underlining the central importance of kidney impairment in this context [117]. Independently from the definition, there are at least three conditions in which the differential diagnosis between PE and CKD is almost impossible: when no data before 20 gestational weeks is available; when CKD flares in pregnancy; when kidney disease develops in pregnancy [116, 118]. According to the widespread pathophysiological view, a placentation defect is at the basis of PE; thus, utero-placental flow analysis may help distinguish PE (impaired flows, and stunted growth) from CKD (normal blood flows and growth) [118–122]. Several clinical and biochemical biomarkers of PE have been suggested and could support the differential diagnosis between PE and CKD [123–131]. The ratio between sFlt-1, a receptor for both vascular endothelial growth factor (VEGF), and PIGF is considered as one of the most promising predictors of PE that may support the differential diagnosis between PE and CKD which, at least in the early stages, is characterized by a normal placentation [116, 118, 132–139].
Kidney biopsy is not recommended in pregnancy, according to a systematic review that highlights risks of severe bleeding complications. However, in early pregnancy, the pros and cons of diagnosis versus empiric therapy should be individually assessed, in particular in cases with progressive kidney function impairment. In late pregnancy, the advantages of an invasive manoeuver versus pre-term delivery should be considered [140]. A kidney biopsy should be performed in patients with persisting proteinuria >3–6 months after delivery (with the timing depending also on other renal parameters) without an established diagnosis of kidney disease. These patients are unfortunately often lost to follow-up, thus limiting the potential advantages of pregnancy as a first occasion for the diagnosis of potentially reversible CKD [32].
The Study Group therefore supports the need for systematic assessment of kidney function following PE, as well as in future pregnancies after PE or HELLP syndrome. While acknowledging the interest in serum biomarkers for the differential diagnosis between PE and CKD, the Study Group underlines that it is based on the results of few studies, mostly from the same groups, and envisages the organization of large multicentre studies on this issue.
Specific issues: hypertension as a complication of CKD and pregnancy
Arterial hypertension is a common complication of pregnancy in CKD, and should be early identified and treated (strong recommendation, indirect evidence from large observational studies).
“Overtreatment” should be avoided, due to the risk of reducing utero-placental flows, and inducing intrauterine growth restriction (strong recommendation, evidence from large observational studies).
Treatment should follow a stepwise approach, starting from the drugs with less contraindications in pregnancy (strong recommendation, based upon systematic reviews and guidelines).
When used for the antiproteinuric and nephron-protective effects, angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) should be discontinued at the first assessment of a positive pregnancy test (strong recommendation for discontinuation, contrasting evidence for the timing).
Target blood pressure values are periodically reviewed in the hypertensive disorders of pregnancy, a setting in which a shared recommendation is to avoid overcorrection of hypertension, for the risk of affecting foetal growth [141–148].
Conversely, blood pressure targets in hypertensive CKD pregnancies are less established. It is presumably wise to avoid overcorrection, but it should also be remembered that hyperfiltration and proteinuria, that are widely described in hypertensive CKD patients, may have a detrimental effect on the kidney function [21].
Considering the recent Control of Hypertension In Pregnancy Study (CHIPS) trial, that revealed a substantial equivalence between “thigh and standard” blood pressure control in pregnancy, our group suggests implementing a strict blood pressure control (‘ideal’ target <130/80 mmHg, acceptable <140/90 mmHg), under careful clinical surveillance, at least in patients with good compliance that control blood pressure at home [149–152].
The main list of anti-hypertensive drugs is reported in Table 1. None is considered as fully safe. The issue of teratogenicity of ACEi and ARBs is still a matter of debate. The Study Group strongly supports early discontinuation of ACEi and ARBs at the first positive pregnancy test (4th–6th gestational week) in proteinuric CKD patients, to maximize the benefits of control of proteinuria, at least in compliant patients [153–158].
Specific issues: proteinuria and low-protein diets
Low-dose acetylsalicylate is indicated in proteinuric patients (as well as in patients with advanced CKD, SLE and immunoglobulin A nephropathy [IgAN]) (strong recommendation, different levels of evidence in the various diseases).
Albumin infusion is not indicated in nephrotic syndrome in pregnancy, since it may increase hyperfiltration and result in a further protein loss (medium recommendation, scattered evidence).
A moderate protein restriction may be of help in counterbalancing the hyperfiltration of pregnancy and may be safely employed in proteinuric patients and in patients with advanced CKD (medium recommendation, evidence from one group only).
The use of low-dose acetylsalicylate for the prevention of PE is suggested by a large body of evidence, in the cases at risk for PE, a category encompassing CKD patients (Table 4) [159, 160]. The timing is controversial. Most guidelines suggest starting therapy after the first trimester, because of the risk of haemorrhage in the case of early foetal loss. From a pathophysiological point of view, however, the effects on placentation should be maximal when aspirin is started early; therefore other authors suggest that an earlier start may be effective in preventing severe and early PE [161–163].
In the absence of clear evidence upon the best timing, the Study Group suggests to identify in each setting a shared policy between Obstetricians and Nephrologists, taking also into account that our patients are more closely followed than the general population, thus potentially minimizing the risks of unexpected haemorrhage.
No study clearly identifies any benefit of albumin infusion in CKD and in PE, even if this empirical therapy is still diffused, with the aim of increasing albumin levels and reducing the risk of placental hypoperfusion. Therefore, the Study Group does not support the systematic use of albumin infusion in pregnant patients with nephrotic syndrome or PE, considering the potential risk of adverse reactions, and the risk of paradoxically increasing proteinuria by enhancing hyperfiltration [164–167].
In the absence of specific anti-proteinuric therapy in pregnancy, two non-randomised Italian reports suggest that a moderate protein restriction (0.6–0.8 g/kg/day), with a vegan-vegetarian diet supplemented with aminoacids and ketoacids, is feasible and safe in pregnant patients with CKD. Interestingly, the prevalence of small for gestational age babies was lower in on-diet patients as compared to patients on an unrestricted diet [168, 169]. The data obtained in a relatively small group of patients are in line with reports on the advantages of vegetal proteins in reducing kidney disease progression in non-pregnant humans, with a systematic review on vegan-vegetarian diets in pregnancy, and with the position of the American Dietary Association, provided that B12, iron and vitamin D are controlled and supplied upon need [170–178].
The Study Group suggests considering the diet option, at least in cases in which hyperfiltration (absolute or relative to the remnant nephrons) is supposed to play an important role, and strongly suggests organising multicentre studies on this issue.
Specific diseases: glomerulonephritis (primary)
The appearance of proteinuria during the third trimester of pregnancy calls for a differential diagnosis between glomerular disease and PE (strong recommendation, scattered evidence).
During pregnancy, glomerulonephritis may appear for the first time or be a relapse or worsening of a previously identified disease: the differential diagnosis is important for management (strong recommendation, evidence from large studies).
Proteinuria and nephrotic syndrome in pregnancy may suggest membranous glomerulonephropathy (MGN), minimal change nephropathy (MCN), or focal segmental glomerulosclerosis (FSGS) (strong recommendation, evidence from observational studies).
In patients with IgAN, the most common glomerulonephritis in the Mediterranean countries, pregnancy is not associated with a faster disease progression. Risk of disease progression after delivery is higher with GFR <70 ml/min/1.73 m2, poorly controlled hypertension, and proteinuria (strong recommendation, evidence from observational studies).
Immunosuppressive therapy for primary glomerulonephritis needs to be re-evaluated in patients who wish to start pregnancy.
Many young women discover haematuria and proteinuria on the occasion of their first urinalysis during pregnancy and these urinary changes are often considered as pregnancy-related missing a diagnosis of underlying glomerular disease.
Microscopic haematuria is very common during pregnancy, detected in about 20 % of women, and disappears in 75 % after delivery [179]. Postpartum follow-up is recommended in women with persistent haematuria and presumed underlying glomerulonephritis, after excluding the presence of infection [180].
The development of proteinuria during pregnancy is often associated with PE. However, as previously discussed, isolated proteinuria or proteinuria with haematuria may reflect the presence of a renal disease. The most common forms of primary glomerulonephritis, in childbearing age, are IgAN, FSGS, MCN, MGN, and membrano-proliferative glomerulonephritis (MPGN); all may present with proteinuria and/or haematuria, occasionally with a rapidly progressive course.
The indication for renal biopsy is limited by the increase in bleeding risk; however, renal biopsy may be considered, in particular in early pregnancy, in the presence of rapidly progressive kidney function impairment or severe nephrotic syndrome [140].
IgA nephropathy
IgAN is the most prevalent primary glomerulonephritis worldwide, most often diagnosed in childbearing age. Proteinuria and/or hypertension in pregnancy are reported in up to 55 % of cases, prematurity in 30 % [181]. Several reports, including an Italian multicentre study on more than 200 pregnancies, suggest that pregnancy does not increase the progression of IgAN, at least in the earlier CKD stages. Favourable prognostic factors are normal blood pressure and GFR equal to or above 70 ml/min before conception [60, 181–186]. Proteinuria at conception was associated with renal function impairment after delivery, stressing the role for its reduction before pregnancy, thus supporting discontinuation of ACEi and ARBs at the first positive pregnancy test (see previous paragraph). In the case of rapid progression, the differential diagnosis may consider both severe PE and a superimposed kidney disease (such as haemolytic uremic syndrome) [187].
Other forms of primary glomerulonephritis
The incidence of primary nephrotic syndrome is approximately 3–5 cases per 100,000 inhabitants per year in children and adults. Albeit rare, all the main causes of primary nephrotic syndrome may present in pregnancy.
Some authors presently classify MCN and FSGS as ‘podocytopathies’ due to the characteristic podocyte damage or dysfunction [188]. Interestingly, podocyte impairment has been described in PE [126]. This may at least partly account for the reported, albeit debated, development of FSGS after PE [188–190]. FSGS associated with PE is characterized by an increase in glomerular size, as is observed in other forms of secondary FSGS such as obesity, in which hyperfiltration plays a central role [191].
While steroids are the first choice therapy in primary FSGS, prolonged treatment at high doses may have a detrimental effect on foetal growth [192]. Since the placental enzyme 11b-hydroxysteroid dehydrogenase 2 (11b-HSD2) protects the foetus reducing the transplacental passage of prednisolone, this drug may be preferred to betamethasone or dexamethasone [193, 194]. In the case of steroid resistance, calcineurin inhibitors may be added (Table 2).
Idiopathic membranous nephropathy (MN) often presents with a nephrotic syndrome; since, in over 70 % of patients with primary disease, autoantibodies against phospholipase A2 receptor 1 (PLA2R) are detectable by commercial kits, diagnosis may be performed without a kidney biopsy, which may, nevertheless, be required after pregnancy for staging [195]. A second target, thrombospondin type-1 domain-containing 7A (THSD7A) has been identified in a cohort of PLA2R-negative patients with MN, but is not yet clinically available [196]. In a study published in 1987, encompassing 33 pregnancies in 24 patients with MN, an increased foetal loss was observed, together with a risk of worsening of renal function [197]. This grim prognosis was not confirmed in a more recent cohort of nine MN patients with 51 pregnancies [198]. While steroids and calcineurin inhibitors are the milestones of treatment in pregnancy, addition of aspirin or low molecular-weight heparin should be considered, according to the clinical conditions.
Few studies and isolated case reports regard pregnancy in patients with primary MPGN, a multifaceted disease presently undergoing reclassification [199]. In a relatively old study on 123 pregnancies in 86 patients with biopsy-proven various glomerular diseases, a higher incidence of complications in MPGN patients versus MGN was observed [200]. However, the outcome was favourable in recent reports, including a case in which the nephrological decision to start steroid therapy instead of pregnancy termination, allowed to safely reach the end of gestation [201–203].
In conclusion, the Study Group calls for attention to the differential diagnosis of glomerulonephritis in pregnancy, and underlines the importance of pregnancy as the first occasion for diagnosis in apparently healthy women. The limits of the current evidence and the need for further studies targeted to the different nephropathies has to be further underlined.
Specific diseases: lupus nephritis as a prototype of systemic immunologic diseases
Before pregnancy, women with SLE should be accurately informed about the risks of pregnancies during active disease (strong recommendation, indirect evidence).
Severe pulmonary hypertension, severe restrictive lung disease, severe heart failure represent absolute contraindications to pregnancy (strong recommendation, scattered evidence).
Severe kidney function impairment (serum creatinine [sCr] >2.5 mg/dl, CKD stage 3–5) represents a relative contraindication to pregnancy (medium recommendation, scattered evidence).
Patients should be in remission for at least 6 months to reduce the risks of SLE flares in pregnancy (strong recommendation, scattered evidence).
Cyclophosphamide, mycophenolate and leflunomide have potential teratogenicity, and patients should be switched early to non teratogenic drugs (strong recommendation, scattered evidence).
Pregnant SLE women should be followed by a multidisciplinary team, in a tertiary care hospital (strong recommendation, indirect evidence).
Changes in immunosuppressive treatment in pregnancy should be limited to SLE flares; choice of treatment should be individualised (strong recommendation, indirect evidence).
Prednisone is considered safe. Intravenous immunoglobulin therapy is an effective and safe treatment for SLE pregnant patients with recurrent spontaneous abortions, especially if associated with antiphospholipid antibodies (strong recommendation, evidence from large series).
Low molecular-weight heparin with or without low-dose aspirin should be given to patients with SLE and antiphospholipid antibodies at high titres or triple positivity (strong recommendation, evidence from large series and RCTs).
An increase in glucocorticoid doses at time of delivery to prevent postpartum flares is a matter of debate (limited evidence).
Follow-up should be intensified postpartum since SLE flares may be more frequent in the first 6–12 months. Follow-up after PE should consider long-term cardiovascular complications (strong recommendation, evidence from large series).
Breastfeeding is allowed if prednisone does not exceed 20 mg/day. Azathioprine and cyclosporine are found in breast milk and lactation is not recommended (strong recommendation, evidence from large series).
SLE is a chronic autoimmune disease, primarily affecting young females with no influence on fertility. Pregnancy in women with SLE, and in those with lupus nephritis in particular, remains a high-risk situation for maternal and foetal complications [204–207]. The paucity of prospective studies on the subject makes evidence-based recommendations difficult; however, a number of retrospective cohort studies suggest that women with stable and prolonged remission of SLE, normal renal function, normal blood pressure and negative antiphospholipid antibodies have a high probability to have positive foetal and maternal outcomes [69, 204–212].
Since pregnancy-related risks are exceedingly high in SLE flares, effective contraceptive counselling is of paramount importance. Two large RCTs demonstrated the safety of oral contraceptives in SLE patients with inactive or mild active disease [213, 214]. However, patients with severe active disease or with antiphospholipid antibodies should not be treated with these drugs.
An extensive clinical, biochemical and immunological evaluation, in particular regarding the extractable nuclear antigen (ENA) profile and presence of antiphospholipid antibodies is necessary before pregnancy, together with a careful re-evaluation of the ongoing treatments [207, 209–211].
Cyclophosphamide should be withdrawn at least 3 months before pregnancy and mycophenolate shifted to azathioprine, as the European Medicine Agency (EMA) recommended in its 23/10/2015 warning. The shift from mycophenolate to azathioprine should be done at least 6 weeks before pregnancy in order to minimize the risk of SLE flares due to this change of therapy [215, 216]. Patients should start the pregnancy with the lowest possible dosage of the allowed drugs. Small birth weight and prematurity are associated with high-dose glucocorticoids, azathioprine and calcineurin inhibitors. Hydroxychloroquine reduces the rate of prematurity, intrauterine growth restriction and the risk of recurrent anti-SSA/Ro-antibodies associated cardiac manifestations in SLE pregnancy, and for these reasons should not be discontinued before pregnancy [217–219]. High-dose immunoglobulins are reported as safe and effective in SLE flares. Intravenous immunoglobulin therapy is an effective and safe treatment for SLE pregnant patients with recurrent spontaneous abortions, especially if associated with antiphospholipid antibodies [220–222].
Whether preeclampsia can be prevented by low dose aspirin is still a matter of debate, but current evidence supports its use in high risk populations [223, 224]. Low molecular-weight heparin with or without low-dose aspirin should be added to patients with SLE and antiphospholipid antibodies at high titres or triple positivity [225, 226].
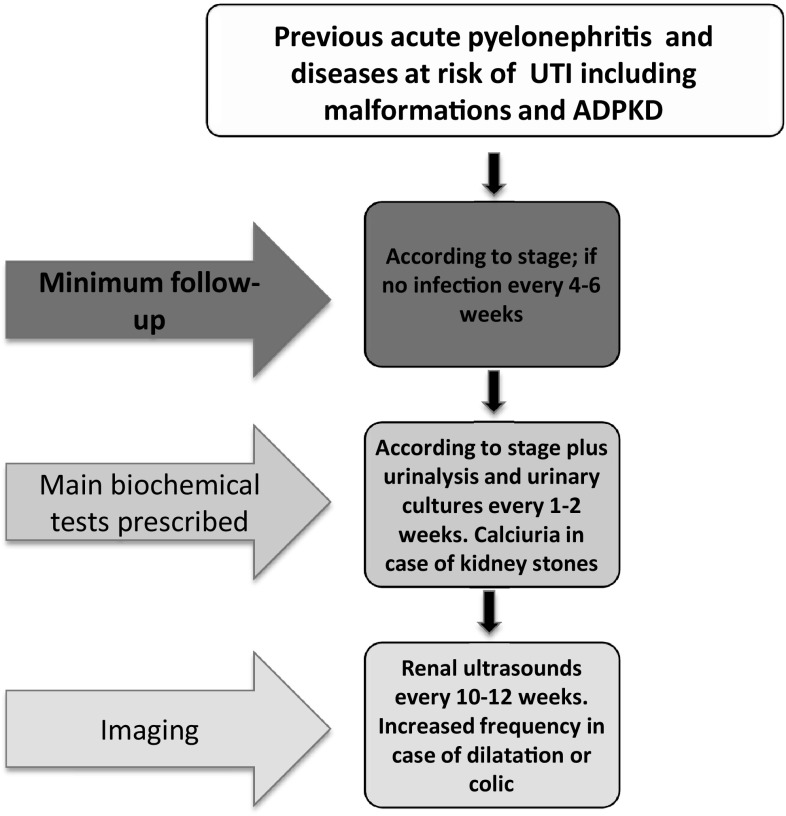
In pregnancy, a strict multidisciplinary follow-up is indicated (Figs. 1, 2, 3, 4).
Fig. 4.
Minimum follow-up for pregnant with previous pyelonephritis
Maternal complications are relatively common: reactivation of lupus nephritis during pregnancy is reported to range between 15 to 30 % in studies published in the last two decades. Severe flares with impairment of renal function are infrequent, accounting for from none to 12 % of all cases [204–212]. The risk of PE is 3–5 times higher in SLE patients than in healthy women [211–227]. Substantial epidemiological data reveal that a history of PE increases the long-term cardiovascular risk by two to four times [228]. A recent meta-analysis on 1000 pregnancies in patients with lupus nephritis shows that arterial hypertension developed in 16.3 % of pregnancies [69]. Pregnancy-related hypertension predisposes to chronic hypertension, premature heart attacks, strokes, and renal complications [228].
Previous lupus nephritis and active lupus at conception are the most important predictors of all maternal complications [212–229]. The presence of antiphospholipid antibodies at high titres predisposes to arterial hypertension, PE, and to thrombotic microangiopathy during pregnancy. As already mentioned, renal function impairment and arterial hypertension at conception are predictors of poor maternal outcome whatever the underlying renal disease [230].
Foetal loss includes spontaneous abortion, stillbirth and neonatal death, and, based on a systematic review of 37 studies of lupus nephritis patients, they are estimated to occur, respectively, in around 16, 3.6, and 2.5 % of pregnancies [69]. Furthermore, based on a recent literature review, the rate of foetal loss in SLE pregnancies significantly decreased from a mean of 43 % in 1960–1965 to 17 % in 2000–2003 but continued to be higher than in the general population [231].
With the progressive reduction of foetal losses, the percentage of pregnancies that ends in premature birth has progressively increased, probably as a result of the immunological and vascular abnormalities associated with SLE. In lupus nephritis, approximately one-third of children are delivered preterm [205–210].
Neonatal lupus and congenital heart block are very rare complications, reported in less than 2 % of pregnancies, and are associated with positive SSA or SSB antibodies [232].
Several predictors of foetal outcome have been identified: high-activity of SLE in the first and second trimesters led to a threefold increase in pregnancy loss [233]. Active lupus nephritis at conception, and hypertension emerged as predictive factors for adverse foetal outcome in our as well as other authors’ experience [206, 227, 234].
As already mentioned, the presence of GFR less than 40 ml/min/1.73 m2 and of proteinuria greater than 1 g/d before conception are strong predictors of poor foetal outcomes whatever the underlying renal disease [52–56]. A relatively old review of 10 studies of 554 women with SLE found that foetal loss ranged from 39 to 59 % in patients with antiphospholipid antibodies versus 18 % in those with negative antiphospholipid antibodies [235].
Overall, as underlined above, history of nephritis, active nephritis or active SLE at conception are the strongest predictors of adverse foetal and maternal outcome [236, 237]. For these reasons, a prerequisite for the success of pregnancy in patients with lupus nephritis is the planning of the pregnancy during periods of disease quiescence.
The study group therefore recommends to pay particular attention to pre-pregnancy counselling and to a strict multidisciplinary follow-up, in specialised Centres, in pregnancy in patients with SLE nephropathy.
Specific diseases: vasculitides
Pregnancies in woman affected by vasculitides are rare. The presence of active disease or of disease diagnosed in pregnancy is associated with a higher risk of adverse pregnancy-related outcomes (strong recommendation, scattered evidence).
A strict, multidisciplinary follow-up should be planned in tertiary care Centres to identify the best, personalised therapeutic strategies and the best balance between pregnancy termination, risks of early delivery and maternal risks (strong recommendation, indirect evidence).
Pregnancies are very rare in women affected by systemic vasculitides. The main reason is that, except for Takayasu disease, the peak age is typically over 40 years; the multi-organ involvement usually discourages the few patients in childbearing age from planning a pregnancy. Furthermore, the most frequently used immunosuppressive drugs, above all cyclophosphamide, contraindicate pregnancy and may induce premature menopause and sterility. However, with earlier diagnosis and new treatments, pregnancies in these patients will probably increase.
Because of the rarity of pregnancy, data on clinical management and maternal and foetal outcomes are based on few series, from experienced referral centres, and on case reports. Within these limits, some important points for risk assessment may be identified [238–245].
With regards to disease activity at conception, three situations are possible: pregnancies electively planned in remission and conducted under a strict multidisciplinary surveillance; unplanned pregnancies in patients on treatment; de novo or relapsing systemic vasculitis during pregnancy.
Pregnancy in patients affected by vasculitis in remission, undergoing planned multidisciplinary follow-up are reported to have good maternal and foetal outcomes, provided treatment is strictly monitored, to avoid relapses. Most published cases fall into this category (usually milder and often localized disease). However, some authors outline specific risks, mainly in the last weeks of pregnancy, including cardiac failure, asthma or decompensating subglottic stenosis.
The materno-foetal risks are higher in patients who get pregnant in a phase of active disease, who relapse or who develop de novo vasculitis during pregnancy. In this setting, ominous maternal and foetal complications have been reported. Among the life-threatening complications, pulmonary haemorrhage, rapidly progressive glomerulonephritis, polyneuritis and myocarditis have been described.
In the setting of kidney involvement and hypertension, the differential diagnosis between vasculitis and PE may be difficult; whatever the cause, hypertension and renal impairment may persist after delivery.
Therapeutic strategies need to be individualised. Patients should be aggressively treated in the case of flares or of active disease. Pregnancy termination or induction of delivery should be considered in these cases. Low-dose acetylsalicylic acid may be indicated for PE prevention. High-dose intravenous (iv) steroids, plasma-exchange, high-dose immunoglobulin, and azathioprine may allow continuation of pregnancy in an attempt to find a balance between maternal and foetal risks [238–245]. For further therapeutic issues, please refer to Tables 1, 2, 3 and 4.
Acknowledging the scarce experience on this issue, the Study Group strongly recommends that patients with systemic vasculitis should be referred to tertiary care Centres to undergo a strict, personalised, multidisciplinary follow-up.
Specific diseases: diabetic nephropathy
Diabetic nephropathy is associated with a higher risk of perinatal death as compared with other primary causes of CKD (evidence from several studies on diabetes, no specific comparison with other causes of CKD).
There may be a higher incidence of malformations in diabetic nephropathy as compared to diabetes per se, in which an in increase in malformation has been reported (important recommendation, one large study only reporting higher risk of malformations in diabetic nephropathy).
Proteinuria may steeply rise in pregnant women with diabetic nephropathy, making the differential diagnosis with PE very difficult (strong recommendation, scattered data).
Prenatal counselling is fundamental to reduce the risks linked to poor glycaemic control and to optimize multidisciplinary follow-up (malformations, prematurity, large for gestational age babies).
The evidence regarding diabetic nephropathy in pregnancy is limited. In a recent systematic review, out of thirty-four papers retrieved, only two reported on more than 100 patients, while most studies described less than 50 cases [28]. When diabetic nephropathy is broadly defined as presence of any sign of renal disease including microalbuminuria, its prevalence ranges from 5 to over 25 % in type 1 diabetic pregnant women.
During pregnancy, diabetes per se has been associated to a variety of complications, including malformations, foetal growth impairment, stillbirth, early mortality and preterm delivery while the incidence of PE has been reported as significantly increased, even if this is always within the limits of the non-univocal definition of this term [28–30, 246–250].
Maternal outcomes are widely scattered and probably reflect several factors: the lack of univocal definitions of diabetic nephropathy, CDK and PE, increasing maternal age over time, different study aims, patients selection and setting of several studies, small sample size and single-centre and care settings.
While the high risk of prematurity, small for gestational age babies, or intrauterine growth restriction is shared also by other kidney disorders and increases along with the progression of kidney disease, stillbirth or foetal death are shared only by SLE, sharing with diabetes the important microvascular damage [62, 242]. The risk is present also in diabetic patients without overt nephropathy, but appears to be increased in the setting of diabetic nephropathy [28–30]. Interestingly, even if in the last period, the risks of stillbirth and foetal death are reduced with respect to the ‘80s (from over 10 to about 5 %), the reduction is much lesser than that observed in the case, for example, of mothers on dialysis, which has decreased by 25 % each decade since the ‘80s, albeit starting from a much higher level [28–30].
Only one recent paper specifically reported on malformations in diabetic nephropathy, described as increased compared to diabetic patients without nephropathy [91]. While these data, obtained in a large cohort, are waiting for further confirmation, a note of caution should probably be made at counselling.
Acknowledging the need for further research in this field, the Study Group suggests a strict multidisciplinary follow-up, paying attention to the concomitant factors, such as hypertension and diabetes, that may further increase pregnancy related complications, and suggests strict follow-up also after delivery, in particular in patients who developed severe proteinuria and hypertension (whether or not labelled as PE) during pregnancy [251–258].
Specific diseases: autosomal-dominant polycystic kidney disease
ADPKD patients may have a higher risk of pyelonephritis in pregnancy and should be strictly controlled for positive urinary cultures (moderate suggestion, scattered evidence).
ADPKD patients may be at higher risk for PE, also when pregnancy starts with normal blood pressure and without proteinuria (moderate suggestion, scattered evidence).
The Study Group does not support a policy of systematic caesarean delivery in ADPKD patients (moderate suggestion, scattered evidence).
While acknowledging the interest in pre-implantation genetic selection, as a potential tool in autosomal recessive PKD, there is no experience on ADPKD, and the problems linked to assisted fertilization procedures have to be carefully weighed up (moderate suggestion, scant evidence).
The evidence on ADPKD in pregnancy is limited, and the largest recently published series encompasses 54 patients only [259]. In keeping with older observations, the current evidence suggests that hypertensive disorders of pregnancy, including PE, are more frequent in ADPKD patients. Preterm delivery and UTIs were also found to be increased, but the relationship between them is not completely clarified [259–262].
The old tenet that women with ADPKD should deliver by caesarean section because of the risk of intracystic bleeding, due to the increased abdominal pressure at parturition, is not supported by current evidence, also considering the risks of a surgical intervention and of urinary catheter. However, some cases have also recently been reported, and the opinion of the Study Group is that the risk of intracystic bleeding should be considered; therefore, we suggest ultrasound monitoring in particular of the largest cysts, also in non-symptomatic patients, at least in the proximity of and immediately after delivery [262–264].
The Group does not presently support the choice of implantation selection procedures, given their non-negligible rate of complications; such an approach has been recently discussed as for autosomal-recessive polycystic kidney disease, where, in spite of the higher severity of the kidney disease, it is likewise controversial [265, 266].
Specific diseases: chronic pyelonephritis, kidney scars and diseases at risk for upper urinary tract infections in pregnancy (including kidney stones)
Asymptomatic bacteriuria, acute cystitis and acute pyelonephritis have been associated with an increased risk of abortion, preterm delivery, low birth weight and perinatal mortality. Gestational hypertension and PE are also increased (relative risk [RR] up to 5) (strong suggestion, from large observational studies).
Since untreated, asymptomatic bacteriuria persists in 60–85 % of cases and progresses to acute cystitis or pyelonephritis in up to 40 % of patients, all cases should be treated (strong suggestion, from large observational studies and RCTs).
Antibiotic therapy allows sterilization of urinary culture in 90 % of patients and reduces the incidence of acute pyelonephritis by 70–80 %, decreasing the incidence of low birth-weight and of preterm birth (strong suggestion, from large observational studies and RCTs).
The optimal treatment for asymptomatic bacteriuria, acute cystitis and acute pyelonephritis is not fully established. Empiric treatment should be tailored to the specific situation and take into account the local resistance patterns (strong suggestion, from large observational studies and RCTs).
Chronic pyelonephritis is now recognized as an umbrella term encompassing many chronic inflammatory conditions even in the absence of a clear history of renal infections [267]. Whatever the cause, kidney scars, identified by imaging techniques, define this condition [268–271]. Risk of bacteriuria is significantly higher in women with a history of UTI in childhood and adolescence, whether or not with kidney scars [271]. The incidence of asymptomatic bacteriuria is estimated to be 2–7 % of all pregnancies (more common in diabetic women: 8–14 %); acute pyelonephritis occurs in 1–2 % of low-risk pregnancies [272–276].
Pregnancy predisposes to UTI for several reasons: the physiological hydroureter of pregnancy, consequent to progesterone-induced hypotonia and decreased peristaltic activity and to the compression of the gravid uterus; pregnancy-induced glycosuria and aminoaciduria may facilitate bacterial colonization, immune-system changes, including reduced response to nitric oxide (NO) and Toll-like receptor 4 (TLR4) [277, 278].
In planning the clinical surveillance, several risk factors should be considered: history of UTI, diabetes, low socioeconomic status, older age, sickle-cell anaemia, urological abnormalities, sexual activity, and multiparity [277–279].
Asymptomatic bacteriuria has been associated with an increased risk of abortion, preterm birth, low birth-weight and perinatal mortality, thus leading to the current practice of repeated urinalysis and urinary cultures in all pregnancies [280–284].
The relationship between asymptomatic bacteriuria, low birth-weight and preterm delivery is however still elusive. Pro-inflammatory cytokines may initiate labour, while microorganisms may produce arachidonic acid, phospholipase A2 and prostaglandins that enhance cervical softening, which stimulates uterine contractions and preterm labour [285]. If untreated, bacteriuria persists in 60–85 % of cases, progressing to cystitis or pyelonephritis in up to 40 % of patients, and increasing the risk of PE [273, 279].
Antibiotic therapy allows sterilization of urinary culture in 90 % of patients and reduces the risks of acute pyelonephritis, along with low birth-weight babies and preterm birth [274, 280, 286–299].
The therapeutic protocols are controversial: single-dose therapy and 3-day courses were used with success in some studies. The pros are better compliance, lesser exposure for the foetus, fewer side effects, lower costs; the con is the higher recurrence rate. On this basis, the Study Group supports standard 6–7 days antibiotic courses until further data are available [300–302]. Follow-up requires at least monthly urine culture and, in case of persistence, suppressive therapy should be considered. For the most commonly used drugs, refer to Table 3.
Acute cystitis is frequently asymptomatic; conversely, acute pyelonephritis usually presents with flank abdominal or pelvic pain, and high fever; severe complications, including septic shock, respiratory distress and acute renal failure are reported in 2–25 % of cases [303–318]. Since Escherichia Coli is involved in about 80 % of the cases and other Gram negative germs in most of the remaining, empirical treatment should be started early on the basis of the local resistance pattern and of the eventual maternal intolerances (Table 3) [319, 320]. Preeclampsia and preterm delivery are reported as increased after UTI in pregnancy, even in the absence of active infection at delivery [321].
Kidney stones
Kidney stones are rare in pregnancy, but they are associated with several negative pregnancy-associated outcomes, including PE and preterm delivery (strong suggestion, evidence from observational studies).
Within the limits of the scarce urethral view, ultrasound should be the first imaging modality, while magnetic resonance imaging (MRI) and computed tomography (CT) scans (II and III trimester) should be limited to selected cases (strong suggestion, scattered evidence).
While most of the stones are passed spontaneously, ureteroscopy is relatively safe in the case of need; small series suggest considering also the option of holmium laser (strong suggestion, large series for uteroscopy, small for holmium laser).
Nephrolithiasis is rare in pregnancy (1:1500–3000), despite several predisposing factors, including urinary stasis, hypercalciuria and increased urinary pH, increased excretion of uric acid, sodium and oxalates, out of proportion with citrate and magnesium excretion [322]. Calcium and phosphate are the most frequent components of kidney stones in pregnancy (up to 75 %) [322–324].
Renal colics are more frequent in the second and third trimester; gross hematuria may occur in about one-third of cases [325–332]. Ultrasounds allow limited visualization of ureteral stones; ureteral jet can discriminate between physiological hydroureter and complete obstruction. Doppler study and resistivity index may help in the diagnosis while transvaginal ultrasounds can highlight stones into the distal ureter [322–327]. In selected cases MRI may be required [328].
In most cases, the dilation of the urinary tract favours stone emission and only symptomatic therapy, based on hydration and analgesia is needed. Ureteral stents, percutaneous nephrostomy or ureteroscopy may be employed in selected cases, as well as holmium laser lithotripsy, according to small patient series [325–331].
Renal stents have been associated with increase in proteinuria to be considered in the differential diagnosis with PE [332]. The association with nephrocalcinosis should be kept in mind for allowing diagnosis of underlying metabolic disorders [333].
In view of the above, considering also the possible association between renal colic, premature rupture of membranes, pregnancy losses and PE, the Study Group suggests a strict multidisciplinary follow-up and personalised therapy for all patients with recurrent UTI or kidney stones [334–337].
Reflux nephropathy
Patients with reflux nephropathy are at higher risk for urinary tract infections even after reflux correction.
Reflux nephropathy shows a complex hereditary pattern and children from affected mothers should be screened before and after birth for UT and/or urinary malformations.
The frequent correction of reflux nephropathy has decreased its importance in pregnancy, and most of the studies related to this condition are relatively old. While the surveillance for infectious complications should follow the strict recommendations already mentioned in the case of recurrent UTI, the disease shows a complex hereditary pattern, and children from affected mothers should be screened before and after birth for UTI and/or urinary malformations [338–340].
Final comment
CKD is increasingly encountered in pregnancy and patients with chronic diseases, including CKD, are increasingly looking for pregnancies. Due to the changes in therapies, and in attitudes towards patients’ self-determination as well as in the definitions of CKD and PE, the evidence on which counselling and follow-up are based is often scattered and fragmentary. This lack of “certitudes” should be kept in mind at counselling, and strongly supports shared choices in the context of multidisciplinary care. Clinical nephrologists need to be continuously updated in this emerging field, and the Study Group supports the establishment of educational programs and of multicentre research studies.
Compliance with ethical standards
Conflict of interest
All authors have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study informed consent is not required.
Footnotes
On behalf of Kidney and Pregnancy Study Group of Italian Society of Nephrology.
An erratum to this article is available at http://dx.doi.org/10.1007/s40620-017-0418-6.
References
- 1. (1975) Pregnancy and renal disease. Lancet 2(7939):801–802 [PubMed]
- 2.Duffy D, Reynolds P. Babies born at the threshold of viability: attitudes of paediatric consultants and trainees in South East England. Acta Paediatr. 2011;100(1):42–46. doi: 10.1111/j.1651-2227.2010.01975.x. [DOI] [PubMed] [Google Scholar]
- 3.Hale TM, Arul M, Veerappan A, Nguyen J, Velastegui Z, Shiffman R, Rajegowda B, Skupski D, Mercado R. Predicting survival of periviable fetuses using NICHD fetal heart rate categories. J Perinat Med. 2011;39(1):47–50. doi: 10.1515/jpm.2010.121. [DOI] [PubMed] [Google Scholar]
- 4.Stephens BE, Tucker R, Vohr BR. Special health care needs of infants born at the limits of viability. Pediatrics. 2010;125(6):1152–1158. doi: 10.1542/peds.2009-1922. [DOI] [PubMed] [Google Scholar]
- 5.Hou SH. Pregnancy in women on haemodialysis and peritoneal dialysis. Baillieres Clin Obstet Gynaecol. 1994;8:481–500. doi: 10.1016/S0950-3552(05)80332-3. [DOI] [PubMed] [Google Scholar]
- 6.Piccoli GB, Conijn A, Consiglio V, et al. Pregnancy in dialysis patients: is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol. 2010;5:62–71. doi: 10.2215/CJN.05660809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hladunewich MA, Hou S, Odutayo A, et al. Intensive hemodialysis associates with improved pregnancy outcomes: a Canadian and United States Cohort Comparison. J Am Soc Nephrol. 2014;25(5):1103–1109. doi: 10.1681/ASN.2013080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toma H, Tanabe K, Tokumoto T, Kobayashi C, Yagisawa T. Pregnancy in women receiving renal dialysis or transplantation in Japan: a nationwide survey. Nephrol Dial Transplant. 1999;14:1511–1516. doi: 10.1093/ndt/14.6.1511. [DOI] [PubMed] [Google Scholar]
- 9.Luders C, Castro MC, Titan SM, et al. Obstetric outcome in pregnant women on long-term dialysis: a case series. Am J Kidney Dis. 2010;56:77–85. doi: 10.1053/j.ajkd.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Jesudason S, Grace BS, McDonald SP. Pregnancy outcomes according to dialysis commencing before or after conception in women with ESRD. Clin J Am Soc Nephrol. 2014;9:143–149. doi: 10.2215/CJN.03560413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccoli GB, Cabiddu G, Daidone G, et al. The children of dialysis: live-born babies from on-dialysis mothers in Italian epidemiological perspective comparing dialysis, kidney transplantation and the overall population. Nephrol Dial Transplant. 2014;29(8):1578–1586. doi: 10.1093/ndt/gfu092. [DOI] [PubMed] [Google Scholar]
- 12.Piccoli GB, Postorino V, Cabiddu G, et al. ‘Kidney and Pregnancy Study Group’ of the ‘Italian Society of Nephrology’. Children of a lesser god or miracles? An emotional and behavioural profile of children born to mothers on dialysis in Italy: a multicentre nationwide study 2000–12. Nephrol Dial Transplant. 2015;30(7):1193–1202. doi: 10.1093/ndt/gfv127. [DOI] [PubMed] [Google Scholar]
- 13.Cabiddu G, Castellino S, Gernone G, et al. Kidney and Pregnancy Study Group of Italian Society of Nephrology. Best practices on pregnancy on dialysis: the Italian Study Group on Kidney and Pregnancy. J Nephrol. 2015;28(3):279–288. doi: 10.1007/s40620-015-0191-3. [DOI] [PubMed] [Google Scholar]
- 14.www.kidney.org. Accessed 20 Sept 2015
- 15.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75:1009–1014. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 16.Smyth A, Radovic M, Garovic VD. Women, kidney disease, and pregnancy. Adv Chronic Kidney Dis. 2013;20:402–410. doi: 10.1053/j.ackd.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davison JM, Lindheimer MD. Pregnancy and chronic kidney disease. Semin Nephrol. 2011;31:86–99. doi: 10.1016/j.semnephrol.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Williams D, Davison J. Chronic kidney disease in pregnancy. BMJ. 2008;33:211–215. doi: 10.1136/bmj.39406.652986.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nevis IF, Reitsma A, Dominic A, et al. Pregnancy outcomes in women with chronic kidney disease: a systematic review. Clin J Am Soc Nephrol. 2011;6:2587–2598. doi: 10.2215/CJN.10841210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccoli GB, Conijn A, Attini R, et al. Pregnancy in chronic kidney disease: need for a common language. J Nephrol. 2011;24:282–299. doi: 10.5301/JN.2011.7978. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JJ, Ma XX, Hao L, Liu LJ, Lv JC, Zhang H. A systematic review and meta-analysis of outcomes of pregnancy in CKD and CKD Outcomes in pregnancy. Clin J Am Soc Nephrol. 2015;10(11):1964–1978. doi: 10.2215/CJN.09250914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lateef A, Petri M. Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol. 2013;27:435–447. doi: 10.1016/j.berh.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanhope TJ, White WM, Moder KG, et al. Obstetric nephrology: lupus and lupus nephritis in pregnancy. Clin J Am Soc Nephrol. 2012;7:2089–2099. doi: 10.2215/CJN.12441211. [DOI] [PubMed] [Google Scholar]
- 24.Vora N, Perrone R, Bianchi DW. Reproductive issues for adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;5:307–518. doi: 10.1053/j.ajkd.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5(5):1178–1185. doi: 10.1681/ASN.V551178. [DOI] [PubMed] [Google Scholar]
- 26.Schneeberger C, Geerlings SE, Middleton P, Crowther CA. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst Rev. 2012;11:CD009279. doi: 10.1002/14651858.CD009279.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Podymow T, August P, Akbari A. Management of renal disease in pregnancy. Obstet Gynecol Clin N Am. 2010;37(2):195–210. doi: 10.1016/j.ogc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Piccoli GB, Clari R, Ghiotto S, et al. Type 1 diabetes, diabetic nephropathy, and pregnancy: a systematic review and meta-study. Rev Diabet Stud. 2013;10:6–26. doi: 10.1900/RDS.2013.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccoli GB, Tavassoli E, Melluzza C, et al. Severe diabetic nephropathy in type 1 diabetes and pregnancy—a case series. Rev Diabet Stud. 2013;10:68–78. doi: 10.1900/RDS.2013.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramham K, Rajasingham D. Pregnancy in diabetes and kidney disease. J Ren Care. 2012;38(Suppl 1):78–89. doi: 10.1111/j.1755-6686.2012.00270.x. [DOI] [PubMed] [Google Scholar]
- 31.Garovic VD. Hypertension in pregnancy: diagnosis and treatment. Mayo Clin Proc. 2000;75(10):1071–1076. doi: 10.4065/75.10.1071. [DOI] [PubMed] [Google Scholar]
- 32.Piccoli GB, Fassio F, Attini R, et al. Pregnancy in CKD: whom should we follow and why? Nephrol Dial Transplant. 2012;27(Suppl 3):iii111–iii118. doi: 10.1093/ndt/gfs302. [DOI] [PubMed] [Google Scholar]
- 33.Morikawa M, Yamada T, Minakami H. Outcome of pregnancy in patients with isolated proteinuria. Curr Opin Obstet Gynecol. 2009;21(6):491–495. doi: 10.1097/GCO.0b013e32833040bf. [DOI] [PubMed] [Google Scholar]
- 34.Smith MC, Moran P, Ward MK, Davison JM. Assessment of glomerular filtration rate during pregnancy using the MDRD formula. BJOG. 2008;115:109–112. doi: 10.1111/j.1471-0528.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 35.Krutzén E, Olofsson P, Bäck SE, Nilsson-Ehle P. Glomerular filtration rate in pregnancy: a study in normal subjects and in patients with hypertension, preeclampsia and diabetes. Scand J Clin Lab Invest. 1992;52:387–392. doi: 10.3109/00365519209088374. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed SB, Bentley-Lewis R, Hollenberg NK, et al. A comparison of prediction equations for estimating glomerular filtration rate in pregnancy. Hypertens Pregnancy. 2009;28:243–255. doi: 10.1080/10641950801986720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alper AB, Yi Y, Rahman M, Webber LS, et al. Performance of estimated glomerular filtration rate prediction equations in preeclamptic patients. Am J Perinatol. 2011;28(6):425–430. doi: 10.1055/s-0030-1268712. [DOI] [PubMed] [Google Scholar]
- 38.Piccoli GB, Attini R, Vigotti FN, et al. Is renal hyperfiltration protective in chronic kidney disease-stage 1 pregnancies? A step forward unravelling the mystery of the effect of stage 1 chronic kidney disease on pregnancy outcomes. Nephrology (Carlton) 2015;20(3):201–208. doi: 10.1111/nep.12372. [DOI] [PubMed] [Google Scholar]
- 39.Gülmezoglu M, Villar J, Hofmeyr J, Duley L, Belizan JM. Randomised trials in maternal and perinatal medicine: global partnerships are the way forward. Br J Obstet Gynaecol. 1998;105(12):1244–1247. doi: 10.1111/j.1471-0528.1998.tb10001.x. [DOI] [PubMed] [Google Scholar]
- 40.Patel D, Nasir S, Elati A, Vernon G, Weeks AD. Historical trends in the timing of informed consent for research into intrapartum complications. BJOG. 2012;119(3):361–365. doi: 10.1111/j.1471-0528.2011.03204.x. [DOI] [PubMed] [Google Scholar]
- 41.Johnson A. Randomised controlled trials in perinatal medicine: 3. Identifying and measuring endpoints in randomised controlled trials. Br J Obstet Gynaecol. 1997;104(7):768–771. doi: 10.1111/j.1471-0528.1997.tb12017.x. [DOI] [PubMed] [Google Scholar]
- 42.Barton S. Which clinical studies provide the best evidence? The best RCT still trumps the best observational study. BMJ. 2000;321(7256):255–256. doi: 10.1136/bmj.321.7256.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devereaux PJ, Yusuf S. The evolution of the randomized controlled trial and its role in evidence-based decision making. J Intern Med. 2003;254(2):105–113. doi: 10.1046/j.1365-2796.2003.01201.x. [DOI] [PubMed] [Google Scholar]
- 44.Barnhart K, Sammel M. The truth is out there: a guide to the pitfalls of interpreting evidence-based medicine in studies of human reproduction. Fertil Steril. 2002;77(2):223–228. doi: 10.1016/S0015-0282(01)03207-1. [DOI] [PubMed] [Google Scholar]
- 45.Feigin VL, Howard G. The importance of epidemiological studies should not be downplayed. Stroke. 2008;39(1):1–2. doi: 10.1161/STROKEAHA.107.503250. [DOI] [PubMed] [Google Scholar]
- 46.La Caze A. Evidence-based medicine must be. J Med Philos. 2009;34(5):509–527. doi: 10.1093/jmp/jhp034. [DOI] [PubMed] [Google Scholar]
- 47.Thompson RP. Causality, mathematical models and statistical association: dismantling evidence-based medicine. J Eval Clin Pract. 2010;16(2):267–275. doi: 10.1111/j.1365-2753.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 48.Mansouri A, Cooper B, Shin SM, Kondziolka D. Randomized controlled trials and neurosurgery: the ideal fit or should alternative methodologies be considered? J Neurosurg. 2015;28:1–11. doi: 10.3171/2014.12.JNS142465. [DOI] [PubMed] [Google Scholar]
- 49.Hou SH, Grossman SD, Madias NE. Pregnancy in women with renal disease and moderate renal insufficiency. Am J Med. 1985;78:185–194. doi: 10.1016/0002-9343(85)90425-5. [DOI] [PubMed] [Google Scholar]
- 50.Jungers P, Forget D, Henry-Amar M, et al. Chronic kidney disease and pregnancy. Adv Nephrol Necker Hosp. 1986;15:103–141. [PubMed] [Google Scholar]
- 51.Jones DC, Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1996;335:226–232. doi: 10.1056/NEJM199607253350402. [DOI] [PubMed] [Google Scholar]
- 52.Bramham K, Briley AL, Seed PT, et al. Pregnancy outcome in women with chronic kidney disease: a prospective cohort study. Reprod Sci. 2011;18:623–630. doi: 10.1177/1933719110395403. [DOI] [PubMed] [Google Scholar]
- 53.Imbasciati E, Gregorini G, Cabiddu G, et al. Pregnancy in CKD stages 3 to 5: fetal and maternal outcomes. Am J Kidney Dis. 2007;49:753–762. doi: 10.1053/j.ajkd.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 54.Epstein FH. Pregnancy and renal disease. N Engl J Med. 1996;335:277–278. doi: 10.1056/NEJM199607253350410. [DOI] [PubMed] [Google Scholar]
- 55.Chopra S, Suri V, Aggarwal N, et al. Pregnancy in chronic renal insufficiency: single centre experience from North India. Arch Gynecol Obstet. 2009;279:691–695. doi: 10.1007/s00404-008-0797-y. [DOI] [PubMed] [Google Scholar]
- 56.Piccoli GB, Attini R, Vasario E, et al. Pregnancy and chronic kidney disease: a challenge in all CKD stages. Clin J Am Soc Nephrol. 2010;5:844–855. doi: 10.2215/CJN.07911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ibrahim HN, Akkina SK, Leister E, et al. Pregnancy outcomes after kidney donation. Am J Transplant. 2009;9:825–834. doi: 10.1111/j.1600-6143.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reisaeter AV, Røislien J, Henriksen T, et al. Pregnancy and birth after kidney donation: the Norwegian experience. Am J Transplant. 2009;9:820–824. doi: 10.1111/j.1600-6143.2008.02427.x. [DOI] [PubMed] [Google Scholar]
- 59.Alsuwaida A, Mousa D, Al-Harbi A, et al. Impact of early chronic kidney disease on maternal and fetal outcomes of pregnancy. J Matern Fetal Neonatal Med. 2011;24:1432–1436. doi: 10.3109/14767058.2011.575483. [DOI] [PubMed] [Google Scholar]
- 60.Limardo M, Imbasciati E, Ravani P, et al. Pregnancy and progression of IgA nephropathy: results of an Italian multicenter study. Am J Kidney Dis. 2010;56:506–512. doi: 10.1053/j.ajkd.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 61.Bramham K, Lightstone L. Pre-pregnancy counseling for women with chronic kidney disease. J Nephrol. 2012;25:450–459. doi: 10.5301/jn.5000130. [DOI] [PubMed] [Google Scholar]
- 62.Piccoli GB, Cabiddu G, Attini R, et al. Risk of adverse pregnancy outcomes in women with CKD. J Am Soc Nephrol. 2015;26(8):2011–2022. doi: 10.1681/ASN.2014050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkhunaizi A, Melamed N, Hladunewich MA. Pregnancy in advanced chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2015;24(3):252–259. doi: 10.1097/MNH.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 64.Singh R, Prasad N, Banka A, Gupta A, Bhadauria D, Sharma RK, Kaul A. Pregnancy in patients with chronic kidney disease: maternal and fetal outcomes. Indian J Nephrol. 2015;25(4):194–199. doi: 10.4103/0971-4065.145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng Z, Minard C, Raghavan R. Pregnancy outcomes in advanced kidney disease. Clin Nephrol. 2015;83(5):272–278. doi: 10.5414/CN108516. [DOI] [PubMed] [Google Scholar]
- 66.Kendrick J, Sharma S, Holmen J, Palit S, Nuccio E, Chonchol M. Kidney disease and maternal and fetal outcomes in pregnancy. Am J Kidney Dis. 2015;66(1):55–59. doi: 10.1053/j.ajkd.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garg AX, Nevis IF, McArthur E, et al. DONOR Network. Gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372(2):124–133. doi: 10.1056/NEJMoa1408932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pagnoux C, Mahendira D, Laskin CA. Fertility and pregnancy in vasculitis. Best Pract Res Clin Rheumatol. 2013;27:79–94. doi: 10.1016/j.berh.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Smyth A, Oliveira GH, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5:2060–2068. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ritchie J, Smyth A, Tower C, et al. Maternal deaths in women with lupus nephritis: a review of published evidence. Lupus. 2012;21:534–541. doi: 10.1177/0961203311434939. [DOI] [PubMed] [Google Scholar]
- 71.Biesenbach G, Stoger H, Zazgornik J. Influence of pregnancy on progression of diabetic nephropathy and subsequent requirement of renal replacement therapy in female type 1 diabetic patients with impaired renal function. Nephrol Dial Transplant. 1992;7:105–109. doi: 10.1093/oxfordjournals.ndt.a092077. [DOI] [PubMed] [Google Scholar]
- 72.Mathiesen ER, Ringholm L, Feldt-Rasmussen B, Clausen P, Damm P. Obstetric nephrology: pregnancy in women with diabetic nephropathy—the role of antihypertensive treatment. Clin J Am Soc Nephrol. 2012;7(12):2081–2088. doi: 10.2215/CJN.00920112. [DOI] [PubMed] [Google Scholar]
- 73.Bramham K, Rajasingham D. Pregnancy in diabetes and kidney disease. J Ren Care. 2012;38(1):78–89. doi: 10.1111/j.1755-6686.2012.00270.x. [DOI] [PubMed] [Google Scholar]
- 74.Hawthorne G. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):77–90. doi: 10.1016/j.bpobgyn.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 75.Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40(4):739–751. doi: 10.1016/j.clp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Asano MK, Dray PB. Retinopathy of prematurity. Dis Mon. 2014;60(6):282–291. doi: 10.1016/j.disamonth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 77.Sutherland MR, Bertagnolli M, Lukaszewski MA, Huyard F, Yzydorczyk C, Luu TM, Nuyt AM. Preterm birth and hypertension risk: the oxidative stress paradigm. Hypertension. 2014;63(1):12–18. doi: 10.1161/HYPERTENSIONAHA.113.01276. [DOI] [PubMed] [Google Scholar]
- 78.Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132(4):741–751. doi: 10.1542/peds.2013-1131. [DOI] [PubMed] [Google Scholar]
- 79.Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci. 2011;18:322–333. doi: 10.1177/1933719111401659. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland MR, Gubhaju L, Moore L, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. 2011;22:1365–1374. doi: 10.1681/ASN.2010121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hodgin JB, Rasoulpour M, Markowitz GS, D’Agati VD. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:71–76. doi: 10.2215/CJN.01700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131:1168–1179. doi: 10.1542/peds.2013-0009. [DOI] [PubMed] [Google Scholar]
- 83.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. 2012;8(5):265–274. doi: 10.1038/nrneph.2012.38. [DOI] [PubMed] [Google Scholar]
- 84.Janvier A, Lorenz JM, Lantos JD. Antenatal counseling for parents facing an extremely preterm birth: limitations of the medical evidence. Acta Paediatr. 2012;101(8):800–804. doi: 10.1111/j.1651-2227.2012.02695.x. [DOI] [PubMed] [Google Scholar]
- 85.Stelloh C, Allen KP, Mattson DL, Lerch-Gaggl A, Reddy S, El-Meanawy A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl Res. 2012;159(2):80–89. doi: 10.1016/j.trsl.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roggero P, Giannì ML, Garbarino F, Mosca F. Consequences of prematurity on adult morbidities. Eur J Intern Med. 2013;24(7):624–626. doi: 10.1016/j.ejim.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Luyckx VA, Brenner BM. Low birth weight, nephron number, and kidney disease. Kidney Int Suppl. 2005;97:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 88.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 89.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics. 2013;131(4):e1240–e1263. doi: 10.1542/peds.2012-2177. [DOI] [PubMed] [Google Scholar]
- 90.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review ant meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–234. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bell R, Glinianaia SV, Tennant PW, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012;55:936–947. doi: 10.1007/s00125-012-2455-y. [DOI] [PubMed] [Google Scholar]
- 92.Piccoli GB, Arduino S, Attini R, et al. Multiple pregnancies in CKD patients: an explosive mix. Clin J Am Soc Nephrol. 2013;8:41–50. doi: 10.2215/CJN.02550312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hladunewich MA, Steinberg G, Karumanchi SA, et al. Angiogenic factor abnormalities and fetal demise in a twin pregnancy. Nat Rev Nephrol. 2009;5:658–662. doi: 10.1038/nrneph.2009.154. [DOI] [PubMed] [Google Scholar]
- 94.Loeffler CL, Macri CJ, Bathgate SL, Freese L, Larsen JW. Autosomal dominant polycystic kidney disease in pregnancy complicated by twin gestation and severe preeclampsia: a case report. J Reprod Med. 2005;50(5):370–372. [PubMed] [Google Scholar]
- 95.Gizzo S, Noventa M, Saccardi C, Paccagnella G, Patrelli TS, Cosmi E, D’Antona D. Twin pregnancy after kidney transplantation: what’s on? A case report and review of literature. J Matern Fetal Neonatal Med. 2014;27(17):1816–1819. doi: 10.3109/14767058.2013.879699. [DOI] [PubMed] [Google Scholar]
- 96.Abou-Jaoude P, Dubourg L, Bessenay L, et al. What about the renal function during childhood of children born from dialysed mothers? Nephrol Dial Transplant. 2012;27:2365–2369. doi: 10.1093/ndt/gfr617. [DOI] [PubMed] [Google Scholar]
- 97.Blowey DL, Warady BA. Outcome of infants born to women with chronic kidney disease. Adv Chronic Kidney Dis. 2007;14:199–205. doi: 10.1053/j.ackd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 98.Banerjee I, Powis S, Shevlin M, Barnes J, Soo A, Sutcliffe AG. Health outcomes of children born to mothers with chronic kidney disease: a pilot study. Pediatr Rep. 2010;2(1):e7. doi: 10.4081/pr.2010.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 100.Masuyama H, Nobumoto E, Okimoto N, Inoue S, Segawa T, Hiramatsu Y. Superimposed preeclampsia in women with chronic kidney disease. Gynecol Obstet Invest. 2012;74(4):274–281. doi: 10.1159/000339935. [DOI] [PubMed] [Google Scholar]
- 101.Brown MA. Pre-eclampsia in 2014: seven ways to make a difference. Pregnancy Hypertens. 2014;4(4):249–252. doi: 10.1016/j.preghy.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Canadian hypertensive diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Disorders of Pregnancy (HDP) Working Group. Pregnancy Hypertens. 2014;4(2):105–145. doi: 10.1016/j.preghy.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Tranquilli AL. Introduction to ISSHP new classification of preeclampsia. Pregnancy Hypertens. 2013;3(2):58–59. doi: 10.1016/j.preghy.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 104.Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Pregnancy Hypertens. 2013;3(1):44–47. doi: 10.1016/j.preghy.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Rezk M, Gamal A, Emara M. Maternal and fetal outcome in de novo preeclampsia in comparison to superimposed preeclampsia: a two-year observational study. Hypertens Pregnancy. 2015;34(2):137–144. doi: 10.3109/10641955.2014.982329. [DOI] [PubMed] [Google Scholar]
- 106.Berks D, Steegers EA, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol. 2009;114(6):1307–1314. doi: 10.1097/AOG.0b013e3181c14e3e. [DOI] [PubMed] [Google Scholar]
- 107.Umans JG. Obstetric nephrology: preeclampsia—the nephrologist’s perspective. Clin J Am Soc Nephrol. 2012;7(12):2107–2113. doi: 10.2215/CJN.05470512. [DOI] [PubMed] [Google Scholar]
- 108.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 109.Arulkumaran N, Lightstone L. Severe pre-eclampsia and hypertensive crises. Best Pract Res Clin Obstet Gynaecol. 2013;27(6):877–884. doi: 10.1016/j.bpobgyn.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 110.Ciantar E, Walker JJ. Pre-eclampsia, severe pre-eclampsia and hemolysis, elevated liver enzymes and low platelets syndrome: what is new? Womens Health. 2011;7(5):555–569. doi: 10.2217/whe.11.57. [DOI] [PubMed] [Google Scholar]
- 111.Al-Safi Z, Imudia AN, Filetti LC, Hobson DT, Bahado-Singh RO, Awonuga A. Delayed post partum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol. 2011;118(5):1102–1107. doi: 10.1097/AOG.0b013e318231934c. [DOI] [PubMed] [Google Scholar]
- 112.Mikami Y, Takagi K, Itaya Y, Ono Y, Matsumura H, Takai Y, Seki H. Post-partum recovery course in patients with gestational hypertension and pre-eclampsia. J Obstet Gynaecol Res. 2014;40(4):919–925. doi: 10.1111/jog.12280. [DOI] [PubMed] [Google Scholar]
- 113.McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010;55(6):1026–1039. doi: 10.1053/j.ajkd.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 114.Wu CC, Chen SH, Ho CH, Liang FW, Chu CC, Wang HY, Lu YH. End-stage renal disease after hypertensive disorders in pregnancy. Am J Obstet Gynecol. 2014;210(2):147. doi: 10.1016/j.ajog.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 115.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83(1):177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 116.http://www.acog.org/Resources_And_Publications/Task_Force_and_Work_Group_Reports/Hypertension_in_Pregnancy. Accessed 15 Sept 2015
- 117.Piccoli GB, Gaglioti P, Attini R, et al. Pre-eclampsia or chronic kidney disease? The flow hypothesis. Nephrol Dial Transplant. 2013;28(5):1199–1206. doi: 10.1093/ndt/gfs573. [DOI] [PubMed] [Google Scholar]
- 118.Parra M, Rodrigo R, Barja P, Bosco C, Fernández V, Muñoz H, Soto-Chacón E. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol. 2005;193(4):1486–1491. doi: 10.1016/j.ajog.2005.02.109. [DOI] [PubMed] [Google Scholar]
- 119.Papageorghiou AT, Roberts N. Uterine artery Doppler screening for adverse pregnancy outcome. Curr Opin Obstet Gynecol. 2005;17(6):584–590. doi: 10.1097/01.gco.0000191898.84567.04. [DOI] [PubMed] [Google Scholar]
- 120.Gaillard R, Arends LR, Steegers EA, Hofman A, Jaddoe VW. Second- and third-trimester placental hemodynamics and the risks of pregnancy complications: the Generation R Study. Am J Epidemiol. 2013;177(8):743–754. doi: 10.1093/aje/kws296. [DOI] [PubMed] [Google Scholar]
- 121.Kane SC, Dennis AT. Doppler assessment of uterine blood flow in pre-eclampsia: a review. Hypertens Pregnancy. 2015;34(4):400–421. doi: 10.3109/10641955.2015.1074244. [DOI] [PubMed] [Google Scholar]
- 122.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 123.von Dadelszen P, Payne B, Li J, PIERS Study Group et al. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377(9761):219–227. doi: 10.1016/S0140-6736(10)61351-7. [DOI] [PubMed] [Google Scholar]
- 124.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garovic VD, Wagner SJ, Turner ST et al (2007) Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol 196(4):320.e1-7 [DOI] [PubMed]
- 126.White WM, Garrett AT, Craici IM, et al. Persistent urinary podocyte loss following preeclampsia may reflect subclinical renal injury. PLoS One. 2014;9(3):e92693. doi: 10.1371/journal.pone.0092693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thangaratinam S, Ismail KM, Sharp S, Coomarasamy A, Khan KS. Tests in Prediction of Pre-eclampsia Severity review group. Accuracy of serum uric acid in predicting complications of pre-eclampsia: a systematic review. BJOG. 2006;113(4):369–378. doi: 10.1111/j.1471-0528.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- 128.Dusse LM, Alpoim PN, Lwaleed BA, et al. Is there a link between endothelial dysfunction, coagulation activation and nitric oxide synthesis in preeclampsia? Clin Chim Acta. 2013;16(415):226–229. doi: 10.1016/j.cca.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 129.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24(9):964–969. doi: 10.1038/ajh.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bilodeau JF. Review: maternal and placental antioxidant response to preeclampsia—impact on vasoactive eicosanoids. Placenta. 2014;35(Suppl):S32–S38. doi: 10.1016/j.placenta.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 131.Gennari-Moser C, Khankin EV, Escher G, et al. Vascular endothelial growth factor-A and aldosterone: relevance to normal pregnancy and preeclampsia. Hypertension. 2013;61(5):1111–1117. doi: 10.1161/HYPERTENSIONAHA.111.00575. [DOI] [PubMed] [Google Scholar]
- 132.Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE (2007) Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol 197(3):244.e1-8 [DOI] [PubMed]
- 134.Duhig KE, Chappell LC, Shennan AH. How placental growth factor detection might improve diagnosis and management of pre-eclampsia. Expert Rev Mol Diagn. 2014;14(4):403–406. doi: 10.1586/14737159.2014.908121. [DOI] [PubMed] [Google Scholar]
- 135.Verlohren S, Herraiz I, Lapaire O et al (2012) The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 206(1):58.e1-8 [DOI] [PubMed]
- 136.Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, EBM CONNECT Collaboration et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012;119(7):778–787. doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 137.Powers RW, Jeyabalan A, Clifton RG, Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units Network et al. Soluble fms-like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS One. 2010;5(10):e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Forest JC, Thériault S, Massé J, Bujold E, Giguère Y. Soluble Fms-like tyrosine kinase-1 to placental growth factor ratio in mid-pregnancy as a predictor of preterm preeclampsia in asymptomatic pregnant women. Clin Chem Lab Med. 2014;52(8):1169–1178. doi: 10.1515/cclm-2013-0955. [DOI] [PubMed] [Google Scholar]
- 139.Gómez-Arriaga PI, Herraiz I, López-Jiménez EA, Gómez-Montes E, Denk B, Galindo A. Uterine artery Doppler and sFlt-1/PlGF ratio: usefulness in diagnosis of pre-eclampsia. Ultrasound Obstet Gynecol. 2013;41(5):530–537. doi: 10.1002/uog.12400. [DOI] [PubMed] [Google Scholar]
- 140.Piccoli GB, Daidola G, Attini R, et al. Kidney biopsy in pregnancy: evidence for counselling? A systematic narrative review. BJOG. 2013;120:412–427. doi: 10.1111/1471-0528.12111. [DOI] [PubMed] [Google Scholar]
- 141.Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:416–438. doi: 10.1016/S1701-2163(15)30588-0. [DOI] [PubMed] [Google Scholar]
- 142.Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;2:CD002252. doi: 10.1002/14651858.CD002252.pub3. [DOI] [PubMed] [Google Scholar]
- 143.Molvi SN, Mir S, Rana VS, et al. Role of antihypertensive therapy in mild to moderate pregnancy-induced hypertension: a prospective randomized study comparing labetalol with alpha methyldopa. Arch Gynecol Obstet. 2012;285:1553–1562. doi: 10.1007/s00404-011-2205-2. [DOI] [PubMed] [Google Scholar]
- 144.Rothberger S, Carr D, Brateng D, Hebert M, Easterling TR. Pharmacodynamics of clonidine therapy in pregnancy: a heterogeneous maternal response impacts fetal growth. Am J Hypertens. 2010;23:1234–1240. doi: 10.1038/ajh.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Collins R, Yusuf S, Peto R. Overview of randomised trials of diuretics in pregnancy. Br Med J. 1985;290:17–23. doi: 10.1136/bmj.290.6461.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Firoz T, Magee LA, MacDonell K, Payne BA, Gordon R, Vidler M, von Dadelszen P. Community Level Interventions for Pre-eclampsia (CLIP) Working Group. BJOG. 2014;121(10):1210–1218. doi: 10.1111/1471-0528.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nabhan AF, Elsedawy MM. Tight control of mild-moderate pre-existing or non-proteinuric gestational hypertension. Cochrane Database Syst Rev. 2011;7:CD006907. doi: 10.1002/14651858.CD006907.pub2. [DOI] [PubMed] [Google Scholar]
- 148.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372(5):407–417. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]
- 149.Magee LA, CHIPS Study Group, et al. von Dadelszen P, et al. Control of hypertension in pregnancy study randomised controlled trial-are the results dependent on the choice of labetalol or methyldopa? BJOG. 2015 doi: 10.1111/1471-0528.13568. [DOI] [PubMed] [Google Scholar]
- 150.Firoz T, Magee LA, MacDonell K, Payne BA, Gordon R, Vidler M, von Dadelszen P, Community Level Interventions for Pre-eclampsia (CLIP) Working Group Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum: a systematic review. BJOG. 2014;121(10):1210–1218. doi: 10.1111/1471-0528.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wise J. Tight blood pressure control during pregnancy offers no clear benefits, study finds. BMJ. 2015;2(350):h549. doi: 10.1136/bmj.h549. [DOI] [PubMed] [Google Scholar]
- 152.Huynh K. Hypertension: tight control of hypertension is safe in pregnant women. Nat Rev Cardiol. 2015;12(4):196. doi: 10.1038/nrcardio.2015.20. [DOI] [PubMed] [Google Scholar]
- 153.Koren G. Hypertension: ACE inhibitor use in pregnancy—setting the record straight. Nat Rev Cardiol. 2011;9(1):7–8. doi: 10.1038/nrcardio.2011.179. [DOI] [PubMed] [Google Scholar]
- 154.Lewis G, Maxwell AP. Should women with diabetic nephropathy considering pregnancy continue ACE inhibitor or angiotensin II receptor blocker therapy until pregnancy is confirmed? Diabetologia. 2014;57(5):1082–1083. doi: 10.1007/s00125-014-3188-x. [DOI] [PubMed] [Google Scholar]
- 155.Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 156.Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343:d5931. doi: 10.1136/bmj.d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vasilakis-Scaramozza C, Aschengrau A, Cabral HJ, Jick SS. Antihypertensive drugs and the risk of congenital anomalies. Pharmacotherapy. 2013;33:476–482. doi: 10.1002/phar.1212. [DOI] [PubMed] [Google Scholar]
- 158.Al-Balas M, Bozzo P, Einarson A. Use of diuretics during pregnancy. Can Fam Physician. 2009;55(1):44–45. [PMC free article] [PubMed] [Google Scholar]
- 159.Henderson JT, Whitlock EP, O'Conner E, Senger CA, Thompson JH, Rowland MG (2014) Low-dose aspirin for the prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Apr. Report No.: 14-05207-EF-1. US Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews [PubMed]
- 160.Roberge S, Villa P, Nicolaides K, Giguère Y, Vainio M, Bakthi A, Ebrashy A, Bujold E. Early administration of low-dose aspirin for the prevention of preterm and termpreeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–146. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 161.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 162.Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–146. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 163.Roberge S, Giguère Y, Villa P, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2012;29(7):551–556. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
- 164.Boldt J. Use of albumin: an update. Br J Anaesth. 2010;104:276–284. doi: 10.1093/bja/aep393. [DOI] [PubMed] [Google Scholar]
- 165.Jouppila P, Jouppila R, Koivula A. Albumin infusion does not alter the intervillous blood flow in severe pre-eclampsia. Acta Obstet Gynecol Scand. 1983;62(4):345–348. doi: 10.3109/00016348309156236. [DOI] [PubMed] [Google Scholar]
- 166.Fliser D, Zurbrüggen I, Mutschler E, et al. Coadministration of albumin and furosemide in patients with the nephrotic syndrome. Kidney Int. 1999;55(2):629–634. doi: 10.1046/j.1523-1755.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 167.Elwell RJ, Spencer AP, Eisele G. Combined furosemide and human albumin treatment for diuretic-resistant edema. Ann Pharmacother. 2003;37(5):695–700. doi: 10.1345/aph.1C320. [DOI] [PubMed] [Google Scholar]
- 168.Piccoli GB, Attini R, Vasario E, et al. Vegetarian supplemented low-protein diets. A safe option for pregnant CKD patients: report of 12 pregnancies in 11 patients. Nephrol Dial Transplant. 2011;26(1):196–205. doi: 10.1093/ndt/gfq333. [DOI] [PubMed] [Google Scholar]
- 169.Piccoli GB, Leone F, Attini R, et al. Association of low-protein supplemented diets with fetal growth in pregnant women with CKD. Clin J Am Soc Nephrol. 2014;9(5):864–873. doi: 10.2215/CJN.06690613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cahill LE, Peng CY, Bankovic-Calic N, Sankaran D, Ogborn MR, Aukema HM. Dietary soya protein during pregnancy and lactation in rats with hereditary kidney disease attenuates disease progression in offspring. Br J Nutr. 2007;97:77–84. doi: 10.1017/S0007114507250470. [DOI] [PubMed] [Google Scholar]
- 171.Barsotti G, Morelli E, Cupisti A, Meola M, Dani L, Giovannetti SA. Low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron. 1996;74:390–394. doi: 10.1159/000189341. [DOI] [PubMed] [Google Scholar]
- 172.Piccoli GB, Ferraresi M, Deagostini MC, et al. Vegetarian low-protein diets supplemented with keto analogues: a niche for the few or an option for many? Nephrol Dial Transplant. 2013;28:2295–2305. doi: 10.1093/ndt/gft092. [DOI] [PubMed] [Google Scholar]
- 173.McMullen S. Soya protein during pregnancy—an opportunity to attenuate the progression of chronic kidney disease? Br J Nutr. 2007;97:4–5. doi: 10.1017/S0007114507257812. [DOI] [PubMed] [Google Scholar]
- 174.Piccoli GB, Clari R, Vigotti FN, et al. Vegan–vegetarian diets in pregnancy: danger or panacea? A systematic narrative review. BJOG. 2015;122(5):623–633. doi: 10.1111/1471-0528.13280. [DOI] [PubMed] [Google Scholar]
- 175.Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109:1266–1282. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 176.Pawlak R, Parrott SJ, Raj S, Cullum-Dugan D, Lucus D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr Rev. 2013;71:110–117. doi: 10.1111/nure.12001. [DOI] [PubMed] [Google Scholar]
- 177.Koletzko B, Bauer CP, Bung P, et al. Nutrition in pregnancy—practice recommendations of the Network “Healthy Start-Young Family Network”. Dtsch Med Wochenschr. 2012;137:1366–1372. doi: 10.1055/s-0032-1305076. [DOI] [PubMed] [Google Scholar]
- 178.Foster M, Herulah UN, Prasad A, Petocz P, Samman S. Zinc status of vegetarians during pregnancy: a systematic review of observational studies and meta-analysis of zinc intake. Nutrients. 2015;7(6):4512–4525. doi: 10.3390/nu7064512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brown MA, Holt JL, Mangos GJ, Murray N, Curtis J, Homer C. Microscopic hematuria in pregnancy: relevance to pregnancy outcome. Am J Kidney Dis. 2005;45(4):667–673. doi: 10.1053/j.ajkd.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 180.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)71003-5. [DOI] [PubMed] [Google Scholar]
- 181.Shimizu A, Takei T, Moriyama T, et al. Effect of kidney disease stage on pregnancy and delivery outcomes among patients with immunoglobulin A nephropathy. Am J Nephrol. 2010;32:456–461. doi: 10.1159/000320730. [DOI] [PubMed] [Google Scholar]
- 182.Ronkainen J, Ala-Houhala M, Autio-Harmainen H, et al. Long-term outcome 19 years after childhood IgA nephritis: a retrospective cohort study. Pediatr Nephrol. 2006;21:1266–1273. doi: 10.1007/s00467-006-0163-x. [DOI] [PubMed] [Google Scholar]
- 183.Abe S. Pregnancy in IgA nephropathy. Kidney Int. 1991;40(6):1098–1102. doi: 10.1038/ki.1991.320. [DOI] [PubMed] [Google Scholar]
- 184.Liu Y, Ma X, Lv J, Shi S, Liu L, Chen Y, Zhang H. Risk factors for pregnancy outcomes in patients with IgA nephropathy: a matched cohort study. Am J Kidney Dis. 2014;64(5):730–736. doi: 10.1053/j.ajkd.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 185.Oh HJ, Han SH, Yoo DE, Kim SJ, Park JT, Kim JK, Yoo TH, Kang SW, Choi KH. Reduced pre-pregnancy proteinuria is associated with improving postnatal maternal renal outcomes in IgA nephropathy women. Clin Nephrol. 2011;76(6):447–454. doi: 10.5414/CN107208. [DOI] [PubMed] [Google Scholar]
- 186.Zand L, Williams A, Babovic-Vuksanovic D, Nwoko R, Cornell L, Garovic V. The Case | Renal dysfunction in a pregnant patient with IgA nephropathy. Kidney Int. 2014;85(6):1477–1478. doi: 10.1038/ki.2013.322. [DOI] [PubMed] [Google Scholar]
- 187.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2(3):529–542. doi: 10.2215/CJN.04121206. [DOI] [PubMed] [Google Scholar]
- 188.Nishimoto K, Shiiki H, Nishino T, et al. Glomerular hypertrophy in preeclamptic patients with focal segmental glomerulosclerosis. A morphometric analysis. Clin Nephrol. 1999;51(4):209–219. [PubMed] [Google Scholar]
- 189.Kwiatkowski S, Kwiatkowska E, Rzepka R, Kurkiewicz V, Mikołajek-Bedner W, Torbè A. Development of a focal segmental glomerulosclerosis after pregnancy complicated by preeclampsia: case report and review of literature. J Matern Fetal Neonatal Med. 2015;29(10):1566–1569. doi: 10.3109/14767058.2015.1053865. [DOI] [PubMed] [Google Scholar]
- 190.Lee HS, Kim TS. A morphometric study of preeclamptic nephropathy with focal segmental glomerulosclerosis. Clin Nephrol. 1995;44(1):14–21. [PubMed] [Google Scholar]
- 191.Kambham N, Markowitz GS, Valeri AM, et al. Obesity related glomerulomegaly: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 192.French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. doi: 10.1016/S0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- 193.Burton PJ, Waddell BJ. Dual function of 11beta-hydroxysteroid dehydrogenase in placenta: modulating placental glucocorticoid passage and local steroid action. Biol Reprod. 1999;60:234–240. doi: 10.1095/biolreprod60.2.234. [DOI] [PubMed] [Google Scholar]
- 194.van Runnard Heimel PJ, Schobben AF, Huisjes AJ, Franx A, Bruinse HW. The transplacental passage of prednisolone in pregnancies complicated by early-onset HELLP syndrome. Placenta. 2005;26(10):842–845. doi: 10.1016/j.placenta.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 195.Ronco P, Debiec H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet. 2015;385(9981):1983–1992. doi: 10.1016/S0140-6736(15)60731-0. [DOI] [PubMed] [Google Scholar]
- 196.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371(24):2277–2287. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Packham DK, North RA, Fairley KF, Whitworth JA, Kincaid-Smith P. Membranous glomerulonephritis and pregnancy. Clin Nephrol. 1987;28(2):56–64. [PubMed] [Google Scholar]
- 198.Malik GH, Al-Harbi AS, Al-Mohaya S, et al. Repeated pregnancies in patients with primary membranous glomerulonephritis. Nephron. 2002;91(1):21–24. doi: 10.1159/000057600. [DOI] [PubMed] [Google Scholar]
- 199.Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 200.Surian M, Imbasciati E, Cosci P, et al. Glomerular disease and pregnancy. A study of 123 pregnancies in patients with primary and secondary glomerular diseases. Nephron. 1984;36(2):101–105. doi: 10.1159/000183126. [DOI] [PubMed] [Google Scholar]
- 201.Malik GH, Al-Mohaya S, Shaikh JF, et al. Repeated pregnancies in patients with non-immunoglobulin a mesangioproliferative glomerulonephritis. Am J Nephrol. 2001;21(5):378–382. doi: 10.1159/000046278. [DOI] [PubMed] [Google Scholar]
- 202.Kurdoglu M, Kurdoglu Z, Adali E, Soyoral Y, Erkoc R. Successful management of membranoproliferative glomerulonephritis type I in pregnancy. Arch Gynecol Obstet. 2010;281(1):105–109. doi: 10.1007/s00404-009-1071-7. [DOI] [PubMed] [Google Scholar]
- 203.De Galasso L, Gigante A, Pirozzi N, et al. Acute renal failure and nephrotic syndrome due to membranoproliferative nephritis during the second trimester of pregnancy. Clin Nephrol. 2011;75(5):480–483. doi: 10.5414/CN106584. [DOI] [PubMed] [Google Scholar]
- 204.Ruiz-Irastorza G, Lima F, Alves J, et al. Increased rate of lupus flare during pregnancy and the puerperium, a prospective study of 78 pregnancies. Br J Rheumatol. 1996;35:133–138. doi: 10.1093/rheumatology/35.2.133. [DOI] [PubMed] [Google Scholar]
- 205.Huong DL, Wechsler B, Vauthier-Brouzes D, Beaufils H, Lefebvre G, Piette JC. Pregnancy in past or present lupus nephritis: a study of 32 pregnancies from a single centre. Ann Rheum Dis. 2001;60:599–604. doi: 10.1136/ard.60.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Moroni G, Quaglini S, Banfi G, et al. Pregnancy in lupus nephritis. Am J Kideny Dis. 2002;40:713–720. doi: 10.1053/ajkd.2002.35678. [DOI] [PubMed] [Google Scholar]
- 207.Moroni G, Ponticelli C. Pregnancy after lupus nephritis. Lupus. 2005;14:89–94. doi: 10.1191/0961203305lu2066oa. [DOI] [PubMed] [Google Scholar]
- 208.Carmona F, Font J, Moga I, Lazzaro I, Cervera R, Pac V, Balasch L. Class III–IV proliferative lupus nephritis and pregnancy: a study of 42 cases. Am J Reprod Immunol. 2005;53:182–188. doi: 10.1111/j.1600-0897.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 209.Zhang L, Abe K, Hashimoto H, Takasaki Y. Pregnancy and fetal outcomes in patients with lupus nephritis. Nihon Rinsho Meneki Gakkai Kaishi. 2007;30:185–192. doi: 10.2177/jsci.30.185. [DOI] [PubMed] [Google Scholar]
- 210.Imbasciati E, Tincani A, Gregorini G, et al. Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome. Nephrol Dial Transplant. 2009;24:519–525. doi: 10.1093/ndt/gfn348. [DOI] [PubMed] [Google Scholar]
- 211.Bramham K, Hunt BJ, Bewley S, Germain S, Calatayud I, Khamashta MA, Nelson-Piercy C. Pregnancy outcomes in systemic lupus erythematosus with and without previous nephritis. J Rheumatol. 2011;38:1906–1913. doi: 10.3899/jrheum.100997. [DOI] [PubMed] [Google Scholar]
- 212.Saavedra MA, Cruz-Reyes C, Vera-Lastra O, et al. Impact of previous lupus nephritis on maternal and fetal outcomes during pregnancy. Clin Rheumatol. 2012;31:813–819. doi: 10.1007/s10067-012-1941-4. [DOI] [PubMed] [Google Scholar]
- 213.Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–2558. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 214.Sanchez-Guerrero J, Uribe AG, Jimenez-Santana L, et al. A trial of contraceptive methods in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2539–2549. doi: 10.1056/NEJMoa050817. [DOI] [PubMed] [Google Scholar]
- 215.Fischer-Betz R, Specker C, Brinks R, Aringer M, Schneider M. Low risk of renal flares and negative outcomes in women with lupus nephritis conceiving after switching from mycophenolate mofetil to azathioprine. Rheumatology (Oxford) 2013;52:1070–1076. doi: 10.1093/rheumatology/kes425. [DOI] [PubMed] [Google Scholar]
- 216.Ponticelli C, Moroni G. Immunosuppression in pregnant women with systemic lupus erythematosus. Expert Rev Clin Immunol. 2015;11:549–552. doi: 10.1586/1744666X.2015.1033404. [DOI] [PubMed] [Google Scholar]
- 217.Leroux M, Desveaux C, Parcevaux M, et al. Impact of hydroxychloroquine on preterm delivery and intrauterine growth restriction in pregnant women with systemic lupus erythematosus: a descriptive cohort study. Lupus. 2015;24(13):1384–1391. doi: 10.1177/0961203315591027. [DOI] [PubMed] [Google Scholar]
- 218.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, et al. Maternal hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation. 2012;126:76–82. doi: 10.1161/CIRCULATIONAHA.111.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Abarientos C, Sperber K, Shapiro DL, et al. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin Drug Saf. 2011;10:705–714. doi: 10.1517/14740338.2011.566555. [DOI] [PubMed] [Google Scholar]
- 220.Kaaja R, Julkunen H, Ammälä P, Palosuo T, Kurki P. Intravenous immunoglobulin treatment of pregnant patients with recurrent pregnancy losses associated with antiphospholipid antibodies. Acta Obstet Gynecol Scand. 1993;72:63–66. doi: 10.3109/00016349309013355. [DOI] [PubMed] [Google Scholar]
- 221.Petri M. Immunosuppressive drug use in pregnancy. Autoimmunity. 2013;36:51–56. doi: 10.1080/0891693031000067296. [DOI] [PubMed] [Google Scholar]
- 222.Perricone R, De Carolis C, Kröegler B, et al. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology (Oxford) 2008;47:646–651. doi: 10.1093/rheumatology/ken046. [DOI] [PubMed] [Google Scholar]
- 223.Coomarasamy A, Honest H, Papaioannou S, Gee H, Khan KS. Aspirin for prevention of preeclampsia in women with historical risk factors: a systematic review. Obstet Gynecol. 2003;101:1319–1332. doi: 10.1016/s0029-7844(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 224.Duley L, Henderson-Smart DJ, Knight M, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;18(2):CD000492. doi: 10.1002/14651858.CD004659.pub2. [DOI] [PubMed] [Google Scholar]
- 225.Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol. 2002;100:408–413. doi: 10.1097/00006250-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 226.Ruffatti A, Salvan E, Del Ross T, et al. Treatment strategies and pregnancy outcomes in antiphospholipid syndrome patients with thrombosis and triple antiphospholipid positivity. A European multicentre retrospective study. Thromb Haemost. 2014;112:727–735. doi: 10.1160/TH14-03-0191. [DOI] [PubMed] [Google Scholar]
- 227.Chakravarty EF, Colon I, Langen ES, et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. 2005;192:1897–1904. doi: 10.1016/j.ajog.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 228.Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. SA Cardiovasc Res. 2014;101:579–586. doi: 10.1093/cvr/cvu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Galdman D, Tandon A, Ibañez D, Urowitz MB. The effect of lupus nephritis on pregnancy outcome and fetal and maternal complications. J Rheumatol. 2010;37:754–758. doi: 10.3899/jrheum.090872. [DOI] [PubMed] [Google Scholar]
- 230.Hou S. Pregnancy in chronic renal insufficiency and end-stage renal disease. Am J Kidney Dis. 1999;33:235–252. doi: 10.1016/S0272-6386(99)70296-9. [DOI] [PubMed] [Google Scholar]
- 231.Clark CA, Spitzer KA, Laskin CA. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol. 2005;32:1709–1712. [PubMed] [Google Scholar]
- 232.Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr. 2003;142:678–683. doi: 10.1067/mpd.2003.233. [DOI] [PubMed] [Google Scholar]
- 233.Clowse ME, Magder LS, Witter F, Petri M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 2005;52:514–521. doi: 10.1002/art.20864. [DOI] [PubMed] [Google Scholar]
- 234.Rahman P, Gladman DD, Urowitz MB. Clinical predictors of fetal outcome in systemic lupus erythematosus. J Rheumatol. 1998;25:1526–1530. [PubMed] [Google Scholar]
- 235.McNeil HP, Chesterman CN, Krilis SA. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/S0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- 236.Hayslett JP, Lynn RI. Effect of pregnancy in patients with lupus nephropathy. Kidney Int. 1980;18:207–220. doi: 10.1038/ki.1980.129. [DOI] [PubMed] [Google Scholar]
- 237.Lateef A, Petri M. Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol. 2013;27:435–447. doi: 10.1016/j.berh.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Doria A, Bajocchi G, Tonon M, Salvarani C. Pre-pregnancy counselling of patients with vasculitis. Rheumatology. 2008;47:iii13–iii15. doi: 10.1093/rheumatology/kem250. [DOI] [PubMed] [Google Scholar]
- 239.Pagnoux C, Le Guern V, Goffinet F, et al. Pregnancies in systemic necrotizing vasculitides: report on 12 women and their 20 pregnancies. Rheumatology. 2001;50:953–961. doi: 10.1093/rheumatology/keq421. [DOI] [PubMed] [Google Scholar]
- 240.Tuin J, Sanders JSF, de Joode AAE, Stegeman C. Pregnancy in women diagnosed with ANCA-Associated Vasculitis: favorable outcome for mother and child. Arthritis Care Res (Hoboken) 2012;64:539–545. doi: 10.1002/acr.21556. [DOI] [PubMed] [Google Scholar]
- 241.Clowse MEB, Richeson RL, Pieper C, Merkel PA, for the Vasculitis Clinical Research Consortium Pregnancy outcomes among patients with vasculitis. Arthritis Care Res. 2013;65(1370):1374. doi: 10.1002/acr.21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Croft AP, Smith SW, Carr S, et al. Successful outcome of pregnancy in patients with anti-neutrophil cytoplasm antibody-associated small vessel vasculitis. Kidney Int. 2015;87:807–811. doi: 10.1038/ki.2014.329. [DOI] [PubMed] [Google Scholar]
- 243.Sangle SR, Vounotrypidis P, Briley A, et al. Pregnancy out come in patients with sistemi vasculitis: a single-centre marched case-control study. Rheumatology (Oxford) 2015;54:1582–1586. doi: 10.1093/rheumatology/kev018. [DOI] [PubMed] [Google Scholar]
- 244.Soh MC, Nelson-Piercy C. High-risk pregnancy and the rheumatologist. Rheumatology (Oxford) 2015;54(4):572–587. doi: 10.1093/rheumatology/keu394. [DOI] [PubMed] [Google Scholar]
- 245.Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117(5):1499–1506. doi: 10.1182/blood-2010-07-295444. [DOI] [PubMed] [Google Scholar]
- 246.Steinke JM, Mauer M. Lessons learned from studies of the natural history of diabetic nephropathy in young type 1 diabetic patients. Pediatr Endocrinol Rev. 2008;5(4):958–963. [PubMed] [Google Scholar]
- 247.Furukawa T, Tokunaga S, Iwashita K, Sato K, Terashima M, Hora K, Oguchi H, Furuta S, Shigematsu H. Diabetic glomerulosclerosis with nephrotic syndrome in early pregnancy. Nippon Jinzo Gakkai shi. 1987;29:541–545. [PubMed] [Google Scholar]
- 248.Biesenbach G, Zazgornik J. Incidence of transient nephrotic syndrome during pregnancy in diabetic women with and without pre-existing microalbuminuria. Br Med J. 1989;299:366–367. doi: 10.1136/bmj.299.6695.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Combs CA, Rosenn B, Kitzmiller JL, Khoury JC, Wheeler BC, Miodovnik M. Early-pregnancy proteinuria in diabetes related to preeclampsia. Obstet Gynecol. 1993;82:802–807. [PubMed] [Google Scholar]
- 250.Coletta J, Simpson LL. Maternal medical disease and stillbirth. Clin Obstet Gynecol. 2010;53(3):607–616. doi: 10.1097/GRF.0b013e3181eb2ca0. [DOI] [PubMed] [Google Scholar]
- 251.Ringholm L, Mathiesen ER, Kelstrup L, Damm P. Managing type 1 diabetes mellitus in pregnancy—from planning to breastfeeding. Nat Rev Endocrinol. 2012;8:659–667. doi: 10.1038/nrendo.2012.154. [DOI] [PubMed] [Google Scholar]
- 252.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193(6):1923–1935. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 253.Mathiesen ER, Ringholm L, Damm P. Pregnancy management of women with pregestational diabetes. Endocrinol Metab Clin North Am. 2011;40(4):727–738. doi: 10.1016/j.ecl.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 254.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S, Guideline Development Group Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207. doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 255.Carr DB, Koontz GL, Gardella C, Holing EV, Brateng DA, Brown ZA, Easterling TR. Diabetic nephropathy in pregnancy: suboptimal hypertensive control associated with preterm delivery. Am J Hypert. 2006;19:513–519. doi: 10.1016/j.amjhyper.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 256.Niensen LR, Damm P, Mathiesen ER. Improved pregnancy outcome in type 1 diabetic women with microalbuminuria or diabetic nephropathy: effect of intensified antihypertensive therapy? Diabetes Care. 2009;32(1):38–44. doi: 10.2337/dc08-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jensen DM, Damm P, Ovesen P, Mølsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Moeller M, Mathiesen ER. Microalbuminuria, preeclampsia, and preterm delivery in pregnant women with type 1 diabetes. Diabetes Care. 2010;33:1. doi: 10.2337/dc09-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Yogev Y, Chen R, Ben-Haroush A, Hod M, Bar J. Maternal overweight and pregnancy outcome in women with type-1 diabetes mellitus and different degrees of nephropathy. J Matern Fetal Neonatal Med. 2010;23(9):999–1003. doi: 10.3109/14767050903544744. [DOI] [PubMed] [Google Scholar]
- 259.Wu M, Wang D, Zand L, et al. Pregnancy outcomes in autosomal dominant polycystic kidney disease: a case-control study. J Matern Fetal Neonatal Med. 2015;29(5):807–812. doi: 10.3109/14767058.2015.1019458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chapman AB, Johnson AM, Gabow PA. Pregnancy outcome and its relationship to progression of renal failure in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994;5(5):1178–1185. doi: 10.1681/ASN.V551178. [DOI] [PubMed] [Google Scholar]
- 261.Vora N, Perrone R, Bianchi DW. Reproductive issues for adults with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51(2):307–318. doi: 10.1053/j.ajkd.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 262.Milutinovic J, Fialkow PJ, Agodoa LY, Phillips LA, Bryant JI. Fertility and pregnancy complications in women with autosomal dominant polycystic kidney disease. Obstet Gynecol. 1983;61(5):566–570. [PubMed] [Google Scholar]
- 263.Backe J, Kristen P, Arlt W, Steck T. Postpartum rupture of a kidney cyst in familial polycystic kidney degeneration and HELLP syndrome. Z Geburtshilfe Neonatol. 1996;200(3):122–126. [PubMed] [Google Scholar]
- 264.Szmigielski W, Klamut M, Caban B, Wieczorek P, Tereszkiewicz J. Superselective arterial embolization in the control of hemorrhage from ruptured cyst in polycystic kidney in pregnancy. Pol Przegl Radiol. 1988;52(2):110–111. [PubMed] [Google Scholar]
- 265.Gigarel N, Frydman N, Burlet P, et al. Preimplantation genetic diagnosis for autosomal recessive polycystic kidney disease. Reprod Biomed Online. 2008;16(1):152–158. doi: 10.1016/S1472-6483(10)60569-X. [DOI] [PubMed] [Google Scholar]
- 266.Banks N, Bryant J, Fischer R, Huizing M, Gahl WA, Gunay-Aygun M. Pregnancy in autosomal recessive polycystic kidney disease. Arch Gynecol Obstet. 2015;291(3):705–708. doi: 10.1007/s00404-014-3445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nicolle LE (2012) Urinary tract infection in adults. In: Brenner and Rector’s the kidney, 9th edn. Saunders. ISBN: 978-1-4160-6193-9, 36, pp 1356–1382
- 268.Piccoli GB, Colla L, Burdese M, et al. Development of kidney scars after acute uncomplicated pyelonephritis: relationship with clinical laboratory and imaging data at diagnosis. World J Urol. 2006;24:66–73. doi: 10.1007/s00345-005-0044-0. [DOI] [PubMed] [Google Scholar]
- 269.Piccoli GB, Consiglio V, Deagostini MC, et al. The clinical and imaging presentation of acute “non complicated” pyelonephritis: a new profile for an ancient disease. BMC Nephrol. 2011;15(12):68. doi: 10.1186/1471-2369-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lee YJ, Lee JH, Park YS. Risk factors for renal scar formation in infants with first episode of acute pyelonephritis: a prospective clinical study. J Urol. 2012;187(3):1032–1036. doi: 10.1016/j.juro.2011.10.164. [DOI] [PubMed] [Google Scholar]
- 271.Raz R, Sakran W, Chazan B, et al. Long-term follow-up of women hospitalized for acute pyelonephritis. Clin Infect Dis. 2003;37:1014–1020. doi: 10.1086/377737. [DOI] [PubMed] [Google Scholar]
- 272.Martinell J, Jodal U, Lidin-Janson G. Pregnancies in women with and without renal scarring after urinary infection in childhood. BMJ. 1990;300:840. doi: 10.1136/bmj.300.6728.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Millar LK, Cox SM. Urinary tract infections complicating pregnancy. Infect Dis Clin N Am. 1997;11:13–26. doi: 10.1016/S0891-5520(05)70339-1. [DOI] [PubMed] [Google Scholar]
- 274.Stamm WE. Management of urinary tract infection in adults. NEJM. 1993;329:1328–1334. doi: 10.1056/NEJM199310283291808. [DOI] [PubMed] [Google Scholar]
- 275.Castellino S, Cabiddu F, Gernone G et al (2013) Rene e Gravidanza: Suggerimenti di pratica clinica e di applicazione delle Linee-guida. G Ital Nefrol, vol S59, Anno 30, Luglio
- 276.Nicolle LE. A practical approach to the management of complicated urinary tract infection. Drugs Aging. 2001;18:243–254. doi: 10.2165/00002512-200118040-00002. [DOI] [PubMed] [Google Scholar]
- 277.Nowicki B. Urinary tract infection in pregnant women: old dogmas and current concepts regarding pathogenesis. Curr Infect Dis Rep. 2002;4(6):529–535. doi: 10.1007/s11908-002-0041-z. [DOI] [PubMed] [Google Scholar]
- 278.Nowicki B, Sledzinska A, Samet A, Nowicki S. Pathogenesis of gestational urinary tract infection: urinary obstruction versus immune adaptation and microbial virulence. BJOG. 2011;118(2):109–112. doi: 10.1111/j.1471-0528.2010.02706.x. [DOI] [PubMed] [Google Scholar]
- 279.Pastore LM, et al. Predictors of urinary tract infection at the first prenatal visit. Epidemiology. 1999;10:282. doi: 10.1097/00001648-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 280.Kass EH. Bacteriuria and pyelonephritis of pregnancy. Arch Intern Med. 1960;105:194. doi: 10.1001/archinte.1960.00270140016003. [DOI] [PubMed] [Google Scholar]
- 281.Naeye RL. Causes of the excessive rates of perinatal mortality and prematurity in pregnancies complicated by maternal urinary-tract infections. N Engl J Med. 1979;300:819. doi: 10.1056/NEJM197904123001503. [DOI] [PubMed] [Google Scholar]
- 282.Mittendorf R. Prevention of preterm delivery and low birth weight associated with asymptomatica bacteriuria. Clin Infect Dis. 1992;14:927. doi: 10.1093/clinids/14.4.927. [DOI] [PubMed] [Google Scholar]
- 283.Patterson TF. Detection, significance and therapy of bacteriuria in pregnancy. Infect Dis Clin N Am. 1997;11:593. doi: 10.1016/S0891-5520(05)70375-5. [DOI] [PubMed] [Google Scholar]
- 284.Delzell JE., Jr Urinary tract infections during pregnancy. Am Fam Physician. 2000;61:713. [PubMed] [Google Scholar]
- 285.Sheiner E, Mazor-Drey E, Levy A. Asymptomatic bacteriuria during pregnancy. J Matern Fetal Neonatal Med. 2009;22(5):423–427. doi: 10.1080/14767050802360783. [DOI] [PubMed] [Google Scholar]
- 286.Smaill FM, Vasquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2007;2:CD000490. doi: 10.1002/14651858.CD000490.pub2. [DOI] [PubMed] [Google Scholar]
- 287.Guinto VT, De Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy (Review) Cochrane Database Syst Rev. 2010;9:CD007855. doi: 10.1002/14651858.CD007855.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Vasquez JC, Abalos E. Treatments for symptomatic urinary tract infections during pregnancy. CochraneDatabase of Systematic Reviews. 2011;1:CD002256. doi: 10.1002/14651858.CD002256.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Gratacòs E. Screening and treatment of asymptomatic bacteriuria in pregnancy prevent pyelonephritis. J Infect Dis. 1994;169:1390. doi: 10.1093/infdis/169.6.1390. [DOI] [PubMed] [Google Scholar]
- 290.Villar J. Nutritional and antimicrobial interventions to prevent preterm birth: an overview of randomized controlled trials. Obstet Gynecol Surv. 1998;53:575. doi: 10.1097/00006254-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 291.NICE clinical guideline 62 – 2008
- 292.Rouse DJ. Screening and treatment of asymptomatic bacteriuria of pregnancy to prevent pyelonephritis: a cost effectiveness and cost benefit analysis. Obstet Gynecol. 1995;86:119. doi: 10.1016/0029-7844(95)00097-B. [DOI] [PubMed] [Google Scholar]
- 293.Honest H, et al. Screening to prevent spontaneous preterm birth: systemtic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess. 2009;13(43):1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- 294.Kass EH. The role of asymptomatic bacteriuria in the pathogenesis of pyelonephritis. In: Quinn EL, Kass EH (eds) Biology of pyelonephritis. In: Quinn EL, Kass EH, editors. Biology of pyelonephritis. Boston: Little, Brown and Co.; 1960. pp. 399–412. [Google Scholar]
- 295.Millar L. Rapid enzymatic urine screening test to detect bacteriuria in pregnancy. Obstet Gynecol. 2000;95:601. doi: 10.1016/s0029-7844(99)00597-9. [DOI] [PubMed] [Google Scholar]
- 296.McNair RD. Evaluation of the centrifuged and Gram-steined smear, urinalysis and reagent strip testing to detect asymptomatic bacteriuria in obstetric patients. Am J Obstet Gynecol. 2000;182:1076. doi: 10.1067/mob.2000.105440. [DOI] [PubMed] [Google Scholar]
- 297.Shelton SD. Urinary interleukin-8 with asymptomatuc bacteriuria in pregnancy. Obstet Gynecol. 2001;97:583. doi: 10.1016/s0029-7844(00)01226-6. [DOI] [PubMed] [Google Scholar]
- 298.Hooton TM. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med. 2000;343(14):992. doi: 10.1056/NEJM200010053431402. [DOI] [PubMed] [Google Scholar]
- 299.Lin K, Fajardo K. U.S. Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults. Ann Intern Med. 2008;149:W20. doi: 10.7326/0003-4819-149-1-200807010-00009-w1. [DOI] [PubMed] [Google Scholar]
- 300.Nicolle LE, Bradley S, Colgan R, et al. IDSA guideline for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 301.Lucas MJ, Cunningham FG. Urinary infection in pregnancy. Clin Obstet Gynecol. 1993;36(4):855–868. doi: 10.1097/00003081-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 302.Cabiddu F, Castellino S, Daidone G, et al. Linee-guida Rene e Gravidanza. G. Ital. Nefrol. 2000;17:24–46. [Google Scholar]
- 303.Widmer M, Gülmezoglu AM, Mignini L, Roganti A (2011) Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database Syst Rev. 12:CD000491. doi:10.1002/14651858.CD000491.pub2 [DOI] [PMC free article] [PubMed]
- 304.Lumbiganon P, Laopaiboon M, Thinkhamrop J. Screening and treating asymptomatic bacteriuria in pregnancy. Curr Opin Obstet Gynecol. 2010;22(2):95–99. doi: 10.1097/GCO.0b013e3283374adf. [DOI] [PubMed] [Google Scholar]
- 305.Estebanez A, Pascual R, Gil V, Ortiz F, Santibáñez M, Pérez Barba C. Fosfomycin in a single dose versus a 7-day course of amoxicillin-clavulanate for the treatment of asymptomatic bacteriuria during pregnancy. Eur J Clin Microbiol Infect Dis. 2009;28(12):1457–1464. doi: 10.1007/s10096-009-0805-6. [DOI] [PubMed] [Google Scholar]
- 306.Keating GM. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs. 2013;73(17):1951–1966. doi: 10.1007/s40265-013-0143-y. [DOI] [PubMed] [Google Scholar]
- 307.Usta TA, Dogan O, Ates U, Yucel B, Onar Z, Kaya E. Comparison of single-dose and multiple-dose antibiotics for lower urinary tract infection in pregnancy. Int J Gynaecol Obstet. 2011;114(3):229–233. doi: 10.1016/j.ijgo.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 308.Schito GC, Naber KG, Botto H, Palou J, Mazzei T, Gualco L, Marchese A. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents. 2009;34(5):407–413. doi: 10.1016/j.ijantimicag.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 309.Rodríguez-Baño J, Alcalá JC, Cisneros JM, et al. Community infections caused by extended-spectrum beta-lactamase-producing E. coli. Arch Intern Med. 2008;168(17):1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 310.Andersen JT. Clarithromycin in early pregnancy and the risk of miscarriage and malformation: a register based nationwide cohort study. PLoS One. 2013;8(1):e53327. doi: 10.1371/journal.pone.0053327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Stein GE. Single-dose treatment of acute cystitis with fosfomycin tromethamine. Ann Pharmacother. 1998;32:215. doi: 10.1345/aph.17227. [DOI] [PubMed] [Google Scholar]
- 312.Crider KS. Antibacterial medication use during pregnancy and risk of birth defect: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978. doi: 10.1001/archpediatrics.2009.188. [DOI] [PubMed] [Google Scholar]
- 313.American College of Obstetricians and Gynecologists Committee on Obstetric Practice ACOG Committee Opinion no 494. Obstet Gynecol. 2011;117(6):1484. doi: 10.1097/AOG.0b013e3182238c57. [DOI] [PubMed] [Google Scholar]
- 314.Nordeng H. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121:306–313. doi: 10.1097/AOG.0b013e31827c5f88. [DOI] [PubMed] [Google Scholar]
- 315.Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343(22):1608–1614. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 316.Bar-Oz B. The safety of quinolones—a meta-analysis of pregnancy outcome. Eur J Obstet Gynecol Reprod Biol. 2009;143(2):75–78. doi: 10.1016/j.ejogrb.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 317.Macejko AM, Schaeffer AJ. Asymptomatic bacteriuria and symptomtic urinary tract infections during pregnancy. Urol Clin North Am. 2007;34:35–42. doi: 10.1016/j.ucl.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 318.Hill JB, Sheffield JS, McIntire DD, Wendel GD., Jr Acute pyelonephritis in pregnancy. Obstet Gynecol. 2005;105(1):18–23. doi: 10.1097/01.AOG.0000149154.96285.a0. [DOI] [PubMed] [Google Scholar]
- 319.Wing DA, Fassett MJ, Getahun D (2014) Acute pyelonephritis in pregnancy: an 18-year retrospective analysis. Am J Obstet Gynecol 210(3):219.e1-6 [DOI] [PubMed]
- 320.Artero A, Alberola J, Eiros JM, Nogueira JM, Cano A. Pyelonephritis in pregnancy. How adequate is empirical treatment? Rev Esp Quimioter. 2013;26(1):30–33. [PubMed] [Google Scholar]
- 321.Minassian C, Thomas SL, Williams DJ, Campbell O, Smeeth L. Acute maternal infection and risk of pre-eclampsia: a population-based case-control study. PLoS One. 2013;8(9):e73047. doi: 10.1371/journal.pone.0073047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Riley JM, Dudley AG, Semins MJ. Nephrolithiasis and pregnancy: has the incidence been rising? Endourol. 2014;28(3):383–386. doi: 10.1089/end.2013.0570. [DOI] [PubMed] [Google Scholar]
- 323.Ross AE, Handa S, Lingeman JE, Matlaga BR. Kidney stones during pregnancy: an investigation into stone composition. Urol Res. 2008;36(2):99. doi: 10.1007/s00240-008-0138-4. [DOI] [PubMed] [Google Scholar]
- 324.Smith CL, Kristensen C, Davis M, Abraham PA. An evaluation of the physicochemical risk for renal stone disease during pregnancy. Clin Nephrol. 2001;55(3):205–211. [PubMed] [Google Scholar]
- 325.Butler EL, Cox SM, Cunningham FG. Symptomatic nephrolithiasis complicating pregnancy. Obstet Gynecol. 2000;96:573. doi: 10.1016/s0029-7844(00)01017-6. [DOI] [PubMed] [Google Scholar]
- 326.McAleer SJ, Loughlin KR. Nephrolithiasis and pregnancy. Curr Opin Urol. 2004;14:123. doi: 10.1097/00042307-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 327.Elgamasy A, Elsherif A. Use of Doppler ultrasonography and rigid ureteroscopy for managing symptomatic ureteric stones during pregnancy. BJU Int. 2010;106:262. doi: 10.1111/j.1464-410X.2009.08950.x. [DOI] [PubMed] [Google Scholar]
- 328.Fulgham PF, Assimos DG, Pearle MS, Preminger GM. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol. 2013;189:1203. doi: 10.1016/j.juro.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 329.Semins MJ, Trock BJ, Matlaga BR. The safety of ureteroscopy during pregnancy: a systematic review and meta-analysis. J Urol. 2009;181:139. doi: 10.1016/j.juro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 330.Watterson JD, Girvan AR, Beiko DT, et al. Ureteroscopy and holmium: YAG laser lithotripsy: an emerging definitive management strategy for symptomatic ureteral calculi in pregnancy. Urology. 2002;60:383. doi: 10.1016/S0090-4295(02)01751-X. [DOI] [PubMed] [Google Scholar]
- 331.Adanur S, Ziypak T, Bedir F, et al. Ureteroscopy and holmium laser lithotripsy: is this procedure safe in pregnant women with ureteral stones at different locations? Arch Ital Urol Androl. 2014;86(2):86–89. doi: 10.4081/aiua.2014.2.86. [DOI] [PubMed] [Google Scholar]
- 332.Piccoli GB, Attini R, Parisi S, et al. Excessive urinary tract dilatation and proteinuria in pregnancy: a common and overlooked association? BMC Nephrol. 2013;27(14):52. doi: 10.1186/1471-2369-14-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Piccoli GB, Attini R, De Pascale A, et al. Protean presentation and multiple challenges of nephrocalcinosis in pregnancy (six pregnancies in four patients) Nephrol Dial Transplant. 2012;27(3):1131–1138. doi: 10.1093/ndt/gfr390. [DOI] [PubMed] [Google Scholar]
- 334.Lewis DF, Robichaux AG, Jaekle RK, et al. Urolithiasis in pregnancy. Diagnosis, management and pregnancy outcome. J Reprod Med. 2003;48:28–32. [PubMed] [Google Scholar]
- 335.Swartz MA, Lydon-Rochelle MT, Simon D, et al. Admission for nephrolithiasis in pregnancy and risk of adverse birth outcomes. Obstet Gynecol. 2007;109:1099. doi: 10.1097/01.AOG.0000259941.90919.c0. [DOI] [PubMed] [Google Scholar]
- 336.Rosenberg E, Sergienko R, Abu-Ghanem S, et al. Nephrolithiasis during pregnancy: characteristics, complications, and pregnancy outcome. World J Urol. 2011;29:743–747. doi: 10.1007/s00345-011-0719-7. [DOI] [PubMed] [Google Scholar]
- 337.Banhidy F, Acs N, Puho EH, et al. Maternal kidney stones during pregnancy and adverse birth outcomes, particularly congenital abnormalities in the offspring. Arch Gynecol Obstet. 2007;275:481–487. doi: 10.1007/s00404-006-0277-1. [DOI] [PubMed] [Google Scholar]
- 338.Jungers P, Houillier P, Chauveau D, et al. Pregnancy in women with reflux nephropathy. Kidney Int. 1996;50(2):593–599. doi: 10.1038/ki.1996.354. [DOI] [PubMed] [Google Scholar]
- 339.North RA, Taylor RS, Gunn TR. Pregnancy outcome in women with reflux nephropathy and the inheritance of vesico-ureteric reflux. Aust N Z J Obstet Gynaecol. 2000;40(3):280–285. doi: 10.1111/j.1479-828X.2000.tb03335.x. [DOI] [PubMed] [Google Scholar]
- 340.www.fda.gov. Accessed 20 June 2014
- 341.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 342.EBPG Expert Group on Renal Transplantation European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.10. Pregnancy in renal transplant recipients. Nephrol Dial Transplant. 2002;17(Suppl 4):50–55. [PubMed] [Google Scholar]
- 343.Østensen M, Khamashta M, Lockshin M, et al. Anti-inflammatory and immunosuppressive drugs and reproduction. Arthritis Res Ther. 2006;8:209. doi: 10.1186/ar1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051–1055. doi: 10.1097/00007890-200104270-00006. [DOI] [PubMed] [Google Scholar]
- 345.Kainz A, Harabacz I, Cowlrick IS, et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation. 2000;70:1718–1721. doi: 10.1097/00007890-200012270-00010. [DOI] [PubMed] [Google Scholar]
- 346.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 347.Sifontis NM, Coscia LA, Constantinescu S, et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation. 2006;82:1698–1702. doi: 10.1097/01.tp.0000252683.74584.29. [DOI] [PubMed] [Google Scholar]
- 348.Anderka MT, Lin AE, Abuelo DN, et al. Reviewing the evidence for mycophenolate mofetil as a new teratogen: case report and review of the literature. Am J Med Genet A. 2009;149:1241–1248. doi: 10.1002/ajmg.a.32685. [DOI] [PubMed] [Google Scholar]
- 349.Framarino-dei-Malatesta M, Derme M, Manzia TM, et al. Impact of mTOR-I on fertility and pregnancy: state of the art and review of the literature. Expert Rev Clin Immunol. 2013;9:781–789. doi: 10.1586/1744666X.2013.824243. [DOI] [PubMed] [Google Scholar]
- 350.Stokholm J, Schjørring S, Pedersen L, et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS One. 2013;8(12):e82932. doi: 10.1371/journal.pone.0082932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Martinez de Tejada B. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Environ Res Public Health. 2014;11(8):7993–8009. doi: 10.3390/ijerph110807993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E (2015) Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst Rev 6:CD002250 [DOI] [PMC free article] [PubMed]
- 353.Nahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: what is and is not known about teratogenic and toxic risks. Obstet Gynecol. 2006;107(5):1120–1138. doi: 10.1097/01.AOG.0000216197.26783.b5. [DOI] [PubMed] [Google Scholar]
- 354.Matuszkiewicz-Rowińska J, Małyszko J, Wieliczko M. Urinary tract infections in pregnancy: old and new unresolved diagnostic and therapeutic problems. Arch Med Sci. 2015;11(1):67–77. doi: 10.5114/aoms.2013.39202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Grandone E, Villani M, Tiscia GL. Aspirin and heparin in pregnancy. Expert Opin Pharmacother. 2015;16(12):1793–1803. doi: 10.1517/14656566.2015.1066335. [DOI] [PubMed] [Google Scholar]
- 356.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7–47. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Reisenberger K, Egarter C, Kapiotis S, et al. Transfer of erythropoietin across the placenta perfused in vitro. Obstet Gynecol. 1997;89(5 Pt 1):738–742. doi: 10.1016/S0029-7844(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 358.Krafft A, Bencaiova G, Breymann C. Selective use of recombinant human erythropoietin in pregnant patients with severe anemia or nonresponsive to iron sucrose alone. Fetal Diagn Ther. 2009;25(2):239–245. doi: 10.1159/000223441. [DOI] [PubMed] [Google Scholar]
- 359.Torrance HL, Benders MJ, Derks JB, et al. Maternal allopurinol during fetal hypoxia lowers cord blood levels of the brain injury marker S-100B. Pediatrics. 2009;124(1):350–357. doi: 10.1542/peds.2008-2228. [DOI] [PubMed] [Google Scholar]
- 360.Hoeltzenbein M, Stieler K, Panse M, et al. Allopurinol use during pregnancy—outcome of 31 prospectively ascertained cases and a phenotype possibly indicative for teratogenicity. PLoS One. 2013;8(6):e66637. doi: 10.1371/journal.pone.0066637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.De-Regil LM, Palacios C, Ansary A et al (2012) Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 2:CD008873 [DOI] [PMC free article] [PubMed]
- 362.Lawlor DA, Wills AK, Fraser A, et al. Association of maternal vitamin D status during pregnancy with bone-mineral content in offspring: a prospective cohort study. Lancet. 2013;381:2176. doi: 10.1016/S0140-6736(12)62203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin D. Washington: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 364.Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]
- 365.Wagner CL, Greer FR, The Section on Breastfeeding and Committee on Nutrition Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 366.ACOG Committee on Obstetric Practice ACOG Committee Opinion No. 495: vitamin D: screening and supplementation during pregnancy. Obstet Gynecol. 2011;118(1):197–198. doi: 10.1097/AOG.0b013e318227f06b. [DOI] [PubMed] [Google Scholar]
- 367.Holick MF, Binkley NC, Bischoff-Ferrari HA et al. (2011) Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline (published correction appears in J Clin Endocrinol Metab 2011;96(12):3908). J Clin Endocrinol Metab. 2011;96(7):1911–1930 [DOI] [PubMed]
- 368.Piccoli GB, Cabiddu G, Attini R, et al. Pregnancy in chronic kidney disease: questions and answers in a changing panorama. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):625–642. doi: 10.1016/j.bpobgyn.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 369.Chakravarty EF, Murray ER, Kelman A, Farmer P. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117(5):1499–1506. doi: 10.1182/blood-2010-07-295444. [DOI] [PubMed] [Google Scholar]
- 370.Klink DT, van Elburg RM, Schreurs MW, van Well GT. Rituximab administration in third trimester of pregnancy suppresses neonatal B-cell development. Clin Dev Immunol. 2008;2008:271363. doi: 10.1155/2008/271363. [DOI] [PMC free article] [PubMed] [Google Scholar]