Abstract
Introduction
A unique anti-interleukin (IL)-13 monoclonal antibody, RPC4046, was generated on the basis of differential IL-13 receptor (R) blockade as assessed in a murine asthma model; the safety, tolerability, pharmacokinetics, and pharmacodynamics of RPC4046 were evaluated in a first-in-human study.
Methods
Anti-IL-13 antibodies with varying receptor blocking specificity were evaluated in the ovalbumin-induced murine asthma model. A randomized, double-blind, placebo-controlled, dose-escalation first-in-human study (NCT00986037) was conducted with RPC4046 in healthy adults and patients with mild to moderate controlled asthma.
Results
In the ovalbumin model, blocking IL-13 binding to both IL-13Rs (IL-13Rα1 and IL-13Rα2) inhibited more asthma phenotypic features and more fully normalized the distinct IL-13 gene transcription associated with asthma compared with blocking IL-13Rα1 alone. In humans, RPC4046 exposure increased dose-dependently; pharmacokinetics were similar in healthy and asthmatic subjects, and blockade of both IL-13Rs uniquely affected IL-13 gene transcription. A minority of participants (28%) had antidrug antibodies, which were transient and appeared not to affect pharmacokinetics. Adverse event profiles were similar in healthy and asthmatic subjects, without dose-related or administration route differences, systemic infusion-related reactions, or asthma symptom worsening. Adverse events were mild to moderate, with none reported as probably related to RPC4046 or leading to discontinuations. Non-serious upper respiratory tract infections were more frequent with RPC4046 versus placebo.
Conclusion
RPC4046 is a novel anti-IL-13 antibody that blocks IL-13 binding to both receptors and more fully blocks the asthma phenotype. These results support further investigation of RPC4046 for IL-13-related allergic/inflammatory diseases (e.g., asthma and eosinophilic esophagitis).
Funding
AbbVie Inc. sponsored the studies and contributed to the design and conduct of the studies, data management, data analysis, interpretation of the data, and in the preparation and approval of the manuscript.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-017-0525-8) contains supplementary material, which is available to authorized users.
Keywords: Asthma, IL-13, Respiratory
Introduction
Interleukin (IL)-13 is known to play a pivotal role in the pathogenesis of T helper cell type 2 (Th2)-mediated inflammatory diseases such as asthma (reviewed by Corren et al. 2013) [1]. IL-13 is a 17-kDa glycoprotein produced predominantly by activated T cells of the Th2 lineage, although it can also be produced by non-T cell populations such as mast cells [1]. The function of IL-13 includes inducing eosinophilic inflammation [2, 3] and promoting immunoglobulin (Ig) isotype switching to IgE in human B cells [4]. Initially, IL-13 was supported as a drug target by virtue of the elevated levels of IL-13 found in the lungs and sputum of patients with asthma [5, 6]. A potential causal relationship was established in preclinical models by demonstrating that recombinant IL-13 challenge to the lungs induces an asthmatic phenotype, namely inducing airway hyperresponsiveness (AHR), mucus production, and inflammatory cellular infiltration, including eosinophilia [7–10]. Neutralization of IL-13 by either soluble IL-13 receptor (R) α2 fusion proteins or an anti-IL-13 antibody has been shown to modulate AHR, mucus production, and cellular infiltration in murine models of allergen-induced asthma [7, 8, 11–13]. In humans, a variant of IL-13, R110Q, is associated with asthma and higher serum IL-13 levels [14]. Several therapeutic antibodies that neutralize IL-13 are currently being tested for the treatment of asthma, including dupilumab, lebrikizumab, and tralokinumab [15]. Several of these agents have demonstrated efficacy in phase 2 studies based on improvement in lung function as measured by forced expiratory volume in 1 s (FEV1) and/or a reduction in exacerbations [16–19].
The receptor system for IL-13 is well described (reviewed in Ingram and Kraft 2012) [20]. IL-13 binds to two cell surface receptors, IL-13Rα1 and IL-13Rα2. IL-13Rα1 interacts with IL-13 with a low affinity (K D ~2–10 nM) with subsequent recruitment of IL-4Rα to form the high-affinity (K D ~0.4 nM) signaling heterodimeric receptor complex [21, 22] that induces signal transduction pathways including signal transducer and activator of transcription 6 (STAT6) and the insulin receptor substrate-2 pathways [23]. IL-13Rα2 binds IL-13 with high affinity (K D ~0.6–1.2 nM) [24]. The function of IL-13Rα2 as a decoy receptor that negatively regulates IL-13 activation of IL-13Rα1/IL-4Rα has been demonstrated in several settings [25]. There are also some reports that suggest IL-13Rα2 may also function as a signaling receptor that induces transforming growth factor β (TGF-β) synthesis and fibrosis via the activator protein 1 (AP-1) pathway in macrophages and possibly other cell types [26].
Thus, blocking of IL-13 function can be achieved with antibodies that either bind IL-4Rα itself, as is the case with dupilumab [27], or that bind IL-13 directly. There are several anti-IL-13 antibodies that block the function of IL-13, either by preventing binding of IL-4Rα to the IL-13/IL-13Rα1 complex (as is the case with lebrikizumab [28] and anrukinzumab [29]) or by interfering with binding to both IL-13Rα1 and IL-13Rα2 [as is the case with tralokinumab [30] as well as RPC4046 (previously known as ABT-308; AbbVie Inc., North Chicago, IL)]. These antibodies inhibit the formation of the high-affinity IL-13Rα1/IL-4Rα signaling complex and also prevent binding of IL-13 to IL-13Rα2 receptor and any IL-13Rα2-driven signaling. RPC4046 is a selective, humanized, recombinant monoclonal antibody against the IL-13 molecule that has a high affinity and potency for both human wild-type and variant IL-13 and blocks binding of IL-13 to both IL-13Rα1 and IL-13Rα2; this antibody was developed for the treatment of Th2-dependent diseases such as asthma and eosinophilic esophagitis (EoE).
We hypothesized that anti-IL-13 antibodies that block binding to both IL-13Rα1 and IL-13Rα2 would have superior efficacy compared with an antibody that blocks binding to IL-13Rα1 alone. Herein, we describe testing this hypothesis in an ovalbumin (OVA)-induced murine asthma model, using surrogate anti-IL-13 antibodies to block binding of IL-13 to either IL-13Rα1 alone or both IL-13Rα1 and IL-13Rα2. We assessed outcomes related to inhibition of the asthma phenotype such as AHR, mucus production, and lung inflammation, as well as IL-13-dependent acidic mammalian chitinase (AMCase) production and gene expression. Subsequently, we performed a first-in-human, single-center, randomized, double-blind, placebo-controlled, three-part dose-escalation study (NCT00986037) in which RPC4046 was administered to healthy volunteers and patients with mild to moderate controlled asthma to assess the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of single escalating intravenous (IV) doses or multiple escalating subcutaneous (SC) doses. Finally, the gene transcription profile was evaluated in the first-in-human study. Some of the results of these studies have been previously reported in the form of abstracts [31–33].
Methods
Preclinical Methods
OVA-Induced Asthma Model
The asthma phenotype was induced as described in the literature [7] in female Balb/c mice (Taconic Biosciences, Hudson, NY, USA) aged 6–8 weeks with the following modification. Endotoxin-free ovalbumin (Sigma-Aldrich, St. Louis. MO, USA) was prepared using Detoxi-Gel™ (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s protocol, and the final preparation contained less than 0.1 endotoxin units/mg protein. Animals were sensitized to OVA on days 0 and 7 by intraperitoneal (IP) injection of 8 µg OVA in 2 mg alum. On days 14 and 16, animals received an intranasal challenge of 0.3 µg OVA in 50 µL sterile phosphate-buffered saline (PBS), and the asthma phenotype was assessed approximately 24 h later on day 17 of the model. Details of assessments of AHR, lung pressure, enzymatic, antibody, and gene expression are in Supplemental Methods. Animal studies were conducted under a program accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal studies were reviewed and approved by AbbVie Bioresearch Center’s Institutional Animal Care and Use Committee.
Antibodies and Steroid Treatments
The dependence of the model on IL-13 was tested by mouse anti-IL-13 antibodies administered by a single IP injection on day 13 (Supplemental Methods). Dexamethasone (3 mg/kg) was administered orally daily on days 13–17 as a positive control.
Statistical Analyses
Data sets from AHR, mucus, cell infiltrate in the bronchoalveolar lavage fluid (BALF), and AMCase were normalized by calculating the percentage of control level induced by OVA challenge and then pooling data from multiple experiments. Statistical significance was determined by one-way analysis of variance with the Dunnett posttest. A P value of less than 0.05 was considered statistically significant. Dose dependence was determined with a posttest for linear trend analysis.
Clinical Methods
Study Design
M10-378 (NCT00986037) was a phase 1, first-in-human, single- and multiple-escalating dose, placebo-controlled, double-blind, randomized, three-part study conducted according to a sequential design. Part 1 of the study consisted of four groups of healthy volunteers with five participants in each group randomized 4:1 to receive a single dose of IV RPC4046 (0.3, 1, 3, or 10 mg/kg) or placebo, respectively, on day 1. Part 2 of the study consisted of three groups of participants with mild to moderate controlled asthma with five participants in each group randomized 4:1 to receive a single IV dose of RPC4046 (0.3, 3, or 10 mg/kg) or placebo, respectively, on day 1. Part 3 of the study consisted of two groups of participants with mild to moderate controlled asthma with six participants assigned to each group and randomized 4:2 to receive three weekly SC injections of RPC4046 (0.3 or 3 mg/kg) or placebo, respectively, on days 1, 8, and 15. Institutional review board (Compass IRB, Mesa, AZ, USA) approval of the protocol and written informed consent from all participants were obtained.
Study Population
Part 1 included adults aged 18–55 years in generally good health and not taking concomitant medications. Parts 2 and 3 included adults aged 18–55 years with mild to moderate controlled asthma diagnosed at least 6 months before screening and having (1) FEV1 of at least 70% at screening and baseline, and (2) a positive methacholine challenge test within at least 12 months or demonstrated airway reversibility in pulmonary function measurements. Further details are in Supplemental Methods.
Pharmacokinetics and Gene Expression Analysis
Details of PK, antidrug antibody (ADA), and gene expression assessments are in Supplemental Methods.
Safety
Adverse events (AEs) and vital sign monitoring was performed at each study visit. Physical examination, electrocardiogram, and laboratory assessments were performed at protocol-specified study visits. Further details are in the Supplemental Methods.
Statistical Analyses
The sample size was determined from the perspective of tolerability, based on the probability that a given AE would not be observed in a group of four participants administered an assigned RPC4046 regimen, relative to the true population incidence rate. For demographic and safety analyses, descriptive statistics were provided by dose level, with participants assigned to placebo combined across groups. For PK analyses, an analysis of variance was performed for PK parameters for each study part. In general, no imputation of data was performed unless the missing values were expected to have an effect on study conclusions or point estimates.
Results
Preclinical Data in Murine Asthma Model: Effects of Blocking Various IL-13Rs
Airway Hyperresponsiveness and Interleukin-13-Dependent Mediators
Consistent with literature reports, in our hands, OVA challenge induced the asthma phenotype as demonstrated by AHR defined as increased airway resistance following methacholine challenge, by cellular infiltrate (predominantly eosinophil) and by mucus hypersecretion (Supplemental Fig. 1). We confirmed the IL-13 dependence of the OVA model with two neutralizing rat anti-mouse IL-13 antibodies that dose-dependently inhibited AHR as well as AMCase, an enzyme that is transcriptionally regulated by IL-13 [34, 35] (Fig. 1a, b). While both antibodies effectively reduced AHR and AMCase levels, the 51D9 antibody blocking IL-13 binding to both IL-13 receptors reduced these endpoints to levels comparable to that achieved by dexamethasone and was more effective than the 48D3 antibody, blocking binding of IL-13 to IL-13Rα1. Surprisingly, while the 51D9 antibody was effective at inhibiting mucus hypersecretion, as measured by levels of murine mucin 5ac (Muc5ac) in the BALF, the 48D3 antibody had no effect on this endpoint (Fig. 1c). This result was confirmed by histologic assessment of goblet cell hyperplasia as measured by area of PAS+ cells within the lung (Fig. 1d). Similar levels of antibody concentrations were detected in the serum as well as the BALF, indicating that the explanation for the differential efficacy was not related to systemic exposure or penetration of the antibodies within the lung (Fig. 1e).
Fig. 1.
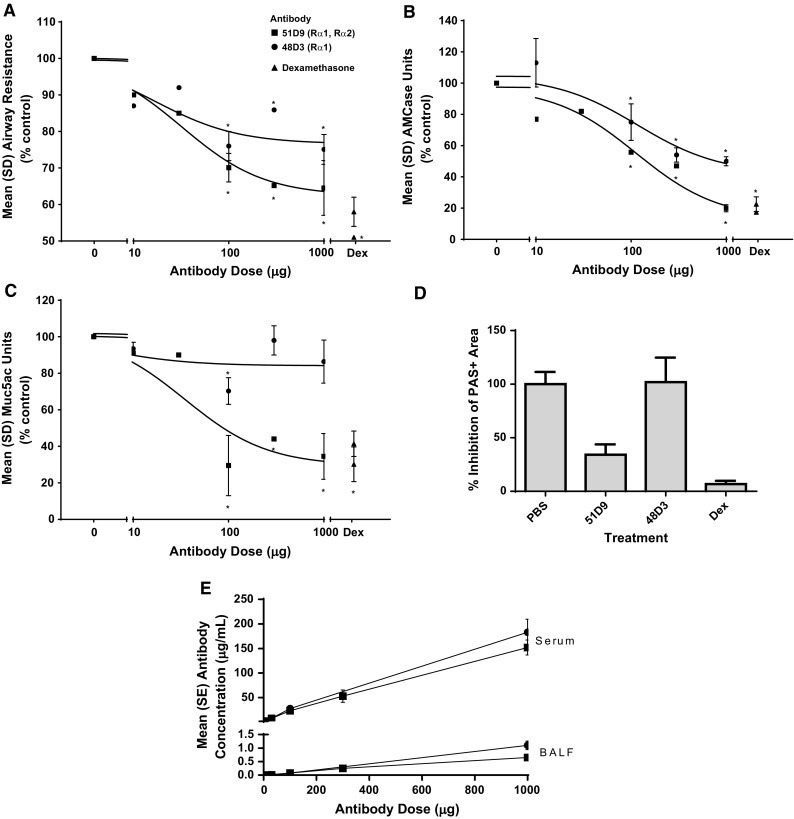
Anti-IL-13 antibody effects in the murine OVA-induced asthma model. Measurement of a AHR, b AMCase levels in the BALF, and c, d mucus production measured by Muc5ac ELISA and histologic assessment by PAS reactivity following administration of antibody 51D9 or 48D3 before OVA challenge. a–c Anti-mIL-13 antibodies that block binding of IL-13 to either IL-13Rα1 alone or both IL-13Rα1 and IL-13Rα2 similarly inhibit AHR and AMCase production, whereas blockade of IL-13Rα2 binding appears to be required for inhibition of mucus production. Pooled data from 3–4 studies; n = 10–30/data point. d Area of mucus-secreting epithelial cells visualized by PAS+ staining in OVA-challenged animals treated with either 51D9, 48D3, or dexamethasome. e Quantification of antibody levels in both serum and BALF of treated mice measured at the termination of the experiment. AHR airway hyperresponsiveness, AMCase acidic mammalian chitinase, ANOVA analysis of variance, BALF bronchoalveolar lavage fluid, Dex dexamethasone, ELISA enzyme-linked immunosorbent assay, IL interleukin, mIL-13 mouse IL-13, Muc5ac mucin 5ac, OVA ovalbumin, PAS periodic acid-Schiff, PBS phosphate-buffered saline, R receptor. *P < 0.05 by one-way ANOVA and the Dunnett posttest
Gene Expression Analysis
Gene expression was analyzed in the lungs of OVA-treated mice (Fig. 2) to understand whether genes were differentially regulated when comparing blockade of IL-13Rα1 alone versus both receptors. In mice treated with 48D3, there was only weak normalization of lung gene expression toward the expression levels found in control PBS-challenged mice. In contrast, there was preferential, strong normalization of lung gene expression in mice treated with 51D9. Thus, blockade of both IL-13Rs resulted in normalization of a unique lung gene expression pattern compared with blockade of IL-13Rα1 alone. Among the genes strongly upregulated by OVA treatment and normalized when both receptors were blocked were Muc5ac and CLCA3, which were also found to be upregulated in equivalent human sputum messenger RNAs (mRNAs; MUC5AC and CLCA1) isolated from asthma patients (Supplemental Table 1). The goal was to understand how this normalization translated to humans by examining key gene expression in a phase 1 study.
Fig. 2.
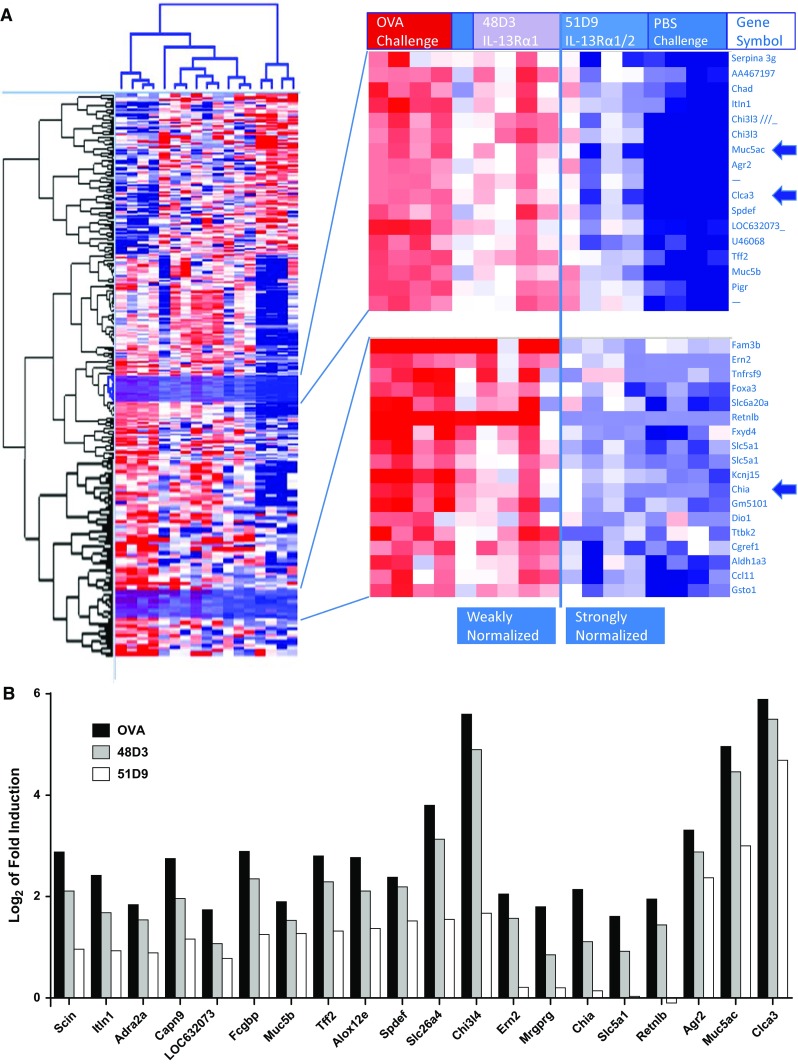
Lung gene expression analysis in murine OVA asthma models. a, left Hierarchical clustering of 361 probe sets showing at least twofold regulation (PBS treated vs OVA treated) with P ≤ 0.05 (the Student t test). a, right Highlight of cluster containing the most highly induced genes by OVA treatment, yet showing preferential normalization in response to 51D9. The positions of CLCA3 (the murine equivalent to human CLCA1) and Muc5ac, which were taken forward into human studies, are indicated. b Highly inducible mRNAs are particularly sensitive to dual receptor blockade. To highlight the effect of dual receptor blockade, the antibody response profile for each of the 20 mRNAs most highly induced in OVA-treated lungs compared with control lungs is indicated. Induced genes were defined as those upregulated at least threefold with P ≤ 0.01 using the Student t test. Values were normalized by subtraction of the PBS control. CLCA calcium-activated chloride channel regulator, IL interleukin, mRNA messenger RNA, Muc5ac mucin 5ac, OVA ovalbumin, PBS phosphate-buffered saline, R receptor
On the basis of these preclinical murine data, we developed a humanized anti-human IL-13 monoclonal antibody, RPC4046, which recognizes both wild-type human IL-13 and the common polymorphic variant R110Q, with binding affinities of 52 and 50 pM, respectively (Supplemental Table 2). RPC4046 fully neutralized IL-13-induced thymus and activation-regulated chemokine from lung epithelial A-549 cells with potencies ranging from 80 to 513 pM. RPC4046 also fully neutralized the bioactivity of the R110Q variant form of IL-13 and natural IL-13 prepared from human T cell culture with similar potency to wild-type recombinant IL-13. RPC4046 completely inhibited human IL-13 binding to both IL-13Rα1 and IL-13Rα2 as measured by enzyme-linked immunosorbent assay (ELISA) with potencies of 352 and 631 pM, respectively.
Before being evaluated in the first-in-human trial, RPC4046 was first evaluated in the murine model of asthma by pharmacologic administration of human IL-13 and measuring effects on the asthma phenotype. RPC4046 administered before human recombinant IL-13 challenge inhibited AMCase by 50% and 95% at RPC4046 exposures of 10 and 151 μg/mL, respectively (Supplemental Fig. 2a). RPC4046 also dose-dependently inhibited AHR (maximal inhibition of 70.5%; Supplemental Fig. 2b) and IL-13-dependent gene expression in BALF, including maximal inhibition of Muc5ac levels by 70.7% and AMCase activity by 54.2% (Supplemental Fig. 2c).
Phase 1 First-in-Human Study with RPC4046
Patient Disposition and Demographics
A total of 47 participants were enrolled in the phase 1 study: 20 in part 1, 15 in part 2, and 12 in part 3. All participants in part 2 completed the study; five participants in part 1 (four withdrew consent, one lost to follow-up) and one participant (lost to follow-up) in part 3 prematurely discontinued the study. All participants who did not complete the study received their assigned dose(s) of study drug and were included in the PK and safety analyses. Patient demographics are summarized in Table 1.
Table 1.
Demographics of healthy volunteers and patients with asthma
| Characteristic | Part 1 | Part 2 | Part 3 | |||
|---|---|---|---|---|---|---|
| Placebo (n = 4) | RPC4046 (n = 16) | Placebo (n = 3) | RPC4046 (n = 12) | Placebo (n = 4) | RPC4046 (n = 8) | |
| Age, mean ± SD (years ) | 36.0 ± 13.1 | 28.1 ± 10.1 | 23.0 ± 2.0 | 33.3 ± 11.9 | 27.5 ± 8.3 | 28.8 ± 7.7 |
| Weight, mean ± SD (kg) | 77.5 ± 5.8 | 78.0 ± 9.1 | 82.3 ± 11.7 | 77.8 ± 11.9 | 73.8 ± 4.5 | 79.3 ± 10.8 |
| Height, mean ± SD (cm) | 176.8 ± 8.7 | 179.7 ± 6.1 | 178.0 ± 3.6 | 172.5 ± 8.2 | 173.3 ± 3.3 | 174.9 ± 6.2 |
| BMI, mean ± SD (kg/m2) | 24.8 ± 1.1 | 24.2 ± 2.9 | 26.0 ± 3.9 | 26.1 ± 3.2 | 24.5 ± 0.6 | 25.9 ± 3.6 |
| Male, n (%) | 4 (100) | 15 (93.8) | 3 (100) | 9 (75.0) | 4 (100) | 7 (87.5) |
| White, n (%) | 2 (50.0) | 8 (50.0) | 3 (100) | 7 (58.3) | 1 (25.0) | 5 (62.5) |
| Black, n (%) | 2 (50.0) | 8 (50.0) | 0 | 3 (25.0) | 2 (50.0) | 0 |
| Asian, n (%) | 0 | 0 | 0 | 2 (16.7) | 1 (25.0) | 3 (37.5) |
BMI body mass index
Pharmacokinetic Assessments
Initial PK analysis following single-dose administration of RPC4046 demonstrated long half-life, low volume of distribution, and high bioavailability. Following a single IV infusion of RPC4046, systemic exposure [area under the plasma concentration versus time curve (AUC) and maximum plasma concentration (C max)] increased in a dose-dependent (Fig. 3a) and dose-proportional manner in healthy volunteers over the 0.3–10 mg/kg range. For patients with asthma, AUC and C max were generally comparable to healthy volunteers; however, these parameters increased in a slightly more (30–40%) than dose-proportional manner over the same 33-fold dose range. Mean terminal half-lives following a single IV administration ranged from about 16–27 days, and the mean volume of distribution ranged from 72.8 to 108.9 mL/kg following IV infusions over the 0.3 to 10 mg/kg dose range.
Fig. 3.
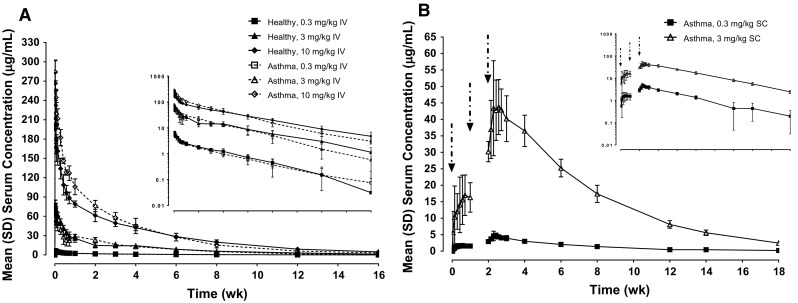
RPC4046 serum concentration–time profiles in healthy participants and patients with asthma. Mean (SD) RPC4046 serum concentration following (a) a single IV infusion in healthy volunteers and asthma patients or (b) three weekly SC doses in patients with asthma (linear and log-linear [inset] scales). Arrows indicate SC injections. IV intravenous, SC subcutaneous
Following multiple dosing (three doses) of RPC4046 SC at doses of 0.3 and 3 mg/kg in patients with asthma in part 3, RPC4046 PK were dose-proportional (Fig. 3b), and these doses resulted in about threefold accumulation after the third dose based on AUC0–168 (AUC in the dosing interval), as expected on the basis of the long half-life observed in healthy volunteers and patients with asthma. The median RPC4046 T max was about 3.5–4.5 days and appeared to be similar following the first and the third dose of RPC4046, as well as across 0.3 and 3 mg/kg SC doses. Mean terminal elimination half-lives were approximately 27 and 24 days following SC administration of 0.3 and 3 mg/kg, respectively. Based on PK modeling of IV and SC PK data, the estimated bioavailability of RPC4046 following SC administration was approximately 70%.
Immunogenicity
Overall, 10 of 36 participants (27.8%; three healthy volunteers and seven asthma patients) had measurable ADAs following either IV infusion (n = 9) or SC injection (n = 1) of RPC4046. For all but one participant with measurable ADAs, ADAs were detected early (approximately 2 weeks following dosing) and were transient in nature, with only a single positive sample. ADAs did not appear to affect the PK of RPC4046, although the number of participants was small.
Gene Expression Analysis
Similar to observations in the murine asthma model, we determined that several mRNAs related to IL-13 mechanism of action, including MUC5AC and CLCA1, were upregulated in induced sputum samples from patients with moderate to severe uncontrolled asthma compared with healthy volunteers (Supplemental Table 1). After 2 days of exposure to RPC4046, patient-level data indicated that the majority of asthma patients experienced net decreases in MUC5AC and CLCA1 mRNA compared with pretreatment levels (Fig. 4 and Supplemental Fig. 3), consistent with mouse data (Supplemental Fig. 2). The percentage decreases observed in those asthma patients experiencing a decrease in MUC5AC or CLCA1 were fairly similar, with a trend for increased responsiveness for both markers at higher doses, and none of the patients showing MUC5AC or CLCA1 increases in the 10-mg dose group. By contrast, most healthy volunteers did not show a decrease in MUC5AC or CLCA1 mRNA levels following RPC4046 administration. Thus, systemic RPC4046 exposure resulted in modulation of IL-13-regulated genes in the human lung in a fashion similar to that in the murine asthma model.
Fig. 4.
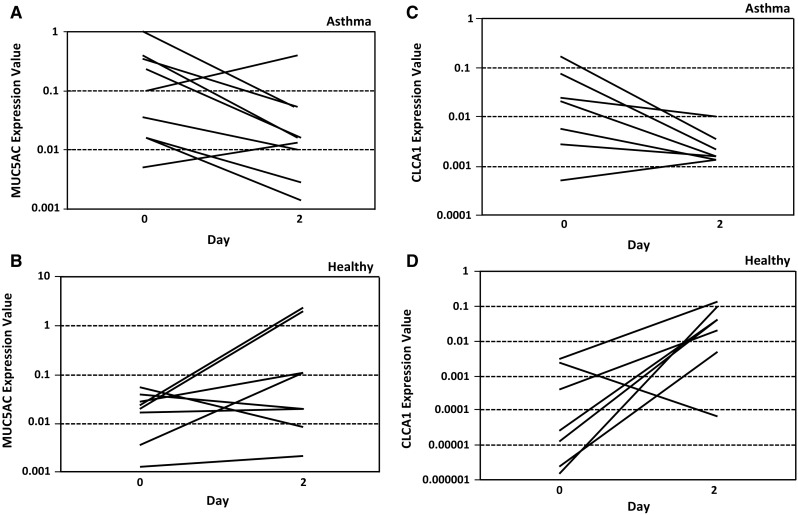
Changes in a, b MUC5AC and c, d CLCA1 mRNA expression levels, in individuals with detectable expression at baseline (day 0), after 2 days of exposure to RPC4046 (all doses). Absolute values in a, c individual patients with asthma and b, d individual healthy subjects. CLCA calcium-activated chloride channel regulator, mRNA messenger RNA, MUC5AC mucin 5AC
Safety
All participants (N = 47) received at least one dose of RPC4046 or placebo and were included in the safety analyses. The AE profile of RPC4046 was similar in healthy volunteers and asthma patients (Tables 2, 3). In part 1, 13 (81.3%) healthy volunteers who received RPC4046 and 2 (50.0%) who received placebo experienced at least one treatment-emergent AE. In part 2, 9 (75.0%) asthma patients who received RPC4046 and 2 (66.7%) who received placebo experienced at least one treatment-emergent AE. In part 3, 6 (75.0%) asthma patients who received RPC4046 and 3 (75.0%) who received placebo experienced at least one treatment-emergent AE. There were no dose-related increases or administration-specific trends in treatment-emergent AEs.
Table 2.
Treatment-emergent adverse events following a single IV infusion of RPC4046 in healthy volunteers and patients with asthma
| MedDRA SOC preferred term, n (%) | Part 1: healthy volunteers | Part 2: patients with asthma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 4) | RPC4046 single IV infusion | Placebo (n = 3) | RPC4046 single IV infusion | ||||||||
| 0.3 mg/kg (n = 4) | 1 mg/kg (n = 4) | 3 mg/kg (n = 4) | 10 mg/kg (n = 4) | Total (N = 16) | 0.3 mg/kg (n = 4) | 3 mg/kg (n = 4) | 10 mg/kg (n = 4) | Total (N = 12) | |||
| Any AE | 2 (50.0) | 3 (75.0) | 4 (100) | 3 (75.0) | 3 (75.0) | 13 (81.3) | 2 (66.7) | 3 (75.0) | 4 (100) | 2 (50.0) | 9 (75.0) |
| URTI | 0 | 2 (50.0) | 2 (50.0) | 0 | 0 | 4 (25.0) | 0 | 2 (50.0) | 1 (25.0) | 0 | 3 (25.0) |
| Viral URTI | 0 | 1 (25.0) | 1 (25.0) | 0 | 1 (25.0) | 3 (18.8) | 0 | 0 | 0 | 0 | 0 |
| Infusion site pain | 0 | 0 | 0 | 1 (25.0) | 0 | 1 (6.3) | 0 | 0 | 0 | 0 | 0 |
| Any AE at least possibly drug related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any severe AE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any SAE | 0 | 1 (25.0)* | 0 | 0 | 0 | 1 (6.3) | 0 | 0 | 0 | 0 | 0 |
| Any AE leading to study drug discontinuation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Any fatal AE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
AE adverse event, IV intravenous, MedDRA Medical Dictionary for Regulatory Activities, SAE serious AE, SOC system organ class, URTI upper respiratory tract infection
* Serious AE was a worsening foot bunion that required hospitalization for bunionectomy; the event was assessed by the investigator as moderate in severity and not related to the study drug
Table 3.
Treatment-emergent adverse events following three weekly subcutaneous doses of RPC4046 in patients with asthma
| MedDRA SOC preferred term, n (%) | Part 3: patients with asthma | |||
|---|---|---|---|---|
| Placebo (n = 4) | RPC4046 3 weekly SC doses | |||
| 0.3 mg/kg (n = 4) | 3 mg/kg (n = 4) | Total (N = 8) | ||
| Any AE | 3 (75.0) | 4 (100) | 2 (50.0) | 6 (75.0) |
| URTI | 1 (25.0) | 3 (75.0) | 0 | 3 (37.5) |
| Viral URTI | 0 | 1 (25.0) | 0 | 1 (12.5) |
| Infusion site pain | 0 | 0 | 0 | 0 |
| Any AE at least possibly drug related | 1 (25.0) | 0 | 0 | 0 |
| Any severe AE | 0 | 0 | 0 | 0 |
| Any SAE | 0 | 0 | 0 | 0 |
| Any AE leading to study drug discontinuation | 0 | 0 | 0 | 0 |
| Any fatal AE | 0 | 0 | 0 | 0 |
AE adverse event, MedDRA Medical Dictionary for Regulatory Activities, SAE serious AE, SC subcutaneous, SOC system organ class, URTI upper respiratory tract infection
All observed events reported with RPC4046 were assessed as mild to moderate in severity, and none were considered probably related to the study drug. In each part of the study, the proportion of participants reporting an upper respiratory tract infection (URTI) or viral URTI was greater among those receiving RPC4046 compared with placebo (Tables 2, 3). No clinically meaningful changes were detected in other safety analyses, including vital signs, electrocardiogram variables, and laboratory measurements. There was only one report of mild infusion site pain in a healthy volunteer receiving IV RPC4046 3 mg/kg that was assessed by the investigator as not related to the study drug. One asthma patient receiving placebo in part 3 experienced a treatment-emergent AE of decreased FEV1, which was assessed as mild in severity and possibly related to the study drug. A review of events that could represent asthma deterioration, including spirometry data, did not suggest worsening of disease among the asthma patients. During all parts of the study, no deaths, discontinuations due to AEs, or other significant AEs occurred. However, one healthy volunteer in the RPC4046 0.3-mg/kg IV group experienced a serious AE of worsening foot bunion requiring hospitalization for bunionectomy; the event was assessed by the investigator as moderate in severity and not related to the study drug.
Discussion
In this manuscript, we describe the biochemical properties of the anti-IL-13 antibody RPC4046, which has high affinity for IL-13 and potently blocks both the wild-type and R110Q variants of IL-13, and describe the binding modality that results in blockade of IL-13 binding to both IL-13Rα1 and IL-13Rα2. A surrogate anti-mouse IL-13 antibody that matched these properties of RPC4046 demonstrated compelling efficacy in the preclinical model of ovalbumin-induced asthma with somewhat improved efficacy compared with an antibody that could only block binding to IL-13Rα1, suggesting that IL-13Rα2 may play a role in the pathogenesis of the asthma phenotype. RPC4046 was safe and well tolerated in a phase 1 study that included both healthy volunteers and patients with asthma; it also demonstrated dose-proportional pharmacokinetic properties and inhibition of IL-13-driven gene transcripts.
We hypothesized that the asthma phenotype would be differentially ameliorated by antibodies that neutralized IL-13 effects on both its receptors, IL-13Rα1 and IL-13Rα2, versus blockade of IL-13Rα1 alone. To test this concept, we generated rat anti-mouse IL-13 (mIL-13) antibodies that exhibit differential blockade of IL-13 to IL-13Rα1 and IL-13Rα2. Indeed, greater efficacy was seen in a murine asthma model when both receptors were blocked compared with blockade of only IL-13Rα1. Both anti-mIL-13 antibodies inhibited AHR in a dose-dependent fashion; however, despite having comparable in vitro affinity and potency, as well as exposure in both lung and serum, blockade of both receptors demonstrated greater overall efficacy and in vivo potency based on inhibition of AHR and neutralization of the IL-13-dependent generation of AMCase activity. Additionally, blocking both receptors reduced mucus production, whereas blockade of IL-13Rα1 alone had little or no such effect across equivalent doses. Both antibodies significantly inhibited BALF AMCase, a biomarker for IL-13 activity in the lungs. Finally, the normalization of an IL-13-dependent gene transcription profile was unique for blockade of both receptors compared with blockade of IL-13Rα1 alone. These data demonstrate that an antibody that blocks IL-13 binding to both IL-13Rα1 and IL-13Rα2 confers greater in vivo efficacy than an antibody that blocks binding to IL-13Rα1 alone; moreover, these data support a distinct role for IL-13Rα2 in mucus hypersecretion in a murine model of asthma.
Several explanations for the greater efficacy observed with the dual Rα1/Rα2 blocking antibody were explored. The simplest explanation was that this antibody had greater penetration into the target tissue, the lung, owing to inherent properties of the protein and this was explored by measuring the exposure of the antibody in both the serum and the BALF. A similar concentration of the two antibodies was observed in serum and the BALF, indicating that this was not the cause of the differential effect on the asthma phenotype. Both antibodies were the same isotype and therefore the differences could not be accounted for with differential Fc effector functions. This led us to explore additional potential explanations related to blocking IL-13 binding to one or both of its receptors, IL-13Rα1 and IL-13Rα2. It is well established that IL-13 binds to IL-13Rα1 which then recruits the IL-4Rα, triggering STAT3-dependent signaling. The role of IL-13Rα2 is less clear. IL-13Rα2 has been shown to function as a decoy receptor that binds and neutralizes IL-13 in vivo. In mice, the circulating levels of IL-13Rα2 are quite high; this has been shown in many animal models, mostly through the use of IL-13Rα2 knockout approaches, which lead to increased levels of IL-13 and exacerbation of IL-13-driven phenotypes including fibrosis [25]. A direct role of IL-13Rα2 has been demonstrated to promote the fibrotic phenotype in a bleomycin-induced model of lung fibrosis [26]. These authors demonstrated increased TGF-β production in macrophages that was dependent on IL-13Rα2 through an AP-1-dependent mechanism. Blockade of IL-13Rα2 in vivo with siRNA resulted in a reduction of TGF-β and fibrosis. We interrogated the murine asthma model for differential regulation of TGF-β and did not find any evidence of differential modulation of that pathway (data not shown). It is possible that blocking a potential IL-13Rα2 signaling pathway in the OVA-induced asthma model is contributing to the enhanced efficacy, perhaps through regulation of goblet cell hyperplasia. However, it is also possible that blockade of IL-13 to IL-13Rα2 preserves the decoy function of the circulating IL-13Rα2 in the mice. Interestingly, the function of IL-13Rα2 in human biology is much less well understood. While it may certainly serve as a decoy receptor, it is predominantly cell-associated, with little soluble IL-13Rα2 present in the serum [36]. Nonetheless, an antibody such as RPC4046 that blocks binding of IL-13 to IL-13Rα2 will preserve the decoy function of this receptor. It has also been suggested that IL-13Rα2 may play a role in the clearance of IL-13 by promoting cellular uptake [37] which was supported by a dose-dependent increase in serum IL-13 levels in healthy subjects dosed with IMA-026 (type 2: Rα1/α2 blocker), whereas accumulation of IL-13 was more modest in subjects dosed with anrukinzumab (type 3: blocks recruitment of IL-4Rα) [29]. Although serum IL-13 levels were not measured in this phase 1 study herein, pharmacodynamic measurements of IL-13-induced gene signatures demonstrated neutralization of IL-13 pathway with RPC4046.
These murine asthma nonclinical data led to the development of the selective, high-affinity humanized anti-IL-13 monoclonal antibody RPC4046, which blocks binding of both wild-type and variant human IL-13 to both IL-13Rα1 and IL-13Rα2. Similar to the data generated with the surrogate antibody 51D9, RPC4046 administered before challenge with human recombinant IL-13 in a murine model of asthma inhibited both AHR and IL-13-dependent gene expression in the BALF, including Muc5ac levels by 70.7% and AMCase activity by 54.2% (Supplemental Fig. 2). Thus, inhibiting IL-13 binding to both IL-13Rs with either 51D9 or RPC4046 effectively inhibited the asthma phenotype in rodent models. Furthermore, 90% inhibition of IL-13-induced AMCase could be seen at an RPC4046 exposure of 151 μg/mL, indicating a high systemic exposure was needed to obtain full inhibition of IL-13 in the lung. Finally, the R110Q variant is associated with the prevalence of asthma [14] and has also been shown to associate with IL-13Rα2 slower than wild type [38]. This raises the possibility that patients with this variant may be less able to neutralize pathogenic IL-13 with IL-13Rα2 and could therefore benefit from an antibody that neutralizes the variant form of IL-13.
In the first-in-human study, RPC4046 demonstrated a PK profile consistent with the low clearance and small volume of distribution of an IgG1 in healthy volunteers and in patients with mild to moderate controlled asthma. Following a single IV infusion or multiple SC injections over the tested dose range, the PK of RPC4046 appeared similar in healthy volunteers and asthma patients. The absolute bioavailability following SC administration was approximately 70%, and the mean terminal elimination half-life was similar (~16–27 days) with either administration route. With a single IV infusion, the systemic exposure (AUC and C max) to RPC4046 increased in a dose-proportional manner over the range of 0.3 to 10 mg/kg for healthy volunteers; however, for patients with mild to moderate controlled asthma, AUC and C max increased in a slightly more (30–40%) than dose-proportional manner over the same 33-fold dose range. For asthma patients administered three weekly SC doses, RPC4046 PK was dose-proportional between the 0.3- and 3-mg/kg doses. The accumulation of RPC4046 was as expected given its half-life: about threefold following three weekly SC doses.
Although 27.8% of participants had measurable ADAs following either IV or SC RPC4046 dosing, it is difficult to compare the relative immunogenicity of this agent to other biologics, as the assays utilized to measure the response vary greatly in sensitivity [39], resulting in increased ability to detect low-titer ADAs. Importantly, the ADA response in this phase 1 study appeared primarily transient and without impact on the PK of RPC4046. Larger studies are needed to make firm conclusions regarding immunogenicity.
Similar results were seen on IL-13-dependent gene transcription in the patients with mild to moderate asthma compared with data in the murine asthma model in that, in most asthma patients with measurable IL-13-dependent mRNA levels at baseline, those treated with RPC4046 experienced a decrease in IL-13-dependent gene transcription exemplified by MUC5AC and CLCA1 mRNA levels on day 2; this decrease was not seen in healthy volunteers. This IL-13 pharmacodynamic assessment suggests that the effects of RPC4046 on MUC5AC and CLCA1 may be specific to asthma patients, for example, being driven by an IL-13-dependent mechanism, whereas the mRNA detectable in healthy volunteers is driven by IL-13-independent processes. The limited study size did not permit a detailed exposure–response relationship, but there was a trend toward more marked decreases in both markers at higher doses (Supplemental Fig. 3), with no patients showing MUC5AC or CLCA1 increases in the 10-mg dose group. These data are similar to what was observed in the murine asthma model in that 50% and 90% inhibition of AMCase was seen with RPC4046 at exposures of 10 and 151 μg/mL, respectively (Supplemental Fig. 2); the C max values for 0.3, 3, and 10 mg/kg IV in the asthma patients were 7, 68, and 292 μg/mL, respectively. The paucity of placebo-treated samples precluded statistical comparisons of the effects of placebo versus RPC4046 in this study, which limits interpretation of the data. However, these data demonstrate that measuring mRNA levels in sputum may be a reasonable, less-invasive approach to assess local IL-13 inhibition within the lungs of asthma patients and warrants further investigation in a larger study.
Finally, RPC4046 was shown to be well tolerated and safe in the first-in-human study when administered as a single dose up to 10 mg/kg IV in healthy volunteers and patients with mild to moderate controlled asthma or as multiple doses up to 3 mg/kg SC in patients with mild to moderate controlled asthma. The AE profile was similar in asthma patients and healthy volunteers. No dose-related increases or administration-specific trends in treatment-emergent AEs were observed. In each part of the study, the proportion of participants reporting a URTI or viral URTI was greater among those receiving RPC4046 compared with those receiving placebo. All of these events were of mild or moderate severity, and none were judged by the investigator to be possibly or probably related to the study drug. There was one report of infusion site pain; no AEs of systemic infusion-related reactions were reported. A review of events that could represent deterioration in asthma, as well as spirometry data, did not suggest a worsening of underlying disease in asthma patients. No participants discontinued the study drug because of a treatment-emergent AE. One patient receiving RPC4046 had a serious AE of worsening bunion and underwent bunionectomy; the event was considered not related to the study drug. No clinically significant trends were detected in other safety analyses, including vital signs, electrocardiogram variables, and laboratory measurements. Taken together, these data indicate that an antibody that neutralizes both IL-13 receptors may provide an improved benefit to human asthma and that further study is warranted.
Besides asthma, the orphan indication of EoE may also be an indication of interest as an emerging worldwide disease that shares a common T helper cell type 2 pathway with asthma and is hypothesized to involve IL-13 in its pathogenesis. Like asthma, EoE is a chronic immune/allergic disease characterized clinically by esophageal dysfunction and histologically by eosinophil-predominant inflammation, with significant local upregulation of cytokines, including IL-13 [40, 41]. Additionally, IL-13 treatment of primary esophageal epithelial cells has been shown to induce a transcript profile that overlaps with the EoE-specific esophageal transcriptome [41]. Although blood levels of eosinophils, eotaxin-3, and IL-5 are elevated in EoE, their sensitivity and specificity are too low to be clinically helpful, and diagnosis requires endoscopy with biopsy analysis. Esophageal biopsies have revealed a 16-fold increase in esophageal IL-13 mRNA in EoE patients [41]. Likewise, the role of IL-13 in chronic tissue remodeling resulting in esophageal strictures is thought to involve IL-13Rα2 [42]. Therefore, as with asthma, blockade of both IL-13 receptors would potentially be advantageous in this condition, as both inflammation and tissue remodeling are thought to have pathophysiologic consequences in this disease [26, 43, 44]. Thus, the properties of RPC4046 in blocking binding of IL-13 to both of its receptors may also make it an effective treatment for EoE. In a recent phase 2 proof-of-concept clinical trial in adults with EoE (NCT02098473), RPC4046 met the primary endpoint of a statistically significant reduction in esophageal eosinophil counts in patients with active EoE [45, 46].
Asthma, similar to many inflammatory disorders, is a heterogeneous disease. Perhaps the most important finding for the continued advancement of the anti-IL-13 class of antibodies was the ability to identify biomarkers of IL-13-driven disease that could identify the patients who would benefit from this class of treatment. Several markers have been reported to stratify patients, including periostin [16], blood eosinophil level [19], and dipeptidyl peptidase IV [18]. This approach was vital to those successful phase 2 studies with this class of agents [16, 17]. However, recently it was reported that lebrikizumab had mixed results in the pivotal phase 3 trials on the primary endpoint of reduction in exacerbations [47]. The reason for failure of one of the trials is unclear [47]. It is important to note that several IL-13 pathway antagonists demonstrated efficacy when patients were stratified for IL-13 pathway markers, whereas prior trials with agents (anrukinzumab and AMG 317) that were not equipped with patient selection markers have failed [48, 49]. It is critically important to understand the differences between these trials to determine whether the inability to reach the primary endpoint of reduced asthma exacerbations was due to operational challenges in the global trial setting or whether the IL-13 mechanism is not sufficiently robust in patients with asthma to confer efficacy.
Conclusion
In summary, RPC4046 is a novel anti-IL-13 monoclonal antibody that blocks IL-13 binding to both of its receptors, generating a unique transcriptional profile at the site of inflammation that may in turn result in improved efficacy in Th2-type allergic diseases such as asthma and EoE. RPC4046 was well tolerated when administered as a single dose up to 10 mg/kg IV in healthy volunteers and patients with asthma or as multiple doses up to 3 mg/kg SC in patients with asthma, and exhibited PK characteristics typical of an IgG1. There were no dose-related increases or administration-specific trends in treatment-emergent AEs. The PK, PD, tolerability, and safety observed support continued evaluation of RPC4046 in EoE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The design, study conduct, analysis, and financial support of the clinical trials were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the content. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Research support was provided by Matthew Perham, Christian Goesse, Richard McCarthy, Christine Grinnel, Jamie Erickson, and Lian Rundell of AbbVie. Medical writing support was provided by Katherine Groschwitz, PhD, and Michael J. Theisen, PhD, of Complete Publication Solutions, LLC, and was funded by AbbVie. The authors acknowledge Sahana Bose and Renee Miller for their contributions to the nonclinical data and Amit Khatri for his contributions to the PK data. Publication charges and open access fees were funded by AbbVie. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Carolyn Cuff is an employee of AbbVie and may hold stock and/or options. Andrew L. Campbell is an employee of AbbVie and may hold stock and/or options. Barbara A. Hendrickson is an employee of AbbVie and may hold stock and/or options. Terry Melim is an employee of AbbVie and may hold stock and/or options. Catherine S. Tripp is a former employee of AbbVie and may hold stock and/or options. Andrew D. Cherniack is a former employee of AbbVie and may hold stock and/or options. Jeff Voss is a former employee of AbbVie and may hold stock and/or options. Chengbin Wu is a former employee of AbbVie and may hold stock and/or options. Kenneth Kim is employed by WCCT Global LLC, which has performed contract research for AbbVie.
Compliance with Ethics Guidelines
Animal studies were conducted under a program accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal studies were reviewed and approved by AbbVie Bioresearch Center’s Institutional Animal Care and Use Committee.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Presently retired: Catherine S. Tripp and Jeff Voss.
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/DB08F06016D8FBF3.
Catherine S. Tripp and Carolyn Cuff contributed equally.
References
- 1.Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep. 2013;13:415–420. doi: 10.1007/s11882-013-0373-9. [DOI] [PubMed] [Google Scholar]
- 2.Horie S, Okubo Y, Hossain M, et al. Interleukin-13 but not interleukin-4 prolongs eosinophil survival and induces eosinophil chemotaxis. Intern Med. 1997;36:179–185. doi: 10.2169/internalmedicine.36.179. [DOI] [PubMed] [Google Scholar]
- 3.Luttmann W, Knoechel B, Foerster M, Matthys H, Virchow JC, Jr, Kroegel C. Activation of human eosinophils by IL-13. Induction of CD69 surface antigen, its relationship to messenger RNA expression, and promotion of cellular viability. J Immunol. 1996;157:1678–1683. [PubMed] [Google Scholar]
- 4.Punnonen J, Aversa G, Cocks BG, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–3734. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 6.Saha SK, Berry MA, Parker D, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kibe A, Inoue H, Fukuyama S, et al. Differential regulation by glucocorticoid of interleukin-13-induced eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in mouse airways. Am J Respir Crit Care Med. 2003;167:50–56. doi: 10.1164/rccm.2110084. [DOI] [PubMed] [Google Scholar]
- 11.Taube C, Duez C, Cui ZH, et al. The role of IL-13 in established allergic airway disease. J Immunol. 2002;169:6482–6489. doi: 10.4049/jimmunol.169.11.6482. [DOI] [PubMed] [Google Scholar]
- 12.Yang G, Li L, Volk A, et al. Therapeutic dosing with anti-interleukin-13 monoclonal antibody inhibits asthma progression in mice. J Pharmacol Exp Ther. 2005;313:8–15. doi: 10.1124/jpet.104.076133. [DOI] [PubMed] [Google Scholar]
- 13.Walter DM, McIntire JJ, Berry G, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 14.Heinzmann A, Mao XQ, Akaiwa M, et al. Genetic variants of IL-13 signalling and human asthma and atopy. Hum Mol Genet. 2000;9:549–559. doi: 10.1093/hmg/9.4.549. [DOI] [PubMed] [Google Scholar]
- 15.Kau AL, Korenblat PE. Anti-interleukin 4 and 13 for asthma treatment in the era of endotypes. Curr Opin Allergy Clin Immunol. 2014;14:570–575. doi: 10.1097/ACI.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 17.Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70:748–756. doi: 10.1136/thoraxjnl-2014-206719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brightling CE, Chanez P, Leigh R, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:692–701. doi: 10.1016/S2213-2600(15)00197-6. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388:31–44. doi: 10.1016/S0140-6736(16)30307-5. [DOI] [PubMed] [Google Scholar]
- 20.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol. 2012;130:829–842. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 21.Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor α chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 22.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 24.Donaldson DD, Whitters MJ, Fitz LJ, et al. The murine IL-13 receptor α2: molecular cloning, characterization, and comparison with murine IL-13 receptor α1. J Immunol. 1998;161:2317–2324. [PubMed] [Google Scholar]
- 25.Mentink-Kane MM, Wynn TA. Opposing roles for IL-13 and IL-13 receptor α2 in health and disease. Immunol Rev. 2004;202:191–202. doi: 10.1111/j.0105-2896.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 26.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13α2 receptor is involved in induction of TGF-β1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 27.Santini G, Mores N, Malerba M, et al. Dupilumab for the treatment of asthma. Expert Opin Investig Drugs. 2017;26:357–366. doi: 10.1080/13543784.2017.1282458. [DOI] [PubMed] [Google Scholar]
- 28.Ultsch M, Bevers J, Nakamura G, et al. Structural basis of signaling blockade by anti-IL-13 antibody lebrikizumab. J Mol Biol. 2013;425:1330–1339. doi: 10.1016/j.jmb.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Tiwari A, Kasaian M, Heatherington AC, Jones HM, Hua F. A mechanistic PK/PD model for two anti-IL13 antibodies explains the difference in total IL-13 accumulation observed in clinical studies. mAbs. 2016;8:983–990. doi: 10.1080/19420862.2016.1172151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popovic B, Breed J, Rees DG, et al. Structural characterisation reveals mechanism of IL-13-neutralising monoclonal antibody tralokinumab as inhibition of binding to IL-13Rα1 and IL-13Rα2. J Mol Biol. 2017;429:208–219. doi: 10.1016/j.jmb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Melim T, Perham M, Murdock S, Goedken E, Wu C, Cuff C. In vivo characterization of ABT-308, a potent anti-IL-13 antibody for the treatment of asthma. Am J Respir Crit Care Med. 2010;181:A4035. [Google Scholar]
- 32.Tripp C, Campbell A, Hendrickson B, et al. The characterization of RPC4046, a novel anti-IL13 monoclonal antibody that blocks binding to IL-13. Ann Allergy Asthma Immunol. 2014;113:A104–A105. [Google Scholar]
- 33.Ying H, Miller R, Bose S, Argiriadi M, Cuff C, Wu C. ABT-308, a highly potent anti-IL-13 therapeutic antibody for the treatment of human asthma. Am J Respir Crit Care Med. 2010;181:A6644. [Google Scholar]
- 34.Miller R, Sadhukhan R, Wu C. Development of an in vitro potency bioassay for therapeutic IL-13 antagonists: the A-549 cell bioassay. J Immunol Methods. 2008;334:134–141. doi: 10.1016/j.jim.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Z, Zheng T, Homer RJ, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Sivaprasad U, Tabata Y, et al. IL-13Rα2 membrane and soluble isoforms differ in humans and mice. J Immunol. 2009;183:7870–7876. doi: 10.4049/jimmunol.0901028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kasaian MT, Raible D, Marquette K, et al. IL-13 antibodies influence IL-13 clearance in humans by modulating scavenger activity of IL-13Rα2. J Immunol. 2011;187:561–569. doi: 10.4049/jimmunol.1100467. [DOI] [PubMed] [Google Scholar]
- 38.Lacy ER. Equilibrium and kinetic analysis of human interleukin-13 and IL-13 receptor alpha-2 complex formation. J Mol Recognit. 2012;25:184–191. doi: 10.1002/jmr.2150. [DOI] [PubMed] [Google Scholar]
- 39.Song S, Yang L, Trepicchio WL, Wyant T. Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. J Immunol Res. 2016;2016:3072586. doi: 10.1155/2016/3072586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blanchard C, Stucke EM, Rodriguez-Jimenez B, et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J Allergy Clin Immunol. 2011;127:208–17.e7. [DOI] [PMC free article] [PubMed]
- 41.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–1300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 42.Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1175–G1187. doi: 10.1152/ajpgi.00313.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vatrella A, Fabozzi I, Calabrese C, Maselli R, Pelaia G. Dupilumab: a novel treatment for asthma. J Asthma Allergy. 2014;7:123–130. doi: 10.2147/JAA.S52387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:197–211, xiii-xiv. [DOI] [PMC free article] [PubMed]
- 45.Dellon E, Collins M, Assouline-Dayan Y, et al. A randomized, double-blind, placebo-controlled trial of a novel recombinant, humanized, anti-interleukin-13 monoclonal antibody (RPC4046) in patients with active eosinophilic esophagitis: results of the HEROES study. [abstract]. Presented at: American College of Gastroenterology Annual Scientific Meeting, 2016; Las Vegas, NV (abstr: Oral 19).
- 46.Hirano I, Collins M, Assouline-Dayan Y, et al. A randomised, double-blind, placebo-controlled trial of a novel recombinant, humanised, anti-interleukin-13 monoclonal antibody (RPC4046) in patients with active eosinophilic oesophagitis: results of the HEROES study. [abstract]. United Eur Gastroenterol J. 2016;2:127 (abstr: OP325).
- 47.Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4:781–796. doi: 10.1016/S2213-2600(16)30265-X. [DOI] [PubMed] [Google Scholar]
- 48.Corren J, Busse W, Meltzer EO, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Rα antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181:788–796. doi: 10.1164/rccm.200909-1448OC. [DOI] [PubMed] [Google Scholar]
- 49.Pfizer. Study evaluating the effect of IMA-638 in subjects with persistent asthma. https://clinicaltrials.gov/ct2/show/NCT00425061. Accessed 14 Feb 2017.
- 50.Goedken ER, O’Brien RF, Xiang T, et al. Functional comparison of recombinant acidic mammalian chitinase with enzyme from murine bronchoalveolar lavage. Protein Expr Purif. 2011;75:55–62. doi: 10.1016/j.pep.2010.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.


