Abstract
Diabetic retinopathy (more specifically diabetic macular edema, DME) is the most common cause of loss of vision in the working population in developed countries. Anti-vascular endothelial growth factor (anti-VEGF) agents considerably changed the treatment algorithms and improved prognosis of center-involving DME. Ranibizumab was the first approved anti-VEGF agent that revolutionized DME treatment. The vast increase in the number of patients undergoing intravitreal treatment and the role of anti-VEGF pharmacotherapy as the mainstay of DME treatment have triggered several challenges. Among them, of considerable interest is the quest for an optimal dosing scheme and the search for combination therapies. Although a significant body of research is directed towards other molecules that could potentially be new therapeutic targets, VEGF inhibition is expected to play an important long-term role in the treatment of DME considering the pathogenesis of the disease. Finally, recent studies revealed that ranibizumab may constitute a significant treatment modality in the management of other diabetic vision-threatening complications including proliferative diabetic retinopathy.
Keywords: Anti-VEGF, Diabetic macular edema, Diabetic retinopathy, Macula exudates, Ophthalmology, Ranibizumab
Introduction
Diabetes mellitus (DM) is a major health concern, especially in the western world where its impact is expected to increase in the near future. It is a well-established risk factor for macrovascular (heart attack, stroke) and microvascular (peripheral neuropathy, nephropathy, retinopathy) complications [1]. In 2016 DM was affecting 415 million people worldwide—a number that will rise to 642 million by 2040 [2].
Diabetic retinopathy (DR) is the main cause of visual loss among the working population in developed countries. Diabetes mellitus adversely affects, through different mechanisms, several parts of the eye and the visual pathway, but in the vast majority of cases vision loss results from DR [3, 4]. Vision-threatening complications of DR include diabetic macular edema (DME; Figs. 1, 2), macular ischemia, vitreous hemorrhage, and tractional retinal detachment. DME is the most common among them [3], with a major impact on the patient’s quality of life [5].
Fig. 1.
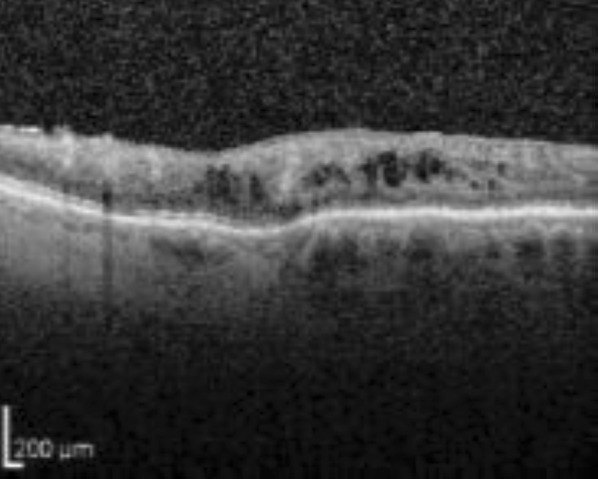
Optical coherence tomography (OCT) scan showing the presence of intraretinal cysts in DME
Fig. 2.
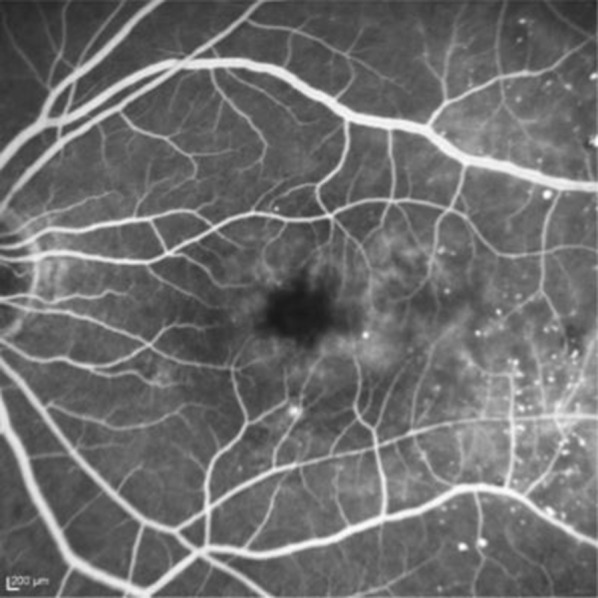
Fundus fluorescein angiography of diabetic macular edema
The main molecular mechanism underlying DME is the disruption of the blood–retinal barrier by phosphorylation of the junctional proteins [6]. The exact molecular trigger had long eluded identification. The possibility of an intraocular substance that promotes vascular growth was proposed [7] and this led to the hypothesis that some soluble vasoproliferative molecules could be the causative agents of DME [8]. The hypothesis was confirmed by the discoveries of the vascular permeability factor [9] and of the vascular endothelial growth factor (VEGF) [10, 11] which were shown by sequencing analysis to be identical molecules. Thus the stage was set for inhibiting the single molecular target which was putatively responsible for the development of DME.
Intravitreal ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA) was the first anti-VEGF agent approved by the US Food and Drug Administration (FDA) for the treatment of DME. This review focuses on the current status of ranibizumab in the treatment of DME. It also attempts to analyze presently unmet needs and future challenges in the anti-VEGF era. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Molecular Mode of Action
VEGF is a dimeric glycoprotein with a molecular weight of 36–46 kDa that consists of seven families: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor (PlGF). Isoforms of VEGF-A have been shown to be the most important promoters of intraocular neovascularization and hyperpermeability. The VEGF165 isoform in particular is the most abundant and most important isoform involved in neovascularization. At the cellular level, the diffusible VEGF molecule binds to and dimerizes three transmembrane receptors (VEGFR-1, VEGFR-2, and VEGFR-3) [12–14]. Although VEGFR-1 binds VEGF165 with greater affinity, it is the VEGFR-2 receptor that regulates the blood–retinal barrier and controls endothelial cell mitogenesis [15].
VEGF is produced by several different cells within the retina (capillary endothelial cells, pericytes, pigment epithelial cells, neurons, and astrocytes). Although all retinal cell types respond to VEGF, the capillary endothelial cell represents the VEGF primary target [16, 17]. Hypoxia-induced upregulation of VEGF breaks down the blood–retinal barrier and increases capillary permeability via VEGF-mediated downregulation of claudin-1. Blocking VEGF with ranibizumab restores claudin-1 levels within 24 h [18, 19]. Vitreous vascular endothelial growth factor levels were found to be significantly higher in eyes with proliferative diabetic retinopathy than in eyes without proliferative diabetic retinopathy [20]. Moreover, VEGF molecular inhibition was proposed for (and proven to be effective in) the treatment of DME. In addition, blocking the VEGF signaling pathway was shown to restore macular anatomy and improve visual acuity in DME patients. This finding resulted in a remarkable treatment algorithm shift for DME patients while it also improved their overall prognosis.
Ranibizumab is a recombinant humanized monoclonal antibody fragment that binds to all isoforms of VEGF-A. Its structure is that of a monoclonal antibody FAB (fragment antigen binding) fragment, which is derived from bevacizumab, a full-length humanized monoclonal antibody against human VEGF. At present, ranibizumab is produced by Escherichia coli cells with the use of recombinant DNA technology. Ranibizumab binds with high affinity to the VEGF-A isoforms (e.g., VEGF110, VEGF121, and VEGF165), thereby preventing binding of VEGF-A to its receptors VEGFR-1 and VEGFR-2. Once VEGF-A is bound to its receptors it promotes endothelial cell proliferation and neovascularization, and leads to vascular leakage by affecting the tight junction proteins [21, 22]. Vascular leakage is the main mechanism that contributes to the development of DME.
Dose and Administration
Ranibizumab is administered as a single intravitreal injection of 0.5 or 0.3 mg. In either case, this corresponds to an injection volume of 0.05 ml of a 10 mg/ml or a 6 mg/ml solution, respectively, by a pre-filled syringe. The FDA-approved dose for DME is 0.3 mg while the 0.5 mg is used in Europe.
General recommendations for the treatment of DME with ranibizumab have been summarized as [22, 23]:
Intravitreal ranibizumab is indicated for center-involving DME while laser photocoagulation may still be the best option in eyes where the center of the macula is not affected or where visual acuity is better than 20/32.
Treatment is initiated with one injection every 4 weeks (which should be the minimum time between two consecutive injections). Several protocols suggest at least three (or even six) consecutive injections initially.
Visual acuity, clinical examination, and imaging (including OCT and angiography) can be used to assess retreatment need in PRN treatment protocols. Monthly retreatment is rarely used in clinical practice.
If, in the physician’s opinion, the patient is not benefiting from continued treatment, ranibizumab should be discontinued. This applies in cases where there is no visual acuity improvement after repeated injections despite the absence of fluid in the macula. This also applies in cases where repeated monthly injections do not result in reduction of retinal fluid and improvement of visual acuity.
Treat-and-extend regimens have been also proposed and in these protocols, once maximum visual acuity is achieved and/or there are no signs of disease activity, the treatment intervals can be extended stepwise until signs of disease activity or visual impairment recur. There are different treat-and-extend protocols proposed in the literature supported by evidence from clinical trials as explained later in this review. If disease activity recurs, the treatment interval should be shortened accordingly [23, 24].
Evidence from Clinical Trials
Several studies have proven the safety and efficacy of ranibizumab for the treatment of DME and resulted in its approval for intraocular use for the treatment of this condition.
In 2010, the DRCR.net study first reports were published comparing:
0.5 mg intravitreal ranibizumab administration with prompt focal/grid laser photocoagulation
0.5 mg intravitreal ranibizumab administration with deferred laser photocoagulation (at least 24 weeks later)
4 mg intravitreal triamcinolone administration with prompt laser photocoagulation
Sham injection with prompt laser photocoagulation
Inclusion criteria were DME with baseline visual acuity between 78 and 24 letters and central subfield thickness on OCT ≥250 μm. Results after the first year showed that ranibizumab combined with either prompt or deferred laser photocoagulation proved to be superior to laser treatment alone in improving best corrected visual acuity (BCVA) (nine letter gain in both ranibizumab groups vs three letter gain in the laser/sham injection group, p < 0.001). The group treated with 4 mg intravitreal triamcinolone did not demonstrate a significant improvement in BCVA compared with laser alone. However, this group did result in a greater reduction in retinal thickness on OCT compared with the laser group. When a subgroup analysis was carried out for the patients that were pseudophakic at baseline, an improvement in BCVA similar to that of the ranibizumab group for those treated with 4 mg triamcinolone with laser was evident. This suggests that the initial finding of no significant BCVA improvement for the whole triamcinolone group may be due to cataract formation/cataract surgery, or both, in phakic patients [25]. The results were similar at the 2-year follow-up point [26]. The 3-year follow-up visual outcome results suggested that photocoagulation therapy at the initiation of intravitreal ranibizumab was not better, or maybe it was worse, when compared to deferring laser treatment for 24 weeks or more. The ranibizumab-treated groups also showed a reduced progression of the overall retinopathy grade [27]. The 5-year follow-up visual acuity outcomes consistently favored the group with deferred laser treatment. However, more injections were needed in that group. The study also concluded that only little additional treatment was needed after the third year [28].
The READ study (Ranibizumab for Edema of the mAcula in Diabetes) compared the effect of:
0.5 mg intravitreal ranibizumab administration (injections at baseline and at months 1, 3, 5)
Laser photocoagulation (at baseline and at 3 months, if needed)
Combined 0.5 mg of ranibizumab administration and laser photocoagulation (photocoagulation and ranibizumab at baseline, and ranibizumab at 3 months if needed)
A total of 126 treatment-naive eyes were included in the READ study. The mean gain in BCVA was significantly better in the ranibizumab monotherapy group at the primary end point of 6 months (+7.24 letters compared to the laser photocoagulation group of −0.43 letters, p = 0.0001 at 6 months). There was no statistically significant difference between the ranibizumab monotherapy group and the combination group. All groups were treated as necessary with ranibizumab after 6 months. The 2-year follow-up results showed that the visual outcomes in the ranibizumab groups were maintained with a PRN regime. Significant visual acuity improvement was also observed in groups 2 and 3. Moreover, data of 101 participants suggested that poor baseline visual acuity (≤20/125) in DME patients predicts poor visual outcome (≤20/100) after 2 years of treatment with ranibizumab and/or focal/grid laser, often due to foveal atrophy and/or persistent edema [29]. The 3-year follow-up outcomes included monthly follow-up and injection if foveal thickness was 250 μm or greater. The authors concluded that more aggressive treatment with ranibizumab during year 3 resulted in better structural and functional outcomes in the ranibizumab group. On the other hand, more extensive focal/grid laser therapy in the other two groups may have reduced the need for more frequent ranibizumab injections [30–32].
The RESOLVE study was a phase II study evaluating ranibizumab in DME. A total of 151 patients were enrolled in the study. The treatment algorithms included three monthly intravitreal injections followed by PRN treatment. The treatment options compared were:
Ranibizumab 0.3 mg
Ranibizumab 0.5 mg
Sham injection
The study protocol suggested that the dose of ranibizumab was doubled after 1 month if edema persisted. Photocoagulation after three injections was included in the study protocol as well. The mean change in visual acuity at month 12 from baseline was 10.3 (±9.1) letters for ranibizumab compared to −1.4 (±14.2) letters for the sham injections reaching statistical significance (p < 0.0001). Mean central retinal thickness reduction in OCT was 194.2 μm with ranibizumab and 48.4 μm for the sham injection (p < 0.0001) [33].
The RESTORE study was one of the main phase III studies for ranibizumab in DME. A total of 345 patients were randomized in a 1:1:1 ratio to receive:
Ranibizumab 0.5 mg monotherapy and sham laser photocoagulation.
Combined ranibizumab 0.5 mg and laser photocoagulation.
Sham injection and laser photocoagulation.
The study included people with type 1 or type 2 diabetes and hemoglobin A1c (HbA1c) lower than 10% (86 mmol/mol). Eyes that were eligible for randomization had visual acuity between 78 and 39 letters measured with the ETDRS (Early Treatment Diabetic Retinopathy Study) charts. Participants who had previous laser photocoagulation were included in the study. Ranibizumab or sham injections were administered monthly for 3 months and subsequently until vision was stabilized for two visits or visual acuity reached 85 letters or more. Treatment with monthly injections was restarted if there was a decrease in visual acuity caused by progression of DME and it was continued until the same criteria were fulfilled. Laser photocoagulation or sham laser photocoagulation was administered on day 1 and repeated at intervals of at least 13 weeks long if that was deemed necessary by the treating clinician. BCVA of eyes randomized to ranibizumab monotherapy rose by a mean average of 6.1 letters and, similarly, BCVA of eyes randomized to ranibizumab plus laser photocoagulation by a mean average of 5.9 letters. On the contrary, eyes randomized to laser photocoagulation alone gained fewer letters (0.8) than eyes randomized to either of the ranibizumab-containing arms (p < 0.001). Most subgroups showed consistent results. However, subjects with a baseline BCVA greater than 73 letters and macular edema with central retinal thickness less than 300 μm did not appear to benefit from treatment with ranibizumab compared to laser photocoagulation [34]. RESTORE study participants were then enrolled in the open-label, multicenter RESTORE extension study which concluded that structural and functional outcomes with ranibizumab were maintained during the follow-up with a progressively declining number of injections [35].
The RETAIN study was a phase IIIb study for ranibizumab in DME. A total of 372 patients were randomized in 1:1:1 ratio to receive:
Ranibizumab 0.5 mg with concomitant laser photocoagulation on a treat-and-extend (TE) regimen
Ranibizumab 0.5 mg monotherapy on a treat-and-extend regimen
Ranibizumab 0.5 mg monotherapy on a PRN regimen
In all groups, ranibizumab was administered monthly until BCVA was stable for at least three consecutive monthly assessments. To those on TE regimen, ranibizumab was administered in 2- to 3-month intervals. In all groups, monthly treatment was re-initiated upon a decrease in BCVA due to DME progression and continued until stable BCVA was reached again. The number of scheduled treatment visits after the initial three injections was about 13 for the two TE regimens and 20 for the PRN regimens. In both TE regimens, more than 70% of patients maintained their BCVA with an average intervisit interval of at least 2 months, suggesting that TE regimens represent a feasible treatment option [36].
The RISE and RIDE studies were two parallel, methodologically identical phase III clinical trials demonstrating that monthly treatment with intravitreal ranibizumab (0.3 or 0.5 mg) resulted in significant visual acuity improvement (>15 ETDRS letters) in a large percentage of patients (compared to sham injections) at the 24-month end point. They were both sham injection-controlled up until month 24 and they both had a total duration of 36 months. Macular focal/grid laser treatment beginning at month 3 of the 24-month treatment period or panretinal photocoagulation (PRP) if needed was also included in the study protocol. Compared to the monthly ranibizumab 0.3 mg administration, no additional benefit was observed for the monthly ranibizumab 0.5 mg treatment.
In RISE, 377 patients were randomized:
To sham injections
0.3 mg of intravitreal ranibizumab administration
0.5 mg of intravitreal ranibizumab administration
At 24 months, 18.1% of the sham-injected patients gained at least 15 letters versus 44.8% for the 0.3 mg and 39.2% for the 0.5 mg ranibizumab patients (p < 0.001).
In RIDE, 382 patients were randomized to receive:
Sham injections
0.3 mg of intravitreal ranibizumab administration
0.5 mg of intravitreal ranibizumab administration
Significantly more ranibizumab-treated patients gained more than 15 letters: 12.3% for the sham-injected patients versus 33.6% for the 0.3 mg patients and 45.7% for the 0.5 mg ranibizumab patients (p < 0.0001). Ranibizumab treatments were associated with significant improvements in macular edema as measured by OCT [37].
From months 25 through 36, patients who previously received sham injections were eligible to receive monthly ranibizumab 0.5 mg and patients originally randomized to monthly ranibizumab administration of 0.3 or 0.5 mg continued to receive their assigned dose. Results suggested that VA outcomes observed at month 24 in patients treated with ranibizumab were maintained with continued treatment through month 36 in both DME studies. Patients in the sham injection arms who received ranibizumab 0.5 mg at month 25 achieved less VA gains compared to patients who underwent ranibizumab treatment at the beginning of the studies [38].
Five hundred adults who completed the 36-month randomized core studies elected to enter the open-label extension study. Eligible patients received 0.5 mg of ranibizumab based on visual acuity or OCT criteria. Results suggested that visual acuity gains already achieved were maintained, even with a marked reduction in treatment frequency. Patients whose treatment was deferred by 2 years (randomized initially to sham injection) did not ultimately achieve the same visual acuity gains [39].
The RELIGHT study was a phase IIIb prospective single-arm study to evaluate ranibizumab 0.5 mg using bimonthly monitoring and individualized retreatment after monthly follow-up for 6 months. The authors concluded that best corrected visual acuity gain achieved during the initial 6 months of monthly follow-up was maintained during the additional 12 months of bimonthly PRN treatment [40].
The READ-3 study was a randomized controlled, double-masked, multicenter clinical trial comparing 2.0 mg of ranibizumab with 0.5 mg of ranibizumab in eyes with central DME. At month 24 no additional benefit was seen for the 2.0 mg group in comparison with the 0.5 mg group [41].
The TREX study is a multicenter, prospective, randomized clinical trial evaluating monthly dosing with a treat-and-extend algorithm using ranibizumab 0.3 mg with and without angiography-guided macular laser photocoagulation. A total of 150 eyes were randomized to receive:
0.3 mg of ranibizumab every 4 weeks
4 monthly injections of ranibizumab 0.3 mg followed by a treat-and-extend algorithm
4 monthly injections of ranibizumab 0.3 mg followed by a treat-and-extend algorithm with angiography-guided macular laser photocoagulation at month 1 and again every 3 months for microaneurysm leakage
At the end of the first year both treat-and-extend groups needed reduced numbers of injections. However, adding angiography-guided laser photocoagulation did not significantly improve outcomes [42].
Further Considerations
Comparison with Other Anti-VEGF Agents
The relative efficacy and safety of the three anti-VEGF agents (ranibizumab, aflibercept, and bevacizumab) used for the treatment of center-involving DME were analyzed by the DRCR network protocol T study. The study design was a randomized trial comparing the three intravitreal agents for the treatment of DME.
The first-year results gained interest among retina specialists. A total of 660 adults (mean age ± SD, 61 ± 10 years) with DME were randomly assigned to receive:
Intravitreal injections of 2.0 mg aflibercept
Intravitreal injections of 1.25 mg bevacizumab
Intravitreal injections of 0.3 mg ranibizumab
Treatment with the three agents was with monthly injections. Focal/grid laser photocoagulation was applied after 6 months, according to the study protocol, if DME persisted. The primary outcome was the mean change in visual acuity at 1 year. Analyzing the entire study population showed differences between the three agents: mean visual acuity score improved by 13.3 letters among patients treated with aflibercept, by 9.7 letters in patients in the bevacizumab group, and by 11.2 letters in the ranibizumab group. The improvement was greater with aflibercept compared to the other two drugs (p < 0.001 for aflibercept vs. bevacizumab, and p = 0.03 for aflibercept vs. ranibizumab), but, as the study authors supported, the results were “driven by the eyes with worse visual acuity at baseline (p < 0.001 for interaction)”. Subgroup analysis showed highly significant differences in the group with worse vision. In patients whose initial visual acuity letter score was 78–69 (equivalent to 20/32–20/40; n = 51% of participants), the mean improvement was 8.0 with aflibercept, 7.5 with bevacizumab, and 8.3 with ranibizumab (p > 0.50 for each pairwise comparison). On the contrary, when the initial letter score was less than 69 (20/50 or worse), the mean improvement was 18.9 with aflibercept, 11.8 with bevacizumab, and 14.2 with ranibizumab (p < 0.001 for aflibercept vs. bevacizumab; p = 0.003 for aflibercept vs. ranibizumab; and p = 0.21 for ranibizumab vs. bevacizumab) [43].
The second-year outcomes did not confirm the first-year results regarding the relative efficacy of aflibercept and ranibizumab. With worse baseline VA (20/50–20/320), mean improvement was 18.1, 13.3, and 16.1 letters for aflibercept, bevacizumab, and ranibizumab, respectively (aflibercept vs. bevacizumab, p = 0.02; aflibercept vs. ranibizumab, p = 0.18; ranibizumab vs. bevacizumab, p = 0.18). With better baseline VA (20/32–20/40), mean improvement was 7.8, 6.8, and 8.6 letters, respectively (p > 0.10 for pairwise comparisons). Superiority of aflibercept over ranibizumab reported after the first year was no longer identified [44]. However a post hoc analysis of the data still suggested, according to the authors, that in eyes with a VA of 20/50 or worse aflibercept has the greatest improvement in VA over 2 years [45].
Management of Hard Exudates
Primary outcomes for all clinical trials focused predominantly on visual acuity and macular thickening measured on OCT. However, one common feature of diabetic maculopathy is hard exudates (HEX). These develop from incomplete clearance of DME [46]. In particular, foveal-plaquing, where exudates congregate at the center of the fovea, is a devastating complication of DME that can cause profound central visual loss [47]. Considering the lack of alternative options, intravitreal pharmacotherapy is the best strategy for the management of hard exudates. A randomized, placebo-controlled clinical trial showed rapid reduction of HEX within 3 months of a single intravitreal injection of triamcinolone acetonide in eyes with DME [48]. Resolution of HEX was also reported after 12 months in patients receiving intravitreal injections of ranibizumab every 4 weeks in the RISE and RIDE clinical trials [49]. A post hoc analysis of a randomized clinical trial comparing Ozurdex 0.7 mg dexamethasone implant (Allergan, Irvine, CA, USA) and intravitreal bevacizumab 1.25 mg showed reduced total area of macular HEX at 12 and 24 months, with no statistically significant difference between the two agents. Ozurdex led to significantly greater regression of HEX from the foveal center at 12 months compared with bevacizumab; however, by 24 months this difference was no longer statistically significant [50]. It has been suggested that in contrast to anti-VEGF agents which, by inhibition of angiogenic activity on endothelial tight junctions, reduce vascular permeability, steroids have anti-inflammatory and angiostatic effects as well and, therefore, they can be more effective in the management of HEX [50].
Cost-Effectiveness of Treatment
Considering the cost of every single anti-VEGF injection and the need for repeated injections over long periods of time, there are considerations regarding the cost-effectiveness of anti-VEGF treatment for DME. The DRCR.net investigators analyzed the relative cost-effectiveness of treating DME for ranibizumab, bevacizumab, and aflibercept. Aflibercept (2.0 mg) and ranibizumab (0.3 mg) were not found to be cost-effective relative to bevacizumab for treatment of DME at present price levels. Likewise, aflibercept was not found to be cost-effective relative to ranibizumab. They concluded that from a societal perspective, bevacizumab as first-line therapy for DME would confer the greatest value, along with substantial cost savings vs the other agents [51].
Ranibizumab for Diabetic Retinopathy
Anti-VEGF treatment has been shown to slow the progression of DR and reduce its complications. It has recently been approved by the FDA as a monthly treatment of all forms of diabetic retinopathy in people with or without diabetic macular edema. The DRCR.net Protocol S study evaluated visual acuity outcomes after intravitreal ranibizumab vs panretinal photocoagulation in proliferative diabetic retinopathy. The results suggest that ranibizumab resulted in visual acuity that was not inferior to that achieved by PRP treatment at 2 years. In addition, it resulted in fewer proliferative retinopathy complications. The authors, however, pointed out that a longer follow-up is needed before the results for this group of patients can be confirmed [52]. Similarly the DRCR.net evaluated the role of ranibizumab in vitreous hemorrhage caused by proliferative diabetic retinopathy. The primary outcome included vitrectomy rate and it was not found to be statistically significant between the ranibizumab and the sham arms. However, complete PRP without vitrectomy by the 16th week was more frequent in the ranibizumab group, while recurrent vitreous hemorrhage developed in fewer ranibizumab treated eyes (6% vs 17%, p = 0.01) [53]. The 1-year follow-up data did not show any clinically relevant differences in the rates of vitrectomy either [54].
Prediction of Response to Anti-VEGF Treatment
Post hoc analysis of data from the DRCR.net Protocol I suggested that BCVA response after three injections is a strong predictor of long-term response after 1 or 3 years. Visual acuity improvement was categorized by the authors into three categories (<5, 5–9, and ≥10 letters). The authors suggested that suboptimal responders could be considered for alternative therapies early during their treatment process [55].
Effect of Treatment on Retinal Non-perfusion
Despite the concerns about the potential deleterious effects of anti-VEGF treatment on retinal perfusion, retrospective analysis of the RISE and RIDE clinical trial data demonstrated that the anti-VEGF treatment actually improved retinal perfusion by slowing down retinal non-perfusion effects. However, this improvement was counteracted by the gradual worsening of perfusion, which is probably due to glucotoxicity [56].
0.3 mg vs. 0.5 mg Dose
The optimal dose of ranibizumab in DME has not been established yet. This reflects the difference between Europe and USA for the approved dose as mentioned before. The RISE and RIDE studies were the only studies providing head-to-head comparison of the 0.3 and 0.5 mg of ranibizumab and placebo. Data suggested that 0.3 and 0.5 mg are equally effective in the treatment of DME [37].
Safety of Treatment
There are several considerations regarding the safety of ranibizumab treatment in diabetic patients. Despite the well-established injection-related risks, there are concerns regarding the vascular safety of treatment.
Major injection-related risks include infective endophthalmitis, sterile endophthalmitis, and retinal detachment. The risk of infective endophthalmitis has been estimated to be 1 in 1700 cases [57]. The risk of rhegmatogenous retinal detachment is even lower and has been calculated to be 1 in 7188 injections [58].
The cardiovascular safety of intravitreal ranibizumab is still controversial. Systemic anti-VEGF has been associated with increased risk of vascular events [59]. A recent pooled analysis of patient level data from randomized clinical trials concluded that ranibizumab is safe and does not increase the risk of systemic vascular events. However, the authors concluded that this may not apply to patients with different characteristics from those included in these studies [60].
Discussion
Several clinical trials conducted worldwide have demonstrated the safety and efficacy of ranibizumab for the treatment of DME. These results have been confirmed in real-life studies based on everyday clinical practice. Moreover, recent clinical trial data show promising results for the use of ranibizumab in the treatment of PDR and its consequences. This could potentially improve the prognosis of the disease and reduce the incidence of severe complications that threaten vision and require vitreoretinal surgery.
Despite the revolutionary outcomes of ranibizumab in the treatment of diabetic maculopathy, several new challenges have developed following its introduction. These include considerations regarding the impact of long-term intravitreal treatment to both society and diabetic patients. Intravitreal treatment requires long-term follow-up of the patient. Currently, the cost and the cost-effectiveness of the treatment, as compared to alternative therapeutic approaches, are being analyzed. Given that there are DME patients who may not respond to ranibizumab, alternative treatments are being investigated while intravitreal steroids still have a role to play. However, VEGF inhibition will always be considered as the mainstay of treatment of established DME considering the significant role that VEGF plays in its pathogenesis. New treatments could potentially provide neutralization of VEGF for longer periods of time and also combine other molecular actions and treatment targets. However, no prospective data support treatment alternatives other than the already widely used intravitreal treatments. Alternative methods of administration are also being investigated [61].
Finally, when treating DME, the systemic nature of diabetes must always be kept in mind by the treating ophthalmologist. Retinal vessel disease represents a small spectrum of diabetes-related vasculopathy. Close cooperation between physicians of different specialties is necessary to maximize the treatment outcomes.
In conclusion, VEGF inhibition plays a key role in the treatment of diabetic maculopathy. Intravitreal ranibizumab in several treatment schemes (treat-and-extend, PRN, monthly) has been shown to be safe and effective in the treatment of DME. Severe ocular complications are rare while there are still considerations regarding the systemic cardiovascular safety in specific groups of patients. VEGF inhibition is also expected to play a significant role in the treatment of diabetic retinopathy in patients with and without DME.
Intravitreal ranibizumab has revolutionized the treatment of diabetic maculopathy over the past few years and it has proven its efficacy both in multiple clinical trials and in everyday clinical practice.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
N. Dervenis and P. Tranos have participated as co-investigators for ranibizumab clinical trials. Athanasia Maria Mikropoulou and Panagiotis Dervenis have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/6B18F0602E5677A8.
References
- 1.Control CfD, Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta: US Department of Health and Human Services; 2014.
- 2.Guariguata L, Whiting D, Hambleton I, Beagley J, Linnenkamp U, Shaw J. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105(6):998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 4.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Davidov E, Breitscheidel L, Clouth J, Reips M, Happich M. Diabetic retinopathy and health-related quality of life. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):267–272. doi: 10.1007/s00417-008-0960-y. [DOI] [PubMed] [Google Scholar]
- 6.Chibber R, Molinatti P, Rosatto N, Lambourne B, Kohner E. Toxic action of advanced glycation end products on cultured retinal capillary pericytes and endothelial cells: relevance to diabetic retinopathy. Diabetologia. 1997;40(2):156–164. doi: 10.1007/s001250050657. [DOI] [PubMed] [Google Scholar]
- 7.Michaelson I. Vascular morphogenesis in the retina of the cat. J Anat. 1948;82(Pt 3):167. [PubMed] [Google Scholar]
- 8.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 9.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161(2):851–858. doi: 10.1016/0006-291X(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 11.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Investig. 1989;84(5):1470. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29(6):789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 13.Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- 14.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947–11954. [PubMed] [Google Scholar]
- 15.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 16.Robbins SG, Conaway JR, Ford BL, Roberto KA, Penn JS. Detection of vascular endothelial growth factor (VEGF) protein in vascular and non-vascular cells of the normal and oxygen-injured rat retina. Growth Factors. 1997;14(4):229–241. doi: 10.3109/08977199709021522. [DOI] [PubMed] [Google Scholar]
- 17.Nomura M, Yamagishi S, Harada S, et al. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270(47):28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- 18.Nishikiori N, Osanai M, Chiba H, et al. Glial cell-derived cytokines attenuate the breakdown of vascular integrity in diabetic retinopathy. Diabetes. 2007;56(5):1333–1340. doi: 10.2337/db06-1431. [DOI] [PubMed] [Google Scholar]
- 19.Deissler HL, Deissler H, Lang GK, Lang GE. VEGF but not PlGF disturbs the barrier of retinal endothelial cells. Exp Eye Res. 2013;115:162–171. doi: 10.1016/j.exer.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Adamis AP, Miller JW, Bernal M-T, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118(4):445–450. doi: 10.1016/S0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 21.Rosenfeld PJ, Schwartz SD, Blumenkranz MS, et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology. 2005;112(6):1048.e4–1053.e4. doi: 10.1016/j.ophtha.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 22.Heier JS, Antoszyk AN, Pavan PR, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113(4):633.e4–642.e4. doi: 10.1016/j.ophtha.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Committee AAoOPPP. Preferred practice pattern guidelines. Comprehensive adult medical eye evaluation—2010. http://aao.org/preferred-practice-pattern/comprehensive-adult-medical-eye-evaluation-octobe. Accessed 26 Feb 2015.
- 24.Modi YS, Pecen PE, Schachat AP. Management of diabetic macular edema. Managing diabetic eye disease in clinical practice. Berlin: Springer; 2015. pp. 81–103. [Google Scholar]
- 25.Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064.e35–1077.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119(11):2312–2318. doi: 10.1016/j.ophtha.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–381. doi: 10.1016/j.ophtha.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Channa R, Sophie R, Khwaja A, et al. Factors affecting visual outcomes in patients with diabetic macular edema treated with ranibizumab. Eye. 2014;28(3):269–278. doi: 10.1038/eye.2013.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology. 2009;116(11):2175.e1–2181.e1. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Do DV, Nguyen QD, Khwaja AA, et al. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013;131(2):139–145. doi: 10.1001/2013.jamaophthalmol.91. [DOI] [PubMed] [Google Scholar]
- 33.Massin P, Bandello F, Garweg JG, et al. Safety and Efficacy of Ranibizumab in Diabetic Macular Edema (RESOLVE Study) A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121(5):1045–1053. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 36.Prünte C, Fajnkuchen F, Mahmood S, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2015;100(6):787–95. [DOI] [PMC free article] [PubMed]
- 37.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 38.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10). [DOI] [PubMed]
- 39.Boyer DS, Nguyen QD, Brown DM, et al. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122(12):2504.e1–2513.e1. doi: 10.1016/j.ophtha.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Pearce I, Banerjee S, Burton BJ, et al. Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT study. Ophthalmology. 2015;122(9):1811–1819. doi: 10.1016/j.ophtha.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 41.Sepah YJ, Sadiq MA, Boyer D, et al. Twenty-four-month outcomes of the Ranibizumab for Edema of the Macula in Diabetes-Protocol 3 with High Dose (READ-3) Study. Ophthalmology. 2016;123(12):2581–2587. doi: 10.1016/j.ophtha.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 42.Payne JF, Wykoff CC, Clark WL, et al. Randomized trial of treat and extend ranibizumab with and without navigated laser for diabetic macular edema: TREX-DME 1 year outcomes. Ophthalmology. 2017;124(1):74–81. doi: 10.1016/j.ophtha.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 43.Diabetic Retinopathy Clinical Research Network. Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;2015(372):1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jampol LM, Glassman AR, Bressler NM, et al. Anti-vascular endothelial growth factor comparative effectiveness trial for diabetic macular edema: additional efficacy post hoc analyses of a randomized clinical trial. JAMA Ophthalmol. 2016;134(12). doi:10.1001/jamaophthalmol.2016.3698. [DOI] [PMC free article] [PubMed]
- 46.Zhang X, Zeng H, Bao S, Wang N, Gillies MC. Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci. 2014;4(1):1. doi: 10.1186/2045-3701-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sigurdsson R, Begg IS. Organised macular plaques in exudative diabetic maculopathy. Br J Ophthalmol. 1980;64(6):392. doi: 10.1136/bjo.64.6.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson J, Kifley A, Zhu M, et al. Rapid reduction of hard exudates in eyes with diabetic retinopathy after intravitreal triamcinolone: data from a randomized, placebo-controlled, clinical trial. Acta Ophthalmol. 2009;87(3):275–280. doi: 10.1111/j.1755-3768.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 49.Domalpally A, Ip MS, Ehrlich JS. Effects of intravitreal ranibizumab on retinal hard exudate in diabetic macular edema: findings from the RIDE and RISE phase III clinical trials. Ophthalmology. 2015;122(4):779–786. doi: 10.1016/j.ophtha.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 50.Mehta H, Fraser-Bell S, Yeung A, et al. Efficacy of dexamethasone versus bevacizumab on regression of hard exudates in diabetic maculopathy: data from the BEVORDEX randomised clinical trial. Br J Ophthalmol. 2015;100(7):1000–1004. [DOI] [PubMed]
- 51.Ross EL, Hutton DW, Stein JD, et al. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134(8):888–896. doi: 10.1001/jamaophthalmol.2016.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137–2146. doi: 10.1001/jama.2015.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhavsar AR, Torres K, Beck RW, et al. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131(3):283. doi: 10.1001/jamaophthalmol.2013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhavsar AR, Torres K, Glassman AR, Jampol LM, Kinyoun JL. Evaluation of results 1 year following short-term use of ranibizumab for vitreous hemorrhage due to proliferative diabetic retinopathy. JAMA Ophthalmol. 2014;132(7):889–890. doi: 10.1001/jamaophthalmol.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol. 2016;172:72–79. doi: 10.1016/j.ajo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121(9):1783–1789. doi: 10.1016/j.ophtha.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Meredith TA, McCannel CA, Barr C, et al. Postinjection endophthalmitis in the comparison of age-related macular degeneration treatments trials (CATT) Ophthalmology. 2015;122(4):817–821. doi: 10.1016/j.ophtha.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer CH, Michels S, Rodrigues EB, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011;89(1):70–75. doi: 10.1111/j.1755-3768.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 59.Scott LJ. Bevacizumab. Drugs. 2007;67(12):1793–1799. doi: 10.2165/00003495-200767120-00009. [DOI] [PubMed] [Google Scholar]
- 60.Zarbin MA, Dunger-Baldauf C, Haskova Z, et al. Vascular safety of ranibizumab in patients with diabetic macular edema: a pooled analysis of patient-level data from randomized clinical trials. JAMA Ophthalmol. 2017 doi: 10.1001/jamaophthalmol.2017.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sarwar S, Hanout M, Sadiq MA, et al. The role of ranibizumab in the management of diabetic retinopathy. Expert Rev Ophthalmol. 2015;10(4):329–340. doi: 10.1586/17469899.2015.1057506. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


