Abstract
Toll-like receptors (TLRs) bind pathogen-specific ligands early in infection, initiating signaling pathways that lead to expression of multiple protective cellular genes. Many viruses have evolved strategies that block the effector mechanisms induced through these signaling pathways, but viral interference with critical proximal receptor interactions has not been described. We show here that the NS3/4A serine protease of hepatitis C virus (HCV), a virus notorious for its ability to establish persistent intrahepatic infection, causes specific proteolysis of Toll-IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF or TICAM-1), an adaptor protein linking TLR3 to kinases responsible for activating IFN regulatory factor 3 (IRF-3) and NF-κB, transcription factors controlling a multiplicity of antiviral defenses. NS3/4A-mediated cleavage of TRIF reduces its abundance and inhibits polyI:C-activated signaling through the TLR3 pathway before its bifurcation to IRF-3 and NF-κB. This uniquely broad mechanism of immune evasion potentially limits expression of multiple host defense genes, thereby promoting persistent infections with this medically important virus.
Keywords: innate immunity, IFN, IFN regulatory factor 3, NF-κB, protease inhibitor
Persistent hepatitis C virus (HCV) infections are associated with cirrhosis and liver cancer and contribute significantly to liver-specific morbidity in human populations (1). Although multiple mechanisms contribute to viral persistence, the ability of the virus to evade early innate immune responses is likely to be particularly important (2). We have shown previously that the serine protease expressed by HCV, NS3/4A, blocks virusinduced activation of IFN regulatory factor 3 (IRF-3) (3), a transcription factor playing a critical role in the induction of type-1 IFNs (4, 5). NS3/4A is a noncovalent enzyme complex that directs posttranslational cleavage of the polyprotein expressed by this positive-strand RNA virus and also possesses RNA helicase activity (6). Inhibition of IRF-3 activation requires only its protease activity and is abrogated by a specific, peptidomimetic protease inhibitor, SCH6 (3). Thus, NS3/4A appears to mediate proteolysis of a cellular protein within an antiviral signaling pathway upstream of IRF-3.
Recent work suggests that viral infections activate IRF-3 through two independent signaling pathways. One pathway involves engagement of Toll-like receptor (TLR) 3 by its specific ligand, dsRNA, whereas a second, recently recognized pathway involves a cellular DExH(D) helicase, retinoic acid-inducible gene I (RIG-I) (7, 8). The activation of either pathway leads to phosphorylation and nuclear translocation of IRF-3 and subsequent expression of multiple protective cellular genes (8–11). Many viruses have evolved strategies that block the effector mechanisms induced through these pathways (12), but viral interference with the critical proximal receptor interactions has not been described. We show that the NS3/4A protease causes specific proteolysis of Toll-IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF or TICAM-1) (13, 14), an adaptor protein linking TLR3 to kinases responsible for activating IRF-3 as well as NF-κB (15). We show that NS3/4A expression from replicating HCV RNA is associated with reduced intracellular abundance of TRIF and inhibition of polyI:C (pIC)-activated signaling through the TLR3 pathway. This mechanism of immune evasion, therefore, limits expression of multiple host defense genes and is likely to contribute to persistent infections with this medically important virus.
Materials and Methods
Cell Cultures, Plasmids, and Promoter Reporter Assays. See Supporting Methods, which is published as supporting information on the PNAS web site.
Protein Expression and Purification. In vitro translation reactions were carried out in Flexi-Rabbit reticulocyte lysates (Promega) programmed with synthetic T7 RNA transcripts and containing [35S]Met. Recombinant His-tagged NS3 protease domain (NS3p), single-chain NS3 (scNS3), and TRIF were expressed in Escherichia coli and isolated from bacterial inclusion bodies. For details, see Supporting Methods.
NS3/4A Cleavage Assays. Products of in vitro translation (1 μl of reaction mix) were incubated at 37°C for 2 h in 30 μl of reaction mixture containing 50 mM Tris·HCl (pH 7.5), 150 mM NaCl, 0.05% Tween 20, 20% glycerol, and 1 mM DTT with 2–3 μM MB–scNS3 (a purified scNS3 protease expressed as a fusion with maltose-binding protein) and the peptidomimetic inhibitor SCH6 (10 μM), as indicated. Purified recombinant TRIF (4 μM) was incubated in 20 μl of reaction mixture with purified scNS3 (1.7 μM) or NS3p (1.7 μM) plus the NS4A peptide KKKGSVVIVGRIILS (250 μM; AnaSpec, San Jose, CA), 50 mM Tris, 0.05% β-maltoside, 50% glycerol, and 10 mM DTT (pH 7.5) at 30°C for 20 h, with or without SCH6. Peptide substrate reactions (60 μl) contained 25 mM Tris, 300 mM NaCl, 5 μM EDTA, 10% glycerol, 0.05% N-β-maltoside (pH 7.5), ≈167 μM TRIF peptide (PPPPPPPPSSTPCSAHL; AnaSpec), and 8.3 μM scNS3 protease (B. Malcolm, Schering–Plough). Reactions were incubated at 30°C. At intervals, 10 μl of the reaction mix was quenched with 10 μl of 1% trifluoroacetic acid and combined with 90 μl of dH2O for analysis by HPLC.
Immunoblots, Immunoprecipitation, and EMSA. See Supporting Methods.
RNA Interference. Cells were transfected with 80 nM short interfering RNA (siRNA) targeting TLR3, TRIF, RIG-I, or a negative control siRNA (Ambion, Austin, TX) as described in Supporting Methods. For promoter assays, cells were cotransfected with reporter plasmids 24 h after siRNA transfection and treated with pIC 24 h later.
Results
NS3/4A Cleaves TRIF at Cys-372 in Vitro. Because dsRNA is a critical intermediate in the replication of HCV (16), we focused on the potential of the NS3/4A protease to interfere with IRF-3 activation through the TLR3 pathway. Because NS3/4A catalyzes trans-cleavages within the HCV polyprotein at Cys–(Ser/Ala) peptide bonds (17, 18), we examined proteins known to be involved in TLR3 signaling for Cys–(Ser/Ala) sequences. TRAF family member-associated NF-κB activator-binding kinase 1 (TBK1) and IκB kinase ε (IKKε) have been implicated in IRF-3 phosphorylation after TLR3 engagement (19, 20). They are linked to TLR3 through the adaptor protein, TRIF (13, 14). Multiple Cys–(Ser/Ala) dipeptides are present in these proteins, but the sequence surrounding one of these (Cys-372–Ser-373 of TRIF) is unique in its remarkable similarity to the NS4B/5A cleavage site in the HCV polyprotein (Fig. 1A). Although this TRIF site lacks the acidic P6 residue conserved in viral cleavage sites, previous studies have shown that an acidic P6 residue is not essential for substrate recognition (6).
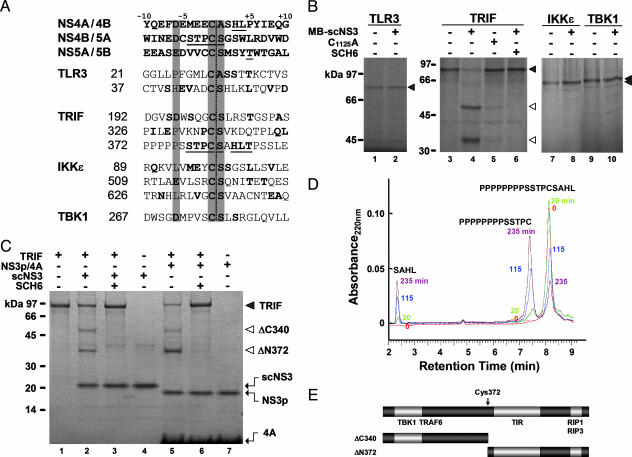
Fig. 1.
TRIF is a substrate for NS3/4A protease. (A) Majority-rule consensus sequences for NS3/4A trans-cleavage sites within the genotype 1b HCV polyprotein, compared with candidate cleavage sites in TLR3, TRIF, IKKε, and TBK1. Conserved Cys–(Ser/Ala) and P6 acidic residues are highlighted. Residues aligning with those in the consensus viral substrates are shown in boldface; viral residues aligning with the potential cleavage site at Cys-372 of TRIF are underlined. (B) TLR3, TRIF, IKKε, and TBK1 synthesized in reticulocyte lysate were incubated with MB–scNS3 or protease-deficient C1125A MB–scNS3 before SDS/PAGE. TRIF cleavage was inhibited by SCH6 (10 μM), a specific ketoamide inhibitor of the protease. (C) Coomassie blue-stained SDS/PAGE gel of reactions containing recombinant TRIF and either scNS3 or NS3p/4A. N-terminal amino acid sequencing confirmed the identity of the ΔN372 product. (D) Cleavage of a TRIF peptide (PPPPPPPPSSTPCSAHL) by scNS3 protease. Cleavage products were analyzed by HPLC at 0, 20, 115, and 235 min. (E) Domain map of TRIF showing the location of NS3/4A cleavage relative to sites of interaction with the TIR domain and recognized signaling partners.
We synthesized these proteins in cell-free translation reactions and incubated the products with a purified scNS3 protease expressed as a fusion with maltose-binding protein (MB–scNS3). An N-terminal extension in scNS3 contains the NS4A cofactor sequence that normally intercalates into NS3 to form the constitutively active protease (21). MB–scNS3 cleaved at a single site within TRIF but did not cause proteolysis of TBK1, IKKε, or TLR3 (Fig. 1B). It is not surprising that scNS3 failed to cleave at Cys–(Ser/Ala) sites in these other proteins, because it mediates proteolysis at only 3 of ≈20 Cys–(Ser/Ala) peptide bonds within the HCV polyprotein. TRIF proteolysis was specific to the NS3/4A protease because negligible cleavage occurred when TRIF was incubated with a C1125A MB–scNS3 mutant deficient in protease activity and incapable of IRF-3 blockade (3) (Fig. 1B, lane 4 vs. lane 5). TRIF cleavage also was inhibited by SCH6 (Fig. 1B, lane 6).
To precisely identify the cleavage site, we expressed TRIF in bacteria and purified it before digestion with recombinant scNS3 or NS3p (residues 3–181) complexed with an NS4A cofactor peptide (6) (Fig. 1C). We observed similar digestion products and confirmed that SCH6 also blocks cleavage of TRIF by NS3p/4A. Immunoprecipitation of tagged TRIF products demonstrated that the more rapidly migrating species was the P′-side, C-terminal fragment (data not shown), and amino acid sequencing demonstrated that its N-terminal sequence was Ser-Ala-His-Leu-Thr. Thus, TRIF is cleaved between Cys-372 and Ser-373 (Fig. 1E), as expected. This finding was confirmed by substituting Cys with Arg at each of the Cys–(Ser/Ala) sequences in TRIF. Such substitutions blocked NS3/4A processing within the viral polyprotein (22) and prevented MB–scNS3-mediated cleavage of TRIF when introduced at Cys-372, but not when introduced at Cys-192 or Cys-326 (Fig. 5A, which is published as supporting information on the PNAS web site). Independently expressed P-side (ΔC340) and P′-side (ΔN372) TRIF digestion products were also resistant to further MB–scNS3 cleavage (Fig. 5A).
scNS3 hydrolyzed a peptide substrate corresponding to residues 360–376 of TRIF (Fig. 1D). Further studies indicated that the eight-residue polyproline sequence upstream of Cys-372 in TRIF contributes to its recognition by NS3/4A but that proteolysis of a short TRIF peptide, PSSTPCSAHL, proceeds with a kcat/Km of 23 ± 3 M-1·s-1 (data not shown), which is ≈50% of the rate of proteolysis of a peptide substrate representing the viral NS4B/5A cleavage site. Similar concentrations of MB–scNS3 were required for in vitro proteolysis of TRIF or a viral NS5AB substrate (Fig. 5B). These data suggest that the intracellular abundance of NS3/4A in HCV-infected cells is likely to be sufficient for TRIF proteolysis.
Signaling Activities of TRIF Cleavage Products. TRIF is essential for dsRNA-stimulated synthesis of type 1 IFNs signaled through the TLR3 pathway, as well as for MyD88-independent activation of NF-κB after engagement of either TLR3 or TLR4 (13, 14, 23–26). NS3/4A cleavage of TRIF could thus impede both IRF-3 and NF-κB activation either by reducing functional TRIF abundance or by generating cleavage products with dominantnegative activity (13). To assess these possibilities, we determined the ability of the TRIF cleavage fragments to initiate or inhibit signaling when expressed ectopically in HEK 293 cells. TRIF overexpression results in activation of both the IFN-β promoter and the NF-κB-dependent PRDII promoter in HEK 293 cells (13, 14) (Fig. 6, which is published as supporting information on the PNAS web site). However, neither the ΔN372 nor ΔC372 cleavage products activated the IFN-β promoter. Only the TIR domain-containing ΔN372 fragment retained the ability to activate NF-κB, consistent with previously described deletion mutants (13, 14, 24). Importantly, neither ΔN372 nor ΔC372 demonstrated dominant-negative activity when coexpressed with intact TRIF (Fig. 6).
NS3/4A Accelerates Degradation of TRIF in Cultured Cells. To determine whether TRIF proteolysis occurs in vivo, we cotransfected HEK 293 cells with vectors expressing NS3/4A and either an N-terminally tagged TRIF (myc-TRIF) or a similarly tagged protease-resistant C372R TRIF mutant (myc-C372R). Both TRIF and the C372R mutant were readily visualized in the absence of NS3/4A expression, but only the myc-C372R mutant was detectable with NS3/4A coexpression (Fig. 2A). Similarly, the abundance of C-terminally tagged TRIF-Flag was markedly reduced in the presence of NS3/4A expression but was restored by treatment with SCH6 (Fig. 7A, which is published as supporting information on the PNAS web site). No digestion products were identified in these experiments, suggesting that they are rapidly degraded after NS3/4A cleavage, despite their stability in reticulocyte lysate (Figs. 1B and 7B). Pulse–chase labeling indicated that myc-TRIF has a relatively brief half-life (<60 min) when ectopically expressed in HEK 293 cells and that its degradation is significantly accelerated with NS3/4A coexpression (Fig. 2B). Similar results were obtained with expression of TRIF-Flag in UNS3-4A-24 osteosarcoma cells that conditionally express NS3/4A under control of a tetracycline-regulated promoter (27) (Fig. 7C). We found no evidence for in vivo NS3/4A-mediated proteolysis of TLR3, TBK1, or IKKε (Fig. 8, which is published as supporting information on the PNAS web site).
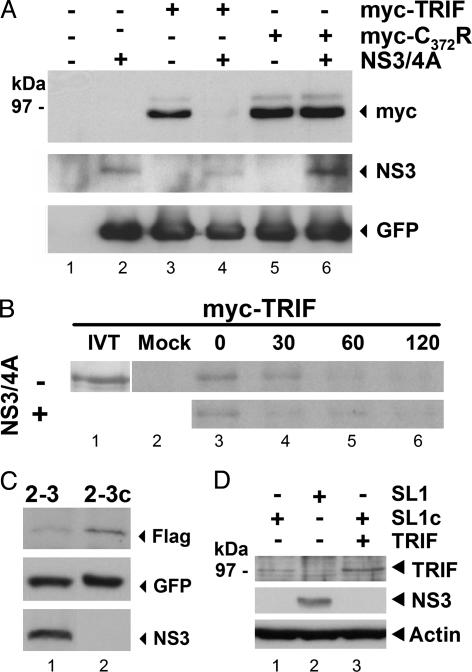
Fig. 2.
NS3/4A mediates in vivo cleavage of TRIF. (A) HEK 293 cells were transfected with vectors expressing N-terminally tagged myc-TRIF or the cleavage-resistant mutant myc-C372R TRIF, NS3/4A (or empty vector), and GFP as a transfection control. (B) Pulse–chase labeling of myc-TRIF expressed in HEK 293 cells in the presence or absence of NS3/4A coexpression. IVT, in vitro synthesized TRIF; Mock, mock-transfected cells. (C) Expression of TRIF-Flag and GFP from cotransfected plasmids in HCV RNA-bearing Huh7 2-3 cells and their IFN-cured progeny, 2-3c cells. TRIF-Flag abundance was quantified relative to GFP by densitometry. (D) Immunoblots showing endogenous TRIF abundance (lanes 1 and 2) in HeLa SL1 cells containing a replicating subgenomic RNA and IFN-cured SL1c cells. SL1c cells in lane 3 were transfected with a vector expressing TRIF to mark its position in SDS/PAGE.
Because cultured cells are poorly permissive for HCV replication, we examined the impact of NS3/4A expression on TRIF abundance in cells supporting autonomous replication of HCV RNAs expressing a selectable marker (28). We compared the abundance of ectopically expressed TRIF-Flag in Huh7 2-3 cells, which express NS3/4A from a replicating genome-length RNA, and in clonally related 2-3c cells from which the viral RNA had been eliminated by prior IFN-α2b treatment (29). Quantitative immunoblot analysis indicated that TRIF-Flag abundance was reduced ≈50% in the 2-3 cells compared with the cured 2-3c cells (Fig. 2C). More importantly, the abundance of endogenous TRIF (normally difficult to detect in immunoblots) was significantly reduced in SL1 cells, a stably selected HeLa cell line supporting replication of a subgenomic HCV replicon (30), compared with clonally related, IFN-cured SL1c cells (Fig. 2D). These results suggest that NS3/4A directs TRIF proteolysis when expressed from replicating viral RNA under conditions in which the protease contributes to a functional viral replicase complex (31).
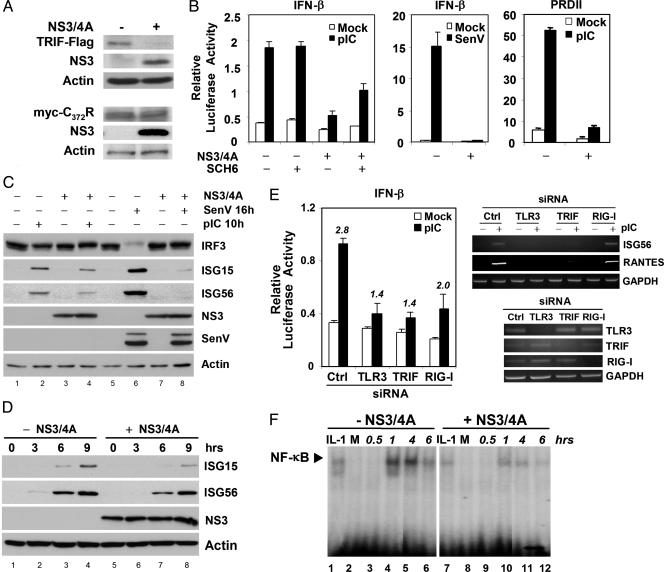
NS3/4A Disrupts TLR3 Signaling. We sought evidence that NS3/4A expression compromises TLR3 signaling by examining its impact on cellular responses to the synthetic dsRNA surrogate pIC. As in HEK and 2-3 cells (Fig. 2), we observed a reduction in the abundance of ectopically expressed TRIF-Flag, but not in the abundance of myc-TRIF-C372R, in UNS3-4A-24 cells conditionally expressing NS3/4A (Fig. 3A). NS3/4A also inhibited pIC activation of the IFN-β promoter in these cells (Fig. 3B Left), and this suppression of the pIC response was partially, but significantly, relieved by treatment of the cells with the protease inhibitor SCH6. NS3/4A also caused a delay in pIC-induced expression of the IRF-3-dependent IFN-stimulated gene (ISG) 56 and ISG15 proteins (Fig. 3 C and D). Importantly, silencing of either TLR3 or TRIF by RNA interference inhibited pIC-induced activation of the IFN-β promoter (Fig. 3E Left) and ISG56 and RANTES (regulated on activation, normal T cell expressed and secreted) transcription in these cells (Fig. 3E Upper Right), confirming that pIC induces these responses through a TLR3- and TRIF-dependent pathway. RIG-I knockdown had substantially less effect on IFN-β promoter activation and no effect on pIC-induced ISG56 and RANTES transcription. pIC-induced activation of the NF-κB-dependent PRDII promoter (Fig. 3B Right), and NF-κB binding to it (Fig. 3F), were also suppressed by NS3/4A expression, consistent with divergence of the TLR3 pathways signaling to NF-κB and IRF-3 at the level of TRIF (15). Together, these data provide strong evidence that NS3/4A expression disrupts the TLR3 pathway in these cells.
Fig. 3.
TRIF proteolysis inhibits pIC-induced activation of IFN-β and PRDII promoters and ISG expression in UNS3-4A-24 cells that conditionally express NS3/4A. (A) Immunoblots showing abundance of transfected TRIF-Flag (Upper) and the cleavage-resistant myc-TRIF-C372R mutant (Lower) with or without NS3/4A expression. (B) IFN-β (Left) and PRDII (Right) promoter activities 8 h after addition of pIC to the media (50 μg/ml). For comparison, IFN-β promoter activity is also shown 16 h after Sendai virus (SenV) infection (Center). Where indicated, cells were treated with 10 μM SCH6. (C) Immunoblot detection of IRF-3, ISG15, and ISG56 after pIC exposure (10 h) or SenV infection (16 h). (D) ISG15 and ISG56 expression with (lanes 5–8) and without (lanes 1–4) NS3/4A expression at various times after the addition of pIC to media. (E) (Left) pIC stimulation of the IFN-β promoter is inhibited by RNA interference silencing of TLR3 and TRIF and, to a lesser extent, RIG-I. Numbers over each column pair indicate the fold-induction of promoter activity by pIC. Ctrl, control siRNA. (Upper Right) RT-PCR assays showing that RNA interference silencing of TLR3 and TRIF, but not RIG-I, prevents pIC induction of ISG56 and RANTES transcription. (Lower Right) RT-PCR detection of TLR3, TRIF, and RIG-I mRNAs after transfection of specific siRNAs. Cells were cultured in the presence of tetracycline (no NS3/4A expression). (F) EMSA demonstrating that NS3/4A expression attenuates pIC stimulated NF-κB binding to the PRDII element. IL-1, IL-1 treatment control; M, mock-treated.
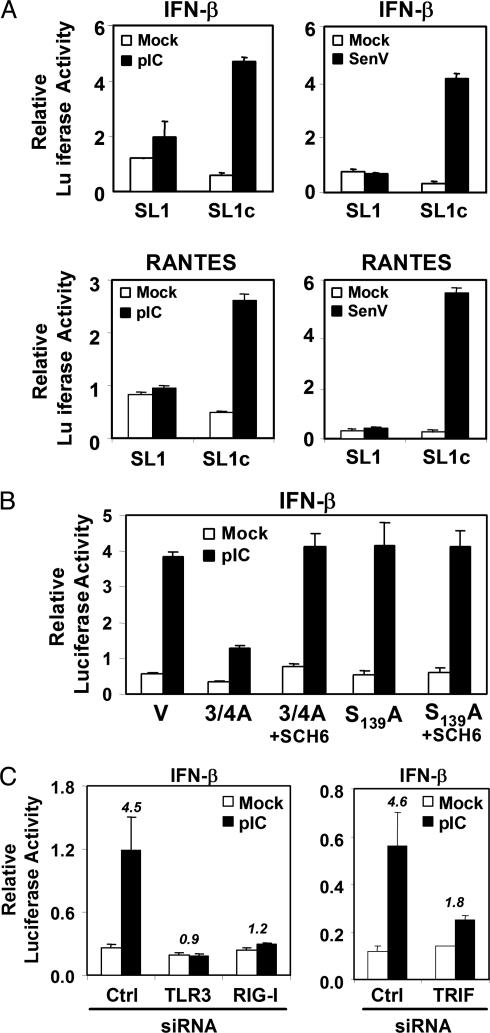
We also found the TLR3 pathway to be compromised in cells supporting active replication of HCV RNA. Huh7 cells neither express TLR3 nor respond to pIC (Fig. 9, which is published as supporting information on the PNAS web site), possibly contributing to their permissiveness for HCV RNA replication (28). They respond to Sendai virus infection through the alternative RIG-I pathway (32). We thus examined HeLa SL1 and SL1c cells for their response to pIC, because the TLR3 pathway is active in HeLa cells (14, 33). Exposure of the cured SL1c cells to extracellular pIC led to substantial increases in IFN-β and RANTES promoter activity, but these responses were ablated in SL1 cells containing replicating viral RNA (Fig. 4A Left). Ectopic expression of the active NS3/4A protease, but not an inactive S139A mutant, also inhibited IFN-β promoter activation by pIC in the cured SL1c cells (Fig. 4B). This inhibition was reversed by SCH6. RNA interference knock-down of TLR3 or TRIF expression ablated pIC-induced activation of the IFN-β promoter (Fig. 4C), confirming that pIC activation occurs in these cells through TLR3 via a pathway that depends on TRIF (14, 25). Although not yet fully understood, RIG-I knock-down also ablated pIC activation of the IFN-β promoter (Fig. 4C), suggesting significant cross-talk between the TLR3 and RIG-I pathways in these cells.
Fig. 4.
The TLR3 pathway is compromised in HeLa SL1 cells containing replicating HCV RNA but not in cured SL1c cells. (A) IFN-β (Upper) and RANTES (Lower) promoter activities in SL1 and SL1c cells, after exposure to extracellular pIC (50 μg/ml) (Left) or SenV infection (Right). (B) Ectopic expression of NS3/4A blocks activation of the IFN-β promoter in SL1c cells exposed to pIC. V, empty vector; 3/4A, NS3/4A; S139A, an active site mutant of NS3/4A. Where indicated, cells were treated with 10 μM SCH6. (C) IFN-β promoter activity in SL1c cells transfected with siRNAs targeting TLR3, TRIF, or RIG-I, with and without pIC stimulation. Ctrl, control siRNA.
These results provide strong evidence that the NS3/4A protease, expressed from replicating viral RNA as in the SL1 cells (Fig. 4), inhibits activation of IRF-3 through the TLR3 pathway by mediating proteolysis of TRIF. This conclusion is consistent with the reduced abundance of endogenous TRIF observed in SL1 cells (Fig. 2D) and the fact that overexpression of TRIF, IKKε, or TBK1 effectively activates the IFN-β promoter in SL1 cells, indicating that NS3/4A disruption of the signaling pathway occurs at or above the level of TRIF (Fig. 10, which is published as supporting information on the PNAS web site). Overexpression of TRIF also partially restores pIC responsiveness to SL1 cells (Fig. 10).
Discussion
Strong IFN responses have been demonstrated in the livers of chimpanzees during acute HCV infection (34, 35). These responses may be explained by the fact that NS3/4A is capable of inhibiting viral activation of IRF-3 and NF-κB only within infected cells. Nonetheless, IRF-3 activation limits the replication of HCV RNA in cultured hepatocytes (3). NS3/4A disruption of signaling pathways that lead to IRF-3 activation may thus contribute to HCV persistence by inhibiting expression of type 1 IFNs and ISGs that restrict viral replication (11, 12). In addition, an impairment in the virus-induced expression of type 1 IFNs and other cytokines could suppress or delay subsequent adaptive CD8+ T cell responses required for elimination of HCV (36, 37). Disruption of virus-activated NF-κB-mediated responses may also promote viral persistence (38). Although the pathways leading to activation of IRF-3 are likely to be redundant in vivo (7, 8), mice defective for the murine homolog of TRIF are compromised in their response to infection with murine cytomegalovirus (26), demonstrating the potential importance of the TLR3–TRIF pathway for control of virus infection.
The cleavage of TRIF by NS3/4A represents a uniquely broad mechanism of viral immune evasion. Poxvirus A52R protein blocks TLR3-mediated activation of NF-κB by associating with IL-1 receptor-associated kinase 2 and TRAF6, key proteins within this TLR3 signaling pathway (39). However, NS3/4A targeting of TRIF represents a more proximal attack on the pathway and inhibits both NF-κB and IRF-3 activation through TLR3 (Fig. 3). The targeting of TRIF by NS3/4A thus has the potential to exert a crippling effect on a multiplicity of protective cellular defense mechanisms. Nonetheless, the cleavage of TRIF is unlikely to account for the NS3/4A inhibition of Sendai virus activation of IRF-3 that we observed previously in Huh7 cells (3). These cultured hepatoma cells are TLR3-deficient (Fig. 9B), perhaps explaining why they are relatively permissive for HCV RNA replication (28). Thus, although we found that NS3/4A expressed from replicating HCV RNA suppresses TLR3 signaling in HeLa cells, further studies will be needed to determine the status of this pathway in infected hepatocytes. Sendai virus activates IRF-3 in Huh7 cells through the RIG-I pathway independently of TRIF, leading us to speculate that the NS3/4A disruption of this pathway represents proteolytic targeting of yet a second signaling molecule (40).
The capacity of the protease active site to accommodate unique substrate interactions with TRIF, and, possibly, a second cellular protein, represents a remarkable example of RNA virus evolution and may account for the unusually shallow active site conformation that distinguishes NS3/4A from other viral proteases (6). It may also explain, in part, the exceptionally potent antiviral efficacy of a specific inhibitor of the NS3/4A protease, BILN 2061, in recent clinical trials (41). Inhibition of the protease may not only suppress replication of the virus, but also restore innate antiviral defenses to the virus in infected hepatocytes.
Supplementary Material
Acknowledgments
We thank those who contributed plasmids, antibodies, cell lines, and reagents. We also thank Z. Chen for excellent technical support. This work was supported by National Institutes of Health Grants U19-AI40035 (to S.M.L.), R21-DA018054 (to K.L.), and U01-AI48235 (to M.G.); the Ellison Medical Foundation (M.G.); and a James W. McLaughlin Fellowship (to J.C.F.). K.L. is the John Mitchell Hemophilia of Georgia Liver Scholar of the American Liver Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HCV, hepatitis C virus; IKKε,IκB kinase σ; IRF-3, IFN regulatory factor 3; NS, nonstructural; NS3p, NS3 protease domain; RIG-I, retinoic acid-inducible gene I; scNS3, single-chain NS3; TBK1, TRAF family member-associated NF-κB activator-binding kinase 1; TLR, Toll-like receptor; TRIF, Toll-IL-1 receptor domain-containing adaptor inducing IFN-β; MB–scNS3, purified scNS3 protease expressed as a fusion with maltose-binding protein; ISG, IFN-stimulated gene; RANTES, regulated on activation, normal T cell expressed and secreted; pIC, polyI:C; siRNA, short interfering RNA.
References
- 1.Seeff, L. B. (1997) Hepatology 26, 21S-28S. [DOI] [PubMed] [Google Scholar]
- 2.Racanelli, V. & Rehermann, B. (2003) Trends Immunol. 24, 456-464. [DOI] [PubMed] [Google Scholar]
- 3.Foy, E., Li, K., Wang, C., Sumter, R., Ikeda, M., Lemon, S. M. & Gale, M., Jr. (2003) Science 300, 1145-1148. [DOI] [PubMed] [Google Scholar]
- 4.Hiscott, J., Pitha, P., Genin, P., Nguyen, H., Heylbroeck, C., Mamane, Y., Algarte, M. & Lin, R. (1999) J. Interferon Cytokine Res. 19, 1-13. [DOI] [PubMed] [Google Scholar]
- 5.Yoneyama, M., Suhara, W. & Fujita, T. (2002) J. Interferon Cytokine Res. 22, 73-76. [DOI] [PubMed] [Google Scholar]
- 6.De Francesco, R. & Steinkuhler, C. (2000) Curr. Top. Microbiol. Immunol. 242, 149-169. [DOI] [PubMed] [Google Scholar]
- 7.Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. (2001) Nature 413, 732-738. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., Taira, K., Akira, S. & Fujita, T. (2004) Nat. Immunol. 5, 730-737. [DOI] [PubMed] [Google Scholar]
- 9.Beutler, B. (2004) Nature 430, 257-263. [DOI] [PubMed] [Google Scholar]
- 10.Peters, K. L., Smith, H. L., Stark, G. R. & Sen, G. C. (2002) Proc. Natl. Acad. Sci. USA 99, 6322-6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandvaux, N., tenOever, B. R., Servant, M. J. & Hiscott, J. (2002) Curr. Opin. Infect. Dis. 15, 259-267. [DOI] [PubMed] [Google Scholar]
- 12.Katze, M. G., He, Y. & Gale, M., Jr. (2002) Nat. Rev. Immunol. 2, 675-687. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto, M., Sato, S., Mori, K., Hoshino, K., Takeuchi, O., Takeda, K. & Akira, S. (2002) J. Immunol. 169, 6668-6672. [DOI] [PubMed] [Google Scholar]
- 14.Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. (2003) Nat. Immunol. 4, 161-167. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, Z., Mak, T. W., Sen, G. & Li, X. (2004) Proc. Natl. Acad. Sci. USA 101, 3533-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlotsky, J. M. (2004) Trends Microbiol. 12, 96-102. [DOI] [PubMed] [Google Scholar]
- 17.Lin, C., Prágai, B. M., Grakoui, A., Xu, J. & Rice, C. M. (1994) J. Virol. 68, 8147-8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinkuhler, C., Urbani, A., Tomei, L., Biasiol, G., Sardana, M., Bianchi, E., Pessi, A. & De Francesco, R. (1996) J. Virol. 70, 6694-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma, S., tenOever, B. R., Grandvaux, N., Zhou, G. P., Lin, R. & Hiscott, J. (2003) Science 300, 1148-1151. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald, K. A., McWhirter, S. M., Faia, K. L., Rowe, D. C., Latz, E., Golenbock, D. T., Coyle, A. J., Liao, S. M. & Maniatis, T. (2003) Nat. Immunol. 4, 491-496. [DOI] [PubMed] [Google Scholar]
- 21.Pasquo, A., Nardi, M. C., Dimasi, N., Tomei, L., Steinkuhler, C., Delmastro, P., Tramontano, A. & De Francesco, R. (1998) Folding Des. 3, 433-441. [DOI] [PubMed] [Google Scholar]
- 22.Kolykhalov, A. A., Agapov, E. V. & Rice, C. M. (1994) J. Virol. 68, 7525-7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzgerald, K. A., Rowe, D. C., Barnes, B. J., Caffrey, D. R., Visintin, A., Latz, E., Monks, B., Pitha, P. M. & Golenbock, D. T. (2003) J. Exp. Med. 198, 1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato, S., Sugiyama, M., Yamamoto, M., Watanabe, Y., Kawai, T., Takeda, K. & Akira, S. (2003) J. Immunol. 171, 4304-4310. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto, M., Sato, S., Hemmi, H., Hoshino, K., Kaisho, T., Sanjo, H., Takeuchi, O., Sugiyama, M., Okabe, M., Takeda, K., et al. (2003) Science 301, 640-643. [DOI] [PubMed] [Google Scholar]
- 26.Hoebe, K., Du, X., Georgel, P., Janssen, E., Tabeta, K., Kim, S. O., Goode, J., Lin, P., Mann, N., Mudd, S., et al. (2003) Nature 424, 743-748. [DOI] [PubMed] [Google Scholar]
- 27.Wolk, B., Sansonno, D., Krausslich, H. G., Dammacco, F., Rice, C. M., Blum, H. E. & Moradpour, D. (2000) J. Virol. 74, 2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohmann, V., Korner, F., Koch, J., Herian, U., Theilmann, L. & Bartenschlager, R. (1999) Science 285, 110-113. [DOI] [PubMed] [Google Scholar]
- 29.Scholle, F., Li, K., Bodola, F., Ikeda, M., Luxon, B. A. & Lemon, S. M. (2004) J. Virol. 78, 1513-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu, Q., Guo, J. T. & Seeger, C. (2003) J. Virol. 77, 9204-9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gosert, R., Egger, D., Lohmann, V., Bartenschlager, R., Blum, H. E., Bienz, K. & Moradpour, D. (2003) J. Virol. 77, 5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumpter, R., Jr., Loo, M. Y., Foy, E., Li, K., Yoneyama, M., Fujita, T., Lemon, S. M. & Gale, M. J., Jr. (2005) J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 33.Iwamura, T., Yoneyama, M., Yamaguchi, K., Suhara, W., Mori, W., Shiota, K., Okabe, Y., Namiki, H. & Fujita, T. (2001) Genes Cells 6, 375-388. [DOI] [PubMed] [Google Scholar]
- 34.Su, A. I., Pezacki, J. P., Wodicka, L., Brideau, A. D., Supekova, L., Thimme, R., Wieland, S., Bukh, J., Purcell, R. H., Schultz, P. G., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigger, C. B., Brasky, K. M. & Lanford, R. E. (2001) J. Virol. 75, 7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwasaki, A. & Medzhitov, R. (2004) Nat. Immunol. 5, 987-995. [DOI] [PubMed] [Google Scholar]
- 37.Hoebe, K. & Beutler, B. (2004) J. Endotoxin Res. 10, 130-136. [DOI] [PubMed] [Google Scholar]
- 38.Santoro, M. G., Rossi, A. & Amici, C. (2003) EMBO J. 22, 2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harte, M. T., Haga, I. R., Maloney, G., Gray, P., Reading, P. C., Bartlett, N. W., Smith, G. L., Bowie, A. & O'Neill, L. A. (2003) J. Exp. Med. 197, 343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foy, E., Li, K., Sumpter, R., Jr., Loo, Y.-M., Johnson, C. L., Wang, C., Fish, P. M., Yoneyama, M., Fujita, T., Lemon, S. M. & Gale, M., Jr. (2005) Proc. Natl. Acad. Sci. USA 102, 2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamarre, D., Anderson, P. C., Bailey, M., Beaulieu, P., Bolger, G., Bonneau, P., Bos, M., Cameron, D. R., Cartier, M., Cordingley, M. G., et al. (2003) Nature 426, 186-189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.