Abstract
Pig embryonic tissues represent an attractive option for organ transplantation. However, the achievement of optimal organogenesis after transplantation, namely, maximal organ growth and function without teratoma development, represents a major challenge. In this study, we determined distinct gestational time windows for the growth of pig embryonic liver, pancreas, and lung precursors. Transplantation of embryonic-tissue precursors at various gestational ages [from E (embryonic day) 21 to E100] revealed a unique pattern of growth and differentiation for each embryonic organ. Maximal liver growth and function were achieved at the earliest teratoma-free gestational age (E28), whereas the growth and functional potential of the pancreas gradually increased toward E42 and E56 followed by a marked decline in insulin-secreting capacity at E80 and E100. Development of mature lung tissue containing essential respiratory system elements was observed at a relatively late gestational age (E56). These findings, showing distinct, optimal gestational time windows for transplantation of embryonic pig liver, pancreas, and lung, might explain, in part, the disappointing results in previous transplantation trials and could help enhance the chances for successful implementation of embryonic pig tissue in the treatment of a wide spectrum of human diseases.
Keywords: fetal, porcine, gestational age, growth potential
The demonstration by Thomson et al. (1) and others (2) that human embryonic stem cells (ESc) can be derived and maintained in culture for prolonged periods of time represents an important milestone in regenerative medicine. In their study, Thomson and colleagues used ESc from the inner cell mass of day-5 human blastocysts that were produced through in vitro fertilization. These cells, maintained in vitro, are pluripotent and can produce cell types of all three embryonic germ layers. Defining the optimal conditions for differentiation of human ESc toward different cell types remains a major challenge. Although the experience of the past decade with mouse ESc suggests that this important goal could be attained, one major obstacle seems to be more difficult: the tumorigenicity of ESc, which leads to the formation of teratomas when implanted into immune deficient mice (3, 4). Current approaches are based on the improvement of terminal differentiation and the separation of differentiated cells (5–11) or on attempts to introduce suicide genes into the residual pluripotent cells as a safety measure in case teratoma occurs (12).
An entirely different approach to address this challenge could be afforded if it were possible to use fully committed embryonic stem cells from later stages of gestation. Organ-committed, rather than pluripotent, embryonic stem/progenitor cells are thought to directly generate many of the entire differentiated cell types in an organ, thereby eliminating the risk of teratoma formation. Embryonic precursor tissues comprised of various types of lineage-restricted progenitor cells may be ideal for organ replacement, provided that successful organogenesis can be achieved after transplantation.
Very recently, using metanephroi as a proof of principle, we were able to define an optimal gestational “window” for the successful organogenesis of human and porcine kidneys. This window, defined as the optimal gestational age at which maximal capacity to grow and differentiate into functional tissue with minimal risk for teratoma formation is established, was shown at 7–8 and 4 weeks of gestation for humans and pigs, respectively (13).
Clearly, this narrow window, which depends not only on the relative level of pluripotent and committed stem cells in the specific tissue but also on the status of stromal elements cotransplanted with the donor precursor cells, might greatly differ among various tissue precursors arising from distant embryonic anatomical sites. In the present study, we expand the scope of this approach by examining various embryonic pig precursor tissues at different gestational stages to define the optimal window for the transplantation of embryonic liver, pancreas, and lung. Considering that early clinical attempts to treat diabetic patients with porcine embryonic pancreatic islets obtained around E80 have failed (14–16) and that, currently, transplantation of isolated embryonic hepatocytes has become an attractive option in the treatment of liver diseases (17–21), the importance of an accurate definition of the optimal window for transplantation cannot be overestimated.
Previous studies have clearly shown that minimal immunogenicity is exhibited by tissues harvested at the earliest available gestational time points (22–24). Thus, once the definition of successful organogenesis is established, the earliest time point in this window is most likely to be preferable for human transplantation.
Our data reveal markedly different windows for liver, pancreas, and lung, in correlation with the emergence of each tissue in normal embryonic development. The definition of such distinct windows could further enhance the potential of pig embryonic tissue as a new source for transplantation.
Methods
Animals. Animals were maintained under conditions approved by the Institutional Animal Care and Use Committee at the Weizmann Institute. Immune-deficient nonobese diabetic (NOD)/severe combined immunodeficient (SCID) mice (Weizmann Institute Animal Breeding Center, Rehovot, Israel) were used at the age of 8–10 weeks as hosts for the transplantation studies. All mice were kept in small cages (up to five animals in each cage), fed sterile food, and given acidulated water containing ciprofloxacin.
Porcine Fetal Tissues. Pig embryos were obtained from the Lahav Institute of Animal Research (Kibbutz Lahav, Israel). The study protocol was approved by ethics committees both in Kibbutz Lahav and at the Weizmann Institute. Pregnant sows were operated on under general anesthesia at various, precise stages of pregnancy (E21, E24, E28, E42, E56, E80, and E100), and embryos were extracted. Warm ischemia time was <10 min, and the embryos were transferred in cold PBS. Pig liver, pancreas, and lung precursors for transplantation were extracted under a light microscope and were kept under sterile conditions at 4°C in RPMI medium 1640 (Biological Industries, Bett HaEmek, Israel) before transplantation. Cold ischemia time until transplantation was <2 h.
Transplantation Procedures. Implantation under the kidney capsule. Implantation of pig embryonic tissue was performed under general anesthesia (2.5% 2,2,2-Tribromoethanol, 97% in PBS, 10 ml/kg i.p.). Host kidney was exposed through a left lateral incision. A 1.5-mm incision was made at the caudal end of the kidney capsule, and a fragment of donor tissue (1–2 mm in diameter) was grafted.
Intrasplenic implantation. Liver-precursor tissue was minced to 1-mm × 1-mm fragments in sterile PBS. Under general anesthesia, the host spleen was exposed through a left lateral incision, and a suspension of liver fragments was injected into the lower pole of the spleen in a total volume of 0.2 ml of PBS. Hemostasis was achieved by suture-ligature proximal to the injection site.
Evaluation of Transplant Growth. The animals receiving implants were killed at 6–8 weeks after transplantation. Kidneys and spleens bearing the transplanted grafts were then removed and either fixed in 4% paraformaldehyde or cryopreserved. Long (L) and short (W) axes of the grafts were measured, and posttransplant size was calculated by multiplying L × W.
Evaluation of Transplant Differentiation. Teratoma was defined when tissue representatives of at least two germ layers were detected in the implants (25, 26). Assessment of graft histology and function was performed by histochemistry and immunohistochemical labeling.
Histochemistry included hematoxylin/eosin (H&E), periodic acid/Schiff (PAS), and Alcian blue staining. For immunohistochemical labeling, the following antibodies were used: goat anti-pig albumin antibody (Bethyl Laboratories, Montgomery, TX), rabbit anti-human α-1-fetoprotein (DAKO), monoclonal anti-human cytokeratin 7 (clone OV-TL 12/30, DAKO), monoclonal anti-human cytokeratin 20 (27) (clone Ks 20.8, DAKO), monoclonal mouse anti-human cytokeratin clone MNF116 (broad-spectrum cytokeratin) (DAKO), polyclonal rabbit anti-human chromogranin A (DAKO), rabbit anti-human glucagons (DAKO), guinea pig anti-rabbit insulin (DAKO), rabbit anti-human pancreatic polypeptide (DAKO), and mouse anti-human Ki67 (clone MIB-1) (DAKO).
Paraffin sections (4 μ) were xylene deparaffinized and rehydrated. Endogenous peroxidase was blocked with 0.3% H2O2 in 70% methanol for 10 min. Antigen-retrieval procedures were performed according to the manufacturer's instructions.
After blocking, both paraffin sections and 6-μ cryosections were incubated with specific first antibody for 60 min. Detection of antibody binding was performed by using the following secondary reagents: DAKO peroxidase EnVision system for the detection of mouse and rabbit antibodies, Histofine simple stain MAX PO (Nichirei, Tokyo) for rat antibodies, and Sigma biotinylated anti-goat antibody (followed by extra avidin peroxidase reagent) for goat antibodies. In all cases, diaminobenzidine was used as a chromogen.
ELISA Measurements of Pig Insulin and Albumin. A porcine/human insulin kit (catalog no. K6219, DAKO), in which the primary pig anti-insulin antibody does not crossreact with mouse insulin, was used to follow pig insulin levels according to the manufacturer's instructions. Pig albumin in mouse serum was measured by standard ELISA procedure using goat anti-pig albumin antibodies (human, mouse, and bovine absorbed), affinity purified, and horseradish-peroxidase conjugated (catalog nos. A100–210A and A100–210P, Bethyl).
Statistical Analysis. Comparisons between groups were evaluated by Student's t test. Data were expressed as mean ± SD and were considered statistically significant if P values were ≤0.05.
Results
Implantation of Embryonic Pig Liver Tissue. Teratoma development vs. organ-specific growth after transplantation of pig liver-precursor tissue. To define the earliest gestational age of liver precursors that are free of the risk of teratoma formation after transplantation, pig embryonic liver-precursor tissue obtained at various gestational time points was implanted into SCID mice, and the developing tissue was examined 6 weeks after transplantation. As shown in Table 1, which summarizes the results of six experiments, a considerable incidence of teratomas was found after kidney-subcapsular or intrasplenic implantation of liver embryonic tissue obtained from E21 donors. Likewise, liver precursors obtained at E24 were also associated with teratoma formation in some of the recipients. In contrast, all transplanted hepatic tissue from E28 donors resulted in organ-specific differentiation. Thus, in 23 mice implanted with E28 liver-precursor tissue, teratoma was not detected.
Table 1. Development of teratoma vs. specific organ growth after transplantation of pig embryonic liver.
| Gestational age
|
|||
|---|---|---|---|
| Growth | E21 | E24 | E28 |
| Teratoma | 10/27 | 3/8 | 0/23 |
| Specific organ growth | 5/27 | 5/8 | 21/23 |
Embryonic liver precursor tissues obtained at various gestational ages were implanted under the kidney capsule. Tissue growth and development were evaluated 6 weeks after implantation as described in Methods.
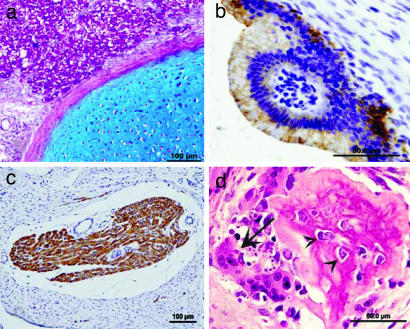
Multiple tissue types were detected in teratomas formed after implantation of E21 and E24 liver implants as shown in Fig. 1. E21 and E24 liver precursors evolved into growths containing, in part, functioning liver cells, but also containing other differentiated-tissue derivatives such as cartilage, bone, striated-muscle fibers, and epithelial elements. When liver was obtained a few days later at E28, organ-specific growth without teratoma was documented.
Fig. 1.
Histology of differentiated elements found in teratomas formed after E21 and E24 pig embryonic liver-precursor transplantations. Teratoma-like structures in liver implants of E21 and E24 are characterized by a mixed growth of various embryonic tissue types. (a) Cartilage stained by Alcian blue and hepatocytes stained by PAS (purple). (b) Columnar epithelia lining cystic structures are decorated by cytokeratine MNF116. (c) Striated-muscle fibers stained by sarcomeric actin. (d) Bone fragment (osteoclast cell is indicated with an arrow, and osteoblasts are indicated with arrowheads) with H&E staining.
E28 liver precursors undergo extensive growth and development at various implantation sites. The growth and functional outcome of pig embryonic liver precursors obtained at minimal teratoma-free gestational age (E28) was followed after kidney-subcapsular or intrasplenic transplantation in NOD/SCID mice. As can be seen in Fig. 2, essential elements of the liver are expressed in both kidney-subcapsular (Fig. 2a) and intrasplenic (Fig. 2b) transplantation. In the kidney-subcapsular grafts, the interface between the kidney and the graft is not sharp, allowing the graft tissue to integrate within the kidney. Similarly, after intrasplenic implantation, liver precursors give rise to large nests of hepatocytes integrated into splenic tissue. In both routes of transplantation, the developing hepatocytes are organized along hepatic cords in typical, lobular structures surrounding a central vein, whereas the portal elements of the liver are evidenced by bile ducts (Fig. 2d) presented in the field. Functional activity of the hepatocytes in the graft is proven by their ability to synthesize albumin (Fig. 2c) and to synthesize and store glycogen (Fig. 2e). Increased proliferative capacity of the hepatic cells is indicated by staining for Ki67 (Fig. 2f).
Fig. 2.
Developmental and functional characteristics of E28 pig liver precursors 6 weeks after implantation. Two sites of implantation are demonstrated under the kidney capsule (a) and intrasplenic (b). H&E staining (a and b) reveals hepatocytes arranged in lobules. Functionality of the hepatocytes is demonstrated by immunohistological staining of pig albumin (c) and PAS staining (e). Bile ducts in the portal regions are made evident by CK-7 staining (d). Proliferating hepatic cells are indicated by Ki67 staining (f, arrows).
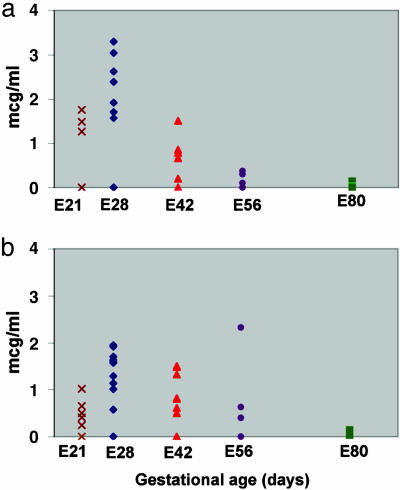
Gestational age strictly determines the functional capacity of embryonic liver grafts. To define the precise time during gestation that affords optimal growth and differentiation into functional hepatocytes, transplantation of liver precursors obtained at various gestational ages (E21, E24, E28, E42, E56, and E80) was performed, and pig-albumin blood levels were measured by highly specific ELISA. Using this assay, we were able to detect pig-albumin secretion as early as 3 days after transplantation of liver embryonic precursors (data not shown). Fig. 3 summarizes the results of nine independent experiments demonstrating pig-albumin secretion 6 weeks after transplantation, either intrasplenic (Fig. 3a) or kidney-subcapsular (Fig. 3b). As can be seen, different levels of pig-albumin secretion are exhibited by grafts obtained at different gestational ages. Grafts from an early gestation stage (E21) secrete low levels of albumin, whereas markedly increased albumin levels are detected in grafts obtained 1 week later (E28). However, the ability of the liver grafts to secrete albumin is reduced upon implantation of tissue from later time points, although implants under the kidney capsule exhibited a slightly less pronounced decline. Thus, rapid deterioration to insignificant albumin levels was documented upon intrasplenic or kidney-subcapsular implantation of liver tissue obtained at E56 or E80, respectively. This correlation between gestational age and growth potential was also supported by the histological findings showing a decline in hepatocyte numbers (data not shown).
Fig. 3.
Pig-albumin levels in serum of SCID mice after transplantation of pig embryonic liver. Donor tissue obtained at various gestational ages was implanted intraspleen (a) and subcapsular (b), and serum pig-albumin levels were documented 6 weeks after transplantation by a specific ELISA, as described in Methods.
Implantation of Embryonic Pig Pancreas Tissue. Teratoma development vs. organ-specific growth after implantation of pig embryonic pancreas. Embryonic pig pancreas-precursor tissues obtained at E24, E28, E42, E56, E80, and E100 were transplanted under the kidney capsule of NOD/SCID mice. Six weeks after transplantation, the implants were harvested and analyzed histologically. A summary of 11 experiments (in each experiment, precursors of two to three different gestational ages were compared) is shown in Table 2.
Table 2. Development of teratoma vs. specific organ growth after transplantation of pig embryonic pancreas.
| Pig pancreas precursors gestational age
|
Histological findings
|
||
|---|---|---|---|
| Ducts and acini | Islets | Teratoma-like structures | |
| E24 | 6/10 | 4/10 | 0/10 |
| E28 | 7/12 | 6/12 | 0/12 |
| E42 | 14/15 | 12/15 | 0/15 |
| E56 | 9/13 | 11/13 | 0/13 |
| E80 | 8/10 | 7/10 | 0/10 |
| E100 | 2/7 | 2/7 | 0/7 |
Embryonic pancreas precursor tissues obtained at various gestational ages were implanted under the kidney capsule. Tissue growth and development were evaluated 6 weeks after implantation as described in Methods.
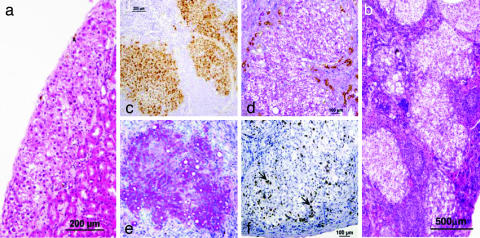
None of the implanted mice exhibited teratoma growth even at the earliest time point at which pancreas embryonic tissue could be precisely collected. Histological analysis of growing embryonic pig pancreatic-precursor tissue revealed that pancreas development after implantation of E24 or E28 tissue is free of teratoma risk and displays marked growth and development, as demonstrated by the presence of fully developed exocrine and endocrine components of the pancreas (Fig. 4a). Pancreatic components are positively stained for pancreatic-specific cytokeratin 20 (Fig. 4b), whereas the donor origin of the pancreatic epithelium is demonstrated by selective staining for cytokeratin MNF116, which is not crossreactive with mouse epithelial cells (Fig. 4c). The functionality of the islets is documented by positive staining for pig insulin, glucagon, and pancreatic polypeptide (Fig. 4 d, e, and f, respectively). Similar to the organization of mature islets, most of the centrally located cells within the developed islets secrete insulin, whereas glucagon secretion is detected mostly in the islets' peripheries. The neuroendocrine origin of the islet cells is supported by positive staining for chromogranin (Fig. 4g).
Fig. 4.
Development of E28 pig pancreas 6 weeks after implantation under the kidney capsule. H&E staining (a) reveals exocrine components (arrows) and endocrine components (arrowhead). Pig pancreatic cytokeratin 20 (b) and broad-spectrum cytokeratin MNF116 (c) and insulin (d), glucagon (e), pancreatic polypeptide (f), and chromogranin (g) are demonstrated by immunohistological staining.
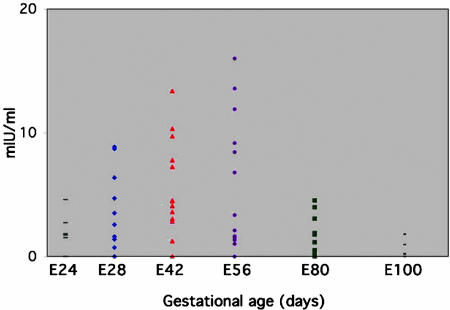
Assessment of the functionality of embryonic pig pancreas implants reveals preferred gestational time points for transplantation. The ability of the grafts obtained at different gestational ages (E24 to E100) to secrete insulin into the serum of NOD/SCID recipient mice was followed by specific ELISA, in which the primary pig anti-insulin antibody does not crossreact with mouse insulin. A summary of the pig-insulin levels detected in mice 6 weeks after transplantation is shown in Fig. 5.
Fig. 5.
Pig-insulin levels in serum of SCID mice after transplantation of pig embryonic pancreas. Donor tissue obtained at various gestational ages was implanted under the kidney capsule, and serum pig-insulin levels were documented 6 weeks after transplantation by specific ELISA, as described in Methods.
Whereas histological assessment could not precisely pinpoint a preferred gestational time point for transplantation, these functional measurements reveal a pattern in which the optimal window for organogenesis is around E42 and E56, with a significant reduction in functional capacity at the very early (E24, P < 0.02) or later (E80, P < 0.003) time points of gestation. A similar conclusion was also suggested by evaluation of the size of the grafts 6 weeks after implantation (Table 3).
Table 3. Graft size and serum levels of pig insulin after transplantation of pig embryonic pancreas.
| Gestational ages
|
||||||
|---|---|---|---|---|---|---|
| Engraftment | E24 | E28 | E42 | E56 | E80 | E100 |
| Graft size, mm2 | 11.3 ± 8.6 | 15.7 ± 11.2 | 33.7 ± 15.5 | 29.3 ± 13.2 | 7.8 ± 8.8 | 0.2 ± 0.07 |
| Pig insulin, μIU/ml | 2 ± 1.5 | 4.2 ± 3.3 | 5.1 ± 4 | 5.9 ± 7.1 | 1.2 ± 1.4 | 0.6 ± 0.7 |
Embryonic pig pancreas tissues obtained at various gestational ages were implanted under the kidney capsule. Tissue growth and serum levels of pig insulin were evaluated 6 weeks after implantation as described in Methods. Data are average ± SD.
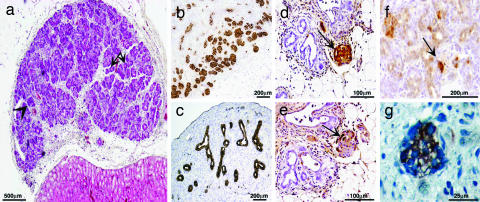
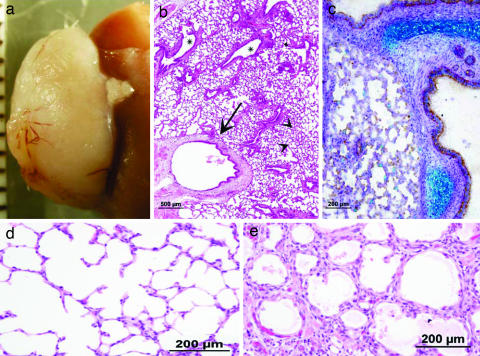
Implantation of Embryonic Pig Lung Tissue. In contrast to liver or pancreas precursors, development of pig lung tissue was not observed after implantation of E24, E28, or even E42 lung precursors, and significant growth can initially be detected only upon implantation of precursor tissue obtained relatively late during gestation, at E56. No teratoma can be detected after implantation of lung tissue obtained at E24, E28, or E42, and only some epithelial cells and fibrosis are found under the kidney capsule at 6 weeks posttransplantation. However, as can be seen in Fig. 6, lung precursors obtained at E56 developed into mature lung tissue containing essential respiratory system elements including different epithelial structures and cartilage (Fig. 6 b and c, respectively). Appropriate types of epithelial cells are detected lining the various lung structures. Importantly, E56 alveoli demonstrate thin interalveolar septa containing capillary plexuses, supported by a minimal amount of fine connective tissue, fulfilling the fine perfusion–ventilation balance required for extrauterine gas exchange (Fig. 6d). Although transplantation of E80 lung precursors also gave rise to lung tissue, the grafts were significantly smaller than those obtained at E56 (P < 0.001). In addition to the reduced growth potential, microscopic findings, including abnormal alveolar-wall thickening and epithelial dysplasia, were evident (Fig. 6e).
Fig. 6.
Development of E56 and E80 pig lung 6 weeks after implantation under the kidney capsule. Impressive growth of transplanted tissue obtained at E56 is macroscopically illustrated (a). Shown are various components of lung tissue (b) including H&E-stained respiratory bronchi (arrow), bronchioles (asterisks), and alveoli (arrowheads). Alcian blue/PAS-decorated cartilage of E56 lung implants are demonstrated (c). Note differences in alveolar-wall structure and thickness of E56 (d) and E80 (e) implants (H&E staining).
Discussion
The potential of embryonic pig tissues as a new source for organ transplantation has been advocated for more than two decades. Clinical applications of this approach can represent an ultimate solution for a wide spectrum of diseases that presently cannot be cured because of the shortage of organs available for transplantation. Among them are such widespread diseases as diabetes, end-stage renal disease, Parkinson's disease, acute and chronic liver failure, and inherited metabolic disorders. The efficacy of committed embryonic pig or human kidney (13, 28), pancreas (29–34), lung (35), heart, or intestine (35, 36) as well as hepatocytes (37–40) or neuronal precursors (41–43), which grew and differentiated upon implantation into SCID or nude mice or rats, has been extensively described. However, preclinical studies in large-animal models, as well as sporadic clinical attempts with neuronal tissue (44–46), pancreas islets (14, 15, 33, 47–50), hepatic tissue (51–54), and intestine (55) met with marginal, if any, success.
In part, this outcome could be attributed to the technical difficulties posed by large-animal models or to the complicated clinical conditions of terminally ill patients in whom this approach was attempted. However, it has also become apparent that several important pieces of information, which could have been provided from the rodent studies, are still missing. In particular, the optimal gestation time for implantation based on the risk for teratoma, growth potential, and immunogenicity, all of which might vary among different organs in fetal development, was not sufficiently characterized. Thus, it is difficult to establish whether the engraftment failure of fetal pig tissues reported in large-animal studies or in humans is mediated only by rejection or could also be attributed to a choice of embryonic tissue with weak growth potential collected at a suboptimal gestation time. This issue is clearly illustrated by our present data, which show that an optimal gestation window for pancreas implantation is afforded between E28 and E56, whereas the growth potential and ability to secrete insulin is significantly reduced beyond E80, at which time most of the pig pancreas transplants in humans were carried out (14).
Similar critical information was afforded by the present experiments with embryonic pig liver and lung, which represent two extremes of the time scale, the former exhibiting a very early gestational window for successful transplantation, whereas the latter becomes relevant only at E56 and later.
Thus, maximal liver-implant growth was achieved at early gestational ages (E21 and E28) followed by a gradual decrease in proliferation capacity and albumin synthesis between E42 and E80. Considering that a high proliferation rate of embryonic liver at E21 was accompanied by an unacceptable risk of teratoma development, our data strongly indicate that the optimal time for pig embryonic liver transplantation is around E28.
Whereas the establishment of the lower limit of the window above which teratoma is least likely to develop is straightforward for all of the tested organ-precursor tissues, defining the upper limit above which potential growth is less than optimal represents a more difficult challenge for organs whose functional performance cannot be established by secretion of a protein into the blood. Thus, the assessment of windows for lung is somewhat more complex compared to the evaluation of pancreas and liver development. However, even with the most conservative interpretation of the histological data, it is apparent that, contrary to the window found for pancreas and liver, early embryonic ages were not favorable for embryonic lung-precursor growth in our model. Thus, development of lung implants obtained at E28 to E42 was not observed, whereas rapidly growing and differentiated lungs that contain all components of the adult respiratory tree, including mature alveoli, were exhibited upon implantation of E56 lung precursors. It should be noted that implants from a later gestational age (E80) show both decreased growth potential and disrupted lung-tissue development with alveolar-wall thickening and epithelial dysplasia.
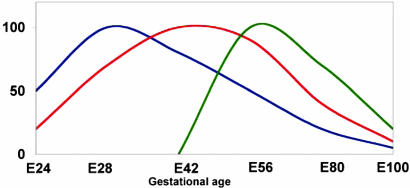
Taken together, all three embryonic precursor tissues exhibit a markedly narrow window, each of which has a different optimum time (Fig. 7). Interestingly, the optimal gestational age represented by each window is in correlation with normal embryonic development, in which liver and pancreas precede the lungs.
Fig. 7.
The relationship between embryonic-precursor gestational age and growth potential is schematically presented. Distinct gestational time windows for implantation of liver (blue), pancreas (red), and lung (green) are demonstrated.
A second intriguing observation is associated with the lack of teratoma formation after implantation of E24 pancreas, in contrast to embryonic liver at the same gestational age, which can still induce teratoma. Likewise, at no examined gestational time points could we find any potential for teratoma in the lung embryonic tissue. This finding could be explained either by the different relative amount of pluripotent and commited stem cells found in the specific developing organ or by the potential restricting activity of stromal elements that might be differentially expressed in various tissues (56). Further studies using phenotypic analysis and in vitro assays are needed to define the levels of pluripotential as opposed to committed stem cells in these early embryonic tissues. Likewise, it is important to define the components of the stromal elements that block differentiation or antagonize differentiation factors.
In conclusion, the present study shows the feasibility of embryonic pig liver and lung implantation, and, in addition, it defines the optimal windows for such transplants. Furthermore, although the potential of pig embryonic pancreas transplantation has been studied and discussed extensively during the past two decades, the present definition of an optimal window might partially explain failures in previous transplantation trials and could improve the future chances for successful clinical applications in the treatment of diabetes.
Studies in large-animal models should define the minimal mass of tissue required, as well as the level of immune suppression needed, to achieve optimal function of such early embryonic precursors.
Acknowledgments
We thank Dorit Natan and Dr. Tamara Berkutzki for excellent assistance and Ronen Borochov for devoted help in the animal facility. This work was supported by Tissera, Inc. (Herzeliya, Israel).
Abbreviations: ESc, embryonic stem cells; En, embryonic day n; NOD, nonobese diabetic; SCID, severe combined immunodeficient; H&E, hematoxylin/eosin; PAS, periodic acid/Schiff.
References
- 1.Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. & Jones, J. M. (1998) Science. 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff, B. E., Pera, M. F., Fong, C. Y., Trounson, A. & Bongso, A. (2000) Nat. Biotechnol. 18, 399–404. [DOI] [PubMed] [Google Scholar]
- 3.Bjorklund, L. M., Sanchez-Pernaute, R., Chung, S., Andersson, T., Chen, I. Y. C., McNaught, K. S. P., Brownell, A.-L., Jenkins, B. G., Wahlestedt, C., Kim, K.-S. & Isacson, O. (2002) Proc. Natl. Acad. Sci. USA 99, 2344–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freed, C. R. (2002) Proc. Natl. Acad. Sci. USA 99, 1755–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eiges, R., Schuldiner, M., Drukker, M., Yanuka, O., Itskovitz-Eldor, J. & Benvenisty, N. (2001) Curr. Biol. 11, 514–518. [DOI] [PubMed] [Google Scholar]
- 6.Ishizaka, S., Shiroi, A., Kanda, S., Yoshikawa, M., Tsujinoue, H., Kuriyama, S., Hasuma, T., Nakatani, K. & Takahashi, K. (2002) FASEB J. 16, 1444–1446. [DOI] [PubMed] [Google Scholar]
- 7.Hori, Y., Rulifson, I. C., Tsai, B. C., Heit, J. J., Cahoy, J. D. & Kim, S. K. (2002) Proc. Natl. Acad. Sci. USA 99, 16105–16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assady, S., Maor, G., Amit, M., Itskovitz-Eldor, J., Skorecki, K. L. & Tzukerman, M. (2001) Diabetes 50, 1691–1697. [DOI] [PubMed] [Google Scholar]
- 9.Jones, E. A., Tosh, D., Wilson, D. I., Lindsay, S. & Forrester, L. M. (2002) Exp. Cell Res. 272, 15–22. [DOI] [PubMed] [Google Scholar]
- 10.Lumelsky, N., Blondel, O., Laeng, P., Velasco, I., Ravin, R. & McKay, R. (2001) Science 292, 1389–1394. [DOI] [PubMed] [Google Scholar]
- 11.Soria, B., Roche, E., Berna, G., Leon-Quinto, T., Reig, J. A. & Martin, F. (2000) Diabetes 49, 157–162. [DOI] [PubMed] [Google Scholar]
- 12.Schuldiner, M., Itskovitz-Eldor, J. & Benvenisty, N. (2003) Stem Cells 21, 257–265. [DOI] [PubMed] [Google Scholar]
- 13.Dekel, B., Burakova, T., Arditti, F. D., Reich-Zeliger, S., Milstein, O., Aviel-Ronen, S., Rechavi, G., Friedman, N., Kaminski, N., Passwell, J. H. & Reisner, Y. (2003) Nat. Med. 9, 53–60. [DOI] [PubMed] [Google Scholar]
- 14.Groth, C. G., Korsgren, O., Tibell, A., Tollemar, J., Moller, E., Bolinder, J., Ostman, J., Reinholt, F. P., Hellerstrom, C. & Andersson, A. (1994) Lancet 344, 1402–1404. [DOI] [PubMed] [Google Scholar]
- 15.Groth, C. G., Korsgren, O., Wennberg, L., Song, Z., Wu, G., Reinholt, F. & Tibell, A. (1998) Transplant. Proc. 30, 3809–3810. [DOI] [PubMed] [Google Scholar]
- 16.Korsgren, O., Groth, C. G., Andersson, A., Hellerstrom, C., Tibell, A., Tollemar, J., Bolinder, J., Ostman, J., Kumagai, M. & Moller, E. (1992) Transplant. Proc. 24, 352–353. [PubMed] [Google Scholar]
- 17.Cantz, T., Zuckerman, D. M., Burda, M. R., Dandri, M., Goricke, B., Thalhammer, S., Heckl, W. M., Manns, M. P., Petersen, J. & Ott, M. (2003) Am. J. Pathol. 162, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laconi, S., Pillai, S., Porcu, P. P., Shafritz, D. A., Pani, P. & Laconi, E. (2001) Am. J. Pathol. 158, 771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oertel, M., Rosencrantz, R., Chen, Y. Q., Thota, P. N., Sandhu, J. S., Dabeva, M. D., Pacchia, A. L., Adelson, M. E., Dougherty, J. P. & Shafritz, D. A. (2003) Hepatology 37, 994–1005. [DOI] [PubMed] [Google Scholar]
- 20.Sandhu, J. S., Petkov, P. M., Dabeva, M. D. & Shafritz, D. A. (2001) Am. J. Pathol. 159, 1323–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sierra, E., Maganto, P., Codesal, J., Mula, N., Cubero, J., Arza, E., Castillo-Olivares, J. L. & Arahuetes, R. M. (2000) Life Sci. 67, 2417–2432. [DOI] [PubMed] [Google Scholar]
- 22.Dekel, B., Burakova, T., Ben-Hur, H., Marcus, H., Oren, R., Laufer, J. & Reisner, Y. (1997) Transplantation 64, 1550–1558. [DOI] [PubMed] [Google Scholar]
- 23.Foglia, R. P., DiPreta, J., Statter, M. B. & Donahoe, P. K. (1986) Ann. Surg. 204, 402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statter, M. B., Foglia, R. P., Parks, D. E. & Donahoe, P. K. (1988) J. Urol. 139, 204–210. [DOI] [PubMed] [Google Scholar]
- 25.Anderson, G. B., BonDurant, R. H., Goff, L., Groff, J. & Moyer, A. L. (1996) Anim. Reprod. Sci. 45, 231–240. [DOI] [PubMed] [Google Scholar]
- 26.Cotran, R. S., Kumar, V., Collins, T. & Robbins, S. L. (1989) Robbins Pathologic Basis of Disease (Saunders, Philadelphia).
- 27.Bouwens, L. (1998) J. Pathol. 184, 234–239. [DOI] [PubMed] [Google Scholar]
- 28.Hammerman, M. R. (2004) Am. J. Transplant. 4, Suppl. 6, 14–24. [DOI] [PubMed] [Google Scholar]
- 29.Castaing, M., Peault, B., Basmaciogullari, A., Casal, I., Czernichow, P. & Scharfmann, R. (2001) Diabetologia 44, 2066–2076. [DOI] [PubMed] [Google Scholar]
- 30.Beattie, G. M., Otonkoski, T., Lopez, A. D. & Hayek, A. (1997) Diabetes 46, 244–248. [DOI] [PubMed] [Google Scholar]
- 31.Amaratunga, A., Khoury, P., Wang, L., Williams, L. & Tuch, B. E. (2003) Xenotransplantation 10, 622–627. [DOI] [PubMed] [Google Scholar]
- 32.Korbutt, G. S., Elliott, J. F., Ao, Z., Smith, D. K., Warnock, G. L. & Rajotte, R. V. (1996) J. Clin. Invest. 97, 2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korsgren, O., Jansson, L., Eizirik, D. & Andersson, A. (1991) Diabetologia 34, 379–386. [DOI] [PubMed] [Google Scholar]
- 34.Rogers, S. A., Chen, F., Talcott, M. & Hammerman, M. R. (2004) Am. J. Physiol. 286, E502–E509. [DOI] [PubMed] [Google Scholar]
- 35.Angioi, K., Hatier, R., Merle, M. & Duprez, A. (2002) J. Surg. Res. 102, 85–94. [DOI] [PubMed] [Google Scholar]
- 36.Lim, F. Y., Kobinger, G. P., Weiner, D. J., Radu, A., Wilson, J. M. & Crombleholme, T. M. (2003) J. Pediatr. Surg. 38, 834–839. [DOI] [PubMed] [Google Scholar]
- 37.Malhi, H., Irani, A. N., Gagandeep, S. & Gupta, S. (2002) J. Cell Sci. 115, 2679–2688. [DOI] [PubMed] [Google Scholar]
- 38.Kokudo, N., Horimoto, H., Ishida, K., Takahashi, S. & Nozawa, M. (1996) Cell Transplant. 5, S21–S22. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi, N., Miyazaki, M., Fukaya, K., Noguchi, H., Tanaka, N. & Namba, M. (2000) Transplant. Proc. 32, 2365–2367. [DOI] [PubMed] [Google Scholar]
- 40.Takebe, K., Shimura, T., Munkhbat, B., Hagihara, M., Nakanishi, H. & Tsuji, K. (1996) Cell Transplant. 5, S31–S33. [DOI] [PubMed] [Google Scholar]
- 41.Larsson, L. C., Corbascio, M., Pearson, T. C., Larsen, C. P., Ekberg, H. & Widner, H. (2003) Transplantation 75, 1448–1454. [DOI] [PubMed] [Google Scholar]
- 42.Larsson, L. C., Frielingsdorf, H., Mirza, B., Hansson, S. J., Anderson, P., Czech, K. A., Strandberg, M. & Widner, H. (2001) Exp. Neurol. 172, 100–114. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong, R. J., Hurelbrink, C. B., Tyers, P., Ratcliffe, E. L., Richards, A., Dunnett, S. B., Rosser, A. E. & Barker, R. A. (2002) Exp. Neurol. 175, 98–111. [DOI] [PubMed] [Google Scholar]
- 44.Deacon, T., Schumacher, J., Dinsmore, J., Thomas, C., Palmer, P., Kott, S., Edge, A., Penney, D., Kassissieh, S., Dempsey, P. & Isacson, O. (1997) Nat. Med. 3, 350–353. [DOI] [PubMed] [Google Scholar]
- 45.Fink, J. S., Schumacher, J. M., Ellias, S. L., Palmer, E. P., Saint-Hilaire, M., Shannon, K., Penn, R., Starr, P., VanHorne, C., Kott, H. S., et al. (2000) Cell Transplant. 9, 273–278. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher, J. M., Ellias, S. A., Palmer, E. P., Kott, H. S., Dinsmore, J., Dempsey, P. K., Fischman, A. J., Thomas, C., Feldman, R. G., Kassissieh, S., et al. (2000) Neurology 54, 1042–1050. [DOI] [PubMed] [Google Scholar]
- 47.Gores, P. F., Hayes, D. H., Copeland, M. J., Korbutt, G. S., Halberstadt, C., Kirkpatrick, S. A. & Rajotte, R. V. (2003) Transplantation 75, 613–618. [DOI] [PubMed] [Google Scholar]
- 48.Hawthorne, W. J., Cachia, A. R., Walters, S. N., Patel, A. T., Clarke, J. E., O'Connell, P. J., Chapman, J. R. & Allen, R. D. (2000) Cell Transplant. 9, 867–875. [DOI] [PubMed] [Google Scholar]
- 49.Reinholt, F. P., Hultenby, K., Tibell, A., Korsgren, O. & Groth, C. G. (1998) Xenotransplantation 5, 222–225. [DOI] [PubMed] [Google Scholar]
- 50.Soderlund, J., Wennberg, L., Castanos-Velez, E., Biberfeld, P., Zhu, S., Tibell, A., Groth, C. G. & Korsgren, O. (1999) Transplantation 67, 784–791. [DOI] [PubMed] [Google Scholar]
- 51.Shimura, T., Takebe, K., Hagihara, M., Kitamura, M., Hiraga, S., Takenoshita, S., Nagamachi, Y. & Tsuji, K. (1996) Transplant. Proc. 28, 1412–1413. [PubMed] [Google Scholar]
- 52.Tsuji, K., Kanai, N., Takebe, K., Shimura, T., Munkhbat, B., Hagihara, M. & Nagamachi, Y. (1997) Transplant. Proc. 29, 3017–3018. [DOI] [PubMed] [Google Scholar]
- 53.Kanai, N., Morita, N., Munkhbat, B., Gansuvd, B., Kise, Y., Sato, K., Takahashi, T., Kakita, A., Nagamachi, Y., Hagihara, M. & Tsuji, K. (1999) Transplant Immunol. 7, 95–99. [DOI] [PubMed] [Google Scholar]
- 54.Habibullah, C. M., Syed, I. H., Qamar, A. & Taher-Uz, Z. (1994) Transplantation 58, 951–952. [DOI] [PubMed] [Google Scholar]
- 55.Zinzindohoue, F., Sarnacki, S., Canioni, D., Brousse, N. & Revillon, Y. (2000) J. Pediatr. Surg. 35, 1728–1732. [DOI] [PubMed] [Google Scholar]
- 56.Zipori, D. (2004) Nat. Rev. Genet. 5, 873–878. [DOI] [PubMed] [Google Scholar]