Abstract
5-lipoxygenase (5-LO) pathway is the major source of potent proinflammatory leukotrienes (LTs) issued from the metabolism of arachidonic acid (AA), and best known for their roles in the pathogenesis of asthma. These lipid mediators are mainly released from myeloid cells and may act as physiological autocrine and paracrine signalling molecules, and play a central role in regulating the interaction between innate and adaptive immunity. The biological actions of LTs including their immunoregulatory and proinflammatory effects are mediated through extracellular specific G-protein-coupled receptors. Despite their role in inflammatory cells, such as neutrophils and macrophages, LTs may have important effects on dendritic cells (DC)-mediated adaptive immunity. Several lines of evidence show that DC not only are important source of LTs, but also become targets of their actions by producing other lipid mediators and proinflammatory molecules. This review focuses on advances in 5-LO pathway biology, the production of LTs from DC and their role on various cells of immune system and in adaptive immunity.
INTRODUCTION
Leukotrienes (LTs) are an important family of eicosanoid lipid mediators derived from the metabolism of arachidonic acid (AA) and associated with asthma and allergic reactions [1]. In contrast to prostaglandins (PGs), which are produced from AA by the action of cyclooxygenase (COX) enzymes, LTs are made predominately by inflammatory cells like polymorphonuclear leukocytes [2], activated macrophages [3], and mast cells [4]. Dendritic cells (DC) are the major players in both innate and adaptive immunity [5]. Despite their important role as the most professional antigen-presenting cells (APC) of the immune system, DC possess the enzymatic machinery to convert AA to proinflammatory LTs [6, 7, 8, 9]. Since, all human DC phenotypes examined until now constitutively express the 5-LO pathway, the role of DC 5-LO-derived lipid mediators in the regulation of the proximal steps of the immune responses was recently suggested [8]. Primary immune response involves phenomena as diverse as antigen uptake by DC, their subsequent migration through multiple tissue barriers, homing in lymphoid organs, and antigen presentation to T cells [8, 9]. LTs could play roles in each of these events. It is known that LTs exert their effects through extracellular G-protein-coupled receptors. Among human tissues studied by Northern blot analysis, LTB4 receptor messenger RNA (mRNA) is strongly expressed in both thymus [10] and lymph nodes (Spanbroek R. unpublished data), while CysLT1 receptor mRNA is expressed in spleen [11]. In addition, 5-LO-deficient mice show altered ovalbumin-dependent cellular and humoral immune responses [12, 13]. These observations provided substantial support for an indispensable role of 5-LO pathway in the regulation of adaptive immunity. Since LTs display a great variety of biological effects, it is not surprising that cellular LT biosynthesis must be tightly regulated. Based in the current knowledge of 5-LO pathway regulation, it becomes obvious that cellular LT biosynthesis is modulated by multiple mechanisms, including gene expression, cytokine effects, enzyme movement, and compartmentalization of the 5-LO pathway. Thus the understanding of the precise regulatory mechanism of the 5-LO activity might provide new concepts for the development of anti-inflammatory drugs.
5-LO PATHWAY AND LT BIOSYNTHESIS
LTs are lipid messengers that play central role in immune responses and tissue homeostasis [14]. Biosynthesis of LTs from AA was initially described in polymorphonuclear leukocytes and monocytes. First described in 1937 as the slow reacting substances of anaphylaxis (SRS-A), these lipid mediators are now known as the cysteinyl LTs (CysLTs), LTC4, LTD4 and LTE4 [15]. Synthesis of LTs can be divided in two pathways: one to create CysLTs and another to create LTB4 (Figure 1). Some of important properties of 5-LO pathway proteins are summarized in Table 1. 5-LO is the key enzyme in LT biosynthesis and is located in the nucleus in some cell types and in the cytosol of others [16]. 5-LO is 72- to 80-kd monomeric soluble protein containing one nonheme iron believed necessary for catalysis [17]. This enzyme possesses an NH2-terminal domain that binds to calcium iron and is essential for nuclear membrane translocation [18]. Active mainly in myeloid cells, such as monocytes, macrophages, B lymphocytes, granulocytes, mast cells, and DC, 5-LO requires Ca2+ and is stimulated by ATP, phosphatidylcholine, lipids, and hydroperoxides [19]. Following cellular activation, 5-LO translocates to the nuclear membrane where it is able to interact with an 18-kd membrane-associated protein referred to as five-lipoxygenase-activating protein (FLAP). FLAP is an AA-binding protein whose function is to optimally present substrate to 5-LO [20]. The first evidence that the 18-kd protein is absolutely required for cellular 5-LO activity comes from transfection studies in human osteosarcoma cell lines [21]. In this system, expression of 5-LO alone resulted in no detectable cellular 5-LO activity following challenge with calcium ionophore A-23187. LT synthesis only occurred when 5-LO and FLAP were co-expressed in these cells. FLAP is expressed on T cells [22], macrophages, and DC [6], but not on erythrocytes or endothelial cells.
Figure 1.
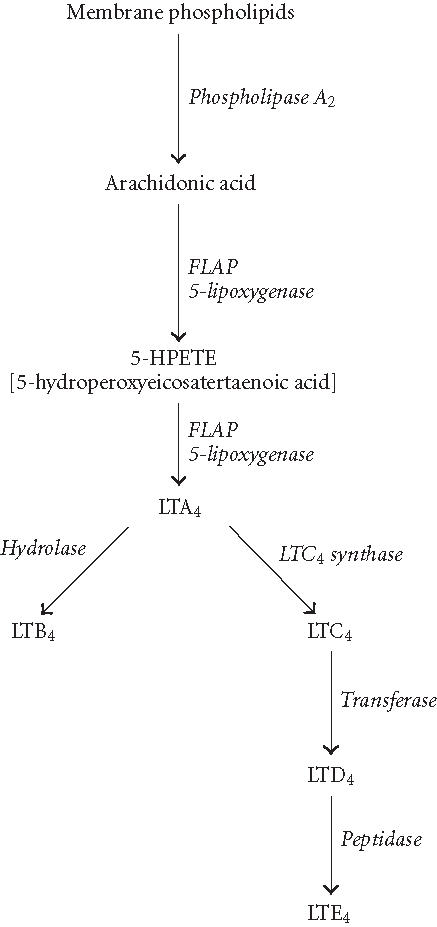
Pathways of LT biosynthesis. Arachidonic acid is liberated from membrane phospholipids by the action of cytosolic phospholipase A2 (cPLA2) and presented to 5-LO enzyme by FLAP. 5-LO enzyme converts arachidonic acid to an unstable intermediate termed 5-hydroperoxyeicosatetraenoic acid (5-HPETE). Subsequently, 5-HPETE is dehydrated to yield the epoxide LTA4, a pivotal intermediate in the biosynthesis of inflammatory and anaphylactic mediators. Enzymatic hydrolysis of LTA4 by LTA4 hydrolase results in the formation of LTB4. Alternatively, LTC4 synthase catalyzes the conjunction of LTA4 with glutathione to form LTC4, which may be converted to LTD4 and LTE4 by the actions of the transferase and peptidase enzymes, respectively.
Table 1.
5-lipoxygenase pathway proteins.
| 5-lipoxygenase | FLAP | LTA4 hydrolase | LTC4 synthase |
|---|---|---|---|
| 78 kd, cytosolic protein | 18 kd, membrane protein | 69 kd, cytosolic protein | 18 kd, membrane protein |
| 673 amino acid | AA-binding protein | Converts LTA4 to LTB4 | Converts LTA4 to LTC4 |
| Nonheme iron | Required for activation of 5-LO | ||
| Translocated to cell membrane after cell activation | |||
| Dioxygenase activity converts AA to 5-HPETE | |||
| Dehydratase activity converts 5-HPETE to LTA4 | |||
Cellular activation by immune complexes, bacterial peptides, and other stimuli elicit a sequence of events that include cytosolic phospholipase A2 (cPLA2) and 5-LO translocation to the nuclear envelope to produce 5-hydroperoxy eicosatetraenoic acid (5-HPETE) from AA. Subsequently, 5-HPETE is dehydrated to yield the epoxide LTA4, a pivotal intermediate in the biosynthesis of inflammatory and anaphylactic mediators. LTA4 undergoes transformation by one or more of three possible fates depending on the cellular context: hydrolysis, conjugation with glutathione, or transcellular metabolism to generate bioactive lipid mediators [23]. In neutrophils and monocytes, LTA4 is converted predominately to the chemoattractant LTB4 by LTA4 hydrolase [24], but in human eosinophils, mast cells, and basophils, LTA4 is conjugated with reduced glutathione by LTC4 synthase to form the first of the CysLTs, LTC4 [25]. After carrier-mediated cellular export, sequential cleavage of the glutathionyl side chain of LTC4 generates the extracellular metabolites LTD4 and LTE4. LTB4 may be degraded by microsomal ω-oxidation and peroxisomal β-oxidation in myeloid cells and hepatocytes. Degradation is accompanied by loss of biological activity. It is important to note that degradative enzymes are increased by the transcription factor peroxisome proliferator-activated receptor α (PPARα) and this nuclear hormone receptor is in turn activated by binding with LTB4 [26]. This feedback loop limits the duration of action of LTB4.
RECEPTORS FOR LEUKOTRIENES
The actions of LTs require the expression of extracellular G-protein-coupled receptors (GPCR) which are members of the rhodopsin-like receptor superfamily. LTB4 is a potent chemoattractant that is primarily involved in inflammation, immune responses, and host defense against infection. LTB4 activates inflammatory cells by binding to its cell-surface receptor (designated BLT) via two known receptors, BLT1 and BLT2, according to the affinity of LTB4 for the receptor. BLT1 has been characterized as a 43-kd GPCR expressed only in inflammatory cells, including neutrophils, alveolar macrophages, and eosinophils and with a high affinity for only LTB4 [27]. The second G-protein-coupled receptor for LTB4, BLT2 receptor, has only recently been described and is a low-affinity receptor expressed more ubiquitously [28, 29]. Both receptors are found in lymph node [30] on the same chromosome, but intriguingly, the promoter region of the BLT1 gene is localized within the open reading frame (ORF) of the BLT2 gene, which encodes a low-affinity receptor for LTB4 [27]. The high-affinity receptor BLT1 mediates chemotaxis, chemokinesis, aggregation, and adherence to endothelium. The low-affinity receptor BLT2 mediates degranulation and oxygen radical release [31]. Overexpression of LTB4 receptors in leukocytes leads to increased neutrophil influx into the lungs after ischemia-reperfusion in mice [32]. It is also important to indicate that LTB4 can bind and activate the intranuclear transcription factor PPARα, resulting in the activation of genes that terminate inflammatory processes [10]. The receptor for cysteinyl LTs, LTC4, LTD4, and LTE4 are named CysLT receptors and are distinct from the LTB4 receptors. Two classes of CysLT receptors have been identified: CysLT1 and CysLT2 according to their sensitivity to selective LT receptor antagonists. CysLT1 has been identified in human smooth muscle cells [11], vascular endothelial cells [33], lung macrophages, and peripheral blood leukocytes. CysLT2 receptor, originally found in pulmonary vein preparations [34] by pharmacological assays, is detected in spleen, Purkinje fibers of the heart, and discreet region of the adrenal gland by molecular methods.
REGULATORY MECHANISMS OF CELLULAR LT BIOSYNTHESIS
Abnormal production of LT contributes to a variety of inflammatory diseases, including asthma, glomerulonephritis, psoriasis, inflammatory bowel diseases, and acute lung injury [35]. Considering their important role in host defense against microbial infections [36], biosynthesis of LTs must be tightly regulated. Two steps seem to be involved in the control of LT biosynthesis, that is, liberation of AA and regulation of 5-LO activity [37]. Moreover, the expression of 5-LO and FLAP, which are subject to regulation in monocytic cells [38], have been considered as the major determinant of cellular LT biosynthesis [39]. 5-LO activity and product generation at sites of inflammation might be modulated in multiple ways. The most obvious would be the recruitment and stimulation of additional inflammatory cells. Another possibility is modulating the expression of enzymatic components through transcriptional or posttranscriptional mechanisms. Recently, it has been reported that cyclic AMP (cAMP) elevating agents, such as PGE2, inhibited LT biosynthesis and 5-LO translocation to the nucleus in cytokine-primed human neutrophils [40]. The intracellular compartmentalization of the 5-LO metabolism affects the integrated output of this biosynthetic pathway [41]. Nuclear import of 5-LO can modulate LT production, since nuclear import can strongly enhance [42] or suppress [43] 5-LO activity. In considering the question of 5-LO translocation and its cellular localization, it is necessary to consider that 5-LO can move in or out of the nucleus in response to in vivo or in vitro experimental conditions [41]. Cytokines and other components may also affect 5-LO pathway, and the expression of 5-LO and FLAP can be controlled by specific molecules. For example, it has been reported that the Th2-derived cytokines, IL-4, and IL-13 inhibited the release of LT from monocytes by decreasing FLAP mRNA and protein levels, whereas, IL-1 and IFNγ (Th1 cytokines) stimulate 5-LO expression [39]. 5-LO activity may be modulated by nitric oxide (NO) since prolonged exposure to lipopolysaccharide inhibits macrophage 5-LO metabolism via induction of NO synthesis [44]. In bone marrow-derived DC, we have recently demonstrated that exogenous or PGE2-induced IL-10 inhibited FLAP expression without any effect on 5-LO enzyme [6]. The regulatory mechanisms of the 5-LO pathway appear to be complex and depends on the composition of the investigated cell populations. Specific control of 5-LO pathway has long been suspected because expression of 5-LO activity is tissue specific and narrowly distributed to inflammatory cells. It is also important to note that there is wide variation in 5-LO activity and in 5-LO and FLAP levels even among different inflammatory cell types.
5-LO PATHWAY AND CELLS OF IMMUNE SYSTEM: WHAT ABOUT DENDRITIC CELLS?
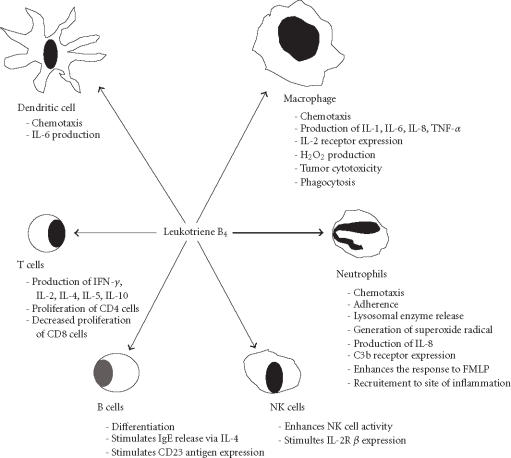
One of the best known and most well-studied LTs is LTB4. LTB4 is produced by many cells of the immune system, including activated neutrophils stimulated with AA or calcium ionophore [45], macrophages, DC, and T cells [46, 47]. This lipid mediators has diverse effects on the function and activities of cells of the immune system (Figure 2). Several observations have suggested that LTB4, in cooperation with monocytes, stimulates T-suppressor-cell functions [48, 49]. These effects appeared to be exerted through inhibition of T CD4+ (helper/inducer) subsets and enhancement of T CD8+ (suppressor/cytotoxic) subsets [50]. The suppression required the presence of monocytes and was induced by the cyclooxygenase inhibitors [51]. It has been suggested that the mechanism of action involved induction of monocytes to produce both prostaglandins (metabolites of cyclooxygenase pathways) and cytokines, such as IFN-γ, IL-2, which in turn lead to the production of IL-1 [52, 53]. Thus, the effects of LTB4 on T lymphocytes are not the result of a direct action on lymphocytes themselves but an indirect action via monocytes. On natural killer (NK) cells, LTB4 significantly augmented NK cell cytotoxicity and stimulated the expression of IL-2 β receptors when added to effector target cocultures [54, 55]. LTB4 is also known to enhance B cell differentiation and secretion of IgE via an IL-4-dependent mechanism [56]. LTB4 causes adhesion and chemotactic movement of leukocytes and stimulates aggregation, enzyme release, and generation of superoxide in neutrophils. Leukotrienes C4, D4, and E4, which are released from the lung tissue of asthmatic subjects exposed to specific allergens, seem to play a pathophysiological role in immediate hypersensitivity reactions. The expression of 5-LO and FLAP genes have been reported in human B cells by reversed transcription chain analysis [22]. Several AA-derived lipid mediators possess immunoregulatory properties in vivo and in vitro. The most interesting example is PGE2, which clearly affects the functions of DC and other cell types of the immune system [57]. In contrast to PGE2, LTB4 is considered as a mainly immunopotentiating mediator, which stimulates the activity of NK cells and B lymphocytes, although it also induces suppressor function among human mononuclear leukocytes. LTB4 can augment proliferation of mixed T lymphocytes and of OKT8+ cells [58]. It also stimulates the release of IL-1 and TNF-α in human monocytes [59], and IL-8 from human polymorphonuclear leukocytes [60].
Figure 2.
Effects of LTB4 on cells of immune system.
Cells that produce LTs or become targets to their actions can also produce other proinflammatory molecules, such as PGs, and various cytokines. For example, the exogenously added or the endogenously produced LTB4 can enhance the release of IL-6 from human monocytes [61] and mice DC [6]. This may constitute an important mean by which monocytes and DC modulate inflammatory responses. Several lines of evidence support an expanded role of the 5-LO pathway in the regulation of antigen-specific adaptive immunity. Epidermal Langerhans cells (LC), a member of the DC family capable of initiating antigen-specific immune responses in naive lymphocytes, markedly express the 5-LO pathway [9]. We have recently demonstrated that mice bone marrow-derived DC (BM-DC) produce LTB4 and other AA metabolites, which have an autocrine actions in APC-mediated immunity [6, 7]. Other investigators have reported the expression of 5-LO in human DC generated from CD34+ hematopoietic progenitors and in lymphoid organs [8]. It has been recently reported that BM-DC express CysLT1 receptors, 5-LO, FLAP, and LTC4 synthase, and produce CysLTs in response to aeroallergen [62]. The produced CysLTs play a crucial role in regulating DC function in a murine model for asthma. The ability of DC to migrate to lymphoid nodes may be affected by 5-LO products since the LTC4 transporter MRP1 may regulate CCL19-dependent mobilization of DC from peripheral tissues to lymph nodes [63]. Taken together, these data suggest that by producing proinflammatory LTs and other components, DC and other APC may play important role in inflammatory processes and allergic diseases.
CONCLUDING REMARKS
The main function of the immune system is the defence of the host against pathogens and tumors. Two major defence systems are involved in host defence against infectious agents and tumors: the innate and adaptive immunity, which differ in the means by which they recognize antigens. However, it is important to indicate that innate immune system activates, orientates, and regulates the adaptive immunity, which through clonal expansion of lymphocytes will respond to pathogen in antigen specific manner. Both innate and adaptive immunity are closely linked, and the interactions between the two systems of the host defence are regulated by a variety of cells and their mediators in particular cytokines and AA-derived lipid mediators, such as LTs.
The development of specific agonists and antagonists for each LT receptors will provide important reagent for further defining the biological and pathological actions of proinflammatory LTs. Other lipoxygenase products will also certainly receive attention in the year to come. To delineate molecular mechanisms within immune responses that are affected by 5-LO pathway products, future studies should be directed toward DC/T lymphocytes interactions, the identification of LT receptor-expressing cells in lymphoid organs, and immune response studies of 5-LO knockout mice. Finally, further studies of LT receptors expression in different types of DC and other APC are needed to truly assess the potential roles of 5-LO pathway in DC biology and APC-mediated immunity.
ACKNOWLEDGMENTS
This work is supported by La Ligue Régionale Contre le Cancer, Comité Départemental des Charentes et de la Gironde.
References
- 1.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 2.Brock T.G, McNish R.W, Coffey M.J, Ojo T.C, Phare S.M, Peters-Golden M. Effects of granulocyte-macrophage colony-stimulating factor on eicosanoid production by mononuclear phagocytes. J Immunol. 1996;156(7):2522–2527. [PubMed] [Google Scholar]
- 3.Chensue S.W, Kunkel S.L. Arachidonic acid metabolism and macrophage activation. Clin Lab Med. 1983;3(4):677–694. [PubMed] [Google Scholar]
- 4.Ramos B.F, Zhang Y, Qureshi R, Jakschik B.A. Mast cells are critical for the production of leukotrienes responsible for neutrophil recruitment in immune complex-induced peritonitis in mice. J Immunol. 1991;147(5):1636–1641. [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Harizi H, Juzan M, Moreau J.F, Gualde N. Prostaglandins inhibit 5-lipoxygenase-activating protein expression and leukotriene B4 production from dendritic cells via an IL-10-dependent mechanism. J Immunol. 2003;170(1):139–146. doi: 10.4049/jimmunol.170.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Harizi H, Gualde N. Dendritic cells produce eicosanoids, which modulate generation and functions of antigen-presenting cells. Prostaglandins Leukot Essent Fatty Acids. 2002;66(5-6):459–466. doi: 10.1054/plef.2002.0383. [DOI] [PubMed] [Google Scholar]
- 8.Spanbroek R, Hildner M, Steinhilber D, et al. 5-lipoxygenase expression in dendritic cells generated from CD34+ hematopoietic progenitors and in lymphoid organs. Blood. 2000;96(12):3857–3865. [PubMed] [Google Scholar]
- 9.Spanbroek R, Stark H.J, Janssen-Timmen U, et al. 5-Lipoxygenase expression in Langerhans cells of normal human epidermis. Proc Natl Acad Sci USA. 1998;95(2):663–668. doi: 10.1073/pnas.95.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387(6633):620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 11.Lynch K.R, O'Neill G.P, Liu Q, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399(6738):789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- 12.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 13.Funk C.D. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta. 1996;1304(1):65–84. doi: 10.1016/s0005-2760(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 14.Funk C.D. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 15.Lewis R.A, Austen K.F, Soberman R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- 16.Peters-Golden M, Brock T.G. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 2001;487(3):323–326. doi: 10.1016/s0014-5793(00)02374-7. [DOI] [PubMed] [Google Scholar]
- 17.Rouzer C.A, Shimizu T, Samuelsson B. On the nature of the 5-lipoxygenase reaction in human leukocytes: characterization of a membrane-associated stimulatory factor. Proc Natl Acad Sci USA. 1985;82(22):7505–7509. doi: 10.1073/pnas.82.22.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X.S, Funk C.D. The N-terminal “beta-barrel” domain of 5-lipoxygenase is essential for nuclear membrane translocation. J Biol Chem. 2001;276(1):811–818. doi: 10.1074/jbc.M008203200. [DOI] [PubMed] [Google Scholar]
- 19.Radmark O. Arachidonate 5-lipoxygenase. Prostaglandins Other Lipid Mediat. 2002;68-69:211–234. doi: 10.1016/s0090-6980(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 20.Mancini J.A, Abramovitz M, Cox M.E, et al. 5-lipoxygenase-activating protein is an arachidonate binding protein. FEBS Lett. 1993;318(3):277–281. doi: 10.1016/0014-5793(93)80528-3. [DOI] [PubMed] [Google Scholar]
- 21.Dixon R.A, Diehl R.E, Opas E, et al. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343(6255):282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- 22.Jakobsson P.J, Steinhilber D, Odlander B, Radmark O, Claesson H.E, Samuelsson B. On the expression and regulation of 5-lipoxygenase in human lymphocytes. Proc Natl Acad Sci USA. 1992;89(8):3521–3525. doi: 10.1073/pnas.89.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gronert K, Clish C.B, Romano M, Serhan C.N. Transcellular regulation of eicosanoid biosynthesis. Methods Mol Biol. 1999;120:119–144. doi: 10.1385/1-59259-263-5:119. [DOI] [PubMed] [Google Scholar]
- 24.Radmark O, Shimizu T, Jornvall H, Samuelsson B. Leukotriene A4 hydrolase in human leukocytes. Purification and properties. J Biol Chem. 1984;259(20):12339–12345. [PubMed] [Google Scholar]
- 25.Yoshimoto T, Soberman R.J, Spur B, Austen K.F. Properties of highly purified leukotriene C4 synthase of guinea pig lung. J Clin Invest. 1988;81(3):866–871. doi: 10.1172/JCI113396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devchand P.R, Keller H, Peters J.M, Vazquez M, Gonzalez F.J, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384(6604):39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 27.Kato K, Yokomizo T, Izumi T, Shimizu T. Cell-specific transcriptional regulation of human leukotriene B4 receptor gene. J Exp Med. 2000;192(3):413–420. doi: 10.1084/jem.192.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276(15):12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]
- 29.Kamohara M, Takasaki J, Matsumoto M, et al. Molecular cloning and characterization of another leukotriene B4 receptor. J Biol Chem. 2000;275(35):27000–27004. doi: 10.1074/jbc.C000382200. [DOI] [PubMed] [Google Scholar]
- 30.Christie P.E, Henderson W.R. Jr. Lipid inflammatory mediators: leukotrienes, prostaglandins, platelet-activating factor. Clin Allergy Immunol. 2002;16:233–254. [PubMed] [Google Scholar]
- 31.Henderson W.R. Jr. The role of leukotrienes in inflammation. Ann Intern Med. 1994;121(9):684–697. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 32.Chiang N, Gronert K, Clish C.B, O'Brien J.A, Freeman M.W, Serhan C.N. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104(3):309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan C.N. Selectivity of recombinant human leukotriene D4, leukotriene B4, and lipoxin A4 receptors with aspirin-triggered 15-epi-LXA4 and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158(1):3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicosia S, Capra V, Accomazzo A.R, et al. Receptors and second messengersfor Cys-leukotrienes. In: Folco G.C, Samuelsson B, Maclouf J.A, Velo G.P, editors. Eicosanoids: From Biotechnology to Therapeutic Applications. New York: Plenum Press; 1996. pp. 127–136. [Google Scholar]
- 35.Goetzl E.J, An S, Smith W.L. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995;9(11):1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- 36.Bailie M.B, Standiford T.J, Laichalk L.L, Coffey M.J, Strieter R, Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157(12):5221–5224. [PubMed] [Google Scholar]
- 37.Steinhilber D. 5-Lipoxygenase: enzyme expression and regulation of activity. Pharm Acta Helv. 1994;69(1):3–14. doi: 10.1016/0031-6865(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 38.Bennett C.F, Chiang M.Y, Monia B.P, Crooke S.T. Regulation of 5-lipoxygenase and 5-lipoxygenase-activating protein. expression in HL-60 cells. Biochem J. 1993;289(pt 1):33–39. doi: 10.1042/bj2890033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nassar G.M, Montero A, Fukunaga M, Badr K.F. Contrasting effects of proinflammatory and T-helper lymphocyte subset-2 cytokines on the 5-lipoxygenase pathway in monocytes. Kidney Int. 1997;51(5):1520–1528. doi: 10.1038/ki.1997.209. [DOI] [PubMed] [Google Scholar]
- 40.Flamand N, Surette M.E, Picard S, Bourgoin S, Borgeat P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol Pharmacol. 2002;62(2):250–256. doi: 10.1124/mol.62.2.250. [DOI] [PubMed] [Google Scholar]
- 41.Peters-Golden M, Brock T.G. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161(pt 2):36–40. doi: 10.1164/ajrccm.161.supplement_1.ltta-8. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh F.H, Lam B.K, Penrose J.F, Austen K.F, Boyce J.A. T helper cell type 2 cytokines coordinately regulate immunoglobulin E-dependent cysteinyl leukotriene production by human cord blood-derived mast cells: profound induction of leukotriene C4 synthase expression by interleukin 4. J Exp Med. 2001;193(1):123–133. doi: 10.1084/jem.193.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brock T.G, Anderson J.A, Fries F.P, Peters-Golden M, Sporn P.H. Decreased leukotriene C4 synthesis accompanies adherence-dependent nuclear import of 5-lipoxygenase in human blood eosinophils. J Immunol. 1999;162(3):1669–1676. [PubMed] [Google Scholar]
- 44.Coffey M.J, Phare S.M, Peters-Golden M. Prolonged exposure to lipopolysaccharide inhibits macrophage 5-lipoxygenase metabolism via induction of nitric oxide synthesis. J Immunol. 2000;165(7):3592–3598. doi: 10.4049/jimmunol.165.7.3592. [DOI] [PubMed] [Google Scholar]
- 45.Ford-Hutchinson A.W, Bray M.A, Doig M.V, Shipley M.E, Smith M.J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- 46.Goetzl E.J. Selective feed-back inhibition of the 5-lipoxygenation of arachidonic acid in human T-lymphocytes. Biochem Biophys Res Commun. 1981;101(2):344–350. doi: 10.1016/0006-291x(81)91266-3. [DOI] [PubMed] [Google Scholar]
- 47.Goodwin J.S, Atluru D, Sierakowski S, Lianos E.A. Mechanism of action of glucocorticosteroids. Inhibition of T cell proliferation and interleukin 2 production by hydrocortisone is reversed by leukotriene B4. J Clin Invest. 1986;77(4):1244–1250. doi: 10.1172/JCI112427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rola-Pleszczynski M, Borgeat P, Sirois P. Leukotriene B4 induces human suppressor lymphocytes. Biochem Biophys Res Commun. 1982;108(4):1531–1537. doi: 10.1016/s0006-291x(82)80081-8. [DOI] [PubMed] [Google Scholar]
- 49.Payan D.G, Goetzl E.J. Specific suppression of human T lymphocyte function by leukotriene B4. J Immunol. 1983;131(2):551–553. [PubMed] [Google Scholar]
- 50.Payan D.G, Missirian-Bastian A, Goetzl E.J. Human T-lymphocyte subset specificity of the regulatory effects of leukotriene B4. Proc Natl Acad Sci USA. 1984;81(11):3501–3505. doi: 10.1073/pnas.81.11.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rola-Pleszczynski M. Differential effects of leukotriene B4 on T4+ and T8+ lymphocyte phenotype and immunoregulatory functions. J Immunol. 1985;135(2):1357–1360. [PubMed] [Google Scholar]
- 52.Rola-Pleszczynski M, Lemaire I. Leukotrienes augment interleukin 1 production by human monocytes. J Immunol. 1985;135(6):3958–3961. [PubMed] [Google Scholar]
- 53.Rola-Pleszczynski M, Chavaillaz P.A, Lemaire I. Stimulation of interleukin 2 and interferon gamma production by leukotriene B4 in human lymphocyte cultures. Prostaglandins Leukot Med. 1986;23(2-3):207–210. doi: 10.1016/0262-1746(86)90187-3. [DOI] [PubMed] [Google Scholar]
- 54.Gagnon L, Girard M, Sullivan A.K, Rola-Pleszczynski M. Augmentation of human natural cytotoxic cell activity by leukotriene B4 mediated by enhanced effector-target cell binding and increased lytic efficiency. Cell Immunol. 1987;110(2):243–252. doi: 10.1016/0008-8749(87)90120-1. [DOI] [PubMed] [Google Scholar]
- 55.Rola-Pleszczynski M, Gagnon L, Sirois P. Leukotriene B4 augments human natural cytotoxic cell activity. Biochem Biophys Res Commun. 1983;113(2):531–537. doi: 10.1016/0006-291x(83)91758-8. [DOI] [PubMed] [Google Scholar]
- 56.Crooks S.W, Stockley R.A. Leukotriene B4. Int J Biochem Cell Biol. 1998;30(2):173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- 57.Harizi H, Grosset C, Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J Leukoc Biol. 2003;73(6):756–763. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 58.Gualde N, Atluru D, Goodwin J.S. Effect of lipoxygenase metabolites of arachidonic acid on proliferation of human T cells and T cell subsets. J Immunol. 1985;134(2):1125–1129. [PubMed] [Google Scholar]
- 59.Dubois C.M, Bissonnette E, Rola-Pleszczynski M. Asbestos fibers and silica particles stimulate rat alveolar macrophages to release tumor necrosis factor. Autoregulatory role of leukotriene B4. Am Rev Respir Dis. 1989;139(5):1257–1264. doi: 10.1164/ajrccm/139.5.1257. [DOI] [PubMed] [Google Scholar]
- 60.McCain R.W, Holden E.P, Blackwell T.R, Christman J.W. Leukotriene B4 stimulates human polymorphonuclear leukocytes to synthesize and release interleukin-8 in vitro. Am J Respir Cell Mol Biol. 1994;10(6):651–657. doi: 10.1165/ajrcmb.10.6.8003341. [DOI] [PubMed] [Google Scholar]
- 61.Rola-Pleszczynski M, Stankova J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood. 1992;80(4):1004–1011. [PubMed] [Google Scholar]
- 62.Machida I, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol. 2004;172(3):1833–1838. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- 63.Robbiani D.F, Finch R.A, Jager D, Muller W.A, Sartorelli A.C, Randolph G.J. The Leukotriene C4 transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103(5):757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]