Abstract
The objective of this study was to evaluate the susceptibility of metallo-β-lactamase (MBL)-producing Acinetobacter baumannii (A. baumannii) clinical isolates to biocides. We also determined the prevalence and correlation of efflux pump genes, class 1 integron and MBL encoding genes. In addition, blaVIM, blaNDM-1, qacE and qacEΔ1 nucleotide sequence analysis was performed and compared to sequences retrieved from GenBank at the National Center for Biotechnology Information database. A. baumannii had a resistance rate to carbapenem of 71.4% and 39.3% and was found to be a MBL producer. The minimum inhibitory concentrations (MICs) of chlorhexidine and cetrimide were higher than the recommended concentrations for disinfection in 54.5% and 77.3% of MBL-positive isolates respectively and their MICs were significantly higher among qac gene-positive isolates. Coexistence of qac genes was detected in 68.1% and 50% of the isolates with blaVIM and blaNDM-1 respectively. There was a significant correlation between the presence of qac genes and MBL-encoding blaVIM and blaNDM-1 genes. Each of the blaNDM-1, blaVIM, qacE and qacEΔ1 DNA sequences showed homology with each other and with similar sequences reported from other countries. The high incidence of Verona integron-encoded metallo-β-lactamases (VIM) and New-Delhi-metallo-β-lactamase (NDM) and qac genes in A. baumannii highlights emerging therapeutic challenges for being readily transferable between clinically relevant bacteria. In addition reduced susceptibility to chlorhexidine and cetrimide and the potential for cross resistance to some antibiotics necessitates the urgent need for healthcare facilities to periodically evaluate biocides efficacy, to address the issue of antiseptic resistance and to initiate a “biocidal stewardship”.
Keywords: Acinetobacter baumannii, MBL encoding genes, qac genes and biocides resistance
1. Introduction
Recently, Acinetobacter baumannii (A. baumannii) has received a lot attention due to its increasing prevalence in hospital environments and its high antimicrobial resistance pattern, including resistance to carbapenems. Moreover, it has been identified as a “Red Alert” to hospitalized immunocompromised patients [1]. Resistance to carbapenems is mainly mediated by carbapenem-hydrolyzing β-lactamases. A variety of β-lactamases which include metallo-β-lactamases (MBL), have emerged as the most worrisome mechanism of resistance among Gram-negative bacteria, which pose a therapeutic challenge to health care settings [2,3,4]. MBL encoding genes, blaIMP, blaVIM, blaGIM, blaSIM, and blaKMH and blaNDM-1, are harbored by class 1 integrons and are found as gene cassettes [5,6]. The VIM-type β-lactamase (Verona integron-encoded metallo-β-lactamases) Acinetobacter spp. was initially described in Europe [7,8] and has been reported worldwide. New-Delhi-metallo-β-lactamase (NDM) is a recently discovered transferable molecular class B β-lactamase, first identified in New Delhi, India [9]. The emergence and dissemination of NDM-producing isolates have been reported in several countries, including USA, Canada, Sweden, UK, Austria, Belgium, France, the Netherlands, Germany, Japan, Australia, Africa and some Middle Eastern countries [3,10]. The blaNDM-1 gene has been detected on different large plasmids, which were readily transferable among bacteria [6]. Most blaNDM-1-positive bacteria are resistant to almost all antibiotics and carry different supplementary mechanisms of resistance [11,12], making blaNDM-1-producing bacteria a significant clinical and public health threat. As a result, reliable detection and surveillance of NDM-producing bacteria is crucial.
Moreover, the co-resistance to antibiotics and biocides might contribute to the epidemic prevalence of resistant strains within healthcare settings [13]. Reduced susceptibility to these antimicrobials has been reported in various Gram-negative bacteria [14,15,16].
One of the biocides resistance mechanisms is the expression of efflux systems involving qac genes (qacE and qacEΔ1). A. baumannii resembling other Gram-negative bacteria harbors multidrug transporter efflux systems. Qac genes are widely propagated in Gram-negative bacteria [17,18] basically due to high spread of plasmid-mediated class 1 integrons, which commonly include qacEΔ1 [17]. Qac genes have been frequently noticed in combination with genes coding for resistance to β-lactams (including carbapenemases), aminoglycosides, trimethoprim, sulphonamides, and chloramphenicol [19,20,21,22,23]. The ability of qac genes to offer resistance to antibiotics remains unspecified. Nevertheless, a close association between resistance to biocides and antibiotics can be explained by the fact that the class 1 integrons (mobile genetic elements) hosts a variety of antibiotic resistance genes [23,24]. Owing to these facts, there is a rational concern that the inadequate use of biocides could select for antibiotic-resistant bacteria in Gram-negative bacteria [25,26].
Although many reports focus on the increasing resistance of A. baumannii strains to antibiotics, few studies have investigated the susceptibility of A. baumannii to biocides [27,28,29]; also, there is little information available that weighs the risks of antibiotic resistance induced by increased resistance to biocides in Egypt. Undoubtedly, understanding A. baumannii susceptibility to disinfectants and its correlation with antibiotic resistance will contribute to the control of this microbe in hospitals.
We aimed to investigate the susceptibility of MBL A. baumannii producer, as one of the troublesome resistant clinical isolates, to biocides. In addition, we evaluate the correlation between carriage rates of qac genes and the prevalence of MBL encoding genes and class 1 integrons.
2. Materials and Methods
2.1. Bacterial Isolates
A total of 56 non-consecutive A. baumannii clinical isolates were included in the present study. The clinical isolates were recovered from different specimens (including blood, pus/wound swabs, throat swabs, chest tube, nasal swabs, sputum and urine) submitted to the Clinical Pathology Department, National Cancer Institute, Cairo, Egypt, for routine culture. The specimens were collected from immunocompromised patients over a period of 9 months from June 2014 to March 2015. Only cases confirmed to be hospital acquired infections were included. Phenotypic identification of isolates was performed using VITEK automated system and the isolates were identified genotypically through detection of the blaOXA-51-like gene by PCR.
2.2. Antimicrobial Susceptibility Testing
Antibiotic sensitivity to 17 antibiotics belonging to five different classes was tested by modified Kirby-Bauer disc diffusion method. The results were interpreted according to Clinical and Laboratory Standards Institute guidelines [30]. An intermediate susceptibility was considered as resistant. The antibiotic discs (Oxoid, UK) used in this study were amoxicillin/clavulanic acid (AMC)—20/10 mcg, amikacin (AK)—30 mcg, cefazoline (KZ)—30 mcg, ceftazidime (CAZ)—30 mcg, cefotaxime (CTX)—30 mcg, cefotetan (CTT)—30 mcg, gatifloxacin (GAT)—5 mcg, tobramycin (TOB)—10 mcg, cefuroxime (CXM)—30 mcg, levofloxacin (LEV)—5 mcg, ceftriaxone (CRO)—30 mcg, meropenem (MEM)—10 mcg, nitrofurantoin (F)—30 mcg, imipenem (IMP)—10 mcg, cefepime (FEP)—30 mcg and trimethoprim/sulfamethoxazole (SXT)—1.25/23.75 mcg, ticarcillin/Clavulinic (TIM)—75/10 mcg, colistin (CL)—10 mcg and tigecycline (TGC)—15 mcg. Escherichia coli ATCC 25922 was used as the reference strain.
2.3. Phenotypic Detection of MBL
E-test MBL strips (AB Biodisk, Solna, Sweden) were used in accordance with the manufacturer’s instructions to investigate MBL production. MIC ratio of imipenem to imipenem/EDTA of 8 or the presence of a phantom zone was taken as a positive result.
2.4. Determination of Disinfectants/Antiseptics Susceptibility by the Broth Macrodilution Method
The biocides selected for testing were the chemical disinfectants/antiseptics recommended for patient-care items and instruments (most widely used biocides). Chlorhexidine (0.3%, CLX), cetrimide (3%), and aqueous povidone-iodine (10% w/v, PVP-I2) solution were included. The MICs of the disinfectants/antiseptics were determined by the broth macrodilution method according to [31]. Each disinfectant solution was diluted by a serial two-fold dilution method using Muller Hinton broth (MHB) (Table 1). The positive control tube contained 0.1 mL inoculum in 1 mL MHB and should show evidence of bacterial growth (turbidity). The negative control tube contained MHB and the disinfectant to be tested and should not show bacterial growth (clear).
Table 1.
The chemical agents, their starting concentrations and dilutions used for minimum inhibitory concentration (MIC) test.
| Chemical Agents | Recommended Dilution * | Starting Concentration | Dilutions |
|---|---|---|---|
| Chlorhexidine digluconate (CLX) | 0.0075% | 0.3% | 0.15%, 0.075%, 0.0375%, 0.0187%, 0.00937%, 0.00468%, 0.00234%, 0.00172% |
| Cetrimide | 0.075% | 3% | 1.5%, 0.75%, 0.375%, 0.187%, 0.094%, 0.046%, 0.023%. |
| Povidone-iodine (PVP-I2) | 5% | 10% | 5%, 2.5%, 1.25%, 0.625%, 0.3125%, 0.156%, 0.078% and 0.039% |
* According to manufacturer instructions.
2.5. PCR and DNA Sequencing
Genomic DNA was extracted from A. baumannii isolates using Qiaamp Mini DNA kit (Qiagen, Hilden, Germany). bla OXA-51-like gene, Intl1, blaVIM, blaNDM-1, blaIMP, blaGIM, blaSIM, blaSPM, qacE, qacΔE1 and cepA were amplified for A. baumannii isolates using the primers listed in Table 2. The primers were prepared by Invitrogen (Grand Island, NY, USA). GoTaq Green Master Mix (Promega, Madison, WI, USA) was used in all PCR assays. The PCR products were separated through a 1.5% agarose gel by electrophoresis. Amplified products were cut and purified using Qiaquick gel purification kit (Qiagen, Hilden, Germany) and sequenced at DNA Biotechnology Facility at University of Connecticut, USA (using the Genetic analyzer 3030X1 (Applied Biosystems, CA, USA) with a big Dye terminator cycle sequencing kit). Sequences in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to identify and compare the genes detected in this study. The blaVIM, blaNDM-1, qacE and qacΔE1 specified DNA amplicon sequences and similar sequences recovered from NCBI GenBank database were used to create the phylogenetic trees in order to understand the genetic relatedness of the study sequences. Alignment of these genes nucleotide sequence was performed using the DNASTAR program (DNASTAR Inc., Madison, WI, USA).
Table 2.
List of primers used in the study.
| Gene Name | Sequence 5′-3′ | PCR Products | Reference | Annealing Temperature |
|---|---|---|---|---|
| qacE | Forward-CCCGAATTCATGAAAGGCTGGCTT Reverse-TAAGCTTTCACCATGGCGTCGG |
350 bp | [14] | 55 °C |
| qacΔE1 | Forward-TAGCGAGGGCTTTACTAAGC Reverse-ATTCAGAATGCCGAACACCG |
300 bp | [32] | 55 °C |
| cepA | Forward-CAACTCCTTCGCCTATCCCG Reverse-TCAGGTCAGACCAAACGGCG |
1058 bp | [33] | 62 °C |
| blaOxa51-like | Forward-TAATGCTTTGATCGGCCTTG Reverse-TGGATTGCACTTCATCTTGG |
353 bp | [34] | 60 °C |
| Intl1 | Forward-GCATCCTCGGTTTTCTGG Reverse-GGTGTGGCGGGCTTCGTG |
457 bp | [35] | 60 °C |
| blaIMP | Forward-CTACCGCAGCAGAGTCT TTG Reverse-AACCAGTTTTGCCTTACCAT |
587 bp | [36] | 55 °C |
| blaNDM-1 | Forward-GGCGGAATGGCTCATCACGA Reverse-CGCAAC ACAGCCTGACTTTC |
287 bp | [6] | 58 °C |
| balVIM | Forward-GATGGTGTTTGGTCGCATA Reverse-CGAATGCGCAGCACCAG |
390 bp | [37] | 52 °C |
| blaSPM | Forward-AAAATCTGGGTACGCAAACG Reverse-ACATTATCCGCTGGAACAGG |
271 bp | ||
| blaGIM | Forward-TCGACACACCTTGGTCTGAA Reverse-AACTTCCAACTTTGCCATGC |
477 bp | ||
| blaSIM | Forward-TACAAGGGATTCGGCATCG Reverse-TAATGGCCTGTTCCCATGTG |
570 bp |
2.6. Statistical Analysis
Statistical analysis was done using SPSS version 20 (IBM®, New York, NY, USA). Chi-square test was performed. A p-value of <0.05 was considered indicative of a statistically significant difference.
3. Results
3.1. Characteristics of Isolates and Their Antibiotics Susceptibility
Out of 56 A. baumannii isolates, 22 were recovered from blood (39.3%), 18 from pus/wound swabs (32.1%), nine from sputum (25%), three from a chest tube (5.4%), two from throat swabs (3.6%), one from a urine specimen (1.8%) and one from a nasal swab (1.8%). Table 3 shows the antimicrobial suscebtipility patterns of 56 A. baumannii isolates. A. baumannii resistance rate to carbapenem (imipenem and meropenem) was 71.4% (40/56) and 39.3% (22/56) were MBL producers. Forty-nine isolates were MDR (87.5%) and seven were extensively drug resistant (XDR) (12.5%). “MDR Acinetobacter spp.” is defined as isolate non-susceptible to at least one agent in three or more antimicrobial categories. “XDR Acinetobacter spp.” is defined as an Acinetobacter spp. isolate that is resistant to all standard antimicrobial agents except colistin or tigecycline [38]. Colistin and tigecycline were totally effective against all tested isolates. All MBL-producing isolates were MDR and 31.8% of them were XDR. Antibiotic susceptibility results for MBL-producing A. baumannii are shown in Table 4.
Table 3.
Antimicrobial susceptibility patterns of Acinetobacter baumannii enrolled in the study.
| Antimicrobial Agent | A. baumannii (56 Isolates) | ||
|---|---|---|---|
| S No (%) | I No (%) | R No (%) | |
| Amikacin | 9 (16.1) | 3 (5.3) | 44 (78.6) |
| Amoxicillin/clavulanic acid | 0 (0.0) | 0 (0.0) | 56 (100) |
| Cefazolin | 0 (0.0) | 0 (0.0) | 56 (100) |
| Cefepime | 4 (7.1) | 0 (0.0) | 52 (92.9) |
| Cefotaxime | 1 (1.8) | 5 (8.9) | 50 (89.3) |
| Cefotetan | 1 (1.8) | 2 (2.6) | 53 (94.6) |
| Ceftazidime | 4 (7.1) | 1 (1.8) | 51 (91.1) |
| Ceftriaxone | 5 (8.9) | 2 (2.6) | 49 (87.5) |
| Cefuroxime | 1 (1.8) | 1 (1.8) | 54 (96.4) |
| Imipenem | 16 (28.6) | 3 (5.3) | 37 (66.1) |
| Levofloxacin | 13 (23.2) | 7 (12.5) | 36 (64.3) |
| Meropenem | 16 (28.6) | 1 (1.8) | 39 (69.6) |
| Gifloatxacin | 8 (14.3) | 5 (8.9) | 33 (58.9) |
| Nitrofurantoin | 0 (0.0) | 0 (0.0) | 56 (100) |
| Ticarcillin/clavulanic acid | 6 (10.7) | 1 (1.8) | 49 (87.5) |
| Tobramycin | 16 (28.6) | 6 (10.7) | 34 (60.7) |
| Trimethoprim/sulfamethoxazole | 4 (7.1) | 0 (0.0) | 52 (92.9) |
| Tigicyclin | 56 (100) | 0 (0.0) | 0 (0.0) |
| Colistin | 56 (100) | 0 (0.0) | 0 (0.0) |
Table 4.
Antibiotic susceptibility, MICs of biocides and PCR results of metallo-β-lactamase (MBL)-positive A. baumannii isolates.
| Isolate Number | Type of Specimen | Biocide Resistance Genes | MIC of Biocide μg/mL | MBL Genes | Intl1 Gene | Antimicrobial Susceptibility | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qacE | qacEΔ1 | cepA | PVP-I2 | CLX | Cetrimide | blaNDM | blaVIM | Ak | AMC | KZ | FEP | CTX | CTT | CAZ | CRO | CXM | GAT | IMP | MEM | LEV | F | TIM | TOB | SXT | TGC | CL | Category | MBL | |||
| 1 | Throat Swab | + | + | - | 0.625 | 0.075 | 0.187 | - | + | + | R | R | R | R | R | R | R | R | R | I | R | R | I | R | R | R | R | S | S | MDR | + |
| 2 | Sputum | + | + | - | 0.625 | 0.0375 | 0.75 | - | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 3 | Throat Swab | + | + | - | 1.25 | 0.009 | 0.049 | + | + | + | R | R | R | R | R | R | R | R | R | R | I | R | R | R | R | R | R | S | S | MDR | + |
| 4 | Blood | + | + | - | 1.25 | 0.0046 | 0.75 | + | + | + | I | R | R | R | R | R | R | R | R | R | R | R | S | R | R | R | R | S | S | MDR | + |
| 5 | Sputum | - | - | - | 1.25 | 0.009 | 0.098 | - | + | + | R | R | R | R | R | R | R | R | R | I | R | R | I | R | R | S | R | S | S | MDR | + |
| 6 | Pus | + | + | - | 0.625 | 0.0375 | 0.098 | + | + | + | S | R | R | R | R | R | R | R | R | R | R | R | I | R | R | S | S | S | S | MDR | + |
| 7 | Blood | + | + | - | 0.625 | 0.009 | 0.75 | - | + | + | R | R | R | R | R | R | R | R | R | I | I | R | I | R | R | I | R | S | S | MDR | + |
| 8 | Sputum | + | + | - | 1.25 | 0.0046 | 0.75 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 9 | Blood | + | + | - | 1.25 | 0.009 | 0.098 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 10 | Pus | - | + | - | 0.625 | 0.075 | 0.187 | + | + | + | S | R | R | R | R | R | R | R | R | S | R | R | R | R | R | R | R | S | S | MDR | + |
| 11 | Pus | - | + | - | 1.25 | 0.009 | 0.75 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 12 | Blood | - | + | - | 1.25 | 0.009 | 0.187 | + | + | + | R | S | R | R | R | R | R | R | R | S | R | R | S | R | R | R | R | S | S | MDR | + |
| 13 | Sputum | - | + | - | 1.25 | 0.0046 | 0.098 | + | + | + | R | R | R | R | R | R | R | R | R | R | I | R | R | R | R | R | R | S | S | MDR | + |
| 14 | Pus | + | + | - | 1.25 | 0.009 | 0.187 | + | + | + | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | MDR | + |
| 15 | Pus | - | - | - | 1.25 | 0.0023 | 0.049 | - | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | R | S | S | MDR | + |
| 16 | Blood | - | - | - | 0.039 | 0.0023 | 0.187 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 17 | Pus | - | - | - | 0.625 | 0.0046 | 0.049 | + | + | + | S | R | R | R | R | R | R | R | R | S | R | R | I | R | R | R | R | S | S | MDR | + |
| 18 | Sputum | - | + | - | 1.25 | 0.0046 | 0.187 | - | + | + | R | R | R | R | R | R | R | R | R | R | R | R | I | R | R | R | R | S | S | MDR | + |
| 19 | Chest Tube | - | - | - | 0.078 | 0.0046 | 0.187 | - | - | + | R | S | R | R | R | I | R | R | R | S | R | R | R | R | R | R | R | S | S | MDR | + |
| 20 | Chest Tube | + | + | - | 0.039 | 0.075 | 0.75 | + | + | + | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | XDR | + |
| 21 | Pus | - | - | - | 0.078 | 0.0023 | 0.049 | - | - | + | R | R | R | R | R | I | R | R | R | R | R | R | I | R | R | R | R | S | S | MDR | + |
| 22 | Nasal Swab | - | - | - | 0.625 | 0.0023 | 0.049 | - | - | + | S | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | S | S | MDR | + |
PVP-I2: Povidone-iodine; CLX: Chlorhexidine; AK: Amikacin; AMC: Amoxicillin/clavulanic acid; KZ: Cefazoline; FEP: Cefepime; CTX: Cefotaxime; CTT: Cefotetan: CAZ: Ceftazidime; CRO: Ceftriaxone; CXM: Cefuroxime; GAT: Gatifloxacin; IMP: Imipenem: MEM: Meropenem: LEV: Levofloxacin; F: Nitrofurantoin; TIM: Ticarcillin/Clavulinic; TOB: Tobramycin; SXT: Trimethoprim/sulfamethoxazole; TGC: Tigecycline; CL: Colistin; R: Resistant, S: Sensitive, I: Intermediate MDR: Multi drug resistant, XDR: Extensive drug resistant.
3.2. Detection of MBL-Encoding Genes
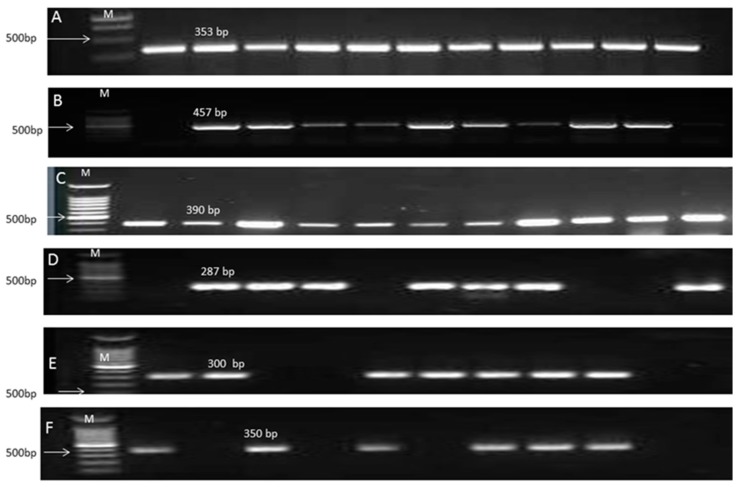
The amplified products of blaOXA-51-like gene, Intl1 gene and MBL-positive genes for the A. baumannii isolate were shown in Figure 1. The intrinsic β-lactamase gene, blaOXA-51-like, was amplified from all A. baumannii isolates. Of the positive MBL isolates, MBL-encoding gene blaVIM was identified in 86.4% (19/22) of MBL-producing isolates and blaNDM-1 was identified in 13 (59.1%) isolates. Coexistence of blaVIM and blaNDM-1 was encountered in 13 isolates (all blaNDM-1-positive isolates). In this study, MBL blaIMP, blaGIM, blaSIM, and blaSPM were not detected. All the MBL-producing isolates were positive for class 1 integron.
Figure 1.
Gel electrophoresis of the PCR amplified products of blaOxa51-like gene, intl1, MBL genes and Qac genes of A. baumannii isolates. (A) PCR amplified products of blaOxa51-like gene with 353 bp amplification fragment. M: 1 Kbp DNA ladder; (B) PCR amplified products of Intl1 gene with 475 bp amplification fragment. M: 100 bp DNA ladder; (C) PCR amplified products of balVIM gene with 390 bp amplification fragment. M: 100 bp DNA ladder; (D) PCR amplified products of blaNDM-1 gene with 287 bp amplification fragment. M: 100 bp DNA ladder; (E) PCR amplified products of qacE gene with 350 bp amplification fragment. M: 100 bp DNA ladder; (F) PCR amplified products of qacEΔ1 gene with 300 bp amplification fragment. M: 100 bp DNA ladder.
3.3. Correlation of Efflux Pump Genes with MIC of Tested Biocides
In the present study, MBL A. baumannii isolates were effectively inhibited by the user’s defined concentrations of PVP-I2, whereas the MICs of 54.5% and 77.3% of the tested isolates for CLX and cetrimide, respectively, were higher than the actual concentrations recommended for disinfection. The amplified products of qac genes were shown in Figure 1E,F.
Among MBL-positive isolates, qacE and qacΔE1 efflux genes were present in 10 (45.5%) and 15 (68%) isolates respectively, whereas cepA gene was not detected. In general, for qac-positive isolates, the MICs ranged from 47 to 750 μg/mL and 470 to 7500 μg/mL for CLX and cetrimide, respectively. For qac-negative isolates, MIC ranged from 23.4 to 94μg/mL and 470 to 1875 μg/mL for CLX and cetrimide, respectively. The MICs of PVP-I2 ranged from 390 to 12,500 μg/mL with both qac-positive and negative isolates (Table 4). There was a significant correlation between qac genes carriage and increased MICs of CLX and cetrimid (p = 0.0096 and 0.0085, respectively) as showed in Table 5.
Table 5.
Correlation between biocides inhibitory concentration and the presence of qac genes.
| Chemical Agent | MIC μg/mL (%) | Number of Isolates (n = 22) | qac Genes | Chi-Square Tests | p-Value | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Povidone-iodine | >50,000 (5%) | 0 | 0 | 0 | - | - |
| <50,000 (5%) | 22 | 15 | 7 | |||
| Chlorhexidine | >75 (0.0075%) | 12 | 11 | 1 | 6.712 | 0.0096 * |
| <75 (0.0075%) | 10 | 4 | 6 | |||
| Cetrimide | >750 (0.075%) | 17 | 14 | 3 | 6.924 | 0.0085 * |
| <50 (0.075%) | 5 | 1 | 4 | |||
* The result is significant when p < 0.05.
3.4. Correlation of blaVIM and blaNDM-1 Genes with qac Genes
As indicated in Table 6, coexistence of blaVIM and qac genes was detected in 15 isolates, while blaNDM-1 was concomitant in 11 isolated with qac genes, and coexistence of the qacE and qacEΔ1 genes was encountered in 10 of the MBL-positive isolates. There is significant correlation between qac and the carriage of both blaVIM and blaNDM-1 genes (p = 0.0064 and 0.0467, respectively).
Table 6.
Correlation between qac genes and blaVIM and blaNDM-1 among MBL-positive A. baumannii isolates.
| MBL Genes | blaVIM | blaNDM-1 | |||
|---|---|---|---|---|---|
| qac Genes | Positive | Negative | Positive | Negative | |
| Positive | 15 | 0 | 11 | 4 | |
| Negative | 4 | 3 | 2 | 5 | |
| p-value | 0.0064 * | 0.0467 * | |||
* The result is significant when p < 0.05.
3.5. Nucleotides Sequence and Phylogenetic Analysis
Sequencing of intrinsic blaVIM and blaNDM-1 confirmed that the nucleotide sequences obtained were identical to genes for VIM and NDM for A. baumannii. In the same way, the nucleotide sequences of class І integrin, qacE and qacEΔ1 also agreed with the results expected by the PCR analysis.
The nucleotide sequences of the blaVIM gene-positive isolates were highly similar, with sequence identities of 90.8–100%. The genetic divergence and homogeneity of the blaVIM sequences are apparent in the phylogenetic tree Figure 2. Among the 19 blaVIM gene sequences, there were nine different clusters. Those from isolates number 14, 18 and 20 were found to form distinct clusters, while six clusters formed from the rest of isolates’ shared similarity.
Figure 2.
Phylogenetic analysis based on blaVIM gene sequences obtained from the nineteen A. baumannii isolates in this study and eight sequences retrieved from GenBank database. Phylogenetic tree was created with DNASTAR MegAlign by ClustalW (weighted) method. ACV; blaVIM gene isolated in this study and the other sequences were taken from Genbank.
blaVIM sequences were found to have genetic relationship with sequences from other countries; one from Korea (GenBank accession number: AF291420.1), two from Greece (GenBank accession numbers: EF690695.1 and EF690596.1), two from India (GenBank accession numbers: JF702919.1 and JF702920.1) and three from Iran (GenBank accession numbers: KU6855081.1, LC107606.1 and LC107421.1).
The nucleotide sequences of the blaNDM-1 gene in 13 out of 22 A. baumannii isolates were highly similar, with sequence identities of 88–100%. blaNDM-1 sequences of isolates number 3, 13 and 20 were found to form distinct clusters whilst the other isolates shared similarity in four diverse clusters. These sequences were closely related to blaNDM-1 sequences from other countries: one from China (GenBank accession number: KP772138), two from Iran (GenBank accession numbers: LC154934.1 and LC154949.1) and two from India (GenBank accession numbers: KU510390 and KU510393). In addition, two sequences reported from Iraq (GenBank accession numbers: KU378644.1 and KU378645.1) were also clearly apparent in the tree (Figure 3).
Figure 3.
Phylogenetic analysis based on blaNDM-1 gene sequences obtained from the thirteen A. baumannii isolates in this study and seven sequences retrieved from GenBank database. Phylogenetic tree was created with DNASTAR MegAlign by ClustalW (weighted) method. NDM; blaNDM gene isolated in this study and the other sequences were taken from Genbank.
The nucleotide sequences of the qacEΔ1 gene (15/22) and qacE gene (10/22) among A. baumannii isolates were highly similar, with sequence identities of 88.9–100% and 88.2–100%, respectively. Phylogenetic analysis based on qacE and qacEΔ1 genes (Figure 4 and Figure 5) showed three and five different clusters respectively. Only isolate number 9 formed distinct cluster of both genes. The nucleotide sequences of qacEΔ1 and qacE shared genetic similarity with sequences from other countries: three from China (GenBank accession numbers: ku133343.1, ku133342.1, ku133337.1) along with one sequence reported from Egypt (GenBank accession number: JX128136.1).
Figure 4.
Phylogenetic analysis based on qacΔE1 gene sequences obtained from the fifteen A. baumannii isolates in this study and four sequences retrieved from GenBank database. Phylogenetic tree was created with DNASTAR MegAlign by ClustalW (weighted) method. DE; qacEΔ1 gene isolated in this study and the other sequences were taken from Genbank.
Figure 5.
Phylogenetic analysis based on qacE gene sequences obtained from the ten A. baumannii isolates in this study and four sequences retrieved from GenBank database. Phylogenetic tree was created with DNASTAR MegAlign by ClustalW (weighted) method. E; qacE gene isolated in this study and the other sequences were taken from Genbank.
4. Discussion
The worldwide increased occurrence of carbapenem-resistant A. baumannii infections in healthcare settings has led to a greater alertness of the threat of hospital acquired infections. In the current study, there was high frequency of carbapenem-resistant A. baumannii strains that may be attributed to the extensive misuse of carbapenems. Resistance to carbapenem in clinical isolates of A. baumannii has been reported in Egypt [39,40,41,42,43]. At this time, there are limited selections of treatment options for carbapenem-resistant A. baumannii infection, according to our results; colistin and tigecycline are considered the last choice to control (100% sensitivity). This finding has been also reported in different studies [4,42,43,44,45].
The high frequency of MBL A. baumannii detected in the study is not common, since two previous separate studies have shown 0% and 2.5% MBL activity among A. baumannii isolated in Egypt [42,43]. Resistance due to MBL production has a potential for rapid dissemination, since it is often plasmid-mediated [40]. The current study showed that all MBL-producing isolates were MDR that exhibited high resistance to beta-lactams, aminoglycosides, and quinolones.
Because of its ability to spread, carbapenem resistance related to VIM and NDM β-lactamase production has become a serious concern. MBL VIM has been reported intermittently in Egypt [40,42]. The nucleotide sequences of the blaVIM gene from the positive isolates were highly similar, with sequence identities of 90.8–100%. blaVIM originated from Greece and South Korea [46]. blaVIM nucleotide sequences showed high similarity with these two countries and other countries like India and Iran. Horizontal transfer of VIM-encoding genes can be inferred from the presence of the same integron in genetically non-related isolates in different species and genera [47]. The most recent MBL identified in A. baumannii are the emerging NDM enzymes. The blaNDM-1 genes have been reported in A. baumannii from India [48] and Germany [49]. Kaase et al. [50] reported the first NDM-producing A. baumannii in Egypt from a patient who transferred from Egypt to Germany without obvious link with the Indian subcontinent. After recent identification of NDM-producing isolates in Iraq [51] and the Sultanate of Oman, the clinical case suggests that NDM-producing bacteria disseminated in the Middle East countries [3]. These isolates were resistant to high levels of all β-lactams, including carbapenems. In our study, 68% of the isolates carried blaNDM-1 gene. The nucleotide sequences of blaNDM-1 gene showed high similarity between isolates (with sequence identities of 88–100%) and with the sequence of blaNDM-1 gene identified from other countries (mainly including India, Iran and Iraq). blaVIM and blaNDM-1 genes are horizontally transferable as they are inserted in integrons, and some of these integrons are located on conjugative plasmids [46]. The large number of trips between the countries combined with the ease of resistance transmission among bacteria led us to consider that our isolates may share genetic similarity of blaVIM and blaNDM-1 with other countries.
The increase in reduced susceptibility to antibiotics is paralleled by a similar trend in reduced susceptibility to biocides [15,52]. Antiseptic resistance has been reported for several agents including quaternary ammonium compounds and biguanides [53]. Interestingly, more than half of the tested isolates showed higher MICs of CLX and cetrimide than the actual concentrations recommended for disinfection. This may be attributed to the extensive use of these types of disinfectants in the routine infection control in Egypt. Moreover, broader and more indiscriminate use of biocides in healthcare facilities could drive the emergence of new genetic elements, with unpredictable consequences for human welfare.
Resistance to biocides is attributable to production of efflux proteins encoded by plasmid-mediated genes such as qac genes [54]. The MICs of CLX and cetrimid significantly correlated to qac genes carriage.
We found that the majority of MBL-positive A. baumannii isolates harbor at least one of the qac genes tested and qacEΔ1 was the most prevalent, with high nucleotide sequence identities between isolates. High levels of qacE∆1 carriage have been reported in clinical isolates of Acinetobacter, and it has been suggested that the increase in both antibiotic and antiseptic resistance in this organism is related to the presence of this gene [55]. Resistance to several antibiotics has frequently been reported in different clinical isolates in association with qac genes [56,57]. The acquisition of efflux pumps and co-selection of antibiotic resistance genes through their linkage with biocide resistance determinants on the same mobile element are the mechanisms by which biocide resistance increases the distribution of antibiotic-resistant bacteria [58,59,60,61].
Increased frequency of biocide resistance genes in A. baumannii from clinical specimens points to the possibility that the hospital atmosphere could apply selective pressure for presence of these strains. Biocide resistance may allow persistence of organisms in the presence of small levels of biocide and contribute to the survival of MDR strains [62]. Different studies have shown that disinfectant-resistance gene expression can be induced by exposure to subinhibitory concentrations of biocides [53]. Reduction in effectiveness can allow more strains harboring qac genes to survive and spread these genes among A. baumannii, which may explain the higher incidence of these resistance genes.
The genetic similarity between isolates was apparent in the nucleotide sequences of blaVIM, blaNDM-1, qacE and qacEΔ1 and suggests a common genetic vehicle related dissemination of the β-lactamase genes and efflux pump genes. Furthermore, these sequences also showed similarities with those previously reported from different geographical areas. The class 1 integrons are the most common mechanism by which bacteria are able to move resistance gene cassettes from one bacterium to another. The importance of horizontal transfer of resistance genes through mobile genetic elements and its relationship with increased incidence of multidrug-resistant A. baumannii in hospitals is a critical issue. We evaluated intl1 gene which comprises the genetic platforms of class 1 integrons (genetic mobile elements). We found that all MBL-producing A. baumannii isolates harbor the intl1 gene. Since efflux genes are located on mobile genetic elements along with specific antibiotic resistance genes [63], cross-resistance and co-resistance may be acquired together [64]. The MBL genes are mostly harbored by class 1 integrons and the integrons may be targeted as epidemiological molecular markers for identifying and surveying MBL-producing Gram-negative bacilli [2].
In conclusion, a prevalence of MBL-producing A. baumannii isolates was observed in this study. The blaVIM-specific amplicons in these isolates were found to be genetically similar to each other. As well, the genetic similarity between isolates was obvious in the nucleotide sequences of blaNDM-1 genes. Furthermore, these sequences also showed similarities with those previously reported from other countries. Thus, this study argues for the urgent implementation of strict control measures to prevent the spread of resistance genes and that it warrants the need for constant surveillance. It is crucial to highlight the need for health care facilities to assess the antimicrobial effectiveness of biocides periodically to overcome dissemination of MBL-producing A. baumannii with reduced sensitivity to biocides. Reduced susceptibility to CHX and cetrimide and the potential for cross resistance to some antibiotics highlights the need to restrict the use of these biocides. Strains harboring qac genes may be more likely to survive the disinfection process and serve as a source of nosocomial outbreaks. The use of biocides may have driven the fixation and spread of the class 1 integrons, and now may contribute to antibiotic resistance.
Acknowledgments
Authors would like to thank Safaa Hassan of Department of Clinical Pathology, National Cancer Institute, Cairo University, Cairo, Egypt, for kindly providing clinical isolates.
Author Contributions
Fatma Alzahraa M. Gomaa and Zeinab H. Helal designed and performed the experiments; Mazhar I. Khan contributed reagents/materials/analysis tools; Fatma Alzahraa M. Gomaa, Zeinab H. Helal and Mazhar I. Khan analyzed the data and wrote the paper.
Conflicts of Interest
There is no conflict of interest. This work received no specific grant from any funding agency.
References
- 1.Chatterjee S., Datta S., Roy S., Ramanan L., Saha A., Viswanathan R., Som T., Basu S. Carbapenem Resistance in Acinetobacter baumannii and Other Acinetobacter spp. Causing Neonatal Sepsis: Focus on NDM-1 and Its Linkage to ISAba125. Front. Microbiol. 2016;7:1126. doi: 10.3389/fmicb.2016.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao W., Hu Z. Integrons: Epidemiological molecular markers for identifying and surveying metallo-β‑lactamase genes in Gram-negative bacilli. Future Microbiol. 2014;9:5–8. doi: 10.2217/fmb.13.21. [DOI] [PubMed] [Google Scholar]
- 3.Zahedi-bialvaei A., Samadi-kafil H., Ebrahimzadeh-leylabadlo H., aghazadeh M., Asgharzadeh M. Dissemination of carbapenemases producing Gram negative bacteria in the Middle East. Iran. J. Microbiol. 2015;7:226–246. [PMC free article] [PubMed] [Google Scholar]
- 4.Moghadam M.N., Motamedifar M., Sarvari J., Sedigh E.S.H., Mousavi S.M., Moghadam F.N. Emergence of multidrug resistance and metallo-beta-lactamase producing Acinetobacter baumannii isolated from patients in Shiraz, Iran. Ann. Med. Health Sci. Res. 2016;6:162–167. doi: 10.4103/2141-9248.183946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh T.R., Toleman M.A., Poirel L., Nordmann P. Metallo-lactamases: The quiet before the storm? Clin. Microbiol. Rev. 2005;18:306–325. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Zhou Z., Jiang Y., Yu Y. Emergence of NDM-1-producing Acinetobacter baumannii in China. J. Antimicrob. Chemother. 2011;66:1255–1259. doi: 10.1093/jac/dkr082. [DOI] [PubMed] [Google Scholar]
- 7.Lauretti L., Riccio M.L., Mazzariol A., Cornaglia G., Amicosante G., Fontana R., Rossolini G.M. Cloning and characterization of blaVIM, a new integron-borne metallo-β-lactamase gene from a Pseudomonas aeruginosa clinical isolate. Antimicrob. Agents Chemother. 1999;43:1584–1590. doi: 10.1128/aac.43.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsakris A., Pournaras S., Woodford N., Palepou M.F., Babini G.S., Douboyas J., Livermore D.M. Outbreak of infections caused by Pseudomonas aeruginosa producing VIM-1 carbapenemase in Greece. J. Clin. Microbiol. 2000;38:1290–1292. doi: 10.1128/jcm.38.3.1290-1292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong D., Toleman M.A., Giske C.G., Cho H.S., Sundman K., Lee K., Walsh T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B.Y., Mcglone S.M., Doi Y., Bailey R.R., Harrison L.H. Economic value of Acinetobacter baumannii screening in the intensive care unit. Clin. Microbiol. Infect. 2011;17:1691–1697. doi: 10.1111/j.1469-0691.2011.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muir A., Weinbren M.J. New Delhi metallo-beta-lactamase: A cautionary tale. J. Hosp. Infect. 2010;75:239–240. doi: 10.1016/j.jhin.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Mochon A.B., Garner O.B., Hindler J.A., Krogstad P., Ward K.W., Lewinski M.A., Rashee J.K., Anderson K.F., Limbago B.M., Humphries R.M. New Delhi metallo-beta-lactamase (NDM-1)-producing Klebsiella pneumoniae: Case report and laboratory detection strategies. J. Clin. Microbiol. 2011;49:1667–1670. doi: 10.1128/JCM.00183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koljalg S., Naaber P., Mikelsaar M. Antibiotic resistance as an indicator of bacterial chlorhexidine susceptibility. J. Hosp. Infect. 2002;51:106–113. doi: 10.1053/jhin.2002.1204. [DOI] [PubMed] [Google Scholar]
- 14.Kucken D., Heinz-Hubert F., Kaukfers P.M. Association of qacE and qacEΔ1 with multiple resistance to antibiotics and antiseptics in clinical isolates of Gram-negative bacteria. FEMS Microbiol. Lett. 2000;183:95–98. doi: 10.1016/S0378-1097(99)00636-9. [DOI] [PubMed] [Google Scholar]
- 15.Higgins C.S., Murtough S.M., Williamson E., Hiom S.J., Payne D.J., Russell A.D., Walsh T.R. Resistance to antibiotics and biocides among non-fermenting Gram-negative bacteria. Clin. Microbiol. Infect. 2001;7:308–315. doi: 10.1046/j.1198-743x.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- 16.Ogbulie J.N., Adieze I.E., Nwankwo N.C. Susceptibility pattern of some clinical bacterial isolates to selected antibiotics and disinfectants. Polish J. Microbiol. 2008;57:199–204. [PubMed] [Google Scholar]
- 17.Kazama H., Hamashima H., Sasatsu M., Arai T. Distribution of the antiseptic-resistance genes qacE and qacEΔ1 in Gram-negative bacteria. FEMS Microbiol. Lett. 1998;159:173–178. doi: 10.1016/S0378-1097(97)00563-6. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y.C., Shih D.Y.C., Wang J.Y., Yang S.S. Molecular characterization of class 1 integrons and antimicrobial resistance in Aeromonas strains from foodborne outbreak-suspect samples and environmental sources in Taiwan. Diagnos. Microbiol. Infect. Dis. 2007;59:191–197. doi: 10.1016/j.diagmicrobio.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Riano I., Moreno M.A., Teshager T., Saenz Y., Dominguez L., Torres C. Detection and characterization of extended-spectrum beta-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 2006;58:844–847. doi: 10.1093/jac/dkl337. [DOI] [PubMed] [Google Scholar]
- 20.Poirel L., Pitout J.D., Nordmann P. Carbapenemases: Molecular diversity and clinical consequences. Future Microbiol. 2007;2:501–512. doi: 10.2217/17460913.2.5.501. [DOI] [PubMed] [Google Scholar]
- 21.Walsh T.R. Clinically significant carbapenemases: An update. Curr. Opin. Infect. Dis. 2008;21:367–371. doi: 10.1097/QCO.0b013e328303670b. [DOI] [PubMed] [Google Scholar]
- 22.Colinon C., Jocktane D., Brothier E., Rossolini G.M., Cournoyer B., Nazaret S. Genetic analyses of Pseudomonas aeruginosa isolated from healthy captive snakes: Evidence of high inter- and intrasite dissemination and occurrence of antibiotic resistance genes. Environ. Microbiol. 2010;12:716–729. doi: 10.1111/j.1462-2920.2009.02115.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao W.H., Chen G.L., Ito R., Kimura S., Hu Z.Q. Identification of a plasmid-borne bla(IMP-11) gene in clinical isolates of Escherichia coli and Klebsiella pneumoniae. J. Med. Microbiol. 2012;61:246–251. doi: 10.1099/jmm.0.035626-0. [DOI] [PubMed] [Google Scholar]
- 24.Weigel L.M., Clewell D.B., Gill S.R., Clark N.C., McDougal L.K., Flannagan S.E., Kolonay J.F., Shetty J., Killgore G.E., Tenover F.C. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 25.Hegstad K., Langsrud S., Lunestad B.T., Scheie A.A., Sunde M., Yazdankhah S.P. Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb. Drug Resist. 2010;16:91–104. doi: 10.1089/mdr.2009.0120. [DOI] [PubMed] [Google Scholar]
- 26.Russell A.D. Do biocides select for antibiotic resistance? J. Pharm. Pharmacol. 2000;52:227–233. doi: 10.1211/0022357001773742. [DOI] [PubMed] [Google Scholar]
- 27.Martro E., Hernandez A., Ariza J., Matas L., Argerich M.J., Martin R., Ausina V. Assessment of Acinetobacter baumannii susceptibility to antiseptics and disinfectants. J. Hosp. Infect. 2003;55:39–46. doi: 10.1016/S0195-6701(03)00220-2. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura-Sato K., Wachino J., Kondo T., Ito H., Arakawa Y. Correlation between reduced susceptibility to disinfectants and multidrug resistance among clinical isolates of Acinetobacter species. J. Antimicrob. Chemother. 2010;65:1975–1983. doi: 10.1093/jac/dkq227. [DOI] [PubMed] [Google Scholar]
- 29.Wisplinghoff H., Schmitt R., Wöhrmann A., Stefanik D., Seifert H. Resistance to disinfectants in epidemiologically defined clinical isolates of Acinetobacter baumannii. J. Hosp. Infect. 2007;66:174–181. doi: 10.1016/j.jhin.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2014. p. 34. Twenty-Fourth Informational Supplement. [Google Scholar]
- 31.Mazzola P.G., Jozala A.F., Novaes L.C., Moriel P., Penna T.C. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009;45:241–248. doi: 10.1590/S1984-82502009000200008. [DOI] [Google Scholar]
- 32.Wang C., Zhan Q., Mi Z., Huang Z., Chen G. Distribution of the antiseptic-resistance gene qacEΔ1 in 331 clinical isolates of Pseudomonas aeruginosa in China. J. Hosp. Infect. 2007;66:93–95. doi: 10.1016/j.jhin.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Fang C.T., Chen H.C., Chuang Y.P., Chang S.C., Wang J.T. Cloning of a cation efflux pump gene associated with chlorhexidine resistance in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2002;46:2024–2028. doi: 10.1128/AAC.46.6.2024-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turton J.F., Woodford N., Glover J., Yarde S., Kaufmann M.E., Pitt T.L. Identification of Acinetobacter baumannii by detection of the blaOXA-51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006;44:2974–2976. doi: 10.1128/JCM.01021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata N., Doi Y., Yamane K., Yagi T., Kurokawa H., Shibayama K., Kato H., Kai K., Arakawa Y. PCR typing of genetic determinants for metallo-β-lactamases and integrases carried by Gram-negative bacteria isolated in Japan, with focus on the class 3 integron. J. Clin. Microbiol. 2003;41:5407–5413. doi: 10.1128/JCM.41.12.5407-5413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senda K., Arakawa Y., Ichiyama S., Nakashima K., Ito H., Ohsuka S., Shimokata K., Kato N., Ohta M. PCR detection of metallo-betalactamase gene (blaIMP) in Gram-negative rods resistant to broad-spectrum beta-lactams. J. Clin. Microbiol. 1996;34:2909–2913. doi: 10.1128/jcm.34.12.2909-2913.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellington M.J., Kistler J., Livermore D.M., Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J. Antimicrob. Chemother. 2007;59:321–322. doi: 10.1093/jac/dkl481. [DOI] [PubMed] [Google Scholar]
- 38.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed S.H., Abdelwahab S.F., Hasanen A.M., Mohammed D.S. Multidrug resistant Egyptian isolates of Acinetobacter baumannii. J. Am. Sci. 2011;7:1013–1019. [Google Scholar]
- 40.Mohamed N.M., Raafat D. Phenotypic and genotypic detection of metallo-betalactamases in imipenem-resistant Acinetobacter baumannii isolated from a tertiary hospital in Alexandria, Egypt. Res. J. Microbiol. 2011;6:750–760. doi: 10.3923/jm.2011.750.760. [DOI] [Google Scholar]
- 41.Al-Hassan L., El Mehallawy H., Amyes S.G. Diversity in Acinetobacter baumannii isolates from paediatric cancer patients in Egypt. Clin. Microbiol. Infect. 2013;19:1082–1088. doi: 10.1111/1469-0691.12143. [DOI] [PubMed] [Google Scholar]
- 42.Fouad M., Attia A.S., Tawakkol W.M., Hashem A.M. Emergence of carbapenem resistant Acinetobacter baumannii harboring the OXA-23 carbapenemase in intensive care units of Egyptian hospitals. Int. J. Infect. Dis. 2013;17:e1252–e1254. doi: 10.1016/j.ijid.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Al-Agamy M.H., Khalaf N.G., Tawfick M.M., Shibl A.M., El Kholy A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int. J. Infect. Dis. 2014;22:49–54. doi: 10.1016/j.ijid.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Al-Sweih N.A., Al-Hubail M., Rotimi V.O. Three distinct clones of carbapenem resistant Acinetobacter baumannii with high diversity of carbapenemases isolated from patients in two hospitals in Kuwait. J. Infect. Public Health. 2012;5:102–108. doi: 10.1016/j.jiph.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Bakour S., Touati A., Sahli F., Ameur A.A., Haouchine D., Rolain J.M. Antibiotic resistance determinants of multidrug-resistant Acinetobacter baumannii clinical isolates in Algeria. Diagn. Microbiol. Infect. Dis. 2013;76:529–531. doi: 10.1016/j.diagmicrobio.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Poirel L., Bonnin R.A., Nordmann P. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species; critical review. IUBMB Life. 2011;63:1061–1067. doi: 10.1002/iub.532. [DOI] [PubMed] [Google Scholar]
- 47.Domingues S., Harms K., Fricke W.F., Johnsen P.J., da Silva G.J., Nielsen K.M. Natural Transformation Facilitates Transfer of Transposons, Integrons and Gene Cassettes between Bacterial Species. PLoS Pathog. 2012;8:e1002837. doi: 10.1371/journal.ppat.1002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karthikeyan K., Thirunarayan M.A., Krishnan P. Coexistence of blaOXA-23 with blaNDM-1 and armA in clinical isolates of Acinetobacter baumannii from India. J. Antimicrob. Chemother. 2010;65:2253–2254. doi: 10.1093/jac/dkq273. [DOI] [PubMed] [Google Scholar]
- 49.Gottig S., Pfeifer Y., Wichelhaus T.A., Zacharowski K., Bingold T., Averhoff B., Brandt C., Kempf V.A. Global spread of New Delhi metallo-b lactamase 1. Lancet Infect. Dis. 2010;10:828–829. doi: 10.1016/S1473-3099(10)70275-9. [DOI] [PubMed] [Google Scholar]
- 50.Kaase M., Nordmann P., Wichelhaus T.A., Gatermann S.G., Bonnin R.A., Poirel L. NDM-2 carbapenemase in Acinetobacter baumannii from Egypt. J. Antimicrob. Chemother. 2011;66:1260–1262. doi: 10.1093/jac/dkr135. [DOI] [PubMed] [Google Scholar]
- 51.Alshara J.M.R., Alsehlawi Z.S.R., Aljameel D.S.A. First Report of New Delhi Metallo-beta-Lactamase (NDM-1) Producing Pseudomonas aeruginosa in Iraq. J. Biol. Agric. Healthc. 2014;4:40–47. [Google Scholar]
- 52.Bjorland J., Steinum T., Kvitle B., Waage S., Sunde M., Heir E. Widespread distribution of disinfectant resistance genes among Staphylococci of bovine and caprine origin in Norway. J. Clin. Microbiol. 2005;43:4363–4368. doi: 10.1128/JCM.43.9.4363-4368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith K., Gemmell C.G., Hunter I.S. The association between biocide tolerance and the presence or absence of qac genes among hospital-acquired and community-acquired MRSA isolates. J. Antimicrob. Chemother. 2008;61:78–84. doi: 10.1093/jac/dkm395. [DOI] [PubMed] [Google Scholar]
- 54.Jaglic Z., Cervinkova D. Genetic basis of resistance to quaternary ammonium compounds—The qac genes and their role: A review. Vet. Med. 2012;57:275–281. [Google Scholar]
- 55.Wang C., Zhan Q., Mi Z., Huang Z., Chen G. Distribution of the antiseptic-resistance gene qacEΔ1 in 283 clinical isolates of Gram-negative bacteria in China. J. Hosp. Infect. 2008;69:394–396. doi: 10.1016/j.jhin.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M., O’Donoghue M.M., Ito T., Hiramatsu K., Boost M.V. Prevalence of antiseptic-resistance genes in Staphylococcus aureus and coagulase-negative staphylococci colonising nurses and the general population in Hong Kong. J. Hosp. Infect. 2011;78:113–117. doi: 10.1016/j.jhin.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 57.Babaei M.R., Sulong A., Hamat R.A., Nordin S.A., Neela V.K. Extremely high prevalence of antiseptic resistant Quaternary Ammonium Compound E gene among clinical isolates of multiple drug resistant Acinetobacter baumannii in Malaysia. Ann. Clin. Microbiol. Antimicrob. 2015;14:11. doi: 10.1186/s12941-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Russell A.D., Tattawasart U., Maillard J.Y., Furr J.R. Possible link between bacterial resistance and use of antibiotics and biocides. Antimicrob. Agents Chemother. 1998;42:2151. doi: 10.1128/aac.42.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langsrud S., Sundheim G., Holck A.L. Cross resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress inducers. J. Appl. Microbiol. 2004;96:201. doi: 10.1046/j.1365-2672.2003.02140.x. [DOI] [PubMed] [Google Scholar]
- 60.Poole K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005;56:20–51. doi: 10.1093/jac/dki171. [DOI] [PubMed] [Google Scholar]
- 61.White D.G., McDermott P.F. Biocides, drug resistance and microbial evolution. Curr. Opin. Microbiol. 2001;4:313–317. doi: 10.1016/S1369-5274(00)00209-5. [DOI] [PubMed] [Google Scholar]
- 62.Boost M.V., Chan J., Shi G., Cho P. Effect of Multipurpose Solutions against Acinetobacter Carrying QAC Genes. Optom. Vis. Sci. 2016;91:272–277. doi: 10.1097/OPX.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T., Tamura Y., Yokota T. Antiseptic and antibiotic resistance plasmid in Staphylococcus aureus that possesses ability to confer chlorhexidine and acrinol resistance. Antimicrob. Agents Chemother. 1988;32:932–935. doi: 10.1128/AAC.32.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wales A.D., Davies R.H. Co-Selection of Resistance to Antibiotics, Biocides and Heavy Metals, and Its Relevance to Foodborne Pathogens; Review. Antibiotics. 2015;4:567–604. doi: 10.3390/antibiotics4040567. [DOI] [PMC free article] [PubMed] [Google Scholar]