Abstract
Introduction
Lenalidomide is an active agent that was approved for use in the EU in 2015 as a first-line therapy for previously untreated, non-transplant eligible multiple myeloma patients. Our objective was to assess the cost impact of lenalidomide when selected as a first-line treatment for transplant-ineligible patients in France, Germany, Italy, Spain, and the United Kingdom (EU5).
Methods
We developed a cost-impact model of the total costs associated with newly diagnosed multiple myeloma over 5 years in the EU5 based on treatment duration and time to progression (TTP) (taken from trial data). We compared a baseline scenario (of current lenalidomide uptake) with two alternative future scenarios. Future Scenario A used an increased uptake of first-line lenalidomide: up to 50% in Year 5. Future Scenario B was similar to the baseline, but included a 20% increased uptake of the triple therapy regimen, carfilzomib, lenalidomide, and dexamethasone (KRd) at second line.
Results
Compared to alternative first-line care pathways, lenalidomide provides a time to progression advantage of up to 5.1 months. In the baseline scenario, the costs per patient were €40,692 in Year 1. Future Scenario A showed an additional expenditure of €867 per patient in Year 1, increasing to €3358 per patient by Year 5, a 2.1% and 8.2% increase from baseline, respectively. However, lenalidomide use was associated with a lower monthly hospitalisation per-patient cost (€813) compared with bortezomib (€1173) and thalidomide (€1532). Future Scenario B was associated with a 29% increase in cost.
Conclusions
Compared to other first line therapies, lenalidomide delays time to progression resulting in associated savings across a patient’s treatment pathway and overall is likely to result in a limited impact on budget. Lenalidomide should, therefore, be considered as a first treatment option for multiple myeloma patients ineligible for transplant.
Funding
Celgene Ltd.
Keywords: Budget impact, Cost impact, First line, Lenalidomide, Multiple myeloma, Newly diagnosed
Introduction
Multiple myeloma is an incurable haematologic cancer, accounting for 1% of all cancers and approximately 10% of all haematologic malignancies [1]. The treatment paradigm of induction followed by maintenance to progression has now been clearly demonstrated to increase progression-free survival (PFS) and overall survival (OS). However, myeloma management is complicated because nearly all patients relapse and become refractory [2]. Clinicians must consider several factors when making treatment decisions: patient preference and access to treatment, age, comorbidities and frailty [3].
Lenalidomide is an efficacious multiple myeloma treatment previously licensed for use in relapsed and refractory disease. It was licensed in the EU as a first-line therapy in 2015, and in light of data from the FIRST study [3, 4], it is likely to become the standard of first-line care for patients with multiple myeloma. In patients with newly diagnosed transplant-ineligible multiple myeloma, continuous lenalidomide–dexamethasone was superior to melphalan, prednisone, and thalidomide (MPT) (72 weeks) for all primary and secondary efficacy endpoints, including OS, at the time of final analysis of primary endpoint, PFS. OS at 4 years was 59% with continuous lenalidomide–dexamethasone, 56% with 18 cycles of lenalidomide–dexamethasone, and 51% with MPT [5]. Further analysis showed that median OS was 58.9, 56.7, and 48.5 months for patients treated with continuous lenalidomide–dexamethasone, 18 cycles of lenalidomide–dexamethasone, and MPT, respectively; for continuous lenalidomide–dexamethasone, this represents a 10.4-month improvement in OS compared with those receiving MPT [6]. The increased survival benefit with lenalidomide was confirmed by Weisel et al., who conducted a network meta-analysis of survival in randomised controlled myeloma trials; lenalidomide was associated with a significant progression-free and overall survival advantage versus other first-line treatments (bortezomib, melphalan, and prednisone [VMP] and MPT) [7]. In addition to its favourable efficacy and acceptable toxicity profile, lenalidomide is an oral therapy requiring fewer hospital visits for treatment administration compared with alternative subcutaneous or intravenous agents [8–10], making this a more acceptable and better tolerated class of drugs.
Within the EU, a lenalidomide doublet regimen will be standard of care for elderly patients and those ineligible for autologous stem cell transplantation, whilst triple therapy regimens combining an immunomodulatory drug and steroid with either a proteasome inhibitor or a monoclonal antibody are anticipated to become new standards of care in the next 5 years for younger, transplant eligible patients [3, 11–14].
In an era of increasingly cost-conscious health systems, economic information, including resource use, plays an important role in market access decision making for innovative medicines. The objective of our study was to assess the cost impact of lenalidomide when selected as a first-line treatment for newly diagnosed multiple myeloma patients in the EU5 (France, Germany, Italy, Spain, and the United Kingdom) versus current treatment options. We hypothesised that lenalidomide is likely to have a marginal incremental cost burden in the EU5 at first-line; the increase in OS and reduced administration costs compared to other first-line therapies will delay those costs incurred by subsequent treatment choices at second and third-line.
Methods
Lenalidomide Efficacy
To demonstrate the average time to progression of an entire multiple myeloma care pathway, treatment sequences that included lenalidomide-based therapy in first line were compared with treatment sequences that included thalidomide- or bortezomib-based regimens as first-line options.
Lenalidomide First-Line Uptake in the EU5
We developed a cost-impact model to estimate the total costs associated with newly diagnosed multiple myeloma over 5 years in the EU5. We based this model on treatment duration and time to progression data from several trials; these outcomes also allowed us to model the efficacy of multiple myeloma treatments. This research is based on previously conducted studies; it does not involve any new studies involving human or animal participants, as conducted by any of the authors. The current EU standard of care for multiple myeloma in autologous stem cell transplant-ineligible patients at first-line is MPT or VMP, as advised by the European Society of Medical Oncology (ESMO) [1]. The model included drug, administration, and hospitalisation costs of first-line treatment and two more additional lines of post-progression treatment for lenalidomide-, thalidomide-, and bortezomib-based regimens, as well as the costs of fourth-line pomalidomide-based regimens, based on expected clinical practice. We sourced hospitalisation costs from a retrospective chart review of Dutch patients with relapsed/refractory multiple myeloma [10]. We adapted these costs to the first-line setting by adjusting for the duration of treatment (DoT) and the time to progression (TTP) as reported in the clinical trials of each regimen (shown in Table 1). It is worth noting that DoT data may also include treatment-free intervals associated with some treatments. The model has been designed using TTP data; time to next treatment data were not available within the trials because of the variability in starting a new treatment line.
Table 1.
Duration of treatment and efficacy of each regimen in each line
| Line | Treatment | Duration of treatment (months) | Progression-free survival (months) | Time to progression (months)a | Source |
|---|---|---|---|---|---|
| 1 | Thalidomide | 15.4 | 21.2 | 23.9 | FIRST trial [5] |
| Bortezomib | 10.6 | 21.7 | 24.0 | VISTA trial [15] | |
| Lenalidomide | 18.4 | 25.5 | 32.5 | FIRST trial [5] | |
| 2 | Thalidomide | 6.4 | 7.3 | 8.0 | Kropff et al. [16] |
| Bortezomib | 7.0 | 8.1 (DoR) | 7.0 | Richardson et al. [17] | |
| Lenalidomide | 12.5 | 14.1 | 17.1 | Stadtmauer et al. [18] | |
| 3 | Thalidomide | 6.4 | 7.3 | 7.0 | Kropff et al. [16] |
| Bortezomib | 4.9 | 7.8 (DoR) | 4.9 | Richardson et al. [17] | |
| Lenalidomide | 9.2 | 9.5 | 10.6 | Stadtmauer et al. [18] | |
| 4b | Pomalidomide | 2.9 | 4.6 | 3.6 | MM-008 trial CSR |
CSR clinical study report, DoR duration of response
aTime to progression given as median
bPomalidomide-based regimens assumed to be used exclusively after third line
Drug costs were calculated according to drug prices and dosing (i.e., the number of doses per month multiplied by the total mg per dose multiplied by the cost per mg). Weighted averages were used to calculate listed drug prices across the EU5. Monthly costs within the line of treatment were equated to the sum of hospital-based costs (visits, admission, procedures, concomitant medication) as published by Gaultney et al. [10]. Hospital costs in the first-line treatment setting were not described by Gaultney et al.; therefore, hospital costs in the second-line treatment setting were adjusted to TTP and applied to first-line use, assuming the same monthly cost during treatment time.
We compared a baseline scenario (of current lenalidomide uptake) with two alternative future scenarios. The baseline scenario reflected the estimated current uptake of first-line lenalidomide (i.e., 11% of the market). For Future Scenario A, we assumed a steadily increasing uptake of first-line lenalidomide: up to 50% in Year 5. The baseline 11% market share for lenalidomide was taken from bortezomib-based and thalidomide-based regimens so that, proportionally, the relative market share of these two comparators remained the same. Table 2 shows the baseline scenario and assumed market share of therapies at first line. Table 3 shows the market share overrides after first-line therapies for Future Scenario A.
Table 2.
Assumed market share of therapies in first-line setting
| Treatment regimen | Model scenario market shares | |||||
|---|---|---|---|---|---|---|
| Baseline scenario (%) | Future Scenario A | |||||
| Year 1 (%) | Year 2 (%) | Year 3 (%) | Year 4 (%) | Year 5 (%) | ||
| Thalidomide | 25 | 19 | 17 | 16 | 15 | 14 |
| Bortezomib | 64 | 53 | 48 | 44 | 40 | 36 |
| Lenalidomide | 11 | 28 | 35 | 40 | 45 | 50 |
Table 3.
Market share overrides after first-line for Future Scenario A
| Treatment regimen | Model scenario market sharesa | |||||
|---|---|---|---|---|---|---|
| After first-line | After second-line | |||||
| THAL (%) | BORT (%) | LEN (%) | THAL (%) | BORT (%) | LEN (%) | |
| Thalidomide | 7 | 14 | 17 | 4 | 21 | 36 |
| Bortezomib | 37 | 25 | 71 | 30 | 13 | 58 |
| Lenalidomide | 56 | 61 | 12 | 66 | 66 | 6 |
BORT bortezomib, LEN lenalidomide, THAL thalidomide
aPomalidomide-based regimens assumed to be used exclusively after third line; some patients were assumed to receive a re-treatment, e.g., MPT after first-line MPT
Scenario Estimating Potential Impact of Triple Therapy
Future Scenario B was identical to baseline scenario, but we also assumed a 20% increased uptake of carfilzomib-based triple therapy (KRd) at second-line. Furthermore, a small percentage of patients in both future scenarios were assumed to receive a re-treatment. For example, VMP after first-line VMP; there is a precedent for this in clinical practice with bortezomib-based regimens [19]. Table 2 shows the baseline scenario and assumed market share of therapies at first-line and Table 3 shows the market share overrides for Future Scenario A. Table 4 shows the market share overrides after first-line therapies for Future Scenario B.
Table 4.
Market share overrides after first-line for Future Scenario B
| Treatment regimen | Model scenario market sharesa | ||||||
|---|---|---|---|---|---|---|---|
| After first-line | After second-line | ||||||
| THAL (%) | BORT (%) | LEN (%) | THAL (%) | BORT (%) | LEN (%) | CAR (%) | |
| Thalidomide | 5 | 11 | 17 | 4 | 21 | 36 | 39 |
| Bortezomib | 26 | 20 | 63 | 30 | 13 | 58 | 61 |
| Lenalidomide | 39 | 39 | 10 | 66 | 66 | 6 | 0 |
| Carfilzomib | 30 | 30 | 10 | 0 | 0 | 0 | 0 |
BORT bortezomib, CAR carfilzomib, LEN lenalidomide, THAL thalidomide
aPomalidomide-based regimens assumed to be used exclusively after third-line; some patients were assumed to receive a re-treatment, e.g. thalidomide after first-line thalidomide
Results
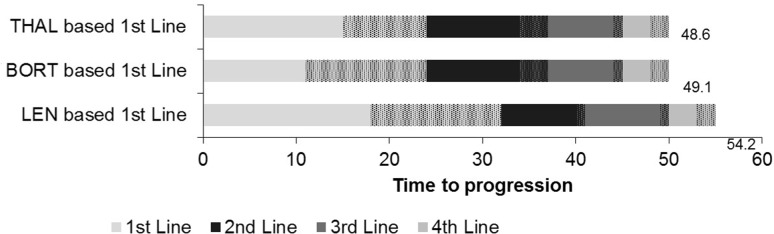
Compared with alternative first-line care pathways, our model results demonstrated that first-line lenalidomide provides a time to progression advantage of up to 5.1 months over a patient’s lifetime (when averaging all treatment pathways), as is shown in Fig. 1.
Fig. 1.
Treatment efficacy (DoT, TTP, months). BORT bortezomib, DoT duration of treatment, LEN lenalidomide, THAL thalidomide, TTP time to progression. Cross-hatching represents the time spent off-treatment before progression. This varies between treatments because they have either a fixed DoT or a dose intervention
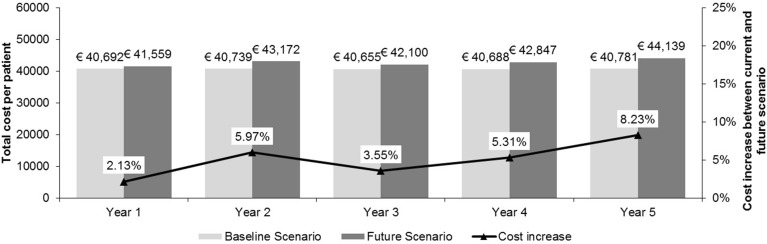
In the baseline scenario, the annual costs per patient were €40,692 in Year 1. In Future Scenario A, we replaced the current first-line standard of care with lenalidomide; in Year 1, this resulted in an additional expenditure of €867 per patient, which represents a 2.1% increase from the baseline scenario. By Year 5, the additional expenditure in Future Scenario A increased to €3358 per patient, an 8.2% increase from baseline. The cost per patient of using lenalidomide at first-line in the EU5 is shown in Fig. 2. Lenalidomide-treated patients had higher monthly drug costs per patient (€5388) compared with patients receiving bortezomib (€3381) or thalidomide (€1742). However, lenalidomide use was associated with a lower monthly hospitalisation cost per patient (€813) compared with bortezomib (€1173) and thalidomide (€1532). Monthly hospitalisation costs were calculated on the basis of “total cost in line of therapy”—this value was derived from the monthly costs within the line of treatment multiplied by the TTP for each treatment arm. Monthly costs within the line of treatment were the sum of hospital based costs published by Gaultney et al. [10].
Fig. 2.
Annual cost per patient receiving first-line lenalidomide in the EU5 for Future Scenario A. EU5 France, Germany, Italy, Spain, and the United Kingdom
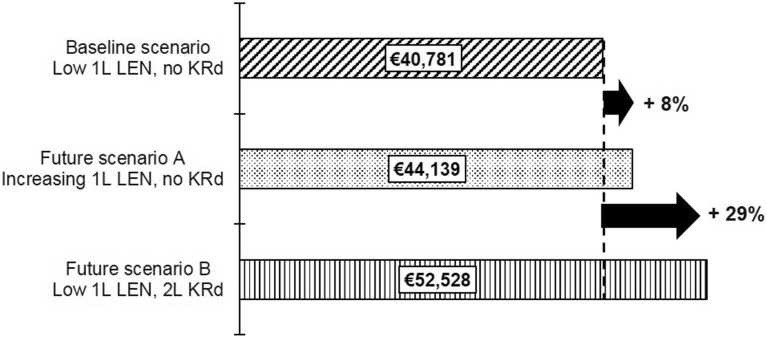
In Future Scenario B, we modelled a 20% uptake of the novel triplet combination KRd at second line; we estimated that the impact on the budget will represent an additional 29% increase over the current baseline scenario. In contrast, modelling a future care pathway with increasing first-line lenalidomide resulted in a budget impact of only 8% over the current baseline, as shown in Fig. 3.
Fig. 3.
Per-patient costs in Year 5 of baseline and future scenarios in the EU5. 1L first-line, 2L second-line, EU5 France, Germany, Italy, Spain, and the United Kingdom, LEN lenalidomide, KRd carfilzomib, lenalidomide, and dexamethasone
Discussion
Our results show that the availability of lenalidomide at first-line has a relatively small impact on budget, i.e., an 8% increase. This increase should be considered both within the context of the increased benefit expected for patients in terms of TTP and OS, and when compared to the potential impact of introducing triple combination therapy with a novel agent as first-line salvage for the 20% of patients we estimated to be fit enough to receive this treatment later in their treatment pathway (29% increase in budget impact). Not all patients are eligible for triple therapy during subsequent lines of treatment; this is especially relevant in the context of an increasingly elderly population. Although there are no direct, randomised data comparing Rd and VMP, lenalidomide is expected to be more effective than current first-line therapies, as suggested by the FIRST trial results and supported by the indirect comparison of treatments conducted by Weisel et al. [5–7]. Using lenalidomide, therefore, delays the time to subsequent therapy because of its improvement in time to progression compared to other first-line therapies, but we are aware that the duration of treatment may vary across countries, which may further affect treatment efficacy. The reduction in requirement for subsequent therapy reduces costs for administration and hospitalisation due to toxicities; this contributes to off-setting the increased drug cost of lenalidomide use. Our results also show that reserving lenalidomide for a later line such as combination with a novel triple therapy combination (KRd) will increase the budget by 29% compared to the current baseline scenario.
Our findings agree with those from Arikian et al., who used US healthcare data to show that, for the first 3 years of treatment, patients who initiated lenalidomide-based treatments had lower mean monthly total costs compared to those who initiated on bortezomib-based treatments [8]. Arikian et al. also demonstrated that the cumulative cost of first-line lenalidomide was approximately $120,000 (€109,000) lower than for first-line bortezomib [8]. These findings further support our recommendation that the most beneficial way to use lenalidomide for patients with newly diagnosed multiple myeloma is at first-line thus capitalising on its increased time to progression/OS and cost impact compared to other choices of first-line treatment.
Our results suggest that compared to other first-line therapies, appropriately selecting patients to receive first-line lenalidomide would have a reduced cost impact, as well as better OS and tolerability. Many of these patients, who are too frail to tolerate triplet combinations upfront, are unlikely to become more suitable upon relapse.
The costs of managing toxicity both to the patient and the healthcare system should also be carefully considered. Myelosuppression, which occurs more frequently with triplet therapies, does have a significant cost impact when treatment with blood products or growth factors is required. One limitation of this study is that it did not assess the costs associated with patient side-effects from treatment. As these data were not available for all comparators, we only assessed the impact on hospitalisation. However, given the relatively similar side-effect profiles between the treatments, this was not believed to significantly affect the conclusions of the model. Another limitation of our study is that data are taken from a variety of sources in order to estimate the likely economic impact of future treatment scenarios. In particular, data for hospitalisation were taken from a retrospective analysis, which increases the risk of bias or confounding.
Further research to evaluate the complete direct and indirect costs of multiple myeloma treatment across all treatment lines would further improve the understanding of the economic impact of this disease. As yet, there are no available real-world data to guide patient selection for therapies to improve both long-term patient outcomes and reduce the impact on EU5 healthcare systems.
Conducting cost-impact analyses may be advantageous for understanding the budget impact implications of new and/or expensive oncology therapies and patient pathways, particularly within disease areas where there are multiple potential sequences of treatments that could present a viable option for patients. Selecting the most appropriate treatment up-front is likely to be even more important in the future, when new, more expensive treatment regimens for relapsed multiple myeloma become available. This will help reduce and optimise the burden to healthcare systems and ensure that these treatments are available to the patients who would benefit the most. Arikian et al. have demonstrated the economic benefits of delaying first progression in multiple myeloma in the US healthcare setting [8]; it is limiting to multiple myeloma patients that this is not an option in the EU. Further research on the benefit to both patients and the healthcare system of delaying first progression is, therefore, also warranted.
Conclusion
Using lenalidomide in the first-line setting delays the time to subsequent therapy compared to other first-line treatments. It offers substantial cost offsets due to the reduced requirement for subsequent therapy, lowering the costs associated with administration and hospitalisation.
Acknowledgements
Sponsorship for this study and article processing charges were funded by Celgene Ltd. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Editorial assistance in the preparation of this manuscript was provided by Ashley Davies and Dawn Lee of BresMed. Support for this assistance was funded by Celgene Ltd.
Disclosures
Sujith Dhanasiri is employed by and has equity in Celgene, who funded the study. Craig J. Gibson is employed by and has equity in Celgene, who funded the study. Chloe Stengel-Tosetti is employed by Dolon, a consultancy who were reimbursed by Celgene for this manuscript work. Prof Steve Schey received an honorarium from Celgene Ltd for participating in an advisory board relating to this work. Luis Felipe Casado Montero received an honorarium from Celgene Ltd for participating in an advisory board relating to this work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies, and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
All data generated or analysed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/FB47F0607D4B49C2.
References
- 1.Moreau P, San Miguel J, Ludwig H, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi133–vi137. doi: 10.1093/annonc/mdt297. [DOI] [PubMed] [Google Scholar]
- 2.Borrello I. Can we change the disease biology of multiple myeloma? Leuk Res. 2012;36(Suppl 1):S3–S12. doi: 10.1016/S0145-2126(12)70003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125(20):3076–3084. doi: 10.1182/blood-2014-09-568915. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Association (EMA). Summary of opinion (post authorization): Revlimid (lenalidomide). 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000717/WC500179310.pdf. Accessed 06 March 2016.
- 5.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 6.Facon T, Hulin C, Dimopoulos MA, et al. Updated overall survival analysis of the FIRST study: continuous lenalidomide plus low dose dexamethasone vs melphalan, prednisone and thalidomide in patients with newly diagnosed multiple myeloma. In: 2015 European Hematology Association annual meeting. Vienna, Austria. 11–14 June 2015. Oral Presentation S105.
- 7.Weisel K, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leuk Lymphoma. 2017;58(1):153–161. doi: 10.1080/10428194.2016.1177772. [DOI] [PubMed] [Google Scholar]
- 8.Arikian SR, Milentijevic D, Binder G, et al. Patterns of total cost and economic consequences of progression for patients with newly diagnosed multiple myeloma. Curr Med Res Opin. 2015;31(6):1105–1115. doi: 10.1185/03007995.2015.1031732. [DOI] [PubMed] [Google Scholar]
- 9.Armoiry X, Fagnani F, Benboubker L, et al. Management of relapsed or refractory multiple myeloma in French hospitals and estimation of associated direct costs: a multi-centre retrospective cohort study. J Clin Pharm Ther. 2011;36(1):19–26. doi: 10.1111/j.1365-2710.2009.01153.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaultney JG, Franken MG, Tan SS, et al. Real-world health care costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J Clin Pharm Ther. 2013;38(1):41–47. doi: 10.1111/jcpt.12020. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Association (EMA). Summary of opinion (initial authorization): Kyprolis (carfilzomib). 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/003790/WC500194117.pdf. Accessed 06 March 2016.
- 12.Amgen Inc. European commission approves Kyprolis® (carfilzomib) for combination use in the treatment of patients with relapsed multiple myeloma. 2015. https://www.amgen.com/media/news-releases/2015/11/european-commission-approves-kyprolis-carfilzomib-for-combination-use-in-the-treatment-of-patients-with-relapsed-multiple-myeloma/. Accessed 06 March 2015.
- 13.European Medicines Association (EMA). Summary for the public: Empliciti. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/003967/WC500206676.pdf. Accessed 27 June 2016.
- 14.European Medicines Association (EMA). EMA fast-tracks treatment of multiple myeloma for approval in EU. 2016. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2016/01/news_detail_002461.jsp&mid=WC0b01ac058004d5c1 Accessed 27 June 2016.
- 15.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 16.Kropff M, Baylon HG, Hillengass J, et al. Thalidomide versus dexamethasone for the treatment of relapsed and/or refractory multiple myeloma: results from OPTIMUM, a randomized trial. Haematologica. 2012;97(5):784–791. doi: 10.3324/haematol.2011.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 18.Stadtmauer EA, Weber DM, Niesvizky R, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur J Haematol. 2009;82(6):426–432. doi: 10.1111/j.1600-0609.2009.01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sood R, Carloss H, Kerr R, et al. Retreatment with bortezomib alone or in combination for patients with multiple myeloma following an initial response to bortezomib. Am J Hematol. 2009;84(10):657–660. doi: 10.1002/ajh.21517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article/as supplementary information files.