Abstract
Bone marrow necrosis (BMN) in acute leukemia is a rare histopathological entity at the time of initial diagnosis. However, it represents an important diagnostic and prognostic challenge. Two cases of BMN are reported: a 44-year-old patient with B cell precursor (BCP) acute lymphoblastic leukemia (ALL) and a 27-year-old man with FAB-M5 acute myeloid leukemia (AML) who both presented with bone marrow failure and extensive necrosis. From these clinical cases, we conducted a brief review of the literature.
Keywords: Acute leukemia, Bone marrow necrosis, Chemotherapy, Prognosis
Introduction
Bone marrow necrosis (BMN) is defined as a necrosis of myeloid tissue and medullary stroma in large areas of hematopoietic bone marrow, without involvement of the cortical bone [6]. It was first described by Wade and Stevenson in 1942 in a patient who had sickle cell disease [7]. It is an uncommon, but not rare cytological and histological entity in both AML and ALL. The clinical symptoms could be very severe with fatigue, bone pains, fever and biological alterations as anemia, thrombocytopenia, leucopenia or leukocytosis, coagulopathy, high LDH level, and/or hypercalcemia [6]. The diagnosis is made by bone marrow aspirates or biopsy, with sometimes the presence of Charcot-Leyden crystals [27]. The treatment is based on the associated pathology. The prognosis is generally poor, depending on age, patient clinical history and severity of the initial pathology. The incidence of BMN, seen across a wide range of malignant and nonmalignant hematological disorders, ranges from 0.5% to 33% [8]. The differences observed in the literature in terms of BMN rates can be explained in part by the use of different grading scales. Paydas et al. described 23 cases (20 adults and three children) of BMN among 1083 bone marrow biopsies that is a prevalence of 2.2% [9]. Badar et al. showed in a more recent report, an incidence of 2.4% in AML and 3.2% in ALL [10]. We report here two cases of bone marrow necrosis (BMN): a 44-year-old patient with B-cell precursor (BCP) acute lymphoblastic leukemia (ALL) and a 27-year-old man with FAB-M5 acute myeloid leukemia (AML) who both presented with bone marrow failure and extensive necrosis. Informed consent was obtained from all patients for being included in the study.
Case Report #1
A 44-year-old man was admitted in April 2014 for lumbar pains related to a paraspinal mass. The patient had no prior medical history. At admission, his complete blood count showed: hemoglobin 129 g/L, platelet count 66 × 109/L, and white blood cell count 4.7 × 109/L with 8% of blast cells.
Bone marrow aspirate revealed a hypocellular marrow with extensive necrosis and pycnotic features in most of the cells (Fig. 1). Bone marrow biopsy confirmed an extensive necrosis (>60%). Peripheral blood flow cytometry analysis revealed a BCP-common ALL profile: CD45dim/SSClo, CD10+hi, CD20+, CD34−, and CD38+hi, CD123−, CD44+hi, CD49f+/− with negativity for myeloid or T lymphoid markers. There was no central nervous system involvement. Cytogenetic analysis did not revealed any abnormality (46, XY on 20 analyzed mitosis). FISH analysis and molecular analyses did not show MLL amplification, BCR-ABL fusion gene, or IKAROS gene deletion. The patient was treated according to the GRAALL-2005 protocol [1]. Corticosensivity and chemosensivity were evaluated on day 8 of treatment. At the end of induction chemotherapy (day 35), a morphologic complete response (CR) was obtained with undetectable minimal residual disease (MRD) based both on flow cytometry and molecular analysis.
Fig. 1.
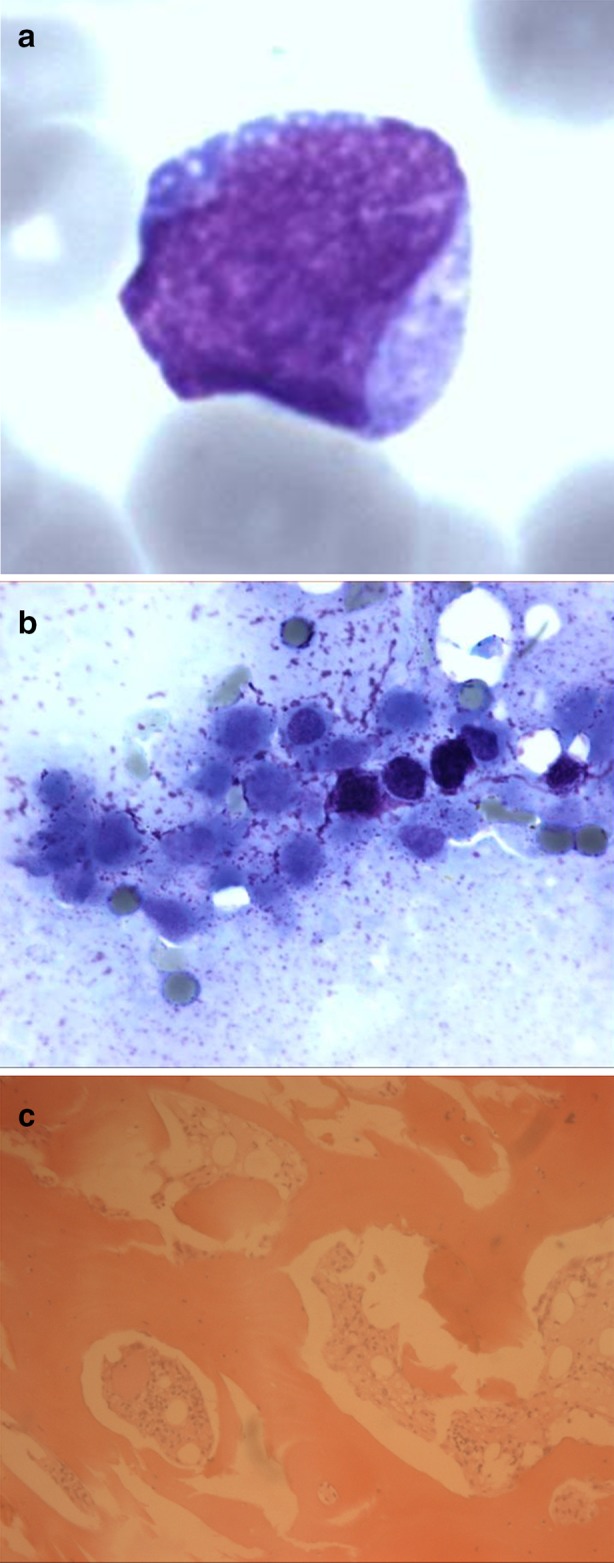
Morphology of leukemia at diagnosis (case report #1: BCP-ALL). a Blood smear showing small to medium size blast cell, with fine and pearly chromatin, evocative of lymphoid blast. b Bone marrow aspirate picture in optic microscopy (May–Grünwald–Giemsa) showing extensive marrow necrosis. Cells cannot be identified. c Bone marrow trephine biopsy showing a fibrotic marrow with a low cellularity but a massive blastic infiltration
The patient received consolidation treatment, without any major complication, but he relapsed at the end of consolidation therapy. Salvage therapy with blinatumomab (a bi-specific monoclonal antibody binding simultaneously to CD3-positive cytotoxic T cells and CD19-positive B cells) was started yielding to a second CR achievement. Further, the patient underwent allogeneic hematopoietic stem cell transplant from a sibling donor, after myeloablative conditioning regimen combining high-dose cyclophosphamide with total body irradiation. The patient relapsed again four months later. The third line therapy consisted in the combination of Hyper-CVAD regimen with epratuzumab (an anti-CD22 monoclonal antibody). Disease progression did not permit obtaining a new response and the patient died 17 months after the initial diagnosis from hemorrhagic and infectious complications.
Case Report #2
A 27-year-old man without significant prior medical history was admitted in December 2014 for intense lumbar pains with right sciatalgia, lumbago and hypoesthesia. The spinal CT scan confirmed L5-S1 discarthrosis. He also presented a numb chin syndrome. At admission, his complete blood count showed: hemoglobin 107 g/L, platelet count 62 × 109/L, and white blood cell count 2.2 × 109/L with 6% of blast cells. Bone marrow aspirate revealed extensive necrosis of hematopoietic tissue (Fig. 2). However, some areas showed intact cells with monoblast morphology, irregular nucleus, fine chromatin, nucleolus, and an intense basophilic cytoplasm. Bone marrow biopsy confirmed an extensive necrosis (>60%), with reticulinic fibrosis (grade 1), necrotic cells with blast morphology, some of which showing erythrophagocytosis features. Bone marrow flow cytometry immunophenotyping showed the following profile: CD45dim/SSClo, CD34−, CD38+hi, CD123+hi, HLADR+hi, CD13+/−, CD33+hi, CD15+hi, CD117−, CD36+, CD4+lo, CD11b+hi and CD56+hi, and negativity for lymphoid markers. A diagnosis of AML of M5 subtype according to FAB classification was established. The cytogenetic analysis revealed a clonal aberration with 47, XX, +8[4]/46, XY[16], classified as intermediate-risk according to the ELN criteria [2]. In molecular biology, there was no EVI1, WT1, FLT3-ITD, MLL nor CEBPA mutations or rearrangements, but oneNPM1 mutation.
Fig. 2.
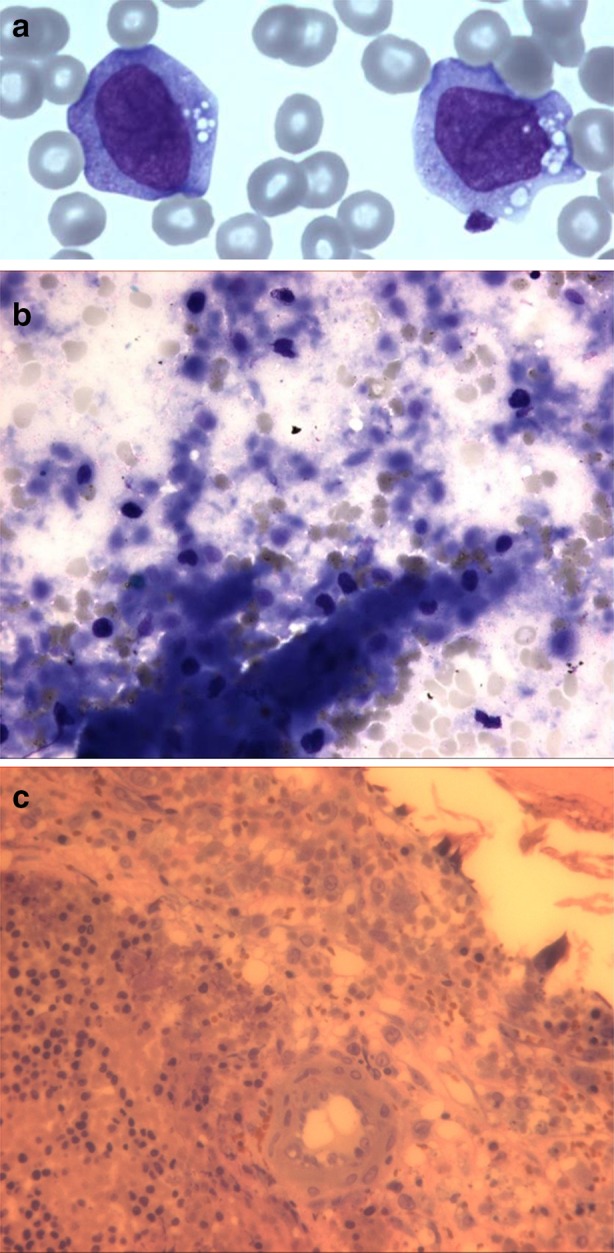
Morphology of leukemia at diagnosis (case report #2: AML). a Blood smear showing an aspect of myeloid blasts with monocytic differentiation. b Bone marrow aspirate picture in optic microscopy (May–Grünwald–Giemsa) showing extensive marrow necrosis. Cells cannot be recognized, showing indistinct outlines and pycnotic nuclei. c Bone marrow trephine biopsy showing a hypercellular marrow with extensive necrosis. Majority of cells are necrotic but however suggestive of blastic cells
The patient received timed sequential induction chemotherapy with two sequences combining daunorubicin with cytarabine according to the ALFA-0702 protocol [3]. Because of central nervous system involvement, treatment was completed by intrathecal chemotherapy administration. A morphologic evaluation at the end of induction therapy showed CR, an hypoplastic bone marrow and the presence of 2–3% of immature cells, confirmed by flow cytometry (MRD = 1%) and persistence of NPM1 positivity. The patient relapsed with extramedullary localizations (leukemia cutis, central nervous system involvement) after consolidation chemotherapy. The patient received two further lines of treatment which did not allow any response [4, 5]. He finally died 10 months after the initial diagnosis.
Discussion
As previously described, BMN is usually observed in the setting of hematological malignancies (60%) or solid tumor (30%) [6, 11]. In a series of 240 BMN, Janssens showed that ALL represented the most common underlying disorder (18%). BMN was also frequently associated with AML (13%). In this series, BMN was observed at the time of diagnosis in 26 of 37 patients (70.3%), and only after induction chemotherapy in one of 37 patients (20.7%), and at the time of recurrence in ten of 37 patients (27%) [6]. In a series of 640 patients, Badar et al. reported that among patients with AML and BMN, 32% had the FAB-M5 subtype, compared to only 10% without BMN.
Clinical symptoms of BMN are non-specific. The most common is bone pain, which is observed in 80% of the cases, and may be disseminated or localized in the lower back [10]. Fever is also often seen in BMN (70% of the cases) [9]. Both symptoms can be related to the importance of tissue necrosis [9, 12]. Laboratory findings often include anemia, thrombocytopenia, elevated lactate deshydrogenase and alkaline phosphatase levels [11, 13].
Few data are currently available in the literature about the prognostic impact of BMN in acute leukemia at the time of diagnosis. Only one large series, describing BMN in 25 AML patients (2.4%) and 20 ALL patients (3.2%), demonstrated a low CR rate and a poor overall outcome [10]. The presence of BMN was associated with a worse outcome among AML patients with a median overall survival (OS) of only 3.7 months. OS in our cases were 17 months and 10 months respectively. In Badar’s study, AML patients had a significantly lower CR rate (32%) than those without BMN (59%) (p = 0.008). Early death was observed in three (12%) and 37 (3.6%) of patients with and without BMN, respectively (p = 0.07). In ALL patients, CR rate was also significantly lower (70% vs 90%, p = 0.01) and 3% vs 1% had early death (p = 0.44) [10].
Prognostic factors in BMN cases mainly depend on patient age and the nature of the underlying disease. The presence of BMN was not reported of poor prognosis in children [14]. Pui et al. examined bone marrow specimens from 1419 children with acute leukemia. BMN was found in seven (0.5%) of them. Six (four with ALL and two with neuroblastoma) remained in long-term remission [15]. However, McFarlane reported only one persistent CR on four patients with BMN [16].
Bone marrow trephine biopsy appeared to be essential for the diagnosis of BMN and for necrosis grading. When bone marrow aspirate is successful, cytology examination shows pycnotic cells, scarcely recognizable in a background of amorphous extracellular eosinophilic material. Histology is more specific and characterized by a combination of gelatinous transformation (hypoplasia and background of amorphous eosinophilic staining material) and necrosis of the myeloid tissue [6]. There is also a disappearance of fat space while cortical bone is preserved. BMN can be evaluated according to three levels, depending on the necrotic area. Grade I (<20%) corresponds to mild necrosis if restricted to a focal area, grade II (20–50%) to moderate necrosis if intermediate involvement is seen, and grade III (>50%) if an extensive involvement of the bone marrow is observed. Moderate (Grade II) and severe (Grade III) bone marrow necrosis are often associated with life threatening illnesses [8].
Physiopathology of BMN is not fully elucidated. BMN usually results from microvascular failure, and is often associated with a hypercellular marrow, except in aplastic anemia where cellularity is generally decreased [11]. When the microvasculature has been restored, the necrotic material may be removed by phagocytosis, followed by reconstitution of bone marrow tissue, with development of proliferating capillaries and fibroblasts. Repopulation of the marrow by a normal hematopoietic tissue is usually the rule, as observed in reported cases of sickle cell anemia [9, 17]. In our cases, bone marrow regeneration was consistently observed after induction therapy. The occurrence of BMN can be explained by hypoxemia following failure of the microcirculation. Mentioned causes are vascular inflammatory lesions mediated by immune complex deposition, or toxins and cytokines (including TNFα) released by tumoral cells [6, 9, 11]. Unexplained physiopathology and poor prognosis is the characteristics of this presentation. In a 10-year retrospective analysis [28] approximately 0.3% of non–site-directed bone marrow cases showed significant necrosis, most of them secondary to metastatic tumor or hematolymphoid malignancy (together, 90% of necrosis cases). In this study, patients with malignancy-associated marrow necrosis had generally a poorer outcome (approximately 55% of mortality on follow-up regardless of tumor type), although this could be related to the poor prognosis of their underlying malignancies. However, some surviving patients with malignancy-associated marrow necrosis can achieve durable remissions (up to 8 years) with return to normal blood counts. We propose that each bone marrow aspirate displaying necrosis features should be completed by bone marrow biopsy morphological examination in order to determine the pattern and stage of cellular necrosis, the search for infectious etiologies, the presence of hematolymphoid malignancy, nonnecrotic tumor cells, Charcot Leyden crystals or other specific morphologic features (Table 1).
Table 1.
Review of the literature
| References | Number of cases | Underlying disease | Treatment | Outcome |
|---|---|---|---|---|
| Niebrugge and Benjamin [14] | 2 cases | ALL | IC |
DFS: 6 months+ DFS: 4 years+ |
| Pui et al. [15] | 7 cases/1419 |
5 ALL 2 neuroblastoma |
IC | ND |
| Forrest et al. [18] | 10 cases/581 |
AL NHL |
IC | ND |
| Janssens et al. [6] | 240 cases |
145 hematologic malignancies 72 solid tumors 22 nonmalignant diseases |
ND | Median OS: 1 to 4 months |
| Paydas et al. [9] | 20 cases/1083 |
3 AML 2 ALL 1 MDS 2 CML 2 HL 2 NHL 3 solid tumors |
ND | OS: few days to few months |
| Eusni et al. [19] | 1 case | ALL | IC | Early death |
| Gérard et al. [10] | 2 cases |
1 AML 1 gastric adenocarcinoma |
HU SC |
Early deaths |
| Osuorji and Goldman [20] | 1 case | AML | IC | ND |
| Lackner et al. [21] | 1 case | HL | IC | ND |
| Shafiq and Ali [22] | 1 case | Sickle cell anemia | SC | ND |
| Koshnaw and Muhealdeen [23] | 1 case | ALL | IC | CR |
| Shapiro et al. [24] | 1 case | Secondary AML | IC | Early death |
| Choudary et al. [11] | 1 case | Myelofibrosis | SC | Early death |
| Badar et al. [13] | 45 cases/1691 |
20 AML 25 ALL |
ND |
Median OS (AML): 3.7 months Median OS (ALL): 61.7 months |
| Daskalakis and Caballero [25] | 1 case | AML | SC | Early death |
| Rashidi et al. [26] | 1 case | AML | IC + ASCT | ND |
| Chambers et al. [12] | 1 case | Secondary AML | SC | Early death |
| Our study | 2 cases |
1 ALL 1 AML |
IC |
OS: 17 months OS: 10 months |
AL acute leukemia, ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, ASCT allogeneic stem cell transplantation, CML chronic myeloid leukemia, CR complete remission, DFS disease-free survival, HL Hodgkin lymphoma, HU hydroxyurea, IC intensive chemotherapy, MDS myelodysplastic syndrome, ND not done, NHL non Hodgkin lymphoma, OS overall survival, SC supportive care
Conclusions
In our institution, only two cases of acute leukemia associated with severe BMN were seen over the last ten years, among more than 1000 newly diagnosed AML and 500 newly diagnosed ALL. Despite intensive therapy, the outcome was rapidly pejorative with relapse and death occurring after few months. In accordance with the literature, BMN may be considered as an adverse prognosis factor. Early identification of BMN in newly diagnosed acute leukemia may be helpful for establishing prognostic stratification and treatment-decision making, especially in patients without any other unfavorable prognostic markers. Given the extreme rarity of this entity, only international collaborative studies could better elucidate the clinical impact of BMN, as an independent prognostic factor in acute leukemia.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Adrien Quintela, Pierre Sujobert, Isabelle Tigaud, Sandrine Hayette, Martine Ffrench, Gilles Salles, Xavier Thomas and Adriana Plesa have nothing to disclose.
Compliance with Ethics Guidelines
Informed consent was obtained from all patients for being included in the study.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/8887F06028F131B8.
References
- 1.Huguet F, Leguay T, Raffoux E, Thomas X, Beljord K, Delabesse E, Chevallier P, Buzyn A, Delannoy A, Chalandon Y, Vernant JP, Lafage-Polchitaloff M, Chevassent A, Lhéritier V, Macintyre E, Béné MC, Ifrah N, Dombret H. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27(6):911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 2.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B. Bloomfield CD; European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommandations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 3.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. doi: 10.1182/blood-2015-08-604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, Legrand O, Thomas X, Turlure P, Reman O, de Revel T, Gastaud L, de Gunzburg N, Contentin N, Henry E, Marolleau JP, Aljijakli A, Rousselot P, Fenaux P, Preudhomme C, Chevret S, Dombret H. Acute Leukemia French Association. Lancet. 2012;379(9825):1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 5.Archimbaud E, Thomas X, Leblond V, Michallet M, Fenaux P, Cordonnier C, Dreyfus F, Troussaud X, Jaubert J, Travade P. Timed sequential chemotherapy for previously treated patients with acute myeloid leukemia: long-term follow-up of the etoposide, mitoxantrone, and cytarabine-86 trial. J Clin Oncol. 1995;13(1):11–18. doi: 10.1200/JCO.1995.13.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Janssens AM, Offner FC, Van Hove WZ. Bone marrow necrosis. Cancer. 2000;88(8):1769–1780. doi: 10.1002/(SICI)1097-0142(20000415)88:8<1769::AID-CNCR3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Wade L, Stevenson L. Necrosis of bone marrow with fat embolism in sickle cell anemia. Am J Path. 1941;17:47–54. [PMC free article] [PubMed] [Google Scholar]
- 8.Maisel D, Lim JY, Pollock WJ, Yatani R, Liu PI. Bone marrow necrosis: an entity often overlooked. Ann Clin Lab Sci. 1988;18(2):109–115. [PubMed] [Google Scholar]
- 9.Paydas S, Ergin M, Balsamisli F, Yavuz S, Zorludemir S, Sahin B, Bolat FA. Bone marrow necrosis: clinicopathologic analysis of 20 cases and review of the literature. Am J Hematol. 2002;70(4):300–305. doi: 10.1002/ajh.10114. [DOI] [PubMed] [Google Scholar]
- 10.Gérard J, Berdin B, Portier G, Godon A, Tessier-Marteau A, Geneviève F, Zandecki M. Bone marrow necrosis in two patients with neoplastic disorders. Ann Biol Clin (Paris). 2007;65(6):636–642. [PubMed] [Google Scholar]
- 11.Choudhary S, Jayaprakash HT, Shiva Kumar BR, Suba G. Bone marrow necrosis: a case report. Int J Sci Stud. 2015;2(10):163–165. [Google Scholar]
- 12.Chambers I, Truong P, Kallail K, Palko W. Extensive bone marrow necrosis and osteolytic lesions in a case of acute myeloid leukemia transformed from polycythemia vera. Cureus. 2016;8(6):e639. doi: 10.7759/cureus.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badar T, Shetty A, Bueso-Ramos C, Cortes J, Konopleva M, Borthalkur G, Pierce S, Huang X, Chen HC, Kadia T, Daver N, Dinardo C, O’Brien S, Garcia-Manero G, Kantarjian H, Ravandi F. Bone marrow necrosis in acute leukemia: clinical characteristic and outcome. Am J Hematol. 2015;90(9):769–773. doi: 10.1002/ajh.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niebrugge DJ, Benjamin DR. Bone marrow necrosis preceding acute lymphoblastic leukemia in childhood. Cancer. 1983;52(11):2162–2164. doi: 10.1002/1097-0142(19831201)52:11<2162::AID-CNCR2820521131>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Pui CH, Stass S, Green A. Bone marrow necrosis in children with malignant disease. Cancer. 1985;56(7):1522–1525. doi: 10.1002/1097-0142(19851001)56:7<1522::AID-CNCR2820560708>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 16.Macfarlane SD, Tauro GP. Acute lymphocytic leukemia in children presenting with bone marrow necrosis. Am J Hematol. 1986;22(4):341–346. doi: 10.1002/ajh.2830220402. [DOI] [PubMed] [Google Scholar]
- 17.Charache S, Page DL. Infarction of bone marrow in the sickle cell disorders. Ann Intern Med. 1967;67(6):1195–1200. doi: 10.7326/0003-4819-67-6-1195. [DOI] [PubMed] [Google Scholar]
- 18.Forrest DL, Mack BJ, Nevill TJ, Couban SH, Zayed E, Foyle A. Bone marrow necrosis in adult acute leukemia and non-Hodgkin’s lymphoma. Leuk Lymphoma. 2002;38(5–6):627–632. doi: 10.3109/10428190009059283. [DOI] [PubMed] [Google Scholar]
- 19.Eusni RM, Hamidah Hussin N, Zarina AL, Rahman J. Bone marrow necrosis preceding infantile acute lymphoblastic leukemia. Malays J Pathol. 2007;29(2):113–117. [PubMed] [Google Scholar]
- 20.Osuorji I, Goldman L. L. G-CSF-associated bone marrow necrosis in AML after induction chemotherapy. Case Rep Hematol. 2012;2012:314278. doi: 10.1155/2012/314278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lackner H, Strenger V, Sovinz P, Beham-Schmid C, Pilhatsch A, Benesch M, Schwinger W, Ulreich R, Schmidt S, Urban C. Bone marrow necrosis in a girl with Hodgkin’s disease. Support Care Cancer. 2012;20(9):2231–2234. doi: 10.1007/s00520-012-1502-z. [DOI] [PubMed] [Google Scholar]
- 22.Shafiq M, Ali N. Bone marrow necrosis—initial presentation in sickle cell anemia. Am J Case Rep. 2013;14:416–418. doi: 10.12659/AJCR.884020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoshnaw NS, Muhealdeen DN. Bone marrow necrosis in an adult patient with precursor B-cell acute lymphoblastic leukaemia at the time of presentation. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-203891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro R, Rizkalla K, Lam S. Extensive bone marrow necrosis in a case of acute myeloid leukemia transformed from a myeloproliferative neoplasm. Case Rep Oncol. 2015;8(2):345–348. doi: 10.1159/000438822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daskalakis M, Caballero M. Bone marrow necrosis in de novo AML. Blood. 2014;123(14):2137. doi: 10.1182/blood-2013-09-522672. [DOI] [PubMed] [Google Scholar]
- 26.Rashidi A, DiPersio JF, Westervelt P, Abboud CN, Romee R. Acute myeloid leukemia presenting with extensive bone marrow necrosis, leukemia cutis and testicular involvement: successful treatment with allogeneic hematopoietic stem cell transplantation. BMT. 2016;51(3):454–455. doi: 10.1038/bmt.2015.272. [DOI] [PubMed] [Google Scholar]
- 27.Khrizman P, Altman JK, Mohtashamian A, Peterson L, Chen YH, Tallman MS. Charcot Leyden crystals associated with acute myeloid leukemia: case report and literature review. Leuk Res. 2010;34(12):8–43. doi: 10.1016/j.leukres.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Wool GD, Deucher A. Bone marrow necrosis: ten-year retrospective review of bone marrow biopsy specimens. Am J Clin Pathol. 2015;143:201–213. doi: 10.1309/AJCP0TN1MCMOLMPK. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


