Abstract
Background
Hepatitis C virus (HCV) is a risk factor for chronic kidney disease (CKD) and end-stage renal disease (ESRD). Direct-acting antiviral agents (DAAs) have improved HCV management in CKD patients, however real-world clinical practice data are limited.
Objective
This study examined the prevalence of CKD among HCV patients receiving oral DAAs in a real-world setting. Comorbidities, early discontinuation rates, and healthcare costs were compared between patients with and without CKD.
Methods
Patients with HCV who were treated with oral DAAs between November 2013 and June 2015, and who were enrolled in a US health plan, were identified. Early discontinuation was calculated based on observed versus expected treatment duration, and expected treatment duration was based on genotype, initial treatment regimen, baseline cirrhosis, and prior treatments. Healthcare costs were calculated during the baseline, treatment, and post-treatment periods.
Results
This study included 3438 patients receiving oral DAAs, of whom 6.9% had a CKD diagnosis. CKD patients were more often male (70.8 vs. 62.9%, p = 0.02) and older (mean age 62.0 vs. 58.8 years, p < 0.001) than non-CKD patients, and had a higher prevalence of most comorbidities. Among early discontinuers, CKD patients were more likely to experience anemia (19.4 vs. 7.7%, p = 0.028).
Conclusions
Few patients with CKD receive DAA treatment for HCV infections. HCV patients with CKD had significantly more comorbidities and higher baseline healthcare costs than patients without CKD. Compared with non-CKD patients, CKD patients were equally likely to discontinue DAA treatment early but had higher rates of anemia. This study highlights the need for more renal-friendly HCV therapies.
Key Points
| Data from observational studies are necessary to bridge the gap between investigation and real-world practice regarding the use of direct-acting antiviral agents (DAAs) and the characteristics and comorbidities of patients prescribed these agents. |
| Few hepatitis C virus (HCV) patients with chronic kidney disease (CKD) [6.9%] received DAA treatment for HCV infections. |
| Patients with CKD had more comorbidities and higher baseline healthcare costs than patients without CKD. |
| While HCV/CKD patients were equally likely to discontinue treatment early compared with non-CKD patients, they experienced significantly higher rates of anemia and slightly higher rates of rash and gastrointestinal complications. |
Introduction
Hepatitis C virus (HCV) is a known risk factor for chronic kidney disease (CKD) and end-stage renal disease (ESRD) [1–4]. HCV is thought to trigger an immune cascade that attacks the kidneys, resulting in glomerulonephritis, and is also associated with insulin resistance and dyslipidemia [5]. In patients with HCV, other comorbidities, such as diabetes, hyperlipidemia, and cirrhosis, as well as male sex and age <50 years, increase the risk of developing CKD [2]. HCV infection in patients with CKD is associated with renal disease progression, and those with more severe CKD have a higher rate of positive anti-HCV antibodies [4]. HCV also increases the risk of developing ESRD, with a 5-year cumulative incidence rate of 52.6% compared with 38.4% in those without HCV [3]. In a study of patients with CKD, HCV infection was associated with decreased kidney function, a progressive loss of kidney function, and a higher mortality risk [6]. While survival of stage 1 and 2 CKD patients infected with HCV matches those without CKD [7], HCV in hemodialysis patients resulted in 1.35 times higher all-cause mortality and 3.82 times higher liver disease-related mortality than those without HCV [8]. HCV infection in CKD patients is also associated with increased healthcare costs and utilization, with further increases in those with ESRD [9].
Patients receiving hemodialysis are at a higher risk for HCV infection, with a prevalence estimate of 8% (based on HCV antibodies) in the US [10]. In dialysis patients, breaches in infection control practices can result in patient-to-patient transmission of HCV [11]; however, the majority of infections are a result of nosocomial transmission (e.g. contaminated multidose medication vials) [12, 13]. Prevalence of HCV in those receiving dialysis has been found to increase with longer hemodialysis duration, male sex, Black ethnicity, concurrent illness (e.g. diabetes, hepatitis B), prior kidney transplant, and alcohol or drug abuse [14].
The goal of HCV treatment is sustained virologic response (SVR), which is associated with reduced mortality and a reduced risk of cirrhosis and hepatocellular carcinoma [15, 16]. In patients awaiting renal transplants, HCV treatment slowed the development of liver disease and reduced the risk of HCV-related post-transplant complications such as diabetes [17] and chronic allograft nephropathy [18]. Treated transplant recipients also have lower rates of glomerulonephritis compared with those who are untreated [19]. The benefits of treatment may extend beyond the liver, with improvements in both cardiovascular and renal outcomes noted in one study of HCV-infected diabetes patients [20].
Historically, treatment of CKD patients with HCV has been pegylated interferon plus ribavirin [21]. In patients with compromised renal function, this regimen is associated with low rates of SVR and early discontinuation due to treatment-related side effects [22]. CKD patients often suffer from multiple comorbidities, making them poor candidates for pegylated interferon therapy. Additionally, pegylated interferon is contraindicated following kidney transplantation due to higher graft rejection rates [23, 24]. Consequently, few advanced CKD patients receive treatment for HCV. In fact, the Dialysis and Practice Patterns Study (DOPPS) found that while 9.5% of dialysis patients were positive for HCV antibodies, only 1% received antiviral therapy [25]. These major limitations highlight the need for interferon-free, renal-friendly, anti-HCV treatments.
Oral direct-acting antiviral agents (DAAs) were introduced in 2011 and have improved the management of HCV in patients with intact kidney function. Sofosbuvir/ledipasvir, sofosbuvir/simeprevir, and ombitasvir/paritaprevir/ritonavir plus dasabuvir have been FDA-approved since 2015 for genotype 1 HCV infections, while velpatasvir/sofosbuvir was recently approved in 2016. These agents have increased SVR rates to >90% and require shorter treatment durations, all with fewer side effects compared with pegylated interferon plus ribavirin [26]; however, few studies have been conducted demonstrating the safety and efficacy of DAAs in patients with CKD. One phase III clinical trial in the severe renal impairment population showed that 99% (n = 115/116) of HCV-infected patients with stage 4–5 CKD treated with grazoprevir/elbasvir achieved SVR and had a low rate of adverse events [27]. Similarly, of patients with stage 4 CKD taking a ombitasvir/paritaprevir/ritonavir regimen, 90% (n = 18/20) experienced SVR, with no reported discontinuations due to treatment side effects [28].
While no large-scale studies have been conducted on sofosbuvir-containing regimens in patients with advanced stage CKD, case series of patients treated with sofosbuvir/simeprevir have shown SVR rates of 89% (n = 8/9) [29] and 100% (n = 17/17) [30]. In the HCV-TARGET study, patients with varying baseline renal function were treated with sofosbuvir-containing regimens [31]. SVR was 88% (n = 15/17) for those with estimated glomerular filtration rate (eGFR) <30 mL/min, and anemia, worsening renal function, and severe adverse events were more frequent in those with reduced kidney function than those with intact function. Despite the promise these results show, physicians still face challenges managing patients with HCV and compromised renal function. Results from clinical trials do not always match outcomes in real-world clinical practice. Data from observational studies are necessary to bridge the gap between investigation and real-world practice regarding the use of DAAs and the characteristics and comorbidities of the patients prescribed them. This study aimed to estimate the prevalence of CKD among HCV patients receiving oral DAAs in a real-world setting. Additionally, comorbidities, early discontinuation rates, potential side effects, and healthcare costs were compared between patients with HCV infection with and without CKD treated with all-oral DAAs.
Methods
Study Design and Data Source
This was a retrospective study using medical and pharmacy claims data, enrollment information, and linked laboratory results from two US administrative health plan databases—the Optum Research Database (ORD) and the Impact National Benchmark Database (Impact). The ORD includes enrollment information, medical and pharmacy claims, and linked laboratory test results for approximately 14 million enrollees in commercial plans and 3 million enrollees in Medicare Advantage with Part D plans annually. The ORD is geographically diverse and representative of the US commercially insured population. The Impact database contains enrollment information, medical and pharmacy claims, and linked laboratory test results for approximately 29.2 million people from 2004 to the present, collected from 16 different healthcare plans serving members across nine census regions. Data extracted for each patient spanned 1 November 2013 through 30 June 2015. Medical claims data included International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, Healthcare Common Procedure Coding System (HCPCS) codes, revenue codes, and paid amounts (combined health plan plus patient paid amounts). Pharmacy claims data included National Drug Codes for filled prescriptions, days supplied, quantity of drug supplied, and paid amounts. Linked outpatient laboratory results were available for a subset of the research database. All study data were accessed using techniques compliant with the Health Insurance Portability and Accountability Act.
Study Population
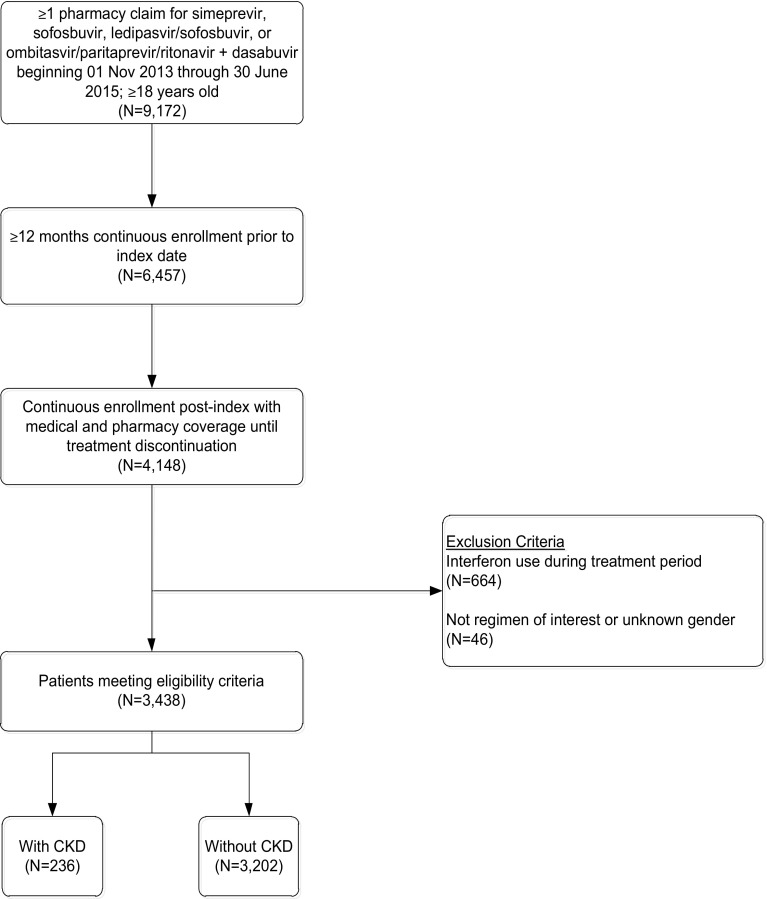
To be included in the study, patients were required to have one or more pharmacy claims for a DAA from 1 November 2013 through 30 June 2015 (Fig. 1). The index date was defined as the pharmacy claim for the most recent DAA treatment regimen during the patient identification period. Patients were required to have continuous enrollment with medical and pharmacy coverage at least 12 months pre-index (baseline period) through treatment discontinuation, and be at least 18 years of age as of the index year. The post-treatment period was initiated based on the run-out of days’ supply of the last prescription fill. Patients with interferon use during the treatment period were excluded from the study. Patients were divided into two cohorts based on whether or not they had a diagnosis of CKD.
Fig. 1.
Patient sample identification. CKD chronic kidney disease
Study Measures
Patient Characteristics
Age, sex, geographic location, health plan type (commercial, Medicare Advantage with Part D prescription coverage), length of pre-index period, and CKD stage were reported as of the index date. HCV genotype and subtype were captured for those with available laboratory results. If multiple genotypes were recorded, the last genotype result measured during the study period was used. The Quan–Charlson comorbidity score was calculated during the pre-index period. The presence of clinically relevant conditions/events during the pre-index period was identified based on ICD-9-CM diagnosis (070.X for HCV and 585.X for CKD) and procedure codes from pre-index medical claims. The oral DAA regimen was determined by pharmacy and medical claims and categorized as the presence/absence of any medication and as counts of individual medications. The following regimens were examined: sofosbuvir/simeprevir, sofosbuvir/simeprevir plus ribavirin, sofosbuvir plus ribavirin, ledipasvir/sofosbuvir, ledipasvir/sofosbuvir plus ribavirin, and ombitasvir/paritaprevir/ritonavir plus dasabuvir ± ribavirin.
Early Discontinuation
Discontinuation was defined as a gap in therapy of ≥30 days for the first gap and ≥14 days for subsequent gaps. The date of discontinuation was defined by the run-out of days’ supply of the last prescription filled prior to the gap in therapy. Discontinuation was determined for each treatment regimen; patients who received ombitasvir/paritaprevir/ritonavir plus dasabuvir were not included in the analysis of early discontinuation due to the small sample size. Patients were removed from analyses of treatment discontinuation at the time they disenrolled from the health plan.
Patients were considered to have discontinued early if the observed treatment duration was shorter than the expected treatment duration. The expected treatment duration was based on 2015 Infectious Diseases Society of America/American Association for the Study of Liver Diseases (IDSA/AASLD) treatment guidelines accounting for HCV genotype, HCV RNA level, treatment status (naive or experienced for 1 or more years), and baseline cirrhosis diagnosis [32]. If genotype was unavailable, expected duration was based on observed treatment duration, treatment regimen, cirrhosis diagnosis, and treatment status (naive or experienced).
The observed treatment duration was calculated from the sum of the total number of days with medication supply for oral DAAs containing sofosbuvir until discontinuation. The date of treatment initiation for each HCV antiviral medication taken during the treatment episode was identified separately and overall for the episode. Among patients who discontinued early, potential side effects were identified in the 4 weeks prior to treatment discontinuation. Potential side effects included anemia, rash, gastrointestinal symptoms (diarrhea and nausea), headache, fatigue, and insomnia, and were identified based on ICD-9-CM diagnosis and procedure codes from the medical claims.
Costs
Healthcare costs were calculated for the baseline, treatment, and post-treatment periods and reported as per-patient-per-month (PPPM). All-cause healthcare costs were calculated as total costs (sum of medical and pharmacy costs) and medical costs (sum of ambulatory, emergency, inpatient, and other medical costs); all costs were inflation-adjusted to 2014 US dollars using the Medical care component from the Consumer Price Index [33].
Statistical Analyses
All study variables were analyzed descriptively. Numbers and percentages were provided for dichotomous and polychotomous variables, while means, medians, and standard deviations were provided for continuous variables. Results were stratified by CKD status, where available. Bivariate comparisons of pre-index characteristics and outcome measures were provided by CKD status, and appropriate tests (e.g. t test, Chi-square test) were used based on the distribution of the measure. Generalized linear models of total, medical, and pharmacy costs during the treatment and post-treatment periods were conducted using a gamma distribution with a log link, and adjusting for baseline costs, observed treatment regimen and duration, patient demographics (age category, sex, insurance type), comorbidities (Quan–Charlson comorbidity score, cirrhosis, HIV, diabetes, cardiovascular disorder, hypertension, hepatitis B, drug abuse, and liver transplant), and prior HCV treatment.
Results
After inclusion and exclusion criteria were applied, the final study sample represented 3438 patients receiving oral DAAs. Approximately 54% of patients had at least 36 months of baseline follow-up. The prevalence of CKD among HCV patients receiving oral DAAs was 6.9% (n = 236) (Fig. 1). In those with known CKD stage, 18.3% were categorized as stage 1 or 2, 52.7% were stage 3, and 29.0% were stage 4 or more advanced (Table 1). Compared with HCV patients without CKD, HCV patients with CKD were more often male (70.8 vs. 62.9%, p = 0.02) and older (mean age 62.0 vs. 58.8 years, p < 0.001). HCV/CKD patients also had higher Quan–Charlson comorbidity scores (6.4 vs. 3.7, p < 0.001), with more frequent diagnoses of anemia (50.0 vs. 17.0%, p < 0.001), cardiovascular disorder (39.4 vs. 14.2%, p < 0.001), cirrhosis or end-stage liver disease [ESLD] (66.5 vs. 50.4%, p < 0.001), chronic obstructive pulmonary disease (COPD)/asthma (55.1 vs. 40.8%, p < 0.001), hypertension (93.6 vs. 63.7%, p < 0.001), diabetes (61.9 vs. 23.4%, p < 0.001), liver transplant (29.7 vs. 4.7%, p < 0.001), fatigue (41.5 vs. 27.6%, p < 0.001), gastrointestinal complications (58.9 vs. 32.4%, p < 0.001), heart failure/rheumatic heart disease (92.4 vs. 67.2%, p < 0.001), hepatitis B (14.0 vs. 7.0%, p < 0.001), hepatocellular carcinoma (13.6 vs. 4.6%, p < 0.001), depression (45.3 vs. 36.0%, p < 0.001), and drug abuse (26.3 vs. 20.1%, p = 0.030) compared with their non-CKD counterparts. Patients were most commonly treated with ledipasvir/sofosbuvir, sofosbuvir + simeprevir, and sofosbuvir + ribavirin (Table 2), with an average treatment length of 3.0 months for CKD patients and 3.1 months for non-CKD patients.
Table 1.
Baseline patient demographics and clinical characteristics
| Demographics | Valid n | HCV with CKD [n = 236] | Valid n | HCV-only [n = 3202] | p value |
|---|---|---|---|---|---|
| Male sex | 236 | 167 (70.76) | 3202 | 2013 (62.87) | 0.02 |
| Age, years [mean (SD)] | 236 | 61.96 (7.74) | 3202 | 58.76 (9.50) | <0.001 |
| Geographic region | 236 | 3202 | |||
| Northeast | 38 (16.10) | 765 (23.89) | 0.007 | ||
| Midwest | 52 (22.03) | 589 (18.39) | 0.166 | ||
| South | 117 (49.58) | 1374 (42.91) | 0.049 | ||
| West | 29 (12.29) | 474 (14.80) | 0.340 | ||
| Insurance type | 236 | 3202 | |||
| Commercial | 106 (44.92) | 2018 (63.02) | <0.001 | ||
| Medicare Advantage | 130 (55.08) | 1184 (36.98) | <0.001 | ||
| Pre-index observation period, months | 236 | 3202 | |||
| ≥12 to <18 | 40 (16.95) | 460 (14.37) | 0.292 | ||
| ≥18 to <24 | 23 (9.75) | 340 (10.62) | 0.743 | ||
| ≥24 to <36 | 46 (19.49) | 611 (19.08) | 0.864 | ||
| ≥36 | 127 (53.81) | 1791 (55.93) | 0.541 | ||
| CKD stage | 186 | – | |||
| 1 | 8 (4.30) | – | – | ||
| 2 | 26 (13.98) | – | – | ||
| 3 | 98 (52.69) | – | – | ||
| 4 | 13 (6.99) | – | – | ||
| 5 | 2 (1.08) | – | – | ||
| ESRD | 39 (20.97) | – | – | ||
| Prior treatment status | 236 | 3202 | |||
| Treatment-experienced | 64 (27.12) | 780 (24.36) | 0.342 | ||
| Genotype | 73 | 1141 | |||
| 1 | 56 (76.71) | 895 (78.44) | 0.162 | ||
| 2 | 10 (13.70) | 172 (15.07) | 0.453 | ||
| 3 | 7 (9.59) | 65 (5.70 | 0.332 | ||
| 4 | 0 (0.00) | 6 (0.53) | 0.506 | ||
| 6 | 0 (0.00) | 1 (0.09) | 0.786 | ||
| Multiple | 0 (0.00) | 2 (0.18) | 0.701 | ||
| Quan–Charlson comorbidity score [mean (SD)] | 236 | 6.38 (2.47) | 3202 | 3.67 (2.11) | <0.001 |
| Comorbidities | 236 | 3202 | |||
| Alcohol abuse | 19 (8.05) | 210 (6.56) | 0.346 | ||
| Anemia and treatment | 118 (50.00) | 543 (16.96) | <0.001 | ||
| Anxiety | 88 (37.29) | 1046 (32.67) | 0.152 | ||
| Cardiovascular disorder | 93 (39.41) | 456 (14.24) | <0.001 | ||
| Cirrhosis | 106 (44.92) | 1064 (33.23) | <0.001 | ||
| COPD/asthma | 130 (55.08) | 1307 (40.82) | <0.001 | ||
| Decompensated cirrhosis/ESLD | 82 (34.75) | 611 (19.08) | <0.001 | ||
| Depression | 107 (45.34) | 1151 (35.95) | 0.005 | ||
| Diabetes | 146 (61.86) | 748 (23.36) | <0.001 | ||
| Drug abuse | 62 (26.27) | 645 (20.14) | 0.030 | ||
| Fatigue | 98 (41.53) | 883 (27.58) | <0.001 | ||
| GI complications | 139 (58.90) | 1038 (32.42) | <0.001 | ||
| Heart failure/rheumatic heart disease | 218 (92.37) | 2153 (67.24) | <0.001 | ||
| Hepatitis B | 33 (13.98) | 223 (6.96) | <0.001 | ||
| Hepatocellular carcinoma | 32 (13.56) | 148 (4.62) | <0.001 | ||
| History of liver transplant | 70 (29.66) | 149 (4.65) | <0.001 | ||
| HIV | 34 (14.41) | 376 (11.74) | 0.213 | ||
| Hypertension | 221 (93.64) | 2041 (63.74) | <0.001 | ||
| Total baseline healthcare costs [mean (SD)] | 236 | 5481.08 (12,651.96) | 3202 | 1922.16 (3768.85) | <0.001 |
| Pharmacy costs | 1195.40 (2222.60) | 812.45 (2110.52) | 0.007 | ||
| Medical costs | 4285.68 (12,359.18) | 1109.71 (2850.60) | <0.001 |
Data are expressed as n (%) unless otherwise specified
CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, ESLD end-stage liver disease, ESRD end-stage renal disease, GI gastrointestinal, HCV hepatitis C virus, SD standard deviation
Table 2.
Distribution of treatment regimen among the HCV-infected patients with CKD and HCV-only cohorts
| Treatment regimen | HCV with CKD [n = 236] | HCV-only [n = 3202] |
|---|---|---|
| Sofosbuvir/simeprevir | 75 (31.78) | 835 (26.08) |
| Sofosbuvir/simeprevir + ribavirin | 10 (4.24) | 127 (3.97) |
| Sofosbuvir + ribavirin | 54 (22.88) | 804 (25.11) |
| Ledipasvir/sofosbuvir | 89 (37.71) | 1394 (43.54) |
| Ledipasvir/sofosbuvir + ribavirin | 5 (2.12) | 30 (0.94) |
| Ombitasvir/paritaprevir/ritonavir + dasabuvir | 3 (1.27) | 12 (0.37) |
Data are expressed as n (%)
CKD chronic kidney disease, HCV hepatitis C virus
Slightly more HCV patients with CKD discontinued DAA treatment early compared with HCV-only patients; however, the results were not statistically significant (15.6% [n = 31/199] compared with 12.0% [n = 324/2695], p = 0.140) (Table 3). Additionally, CKD stage did not impact early discontinuation rates (17.7% in stage 1–3 vs. 8.3% in stage 4 ESRD, p = 0.133). While patients with and without CKD were equally likely to discontinue early, more patients with CKD had reported potential side effects. Insomnia and gastrointestinal symptoms were most commonly reported overall (Table 4). Patients with CKD were significantly more likely to experience anemia compared with those without CKD (19.4 vs. 7.7%, p = 0.028), while gastrointestinal symptoms (32.3 vs. 17.9%, p = 0.052) and rash (22.6 vs. 11.1%, p = 0.062) trended towards significance.
Table 3.
Adherence to expected treatment durationa,b
| Total [n = 2894] | HCV with CKD [n = 199] | HCV-only [n = 2695] | p value | |
|---|---|---|---|---|
| Discontinued early | 355 (12.27) | 31 (15.58) | 324 (12.02) | 0.140 |
| Completed as expected | 2432 (84.04) | 159 (79.90) | 2273 (84.34) | 0.099 |
| Extended beyond expected | 107 (3.70) | 9 (4.52) | 98 (3.64) | 0.523 |
Data are expressed as n (%)
CKD chronic kidney disease, HCV hepatitis C virus
aIncludes only those patients with known expected treatment duration
bPatients who received ombitasvir/paritaprevir/ritonavir were excluded from the analysis due to the small sample size
Table 4.
Potential side effects among patients who discontinued DAA therapy earlya
| HCV with CKD [n = 31] | HCV-only [n = 324] | p value | |
|---|---|---|---|
| Any potential side effect | 17 (54.84) | 149 (45.99) | 0.345 |
| Anemia | 6 (19.35) | 25 (7.72) | 0.028 |
| Rash | 7 (22.58) | 36 (11.11) | 0.062 |
| Gastrointestinal | 10 (32.26) | 58 (17.90) | 0.052 |
| Fatigue | 1 (3.23) | 27 (8.33) | 0.313 |
| Insomnia | 11 (35.48) | 72 (22.22) | 0.096 |
| Headache | 0 (0.00) | 9 (2.78) | 0.347 |
Data are expressed as n (%)
CKD chronic kidney disease, DAA direct-acting antiviral, HCV hepatitis C virus
aPatients who received ombitasvir/paritaprevir/ritonavir were excluded from the analysis due to the small sample size
Total unadjusted PPPM healthcare costs in the baseline period were higher in HCV/CKD patients than HCV-only patients ($5481 vs. $1922, p < 0.001), with medical costs almost four times higher ($4286 vs. $1110, p < 0.001) in patients with CKD compared with those without (Table 1). Total healthcare costs were also higher among HCV/CKD patients than HCV-only patients during both the treatment and post-treatment periods (average post-treatment follow-up was 5.8 months in CKD patients and 5.3 months in non-CKD patients). Compared with HCV-only patients, patients with CKD experienced higher PPPM pharmacy costs during the post-treatment period ($793 vs. $454, p = 0.005), but experienced similar costs during the treatment period ($39,988 vs. $38,773, p = 0.083), representative of the cost of DAA therapy. Medical costs were double in the treatment period ($2365 vs. $1069, p < 0.001) and four times higher in the post-treatment period ($4087 vs. $958, p = 0.023) compared with patients without CKD (Table 5). While total adjusted PPPM healthcare costs in the post-treatment period were higher among HCV/CKD patients than HCV-only patients ($2056 vs. $1526), the results were not statistically significant (cost ratio = 1.35, p = 0.105) (Table 5). Significant predictors of higher costs were higher baseline costs, a sofosbuvir + simeprevir 12-week regimen (cost ratio = 1.36, p = 0.012), all durations of sofosbuvir + simeprevir + ribavirin (cost ratio = 1.85, p = 0.007), a sofosbuvir + ribavirin 8-week regimen (cost ratio = 2.214, p = 0.034), cirrhosis (cost ratio = 1.264, p = 0.020), and HIV infection (cost ratio = 1.864, p = 0.014), while prior HCV treatment was associated with lower costs (cost ratio = 0.698, p < 0.001) in the post-treatment period (data not shown). Adjusted total costs in the treatment period ($40,383 vs. $39,988, cost ratio = 1.01, p = 0.352) were also not significantly different for HCV/CKD patients versus HCV-only patients (Table 5). Higher baseline costs were significantly associated with higher total costs, as were HIV infection (cost ratio = 1.031, p = 0.038) and diabetes (cost ratio = 1.014, p = 0.025), while liver transplantation (cost ratio = 0.976, p = 0.047) and prior HCV treatment (cost ratio = 0.987, p = 0.038) were associated with lower total costs during the treatment period (data not shown).
Table 5.
Unadjusted and adjusted PPPM treatment and post-treatment period healthcare costs (US$)
| Costs (PPPM) | Unadjusted | p value | Adjusted | p value | ||
|---|---|---|---|---|---|---|
| HCV with CKD [n = 236] | HCV-only [n = 3202] | HCV with CKD [n = 236] | HCV-only [n = 3202] | |||
| Treatment-period costs | ||||||
| Total healthcare costs | 42,353 | 39,842 | 0.003 | 40,383 | 39,988 | 0.352 |
| Medical costs | 2365 | 1069 | <0.001 | 1432 | 1112 | 0.228 |
| Pharmacy costs | 39,988 | 38,773 | 0.083 | 38,749 | 38,865 | 0.704 |
| Total HCV-related costs | 39,775 | 38,668 | 0.134 | 38,650 | 38,751 | 0.784 |
| Medical costs | 647 | 403 | 0.201 | 504 | 378 | 0.260 |
| Pharmacy costs | 39,128 | 38,264 | 0.217 | 38,236 | 38,330 | 0.750 |
| Post-treatment costs | ||||||
| Total healthcare costs | 4879 | 1412 | 0.012 | 2056 | 1526 | 0.105 |
| Medical costs | 4087 | 958 | 0.023 | 1488 | 1026 | 0.123 |
| Pharmacy costs | 793 | 454 | 0.005 | 436 | 478 | 0.525 (0.559)a |
| Total HCV-related costs | 2356 | 279 | 0.107 | 438 | 280 | 0.171 |
| Medical costs | 2356 | 279 | 0.107 | 438 | 280 | 0.171 |
| Pharmacy costs | 0 | 0 | – | 0 | 0 | – |
CKD chronic kidney disease, HCV hepatitis C virus, PPPM per-patient-per-month
a14.1% of individuals had zero post-treatment pharmacy costs. Adjusted costs were calculated using a two-part (logistic/gamma) model. Expected costs were calculated at the individual level [P positive cost × predicted cost]. Adjusted costs are the mean of these expected costs
Discussion
This retrospective study evaluated patient characteristics, discontinuation, and costs in CKD patients infected with HCV and treated with oral DAAs. Our results provide important insights into this difficult-to-treat population in a real-world setting. A relatively low number of patients with HCV and CKD were treated with oral DAAs. CKD patients were older and sicker than their non-CKD counterparts, with higher rates of cirrhosis and ESLD, anemia, cardiovascular disorder, fatigue, heart failure/rheumatic heart disease, COPD/asthma, hypertension, diabetes, liver transplant, hepatitis B, hepatocellular carcinoma, and depression. While patients with and without CKD were equally likely to discontinue treatment early, CKD patients were more likely to experience potential side effects, particularly anemia and GI symptoms. Actual healthcare costs during the treatment and post-treatment periods were significantly higher among CKD patients than non-CKD patients; however, after adjustment for baseline costs, DAA treatment regimen and duration, as well as patient characteristics and comorbidities, costs during the treatment and post-treatment periods were no longer different between the cohorts.
During the study period (November 2013 to June 2015), 6.9% of CKD co-infected HCV patients were treated with oral DAAs, which is lower than prevalence estimates reported in previous studies. In a study in Taiwan, 16.5% of study participants seropositive for HCV had CKD [1], while a US study found the unadjusted prevalence of HCV infection in patients receiving hemodialysis to be 14.4% [14]. Similarly, a recent study of HCV patients reported 14.4% had stage 3–5 CKD [34]. Patients with more advanced CKD have higher rates of HCV infection, with one study reporting a prevalence of 8.5% in those with stage 1 CKD and 14.5% in those with stages 4–5 [4]. Our study included patients with varying renal function, not just those with advanced CKD, which may partially explain the lower prevalence we observed. Additionally, our study included only patients treated with oral DAAs, indicating that a relatively low number of CKD patients infected with HCV are treated with oral DAAs.
In this study, patients with CKD were slightly more likely to discontinue treatment early than non-CKD patients (15.6 vs. 12.0%); however, the results were not significant. This is similar to findings from the HCV-TARGET study, with slightly higher early discontinuation rates in patients with eGFR <45 mL/min (4.1 vs. 2.5%, p = nonsignificant) [31]. The early discontinuation rates reported in this study are higher than those reported in previous case series and clinical trial results. This is likely a direct result of the definition based on AASLD guidelines used to identify the expected treatment duration in the claims data. Since early discontinuation was calculated comparing the observed treatment duration with the expected treatment duration, patients were categorized as being an early discontinuer compared with guidelines.
While HCV patients with and without CKD were equally likely to discontinue early, more patients with CKD were anemic. In those who discontinued early, evidence of anemia was observed in 19.4% of CKD patients and 7.7% of those without CKD (p = 0.028). While not significant, CKD patients also more frequently experienced gastrointestinal symptoms (32.3 vs. 17.9%, p = 0.052) and rash (22.6 vs. 11.1%, p = 0.062) compared with non-CKD patients, which may be clinically relevant. Similarly, the HCV-TARGET study also found higher rates of treatment side effects among patients with renal insufficiency (eGFR <45 mL/min) than those with normal renal function (21.9 vs. 6.3%, p < 0.001). Even after removing regimens with ribavirin, a higher rate of anemia (10.0 vs. 0.01%) and worsening renal function (20.0 vs. 0.01%) was still found in those with an eGRF <45 mL/min [31]. Anemia, as a result of decreased erythropoietin production, and nausea are both known complications of CKD and it is unknown whether they were caused by treatment with DAAs alone.
In this study, HCV patients with CKD had significantly higher healthcare costs than patients without CKD ($5481 vs. $1922) following treatment with DAAs. These results are similar to a recent study comparing costs in HCV patients with and without ESRD CKD with costs in patients without CKD in a commercially insured and Medicare population [9]. Costs during the 1-year follow-up period were significantly higher in commercially insured patients with non-ESRD CKD ($3720) and ESRD ($8117) compared with patients with no CKD ($1085). Patients with Medicare Advantage insurance experienced a similar pattern of costs.
Considering the incremental burden of CKD from HCV along with hepatic and extrahepatic manifestations, more renal-friendly treatment of HCV is imperative. Recently, the 2016 AASLD guidelines recommended additional DAA treatment options for patients with renal impairment, including those with severe renal impairment and ESRD [32]. These treatment options should take into consideration the higher prevalence of critical comorbidities identified in this study that can impact adherence to treatment, including adverse events while receiving treatment. These additional comorbidities highlight the need to identify CKD patients as a special population that may require additional monitoring to achieve safe and effective HCV treatment outcomes.
Limitations
Claims data offer the advantage of large sample sizes of patients with diverse medical histories; however, certain limitations inherent to claims-based analyses should be considered when interpreting the results of this study. The presence of a medical or pharmacy claim is not proof-positive for the presence of disease or that the medication was consumed or taken as prescribed. Cost estimates using data aggregated from claims paid by a single health payer may underestimate total direct healthcare expenditures for patients with multiple payers (e.g. Medicare, managed care beneficiaries) and do not include information relating to non-medical costs associated with patient or societal expenditures, such as transportation for treatment or missed work days. Misclassification may exist because baseline medical history information was limited to at least 1 year; however, more than half of the patients had at least 3 years of baseline follow-up. Treatment-naive status may be misidentified as the definition relies on the absence of evidence of prior treatments in the claims data, impacting the determination of treatment duration. There are limitations as to the generalizability of the results of this study in that the study data are from a commercial and Medicare Advantage population and may not be generalizable to other populations. Lastly, our inability to detect deaths, particularly in this advanced population, may have resulted in misclassification of some patients as early discontinuers.
Conclusions
Standard treatment with pegylated interferon and ribavirin is associated with a host of complications in patients with renal impairment. The introduction of DAAs has provided an interferon-free treatment option that has shown higher rates of SVR with fewer potential side effects than interferon therapy in clinical trial data for this population; however, distinct recommendations in support of DAAs in renal-insufficient patients did not exist. Patients with CKD had more comorbidities and higher baseline healthcare costs than patients without CKD. While HCV/CKD patients were equally likely to discontinue DAA treatment early compared with non-CKD patients, they experienced significantly higher rates of anemia and slightly higher rates of rash and gastrointestinal complications that may be clinically relevant. This study highlights the need for more renal-friendly HCV therapies.
Compliance with Ethical Standards
Conflicts of interest
Michael Hull, Jeffrey McPheeters, and Kay Schwebke are employees of Optum, while Amy Puenpatom is an employee of Merck & Co., Inc.
Funding
This study was funded in full by Merck & Co., Inc. Writing support was provided by Deja Scott-Shemon of Optum and funded by Merck & Co., Inc.
Compliance with ethics guidelines
No identifiable protected health information was used in this study, and thus it did not require Institutional Review Board approval or waiver of authorization.
References
- 1.Li WC, Lee YY, Chen IC, Wang SH, Hsiao CT, Loke SS. Age and gender differences in the relationship between hepatitis C infection and all stages of Chronic kidney disease. J Viral Hepat. 2014;21(10):706–715. doi: 10.1111/jvh.12199. [DOI] [PubMed] [Google Scholar]
- 2.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85(5):1200–1207. doi: 10.1038/ki.2013.455. [DOI] [PubMed] [Google Scholar]
- 3.Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, et al. Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis. PloS One. 2014;9(6):e100790. doi: 10.1371/journal.pone.0100790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ. Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: a cross-sectional study. Am J Kidney Dis. 2010;56(1):23–31. doi: 10.1053/j.ajkd.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Dai CY, Yeh ML, Huang CF, Hou CH, Hsieh MY, Huang JF, et al. Chronic hepatitis C infection is associated with insulin resistance and lipid profiles. J Gastroenterol Hepatol. 2015;30(5):879–884. doi: 10.1111/jgh.12313. [DOI] [PubMed] [Google Scholar]
- 6.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, et al. Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology. 2015;61(5):1495–1502. doi: 10.1002/hep.27664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: a link with cardiovascular mortality? J Viral Hepat. 2012;19(9):601–607. doi: 10.1111/j.1365-2893.2012.01633.x. [DOI] [PubMed] [Google Scholar]
- 9.Solid CA, Peter SA, Natwick T, Guo H, Collins AJ, Arduino JM. Impact of renal disease on patients with hepatitis C: a retrospective analysis of disease burden, clinical outcomes, and health care utilization and cost. Nephron. 2017 doi: 10.1159/000454684. [DOI] [PubMed] [Google Scholar]
- 10.Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52–61. doi: 10.1111/j.1525-139X.2005.18108.x. [DOI] [PubMed] [Google Scholar]
- 11.Rao AK, Luckman E, Wise ME, MacCannell T, Blythe D, Lin Y, et al. Outbreak of hepatitis C virus infections at an outpatient hemodialysis facility: the importance of infection control competencies. Nephrol Nurs J. 2013;40(2):101–110. [PubMed] [Google Scholar]
- 12.Savey A, Simon F, Izopet J, Lepoutre A, Fabry J, Desenclos JC. A large nosocomial outbreak of hepatitis C virus infections at a hemodialysis center. Infect Control Hosp Epidemiol. 2005;26(9):752–760. doi: 10.1086/502613. [DOI] [PubMed] [Google Scholar]
- 13.Arenas MD, Sanchez-Paya J, Munoz C, Egea JJ, Martin F, Gil MT, et al. Nosocomial transmission of the hepatitis C virus in hemodialysis: monitors, personnel, or both? [in Spanish] Nefrologia. 2001;21(5):476–484. [PubMed] [Google Scholar]
- 14.Fissell RB, Bragg-Gresham JL, Woods JD, Jadoul M, Gillespie B, Hedderwick SA, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: the DOPPS. Kidney Int. 2004;65(6):2335–2342. doi: 10.1111/j.1523-1755.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 15.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45(3):579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 16.Hung CH, Lee CM, Lu SN, Wang JH, Hu TH, Tung HD, et al. Long-term effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. J Viral Hepat. 2006;13(6):409–414. doi: 10.1111/j.1365-2893.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- 17.Gursoy M, Guvener N, Koksal R, Karavelioglu D, Baysal C, Ozdemir N, et al. Impact of HCV infection on development of posttransplantation diabetes mellitus in renal allograft recipients. Transplant Proc. 2000;32(3):561–562. doi: 10.1016/S0041-1345(00)00890-3. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud IM, Sobh MA, El-Habashi AF, Sally ST, El-Baz M, El-Sawy E, et al. Interferon therapy in hemodialysis patients with chronic hepatitis C: study of tolerance, efficacy and post-transplantation course. Nephron Clin Pract. 2005;100(4):c133–c139. doi: 10.1159/000085442. [DOI] [PubMed] [Google Scholar]
- 19.Cruzado JM, Casanovas-Taltavull T, Torras J, Baliellas C, Gil-Vernet S, Grinyo JM. Pretransplant interferon prevents hepatitis C virus-associated glomerulonephritis in renal allografts by HCV-RNA clearance. Am J Transplant. 2003;3(3):357–360. doi: 10.1034/j.1600-6143.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YC, Lin JT, Ho HJ, Kao YH, Huang YT, Hsiao NW, et al. Antiviral treatment for hepatitis C virus infection is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology. 2014;59(4):1293–1302. doi: 10.1002/hep.26892. [DOI] [PubMed] [Google Scholar]
- 21.Omata M, Kanda T, Yu ML, Yokosuka O, Lim SG, Jafri W, et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6(2):409–435. doi: 10.1007/s12072-012-9342-y. [DOI] [PubMed] [Google Scholar]
- 22.Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of hepatitis C in chronic kidney disease. Kidney Int Suppl. 2008;109:S1–S99. doi: 10.1038/ki.2008.81. [DOI] [PubMed] [Google Scholar]
- 23.Baid S, Tolkoff-Rubin N, Saidman S, Chung R, Williams WW, Auchincloss H, et al. Acute humoral rejection in hepatitis C-infected renal transplant recipients receiving antiviral therapy. Am J Transplant. 2003;3(1):74–78. doi: 10.1034/j.1600-6143.2003.30113.x. [DOI] [PubMed] [Google Scholar]
- 24.Weclawiack H, Kamar N, Mehrenberger M, Guilbeau-Frugier C, Modesto A, Izopet J, et al. Alpha-interferon therapy for chronic hepatitis C may induce acute allograft rejection in kidney transplant patients with failed allografts. Nephrol Dial Transplant. 2008;23(3):1043–1047. doi: 10.1093/ndt/gfm678. [DOI] [PubMed] [Google Scholar]
- 25.Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am J Nephrol. 2013;38(5):405–412. doi: 10.1159/000355615. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez JA, Lawitz EJ, Poordad F. Interferon-free, direct-acting antiviral therapy for chronic hepatitis C. J Viral Hepat. 2015;22(11):861–870. doi: 10.1111/jvh.12422. [DOI] [PubMed] [Google Scholar]
- 27.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386(10003):1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 28.Pockros P, Reddy K, Mantry P, et al. Safety of ombitasvir/paritaprevir/ritonavir plus dasabuvir for treating HCV GT1 infection in patients with severe renal impairment or end-stage renal disease: the RUBY-I study [abstract] J Hepatol. 2015;62:S257. doi: 10.1016/S0168-8278(15)30147-1. [DOI] [Google Scholar]
- 29.Czul F, Schiff E, Peyton A, Levy C, Hernandez M, Jeffers L, et al. First ribavirin-free sofosbuvir and simeprevir treatment of hepatitis C genotype 1 patients with severe renal impairment (GFR <30 mL/min) or dialysis [abstract] J Hepatol. 2015;62:S670–S671. doi: 10.1016/S0168-8278(15)31080-1. [DOI] [Google Scholar]
- 30.Nazario HE, Ndungu M, Modi AA. Sofosbuvir and simeprevir in hepatitis C genotype 1- patients with end-stage renal disease on hemodialysis or GFR <30 mL/min. Liver Int. 2016;36:798–801. doi: 10.1111/liv.13025. [DOI] [PubMed] [Google Scholar]
- 31.Saxena V, Koraishy FM, Sise ME, Kim JK, Schmidt M, Chung RT, et al. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C infected patients with reduced renal function. Liver Int. 2016;36:807–816. doi: 10.1111/liv.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Association for the Study of Liver Diseases/Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://hcvguidelines.org/sites/default/files/HCV-Guidance_October_2016_a.pdf. Accessed 27 Oct 2016. [DOI] [PMC free article] [PubMed]
- 33.US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Medical Care. Series ID: SUUR0000SAM. 2012. http://data.bls.gov/cgi-bin/surveymost?su. Accessed 20 Jan 2016.
- 34.Butt AA, Yan P, Shaikh OS, Chung RT, Sherman KE, ERCHIVES study Sofosbuvir-based regimens in clinical practice achieve SVR rates closer to clinical trials: results from ERCHIVES. Liver Int. 2016;36(5):651–658. doi: 10.1111/liv.13036. [DOI] [PubMed] [Google Scholar]