Abstract
Genes introduced into higher plant genomes can become silent (gene silencing) and/or cause silencing of homologous genes at unlinked sites (homology-dependent gene silencing or HDG silencing). Mutations of the HOMOLOGY-DEPENDENT GENE SILENCING1 (HOG1) locus relieve transcriptional gene silencing and methylation-dependent HDG silencing and result in genome-wide demethylation. The hog1 mutant plants also grow slowly and have low fertility and reduced seed germination. Three independent mutants of HOG1 were each found to have point mutations at the 3′ end of a gene coding for S-adenosyl-l-homocysteine (SAH) hydrolase, and hog1-1 plants show reduced SAH hydrolase activity. A transposon (hog1-4) and a T-DNA tag (hog1-5) in the HOG1 gene each behaved as zygotic embryo lethal mutants and could not be made homozygous. The results suggest that the homozygous hog1 point mutants are leaky and result in genome demethylation and poor growth and that homozygous insertion mutations result in zygotic lethality. Complementation of the hog1-1 point mutation with a T-DNA containing the gene coding for SAH hydrolase restored gene silencing, HDG silencing, DNA methylation, fast growth, and normal seed viability. The same T-DNA also complemented the zygotic embryo lethal phenotype of the hog1-4 tagged mutant. A model relating the HOG1 gene, DNA methylation, and methylation-dependent HDG silencing is presented.
INTRODUCTION
The literature of gene and homology-dependent gene (HDG) silencing is complex, and authors studying different genes and transgenes have found very different phenomena. In some cases, the silencing appears transcriptional and in other cases posttranscriptional. Sometimes silencing phenomena are associated with DNA methylation, and in a subset of such cases demethylation results in the relief of silencing, suggesting a causal role for DNA methylation in such silencing (Furner et al., 1998). Hairpin constructs directed to the coding sequence result directly in HDG silencing of endogenous genes by producing double-stranded RNA and act posttranscriptionally (Wesley et al., 2001). By contrast, hairpin constructs derived from promoter regions target DNA methylation to homologous DNA sequences where it can result in transcriptional gene silencing (Mette et al., 2000; Sijen et al., 2001; Aufsatz et al., 2002). The most incisive studies in this area have been genetic, and several authors have selected Arabidopsis thaliana mutants impaired in silencing or HDG silencing of transgenes and endogenous genes. Some of these mutant genes have been identified at a molecular level and they fall into two groups: those affecting the metabolism of RNA, SGS2/SDE1 (Dalmay et al., 2000; Mourrain et al., 2000), SGS3 (Mourrain et al., 2000), AGO1 (Fagard et al., 2000), SDE3 (Dalmay et al., 2001), HEN1 (Boutet et al., 2003), and WEX (Glazov et al., 2003), and those affecting chromatin. The latter group can be further subdivided into genes corresponding to mutants impaired in DNA methylation, including DDM1 (Vongs et al., 1993; Scheid et al., 1998; Jeddeloh et al., 1999; Morel et al., 2000), MET1 (formerly known as DDM2; Vongs et al., 1993; Morel et al., 2000), CMT3 (Lindroth et al., 2001), DRM1 and DRM2 (Cao and Jacobsen, 2002), KYP (Jackson et al., 2002), AGO4 (Zilberman et al., 2003), HDA6/SIL1 (Aufsatz et al., 2002; Probst et al., 2004), and DRD1 (Kanno et al., 2004), and genes corresponding to mutants that are not, including MOM (Amedeo et al., 2000). The observation that different genetic screens give different spectra of mutants suggests that that there are multiple factors contributing to the phenomenon, and the relative importance of the three groups of genes varies with the screen employed.
The methylation-deficient mutants of Arabidopsis define a structurally diverse group of genes with mutants showing a similar molecular phenotype (i.e., decreases in the level of total methylation in genomic DNA). The decreased DNA methylation mutants (ddm1 and met1) were originally identified in a direct screen for lines with decreased DNA methylation (Vongs et al., 1993) and subsequently shown to affect gene silencing (Jeddeloh et al., 1999; Morel et al., 2000). The chromomethylase3 (cmt3) mutant was identified in a reversion screen and appears to be impaired in the methylation of CpXpG sites (Lindroth et al., 2001). Other authors have reported that cmt3 mutants are also impaired in nonsymmetric DNA methylation (Bartee et al., 2001). The kryptonite (kyp) mutation gives a similar pattern of methylation deficiency to cmt3 mutants, but the effect appears to be indirect as the locus encodes an enzyme affecting histone methylation (Jackson et al., 2002). The four recessive mutants (ddm1, met1, cmt3, and kyp) all have global effects on DNA methylation. By contrast, posttranscriptional gene (PTG) silencing mutants impaired in aspects of RNA metabolism also have effects on DNA methylation; however, it is typically restricted to the transgenes showing silencing (Elmayan et al., 1998; Dalmay et al., 2000; Fagard et al., 2000; Mourrain et al., 2000).
Some years ago, we initiated a study of HDG silencing of the chalcone synthase gene (CHS) of Arabidopsis (Davies et al., 1997). Chalcone synthase is the first enzyme of the pathway leading to the synthesis of the purple pigment anthocyanin. Arabidopsis plants usually contain little anthocyanin, but under inducing conditions (bright white light or high sucrose in vitro) they show induction of CHS, anthocyanin biosynthesis, and a purple phenotype. In Arabidopsis, CHS is coded by a single gene at the TT4 locus, and tt4 mutants have yellow seeds and remain green under inducing conditions. The HDG silencing of CHS was initiated by introducing the intact genomic copy of CHS into wild-type Arabidopsis. The great majority of transgenic plants showed no obvious phenotype, but three lines showed reduced anthocyanin. The most extreme line (line C) contains multiple scrambled highly methylated copies of the T-DNA inserted at a single site and no copies at other sites. The C-insert line had yellow seeds and trace amounts of anthocyanin in the leaves under inducing conditions. Loss of the C-insert resulted in reversion to a purple phenotype under inducing conditions, and in such lines the CHS copy at TT4 was found to be hypermethylated. The result implies that the presence of the C-insert can cause methylation of the unlinked TT4 locus in trans presumably in a homology-dependent manner. The genetics of the C-insert were unusual in that hemizygotes produced from crosses were variegated (purple/green), whereas hemizygotes derived from self-fertilization of hemizygotes were a uniform green in color. This effect appears to be attributable to the time taken for the naïve TT4 allele from the wild-type parent to become silent. The time taken to become silent may reflect the period necessary for methylation of the naïve wild-type allele because crosses to C-insert lines using methylated TT4 alleles gave uniform green progeny. In marked contrast with the complex genetics of the C-insert, other researchers have reported that CHS silencing using a hairpin construct derived from transcribed regions is inherited as a simple Mendelian dominant trait in Arabidopsis (Wesley et al., 2001).
Subsequently, in a study on genetic modifiers of C-insert–induced HDG silencing of TT4, we reported that the ddm1 mutation relieves the silencing, suggesting that hypermethylation of the C-insert and/or the TT4 locus was necessary for the HDG silencing to occur (Furner et al., 1998). In the same study, a new mutant was described that reactivated CHS expression and anthocyanin biosynthesis in C-insert homozygotes. The mutant was called homology-dependent gene silencing1 (hog1) and is here referred to as hog1-1. The hog1-1 mutant line showed demethylation of the three transgenes on the C-insert and of the rDNA arrays, suggesting a defect in global DNA methylation (Furner et al., 1998). The hog1-1 mutant showed broadly similar genetics to the ddm1 mutant but was nonallelic. The precise details of silencing by the C-insert have not been worked out, and attempts to detect short interfering RNAs (siRNAs) in this line have not yet been successful (H. Vaucheret, unpublished data). In this study, we have performed a more detailed genetic analysis of C-insert silencing and identified the gene encoding HOG1. The defects in the line have been examined in greater detail, and four new hog1 alleles are described. A model integrating the genetic and molecular data on HOG1 and other methylation-related genes and their effects on HDG silencing are presented.
RESULTS
Genetics of HDG Silencing
In an earlier study, we reported that hog1 and ddm1 were both monogenic recessive modifiers of C-insert silencing (Furner et al., 1998). To extend these observations, a series of crosses were made between C-insert homozygotes and seven other mutants reported to be impaired in gene silencing and/or DNA methylation. The phenotypes of the F2 generation plants were scored under inducing conditions (Table 1). As a control, similar crosses were made to an Arabidopsis line homozygous for a hairpin insert directed against the 3′ end of CHS reading frame (Wesley et al., 2001), here referred to as the V-insert. This line produces CHS siRNAs (Wesley et al., 2001), resulting in a form of posttranscriptional silencing called inverted repeat posttranscriptional gene (IR-PTG) silencing (Béclin et al., 2002). In the control crosses of the C- and V-insert lines to wild-type plants, the F2 segregations were each a good fit to the 1:3 (purple:green) ratio and significantly lower than a 7:9 ratio expected for a modifier of transgene silencing. All the crosses of the V-insert line to all nine putative modifiers gave a 1:3 ratio, suggesting that none of the mutants had an effect on silencing by the hairpin construct. The genetic interactions of the C-insert with the silencing modifiers were more complex. Crosses to the kyp-2 mutant gave ambiguous results that could fit either segregation. The remaining mutants fell into two groups: those that failed to modify C-insert silencing and segregated 1:3 (sgs1-1, sgs2-1, sgs3-1, and ago1-27) and those that effectively modified C-insert silencing and segregated 7:9 (ddm1-2, met1-1, cmt3-7, and hog1-1).
Table 1.
Interactions among CHS Silencing, the V- and C-Inserts, and Nine Putative Genetic Modifiers of Gene Silencing
| C-Insert
|
V-Insert
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Allele | Green | Purple | χ3:12 | χ9:72 | Green | Purple | χ3:12 | χ9:72 |
| SGS1 | sgs1-1 | 74 | 23 | 0.09NS | 15.83S | 123 | 33 | 1.23NS | 32.37S |
| SGS2 | sgs2-1 | 73 | 27 | 0.21NS | 11.40S | 115 | 49 | 2.08NS | 12.82S |
| SGS3 | sgs3-1 | 73 | 24 | 0.00NS | 14.24S | 126 | 35 | 0.91NS | 31.70S |
| AGO1 | ago1-27 | 68 | 20 | 0.24NS | 15.80S | 117 | 39 | 0.00NS | 22.29S |
| KYP | kyp-2 | 32 | 16 | 1.78NS | 2.12NS | 66 | 16 | 1.32NS | 19.57S |
| DDM1 | ddm1-2 | 42 | 41 | 26.35S | 1.08NS | 93 | 28 | 0.22NS | 20.88S |
| MET1 | met1-1 | 43 | 37 | 19.27S | 0.20NS | 82 | 25 | 0.15NS | 18.07S |
| CMT3 | cmt3-7 | 47 | 36 | 14.94S | 0.00NS | 69 | 20 | 0.30NS | 16.37S |
| HOG1 | hog1-1 | 46 | 34 | 13.07S | 0.05NS | 68 | 18 | 0.76NS | 18.20S |
| WT | – | 64 | 27 | 1.06NS | 7.33S | 64 | 19 | 0.20NS | 14.67S |
The results presented are the segregations of green (CHS silenced) and purple (CHS active) seedlings in the F2 generation derived from crosses homozygous for the respective insert and mutant. The inserts are dominant and the modifiers are (when effective) recessive in the F2 generation. Failure of a putative modifier mutation to affect CHS silencing results in a 3:1 (green:purple) ratio because of the segregation of the insert alone. Success of a putative modifier mutation in relieving C-insert silencing results in a 9:7 (green:purple) ratio because of the superimposition of the two independent segregations. Each set of observations was tested for fit to each ratio using corrected χ2. Values of χ2 > 3.84 indicate <5% frequency by chance alone and are marked as significant (S), and values lower than this are marked as not significant (NS).
Genetics of the hog1 Point Mutants
The HOG1 gene was initially identified by a single monogenic recessive mutation, hog1-1 (Furner et al., 1998). Four new hog1 alleles are described in this article. The hog1-1 mutant was isolated from a heavily ethyl methanesulfonate (EMS)–mutagenized population, and the homozygous line was both slow growing and showed low fertility (Furner et al., 1998). In crosses to the parental C-line, the F2 generation segregates one-quarter purple hog1-1 homozygotes (Furner et al., 1998). Such plants are also slow growing and show low fertility, suggesting these traits are a property of the homozygous mutant or other mutations closely linked to the hog1-1 mutation. Typically, hog1-1 homozygotes flower approximately a week later than wild-type controls with a similar number of leaves. Female fertility seems more impaired than male fertility, and crosses using hog1-1 as female parent often fail to set seed irrespective of the genotype of the male parent. Light microscopy of hog1-1 pollen showed no obvious pollen defects (data not shown). Seed viability and germination rate were found to be low particularly on agar plates. In the initial report, we found that the hog1-1 homozygotes showed demethylation of the C-insert (Furner et al., 1998). The purple plants in the F2 generation of the cross between the hog1-1 line and the C-insert line also showed C-insert demethylation, whereas wild-type segregants did not (as judged from DNA gel blots prepared with methylation-sensitive restriction enzymes; data not shown). The result suggested cosegregation of hog1-1 and DNA demethylation and implied that the observed DNA demethylation might play a causal role in the relief of HDG silencing of TT4 and the restoration of anthocyanin biosynthesis.
Two more hog1 alleles (hog1-2 and hog1-3) were recovered from further screens of EMS-derived M1 seedlings (see Methods). They also show low fertility and are slow growing compared with the parental line. The induced purple phenotype is weaker in the two new mutants (compared with hog1-1), implying that they are less effective in relieving C-insert–induced silencing of TT4. A direct determination of the amount of 5-methyl-cytosine in extracted DNA was employed to assess the methylation state of the genomes of the three hog1 point mutants (Table 2). The method depends on end-labeling genomic DNA cut with TaqI (site T/CGA) using T4 polynucleotide kinase and allows quantification of the labeled cytosine or 5-methyl-cytosine at the site (Vongs et al., 1993). The data should be interpreted with caution because they depend on the methylation state of the cytosine of the TaqI site. The sequence context of this site is CpG, and the results obtained may not be typical of cytosine in other sequence contexts (CpXpG and nonsymmetric). The wild-type plants had 23% of total cytosine as 5-methyl-cytosine, and in the hog1-1 mutant this was reduced to 10%. The other two hog1 mutants were intermediate, and the ddm1-2 mutant showed an even greater reduction to 8%. When the levels of 5-methyl-cytosine are compared as a percentage of the wild-type level, the hog1-1 mutant had 45% and the ddm1-2 mutant 33%. The results are consistent with genome-wide demethylation of CpG sites in the hog1 mutants, and the mutant with the strongest CHS reactivation phenotype (hog1-1) showed the greatest demethylation.
Table 2.
Determination of Methylation Levels in Total Genomic DNA in the Wild Type and a Variety of Homozygous Mutantsa
| CpG Methylation in TaqI Sites of Genomic DNA
| ||||
|---|---|---|---|---|
| Wild Type | hog1-1 | hog1-2 | hog1-3 | ddm1-2 |
| 5-Methyl-cytosine percentage of labeled cytosine | ||||
| 23.0 ± 2.8 | 10.3 ± 0.6 | 16.7 ± 1.0 | 15.8 ± 2.2 | 7.5 ± 1.1 |
| 5-Methyl-cytosine percentage of wild-type level | ||||
| 100.0 | 44.7 | 72.6 | 68.7 | 32.6 |
The method employed measures DNA methylation in the context CpG of the TaqI site. Values are averages ± se (n = 5).
To test whether the hog1-1 mutation also relieves transcriptional gene silencing, hog1-1 and C-insert double homozygote plants were crossed to the transcriptional gene silenced L5 line (formerly known as the Arabidopsis 6b5 line). This line is homozygous for a transcriptionally inactive β-glucuronidase (GUS) gene on a T-DNA insert, and the silent gene can be reactivated by ddm1 and met1 (Morel et al., 2000), mom1 (Amedeo et al., 2000), and sil1 (Probst et al., 2004). The F2 generation plants from the cross were stained for GUS activity, and the following numbers of plants were observed: 57 unstained and 15 blue. This segregation is in good agreement with the expected 13:3 ratio for a recessive modifier of a silent transgene (χ13:32 0.09NS). The reactivation of the silent GUS gene is attributable to the hog1-1 mutation because control crosses of the C-insert line (which is wild-type at the HOG1 locus) to the L5 line gave 72 unstained plants in the F2 generation. In the F3 generation of the hog1-1 (C/C) to L5 cross, a family was identified that segregated only hygromycin-sensitive and GUS-positive seedlings. This line is referred to as L5-hog1-1 and is homozygous for both the L5-insert and the hog1-1 mutation but lacks the C-insert. This L5-hog1-1 line also shows poor female fertility and slow growth compared with the L5 line, implying that these traits are coded by or are tightly linked to the hog1-1 mutation.
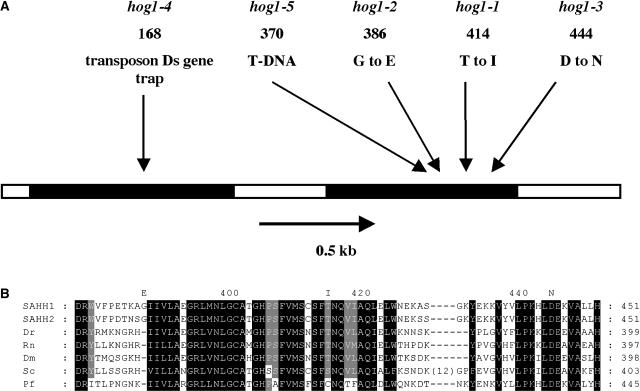
The hog1-1 mutation was mapped to a region on chromosome 4 between mi128 and mi112 and close to m326 (see Methods). We examined the genomic sequence of the interval for candidate genes in which mutations might result in genome-wide hypomethylation. The most obvious candidate genes in the region were two genes with DNA methylase homology (AT4G13610 and AT4G14140). In between these two genes was another, less obvious candidate gene (AT4G13940) encoding a predicted 485-residue-long protein with homology to S-adenosyl-homocysteine hydrolase (EC 3.3.1.1), here designated as SAH hydrolase 1. The physiological relevance of SAH hydrolase resides in its role in the removal of SAH, the by-product of trans-methylation reactions where S-adenosyl methionine (SAM) is the group donor. SAH is a strong inhibitor of SAM-dependent methyltransferases, and it appears that the relative levels of SAH and SAM are critical in the methylation of DNA, proteins, pectins, and other small molecules in the cell (Moffatt and Weretilnyk, 2001). Sequencing of the AT4G13940 gene from the wild-type and the hog1-1 homozygote genomes revealed a point mutation in the latter (see Figure 1). The observation of the mutation in the 3′ end of the gene coding for SAH hydrolase 1 suggests that this enzyme is the product of the HOG1 gene. Sequencing of the AT4G13940 gene in both the hog1-2 and hog1-3 homozygous mutant lines showed that each contains a point mutation in the 3′ end region, leading to substitutions in the predicted SAH hydrolase primary structure (Figure 1).
Figure 1.
Location of Mutations of the HOG1 Gene (AT4G 13940) Coding for SAH Hydrolase.
(A) Coordinates are given in amino acids from the start of the protein. The specific amino acid change is indicated for each of the three point mutants. The positions of the insertions are indicated for the two tagged mutants. The 5′ untranslated region, 3′ untranslated region, and the unique intron (according to The Arabidopsis Information Resource [TAIR] annotation) are indicated by white boxes and exons by black boxes. The arrow indicates the direction of transcription.
(B) Partial alignment of SAH hydrolases of Arabidopsis with other eukaryotic hydrolases. The alignment is representative of hydrolases of the eukaryotic type as defined by Kurowski and Bujnicki (2003) and corresponds to the region to which the hog1 substitutions map. The substituted residues are indicated in the top row; numbering corresponds to SAH hydrolase 1. SAHH1, SAH hydrolase 1; SAHH2, SAH hydrolase 2; Dr, Danio rerio (GI 37,681,725); Rn, Rattus norvegicus (GI 38,197,378); Dm, Drosophila melanogaster (GI 7,293,098); Sc, Saccharomyces cerevisiae (GI 2,950,505); Pf, Plasmodium falciparum (GI 627050). An insertion in the D. melanogaster sequence is indicated. Invariant and highly conserved residues are highlighted.
Genetics of the hog1 Insertion Mutants
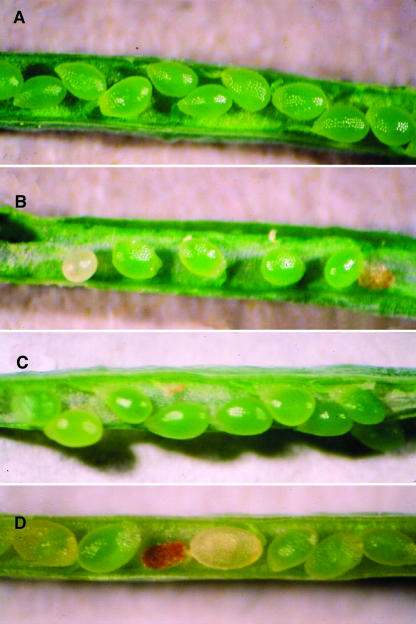
To obtain a null allele of HOG1, a variety of databases were examined for reports of insertion events affecting HOG1. A Dissociator (Ds) gene trap (transposon conferring kanamycin resistance) inserted in the first exon of HOG1 was found on the Arabidopsis thaliana Insertion Database (ATIDB) site (GT1724; see Figure 1). Seeds of this line, here renamed hog1-4, were obtained, and 99 families each derived from a single plant of this line were plated on kanamycin to look for a homozygous resistant line, but none were identified (60 families were segregating and 39 families were entirely sensitive). The absence of the homozygous kanamycin-resistant class was unexpected, and there are three possible explanations: lethality or transmission failure in the male gametophyte, lethality or transmission failure in the female gametophyte, or zygotic lethality. Fully penetrant single sex gametophytic lethality should segregate 1:1 (resistant:sensitive), and zygotic lethality should segregate 2:1 (resistant:sensitive). The observations are a good fit to zygotic lethality (χ2:12 1.38NS) and a poor fit to single sex fully penetrant gametophytic lethality (χ1:12 4.89S). They could, however, fit gametophytic lethality with incomplete penetrance. To investigate the possibility of transmission defects associated with the hog1-4 insert, controlled reciprocal crosses were made between hemizygous kanamycin-resistant hog1-4 plants and wild-type plants and the resultant F1 seeds germinated on kanamycin. In crosses using hog1-4 hemizygote as pollen parent, 228 resistant and 222 sensitive seedlings were found (χ1:12 0.11NS), and in the reciprocal cross, 160 resistant and 182 sensitive seedlings were found (χ1:12 1.29NS). Transmission of the hog1-4 insert was entirely normal in crosses in both directions. In self-fertilized hog1-4 hemizygotes, 129 resistant and 65 sensitive plants were found. This is significantly different from the expectation of a simple Mendelian dominant trait (χ3:12 7.04S) and a good fit to a fully penetrant zygotic lethal trait (χ2:12 0.00NS). Taken together, the results suggest that hog1-4 is a fully penetrant zygotic lethal trait with normal male and female transmission. Upon dissection, immature siliques of self-fertilized hog1-4 hemizygous plants were found to contain approximately one-quarter aborted seeds. The stage of failure was variable and some affected seeds were nearly mature, whereas others appeared to have failed early in embryonic development (Figure 2B). Wild-type controls had very low levels of seed abortion (Figure 2A). These observations indicate that hog1-4 results in embryo lethality, and null mutations of HOG1 are homozygous lethal. Although attractive, this is not the only possible explanation of the observations. The hog1-4 allele may be in some way exceptional and/or be linked to some other defect that cannot be made homozygous. The alternatives were tested with a T-DNA insert (NS023915) affecting the second exon of HOG1, found on the Signal database and ordered from the ABRC (via the Nottingham Arabidopsis Stock Centre [NASC]; see Figure 1) and renamed hog1-5. Because the kanamycin resistance gene in this line was inactive and could not be scored, the genotypes of 45 individual plants grown from seeds segregating for hog1-5 were tested in batches of their progeny by a PCR-based method (see Methods). In the sample, 25 parent plants were heterozygous for hog1-5, 20 were wild-type, and 0 were homozygous for hog1-5. The absence of hog1-5 homozygotes is significant (χ1:32 8.07S), and the numbers of hog1-5 segregating and wild-type families can be fitted to the 2:1 ratio expected of a zygotic lethal trait (χ2:12 2.03NS). Overall, the genetics of hog1-5 resemble those of hog1-4: both are zygotic lethal traits that cannot be made homozygous. The occurrence of two exceptional alleles is unlikely, and a more likely explanation is that null mutants deficient in SAH hydrolase activity are embryonic lethal. A similar deficit of homozygous mutant plants has been reported for tagged alleles of the MET1 locus coding DNA methylase 1 (Saze et al., 2003). To look for an embryo abortion phenotype in plants hemizygous for an insertion allele of MET1, a T-DNA tagged allele was ordered from NASC (stock number N576522). The identity of the insert was confirmed and renamed met1-7. Siliques of hemizygous kanamycin resistant plants were opened and examined for the presence of aborted seeds. A similar range of seed abortion phenotypes was found (Figure 2D).
Figure 2.
Zygotic Lethality in the hog1-4 and met1-7 Mutants.
(A) Wild-type (HOG1) silique.
(B) Silique from a hemizygous hog1-4/HOG1 plant. Note the presence of aborted hog1-4 homozygote seeds.
(C) Silique from a plant hemizygous for both hog1-4 and the complementing SAH hydrolase T-DNA. Note the decrease in zygotic lethality.
(D) Silique from a hemizygous met1-7/MET1 plant. Note the presence of aborted met1-7 homozygote seeds.
SAH Hydrolase Activity and SAH and SAM Levels in the hog1-1 Point Mutant
The possibility that the mutant phenotypes are a result of SAH hydrolase deficiency was tested directly by measuring SAH hydrolase activity in the aerial organs of the wild-type, C-insert homozygotes and hog1-1 plants (homozygous for the C-insert; Table 3). The levels of SAH hydrolase were reduced in both leaves and buds/flowers tested of the hog1-1 mutant compared with both wild-type and C-insert homozygote controls. The SAH hydrolase activity varied from ∼35 (leaves) to 49% (buds/flowers) of the C-insert control. Because SAH hydrolase activity was found in the most phenotypically severe point mutant (hog1-1), either the allele codes for an enzyme with some residual activity or the observed activity is dependent on another locus. The genome sequence of Arabidopsis was examined, and a second gene encoding an open reading frame homologous to SAH hydrolases was identified on chromosome 3 (AT3G23810) and will be referred to as the SAH hydrolase 2 gene (see below).
Table 3.
Direct Measurement of Total SAH Hydrolase Activity in Crude Extracts from Various Tissues of Bolting Plants of the Wild Type, C-Insert Homozygotes, and hog1-1 and C-Insert Double Homozygotes
| SAH Hydrolase Activity (nmol/min/mg Protein)
| ||||
|---|---|---|---|---|
| Organ | Wild Type(HOG1)a | C-Insert Homozygote (HOG1)a | C-Insert hog1-1 Homozygotea | hog1-1/C-Insert × 100 (%) |
| Leaves | 3.11 ± 0.40 | 2.99 ± 0.17 | 1.04 ± 0.08 | 35 |
| Buds/flowers | 10.35 ± 0.09 | 13.11 ± 1.62 | 6.37 ± 0.54 | 49 |
Values are averages ± se (n = 4).
Reducing the level of SAH hydrolase activity should increase the levels of the SAH and decrease the ratio of SAM:SAH. To assess these predictions, the levels of SAH, SAM, and the SAM:SAH ratio were determined (Table 4). The hog1-1 homozygote shows a similar SAM level to the C-line and a slight increase in SAH. Perhaps the more important figure is the shift in the SAM:SAH toward SAH, and the ratio is 86% of the level of the parental line. The shift in SAM:SAH ratios in the extracts is relatively small, but it should be kept in mind that the figures are for a single time point, and only leaves were assayed. It may be that the shift in the ratio is greater at particular times or in particular tissues.
Table 4.
Quantification of SAM and SAH in Leaves of the Wild Type, C-Insert Homozygotes, and hog1-1 C-Insert Double Homozygote Leaves
| Metabolite Pools in Various Lines (pmol/mg)
|
||||
|---|---|---|---|---|
| Plant Line | SAMa | SAHa | SAM:SAHa | hog1-1/C-Insert × 100 (%) |
| Wild type | 3.34 ± 0.48 | 0.14 ± 0.02 | 25.38 ± 0.89 | – |
| C-insert homozygote | 4.48 ± 0.16 | 0.21 ± 0.19 | 23.94 ± 0.95 | – |
| C-insert-hog1-1 homozygote | 4.60 ± 0.82 | 0.22 ± 0.05 | 20.44 ± 0.83 | 86% |
Values are averages ± se (n = 3).
Characterization of the Second Gene Coding for SAH Hydrolase
The SAH hydrolase 2 gene occurs in a syntenic region to the chromosome 4 gene, and their predicted protein products have 96% identity. No notable difference was found upon comparison with all other predicted primary structures, plant SAH hydrolases representing nine species that were found to be very highly conserved, all sharing the same length (485 residues) and >87% identity in pairwise comparisons (data not shown). Superficial inspection of the AT3G23810 sequence found it to be both intact and without any obvious molecular defects, containing a single intron at an equivalent location with SAH hydrolase 1.
No point mutants of the chromosome 3 gene were known, but a T-DNA insert in the second intron at the 3′ end of the gene that would be expected to disrupt it is reported at GABI-Kat (139A12; Li et al., 2003). As the corresponding seeds were initially segregating for the tagging insert, a generation was grown up and self-fertilized, single plants harvested, and seed germinated on plates containing sulfonurea to identify a homozygous line. Four fully sulfonurea-resistant (homozygous), 17 segregating, and eight fully sensitive families were identified as expected for segregation of a dominant monogenic trait (1:2:1). Samples of genomic DNA from these families were analyzed with DNA gel blots using an SAH hydrolase probe, and the results were consistent with disruption of SAH hydrolase 2 in the homozygote sulfonurea-resistant class (data not shown). The homozygous class showed normal growth and fertility and were indistinguishable from control plants. The homozygous T-DNA insert into AT3G23810 will be referred to as the SAH hydrolase 2–tagged mutant line.
We examined the SAH hydrolase 2–tagged mutant for traits resembling those of the hog1-1, but none were found. The L5 (transcriptionally inactive GUS) line was crossed to the homozygous SAH hydrolase 2–tagged mutant line and seedlings of the F2 generation stained for GUS. No blue staining plants were recovered in 83 progeny examined. The results suggest that the mutation cannot reactivate the silent GUS transgenes in the L5 line. Crosses of the SAH hydrolase 2–tagged mutant line were made to plants homozygous for the C-insert, and 15 purple and 41 green plants were found in the F2 generation. The observations are a good fit to the segregation of the C-insert as a simple Mendelian dominant trait (χ1:32 0.02NS) and a poor fit to the independent segregation of a recessive C-insert modifier (χ7:92 5.88S). DNA gel blot assessment of rDNA methylation of the SAH hydrolase 2–tagged mutant line using the methylation-sensitive restriction enzymes MspI and HpaII showed that the pattern of hybridization was indistinguishable from the wild type, and the rDNA arrays were highly methylated in both (data not shown).
Complementation of the hog1 Mutations with a Genomic Clone Containing the Gene Coding for SAH Hydrolase 1
The sequencing of the three hog1 point mutants suggests HOG1 codes for SAH hydrolase 1 but falls short of a rigorous molecular proof. To prove SAH hydrolase 1 complements and corresponds to a mutant of the HOG1 gene, a genomic clone corresponding to the wild-type region coding for the enzyme was introduced into L5-hog1-1 plants using Agrobacterium tumefaciens–mediated gene transfer (see Methods). Seeds were harvested from 20 individual self-fertilized Basta-resistant plants and germinated and the seedlings stained for GUS. The L5-hog1-1 line stains for GUS, and complementation of the mutation was expected to result in reversion to a nonstaining phenotype resembling the parental L5 line. Five transgenic lines were identified that showed greatly reduced GUS staining in the T1 or T2 generation. The slow and variable resilencing of the L5 insert observed in these transgenic HOG1 lines is consistent with the original observation that multiple generations are necessary before the L5 line reaches a complete and stable silent state (Probst et al., 2004). Recovery of the genome from hypomethylation is typically slow (Vongs et al., 1993). In general, staining decreased in the five complementing lines in succeeding generations. The fertility of all five complementing lines was higher than the L5-hog1-1 line, but some reduction in fertility (compared with L5) was noted in one of them. By T3, two independent lines showing no detectable GUS staining in seedlings were identified. One of the two lines was arbitrarily chosen for subsequent work. This line was initially heterozygous for the insert and segregated 65 Basta-resistant and 12 Basta-sensitive seedlings (χ3:12 3.16NS). From the progeny of self-fertilized individual plants, a homozygous line that was both nonstaining and 100% Basta resistant was identified. This line was presumed to be homozygous for the L5 insert, the hog1-1 mutation, and the complementing T-DNA coding for SAH hydrolase 1 (this line is subsequently referred to as the L5-hog1-1-complementation line). Its existence proves that HOG1 codes for SAH hydrolase 1 and complementation of the defect restores the normal gene silencing of the L5 insert (the general properties of the line are given in Table 5). To assess whether the complementation line also restores HDG silencing to hog1-1 mutants, plants homozygous for the C-insert and hog1-1 were crossed to either the L5-hog1-1 line or the L5-hog1-1-complementation line and the F1 generation placed under CHS-inducing conditions. Twenty plants were examined/crossed and all plants from the first cross were purple in phenotype as expected for plants homozygous for the hog1-1 mutation and heterozygous for the C-insert. The second cross should give plants of the same genotype except that they should also be heterozygous for the correcting insert. All 20 plants showed the expected uniform green phenotype. The result shows that the complementing insert also relieves the defect in HDG silencing of CHS by the hog1-1 mutation in the C-insert background. The L5-hog1-1-complementation line plants were healthier and faster growing than the L5-hog1-1 line. They flowered at the same time as the L5 (HOG1) plants and grew faster, and seed germination was increased (Table 5).
Table 5.
A Comparison of L5, L5-hog1-1, and L5-hog1-1-Complementation Lines with Respect to a Variety of Traits Affected by the hog1-1 Mutation
| Line | GUS mRNA | GUS Staining | GUS Methylation | rDNA Methylation | Seed Germination (% ± se) | Growth Rate | Flowering Time | Fertility |
|---|---|---|---|---|---|---|---|---|
| L5 | No | No | High | High | High (99.3 ± 0.5) | Fast | Early | High |
| L5-hog1-1 | Yes | Yes | Low | Low | Reduced (85.9 ± 2.5) | Slow | Late | Low |
| L5-hog1-1- complemented | No | No | High | High | (99.8 ± 0.3) | Fast | Early | Variable |
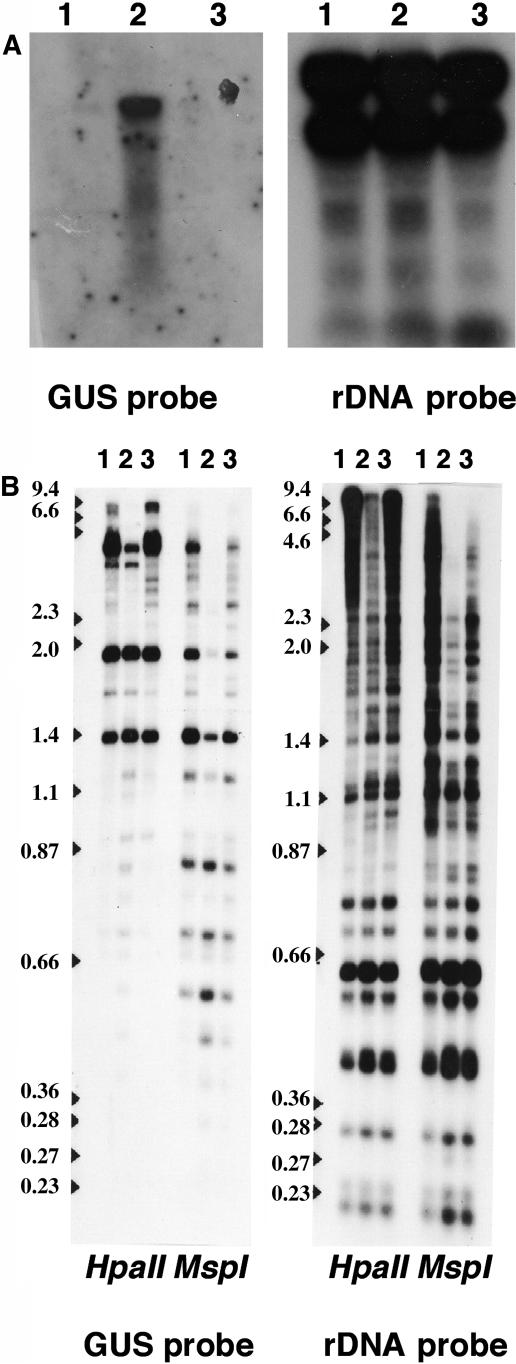
The hog1-1 mutant was shown to be deficient in the DNA methylation of the C-insert–encoded CHS gene and rDNA sequences using DNA gel blots and the methylation-sensitive restriction endonucleases MspI and HpaII (Furner et al., 1998). A similar analysis was performed on the L5 line, the L5-hog1-1 line, and the L5-hog1-1-complementation line and is shown in Figure 3B. The L5 insert-encoded GUS gene and the rDNA arrays are poorly digested in the L5 line by both enzymes, suggesting, as expected, that both sequences are highly methylated at CpG and CpXpG sites. By contrast, the L5-hog1-1 line DNA shows greater digestion with both enzymes and both probes, suggesting that the hog1-1 mutation had resulted in a global demethylation affecting both CpG and CpXpG sites. The demethylation is largely restored in the L5-hog1-1-complementation line and both probes and both enzymes show reduced digestion and correspondingly increased methylation of both CpG and CpXpG sites.
Figure 3.
Complementation of the hog1-1 Mutation.
(A) RNA gel blots of total RNA prepared from the L5 (lane 1), L5-hog1-1 (lane 2), and L5-hog1-1-complementation (lane 3) lines. The probes are indicated at the bottom of the panel.
(B) DNA gel blots of total genomic DNA prepared from L5 (lane 1), L5-hog1-1 (lane 2), and L5-hog1-1-complementation lines (lane 3) cut with methylation-sensitive restriction endonucleases as indicated. The DNA was also cut with HindIII. The probes are indicated at the bottom of the panel, and the numbers at left are the molecular weights in kilobases.
RNA gel blots of total RNA extracted from the three lines were probed with GUS and, after autoradiography, stripped and reprobed with an rDNA probe as a loading control (Figure 3A, Table 5). GUS transcripts were only detected in the L5-hog1-1 line. The GUS mRNA levels agreed with the GUS staining pattern as only the GUS staining line showed any GUS mRNA. The rRNA levels and intactness in the three preparations are similar, indicating that the loading was also similar.
The hog1-4 mutant contains a kanamycin resistance gene inserted into the HOG1 gene, and as the mutant codes a zygotic (sporophytic) lethal trait, it is associated with the segregation of 25% aborted embryos and a 2:1 segregation ratio for kanamycin (resistant:sensitive). If these traits are a result of the inability to produce the SAH hydrolase 1, they should be complemented in the presence of the L5-hog1-1-complementation line T-DNA containing the intact SAH hydrolase 1 gene. To accomplish this, the homozygous L5-hog1-1-complementation line was crossed to a plant hemizygous for hog1-4. The F1 plants segregated 1:1 for kanamycin resistance coded by hog1-4. The siliques of the kanamycin-resistant plants (triple transgenic heterozygous class) were opened and the number of aborted and wild-type seeds counted in the kanamycin resistant plants. Because the plants were hemizygous for two T-DNAs affecting SAH hydrolase 1, complementation of the zygotic lethal phenotype was expected to depend on both complementation and segregation. This was expected to shift the proportion of aborted seeds from 1:4 to 1:15, presuming the T-DNAs segregated independently and complementation was epistatic to lethality (Figure 2C). We observed 96 wild-type seeds and six aborted seeds. This is a good fit to the expectation for complementation (χ15:12 0.00NS) and a poor fit to the uncomplemented ratio (χ3:12 18.88S). Approximately three-quarters of the homozygous hog1-4 embryos that would otherwise have aborted were rescued by the SAH hydrolase 1 gene on the T-DNA. Given the effectiveness of the rescue, the segregation of hog1-4–encoded kanamycin resistance was expected to shift from 2:1 to 11:4 (1 in 16 dies). To test this, F2 seeds from the cross were plated on kanamycin and the phenotypes (resistant:sensitive) scored after germination. Exactly 704 resistant and 301 sensitive plants were scored. The result is significantly higher than the 2:1 ratio (χ2:12 5.02S) but falls significantly short of the 11:4 ratio (χ11:42 5.37S). The small deficit of the tagged kanamycin-resistant (presumably hog1-4/hog1-4 class) might be because of failures in germination, segregation of other traits (L5 and hog1-1), or background effects.
DISCUSSION
Silencing-Related Mutants and CHS Silencing
Gene silencing and HDG silencing are complex and overlapping phenomena, and a single, simple unifying mechanism for the huge diversity of related observations seems unlikely. Two superficially unrelated silencing phenotypes involve the same genes when they can both be reverted by mutations in those genes. Conversely, if a particular mutation results in phenotypic reversion of a silencing phenotype but has no effect on a second silencing phenotype, the two silencing processes must be to some extent separate and involve at least one distinct step. We have previously shown that hog1-1 and ddm1-2 are both monogenic recessive second site modifiers of CHS silencing by the C-insert (Furner et al., 1998). In this study, we have extended this analysis to seven more mutants impaired in either some aspect of DNA methylation or PTG silencing. The interactions between the C-insert, CHS silencing, and the various silencing mutants are consistent in that mutants impaired in global DNA methylation relieve silencing and those which are impaired in PTG silencing do not. The C-insert–induced CHS silencing was relieved by ddm1-2, met1-1, cmt3-7, and hog1-1 homozygous backgrounds (the results with kyp-2 were ambiguous) but not in sgs1-1, sgs2-1, sgs3-1, and ago1-27 homozygous backgrounds. The former group has defects in CpG, CpXpG, and nonsymmetric cytosine methylation (Vongs et al., 1993; Furner et al., 1998; Lindroth et al., 2001), and the latter group have defects in sense posttranscriptional gene (S-PTG) silencing and defects in DNA methylation restricted to the transgenes (Elmayan et al., 1998; Fagard et al., 2000; Mourrain et al., 2000; Morel et al., 2002). In the control crosses to the CHS hairpin construct (Wesley et al., 2001), none of the mutants tested had any discernable effect on IR-PTG silencing, implying that the corresponding genes are not required for hairpin-induced silencing. The absence of any effect of mutants impaired in S-PTG silencing on IR-PTG silencing of CHS is in agreement with earlier studies on hairpin constructs and S-PTG silencing mutants (Béclin et al., 2002).
The contrasting result for methylation mutants and the two inserts suggest that the V-insert triggers IR-PTG silencing, which is supported by the detection of CHS siRNAs in this line, whereas the complex and scrambled C-insert could trigger RNA-directed DNA methylation and transcriptional gene silencing. Therein, no direct evidence on the nature of silencing in the C-line but RNA-directed promoter methylation is plausible, owing to the presence of the CHS promoter in the C-insert. We were not able to detect siRNAs corresponding to the CHS promoter in the C-line (data not shown), perhaps because the CHS transgene is driven by the authentic CHS promoter that is transcribed at a much lower level than the 35S promoter that is commonly used to transcribe promoter hairpin constructs (Mette et al., 2000; Sijen et al., 2001; Aufsatz et al., 2002).
The hog1 Mutants
The first allele of the HOG1 gene (hog1-1) was isolated some time ago as a suppressor of C-insert silencing (Furner et al., 1998). Backcrossing hog1-1 into the unmutagenized C-insert background revealed that the mutant was associated with slow growth, poor germination, and infertility. Here, we report two further alleles (hog1-2 and hog1-3) isolated that have similar phenotypes to hog1-1, indicating that these are attributable to mutations in HOG1 rather than to linked traits in the same chromosome region. The hog1-1 mutation was crossed into a background containing a transcriptionally silent GUS gene on a T-DNA insert (L5-insert), and reactivation of the gene occurred. The result was not unexpected because this transcriptionally silent GUS insert can be reactivated by the ddm1 and met1 mutations (Morel et al., 2000). A GUS staining Arabidopsis line homozygous for both the L5 insert and the hog1-1 mutation (L5-hog1-1) was identified. This shows that the hog1-1 mutation can relieve transcriptional gene silencing.
The genetics of both hog1-4 and hog1-5 insertion mutants were unusual in that the inserts could not be made homozygous and they segregated 2:1 (hemizygous:wild type). These observations are compatible with zygotic lethality, and aborted seed were found in siliques of self-fertilized hog1-4 hemizygotes. That both inserts behave as recessive zygotic lethal traits implies that a complete knockout of HOG1 and absence of SAH hydrolase 1 is lethal. Another T-DNA insert into the SAH hydrolase 1 gene has been isolated in a screen for embryonic lethal mutants (http://www.seedgenes.org/). The mutation was called emb1359, and in this article, it will be termed hog1-6. Like hog1-4, hemizygotes for the hog1-6 mutation show approximately one-quarter aborted seeds with a variable stage of developmental arrest. In most mutant seed, no embryo was detected, a large group arrested at the globular embryo stage, and a few embryos showed later arrest. All three inserts into HOG1 have a similar zygotic lethal phenotype for homozygotes, and all three point mutants were homozygous viable with methylation defects. Given the screen used, it is unlikely that fully penetrant point mutants could have been recovered as a result of the zygotic lethal phenotype.
The normal transmission of hog1-4 through pollen and ovules implies that SAH hydrolase 1 deficiency is not lethal in either gametophyte. Deficits in the frequency of heterozygotes in crosses involving tagged alleles of MET1 suggest that gametophytic lethality/nontransmission can be induced by genome-wide hypomethylation (Saze et al., 2003). It seems that either SAH hydrolase 1 activity is not required during gametophytic development or that sufficient levels of enzyme are made in the parent sporophyte to allow normal gametophytic growth and development, but this is insufficient early in zygotic development, resulting in lethality of homozygotes.
The hog1 Mutants, SAH Hydrolase Deficiency, and Genome-Wide Demethylation
Genetic mapping localized the hog1-1 mutation to an interval on chromosome 4 that included the gene coding for SAH hydrolase 1. The gene was sequenced in three mutant alleles of HOG1, and each had a different point mutation in its 3′ end, implying that HOG1 codes for the SAH hydrolase 1 enzyme. Assays of the SAH hydrolase activity in extracts from bolting plants showed that hog1-1 homozygotes only had ∼35 to 50% the activity of wild-type plants. Plants homozygous for hog1-1 also showed a decreased SAM:SAH ratio consistent with a reduced ability to degrade SAH and raising the possibility of inhibition of SAM-dependent reactions.
Genomic DNA isolated from mature tissue of all three hog1 point mutants was found to be hypomethylated (compared with the wild type), and this seems to be a consequence of SAH hydrolase deficiency and lower SAM:SAH ratios. The biological role of the enzyme is the degradation of SAH, the by-product of trans-methylation reactions using SAM, to adenosine and homocysteine. SAH is a potent inhibitor of SAM-dependent methyltransferases, and it seems likely that the relative levels of SAH and SAM are critical in the methylation of a broad array of substrates, including DNA, proteins, lipids, pectins, and several small molecules (Moffatt and Weretilnyk, 2001). The adenosine derived from SAH is further metabolized by adenosine kinase (ADK), and transgenic plants with greatly reduced ADK activity show increased SAH levels and decreased transmethylation reactions (Moffatt et al., 2002). Tobacco (Nicotiana tabacum) plants engineered to produce antisense SAH hydrolase transcripts show stunting, floral abnormalities, and demethylation of repetitive DNA as judged from DNA gel blots with methylation-sensitive enzymes (Tanaka et al., 1997). The drug (S)-9-(2,3-dihydroxypropyl)adenine inhibits SAH hydrolase and results in hypomethylation and reactivation of a methylated and silent neomycin phosphotransferase in transgenic callus cultures of tobacco (Kovarik et al., 2000). An earlier study using the same drug found increased SAH:SAM ratios and genomic hypomethylation in tobacco suspension cultures (Fojtová et al., 1998).
Taken together, the above results suggest that compromising either the SAH hydrolase or ADK may result in SAH buildup, resulting in inhibition of methylation reactions and demethylation of the genomic DNA. These observations probably oversimplify a complex situation. It remains undetermined if the observed hypomethylation effects reflect direct inhibition of DNA methylases by the low SAM:SAH ratios or are an indirect effect triggered by the altered SAH levels or reduction in SAH hydrolase. It is noteworthy that in contrast with the small shift in the SAM:SAH ratio in the hypomethylated hog1-1 plants, hypomethylation of the tobacco genome in suspension cultures induced by (S)-9-(2,3-dihydroxypropyl)adenine only occurred when the SAM:SAH ratio decreased by 300-fold relative to untreated material (Fojtová et al., 1998). This apparent discrepancy likely reflects metabolic as well as developmental differences between these two systems, which can be anticipated to affect the impact of SAH hydrolase deficiency. For example, the tobacco cultures experienced no toxic effects when SAH levels were increased 100- to 1000-fold, whereas Arabidopsis ADK-deficient lines with 50-fold higher SAH levels are severely compromised. A critical consideration in the extent of hypomethylation may be precisely when and where SAM:SAH ratios are reduced. For example, low SAM:SAH ratios in tissue undergoing DNA replication might be expected to induce demethylation, whereas no demethylation would be expected in tissue that had ceased replication. Demethylation of the genome in mature leaves might reflect SAM:SAH ratios in the dividing cells of shoot apical meristem and leaf primordia rather than the leaf itself. The shift in SAH:SAM ratios is small in the mature leaf tissue of hog1-1 plants, whereas genomic DNA prepared from leaves is extensively hypomethylated. Once established, hypomethylation can persist for generations (Vongs et al., 1993), and it is possible that hypomethylation provoked by low SAM:SAH ratios in earlier generations and/or early in zygotic development are reflected in hypomethylation of the genomic DNA of the mature organs. Given the above considerations, it seems the SAM:SAH ratio affects DNA methylation, but it is less clear when and where such an effect is manifested.
Point mutants of SAH hydrolase 1 show reduced fertility, poor seed germination, and slow growth, but it is unclear if these problems are a direct result of SAH hydrolase deficiency or an indirect result mediated by genome hypomethylation. Similarly, insertion mutants of SAH hydrolase 1 are lethal during embryogenesis, but it is unclear whether this is a direct or indirect effect. The result suggests an absolute requirement for SAH hydrolase 1 during embryogenesis but do not preclude an absolute requirement later in development (at stages that are not reached in the homozygotes). The viability of the homozygous point mutants isolated implies that their protein products retain some SAH hydrolase activity. SAH hydrolases are well conserved proteins, and it is possible to predict the tertiary structure of SAH hydrolase 1 to a reasonable degree of confidence by comparative modeling to the known structure of its rat homolog (Hu et al., 1999) and with which it shares 63.4% identity (P. Rocha, unpublished data). Each of the substitutions resulting from the hog1 point mutations were found in a distinct region of the predicted three-dimensional structure: the G386E (hog1-2) maps to the cofactor binding domain, and both the D444N (hog1-3) and T414I (hog1-1) substitutions are located in the substrate binding domain. None of the substitutions affects residues corresponding to those known or predicted to be involved in substrate or cofactor binding in the rat homolog (Hu et al., 1999; Komoto et al., 2000). However, the affected residue in hog1-1 is in close proximity to the hinge region and the C-terminal domain of the enzyme. It may be that the alternation between open and closed conformations of the enzyme during its active cycle may be partially compromised in hog1-1.
A T-DNA Tag in the Gene Coding for SAH Hydrolase 2 Is Aphenotypic
The SAH hydrolase 2 gene could represent another possible modifier of gene silencing or HDG silencing. However, plants homozygous for its T-DNA insertion mutation, unlike hog1 point mutants, were not impaired in any aspect of growth, fertility, DNA methylation, L5 transcriptional gene silencing, or C-insert–encoded HDG silencing of TT4. Because the insert occurs late in the gene, it may result in a truncated SAH hydrolase 2 with some activity. Alternatively, the wild-type gene may be comparatively inactive (relative to SAH hydrolase 1), and knocking it out has few, if any, consequences for DNA methylation, gene silencing, or the plant phenotype. Looking at other insertion mutants earlier in the gene and assessing the phenotype could test the former hypothesis. The second hypothesis contains an implication that SAH hydrolase 1 is the more active of the two enzymes and that the residual SAH activity in the hog1-1 mutant could be mostly or entirely because of the leakiness of the allele. Suggestively, the Munich Information Center for Protein Sequences Web site lists 191 ESTs corresponding to SAH hydrolase 1 and only 31 for SAH hydrolase 2. Presuming the cDNA libraries on which this is based were unbiased, the observations suggest that 80% of the SAH hydrolase mRNA comes from HOG1. In agreement with this observation, gene chip data from NASC suggest that SAH hydrolase 1 mRNA is approximately five times more abundant (on average) than SAH hydrolase 2 mRNA in a large number of hybridization experiments. The high conservation of the structural region of SAH hydrolase 2 relative to SAH hydrolase 1 (>70% identity), as well as of its encoded product, and the documented transcription of the corresponding region suggest that the gene is functional in SAH metabolism. Its activity may be developmentally or physiologically restricted. We note that despite the high sequence conservation between the structural portions of the SAH hydrolases 1 and 2 genes (>70%), hog1-4, hog1-5, and hog1-6 are zygotic lethal, indicating that SAH hydrolase 2 alone is insufficient to support life during embryo development, and SAH hydrolase 1 may be the major and perhaps the only active SAH hydrolase in embryos. Arabidopsis is a cryptic tetraploid, and the genome is a composite of the two ancestral genomes (Arabidopsis Genome Initiative, 2000). In the two progenitor species, both SAH hydrolase 1 and SAH hydrolase 2 must each have been sufficient to support life at all stages of the life cycle, and this property must have been lost from SAH hydrolase 2 after the polyploidization.
Complementation of the hog1 Mutant with a Genomic Clone Corresponding to SAH Hydrolase 1
In two of the transformants of the L5-hog1-1 line with the wild-type SAH hydrolase 1, the GUS staining was completely lost because of complementation of the hog1-1 mutation. One of these lines was made homozygous for the new insert and termed the L5-hog1-1-complementation line. The correcting T-DNA restores DNA methylation, absence of GUS RNA, absence of GUS staining (gene silencing), C-insert–coded HDG silencing of TT4, wild-type growth rate, normal fertility, and increased seed viability. The SAH hydrolase 1 containing T-DNA also complements the zygotic lethality of the tagged hog1-4 allele, implying that the zygotes die from SAH hydrolase deficiency and not from some aspect of the mutation unrelated to SAH hydrolase. The zygotic lethality of the hog1-4 allele is associated with a reduced segregation ratio of the insert-encoded kanamycin resistance, and the segregation is partially normalized by the SAH hydrolase 1 T-DNA (presumably because the hog1-4 homozygotes are rescued by it). The observation implies that the deficit of kanamycin-resistant plants in hog1-4 crosses is also an indirect consequence of SAH hydrolase deficiency. The SAH hydrolase 1 gene supplied on a T-DNA can complement the defects of the hog1 point and insertion mutants, and it may be that any SAH hydrolase gene, including SAH hydrolase 2, expressed by the authentic promoter in the appropriate tissues and organs could do this.
Lethality in Methylation Mutants
These observations suggest why the hog1 point mutants result in DNA demethylation but not why the hog1 tagged mutants die. Given the rather general nature of the defect and the broad range of potential methylation targets, it seems likely that at very low SAM:SAH ratios, insufficient methylation of one or more targets results in lethality. DNA methylation may be a major contributing factor as plants homozygous for extreme alleles of ddm1 are unhealthy and have 30% wild-type levels of DNA methylation (Table 2; Vongs et al., 1993). Point mutants of the major maintenance DNA methylase MET1 are typically viable, but T-DNA insertions into the same gene can show zygotic lethality, reduced transmission, and a profound deficit of the homozygous class (Saze et al., 2003). This result suggests that the point mutants are leaky, and the complete loss of the major maintenance methylase is incompatible with normal zygotic development. The similarity of the effect of point mutants and T-DNA tags in HOG1 and MET1 may be a consequence of a common problem. Point mutants in both genes result in measurable hypomethylation and a reduction in plant vigor, and insertion (null) mutants presumably cause more extreme hypomethylation, zygotic lethality, and the presence of aborted seeds. In the case of inserts in the HOG1 gene, it cannot be proven that the lethality is a direct result of DNA hypomethylation as the increased levels of SAH might poison other SAM-dependent reactions affecting other targets, such as protein methylation. The hog1-1 mutant is pleiotropic with several unrelated phenotypes, most of which are not shared with other methylation mutants, some of which may be attributable to hypomethylation of non-DNA targets. Silenced ADK Arabidopsis lines (Moffatt et al., 2002) and antisense SAH hydrolase tobacco lines (Tanaka et al., 1997) have distinct and different phenotypes, but neither particularly resembles hog1-1 homozygotes. It may be that subtle metabolic differences between mutants and transgenics result in substantial phenotypic disparities. The lethality of the tagged hog1 alleles was a surprise given the data from the antisense lines, but antisense transgenic lines are dominant and dominant lethal traits are lost as soon as they arise.
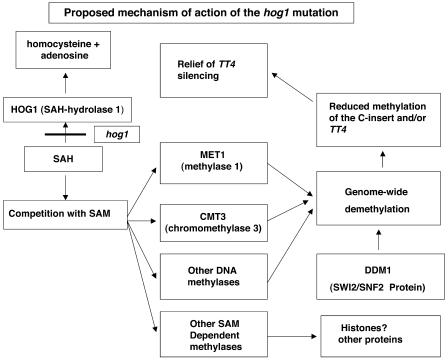
A Model for hog1, DNA Methylation, and C-Insert Silencing
The downstream targets of SAH buildup that affect DNA methylation might be expected to include the known DNA methylases. Because the met1 and cmt3 mutants have the same effect on C-insert silencing as hog1 (Table 1), both are good downstream candidates (this is illustrated in Figure 4). The ddm1 mutant also relieves DNA methylation and C-insert silencing but has been placed to one side because the defect in DNA methylation appears to be an indirect consequence of the genetic lesion (Jeddeloh et al., 1999). In addition, SAH levels may directly or indirectly affect DNA methylation via its effects on the activities of other SAM-dependent methylases of DNA or of other substrates, as illustrated. All four mutants converge on genome-wide hypomethylation (this includes the C-insert). The methylated C-insert produces a signal that results in silencing and DNA methylation of TT4. It is unclear whether it is the demethylation of the C-insert or of TT4 that is critical in the relief of HDG silencing. Because hypermethylated TT4 alleles are expressed and the ongoing presence of the C-insert is required to maintain the silencing, hypermethylation of TT4 alone is not sufficient for silencing (Davies et al., 1997).
Figure 4.
An Integrated Model to Explain the Mechanism of Action of the hog1 Mutants on C-Insert–Induced Silencing of the TT4 Locus.
Also indicated in the model are proposed roles for genes corresponding to other mutants impaired in DNA methylation (ddm1, met1, and cmt3) that also relieve C-insert silencing.
The observations presented are compatible with models of gene silencing where methylated inserts produce a signal that targets both methylation and gene silencing to themselves and homologous targets at distant sites (Furner et al., 1998). In single element transcriptional gene silencing (like L5), mutation-induced hypomethylation is sufficient to allow transcription and gene expression. The insert retains the capacity to resilence once the methylation mutation is corrected or crossed away. In two-element systems (C-insert and TT4) the biological role of the DNA methylation is much less clear. One possibility is that transcripts from the methylated C-insert are in some way different from those of the unmethylated C-insert. The former might effectively target HDG silencing while the latter do not. The genomes of higher plants do not normally contain transgenes, but they do contain very large numbers of transposons, retrotransposons, repeat families, pseudogenes, and repeated genes. The SAH hydrolase gene is not directly involved in either gene silencing or HDG silencing, but mutations in HOG1 destabilize maintenance methylation and have the potential to affect expression of thousands of sequences that are normally under the control of silencing and HDG silencing systems.
METHODS
Growth of plants, selection of mutants, anthocyanin induction, genetics, DNA gel blots, and RNA gel blots were largely performed as before (Davies et al., 1997; Furner et al., 1998).
Determination of 5-Methyl-Cytosine Levels in DNA
The measurement of the DNA methylation in total DNA was based on the method published by Vongs et al. (1993). The enzyme TaqI was used to generate CpG and mCpG ends to label. Chromatography plates were autoradiographed and the spots cut out and counted by scintillation.
Mapping and the hog1-1 Mutation
To identify the HOG1 gene, we set about mapping the hog1-1 mutation in crosses between the hog1-1 and C-insert double homozygote in the Landsberg erecta background to wild-type (HOG1) untransformed Columbia plants. As both the C-insert and the hog1-1 mutation were segregating in the cross, a 7:9 ratio of purple-to-green plants was found in the F2 generation. The purple class was a composite of two classes, 4/7 had no T-DNA, and 3/7 had at least one copy of the C-insert and were also homozygous for the hog1-1 mutation. restiction fragment length polymorphism and cleaved-amplified polymorphic sequence markers were used to analyze the T-DNA containing class. It was previously determined that the C-insert was at the top of chromosome 3 (data not shown). The DNA from 72 families was assessed with a variety of molecular probes covering the entire genome looking for regions with an excess of Landsberg markers that were not linked to the C-insert. The markers were based on information from TAIR and/or NASC Web sites. For restiction fragment length polymorphism markers, the corresponding clones were obtained from ABRC, and for cleaved-amplified polymorphic sequence markers, appropriate primers were synthesized. Once preliminary evidence of linkage of hog1-1 to probes on chromosome 4 was found, a detailed analysis was undertaken with more probes in the region.
Generation of Modifiers of C-Insert Silencing of TT4
The hog1-2 and hog1-3 modifiers were obtained in genetic screens of EMS-mutagenized populations largely as described by Furner et al. (1998). Seedlings were induced to produce anthocyanin with 2% sucrose (Davies et al., 1997) and purple individuals rescued for further analysis. Homozygotes of these mutants fail to complement the original hog1-1 mutant, giving a uniform purple F1 generation in crosses, and no green segregants were found in the F2 generation.
Determination of the hog1-5 Genotype in Segregating Populations
To assess the genotype of individuals in the population segregating for hog1-5 in their seedling progeny, the presence of the wild-type allele was tested by PCR amplification with primers (SALK 023915 LP 5′-CCGTCACTCACTCCCTGATGG-3′) and (SALK 023915 RP 5′-CAAGCACAACCTTCAAAAGCCA-3′); the hog1-5 allele was detected with primers (SALK LBb1 5′-GCGTGGAAAAACCGCTTGCTGCAACT-3′) and (SALK 023915 LP).
Sequencing the hog1 Alleles
Once it appeared that the gene coding for SAH hydrolase 1 was a candidate for the HOG1 gene, specific primers were designed to amplify the gene and to sequence the wild-type and mutant alleles. Because the gene was comparatively long, it was amplified in two segments. Segment one was amplified with SAHH-13511F (5′-ACGACTCTGTCACCAAGAGC-3′) and SAHH-14592R (5′-AAGTAGATCCGAACCTGAACC-3′), and segment 2 was amplified with SAHH-12515F (5′-AACTACGATCTACGATTCACTG-3′) and SAHH-13818R (5′-AACCATACAAGTTGTCGAACTG-3′). Sequencing was performed with the end primers and further primers internal to the amplified regions. All mutations were sequenced on both strands.
SAH Hydrolase Assay and SAM and SAH Determination
The assay was performed on extracts of various tissues of bolting plants as described by Moffatt et al. (2002). Pools of SAH and SAM were measured as previously described (Moffatt et al., 2002).
Complementation of the hog1-1 Mutation
The BAC clone T20K8 spanning the gene coding for SAH hydrolase 1 was obtained from the ABRC. An 8.5-kb ClaI containing the entire SAH hydrolase 1 gene was subcloned into pBluescript KS+. The plasmid was then linearized with NotI and ligated into pEC2 (Cartea et al., 1998). The resulting plasmid was transformed into Agrobacterium tumefaciens, and Arabidopsis thaliana transformation was performed using vacuum infiltration of flowers (Bechtold and Pelletier, 1998). The GUS staining L5-hog1-1 line was treated with Agrobacterium and the T1 generation seedlings sprayed with Basta (100 μg/mL) to select transformants. The surviving plants were allowed to flower and self-fertilize. Complementation of the hog1-1 mutation was assessed by staining T1 tissue and T2 seedlings for GUS.
Biological Materials
The C-insert transgenic (N1695), the hog1-1 point mutants (N1892), hog1-2 (NS9392), hog1-3 (NS9393), and the hog1-5 tagged mutant (NS023915) are available from NASC. The tagged met1-7 allele is available from NASC (stock number N576522).The SAH hydrolase 2–tagged mutant (T-DNA inserted in AT3G23810) can be obtained from GABI-Kat under the name 139A12 and the hog1-4 DS tag (GT1724) from the Cold Spring Harbor Laboratory.
Acknowledgments
We would like to thank the Biotechnology and Biological Science Research Council for funding the work, Noel Cogan for sequencing the hog1-3 allele, and four unnamed reviewers for advice and help revising the manuscript. We would also like to thank the following organizations and individuals for seeds and/or bioinformatics support: NASC, ABRC, TAIR, GABI-Kat, SIGnAL, ATIDB, Steve Jacobsen, Eric Richards, Varsha Wesley, and Peter Waterhouse.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ian Furner (ijf@mole.bio.cam.ac.uk).
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.028332.
References
- Amedeo, P., Habu, Y., Afsar, K., Scheid, O.M., and Paszkowski, J. (2000). Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 405, 203–206. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Aufsatz, W., Mette, M.F., van der Winden, J., Matzke, M., and Matzke, A.J. (2002). HDA6, a putative histone deacetylase needed to enhance DNA methylation induced by double-stranded RNA. EMBO J. 21, 6832–6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartee, L., Malagnac, F., and Bender, J. (2001). Arabidopsis cmt3 chromomethylase mutations block non-CG methylation and silencing of an endogenous gene. Genes Dev. 15, 1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82, 259–266. [DOI] [PubMed] [Google Scholar]
- Béclin, C., Boutet, S., Waterhouse, P., and Vaucheret, H. (2002). A branched pathway for transgene-induced RNA silencing in plants. Curr. Biol. 12, 684–688. [DOI] [PubMed] [Google Scholar]
- Boutet, S., Vazquez, F., Liu, J., Béclin, C., Fagard, M., Gratias, A., Morel, J.B., Crete, P., Chen, X., and Vaucheret, H. (2003). Arabidopsis HEN1: A genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr. Biol. 13, 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., and Jacobsen, S.E. (2002). Role of the arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr. Biol. 12, 1138–1144. [DOI] [PubMed] [Google Scholar]
- Cartea, M.E., Migdal, M., Galle, A.M., Pelletier, G., and Guerche, P. (1998). Comparison of sense and antisense methodologies for modifying the fatty acid composition of Arabidopsis thaliana oilseed. Plant Sci. 136, 181–194. [Google Scholar]
- Dalmay, T., Hamilton, A., Rudd, S., Angell, S., and Baulcombe, D.C. (2000). An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101, 543–553. [DOI] [PubMed] [Google Scholar]
- Dalmay, T., Horsefield, R., Braunstein, T.H., and Baulcombe, D.C. (2001). SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J. 17, 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, G.J., Sheikh, M.A., Ratcliffe, O.J., Coupland, G., and Furner, I.J. (1997). Genetics of homology-dependent gene silencing in Arabidopsis; a role for methylation. Plant J. 12, 791–804. [DOI] [PubMed] [Google Scholar]
- Elmayan, T., Balzergue, S., Beon, F., Bourdon, V., Daubremet, J., Guenet, Y., Mourrain, P., Palauqui, J.C., Vernhettes, S., Vialle, T., Wostrikoff, K., and Vaucheret, H. (1998). Arabidopsis mutants impaired in cosuppression. Plant Cell 10, 1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagard, M., Boutet, S., Morel, J.B., Bellini, C., and Vaucheret, H. (2000). AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc. Natl. Acad. Sci. USA 97, 11650–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojtová, M., Kovarik, A., Votruba, I., and Holý, A. (1998). Evaluation of the impact of S-adenosylhomocysteine pools on cytosine methylation of the tobacco genome. Eur. J. Biochem. 252, 347–352. [DOI] [PubMed] [Google Scholar]
- Furner, I.J., Sheikh, M.A., and Collett, C.E. (1998). Gene silencing and homology-dependent gene silencing in Arabidopsis: Genetic modifiers and DNA methylation. Genetics 149, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazov, E., Phillips, K., Budziszewski, G.J., Meins, F., Jr., and Levin, J.Z. (2003). A gene encoding an RNase D exonuclease-like protein is required for post-transcriptional silencing in Arabidopsis. Plant J. 35, 342–349. [DOI] [PubMed] [Google Scholar]
- Hu, Y., Komoto, J., Huang, Y., Gomi, T., Ogawa, H., Takata, Y., Fujioka, M., and Takusagawa, F. (1999). Crystal structure of S-adenosylhomocysteine hydrolase from rat liver. Biochemistry 38, 8323–8333. [DOI] [PubMed] [Google Scholar]
- Jackson, J.P., Lindroth, A.M., Cao, X., and Jacobsen, S.E. (2002). Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416, 556–560. [DOI] [PubMed] [Google Scholar]
- Jeddeloh, J.A., Stokes, T.L., and Richards, E.J. (1999). Maintenance of genomic methylation requires a SWI2/SNF2-like protein. Nat. Genet. 22, 94–97. [DOI] [PubMed] [Google Scholar]
- Kanno, T., Mette, M.F., Kreil, D.P., Aufsatz, W., Matzke, M., and Matzke, A.J. (2004). Involvement of putative SNF2 chromatin remodeling protein DRD1 in RNA-directed DNA methylation. Curr. Biol. 14, 801–805. [DOI] [PubMed] [Google Scholar]
- Komoto, J., Huang, Y., Gomi, T., Ogawa, H., Takata, Y., Fujioka, M., and Takusagawa, F. (2000). Effects of site-directed mutagenesis on structure and function of recombinant rat liver S-adenosylhomocysteine hydrolase. J. Biol. Chem. 275, 32147–32156. [DOI] [PubMed] [Google Scholar]
- Kovarik, A., Van Houdt, H., Holy, A., and Depiker, A. (2000). Drug induced hypomethylation of a posttranscriptionally silenced transgene locus of tobacco leads to partial release of silencing. FEBS Lett. 467, 47–51. [DOI] [PubMed] [Google Scholar]
- Kurowski, M.A., and Bujnicki, J.M. (2003). GeneSilico protein structure prediction meta-server. Nucleic Acids Res. 31, 3305–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Rosso, M.G., Strizhov, N., Viehoever, P., and Weisshaar, B. (2003). GABI-Kat SimpleSearch: A flanking sequence tag (FST) database for the identification of T-DNA insertion mutants in Arabidopsis thaliana. Bioinformatics 19, 1441–1442. [DOI] [PubMed] [Google Scholar]
- Lindroth, A.M., Cao, X., Jackson, J.P., Zilberman, D., McCallum, C.M., Henikoff, S., and Jacobsen, S.E. (2001). Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 92, 2077–2080. [DOI] [PubMed] [Google Scholar]
- Mette, M.F., Aufsatz, W., van der Winden, J., Matzke, M.A., and Matzke, A.J. (2000). Transcriptional silencing and promoter methylation triggered by double-stranded RNA. EMBO J. 19, 5194–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt, B.A., Stevens, Y.Y., Allen, M.S., Snider, J.D., Pereira, L.A., Todorova, M.I., Summers, P.S., Weretilnyk, E.A., Martin-McCaffrey, L., and Wagner, C. (2002). Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol. 128, 812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt, B.A., and Weretilnyk, E.A. (2001). Sustaining S-adenosyl-L-methionine-dependent methyltransferase activity in plant cells. Physiol. Plant. 113, 435–442. [Google Scholar]
- Morel, J.-B., Mourrain, P., Beclin, C., and Vaucheret, H. (2000). DNA methylation and chromatin structure affect transcriptional and post-transcriptional transgene silencing in Arabidopsis. Curr. Biol. 10, 1591–1594. [DOI] [PubMed] [Google Scholar]
- Morel, J.B., Godon, C., Mourrain, P., Beclin, C., Boutet, S., Feuerbach, F., Proux, F., and Vaucheret, H. (2002). Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell 14, 629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourrain, P., et al. (2000). Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101, 533–542. [DOI] [PubMed] [Google Scholar]
- Probst, A.V., Fagard, M., Proux, F., Mourrain, P., Boutet, S., Earley, K., Lawrence, R.J., Pikaard, C.S., Murfett, J., Furner, I.J., Vaucheret, H., and Scheid, O.M. (2004). Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 16, 1021–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze, H., Scheid, O.M., and Paszkowski, J. (2003). Maintenance of CpG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat. Genet. 34, 65–69. [DOI] [PubMed] [Google Scholar]
- Scheid, O.M., Afsar, K., and Paszkowski, J. (1998). Release of epigenetic gene silencing by trans-acting mutations in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen, T., Vijn, I., Rebocho, A., van Blokland, R., Roelofs, D., Mol, J.N., and Kooter, J.M. (2001). Transcriptional and posttranscriptional gene silencing are mechanistically related. Curr. Biol. 11, 436–440. [DOI] [PubMed] [Google Scholar]
- Tanaka, H., Masuta, C., Uehara, K., Katoaka, J., Koiwai, A., and Noma, N. (1997). Morphological changes and hypomethylation of DNA in transgenic tobacco expressing antisense RNA of the S-adenosyl-L-homocysteine hydrolase gene. Plant Mol. Biol. 35, 981–986. [DOI] [PubMed] [Google Scholar]
- Wesley, S.V., et al. (2001). Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J. 27, 581–590. [DOI] [PubMed] [Google Scholar]
- Vongs, A., Kakutani, T., Martienssen, R.A., and Richards, E.J. (1993). Arabidopsis thaliana DNA methylation mutants. Science 260, 1926–1928. [DOI] [PubMed] [Google Scholar]
- Zilberman, D., Cao, X., and Jacobsen, S.E. (2003). ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299, 716–719. [DOI] [PubMed] [Google Scholar]