Abstract
This review provides a comprehensive survey of proprietary drug discovery and development efforts performed by Indian companies between 1994 and mid‐2016. It is based on the identification and detailed analysis of pharmaceutical, biotechnology, and contract research companies active in proprietary new chemical entity (NCE) research and development (R&D) in India. Information on preclinical and clinical development compounds was collected by company, therapeutic indication, mode of action, target class, and development status. The analysis focuses on the overall pipeline and its evolution over two decades, contributions by type of company, therapeutic focus, attrition rates, and contribution to Western pharmaceutical pipelines through licensing agreements. This comprehensive analysis is the first of its kind, and, in our view, represents a significant contribution to the understanding of the current state of the drug discovery and development industry in India.
Keywords: drug development, drug discovery, India, licensing, pharmaceutical R&D pipeline
1. Introduction
In 1997, DRF‐2593, later known as balaglitazone, a small‐molecule peroxisome proliferator‐activated receptor γ (PPARγ) agonist discovered at Dr. Reddy's Laboratories (DRL) in Hyderabad, became the first preclinical‐stage compound discovered at an Indian pharmaceutical company to be licensed to a Western company, Novo Nordisk.1 This deal was hailed by many at that time as only the first step on the way to what was expected to become a growing flow of successful drugs from India. However, by 2009, DRL had terminated all its internal discovery activities, without having launched a single new drug.2 This exit from early in‐house pharmaceutical research activities followed the one in 2008 by Ranbaxy Laboratories, and preceded by five years the one of a third major Indian company, Piramal Enterprises, in 2014.3, 4 Other Indian companies have abandoned, significantly decreased, or postponed their research efforts in recent years, or are experiencing slow progress, if any, with major pipeline products. Proprietary drug discovery activities in India had been initiated since the mid‐1990s by companies that had been active in the generics business, often for several decades. At that time, India was poised to become the drug discovery powerhouse of the world, as it had become its generics pharmacy. We know today that this grand vision did not materialize. But does this mean the end of the drug discovery industry in India, the “Death of a Dream” as some have claimed?5 We think this deserves a more thorough analysis.
Drug discovery and pharmaceutical R&D in India has been reviewed and analyzed previously, but generally from specific points of view, such as its historical background,6, 7, 8 the fate of the big pharma companies,5, 9 or the impact of process versus product patent output as a consequence of the Agreement on Trade‐Related Aspects of Intellectual Property Rights (the so‐called TRIPS agreement).10 Other authors have analyzed its socioeconomic impact and public policy implications and recommendations,11, 12, 13, 14, 15 drug development in India and in comparison with other emerging countries,16, 17 drug discovery in private companies and in publicly funded institutions,18, 19 current changes and opportunities,20, 21 or out‐licensing deals.22 We recently reviewed contract and collaborative research alliances between Indian and global pharmaceutical companies.23 Although these components represent valuable contributions to the understanding of India's current pharmaceutical industry environment, they lack a comprehensive analysis of the overall scientific productivity of proprietary drug discovery in India, its ongoing achievements in contributing to the global drug discovery and development pipeline, but also its challenges, the knowledge of which is required if one wants to better understand the country's current situation.
In this review, we attempt to fill this gap by describing and analyzing in a comprehensive manner the contributions to proprietary drug discovery by Indian companies, and by highlighting how the landscape of industrial pharmaceutical research in India, as we know it today, has evolved from its beginnings over two decades ago, into the current ensemble of pharmaceutical and biotechnology companies.
2. Analysis Data and Methods
In our analysis we aim to cover all proprietary research by Indian companies on new chemical entities (NCEs) that have reached preclinical or clinical development stages between its beginnings in 1994 and mid‐2016. We set out by identifying and screening today's top‐100 Indian pharmaceutical and biotechnology companies, as assessed from their latest available annual revenue figures, that is, annual reports of public companies, and various sources for privately held companies (Supporting Information Table 5).24, 25, 26, 27 We included former top‐100 companies with contributions to drug discovery, that have been acquired since, that is, three companies. These have been complemented by all early‐stage companies identified by an extensive analysis of Indian drug development activities. We have included several companies headquartered outside of India, but with Indian founders, and in general discovery and development activities run out of India. Not included in the analysis are Indian subsidiaries of multinational companies, although one company is specifically mentioned in the text for its contributions (AstraZeneca India). We have focused our work specifically on small‐molecule drug discovery, and have excluded biologics, vaccines, botanical drugs, herbal extracts, or combination preparations of existing commercial drugs, and repositioned existing products. Prodrugs or polymer‐supported forms of marketed drugs, as well as peptides were only included if they were part of broader NCE drug discovery efforts. Compounds that were in‐licensed for clinical development purposes from external sources are mentioned in text and tables as integral part of company backgrounds, although they are not included in calculations nor figures. Not included either are drug discovery projects at Indian academic and public research institutions, unless these were done in collaboration with pharma companies and led to joint patent applications. More comprehensive reports on these have been published elsewhere.8, 18, 19, 28, 29, 30, 31
For each company, we have analyzed annual reports, as available from company websites or from service providers,32 company or investor presentations, web pages, press releases, and published patent applications.33 We have compiled all available information, including, where available, compound codes, therapeutic areas, modes of action, years in which development phases were initiated, highest stage of development reached, current status of development, and eventually status of partnerships or licensing deals with global pharmaceutical companies. Our analysis reflects the status of the drug discovery pipeline in India by mid‐2016.
Several clarifications and limitations regarding our approach are noteworthy at the outset. First, companies were identified based on their publicly disclosed scientific output in terms of development compounds or patent applications. Although we are confident to have included all relevant companies and compounds in our analysis, we cannot exclude minor omissions. Should this be the case, the overall impact on our analysis would be small, given the large number of companies and compounds included in this review.
Second, timelines were assessed based on the years during which the information was disclosed. As this includes annual reports, actual decision dates can be offset by up to a full year. Further, as the term “preclinical” can represent stages from early research to preclinical studies enabling Investigational New Drug (IND) applications, we have made efforts to include only compounds beyond the early discovery stage, that is, our inclusion criteria have been stricter than in an earlier preliminary short report.34 Also, as the termination of development is not always publicized, we assumed it occurred during the year in which the compound disappeared from company reports or presentations.
Finally, although considerable efforts have been made to identify all relevant documents, limitations in the amounts of publicly available information, and time‐lags between conducting research and disclosing the results publicly, means that some projects might not have been captured correctly in our analysis. Despite these limitations, we strongly believe the data presented provides valuable information, as it gives for the first time a comprehensive view over two decades of proprietary Indian pharmaceutical R&D.
3. Drug Discovery in India
3.1. Historical background
India's oldest pharmaceutical companies were established at the turn of the past century, such as Bengal Chemicals & Pharmaceuticals Ltd. (1901), or Alembic Chemical Works (1907) to manufacture quality chemicals, pharmaceuticals and home products.12 The beginning of drug discovery in the country can be traced back to the second decade of the twentieth century, with work on drugs for visceral leishmaniasis, also known as kala‐azar, by Upendranath Brahmachari at the Campbell Medical College, Calcutta, one of the oldest medical schools to teach European medicine in India, which led to “urea stibamine”, introduced in 1922.8, 35 The drug was designed to decrease the toxicity of known inorganic pentavalent antimony salts by their incorporation into organometallic aniline derivatives, inspired by Ehrlich's salvarsan, an organoarsenic compound for the treatment of syphilis, that had been introduced in 1910. Urea stibamine was only the second successful anti‐infective agent to be developed in the world, saved countless patients in the 1920–1930s, and paved the way for the development of more recent antimonials such as sodium stibogluconate, or meglumine antimoniate, both on the World Health Organization (WHO) list of essential medicines.35, 36 Although not a drug, oral rehydration therapy (ORT), that is, drinking water with controlled amounts of salt and sugar, considered as “potentially the most important medical advance of the [20th] century”,37 was first discovered by H. N. Chatterjee, a medical practitioner working on cholera patients in Calcutta. Despite being published 1953 in The Lancet, it was unfortunately ignored, only to be rediscovered in 1968 by Western scientists.38
During the early years after independence, the Government of India, through its Council of Scientific and Industrial Research (CSIR), established the Central Drug Research Institute (CDRI) in Lucknow to lead the country's efforts in drug research and development (1951),8, 39, 40 followed later by other public institutions such as the Regional Research Laboratories in Hyderabad (1956, now Indian Institute of Chemical Technology, IICT),41 and in Jammu (1957, now Indian Institute of Integrative Medicine, IIIM).42 CDRI′s main focus was to identify lead molecules for tropical diseases and population control measures from medicinal plants, initially relying on the country's traditional systems of medicine such as Ayurveda and Unani, but later expanded to include other plants, and synthetic small molecules. The knowledge of ayurvedic remedies resulted in a number of drugs or standardized extracts with identified active compounds: “gum guggulu”, derived from Commiphora mukul, led to the identification of guggulsterones, antagonists of the farnesoid X receptor (FXR) receptor with lipid‐lowering properties, approved 1986 in India as an extract under the name Gugulipid;40, 43 “brahmi”, prepared from Bacopa monnieri, gave triterpenoid glycoside modulators of the serotonin system as memory enhancers,44 launched in 1996 as a standardized extract under the name Memory plus.40 The search for antimalarials, based on the investigation of Artemisia annua, used in traditional Chinese medicine, resulted in arteether, a drug able to cure multidrug‐resistant or chloroquine‐resistant Plasmodium falciparum, approved in 1998.40 Further research efforts in India to discover new drugs from plants have been reviewed recently.45, 46 In the area of small‐molecule drug discovery, centchroman (ormeloxifene), a selective estrogen receptor modulator, and the world's first nonsteroidal oral contraceptive, introduced in 1990, remains among CDRI′s major achievements.47 The institute's current challenges such as a decrease in the output of new drugs have been discussed recently, and potential opportunities include the revival of natural products chemistry, re‐emphasizing phenotypic assays, and strengthening mode of action and target identification capabilities.19 Notable discoveries made at other public laboratories are enfenamic acid, an anti‐inflammatory agent (IICT, 1980),7 or more recently risorin, a combination preparation for tuberculosis (IIIM, 2009).48 A list of projects toward drug discovery research at additional CSIR‐funded institutions is available at the Council's website.49
In these early years, the pharmaceutical industry as a whole, including during the two decades after India's independence, was dominated by multinational companies, that imported their bulk products, even after the setup in 1960 of public sector companies, such as Hindustan Antibiotics Ltd., and Indian Drugs and Pharmaceuticals Ltd. Notable discoveries made by multinational companies in India were for example reserpine, an indole alkaloid with antipsychotic and antihypertensive properties isolated from Rauwolfia serpentina at the Ciba Research Centre in Mumbai (then Bombay, 1952),50 or forskolin, a diterpenoid adenylate cyclase activator (1974), co‐discovered independently as coloneol at CDRI,45 and flavopiridol, the first cyclin‐dependent kinase inhibitor, derived from the natural product rohitukine (1990s), both at the Hoechst Bombay Research Centre.51
This changed radically with the Indian Patent Act of 1970, which abolished product patents for pharmaceutical ingredients, recognizing only patents with a decreased term (5–7 years) for process improvements (also called reverse engineering, allowing to copy foreign patented drugs), which was much less challenging than innovative drug discovery. The ensuing rise of local competitors, the loss of royalties from product patents, and new laws limiting the price of certain drugs, as well as decreasing the ownership of multinational companies in their Indian enterprises to a maximum of 40 %, led most multinational companies to leave India.52 This all strengthened the growing local generics industry, which became “the world's pharmacy”, according to Médecins Sans Frontières (or Doctors Without Borders) accounting currently for 10 % of the global pharmaceutical production, and 20 % of global exports of generics in volume terms.53
India's “New Economic Policy” of 1991, aiming to liberalize the country's economic policies, and its joining the World Trade Organization (WTO) in 1995, which included the signature of the agreement on TRIPS, with a ten year transition period from 1995 to 2005, and the Indian Patents (Amendment) Act 2005, opened the country again to product patents, and consequently to pharmaceutical innovation.10 The main industry players recognized the unique opportunities offered by these changes.20
To reflect the significant changes that the entire industry went through over two decades, we have chosen to present our data by company in a chronological order, starting with the large Indian pharma companies that were the first to enter the field (Table 1). These were followed by contract research organizations (CROs), a limited number of which initiated also proprietary projects, and more recently, by biotechnology and startup companies specializing in integrated proprietary drug discovery.
Table 1.
Drug discovery activities at major Indian pharmaceutical companies 1994–2016.
| Entry | Company | Year drug discovery initiated | Year company established | Rank[a] | Estimated drug discovery or R&D headcount (year) | Total pipeline compounds[b] | Status 2016 |
|---|---|---|---|---|---|---|---|
| 1 | Dr. Reddy's Laboratories | 1994 | 1984 | 2 | 320 in NCE R&D (2005) | 27 | Exited drug discovery in 2009 |
| 2 | Ranbaxy (now part of Sun Pharma) | 1995 | 1961 | (−)[c] | 280 in “New Drug Discovery Research”; 1400 in R&D (2008) | 16 | Exited drug discovery in 2008 after acquisition by Daiichi‐Sankyo |
| 3 | Torrent Pharmaceuticals | 1997 | 1972 | 9 | 130 in NCE discovery research (2008–14) | 6 | Active NCE R&D, three compounds in pipeline |
| 4 | Wockhardt Ltd. | 1997 | 1967 | 13 | 850 in R&D (2015) | 15 | Active NCE R&D, five compounds in pipeline |
| 5 | Piramal Life Sciences | 1998 | 1988 | 17 | 360 in NCE R&D (2011) | 16 | Exited drug discovery in 2014 |
| 6 | Dabur Research Foundation (now Fresenius‐Kabi Oncology) | 1998 | 1886 | (−)[d] | 20 in oncology drug discovery (2001) | 0 | Exited drug discovery in 2010 after acquisition by Fresenius‐Kabi |
| 7 | Sun Pharma | 1999 | 1993 | 1 | 275 at SPARC R&D (2015) | 8 | Active NCE R&D, three compounds in pipeline |
| 8 | Alembic Ltd. | 1999 | 1907 | 20 | NA | 0 | Collaboration with NCL (1999‐2003); investment in Incozen Therapeutics and Rhizen Pharmaceuticals (2008) |
| 9 | Zydus Cadila (Cadila Healthcare) | 2000 | 1995 | 6 | 250 in NCE R&D (2016) | 15 | Active NCE R&D, one compound launched (2013), four compounds in pipeline |
| 10 | Cadila Pharmaceuticals | 2000 | 1995 | 34 | NA | 0 | Develops mainly combination preparations; drug discovery collaboration with IIIM |
| 11 | FDC Ltd. | 2000 | 1940 | 42 | NA | 1 | Patent filings (2002–13) with National Chemical Laboratory (Pune), no internal drug discovery |
| 12 | JB Chemicals & Pharmaceuticals | 2000 | 1976 | 37 | NA | 1 | Exited drug discovery in 2006 |
| 13 | Cipla | 2000 | 1935 | 5 | NA | 0 | Patent filings (2000–01) with University of Mumbai, no internal drug discovery |
| 14 | Glenmark Pharmaceuticals | 2001 | 1977 | 8 | 300 NCE R&D (2015) | 19 | Active NCE R&D, four compounds licensed (all failed), one under option agreement (ongoing), one additionalcompound in pipeline |
| 15 | Lupin Ltd. | 2001 | 1972 | 3 | ∼130 in NCE drug discovery, 320 in R&D (2015) | 7 | Active NCE R&D, four compounds in pipeline |
| 16 | Reliance Life Sciences | 2001 | 2001 | 58 | NA | 2 | Exited NCE R&D in 2010 |
| 17 | Orchid Pharma | 2002 | 1992 | 47 | 130 in drug discovery (2013) | 5 | Halted drug discovery in 2014; one compound licensed to Allecra (2013), development ongoing |
| 18 | Suven Life Sciences | 2003 | 1989 | 59 | 120 in NCE R&D, out of 386 in R&D (2016) | 15 | Active NCE R&D, eleven compounds in pipeline |
| 19 | Natco Pharma | 2004 | 1981 | 38 | 15 in oncology drug discovery (2014) | 3 | Active NCE R&D, two compounds in pipeline |
| 20 | Panacea Biotech | 2005 | 1984 | 51 | 110 in drug discovery (2013) | 4 | Halted internal drug discovery in 2014; two compounds in pipeline |
| 21 | Matrix Laboratories (now Mylan) | 2005 | 2000 | (−)[e] | 18 in drug discovery (2006) | 2 | Exited drug discovery after acquisition by Mylan (2006) |
| 22 | Hetero Drugs | 2006 | 1993 | 7 | NA | 2 | NCE drug discovery mainly targeting HIV, also HCV, diabetes and cancer |
| 23 | Jubilant Life Sciences | 2007 | 1978 | 11 | NA | 3 | Active NCE R&D, three compounds in pipeline (one licensed in 2016) |
| 24 | Elder Pharmaceuticals | 2008 | 1989 | 44 | NA | 0 | Patents filed with Poona College of Pharmacy (2008), no internal drug discovery |
| 25 | IPCA Laboratories | 2009 | 1949 | 22 | NA | 0 | Claimed two compounds in pipeline (2012), but no development reported |
| 26 | Mankind Pharma | 2011 | 1995 | 15 | NA | 0 | No published NCE patent applications |
| 27 | Alkem Laboratories | 2013 | 1973 | 12 | 20 in drug discovery (2014) | 1 | Exited drug discovery prior to going public in 2015 |
| 28 | Emcure | 2014 | 1981 | 26 | NA | 0 | One published NCE patent application |
| Total: 168 |
[a] Ranking by 2016 revenue or latest available figure (Supporting Information Table 5). [b] Status mid‐2016. [c] Ranked #1 in acquisition year 2008. [d] Ranked #37 in acquisition year 2008. [e] Ranked among top‐10 in acquisition year 2006.
3.2. Initiation of drug discovery at large pharmaceutical companies
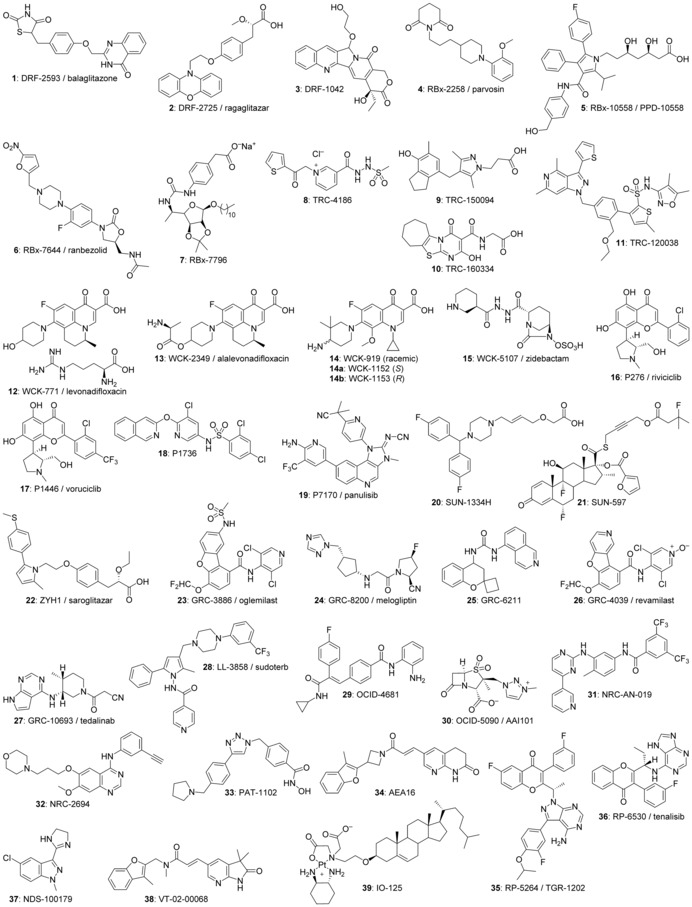
Dr. Reddy's Laboratories (DRL), established in 1984 for the manufacturing of active pharmaceutical ingredients (APIs), and with over 21 000 employees worldwide, had been the first Indian company to launch drug discovery research in 1994.32, 54 Between 1995 and 2009, DRL reported 27 development compounds, of which 12 reached the clinical development stage (Supporting Information Table 6a, entries 1–27). It was the first Indian company to out‐license, in 1997, a molecule, balaglitazone 1 (DRF‐2593), a thiazolidinone (or glitazone)‐type PPARγ agonist for the treatment of diabetes,55, 56 and in 1998 a second molecule, ragaglitazar 2 (DRF‐2725), a dual PPAR α/γ insulin sensitizer for metabolic disorders,56, 57 to a Western company, Novo Nordisk (Figure 1). Novo Nordisk eventually abandoned 2 in 2002, when bladder tumors were identified in rats during toxicology studies, and returned 1 to DRL in 2004. In 2005 DRL partnered with Rheosciences for the further development of 1, which in 2007 became the first Indian compound to reach the level of Phase 3 studies and were completed with DRL′s internal funds. Although positive clinical data were reported in 2010, the compound was abandoned in 2011, after Avandia (rosiglitazone), another compound of the glitazone family developed by GlaxoSmithKline (GSK), had been linked to apparent risks for increased heart attacks, and banned from use in Europe and India in 2010. DRL found additional partners in Novartis (2001) for the development of DRF‐4158, a dual PPARα/γ agonist and hydroxymethyl‐glutaryl‐coenzyme A (HMG‐CoA) reductase inhibitor for the treatment of diabetes (returned to DRL and abandoned in 2003),58 and in Clintech (2006) for DRF‐1042 (3), an orally active camptothecin analogue to treat solid tumors (abandoned in 2007).59, 60 In 2005, a joint venture led to the creation of Perlecan Pharma,61 with the aim to develop four DRL compounds: RUS‐3108, a perlecan inducer for the treatment of atherosclerosis;62 DRF‐10945, a non‐fibrate predominantly PPARα agonist for the treatment of metabolic disorders;61 DRL‐11605 a pan‐PPARα/δ/γ agonist for the treatment of obesity and dyslipidemia;61 and DRL‐16536, an AMP‐activated protein kinase (AMPK) modulator for metabolic disorders.63 All four compounds failed in preclinical or early clinical studies and were subsequently abandoned. In 2008, the joint venture was terminated, and DRL had to buy back the shares. The company entered into a collaboration with Argenta Discovery to develop novel treatments for chronic obstructive pulmonary disease (COPD), which led in 2007 to a clinical development compound,64 a PPARγ agonist, which was, however, abandoned the following year. Other compounds reached the clinical development stage at DRL, and all failed, including a second camptothecin derivative, DRF‐1644,65 (abandoned in 2005), and two compounds with an undisclosed mechanism of action for the treatment of dyslipidemia, DRL‐21994 and DRL‐21995.54 Fourteen more compounds did not pass the preclinical development stage.54 After these repeated failures, DRL announced in 2009 the closure of its research activities in Hyderabad, shifting its R&D focus to new drug delivery systems, improved generics and biosimilars.54 The company continued limited development activities, including on DRL‐17822, an orally active cholesteryl ester transfer protein (CETP) inhibitor for the treatment of dyslipidemia,66 which was, however, abandoned 2013 in Phase 2.
Figure 1.
Examples of development compounds.
Ranbaxy Laboratories, since 2015 part of Sun Pharma, was established in 1961, and employed more than 14 000 people in 2014.32, 67 The decision in the early 1990s to become a research‐based company led to the establishment of a new research center, and to the start of drug discovery projects in 1995, which grew to 280 scientists in 2008.67 The company's first proprietary molecule RBx‐2258 (parvosin) 4,67, 68 an adrenergic α1 receptor antagonist for the treatment of benign prostatic hyperplasia, reached clinical Phase 1 stage in 1999 (Supporting Information Table 6a entries 28–43). In 2002, Ranbaxy licensed out 4 to Schwarz Pharma, but the compound was stopped shortly after in Phase 2 development due to unconvincing clinical results. In 2003, Ranbaxy partnered with Medicines for Malaria Venture (MMV) for the development of arterolane or OZ277, subsequently called RBx‐11160, for the treatment of Plasmodium falciparum malaria, and was subsequently granted a worldwide license (Supporting Information Table 6b, entry 1).69, 70, 71 The compound reached the Indian market in April 2012 as Synriam, a combination of arterolane maleate and piperaquine phosphate, the first medicine developed (although not invented) by an Indian company.72 In 2003, Ranbaxy signed a deal with GlaxoSmithKline (GSK) for the discovery of treatments for respiratory diseases, which led to RBx‐10017609, a dual matrix metalloproteinase‐9 and ‐12 (MMP‐9/MMP‐12) inhibitor,73 which reached Phase 2 clinical studies, but has been abandoned since. In 2007, Ranbaxy out‐licensed its HMG‐CoA reductase inhibitor RBx‐10558 5 to Pharmaceutical Product Development (PPD) for the treatment of hypercholesterolemia.74 PPD, and subsequently its spin‐off Furiex undertook clinical studies, but further development of the compound was abandoned in 2011 due to lack of efficacy in Phase 2 studies.75 In 2008, Ranbaxy partnered with Merck Sharp and Dohme (MSD) for the development of antibacterials and antifungals, but the deal was called off in 2011. Ranbaxy's discovery group produced other proprietary molecules in a variety of therapeutic areas which reached the stage of clinical development: RBx‐4638 or clafrinast,76, 77 a very late antigen‐4 (VLA‐4) antagonist to treat asthma and COPD; RBx‐7644 (ranbezolid) 6, an oxazolidinone‐type protein synthesis inhibitor against bacterial infections;78 RBx‐7796 7, a 5‐lipoxygenase (5‐LO) inhibitor against asthma and allergic rhinitis;79 and RBx‐9841, a muscarinic acetylcholine M3 receptor inhibitor against urinary incontinence.80 Nine additional compounds were stopped at the preclinical development stage. In mid‐2008, however, Daiichi‐Sankyo acquired a majority stake in Ranbaxy, which led to the termination of in‐house research projects. In 2015 Daiichi‐Sankyo sold the generics business of Ranbaxy to Sun Pharma, and in January 2017 decided to close the remaining R&D site in Gurgaon.67, 81
Torrent Pharmaceuticals Ltd. was incorporated 1972 with currently over 10 000 employees, and focuses on manufacturing and distributing generics, as well as on contract manufacturing, in particular for the production of insulin for Novo Nordisk.32, 82 The company inaugurated its Torrent Research Centre (TRC) 1996 in Ahmedabad, and launched in‐house drug discovery projects the following year, with a focus on metabolic disorders and related diseases, including cardiovascular disorders, ischemia, and later COPD. In addition to novel NCE research, Torrent's R&D group focused its efforts on process and formulation development for existing APIs, and grew rapidly to around 800 people, including 130 in discovery research, with so far three clinical stage compounds (Supporting Information Table 6a, entries 44–49). TRC‐4186 8,16, 83, 84 an advanced glycation end‐product (AGE) breaker compound, to which Novartis took an option in 2002, but returned the compound in 2005. Torrent currently develops the compound on its own, and completed Phase 2 clinical studies in 2015. TRC150094 9, a mimetic of diiodothyronine (T2) (T2 mimetic),16, 85 for the treatment of cardiometabolic risks is currently undergoing Phase 2 studies. TRC160334 10, a hypoxia‐inducible factor (HIF) hydroxylase inhibitor to treat kidney failure and intestinal bowel disease (IBD), is undergoing Phase 1 studies.86 Two more compounds failed at the preclinical stage: TRC‐282,87 a NO donor for cardiovascular disorders, and TRC‐8156,88 a dipeptidyl peptidase‐IV (DPP‐IV) inhibitor for the treatment of diabetes. AstraZeneca initiated in 2005 a cost sharing collaboration with Torrent for the development of drugs against hypertension, in which the intellectual property (IP) belonged to Torrent. This led to a patent application on dual angiotensin II receptor type 1 (AT1 receptor) and endothelin type A (ETA) receptor antagonists.23 Of these one compound, possibly TRC 120038,89 11 entered preclinical toxicology studies in 2008, although no further progress has been reported since.
Wockhardt Ltd., headquartered in Mumbai, was founded in 1967, and manufactures and markets generics and biosimilars with currently about 10 000 employees.32, 90 The company initiated drug discovery in 1997, focusing on treatments for bacterial infections, and brought 15 compounds into development, including salts and single enantiomers, of which five progressed into the clinic (Supporting Information Table 6a, entries 50–64): WCK‐771 or levonadifloxacin 12, specifically developed for use in parenteral administration,91, 92 is the arginine salt of the S enantiomer of the fluoroquinoline class antibiotic nadifloxacin, a DNA gyrase inhibitor developed and launched in 1993 by Otsuka for the topical treatment of acne. As such, it is not an NCE, but we have included it in the analysis because the compound is an important part of the company's discovery platform, which includes derivatives and prodrugs; WCK‐2349 or alalevonadifloxacin 13,93 an oral amino acid ester prodrug of WCK‐771 in Phase 2 in India; WCK‐1152 14,91 also a fluoroquinoline (abandoned); WCK‐4873 or nafithromycin, a ketolide‐type protein synthesis inhibitor completed Phase 1 studies in 2015 in the US;90, 94, 95 and WCK‐5107 (or zidebactam 15, a dual penicillin‐binding protein 2 (PBP2) and β‐lactamase inhibitor.96 Five compounds or combination preparations received recently the qualified infectious disease product (QIDP) status,94 which allows fast‐track development and review of the drug application by the US Food and Drug Administration (FDA), and is granted to drugs acting against pathogens which have a high degree of unmet needs, such as methicillin‐resistant Staphylococcus aureus (MRSA).
Piramal Enterprises Ltd., as the company has been known since 2012, was established in 1988 as Nicholas Piramal India Ltd., and entered drug discovery research in 1998 with the acquisition of the Hoechst Research Centre in Mumbai.32, 97 Piramal's R&D activities, focusing on oncology, inflammation, metabolic disorders and infections, grew to over 460 scientists in 2012, of which 360 were in NCE discovery research. The company filed its first patent application in 2002 on inhibitors of cyclin‐dependent kinases (CDK) for the treatment of cancer, and in 2005 initiated its first clinical Phase 1 studies on P276 (riviciclib) 16,98 a novel CDK4 inhibitor, terminated in 2013 after a setback in Phase 2 clinical trials (Supporting Information Table 6a, entries 65–80). P1446 or voruciclib 17,98 a second CDK4 inhibitor, entered clinical development in 2008. In January 2007 Piramal announced a collaboration with Eli Lilly & Co. on the preclinical and clinical development of two in‐licensed Lilly development candidates for the treatment of metabolic disorders: P1201 (mode of action not disclosed) entered Phase 1 in Europe, was stopped in 2013,97 and P2202, a 11β‐hydroxysteroid dehydrogenase type 1 (βHSD1) inhibitor, which entered Phase 1 in Europe in 2009, reached Phase 2 in India and Canada, but has been abandoned since (Supporting Information Table 6b entries 2–3).97 In 2007, Piramal engaged in an integrated oncology drug discovery collaboration with MSD to discover and develop novel treatments for cancer up to clinical proof‐of‐concept studies, which led to PL225B,99, 100, 101 an oral insulin‐like growth factor 1 receptor (IGF1R) inhibitor, structurally possibly related to sulfonyl‐indole derivatives which reached Phase 1,102 but which has probably been abandoned in 2014. A second IGF1R project (“Target Y”) did not pass the candidate selection stage. Piramal's natural products collection, with more than 53 000 microbial strains and 7300 plants and extracts (2011 figures) from diverse habitats,97 has been made available for screening to external partners, of which two, Pierre Fabre and Oncotest, have been disclosed publicly.103, 104 In addition to five compounds which were abandoned in preclinical studies, several other Piramal compounds entered clinical trials: P1736 18, a non‐thiazolidinedione insulin sensitizer for the treatment of diabetes, discovered using phenotypic screening;105 P1961A, a dual CDK4/HIF1α inhibitor for the treatment of cancer;97 P2745, a potent inhibitor of the transforming growth factor β (TGF‐β) pathway for the treatment of hematological malignancies (abandoned in Phase 1 in 2013);[106] P3914, a dual‐action naproxen‐based cyclooxygenase inhibitor linked to an NO donor for the treatment of pain;107, 108 P7170, or panulisib 19,109, 110 a phosphoinositide 3‐kinase (PI3K)/mammalian target of rapamycin (mTOR) and Alk‐1 inhibitor for the treatment of cancer and inflammation, partnered with MSD; P7435,97 a potent and highly selective, oral diacylglycerol O‐acyltransferase 1 (DGAT1) inhibitor for the treatment of diabetes; and P11187,97 a G‐protein‐coupled receptor 40 (GPR40) agonist against metabolic disorders. After a name change to Piramal Healthcare Ltd. in 2007, the company evolved rapidly over the following years, by selling in 2010 its generics business to Abbott, by acquiring in 2011 Oxygen Healthcare for drug discovery services, rebranded as Piramal Discovery Solutions, and in 2012 Bayer's molecular imaging development portfolio which became Piramal Imaging.97 In August 2014, Piramal announced the closure of its drug discovery activities in Mumbai, essentially terminating all early‐stage R&D activities, affecting close to 200 scientists.111 An attempt was made to out‐license the five at that time remaining Phase 1 compounds, but most were later abandoned, and only the DGAT1 inhibitor and the GPR40 agonist completed Phase 1 studies in 2014, although no further information has been published.97 Two early candidate compounds were licensed to Krish Biotech in 2015 (see below).
Dabur Research Foundation (DRF) was incorporated in 1979 as the healthcare R&D branch of Dabur, a company active in ayurvedic medicines and consumer goods in India since 1886. DRF established preclinical labs in 1994, and initiated drug discovery research in 1998.32, 112 The company filed a series of patent applications in 2000 on the use of neuropeptide analogues for the treatment of cancer, but did never developed a proprietary NCE, as DRF‐7295,113 Dabur's first so‐called NCE, was actually a mixture of peptides derived from vasoactive intestinal peptide (VIP), bombesin, substance P and somatostatin, known to be overexpressed in various cancers, which entered Phase 1 clinical trials as a potential anticancer vaccine in India in 2002, followed by reportedly successful Phase 2 trials initiated in 2004. However, no progress has been reported since 2008, and it has most likely been abandoned. When in 2008 Fresenius Kabi acquired Dabur Pharma, the Dabur Research Foundation was spun out and became an independent drug discovery services company in 2010.
Sun Pharma was founded in 1983 in Vapi, and established its first research center in 1993. With the recent acquisition of Ranbaxy from Daiichi‐Sankyo, and more than 30 000 employees in 2016, Sun has become India's number one pharmaceutical company, and the world's fifth largest global specialty generics company.114 Sun entered drug discovery in 1999, working on NCEs and new drug delivery systems (NDDS). The research department, by then 80 scientists, moved into a new R&D center in 2004. In 2007, the research department was established as an independent company, Sun Pharma Advanced Research Company (SPARC) with more than 120 scientists, and has grown since to 275 scientists.115 Sun's research has produced so far eight development compounds, of which three reached the clinical stage (Supporting Information Table 6a, entries 81–88). SUN‐1334H 20,116, 117 a potent antihistamine, reached Phase 2 clinical trials in the US, but has been abandoned in 2013. Prodrugs with undisclosed structures of baclofen (SUN‐09, reached Phase 1 in India) and gabapentin (SUN‐44, preclinical stage), but have not been progressed further.118 Sun has been working on so‐called “soft” corticosteroids,119 with hydrolytically and metabolically labile substituents, including SUN‐461, abandoned in Phase 1,114 and SUN‐597 21,120, 121, 122 which is undergoing Phase 1 studies for dermatology, but has been abandoned for nasal and inhalation indications. SUN‐L731, an oral leukotriene D4 (LTD4) antagonist,123 entered preclinical development, but has been abandoned in 2016. The company has also been developing preclinical stage mutant‐selective Bcr‐Abl tyrosine kinase inhibitors,124, 125 such as SUN‐K706, devoid of vascular endothelial growth factor receptor 2 (VEGFR‐2) inhibition and presumably less likely to lead to side effects observed with other compounds of the family, in particular arterial thrombosis,115 and, more recently SUN‐K954, a follow‐on compound with a higher selectivity toward the T351M mutant.126 Sun Pharma filed patent applications with Bioprojet (France) on S1P1 receptor agonists to treat transplant rejection, autoimmune disorders, and inflammation,127, 128, 129 although no development activity on these compounds has been identified.
Alembic Ltd. is one of the oldest pharmaceutical and chemical companies in India, with its foundation in 1907 to manufacture tinctures and alcohol.32, 130 The company grew since the 1960s with the manufacturing and commercialization of antibiotics, including penicillins, cephalosporins and macrolides. Alembic entered proprietary drug discovery in 1999 through a collaboration with the National Chemical Laboratory (NCL) in Pune on antimicrobial macrolides, a project which has likely been abandoned around 2003, after a strategic switch of the company to contract research under the name of BioArc Research Solutions. In 2008 Alembic invested in two drug discovery companies, Incozen Therapeutics in Hyderabad, and Rhizen Pharmaceuticals in Switzerland (see below). Two years later, it split off its pharma business under the name of Alembic Pharmaceuticals Ltd., keeping bulk drug manufacturing and real estate activities under Alembic Ltd.
Zydus Cadila Healthcare (or Cadila Healthcare), the pharmaceutical arm of what became known as the Zydus Cadila group, headquartered in Ahmedabad, was initially founded as Cadila Laboratories in 1952, and was incorporated in 1995 after separating from Cadila Pharmaceuticals (see below). The company had over 15 000 employees worldwide in 2016, with operations in generics, active pharmaceutical ingredients, animal healthcare products, as well R&D in the areas of NCEs, formulation, biosimilars and vaccines.32, 131 The Zydus Research Centre, established in 2000 in Ahmedabad, houses around 400 scientists, out of which about 250 dedicated to NCE research. Zydus Cadila initiated its NCE discovery programs in 2000 with a strong focus on metabolic disorders and, to a lesser extent, inflammatory diseases, and disclosed a total of 15 drug candidates, of which 13 moved into clinical development stages (Supporting Information Table 6a, entries 89–103). ZYH1 (INN saroglitazar, trademark Lipaglyn) 22,132 a PPARα/γ agonist, was approved for the treatment of diabetic dyslipidemia in 2013 in India as the first NCE discovered and developed by an Indian company. It is currently under development for other indications, including nonalcoholic steatohepatitis (NASH). Zydus Cadila currently has one compound in Phase 2 studies in India (ZYH7, a PPARα agonist),131 and two at Phase 1 level: ZYDPLA1, a once‐a‐week DPP‐IV inhibitor for diabetes,133 in the US, and ZYAN1, a hypoxia‐inducible factor‐prolyl hydroxylase (HIF‐PH) inhibitor for the treatment of anemia,134 commonly observed in chronic kidney disorder. In addition, ZYTP1, a poly(ADP‐ribose) polymerase (PARP) inhibitor has recently been approved for the initiation of Phase 1 trials in India.135 Nine additional compounds progressed into clinical studies, but most were abandoned during Phase 1 studies. The majority focused on treating diabetes via different modes of action: ZYH2, a dual PPARα/γ agonist;131 ZYO1, a cannabinoid receptor type 1 (CB1) antagonist,136 stopped in Phase 2; ZYD1, a peptidic GLP‐1 agonist;137 ZYOG1, an oral peptidomimetic GLP‐1 agonist;138 ZYGK1, a glucokinase activator;139 and ZYG19, a G‐protein‐coupled receptor 119 (GPR119) agonist.140 Others targeted dyslipidemia (ZYT1, a thyroid hormone receptor β (TR‐β) agonist),141 inflammation and pain (ZYI1, mode of action not disclosed, abandoned in Phase 2),142 or osteoporosis (ZYPH0907, an oral parathyroid hormone (PTH) receptor agonist).143 The company also entered several collaborative drug discovery alliances,23 including with Karo Bio on inflammatory disorders (2008) which led to a patent application,144 and Lilly on cardiovascular disorders (2009),145 although both collaborations have been terminated since.
Cadila Pharmaceuticals, the privately owned sister company after the parent company's split‐up into two separate entities in 1995, has been considerably less involved in NCE research.146, 147 It developed for example, Polycap, a fixed‐dose combination treatment based on existing drugs to treat heart attacks, Mycidac‐C, a lung cancer vaccine, or Risorin to treat tuberculosis with a combination of rifampicin and piperine, an alkaloid extracted from pepper whose bioavailability enhancing properties had been discovered at the Regional Research Laboratory, now Indian Institute of Integrative Medicine (IIIM), Jammu.18 The company also engaged in preclinical and clinical development projects with Western biotechnology companies.148
FDC Ltd. manufactures and sells APIs and formulations, and initiated efforts in R&D around 2000, focusing on academic collaborations, in particular with the National Chemical Laboratory (NCL) in Pune, on antifungals, which has led to a series of joint patent applications, including on Fluconazole analogues.32, 149, 150, 151, 152 No progress, however, has been reported on NCL‐FDC‐101, an early‐stage compound disclosed in 2006, which is likely to have been abandoned, as no reference has been made to drug discovery in annual reports since 2013 (Supporting Information Table 6a, entry 104).149
JB Chemicals & Pharmaceuticals Ltd. (JBCPL), a producer of generics and bulk drugs established in 1976, initiated NCE research around the year 2000, working on NSAIDS including cyclooxygenase‐2 (COX‐2) inhibitors for the treatment of inflammation;32, 153, 154, 155 In 2004 the company reported three compounds in preclinical development, including JB‐7/G (Supporting Information Table 6a, entry 105).156 In the same year, however, work on COX‐2 inhibitors was badly affected worldwide by the withdrawal by MSD of rofecoxib (trademark Vioxx) due to the increased risk of cardiovascular side effects, and development of JB‐7/G was discontinued. JBCPL abandoned NCE drug discovery subsequently, as no further research projects have been mentioned in annual reports, and no further NCE patents have been filed since 2006.
Cipla Ltd., founded in 1935, is the world's largest manufacturer in terms of volume of antiretroviral drugs to fight HIV.157 The company did not invest in internal NCE research, but licensed two compounds for commercialization from Central Drug Research Institute (CDRI), that is, gugulipid to treat hyperlipidemia (1987),39 and chandonium iodide, a neuromuscular blocking agent (1994).8 A collaboration around the year 2000 with University of Mumbai led to patents on antihistamines,158 and antibacterials,159 but these compounds were abandoned later, without any information being disclosed on their development status. Cipla invested in stem cell research in 2010 through a strategic alliance with Stempeutics Research, and in 2014 launched its business‐incubating unit, Cipla New Ventures, through which it invested in Chase Pharmaceuticals Corporation, an early‐stage US‐based development company, to finance Phase 2 studies of the company's lead Alzheimer's disease (AD) treatment drug CPC‐201.160
Glenmark Pharmaceuticals, was founded in 1977 for the manufacturing of APIs and generics, and employs over 12 000 people.32, 161 In 2001, the company initiated small‐molecule drug discovery in the in newly established R&D center in Navi Mumbai, focusing with currently around 300 scientists on metabolic disorders and airway diseases, and in 2004 inaugurated its biologics research center in Switzerland,32, 161 with currently 50 researchers. Glenmark's small‐molecule research has delivered so far 19 development compounds, of which eight moved into clinical trials, including several phosphodiesterase type 4 (PDE4) inhibitors for airway diseases (Supporting Information Table 6a, entries 106–124).162 One of these, oglemilast (GRC‐3886)163, 164 23 was Glenmark's first compound to be out‐licensed, to Forest Laboratories in 2004 for the North American market, followed in 2005 with Teijin for Japan. In 2009 the compound was abandoned in Phase 2. Glenmark has been successful in out‐licensing the rights to further compounds: to Merck KGaA in 2006 for melogliptin (GRC‐8200) 24,165, 166 a DPP‐IV inhibitor for type 2 diabetes, which was abandoned after Phase 2 studies in 2011; to Lilly in 2007 for GRC‐6211 25,167, 168 a transient receptor potential cation channel type V1 (TRPV1) antagonist for various diseases, stopped in Phase 2 studies the following year; more recently, to Sanofi in 2010 for GRC‐15300 (or SAR292833), globally the first transient receptor potential cation channel type V3 (TRPV3) antagonist to enter clinical trials, for osteoarthritic pain.169, 170 In 2012 Glenmark signed an option agreement with Forest Laboratories for the discovery and development of novel microsomal prostaglandin E synthase‐1 (mPGES1) inhibitors for the treatment of chronic inflammatory conditions, with GRC‐27864 currently in Phase 1.171, 172, 173, 174 Additional compounds which reached the clinical stage are revamilast (GRC‐4039) 26,175, 176 also a PDE4 inhibitor, stopped in 2012 at the Phase 2 stage; tedalinab (GRC‐10693) 27,177, 178 a cannabinoid CB2 receptor agonist for the treatment of inflammatory and neuropathic pain (abandoned in Phase 1 in 2011); and GRC‐17536,179 a transient receptor potential cation channel, subfamily A, member 1 (TRPA1) inhibitor for respiratory disorders, that reached Phase 2 studies,180 exploring a potassium salt and prodrugs. Glenmark also actively in‐licensed development compounds for the markets in India and other emerging countries. This includes, in 2005 Napo Pharmaceutical's antidiarrheal product crofelemer, a purified oligomeric proanthocyanidin (M r up to 9 kDa) which blocks two unrelated chloride channels in the gut, approved in 2012,23 or monoclonal antibodies to build up a biologics pipeline (Supporting Information Table 6b entry 4).161
Lupin Ltd., founded in 1968, is one of the world's largest producers of generic antituberculosis drugs with more than 16 000 employees worldwide.32, 181 Lupin opened its R&D center in 2001 in Pune, with a focus on NCE drug discovery, process chemistry for generics, research in dosage forms, and advanced drug delivery systems. Lupin's first therapeutic focus was broad and included cardiovascular (antimigraine), anti‐infectives (antituberculosis, bacterial resistance), respiratory (antiasthma) and dermatological (psoriasis) diseases. The company invested significantly into the development of herbal drugs, and claims to have identified the active constituent of Desoris, a purified arabinoglycan–protein molecule code‐named LL‐4218 (desoside‐P)182 which was brought into Phase 2 (Supporting Information Table 6a, entries 125–131). Two further molecules were abandoned: sudoterb (LL‐3858) 28,183, 184 a novel small‐molecule antibacterial agent that completed Phase 2 studies in India in late 2013, and LL‐6531, a preclinical stage PPAR modulator.181 Lupin's R&D was entirely restructured in 2010, with the launch of new projects in the therapeutic areas of metabolic and endocrine disorders, pain and inflammation, autoimmune diseases, central nervous system (CNS) including cognition deficits, oncology and antivirals. In 2015 the company's R&D department counted over 300 scientists, of which an estimated 130 in NCE drug discovery. Among the new projects, four are undergoing clinical development in European countries,181 and are available for out‐licensing: LND101001, a Phase 2 α7 nAChR modulator for cognitive deficits such as in AD,185, 186 LNP1892, a Phase 1 calcium‐sensing receptor (CaSR) modulator for the treatment of primary hyperparathyroidism,187 LNP3794, a mitogen‐activated protein kinase kinase (MEK) inhibitor, which completed a Phase 1 study in terminally ill patients in the UK,188 and LNP1955, a calcium release‐activated channel (CRAC) modulator for the treatment of autoimmune diseases such as rheumatoid arthritis and psoriasis, which has successfully completed Phase 1 studies, and is entering Phase 2 proof‐of‐concept studies.189
Reliance Life Sciences was established in 2001 as the biopharmaceutical arm of Reliance Industries, one of India's largest industrial conglomerates.190 In addition to working on stem cell technologies, since 2002, the company filed patent applications from 2005 to 2007 on small‐molecule compounds to treat lipase‐mediated diseases, inflammation and cancer.191, 192, 193 These included early‐stage compounds such as RSCL‐0409, a glucodisaccharide,194 or RSCL‐0520, a phenanthrene derived from Eulophia ochratea,195 both inhibiting Toll‐Like receptor (TLR) signaling pathways (Supporting Information Table 6a, entries 132–133). The compounds are likely to have been abandoned around 2010 when the company's research focus shifted to siRNA‐mediated approaches to treat cancer.196
Orchid Chemicals & Pharmaceuticals, or Orchid Pharma since its recent name change in 2015, was established in 1992 in Chennai to manufacture antibiotics, and entered drug discovery in 2001 with projects in the areas of anti‐infectives and treatments for pain.32, 197 In 2002, the company engaged in a joint venture to develop US‐based firm Bexel Biotechnology's BLX‐1002, an oral, non‐PPAR AMPK activator for the treatment of diabetes,198 later repositioned for NASH (2012), but no further progress has been reported recently.197 In 2008, Orchid invested in Diakron Pharmaceuticals, a US‐based company that had an exclusive license to MSD′s investigational oral anticoagulant drug, a direct thrombin inhibitor later known as DPOC‐4088 (or DP‐4088),199 which reached Phase 1 clinical studies in Europe in 2012 (Supporting Information Table 6b, entries 5–6).200 The company's own internal discovery efforts had a broad therapeutic focus, covering infectious diseases, inflammation, pain, oncology, metabolic disorders, and CNS diseases. OCID‐2987,197, 201 a PDE4 inhibitor for the treatment of inflammatory disorders such as COPD, completed successfully Phase 1 studies in Europe in 2012, and OCID‐4681 29,202, 203 a histone deacetylase (HDAC) inhibitor for cancer had received approval in 2011 for Phase 1 studies for solid tumors in India, but we assume both have been abandoned, as cancer and inflammation are not mentioned in the company's latest annual reports.197 Two additional compounds were abandoned at the preclinical stage: OCID‐5005, a STAT‐3/IL‐6 inhibitor for oncology, and a unnamed Th1/Th2 cytokine synthesis inhibitor for inflammation (Supporting Information Table 2a, entries 134–138).197 Financial issues led Orchid, as of 2009, to sell parts of its business to Hospira (now part of Pfizer). As a consequence, no progress has been reported on its discovery programs since 2010, and no further NCE patent application has been published since 2012. However, in 2013 Orchid licensed its broad‐spectrum β‐lactamase inhibitor OCID‐5090, a zwitterionic N‐methylated tazobactam derivative, to the German Allecra Therapeutics for a 20 % stake in the company, for use in combination with antibiotics to treat multidrug‐resistant gram negative bacteria.204, 205, 206, 207 Allecra's lead compound AAI202, a combination of cefepime and AAI101/OCID‐5090 30, is currently in Phase 1 studies in France.208, 209
Suven Life Sciences Ltd., incorporated in 1989 as Suven Pharma, with the goal to offer contract research and manufacturing services, changed its name in 2003, the year it initiated internal drug discovery efforts, focusing exclusively on the central nervous sytem (CNS).32, 210 The company entered into a global collaborative research partnership in 2006 with Eli Lilly, followed by a second deal in 2008, although nothing has been disclosed on the outcome of this collaboration.211 Suven filed its first IND application in 2007 for lead compound SUVN‐502, a serotonin 5‐HT6 receptor antagonist for the treatment of mild cognitive impairment associated with CNS diseases such as AD, Parkinson's disease (PD) or schizophrenia. Phase 1 studies were completed in 2009, and after unsuccessful attempts to out‐license the compound, Suven initiated Phase 2a trials on its own in 2015 (Supporting Information Table 6a, entries 139–153).212, 213, 214 SUVN‐G3031, a histamine H3 receptor antagonist for cognitive impairment completed Phase 1 studies in 2015 in the US.212, 215 SUVN‐D4010, a partial 5HT4 agonist for the same indication entered Phase 1 trials in the US in in 2015.212, 216 Preclinical stage compounds include α4 β2 nAChR antagonists such as SUVN‐911, or cannabinoid CB2 receptor agonists.212, 217
Natco Pharma was incorporated in 1981 with the objective to manufacture conventional and controlled release generics, and inaugurated in 1997 the Natco Research Centre (NRC) in Hyderabad.218 In 2003 Natco launched its oncology division with generic imatinib (Gleevec), and initiated in‐house discovery research, with a first patent filing in 2004 on Bcr‐Abl kinase inhibitors. In 2012 Natco was awarded the first Indian compulsory license for Bayer's and Onyx Pharmaceuticals’ sorafenib. The company has currently two compounds in clinical development: NRC‐AN‐019 31,219 an analogue of imatinib, which received orphan drug status for chronic myelogenous leukemia (CML), glioma and pancreatic cancer in 2011 by the FDA (undergoing Phase 2 trials in India), and NRC 2694 32,220 an erlotinib (Tarceva) analogue EGFR kinase inhibitor in Phase 1 trials in India for late‐stage solid tumors (Supporting Information Table 6a, entries 154–155). NRC‐AN‐015, an earlier Bcr‐Abl tyrosine kinase inhibitor, has been abandoned at the preclinical stage.218
Panacea Biotec, founded 1984, has become one of the top‐10 vaccine producers in India, in addition to manufacturing APIs, and the marketing of generics.221 The company has notably played a key role worldwide in polio eradication campaigns. It currently counts around 2500 employees, down from 3300 in 2014, of which 230 were in R&D. It has four R&D centers, including the LAKSH Drug Discovery R&D Centre in Mohali, Punjab, established in 2005 focusing on metabolic disorders, diabetes and infectious diseases. In 2007 CNS diseases were added as an indication through a collaboration with Punjab University, and the NCE discovery group grew to over 100 people in 2011. The company started filing NCE patents as of 2008, in particular on inhibitors of DPP‐IV and of sodium–glucose co‐transporter‐2 (SGLT2) for the treatment of diabetes, and on novel oxazolidinone antibacterial agents to treat infectious diseases. Its research efforts have led to four development compounds, of which PBL‐1427, a DPP‐IV inhibitor reached Phase 1 clinical trials in India in 2012 (Supporting Information Table 6a, entries 157–160).222, 223 Panacea's turnover in fiscal year 2014–2015 decreased to less than half of what is was in 2010‐2011, after the company was hit by quality management issues with its vaccine manufacturing. As a consequence of the severe drop in income, internal R&D was decreased, in‐house drug discovery was halted, and attempts were made to out‐license or partner existing internal projects. In 2014 the company adopted a new, integrated contract research service model under the brand name of Panacea Life Sciences. Panacea's current pipeline includes, in addition to PBL‐1427, one additional unnamed compound at the preclinical stage.221
Matrix Laboratories was set up in 2000 to manufacture generic APIs, and grew by domestic and international acquisitions to become in 2006 ranked 10 of Indian pharmaceutical companies in terms of market capitalization.32, 224 At the end of 2004, Matrix signed a collaboration agreement with aRigen, a Japanese biotech company focusing on anti‐infectives research, to supply compounds for screening. During the following year, the company initiated internal drug discovery programs targeting asthma and metabolic disorders, later expanding into treatments of pain. Matrix′s first patent applications were filed in 2006 on DPP‐IV inhibitors, with the lead compound MX‐6001, and phosphodiesterase (PDE) inhibitors, including PDE4 inhibitor MX‐4007, followed in 2008 on vanilloid receptor modulators (Supporting Information Table 6a, entries 161–162).225, 226, 227, 228 In 2006, Matrix, at that time with 2000 employees, of which 200 scientists in R&D, was acquired by Mylan. Drug discovery and development was subsequently abandoned, and did not appear in later annual reports of the company.
Hetero Drugs is one of the top‐10 pharmaceutical companies, and the largest privately held Indian pharmaceutical company.229 Founded in 1993, with currently over 15 000 employees, it is the world's largest manufacturer of antiretroviral drugs, accounting for a 25 % share of the global antiretroviral production. Hetero Research Foundation (HRF), the R&D arm of the Hetero Group companies, employs 400 scientists focusing on process and analytical R&D, and more recently on discovery research. The company's main areas of discovery, initiated around 2006 include treatments against human immunodeficiency virus (HIV), hepatitis C virus (HCV), cancer and diabetes. HRF has been particularly active in anti‐HIV drug discovery, where it started filing patents in 2008 on compounds with different mechanisms of action, including nucleosides,230 peptidomimetic protease inhibitors,231 and more recently on triterpene maturation inhibitors.232 In 2012 HRF claimed to have two novel compounds ready to enter Phase 1 clinical studies (Supporting Information Table 6a, entries 163–164).233 However, no information has been publicly disclosed on the progress of these compounds.
Jubilant Life Sciences is the pharmaceuticals and life sciences arm of the vast Jubilant Bhartia Group, a conglomerate with over 36 000 employees encompassing diverse sectors such as oil, gas, automobiles, aerospace, food, agrochemicals, and polymers. Jubilant entered the drug discovery services business in 2003.234 With 6100 employees worldwide, of which around 700 in discovery services, the company has developed from a manufacturer of bulk chemicals, incorporated in 1978, into one of the main research and development service providers in India. The company has been engaged in multiple drug discovery collaborations, including Lilly, Amgen, Forest, Orion, Endo, or Johnson & Johnson (J&J), and in 2016, initiated a strategic alliance with Sanofi to discover drugs for metabolic disorders.23, 235 More recently Jubilant started transforming itself into a dual‐business model company, with the creation in 2007 of Jubilant Innovation, the company's branch for the discovery and development of proprietary or co‐owned molecules. The first effort to enter drug development, was by partnering with CGI Pharmaceuticals in 2008 for the development of CGI‐1842 (subsequently known as JI‐101), a tyrosine kinase inhibitor targeting selectively vascular endothelial growth factor receptor type 2 (VEGFR2), ephrin type‐B receptor 4 (EphB4) and platelet‐derived growth factor receptor β (PDGFRβ), which reached a Phase 1/Phase 2 stage trial in solid tumors in the US in 2012, but has been abandoned since for lack of efficacy (Supporting Information Table 6b, entry 7).236 Since 2014, the company has been disclosing its proprietary oncology‐focused drug discovery projects, on EGFR kinase inhibitors (JIEM‐0186),237 dual lysine‐specific histone demethylase (LSD1)‐histone deacetylase inhibitors (JBI‐097),238, 239 and bromodomain and extra‐terminal motif (BET) inhibitors (JBET‐050).240 Jubilant's BET BRD4 inhibitor program, including lead compound CK‐103, has recently been licensed to Checkpoint Therapeutics (2015) (Supporting Information Table 6a, entries 165–167).241
Elder Pharmaceuticals, founded 1989, and active in the manufacturing of APIs and the distribution of products for women's healthcare, wound care and nutraceuticals, filed two patents in 2008 with Poona College of Pharmacy on anti‐inflammatory agents,242 and on thiazolidinone compounds for the treatment of diabetes.243 The company has had serious financial issues, and is currently facing bankruptcy.244 No information is available in annual reports on any internal drug discovery or development activities.32, 245
IPCA Laboratories, a manufacturer of APIs and generic formulations, and market leader in India for antimalarials, entered NCE research by licensing two antimalarial ozonide development compounds from the Indian Central Drug Research Institute. CDRI‐97/78 (in‐licensed in 2007) is currently undergoing Phase 1 studies, and CDRI‐99/411 (in‐licensed in 2008) is likely to have been abandoned at the preclinical stage (Supporting Information Table 6b, entries 8–9).246 The company initiated in‐house drug discovery projects in 2009 to develop treatments for pain, ulcers, and malaria and thrombosis, and claimed to have two compounds in their pipeline for thrombosis and malaria in 2012, but no internal compound appears to have passed the research stage so far.247, 248
Mankind Pharma, founded in 1995, has become one of the top‐five privately held Indian pharma companies. It currently counts more than 11 000 employees, of which 200 in its R&D Centre in Manesar, established in 2011, focusing on pharmaceutical development and new drug discovery research.249 The company claims to be active in five new drug discovery projects in the areas of diabetes, arthritis and angina, but no information on the research programs has been disclosed, nor has there been any published NCE patent application.
Alkem Laboratories was founded in 1973, and until recently a privately held pharmaceutical company.250 Alkem initiated drug discovery efforts in 2012 in the area of infectious diseases.251 A more recent focus has been on the development of cathepsin K inhibitors for the treatment of osteoporosis, with Alkem‐43 as a potential candidate compound (Supporting Information Table 6a, entry 168).252, 253, 254 Alkem closed its drug discovery efforts in preparation of its successful initial public offering in 2015, and has attempted to license the project.
Emcure Pharmaceuticals was incorporated in 1981, and counts among the top‐30 Indian pharmaceutical companies with over 9000 employees.255 The R&D team counts 400 scientists, focusing on API development and formulations research. Around 2014, the company started a “New Drug Discovery Research” group, with so far a single published patent application on acid secretion inhibitors.256 No further information is currently available on Emcure's drug discovery efforts.
Finally, and although not included in our analysis as the Indian subsidiary of a multinational company, it is worth mentioning the contributions, primarily driven by Indian scientists, of what has been considered by some in the country as an “iconic lab”, AstraZeneca India.257 At a time when the R&D centers of other Western companies had left the country, AstraZeneca, formed by the merger of Astra and Zeneca in 1999, chose to build on the existing Astra Research Centre India (ARCI) in Bangalore.258 Established in 1987 as a nonprofit organization to address the problem of infectious diseases in developing countries, the company initially developed diagnostic tools to identify parasitic diseases, soon followed by drug discovery projects in collaboration with Astra Sweden in the field of antimalarial and antimycobacterial agents.259 The merger resulted, in addition to a change in name to AstraZeneca India Pvt. Ltd., in a modified remit with a focus on antituberculosis drug discovery. The Bangalore group successfully delivered its first development compound, AZD5847, a novel oxazolidinone antibiotic, that entered Phase 1 studies in 2009.258, 260, 261 This was followed in October 2014, through a collaboration with MMV initiated in 2010, by MMV253 (or AZ13721412), a triaminopyrimidine V‐type H+‐ATPase inhibitor, a novel class of fast‐killing and long‐acting antimalarials, as preclinical development compound in 2014.262, 263 The collaboration with TB Alliance, also initiated in 2010, yielded a third development candidate, TBA‐7371, a potent inhibitor of DprE1, currently undergoing preclinical development.264, 265 By January 2014 however, AstraZeneca had announced the closure of its Bangalore R&D center, impacting 168 employees in drug discovery and process R&D.257 The company continued the clinical development of AZD5847, which entered Phase 2 studies in 2012, but was abandoned in 2015.258 TB Alliance found a partner in Lilly for the further preclinical development of TBA‐7371, and MMV recovered the rights to MMV253, which was partnered with another Indian company, Zydus Cadila.266
3.3. From contract research to proprietary discovery projects
India became a hub for drug discovery collaborations in the late‐1990s when global pharmaceutical and biotechnology companies started outsourcing non‐IP‐sensitive chemistry such as chemical libraries, intermediates and reference compounds. Cost arbitrage, together with the availability of synthetic chemistry expertise, and existing knowhow from the generics development and manufacturing business, had largely been the drivers initially, and still are, to a large extent, even though Western companies are looking increasingly for added value beyond cheap labor. The service offerings expanded beyond chemistry into biology and pharmacology, and a number of deals gradually evolved into IP‐generating collaborations with Indian inventors, and often into collaborative, integrated drug discovery alliances, with, in selected cases, the elements of risk and reward sharing.
In addition to a growing network of smaller companies, this led to the rise of several large contract research companies. Those that did not initiate proprietary in‐house projects are not included in our analysis, although some of the largest deserve being mentioned for their contributions in support of global drug discovery projects.23 Syngene International Ltd., founded in 1994, is the contract research subsidiary of Biocon, and has engaged in collaborations with a number of pharmaceutical and biotechnology companies, of which by far the largest, with over 550 scientists, has been signed in 2007 with Bristol‐Myers Squibb (BMS), covering a broad range of integrated drug discovery and development services.23, 267 This was followed by Abbott Nutrition (2010), Baxter (2014), and recently by Amgen (2016), all with dedicated centers.268 TCG Lifesciences (TCGLS) was established as Chembiotek Research in 1998 and started offering chemistry services from its facilities in Kolkata in 2001, then expanded into multiple service areas, including biology, pharmacology, DMPK, clinical services and bioinformatics.269 Pfizer chose the company at the end of 2009 as a partner for integrated drug discovery services after its acquisition of Wyeth, and terminated the acquired company's existing large‐scale alliance with GVK Bio which had been in place since 2006.23, 270 Sai Life Sciences was established in 1999, and steadily expanded its chemistry capabilities in drug discovery, process R&D, and manufacturing, and more recently included biology and DMPK.271 Since 2009, the company has been involved in a discovery chemistry collaboration with UCB.272 A more limited number of CROs took the risk of adopting a dual business model, by initiating proprietary projects in addition to external discovery services (Table 2).
Table 2.
Proprietary drug discovery activities at Indian biotech companies.
| Entry | Company | Year[a] | Development compounds | Estimated headcount | Status 2016 |
|---|---|---|---|---|---|
| a) Contract research companies with proprietary projects | |||||
| 1 | Advinus | 2005 | 9 | NA | NCE R&D in multiple therapeutic areas, one compound in Phase 2, recent emphasis on drug discovery services |
| 2 | Anthem Biosciences | 2009[b] | 2 | NA | No ongoing internal drug discovery, isolated IP |
| 3 | Aurigene | 2010[c] | 12 | NA | NCE R&D with a strong focus on oncology, four compounds out‐licensed and two under option agreement |
| 4 | GVK Bio | 2014[c] | 1 | NA | Limited proprietary drug discovery activity |
| b) Biotech companies | |||||
| 5 | Kareus Therapeutics | 2007 | 2 | >10 | NCE R&D to treat CNS diseases and diabetes |
| 6 | Connexios Life Sciences | 2008[c] | 4 | 180[d] | NCE R&D focusing on metabolic diseases, one compound out‐licensed, currently focus on development activities |
| 7 | Rhizen Pharmaceuticals / Incozen Therapeutics | 2008 | 6 | 40[e] | NCE R&D for oncology and inflammation, two compounds out‐licensed, one undergoing Phase 3 studies |
| 8 | Sphaera Pharma | 2008 | 2 | NA | NCE R&D in oncology and infectious diseases |
| 9 | Curadev | 2010 | 1 | 50 | NCE R&D in oncology, one compound out‐licensed |
| 10 | Shantani Proteome Analytics | 2010 | 1 | <10 | Limited proprietary drug discovery activity |
| 11 | Vyome Biosciences | 2010 | 2 | 30 | NCE R&D based on cleavable linker and nanoparticle technologies to treat infections |
| 12 | Vitas Pharma | 2011 | 1 | 10 | NCE R&D to treat drug‐resistant nosocomial infections |
| 13 | Invictus Oncology | 2011 | 1 | 30 | Platinum‐based nanotherapeutics to treat cancer |
| 14 | Krish Biotech | 2015[b] | 2 | NA | NCE R&D in multiple therapeutic areas, two compounds in‐licensed from Piramal |
| Total: 46 | |||||
[a] Year company established unless specified otherwise. [b] Year proprietary patent filed. [c] Year proprietary drug discovery initiated. [d] Figure for year 2014. [e] Estimated combined headcount Rhizen/Incozen (2014).
Advinus Therapeutics, backed financially by the Tata Group, was established in 2005 by the former heads of research and drug discovery, respectively, at Ranbaxy, who had held previous scientific positions at Bristol‐Myers Squibb.273 The company was launched with a dual business model, having pharmaceutical and agrochemical development services in Bangalore, and a drug discovery research center in Pune, focusing on metabolic, inflammatory, and neglected diseases. Advinus runs both proprietary drug discovery projects with the aim to out‐license preclinical drug candidates, and collaborative discovery and development projects. The company entered into collaborations with major companies including MSD, Novartis, J&J, Celgene and Takeda.274 Advinus has been working on a pipeline of proprietary drug discovery projects, with its most advanced compound, GKM‐001, a glucokinase activator having successfully completed a 14‐day Phase 2 proof‐of‐concept study,275, 276, 277 and backup compound GKM‐002 at the preclinical level.278, 279 So far, the company has been unable to find a partner for a further development of these compounds. A range of preclinical compounds, initially developed for various indications such as COPD, IBD, Parkinson's Disease, or inflammatory and autoimmune disorders,280 have been repositioned more recently for immuno‐oncology, including adenosine A2A and A2B receptor antagonists;281, 282 Janus kinase and BTK inhibitors (Supporting Information Table 2a, entries 169–177).278, 279, 283, 284, 285, 286 However, after the departure of Advinus’ managing director in May 2016,287 a shift of the company's emphasis more toward discovery services, followed by a restructuring of its operations, the future of these compounds has become uncertain.288
Anthem Biosciences, incorporated in 2006 and headquartered in Bangalore, is a biotech company offering drug discovery and development, as well as process research and manufacturing services.289 Although the company does not work on proprietary drug discovery projects, it owns intellectual property rights to novel HDAC inhibitors for cancer therapy from an earlier collaboration with Portsmouth Technologies, a virtual drug discovery company,290 and has plans to partner candidate compounds PAT‐1102 33, and PAT‐1118 for preclinical development (Supporting Information Table 2a, entries 178–179).291, 292
Aurigene Discovery Technologies was established 2002 in Bangalore as a subsidiary of Dr. Reddy's Laboratories, to provide services in medicinal chemistry, structural biology and structure‐based drug design.293 The company rapidly evolved away from pure functional services toward integrated, collaborative, risk‐sharing drug discovery alliances, and grew in size, including in 2009 with the absorption of a development group in Hyderabad after Dr. Reddy's termination of all in‐house R&D projects. With over 500 scientists, Aurigene's current therapeutic focus lies in immuno‐oncology, epigenetics, precision oncology and selected targets for inflammatory disorders. Aurigene has entered into a large number of collaborations, including with Novartis, Merck KGaA, Debiopharm, Endo Therapeutics, and Asana Biosciences, which all successfully delivered development candidates.23 The company initiated proprietary in‐house drug discovery projects in 2010,294 using both small‐molecule and peptidic or peptidomimetic approaches, and announced its first licensing agreement with Debiopharm on Debio‐1142, an inhibitor of an undisclosed oncology pathway (2011) (Supporting Information Table 6a, entries 180–191).295 Aurigene has developed a range of inhibitors which block the signaling pathway of PD‐1, or Programmed cell death 1, an immunoreceptor which plays an important role in negatively regulating immune responses. Sequences of the extracellular domain of PD‐1 that are critical for ligand–receptor interaction, served as starting points for the investigation of 7‐ to 30‐mer peptides derived from human and murine PD‐1 sequences, leading to the discovery of the 29‐mer AUNP‐12/W016A, licensed to Pierre Fabre Médicaments (2014, but returned at the end of 2015).296, 297 Aurigene has also identified shorter peptides and small‐molecule peptidomimetics, licensed to Curis, including CA‐170/AUPM‐170, a dual PD‐L1/V‐domain Ig suppressor of T‐cell activation (VISTA) inhibitor, which entered Phase 1 mid‐2016, and CA‐327/AUPM‐327, a dual PD‐L1/T‐cell immunoglobulin and mucin‐domain‐containing molecule‐3 (TIM‐3) inhibitor, currently at a preclinical development stage. In addition, the agreement with Curis includes CA‐4948/AU‐4948), an orally active interleukin‐1 receptor‐associated kinase 4 (IRAK‐4) inhibitor for precision oncology.298, 299, 300 Aurigene is also targeting epigenetic regulation mechanisms, for example with pan‐BET inhibitors (BRD4/ BRD2/BRD3), such as ODM‐207, a quinolin‐2(1H)‐one derivative which it licensed to Orion, and for which IND enabling studies are currently being pursued.301, 302, 303 Additionally, the companies are also exploring selective BET inhibitors. Other projects continue internally with a strong focus on oncology, including with nicotinamide phosphoribosyltransferase (NAMPT) inhibitors such as AU‐4869,304 covalent K‐Ras inhibitors,305 or CDK7 inhibitors,306 and RAR‐related orphan receptor γ (RORγ) inverse agonists for the treatment of inflammatory disorders.307 Additional approaches targeted the treatment of infections with PD‐1 inhibitors,293 or with FabI (enoyl‐acyl carrier protein (ACP) reductase) inhibitors such as AEA16 34.308
GVK Biosciences, set up in 2001, has become one of the largest contract research organizations and has been engaged in major drug discovery service collaborations with companies such as Wyeth, Endo, or Medivir.309 In 2013, GVK Bio formed a joint venture with Onconova for lead optimization and subsequent development for IND filing on two early research compounds,309 including GBO‐006‐1 (previously ON‐1231320), a PLK2 inhibitor for the treatment of breast cancer, which appears to have been discontinued. Around 2014, GVK Bio initiated internal projects, with the aim to license these at an early stage to potential clients. As a first example, GVK‐TrkI, a selective tropomyosin receptor kinase A (TrkA) inhibitor for the treatment of cancer, progressed into preclinical development (Supporting Information Table 6a, entry 192).310, 311 A second project with GVK01406, a compound targeting PI3Kβ inhibition, reached the lead optimization stage.312
3.4. The rise of drug discovery at biotechnology companies
The launch of startups and small biotechnology companies with the aim to generate IP and licensing revenues by discovering new drugs and by partnering these for development, is a more recent addition to the country's pharmaceutical environment. This is due to the significantly higher risk involved, which some companies compensate in part by generating a revenue stream from offering, in addition to discovery collaborations, a range of discovery and development services to Indian and global companies.
Kareus Therapeutics was established in 2007 by former members of Dr. Reddy's Laboratories, and runs currently as a virtual company headquartered in Switzerland.313 The company's activities focus on CNS diseases, diabetes and chronic pain. Kareus currently has two compounds in development in the US, namely KU‐046, in Phase 1 studies for the treatment of Alzheimer's disease,314 exploring further options for multiple sclerosis and fragile X syndrome, claimed to be acting by dual targeting of oxidative stress and energy deficiency by linking niacin derivatives and redox‐active aromatic compounds, and KU‐5039 for diabetes,315 likely to be a fatty acid analogue activator of AMPK (Supporting Information Table 6a, entries 193–194).
Connexios Life Sciences was established in 2003, backed financially by the venture investing arm of a co‐founder of Infosys, one of India's largest IT service companies.316 The company focused initially on developing systems biology approaches, databases and cell‐based assays, but in 2008, took the strategic decision to launch full‐scale drug discovery operations, specializing in metabolic diseases such as type 2 diabetes. Connexios’ most advanced compounds are CNX‐012‐570, an AMPK activator for the treatment of type 2 diabetes, recently licensed to Boehringer Ingelheim,317, 318 CNX‐011‐67, a GPR40 agonist,319 for which the company has been looking for development partners since 2012; CNX‐013‐B2, an activator of retinoid X receptor (RXR) α,β,γ;320 and CNX‐010‐49, a βHSD inhibitor (Supporting Information Table 6a, entries 195–198).321 Connexios counted around 200 scientists in discovery before it restructured its activities to focus on its development program, and no recent update has been given on the fate of the company's earlier stage compounds.
Rhizen Pharmaceuticals S.A. was incorporated in Switzerland, with the aim to develop and partner compounds originating from Incozen, a biotech company founded in 2008 in Hyderabad to discover novel treatments for oncology and inflammation (Supporting Information Table 6a, entries 199–204).322, 323 Both companies were established with funding from Alembic Ltd. (see above). In 2012 RP‐5264 (now TGR‐1202) 35, a selective PI3Kδ inhibitor for the treatment of hematological lymphomas,324 was licensed to TG Therapeutics, and has recently entered Phase 3 studies as a combination treatment together with TGR‐1101, an anti‐CD20 monoclonal antibody (mAb) for chronic lymphocytic leukemia. Phase 2 studies with TGR‐1202 were still ongoing as a stand‐alone treatment in 2016.325, 326 Recently RP‐6530, or tenalisib 36, a dual PI3Kγ/δ inhibitor for the treatment of hematological malignancies, entered Phase 1 studies.324, 327, 328 Further compounds reached different stages of preclinical evaluation,329 including RP‐1400, a c‐Met kinase inhibitor,330 abandoned in 2015, and RP‐3128, a CRAC inhibitor.331 RP‐6503, an inhaled dual PI3Kγ/δ inhibitor, was licensed to Novartis at the end of 2015 for the treatment of airway diseases, and is potentially structurally related to a recently patented single enantiomer.332 In 2016 Rhizen disclosed PR10107, a glutaminase inhibitor for the treatment of cancer.329 In addition, the company is developing RV1001, a PI3Kδ inhibitor for veterinarian use in canine lymphoma (Phase 2).329
Sphaera Pharma was launched in 2008, with laboratory space in Manesar, Haryana, and headquarters in Singapore.333 The company filed a first patent in 2011 on PI3K/mTOR inhibitors,334 of which one, SPR965, has reached the preclinical evaluation stage.335, 336 The compound is likely to be structurally related, if not identical to a compound recently described in a publication.337 In 2015, Sphaera announced a collaboration with the International Centre for Genetic Engineering and Biotechnology (ICGEB), funded by the Wellcome Trust for the development of inhibitors of niacin receptor 1 (NIACR1), also known as GPR109A, to treat multidrug‐resistant infections, that led to SPR113 which entered preclinical development (Supporting Information Table 6a, entries 205–206).338, 339, 340
Curadev was founded 2010 in Noida, and currently employs around 50 people.341 The company has integrated drug discovery activities, offering services and in parallel working on proprietary projects. In 2010, Curadev entered into a research collaboration with Endo Pharmaceuticals for cancer up to candidate selection stage, and in 2011, a collaboration with Medivation. Curadev's most advanced internal program on dual indoleamine 2,3‐dioxygenase (IDO)/tryptophan‐2,3‐dioxygenase (TDO) inhibitors, originating from its Endo partnership, is at the preclinical development stage. Its lead compound CRD1152 (now RG70099) has been licensed to Hoffmann‐La Roche in April 2015 (Supporting Information Table 6a, entry 207).342, 343, 344, 345
Shantani Proteome Analytics, founded in 2010 in Pune, offers chemical proteomics services in target identification, toxicity profiling, or drug repurposing.346 More recently the company entered the field of drug discovery with a proprietary project to treat type 2 diabetes, and a first‐in class lead compound at the preclinical stage.347, 348 This is likely related to a recently published patent application by Shantani on indazole compounds including NDS100179 37, together with the Council of Scientific and Industrial Research (CSIR) and the National Chemical Laboratory (Supporting Information Table 6a, entry 208).349, 350
Vyome Biosciences was founded in 2010, and currently consists of a team of around 30 scientists.351 The company focuses on dual‐action compounds joining two known antibiotics by a chemical linker,352 and on conjugate‐based antifungal and antibacterial products, by derivatizing existing drugs with a hydrolytically or enzymatically labile linker and a carrier, including fatty acids, surfactants or polymers to formulate the drug into nanoparticles.353 VB‐1953, a dual‐acting compound likely to combine a nitro‐imidazole antibiotic and a fluoroquinolone joined with a linker moiety, is Vyome's first NCE to be approved by the FDA for Phase 1 studies (Supporting Information Table 6a, entries 209–210).354 A second, unnamed compound recently entered preclinical studies.355
Vitas Pharma is a small startup drug discovery company founded in 2011, based in the Technology Business Incubator at the University of Hyderabad, with currently about 10 people.356, 357 The company focusses on developing treatments for infectious diseases, particularly, drug‐resistant nosocomial infections. The most advanced compound, presumably VT‐02‐00068 38 or an analogue thereof,358 prevents fatty acid biosynthesis, a vital metabolic pathway in bacteria, by inhibiting the enzyme FabI, and is currently undergoing safety and toxicity testing (Supporting Information Table 6a, entry 211).359 It is equally potent against diverse MRSA strains.
Invictus Oncology, incorporated in 2011 and based in New Delhi, develops nanotherapeutics for cancer treatment.360 Invictus has licensed the rights to a technology developed at Harvard Medical School, consisting in decreasing the toxicity of platinum‐based anticancer drugs by conjugating them to cholesterol, and assembling them into nanoparticles.361 As tested on cisplatin, the increased nanoparticle size allows the drug to enter cancer cells, which have wide pores on their surface, but not normal cells, with reduced pore sizes. The company is also applying the technology to its own new platinum drugs as well as antibody drug conjugates. IO‐125 39, the company's lead compound, is undergoing preclinical development studies (Supporting Information Table 6a, entry 212).362, 363
Krish Biotech was established in 2009 near Kolkata to offer preclinical services including analytical and toxicological testing.364 In 2015, the company initiated drug discovery efforts, led by former Piramal scientists, in the areas of metabolic disorders, oncology, immunology, pain and inflammation. With the aim of building a proprietary research pipeline, including through the acquisition of external compounds, Krish Biotech in‐licensed two preclinical stage compounds from Piramal: KBR1001 (or KBRPL1001), a RORγt antagonist for the treatment of autoimmune and inflammatory disorders,365 and KBR2001 or (KBRPL2001), a GPR120 agonist for diabetes and metabolic disorders.366 Three additional projects targeting oncology and pain are currently at the discovery stage (Supporting Information Table 6a, entries 213–214).367
4. Results and Discussion
4.1. Proprietary drug discovery at Indian companies
Our analysis identified 28 major Indian pharmaceutical companies and 14 biotech and startup companies that have reported proprietary NCE R&D with preclinical or clinical development compounds between 1994 and mid‐2016 (Tables 1 and 2).
Among top‐100 pharma companies, overall R&D size and productivity vary considerably, however, and only 12 companies have engaged in long‐term and large scale drug discovery efforts, and have produced a significant number of development compounds (Supporting Information, Tables 6–8). For the majority, drug discovery remained a rather limited activity, as illustrated for example, by isolated patenting of internal research results, or by academic collaborations.
Few CROs have ventured successfully into proprietary research. Advinus had been a pioneer in this field since its inception in 2005, although the failure to license out a single of its internal compounds has recently led to a refocus of the company's activities, and it remains to be seen if the current discovery pipeline survives. Aurigene is currently leading the way, with a strong proprietary portfolio and four out‐licensed compounds, and two more under an option agreement. The number of biotech companies and startups with proprietary development compounds remains small with 10 companies.
Since the initiation of drug discovery by Dr. Reddy's Laboratories in 1994, Indian companies have disclosed a total of 214 proprietary preclinical and clinical stage development compounds, of which 168 originated from large pharma companies, and 46 from contract research and biotech companies. Of these, 83 compounds were progressing in the pipeline by mid‐2016 according to publicly available information (Figures 2 and 3, Supporting Information Tables 8a and 8b). Given the inherent fluctuations of R&D pipelines, this number is likely to evolve as more information becomes available on existing pipelines (e.g., Advinus, Connexios), and as additional development compounds will be disclosed.
Figure 2.
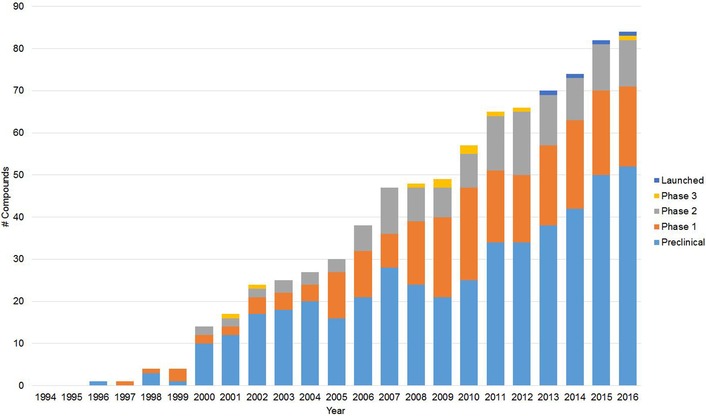
Development compounds at Indian pharmaceutical and biotechnology companies.
Figure 3.
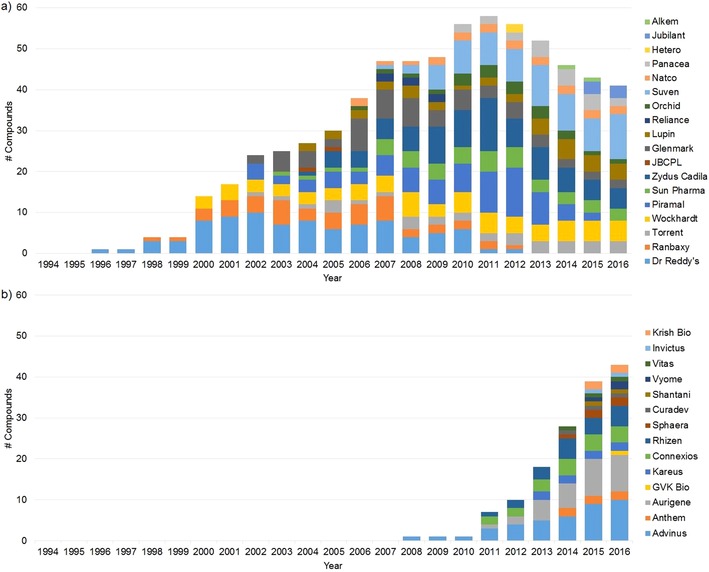
a) Development compounds at major Indian pharmaceutical companies. b) Development compounds at Indian biotech companies.
Despite this significant number of compounds, Zydus Cadila's saroglitazar, launched in 2013, remains so far the only compound that was entirely discovered and developed by an Indian company. Three more compounds reached the level of Phase 3 studies: Dr. Reddy's ragaglitazar and balaglitazone, both discontinued, and more recently, Rhizen's RP5264/TGR‐1202, in combination with an anti‐CD20 mAb, licensed to TG Therapeutics. Phase 2 stage compounds peaked in 2012 with 15 compounds, but this figure has decreased slightly since. The number of Phase 1 compounds has been quite stable with about 20 compounds each year since 2009, but with a notable recent increase in compounds from biotechnology companies (from 2 % of the total pipeline in 2009 to 31 % in 2016). Preclinical stage compounds have more than doubled since 2009, again driven largely by biotech companies (from 4 % in 2008 to 71 % in 2016).
At the major pharmaceutical companies, many internal R&D efforts have not been met with the expected success, as seen from those companies that have abandoned drug discovery in the meantime, be it after being acquired (Dabur, Matrix, Ranbaxy), before going public (Alkem), or after failing to deliver commercial compounds despite significant investments (Dr. Reddy's, Piramal). Other companies have significantly reduced the number of NCEs in their pipeline, including Zydus Cadila (from 13 in 2011 to 5 in 2016), Glenmark (from 8 in 2006 to 2 in 2016), and Sun (from 5 in 2012 to 3 in 2016). One significant exception to this is Lupin Pharma, which restructured completely its R&D organization in 2010 and launched a range of new NCE projects, with currently 4 compounds undergoing clinical development. As an overall result, however, the combined pipeline contribution by major companies has fallen from a peak in 2011 with 58 compounds (89 % of total pipeline), to 41 compounds (or 49 % of total pipeline) in 2016.
Biotechnology and startup companies, in contrast, have grown in number, size and research output, as illustrated by an increasing number of early development stage compounds, led currently by Aurigene. Several of the more recently founded companies have also been successful in generating preclinical compounds that attracted global partners, such as Rhizen Pharmaceuticals, Connexios, and Curadev. The increase in development compounds coming out of India is thus currently entirely driven by R&D focused biotech companies, which for the first time surpassed pharma companies with 42 compounds in 2016, growing from 2 % of the overall pipeline in 2009 to 51 % in 2016.
Whereas some of the major Indian pharmaceutical companies have the ambition to develop and market their own drugs, high costs and long timelines, combined with the lack of experience in clinical development lead most, and in particular smaller biotech companies, to prefer out‐licensing deals to finance part or all of their development costs.22 This is illustrated by the fact that 23 small‐molecule compounds have been licensed to global pharmaceutical companies or have been under option agreements over the past two decades (Table 3).
Table 3.
Licensing and option agreements.
| Company | Compound | Mode of action/therapeutic area | Status mid‐2016 (highest phase reached) |
|---|---|---|---|
| Dr. Reddy's Laboratories | balaglitazone (DRF‐2593), 1 | PPARγ agonism/diabetes | Licensed to Novo Nordisk (1997–2004); Rheosciences (2005–2010); DRL owns development in 2010, Phase 3—stop 2011 |
| ragaglitazar (DRF‐2725), 2 | Dual PPARα/γ agonism/diabetes | Licensed to Novo Nordisk (1998‐2002), Phase 3—stop 2002 | |
| DRF‐4158 (LBL‐752) | Dual PPARα/γ agonism, HMG‐CoA reductase inhibition/metabolic disorders | Licensed to Novartis (2001), Preclinical—stop 2003 | |
| Ranbaxy | RBx‐2258 (SPM‐969), parvosin, pamirosin, 4 | α1/δ‐Adrenoceptor antagonism/benign prostatic hyperplasia | Licensed to Schwarz Pharma (2002), Phase 2—stop 2004 |
| RBx‐10558, PPD‐10558, 5 | HMG‐CoA synthase inhibition/hypercholesterolemia | Licensed to Furiex Pharma/PPD (2007); Phase 2—stop 2011 | |
| Torrent | TRC‐4186, 8 | AGE breaker/diabetes‐related cardiovascular disorders | Option agreement with Novartis (2002–2005); Phase 2 completed 2015 |
| Glenmark | GRC‐3886 (oglemilast), 23 | PDE‐4 inhibition/asthma, COPD | Licensed to Forest (2004), Teijin (2005), Phase 2—stop 2009 |
| GRC‐6211, 25 | TRPV1 antagonism/pain, migraine, incontinence, asthma | Licensed to Lilly (2007), Phase 2—stop 2008 | |
| GRC‐8200 (melogliptin), 24 | DPP‐IV inhibition/diabetes | Licenced to Merck KGaA (2006), returned 2008, Phase 2—stop 2011 | |
| GRC‐15300 (SAR292833) | TRPV3 antagonism/pain | Licensed to Sanofi (2010), Phase 2—stop 2014 | |
| GRC‐27864 | mPGES‐1 inhibition/inflammation, pain | Option agreement with Forest Labs (2012), Phase 1—ongoing | |
| Orchid | OCID‐5090/AAI101, 30 | β‐lactamase inhibition/bacterial infections | Licensed to Allecra Therapeutics (2013), Phase 1, France—ongoing |
| Jubilant | CK‐103 (JBET‐050) | BET BRD4 bromodomain inhibition/cancer | Licensed to Checkpoint Therapeutics (2016), preclinical—ongoing |
| Aurigene | Debio‐1142 | Kinase inhibition/cancer | Licensed to Debiopharm (2011), preclinical—stop 2014 |
| AUNP‐012 (W014A) | PD‐1 inhibition/cancer | Licensed to Pierre Fabre (2014)—preclinical stop 2015 | |
| ODM‐207 | BET bromodomain inhibition/cancer | Option agreement with Orion Pharma (2014), preclinical—ongoing | |
| CA‐170 (AUPM‐170) | Dual PD‐L1/Vista inhibition/cancer | Licensed to Curis (2015)—preclinical ongoing | |
| CA‐327 (AUMP‐327) | Dual PD‐L1/Tim‐3/cancer | Option agreement with Curis (2015), preclinical—ongoing | |
| CA‐4948 (AU‐4948) | IRAK‐4 inhibition/cancer | Licensed to Curis (2015), preclinical—ongoing | |
| Connexios | CNX‐012‐570 | AMPK activation/diabetes | Licensed to Boehringer Ingelheim (2014), preclinical—ongoing |
| Rhizen | TGR‐1202 (RP5264), 35 | Selective PI3Kδ kinase inhibition/cancer | Licensed to TG Therapeutics (2012), Phase 2/3—ongoing |
| RP‐6503 | Dual PI3Kγ/δ kinase inhibition/asthma, COPD | Licensed to Novartis (2015), preclinical—ongoing | |
| Curadev | RG70099 (CRD1152) | Dual IDO/TDO inhibition/cancer | Licensed to Roche (2015), preclinical—ongoing |
Initially dominated by major pharma companies, in particular DRL, Ranbaxy and Glenmark, the out‐licensing model experienced several resounding failures, including in Phase 2 studies, which led some analysts to claim the death of the Indian out‐licensing model.14 Whereas the steady decrease of licensed compounds appears to confirm this for large Indian pharma companies, the reverse is true for biotech companies with 10 licensing or option agreements since 2011, largely led by Aurigene, and illustrates the continuing attractiveness of Indian development compounds for global companies.
4.2. Development compounds by indication and target class
Counting all, including multiple indications targeted by these development compounds, endocrinology and metabolic disorders, together with oncology dominate the therapeutic areas, representing almost half of all indications covered by drug discovery projects in India, followed by infections, immunological and rheumatological diseases, neurology and pulmonary and respiratory diseases. All remaining disease areas combined represent less than 10 % (Figure 4, Supporting Information Table 8c). This distribution is clearly quite different from the burden of diseases in India itself, where the leading causes are maternal and neonatal conditions, followed by cardiovascular diseases and diabetes, various infectious diseases, neuropsychiatric conditions, respiratory diseases and cancer, and corresponds more to the needs resulting from the leading Western diseases, that is, diabetes and cardiovascular disorders, cancer and neuropsychiatric conditions.368
Figure 4.
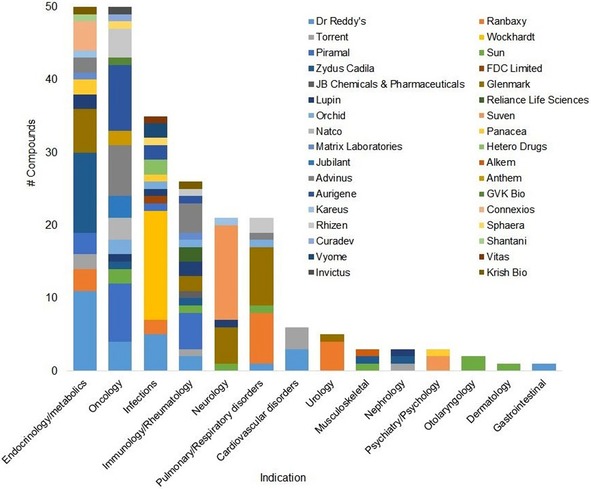
Development compounds by indication.
With the exception of startups, few Indian companies have specialized entirely in particular disease areas, such as Wockhardt in anti‐infectives, Suven in CNS diseases, Natco and Jubilant in oncology, or at least partially, such as Zydus Cadila in metabolic disorders, or Aurigene with a strong focus on oncology. The majority of Indian companies have opted to spread their efforts over multiple therapeutic areas (Figure 5, Supporting Information Table 8d).
Figure 5.
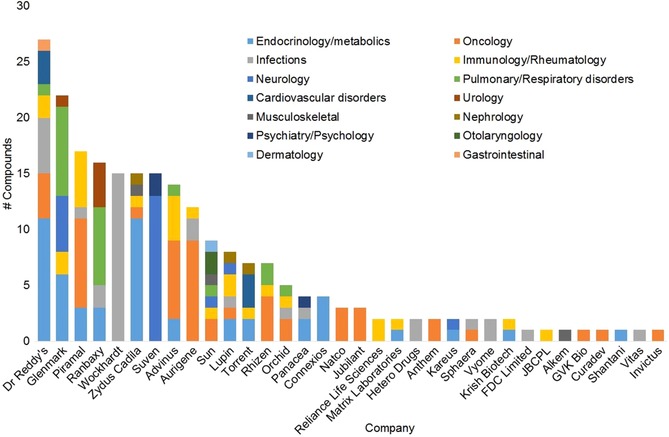
Therapeutic indications by company.
If one groups all development compounds by target class, including multiple modes of action, the top‐3 of the resulting 23 classes, that is, GPCRs, kinases, and nuclear signaling pathways, represent about half of all targets (46 %) (Figure 6, Supporting Information Table 8e). Few companies have focused on specific targets, such as Suven on 5‐HT6 receptors, or Wockhardt on fluoroquinoline and oxazolidine type antibiotics, and some others have a bias toward certain target classes, such as Aurigene and its kinase platform, but in general companies adopted a broad approach with multiple target types.
Figure 6.
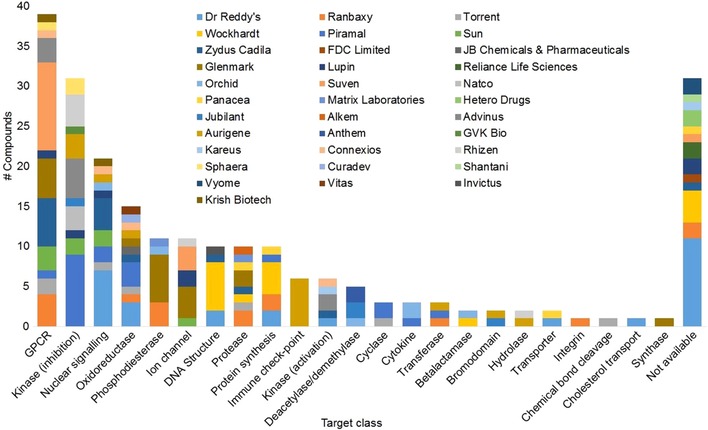
Development compounds by target class.
4.3. Novelty of projects
Of all new FDA‐approved NCEs between 1999 and 2013, almost one third are first‐in‐class compounds, as modulators of an until then unprecedented target or biological pathway,369 and the share of potentially first‐in‐class drugs in the current global pipeline has been estimated to be on average 70 %.370 India's past and current discovery pipeline is far from reaching comparable figures, as the vast majority of modes of action have been extensively targeted previously by other companies, such as PDE4 inhibitors, DPP‐IV inhibitors, kinase inhibitors, or oxazolidinone and fluoroquinolinone antibiotics. Lack of novelty, or lack of differentiation from existing development compounds, are therefore likely to be key factors that prevented in a number of cases Indian companies from finding global development partners.
However, this does not mean that Indian companies are not innovating in drug discovery. Even though Zydus Cadila's saroglitazar has only been one of many glitazars that entered development, it has been the first one to reach the market in 2013. Fifteen years earlier, Dr. Reddy's had been among the first companies to work on glitazars, with ragaglitazar reaching Phase 3 trials before being abandoned. Glenmark's GRC‐15300 had been the first TRPV3 inhibitor to enter clinical trials, and the company's GRC‐17536 has the potential to become a first‐in class TRPA1 inhibitor for the treatment of pain.161 Advinus’ glucokinase activator, although only one out of more than 20 compounds that have entered clinical trials, is an orally available, potential first‐in‐class drug with the advantage over earlier, discontinued development compounds of being liver‐selective with a lower hypoglycemia risk.371 Sun's Bcr‐Abl tyrosine kinase inhibitors SUN‐K706 and follow‐on compound SUN‐K954 are active against native Bcr‐Abl as well as Abl T315I mutations, the most resistant form of mutation in leukemic cells, and are more selective with respect to a range of other kinases inhibited by ponatinib, the currently used benchmark inhibitor, which might contribute to that compound's toxicity profile which limits its clinical use.126 Whereas several of Aurigene's programs aim at developing best‐in‐class compounds with significant improvements over competitors (for example, its inhibitors of BET, NAMPT, or FabI), the company's PD‐1 inhibitors licensed to Curis are the first highly potent and orally available small‐molecule compounds targeting Programmed cell death‐1. CA‐170, which has recently been nominated as development candidate, is a first‐in‐class dual‐acting molecule targeting both Programmed cell death ligand‐1 (PD‐L1) and V‐domain immunoglobulin suppressor of T‐cell activation (VISTA).299, 300 Jubilant's JIEM‐0186 is specifically targeting non‐small cell lung cancer associated with EGFR mutants L858R or T790M, and shows a high selectivity against cells carrying the wild‐type EGFR.237 Among the smaller companies, Curadev is targeting a highly competitive area with its potentially first‐in‐class dual IDO and TDO inhibitors,372 and Shantani, based on the use of its proteomics platform, has reported an early development compound with a novel mechanism of action for the treatment of type 2 diabetes.348
4.4. Success rates and timelines
Success rates and time spent by development phase have been widely used as parameters to quantify efficiency in pharmaceutical R&D.373, 374, 375 Current success rates by phase are defined as the number of development compounds that advance to the next phase, divided by the number of compounds that entered the phase from which is subtracted the number of compounds of yet unknown fate, for example, those in ongoing studies.
We have compiled available data (Supporting Information Table 7) on attrition and timelines for the 214 compounds that entered preclinical development, of which 82 progressed to Phase 1, 34 to Phase 2, and 4 to Phase 3, with 1 compound launched. Although the very low number of drug candidates reaching Phase 3, together with the lack of precision in collection timelines do not allow us to generate statistically significant values for all variables, we still believe our analysis to be of relevance, as it illustrates for the first time ever efficiency trends in Indian drug discovery, which allow a comparison with industry figures (Table 4, Supporting Information Table 8f).
Table 4.
Attrition rates and timelines.
| Indian companies | Industry average | |
|---|---|---|
| Success rates [%] | ||
| Preclinical | 50.3 (82/163) | 63–69 |
| Phase 1 | 54.0 (34/63) | 48–64 |
| Phase 2 | 17.4 (4/23) | 29–34 |
| Phase 3 | 33.3 (1/3) | 60–70 |
| Timelines [years] | ||
| Preclinical | 1.9 | 1 |
| Phase 1 | 2.5 | 1.5 |
| Phase 2 | 3.4 | 2.5 |
| Phase 3 | 3.0 | 2.5 |
With the exception of Phase 1 trials, for which the success rate of 54 % lies well within the range reported for industry, the chances for successfully transitioning to higher phases are considerably lower at Indian companies than the industry average. The very low success rate of 17.4 % during Phase 2 trials could hint at an overall less efficient selection process during earlier phases, and therefore expensive terminations in advanced stages. This is particularly true for the three major companies that abandoned drug discovery (Dr. Reddy's, Ranbaxy and Piramal), with 9 out of 11 compounds failing in Phase 2, and none completing successfully Phase 3 studies, where 2 out of 2 failed. These lower success rates are compounded by the increased time spent by phase, especially at the earlier stages. Even if the nearly twofold increase in time spent at the preclinical level could be in part the result of capturing with too little precision this first stage of development, clinical phases, too, take considerably longer to complete, and make the drug discovery process at Indian companies considerably less efficient than the industry average. It remains to be seen if the large number of compounds that have entered development between 2007 and 2016, and of which many are currently progressing at the early stages, will be able to modify this trend significantly. It would also be interesting to compare the Indian success rates to those of other emerging countries, but these are to our knowledge not yet available in the public domain.
4.5. Drug discovery and R&D investments
All major Indian pharmaceutical companies with significant drug discovery and development activities have reported considerable R&D expenditures in their annual reports, usually in the range of 5–10 % of revenues. However, it must be pointed out that NCE research is in general only a minor component of this, and that the bigger part goes toward generics development, formulation and drug delivery technologies, or process R&D. This can be assessed from those cases where drug discovery expenditure is specifically mentioned, or can be calculated from available data. Based on these, we estimate that an average of 20–30 % of the total R&D budget is dedicated to NCE research and development, even though some analysts estimate that in specific cases as little as 10 % goes to new discovery research.5 Assuming even an average spending on NCE R&D of 25 % of total R&D expenditures, this brings the overall investment in new drug discovery and development down to around 2 %, about one tenth of the average spend on R&D by the US pharmaceutical industry, with about 20 % of sales.376
5. Conclusions and Outlook
Since the initiation of drug discovery by major companies in the 1990s, Indian pharmaceutical R&D efforts have resulted in over 200 preclinical‐ and clinical‐stage development compounds, of which only one has reached the market thus far. This illustrates the tremendous accumulation of R&D capacity and of know‐how that has occurred over a time span of two decades, and at the same time its weakness in terms of efficiency. With slightly over 80 active pipeline compounds, India is far from being the drug discovery powerhouse it once had the ambition to become. The fact that several of the large investors in pharmaceutical R&D have closed down their drug discovery units in the meantime, and that others have decreased their pipeline, shows to what extent the industry, which had been familiar with the generics business, had underestimated the challenges that come with innovative NCE discovery and development.
This is likely due to a range of factors. One is a skill gap. The Indian Patent Act of 1970, with its focus on process patents and generics manufacturing, despite the social good it did in India, and arguably worldwide, by making good quality generics affordable to poor countries, removed all incentives to discover new medicines.20 The consequence was a rapid loss of skills, as Western companies closed their research departments, and as drug discovery‐related disciplines, including medicinal chemistry, biology and pharmacology, declined in the Indian science education system.20 These skills were again built up gradually by the pioneer pharma companies, and accelerated with the rise of contract research companies, as these evolved from custom synthesis, into higher added value services including medicinal chemistry, discovery biology, in vivo pharmacology, DMPK, and early toxicology models.20 This has been possible at the expense of in‐house training, since a recent industry survey estimated that 66 % of the existing manpower is not industry ready, highlighting the misfit between academic training and industry needs.377
Some of the basic problems that need to be addressed in order to strengthen weaknesses in the scientific education system have been summarized in recent reports.377, 378, 379, 380 A certain number are due to internal issues, such as inadequate facilities and quality of teaching, bureaucracy and political influence. Others are a consequence of the historical deficiency of interactions between industry and academia or public research institutions, because existing collaborations are still considered by a large majority as “limited”, and by only 10 % as “good”, due to the lack of interest for “applied science”, profound distrust, or different priorities and key performance indicators, such as publications in academia versus patents and commercialization in industry.19, 377, 381
All prevent science from being considered as an attractive career path, with as a consequence a severe brain drain, as 40 % of India‐born researchers were working overseas in 2011.382 A series of initiatives have been launched, aiming to attract experienced returnees, that is, researchers who have spent part of their scientific career abroad, back into the country with a range of fellowships in all areas of science and biotechnology. With an estimated figure of 750 fellowships since 2006, these are, however, likely to be insufficient for the country's needs.383, 384, 385, 386
The industry itself has also evolved, but needs to progress further. There has been a gradual shift in contributions to the growing pipeline away from the established pharmaceutical companies, whose contribution peaked in 2011, toward smaller, research‐intensive biotechnology companies. These do not only benefit from the availability of trained scientists with relevant industrial experience in the country, but also tend to focus on specific disease areas or target classes, which gives them the opportunity to carve out niches to be successful in their particular areas of expertise, as illustrated by a number of recent licensing deals. The industry has started to integrate the importance of innovation, and is moving away from low‐risk follow‐on projects, which certainly decreased the risk, but on the other hand led to molecules without preclinical and clinical advantages over existing development compounds, and were therefore difficult to out‐license. Current projects are targeting increasingly best‐in‐class compounds that address specific issues of compounds currently progressing in the global pipeline, if not compounds that have the potential to become first‐in‐class drugs.
There are, however, other weaknesses that need to be addressed. Overall investment in R&D, and more specifically in NCE drug discovery, needs to be increased, as it has been lagging far behind global industry average. More biotechnology companies need to be created, and funded appropriately. The US is home to over 40 pharmaceutical companies and an estimated 1700 biotech companies, which dwarfs India's life sciences landscape.387 India is, however, making progress in putting in place an ecosystem which is more conducive to biotechnology entrepreneurship, through a series of grant schemes to foster innovation, and bio‐incubators which provide space, access to scientific equipment, connections to industry and mentorship for IP management to startup companies.388 The implementation of biotech parks and clusters, in particular, to promote research and innovation, by establishing stronger links between institutions active in life sciences R&D, biotech and pharma companies, innovative small and medium size enterprises, has over the past years proven extremely valuable to attract companies, for example, in the area around Bangalore with around 200 companies, or Genome Valley in the vicinity of Hyderabad, with over 150 enterprises. Several more are in the planning phase.389, 390
The Indian Government aims to stimulate the launch of 2000 startups in life sciences over the coming five years.391 It is, however, uncertain how many of these will venture into proprietary NCE projects, as so far drug discovery has been considered the least attractive from an investment standpoint, ranking last of 12 options, far behind diagnostics, medical devices, or discovery services.377 This low attractiveness is compounded by structural weaknesses across the entire sector, such as insufficient understanding of IP protection, regulatory uncertainty regarding clinical trials, unethical practices, or pricing uncertainties.380, 392 All these contribute to raising barriers, and therefore need to be fixed.
It remains to be seen if and how fast industry initiatives and government efforts will be able to bring about the required changes. In the meantime, the country has already scaled back its highly ambitious, if not unrealistic, goal, which in 2010 had been part of the government's “Pharma Vision 2020”, to have “one out of five to ten new drugs discovered in the world originating from India by 2020”,393 which would have represented an average of at least three to six new medical entities (NMEs) per year, to a more realistic, but still highly ambitious target of “one NME per year and 10–12 incremental innovation launches per year by 2030”.394
The coming years will be critical. “Success will breed success”, a statement made by one of the industry leaders at a recent biotechnology convention in Hyderabad, might be true, but it still requires success stories to initiate, then to fuel the process.
Conflict of interest
The author declares no conflict of interest.
Biographical Information
Edmond Differding studied chemistry and received his PhD from Université catholique de Louvain, in Louvain‐la‐Neuve, Belgium. He was a postdoctoral fellow and Fulbright Scholar at the Massachusetts Institute of Technology, and holds a Master's Degree in Drug Design from ENSCL/University Lille II, France. After working as a researcher with Ciba‐Geigy (now Novartis) in Switzerland, he joined UCB Pharma in Belgium in 1991, where he subsequently became Vice President of Global Chemistry. His interest in India stems from a two‐year stay in Mumbai, from 2008 to 2010. He is currently Owner and Managing Director of Differding Consulting s.p.r.l. (Louvain‐la‐Neuve, Belgium), a consultancy firm established in 2010 that specializes in pharmaceutical R&D.

Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
E. Differding, ChemMedChem 2017, 12, 786.
References
- 1. Reddy K. A., An Unfinished Agenda: My Life in the Pharmaceutical Industry, Penguin Books India Pvt. Ltd., Gurgaon, 2015. [Google Scholar]
- 2. Dr. Reddy's Announces Reorganisation of its Drug Discovery Biz, Business Standard May 21, 2009 (accessed December 10, 2016): http://www.business-standard.com/article/companies/d.r.-reddy-s-announces-reorganisation-of-its-drug-discovery-biz-109052100164_1.html.
- 3. Daiichi-Sankyo Acquires Ranbaxy in $4.6B Deal, J. Carroll, FierceBiotech June 11, 2008 (accessed December 10, 2016): http://www.fiercebiotech.com/biotech/daiichi-sankyo-acquires-ranbaxy-4-6b-deal.
- 4. Piramal to Exit Drug Discovery Business, C. H. Unnikrishnan, LiveMint August 28, 2014, (accessed December 10, 2016): http://www.livemint.com/Companies/7weGbimcrdp7lrKH0YaSnL/Piramal-to-exit-drug-discovery-business.html.
- 5. Death of a Dream, N. Bisserbe, Businessworld, New Delhi February 1, 2010, 29, 32–38, (accessed September 30, 2015), http://old.businessworld.in/news/business/pharma/death-of-a-dream/460435/page-1.html.
- 6. Nagarajan K., Arya V. P., J. Sci. Ind. Res. 1982, 41, 232–240. [Google Scholar]
- 7.“Medicinal Chemistry Research in India”: Singh H., Chawla A. S., Kapoor V. K. in Progress in Medicinal Chemistry, Vol. 22 (Eds.: G. P. Ellis, G. B. West), Elsevier, Amsterdam, 1985, pp. 244–266. [DOI] [PubMed] [Google Scholar]
- 8. Singh H., Indian J. Hist. Sci. 2014, 49, 413–423. [Google Scholar]
- 9.Made in India, N. Datta, OutlookBusiness, Mumbai, Outlook Publishing (India) Pvt. Ltd., April 2010, April Issue, 42–55.
- 10. Kiran R., Mishra S., Eurasian J. Bus. Econ. 2011, 4, 53–67. [Google Scholar]
- 11. Five Years Into the Product Patent Regime: India's Response, S. Chaudhuri, C. Park, K. M. Gopakumar, United Nations Development Programme 2010, (accessed December 7, 2016): http://apps.who.int/medicinedocs/documents/s17761en/s17761en.pdf.
- 12.R. K. Joseph, The R&D Scenario in Indian Pharmaceutical Industry, Vol. RIS-DP #176, Research and Information System for Developing Countries, New Delhi, 2011.
- 13. Akhtar G., IOSR J. Hum. Soc. Sci. 2013, 13, 51–66. [Google Scholar]
- 14. Joseph R. K., Pharmaceutical Industry and Public Policy in Post-Reform India, Routledge, New Delhi, 2015. [Google Scholar]
- 15. Pharmaceutical Innovation and Contribution of In-House R&D of Domestic Firms After TRIPS in India, Working Paper 189, Institute for Studies in Industrial Development, New Delhi, D. Abrol, N. Singh, March 2016, (accessed April 24, 2017): http://isid.org.in/pdf/WP189.pdf.
- 16. India, M. Lansdell, in Innovative Drug Discovery In Emerging Markets, Scrip Insights Report Published by Business Insights, 2010, pp. 105–169.
- 17. Rezaie R., McGahan A. M., Daar A. S., Singer P. A., Nat. Biotechnol. 2012, 30, 923–926. [DOI] [PubMed] [Google Scholar]
- 18. Balganesh T., Kundu T. K., Chakraborty T. K., Roy S., ACS Med. Chem. Lett. 2014, 5, 724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dikshit M., Dikshit D. K., Curr. Sci. 2016, 111, 252–255. [Google Scholar]
- 20. Vishwakarma R., Curr. Sci. 2014, 107, 335–336. [Google Scholar]
- 21. Vishwakarma R., ACS Med. Chem. Lett. 2017, 8, 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pharmaceutical Research and Licensing Deals in India, B. P. Thomas, P. K. Chugan, D. K. Srivastava, (Ed.: Macro and Micro Dynamics for Empowering Trade Industry and Society), Excel India Publishers, New Delhi, for: Institute of Management, Nirma University, Ahmedabad, New Delhi, India, January 1, 2016, pp. 13–21.
- 23. Differding E., ChemMedChem 2014, 9, 43–60, and references therein. [DOI] [PubMed] [Google Scholar]
- 24. Top Pharmaceutical Companies in India by Sales 2016, MoneyControl, 2016, (accessed November 8, 2016): http://www.moneycontrol.com/stocks/marketinfo/netsales/bse/pharmaceuticals.html.
- 25. Unlisted Companies India 2012, Business Standard, (accessed April 13, 2017): http://www.business-standard.com/india/bs1000_12/pdf/bs-1000-2012_04.pdf.
- 26. Unlisted Companies India 2015, Business Standard, (accessed April 18, 2017): http://www.business-standard.com/content/general_pdf/bs1000-2015_04.pdf.
- 27. Unlisted Companies India 2016, Business Standard, (accessed December 7, 2016): http://bsmedia.business-standard.com/_media/bs/data/general-file-upload/2016-02/bs-1000–03.pdf.
- 28. Annual Reports 2007–2016, CSIR Central Drug Research Institute, (accessed November 24, 2016): http://www.cdriindia.org/annualreports.htm.
- 29. Dutta G. P., Proc. Indian Natn. Sci. Acad. 2016, 82, 31–52. [Google Scholar]
- 30. Biennial Reports, CSIR-Indian Institute of Integrative Medicine, (accessed April 26, 2017): http://www.iiim.res.in/annualreport.php.
- 31. CSIR-IICT: Celebrating Seven Decades of Service to the Nation, CSIR-Indian Institute of Chemical Technology, 2015, (accessed April 26, 2017): http://www.iictindia.org/htm/Resources/BiennialReports/70Year/IICT%20Book-2015.compressed.pdf.
- 32. Database of Company Financial Annual Reports, ReportJunction, (accessed 2014–2017): http://www.reportjunction.com/.
- 33. Free Patents On Line—Patent Search Tool, (accessed 2008–2017): http://www.freepatentsonline.com.
- 34. Innovative Drug Discovery in India—The Growing Indian R&D Pipeline, E. Differding, 2013, (accessed December 7, 2016): http://www.differding.com/data/Indian_drug_discovery_and_development_pipeline_2013.pdf.
- 35. Use of Antimony in the Treatment of Leishmaniasis: Current Status and Future Directions, Haldar A. K., Sen P., Roy S., Mol. Biol. Int. 2011, 571242; (accessed April 13, 2017): https://doi.org/10.4061/2011/571242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Essential Medicines, World Health Organization, 2017, (accessed April 13, 2017): http://www.who.int/medicines/services/essmedicines_def/en/.
- 37. ORS: The Medical Advance of the Century, UNICEF, The State of the World's Children, 1996, (accessed April 13, 2017): https://www.unicef.org/sowc96/joral.htm.
- 38. Ruxin J. N., Med. Hist. 1994, 38, 363–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.“Drug Discovery and Development: India” Gupta C. M., in Examples of the Development of Pharmaceutical Products from Medicinal Plants, Vol. 10 (Ed.: UNDP Special Unit for South–South Cooperation), 2005, pp. 29–44; (accessed December 10, 2016): http://unossc1.undp.org/GSSDAcademy/SIE/Docs/Vol10/V10_S1_drug.pdf. [Google Scholar]
- 40.P. McGrath, Central Drug Research Institute, Lucknow, India—Excellence in Science: Profiles of Research Institutions in Developing Countries, The World Academy of Sciences, Trieste, 2007, (accessed April 13, 2017): https://twas.org/sites/default/files/twas_packard_cdri.pdf.
- 41. Website, CSIR-IICT, 2017, (accessed April 18 2017): http://www.iictindia.org/.
- 42. Website, CSIR-IIIM, 2017, (accessed November 28, 2016): http:///www.iiim.res.in/.
- 43. Pharmacology and Phytochemistry of Oleo-Gum Resin of Commiphora wightii (Guggulu), P. Sarup, S. Bala, S. Kamboj, Scientifica 2015, 2017, 1–14 (accessed April 13 2017): https://doi.org/2010.1155/2015/138039. [DOI] [PMC free article] [PubMed]
- 44. Molecular and Functional Characterization of Bacopa monnieri: A Retrospective Review, K. E. Rajan, J. Preethi, H. K. Singh, Evid. Based Complement. Alternat. Med 2015, 2015, 1–12, (accessed April 13 2017): https://doi.org/2010.1155/2015/945217. [DOI] [PMC free article] [PubMed]
- 45. Nagarajan K., Indian J. Hist. Sci. 2014, 49, 377–398. [Google Scholar]
- 46. Bhutani K. K., Gohil V. M., Indian J. Exp. Biol. 2010, 48, 199–207. [PubMed] [Google Scholar]
- 47. Centchroman, CDRI, (accessed April 13, 2017): http://cdri.res.in/centchroman.aspx.
- 48. Risorine—A Novel CSIR Drug Curtails TB Treatment, in CSIR News: Progress, Promise and Prospects 60 (5–6) pp. 52–54, CSIR, March 2010, (accessed April 18 2017): http://www.niscair.res.in/sciencecommunication/rndnewsletters/csirnews2k10/csirnews_mar10.pdf.
- 49. List of CSIR Knowledgebase for Industries, CSIR, 2017, (accessed April 18, 2017): http://www.csir.res.in/csir-knowledgebase.
- 50. Raviña E., The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs, Wiley-VCH, Weinheim, 2011. [Google Scholar]
- 51.“Rohitukine and Forskolin”: de Souza N. J. in Human Medicinal Agents from Plants, ACS Symposium Series, Vol. 534, American Chemical Society, Washington, DC, 1993, Chap. 22, pp. 331–340. [Google Scholar]
- 52. Aurigene Discovery Technologies—London Business School Case Study, N. Gelli, C. Tummalapalli, London Business School 2003, (accessed December 7, 2016): http://faculty.london.edu/ppuranam/assets/documents/AURIGENE_case-1.pdf.
- 53. Pharmaceuticals, India Brand Equity Foundation (IBEF), 2015, (accessed December 7, 2016): http://www.ibef.org/download/Pharmaceuticals-March-2015.pdf.
- 54. Annual Reports 1997–2016, Dr. Reddy's Laboratories, (accessed December 10, 2016): http://www.drreddys.com/investors/reports-and-filings/annual-reports/.
- 55. Agrawal R., Jain P., Dikshit S. N., Mini-Rev. Med. Chem. 2012, 12, 87–97. [DOI] [PubMed] [Google Scholar]
- 56. Maji D., Samanta S., Asian J. Pharm. Clin. Res. 2015, 8, 26–31. [Google Scholar]
- 57. Chakrabarti R., Vikramadithyan R. K., Misra P., Hiriyan J., Raichur S., Damarla R. K., Gershome C., Suresh J., Rajagopalan R., Br. J. Pharmacol. 2003, 140, 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. New Heterocyclic Compounds and Their Use in Medicine, Process for their Preparation and Pharmaceutical Compositions Containing Them, Lohray V. B., Lohray B. B., Paraselli R. B., Ramanujam R., Chakrabarti R., (Dr. Reddy's Research Foundation, Hyderabad, India), PCT Pat. Appl. WO1999/008501 A2, February 25, 1999.
- 59. Rajagopal S., Kumar R. A., Subramanya D., Sharma V. M., Sastry T. V. R. S., Houghton P. J., Venkateshwaralu A., Rajagopalan R., Cancer Res. 2004, 64, 710. [Google Scholar]
- 60. Pharmaceutical Compositions of [5(S)-(2′-Hydroxyethoxy)-20(S)-Camptothecin, Nekkanti V. K., Karatgi P. J., Paithankar M., Pillai R. S., Venkateswarlu A., Shanvas A., Kumar R. A., Ramesh M., Raju S., Subrahmanyam D., Sriram R., (Dr. Reddy's Laboratories, Hyderabad, India), US Pat. Appl. US2011/0177161 A1, July 21, 2011.
- 61. Dr. Reddy's Announces the Formation of Perlecan Pharma, Dr. Reddy's Laboratories BusinessWire, September 28, 2005, (accessed December 7, 2016): http://www.businesswire.com/news/home/20050927006290/en/Dr.-Reddys-Announces-Formation-Perlecan-Pharma-Perlecan#.VFofh00tC70.
- 62. Heterocyclic Compounds That Block the Effects of Advanced Glycation End-Products (AGE), Yeleswarapu K. R., Manojit P., Manohar S. Vedula, Akella V., Pillarisetti S., Padakanti S., Kalleda S. R., (Dr. Reddy's Laboratories Ltd., Hyderabad, India), PCT Pat. Appl. WO2005/040163 A1, May 6, 2005.
- 63. New Anti-Diabetic Agent from Reddy's Labs Being Tested, Dr. Reddy's Laboratories, OneIndia, December 26, 2006, (accessed December 7, 2016): http://www.oneindia.com/2006/12/26/new-anti-diabetic-agent-from-reddys-labs-beibng-tested-1167147168.html.
- 64. Argenta Discovery and Dr. Reddy's Progress Pre-Clinical Anti-Inflammatory Candidate to Treat Chronic Respiratory Disease, Argenta Discovery, November 26, 2007, (accessed December 7, 2016): http://www.mvm.com/upload/news/downloads/26112007Argenta%20Press%20Release.pdf.
- 65. Dr. Reddy's Cancer Molecule Completes Phase-I Trials, The Hindu BusinessLine, May 14, 2002, (accessed December 7, 2016): http://www.thehindubusinessline.com/2002/05/14/stories/2002051401130400.htm.
- 66. Substituted Heterocyclic Amine Compounds as Cholesteryl Ester-Transfer Protein (CETP) Inhibitors, Boruah A., Alikunju S., (Dr. Reddy's Laboratories, Hyderabad, India), PCT Pat. Appl. WO2013/024358 A2, February 21, 2013.
- 67. Annual Reports 1997–2014, Ranbaxy Laboratories, (accessed 2008–2014): http://www.ranbaxy.com.
- 68. Drug Profile RBx-2258, Adis Insight, (accessed December 7, 2016): http://adisinsight.springer.com/drugs/800011920.
- 69. Dong Y., Wittlin S., Sriraghavan K., Chollet J., Charman S. A., Charman W. N., Scheurer C., Urwyler H., Tomas J. S., Snyder C., Creek D. J., Morizzi J., Koltun M., Matile H., Wang X., Padmanilayam M., Tang Y., Dorn A., Brun R., Vennerstrom J. L., J. Med. Chem. 2010, 53, 481–491. [DOI] [PubMed] [Google Scholar]
- 70. Ranbaxy Laboratories Limited (RLL) Collaborates with Medicines for Malaria Venture (MMV), Asia Pac. Biotech News 2006, 10, 34–35, (accessed April 30, 2017): http://www.asiabiotech.com/publication/apbn/10/english/preserved-docs/1001/0034_0035.pdf.
- 71. Kreidenweiss A., Mordmüller B., Krishna S., Kremsner P. G., Antimicrob. Agents Chemother. 2006, 50, 1535–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ranbaxy Launches Synriam—India's First New Drug, (accessed December 7, 2016): http://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/005898.html.
- 73. 5-Phenylpentanoic Acid Derivatives as Matrix Metalloproteinase Inhibitors for the Treatment of Asthma and Other Diseases, Palle V. P., Sattigeri V. J., Khera M. K., Voleti S. R., Ray A., Dastidar S. G., (Ranbaxy Laboratories Ltd., New Delhi, India), PCT Pat. Appl. WO2006/090235 A1, August 31, 2006.
- 74. HMG-CoA Reductase Inhibitors, Kumar Y., Aryan R. C., Sattigeri J., Salman M., Shankar G., Bhushan K. H., Pandya B. R., Sharma R., (Ranbaxy Laboratories Ltd., Gurgaon, Haryana, India), PCT Pat. Appl. WO2004/005250 A1, January 15, 2004.
- 75. Combination Therapies, Davenport J. M., Covington P., McIntyre G., (Furiex Pharmaceuticals, Inc., Morrisville, NC, USA), PCT Pat Appl. WO2011/153247 A1, December 8, 2011.
- 76. Dioxolane Derivatives As Cell Adhesion Inhibitors, Palle V. P., Sattigeri V. J., Salman M., Soni A., Ray A., Dastidar S. G., (Ranbaxy Laboratories Ltd., New Delhi, India), PCT Pat. Appl. WO2005/026163 A1, March 24, 2005.
- 77. Heterocyclic Derivatives as Cell Adhesion Inhibitors, Sattigeri V. J., Palle V. P., Soni A., Naik K. P., Ray A., Dastidar S. G., (Ranbaxy Laboratories Ltd., Gurgaon, Haryana, India), PCT Pat. Appl. WO2006/090234 A1, August 31, 2006.
- 78. Das B., Rudra S., Yadav A., Ray A., Rao A. V. S. R., Srinivas A. S. S. V., Soni A., Saini S., Shukla S., Pandya M., Bhateja P., Malhotra S., Mathur T., Arora S. K., Rattan A., Mehta A., Bioorg. Med. Chem. Lett. 2005, 15, 4261–4267. [DOI] [PubMed] [Google Scholar]
- 79.“5-Lipoxygenase Pathway: A Validated Route to Drug Discovery”: Pergola C., Werz O. in Obstructive Airway Diseases—Role of Lipid Mediators (Eds.: A. Ray, P. K. Srivastava), CRC, Boca Raton, 2012, pp. 73–100. [Google Scholar]
- 80. Xanthine Derivatives as Muscarinic Receptor Antagonists, Mehta A., Srivastava S. K., Gupta J. B., (Ranbaxy Laboratories Ltd., New Delhi, India), PCT Pat. Appl. WO2004/056810 A1, July 8, 2004.
- 81. Tremblay J.-F., Chem. Eng. News 2017, 95 (3), 11. [Google Scholar]
- 82. Annual Reports 1997–2016, Torrent Pharmaceuticals, (accessed 2010–2016): http://www.torrentpharma.com/archive_investor_presentation.php.
- 83. Joshi D., Gupta R., Dubey A., Shiwalkar A., Pathak P., Gupta R., Chauthaiwale V., Dutt C., J. Cardiovasc. Pharmacol. 2009, 54, 72–81. [DOI] [PubMed] [Google Scholar]
- 84. Drug Profile TRC 4186, Adis Insight, (accessed December 12, 2016): http://adisinsight.springer.com/drugs/800017743.
- 85. Cioffi F., Zambad S. P., Chhipa L., Senese R., Busiello R. A., Tuli D., Munshi S., Moreno M., Lombardi A., Gupta R. C., Chauthaiwale V., Dutt C., de Lange P., Silvestri E., Lanni A., Goglia F., FASEB J. 2010, 24, 3451–3461. [DOI] [PubMed] [Google Scholar]
- 86. Jamadarkhana P., Chaudhary A., Chhipa L., Dubey A., Mohanan A., Gupta R., Deshpande S., Am. J. Nephrol. 2012, 36, 208–2018. [DOI] [PubMed] [Google Scholar]
- 87. Benzofuroxan Derivatives, Their Therapeutic Uses and Pharmaceutical Compositions, Sankaranarayanan A., (Torrent Pharmaceutical Ltd., Gujarat, India), US Pat. US6.232.331 B1, May 15, 2001.
- 88. Design and Synthesis of Novel Thiazolidine and Pyrrolidine Derivatives as DPP-IV Inhibitors, R. C. Gupta, L. Chhipa, A. B. Mandhare, S. S. Nadkarni, D. Joshi, S. Zambad, P. Pathak, V. Chauthaiwale, C. Dutt, (accessed December 7, 2016): http://www.eposters.net/pdfs/design-and-synthesis-of-novel-thiazolidine-and-pyrrolidine-derivatives-as-dpp-iv-inhibitors-.pdf.
- 89. Mohanan A., Gupta R., Dubey A., Jagtap V., Mandhare A., Gupta R. C., Chauthaiwale V., Dutt C., Int. J. Hypertens. 2011, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Annual Reports 1997–2016, Wockhardt Ltd., (accessed 2010–2016): http://www.wockhardt.com/investor-connect/annual-reports.aspx.
- 91. Al-Lahham A., de Souza N. J., Patel M., Reinert R. R., J. Antimicrob. Chemother. 2005, 56, 1130–1133. [DOI] [PubMed] [Google Scholar]
- 92. de Souza N. J., Gupte S. V., Deshpande P. K., Desai V. N., Bhawsar S. B., Yeole R. D., Shukla M. C., Strahilevitz J., Hooper D. C., Bozdogan B., Appelbaum P. C., Jacobs M. R., Shetty N., Patel M. V., Jha R., Khorakiwala H. F., J. Med. Chem. 2005, 48, 5232–5242. [DOI] [PubMed] [Google Scholar]
- 93. de Souza Mendes C., de Souza Antunes A., Antibiotics 2013, 2, 500–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wockhardt Receives Qualified Infectious Disease Product (QIDP) Designation for its New Drug WCK-4873 from the USFDA, May 21, 2015, (accessed December 7, 2016): http://www.wockhardt.com/pdfs/Press%20Release.pdf.
- 95. Nafithromycin, WHO Drug Information 2015, 29 (4), 545–546, (accessed December 14, 2016): http://www.who.int/medicines/publications/druginformation/issues/PL_114.pdf?ua=1.
- 96.WCK-5222 (Cefepime–Zidebactam) In Vitro Time–Kill Studies against Pseudomonas aeruginosa and Acinetobacter baumannii Isolates with Characterized β-Lactamases, M. D. Huband, M. Castanheira, R. N. Jones, P. R. Rhomberg, A. A. Watters, H. S. Sader, Microbe 2016, Boston, June 16–20, 2016, (accessed January 18, 2017): https://www.jmilabs.com/data/posters/Microbe16-WCK-5222-Friday-477.pdf.
- 97. Annual Reports 1997–2016, Piramal, (accessed December 7, 2016): http://www.piramal.com/investors/financial-reports#parentVerticalTab2.
- 98. Butler M. S., Robertson A. A., Cooper M. A., Nat. Prod. Rep. 2014, 31, 1612–1661. [DOI] [PubMed] [Google Scholar]
- 99. Abdel-Magid A. F., ACS Med. Chem. Lett. 2013, 4, 20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Insulin-Like Growth Factor-1 Receptor Inhibitors, Balachandran S., Dinsmore C. J., Roychowdhury A., Sharma R., Vishwakarma R. A., (Merck, Sharp and Dohme, Rahway, NJ, USA & Piramal Healthcare, Mumbai, India), PCT Pat. Appl. WO2012/145471 A1, October 26, 2012.
- 101. Insulin-Like Growth Factor-1 Receptor Inhibitors, Roychowdhury A., Sharma R., Mascarenhas M., B-Rao C., Patil S. M., Chavan S., Lad N., Khanna S., Dinsmore C. J., (Merck Sharp & Dohme Corp., Rahway, NJ, USA, Piramal Enterprises Ltd., Mumbai, India), PCT Pat. Appl. WO2013/148227 A1, October 3, 2013.
- 102. A Crystalline Form of a Salt of a Morpholino Sulfonyl Indole Derivative and a Process for its Preparation, Chennamsetty S. M. B., Joshi K., Chinchwade Y., Hulawale Y., Paramasivan S., Sivakumar M., Hariharan S., (Piramal Healthcare Ltd., Mumbai, India), PCT Pat. Appl. WO2012/143879 A1, October 26, 2012.
- 103. Nicholas Piramal and Pierre Fabre Laboratories Sign Agreement on Research in Oncology, Nicholas Piramal India Ltd., January 25, 2008, (accessed December 10, 2016): http://m.piramal.com/sites/default/files/pdf/Pierre-Fabre-Oncology-RnD-Agr.pdf.
- 104. Macrocyclic Lactone Derivatives for the Treatment of Cancer, Abdul S. M., Mishra P. D., Vishwakarma R., Fiebig H.-H., Kelter G., (Piramal Life Sciences Ltd., Mumbai, India, Oncotest Gmbh, Freiburg, Germany), PCT Pat. Appl. WO2011/061666 A1, May 26, 2011.
- 105. Anthony J., Kelkar A., Wilankar C., Ranjith V., Bhumra S. K., Mutt S., Deka N., Sivaramakrishnan H., Sharma S., Marita A. R., PLoS One 2013, 8, e77946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Oxadiazole Compounds, Their Preparation and Use, Sivakumar M., Joshi K. S., Aware V. S., Sarde A. G., Bagul S. M., Manohar S. M., (Piramal Life Sciences Ltd., Mumbai, India), PCT Pat. Appl. WO2011/104680 A1, September 1, 2011.
- 107. Nitric Oxide Releasing Prodrugs of Therapeutic Agents, Satyam A., (Piramal Life Sciences Ltd., Mumbai, India), US Pat. Appl. US2011/0263526 A1, October 27, 2011.
- 108. Study of P3914 to Evaluate the Safety, Tolerability, Food Effect & Pharmacokinetics in Healthy Male Subjects and Efficacy & Safety of P3914 in Patients with Acute Dental Pain, ClinicalTrials.gov, Bethesda, MD (USA): US National Library of Medicine, NLM Identifier: NCT01339156, Piramal Enterprises Ltd., (accessed April 18, 2017): https://clinicaltrials.gov/ct2/show/NCT01339156.
- 109.
- 109a. Substituted Imidazoquinoline Derivatives as Kinase Inhibitors, Kumar S., Sharma R., Zahler R., Sahu B., Agarwal V. R., Naik N., (Piramal Enterprises Ltd., Mumbai, India), US Pat. Appl. US2013/0116248 A1, May 9, 2013;
- 109b. Panulisib, WHO Drug Information 2014, 28 (1), 99–100, (accessed December 7, 2016): http://www.who.int/medicines/publications/druginformation/innlists/RL71.pdf?ua=1.
- 110. Clinical Study of Oral PI3K/mTOR Inhibitor in Patients with Advanced Refractory Solid Tumors, ClinicalTrials.gov, Bethesda, MD (USA): US National Library of Medicine, NLM Identifier: NCT01762410, Piramal Enterprises Ltd., (accessed December 13, 2016): https://clinicaltrials.gov/ct2/show/NCT01762410.
- 111. Piramal Life Sciences Exits Drug Discovery Business, M. Terry, BioSpace, August 28, 2014, (accessed April 14 2017): http://www.biospace.com/News/piramal-life-sciences-exits-drug-discovery/344587.
- 112. Annual Reports 1999–2009, Dabur, (accessed 2010–2016): http://www.dabur.com.
- 113. Peptide Combination for the Treatment of Cancer, Burman A. C., Mukherjee R., Prasad S., Jaggi M., Singh A. T., (Dabur Oncology PLC, Bordon Hampshire, UK), PCT Pat. Appl. WO2003/002203 A1, January 9, 2003.
- 114. Annual Reports 2000–2016, Sun Pharma, (accessed 2010–2016): http://www.sunpharma.com/investors/annualreports.
- 115. Annual Reports 2007–2016, SPARC, (accessed December 14, 2016): http://www.sunpharma.in/annual-reports.htm.
- 116. Mandhane S. N., Ayer U. B., Midha A. S., Rao C. T., Rajamannar T., Drugs R&D 2008, 9, 93–112. [DOI] [PubMed] [Google Scholar]
- 117. 4-(Diarylmethyl)-1-piperazinyl Derivatives, Midha A. S., Chokshi H. A., Chitturi T. R., Thennati R., (Sun Pharmaceutical Industries Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2003/079970 A2, October 2, 2003.
- 118. Novel Prodrugs, Thennati R., Samanta B., Pal R. K., Kilaru S., Jivani J., Kumbhani S. A., Adhyapak J. P., (Sun Pharma Advanced Research Company Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2008/111096 A2, September 18, 2008.
- 119. Novel 11β-Hydroxyandrosta-4-ene-3-ones, Patel J. R., Patel G. C., Sheth G. S., Shah S. R., Mandhane S. N., Chitturi T. R., Thennati R., (Sun Pharmaceutical Industries Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2007/099548 A2, September 7, 2007.
- 120. Badorrek P., Hohlfeld J. M., Krug N., Joshi A., Raut A., Ann. Allergy Asthma Immunol. 2015, 115, 325–329. [DOI] [PubMed] [Google Scholar]
- 121. Thennati R., Khanna A., Khanna M., Sonaiya T., Mehta T., Mehta K., Shahi P., Patel J., Clin. Pharmacol. Drug Dev. 2014, 3, 428–438. [DOI] [PubMed] [Google Scholar]
- 122. Methods for the Treatment of Inflammatory Conditions Using S-[4-(3-fluoro-3-methylbutyryloxy)but-2-ynyl]6α,9α-difluoro-17α-(furan-2-yl)carbonyloxy-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, Patel J. R., Patel G. C., Sheth G. S., Mandhane S. N., Rao C. T., Thennati R., (Sun Pharma Advanced Research Company Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2014/192027 A1, December 4, 2014.
- 123. Cysteinyl Leukotriene Antagonists, Rathod R., Bhatt T., Joshi K., Dole B., Murty K. V. S. N., Thennati R., (Sun Pharma Advanced Research Company Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2013/051024 A2, April 11, 2013.
- 124. Novel Hydrazide Containing Tyrosine Kinase Inhibitors, Sengupta P., Puri C. S., Chokshi H., Chitturi T. R., Thennati R., (Sun Pharma Advanced Research Company Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2009/109991 A2, September 11, 2009.
- 125. Diarylacetylene Hydrazide Containing Tyrosine Kinase Inhibitors, Sengupta P., Chokshi H. A., Puri C. S., Chimanwala S. Y., Mehta V. A., Desai D. M., Chitturi T. R., Thennati R., Atkinson J. D. M., (Sun Pharma Advanced Research Company Ltd., Andheri, Mumbai, India), PCT Pat. Appli. WO2012/098416 A1, July 26, 2012.
- 126. Investor Update on R&D Pipeline, Sun Pharma, August 4, 2016, (accessed December 10, 2016): http://www.sunpharma.in/SPARC_Investor%20Presention_4th%20August_Final.pdf.
- 127. Novel Dicarboxylic Acid Derivatives, Capet M., Levoin N., Berrebi-Bertrand I., Poupardin O., Robert P., Schwartz J.-C., Lecomte J.-M., Rajamannar T., Pal R. K., Samanta B., Jivani J. K., Panchal B., Bhatt I. H., Aradhye J. D., (Bioprojet, Paris, France; Sun Pharmaceutical Industries Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2006/043149 A2, April 27, 2006.
- 128. Novel Dicarboxylic Acid Derivatives as S1P1 Receptor Agonists, Capet M., Levoin N., Berrebi-Bertrand I., Robert P., Schwartz J.-C., Lecomte J.-M., Aradhye J. D., Pillai M. N., Panchal B. M., Jivani J. K., Samanta B., Pal R. K., Thennati R., (Bioprojet, Paris, France; Sun Pharma Advanced Research Company Ltd., Anheri, Mumbai, India), PCT Pat. Appl. WO2008/152149 A1, December 18, 2008.
- 129. Novel Piperidinyl Monocarboxylic Acids as S1P1 Receptor Agonists, Capet M., Berrebi-Bertrand I., Robert P., Schwartz J.-C., Lecomte J.-M., Thennati R., Pal R. K., Samanta B., Pillai M. N., Desai J. N., Rana D. C., Prajapati K. D., Pathak S. P., Panchal B. M., Aradhye J. D., (Bioprojet, Paris, France; Sun Pharma Advanced Research Company Ltd., Andheri, Mumbai, India), PCT Pat. Appl. WO2012/140020 A1, October 18, 2012.
- 130. Annual Reports 1999–2016, Alembic Pharmaceuticals, (accessed 2010–2016): http://www.alembic-india.com/.
- 131. Annual Reports, Corporate Presentations, Website 2000–2016, Cadila Healthcare, (accessed 2008–2016): http://zyduscadila.com/.
- 132.“To Market, to Market – 2013”: Bronson J., Black A., Dhar M., Ellsworth B., Merritt J. R. in Annu. Rep. Med. Chem., Vol. 49 (Ed.: M. C. Desai), Elsevier, Amsterdam, 2014, pp. 437–508. [Google Scholar]
- 133. 2-Phenyl-5-heterocyclyl-tetrahydro-2H-pyran-3-amine Compounds for Use in the Treatment of Diabetes and its Associated Disorders, Desai R. C., Bahekar R., Jadav P., Goswami A., P. Patel, (Cadila Healthcare Ltd., Ahmedabad, Gujarat, India), PCT Pat. Appl. WO2014/061031 A1, April 24, 2014.
- 134. Novel Quinolone Derivatives, Desai R. C., Pandya V., Patel P. R., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2014/102818 A1, July 3, 2014.
- 135. Minutes of the IND Committee Meeting Held on December 16, 2015, Central Drugs Standard Control Organization, 2015, (accessed December 10, 2016): http://www.cdsco.nic.in/writereaddata/IND%20Committee%20Meeting%20Minutes%20held%20on%2016_12_2015.pdf.
- 136. Novel Heterocyclic Compounds, Lohray B. B., Lohray V. B., Srivastava B., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2006/025069 A2, March 9, 2006.
- 137. Antidiabetic Compounds, Bahekar R. H., Lohray B. B., Lohray V. B., Jain M. R., Banerjee K. M., Patel P. R., (Cadila Healthcare Ltd., Ahmedabad, Gujarat, India), PCT Pat. Appl. WO2008/062457 A2, May 29, 2008.
- 138. Short Chain Peptidomimetics Based Orally Active GLP-1 Agonist and Glucagon Receptor Antagonist, Bahekar R., Jain M. R., Patel P. R., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2011/048614 A2, April 28, 2011.
- 139. Substituted Benzamide Derivatives as Glucokinase (GK) Activators, Kharul R., Jain M. R., Patel P. R., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2011/013141 A2, February 3, 2011.
- 140. Novel GPR119 Agonists, Pingali H., Zaware P., Jain M., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2010/146605 A1, December 23, 2010.
- 141. Selective TR-β1 Agonist, Raval S., Raval P., Lohray B. B., Lohray V. B., Patel P. R., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2008/062469 A2, May 29, 2008.
- 142. Substituted Hydroxamic Acid Derivatives as TNF Inhibitors, Lohray B. B., Lohray V. B., Jain M. R., Thombare P. S., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2005/077937 A1, August 25, 2005.
- 143. Short-Chain Peptides as Parathyroid Hormone (PTH) Receptor Agonists, Bahekar R., Jain M. R., Patel P. R., (Cadila Healthcare Ltd., Ahmedabad, India), PCT Pat. Appl. WO2010/128521 A2, November 11, 2010.
- 144. Novel Compounds as Modulators of Glucocorticoid Receptors, Thombare P. S., Goswami A., Zamaratski E., Hansson T., Thomas N., (Cadila Healthcare Ltd., Karo Bio AB), PCT Pat. Appl. WO2011/016050 A2, February 10, 2011.
- 145. Zydus Cadila in Research Pact With Eli Lilly, Livemint, March 30, 2009, (accessed December 10, 2016): http://www.livemint.com/Companies/5prChqQlQtxDgJ3eMCE78I/Zydus-Cadila-in-research-pact-with-Eli-Lilly.html.
- 146. Cadila Pharmaceuticals Website, (accessed December 14, 2016): http://cadilapharma.com/.
- 147. Cadila Pharmaceuticals Champion Brands, (accessed December 10, 2016): http://cadilapharma.com/champion-brands/.
- 148. Cadila Pharmaceuticals Alliances, (accessed December 7, 2016): http://cadilapharma.com/alliances/.
- 149. Annual Reports 1999–2016, FDC Ltd., (accessed December 13, 2016): http://www.fdcindia.com/financial-report.php.
- 150. Borate H. B., Sawargave S. P., Chavan S. P., Chandavarkar M. A., Iyer R., Tawte A., Rao D., Deore J. V., Kudale A. S., Mahajan P. S., Kangire G. S., Bioorg. Med. Chem. Lett. 2011, 21, 4873–4878. [DOI] [PubMed] [Google Scholar]
- 151. Thiophene-Containing Analogues of Fluconazole as Antifungal Agents and Process Thereof, Borate H. B., Sawargave S. P., Maujan S. R., Chandavarkar M. A., Vaiude S. R., Joshi V. A., (Council of Scientific & Industrial Research, New Delhi, FDC Ltd., Mumbai, India), PCT Pat. Appl. WO2010/046912 A2, April 29, 2010.
- 152. Enantiomers of Fluconazole Analogues Containing Thieno[2,3-d]pyrimidin-4(3H)-one Moiety as Antifungal Agents, Borate H. B., Maujan S. R., Sawargave S. P., Chavan S. P., Chandavarkar M. A., Iyer R. R., Nawathye V. V., Chavan G. J., Tawte A. C., Rao D. D., (FDC Ltd., Mumbai, Council of Scientific & Industrial Research, New Delhi, India), PCT Pat. Appl. WO2012/123952 A1, September 20, 2012.
- 153. JB Chem to Set Up Research Unit in Pune, The Hindu Business Line, August 5, 2003, (accessed December 7, 2016): http://www.thehindubusinessline.com/bline/2003/08/05/stories/2003080501890300.htm.
- 154. Heterocyclic Compounds for Therapeutic Use, Shrikhande A. A., Doshi M. M., Mody S. B., (J. B. Chemicals & Pharmaceuticals, Mumbai, Maharashtra, India), PCT Pat. Appl. WO2002/074235 A2, September 26, 2002.
- 155. Annual Reports 1997–2016, JB Chemicals & Pharmaceuticals, (accessed December 7, 2016): https://www.jbcpl.com/investors/annual.html.
- 156. JB Chemicals Files for Patent on 20 NCEs in NSAIDs Slot, The Financial Express, February 11, 2002, (accessed December 10, 2016): http://www.financialexpress.com/archive/jb-chemicals-files-for-patent-on-20-nces-in-nsaids-slot/37338/.
- 157. Cipla Ltd. Website, (accessed December 14, 2016): http://www.cipla.com/en/.
- 158. Antihistaminic Compounds, Hamied Y. K., Kulkarni V. M., (Cipla Ltd., Mumbai, India), PCT Pat. Appl. WO2001/79188 A1, October 25, 2001.
- 159. Oxazolidinone Derivatives as Antibacterial Agents, Hamied Y. K., Kulkarni V. M., (Cipla Ltd., Mumbai, India), PCT Pat. Appl. WO2003/011859 A2, February 13, 2003.
- 160. Annual Reports 1999–2016, Cipla Ltd., (accessed December 10, 2016): http://www.cipla.com/en/investor-information/annual-report-and-chairman-s-speech.html.
- 161. Annual Reports 2001–2016, Glenmark Pharmaceuticals, (accessed 208–2010): http://www.glenmarkpharma.com/investors/reports-presentation.
- 162. Glenmark: Will Innovation Pay?, D. Swamy, 2012, (accessed December 10, 2016): http://forum.valuepickr.com/t/glenmark-will-innovation-pay/286.
- 163. Pagès L., Gavaldà A., Lehner M. D., Expert Opin. Ther. Pat. 2009, 19, 1501–1519. [DOI] [PubMed] [Google Scholar]
- 164. Dyke H. J., Expert Opin. Ther. Pat. 2007, 17, 1183–1189. [DOI] [PubMed] [Google Scholar]
- 165. Novel Dipeptidyl Peptidase IV Inhibitors, Pharmaceutical Compositions Containing them, and Process for their Preparation, Thomas A., Gopalan B., Lingam P. R. V. S., Shah D. M., (Glenmark Pharmaceuticals S.A., Neuchâtel, Switzerland), PCT Pat. Appl. WO2006/040625 A1, April 20, 2006.
- 166. Perspective A., Kushwaha R. N., Haq W., Katti S. B., Curr. Med. Chem. 2014, 21, 4013–4045. [DOI] [PubMed] [Google Scholar]
- 167. Substituted Benzofused Derivatives and their Use as Vanilloid Receptor Ligands, Gharat L. A., Joshi U. M., Joshi N. K., (Glenmark Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), PCT Pat. Appl. WO2007/042906 A1, April 19, 2007.
- 168. Gunthorpe M. J., Chizh B. A., Drug Discovery Today 2009, 14, 56–67. [DOI] [PubMed] [Google Scholar]
- 169. Sanofi-Aventis et Glenmark Pharmaceuticals Signent un Accord de Licence Pour le Traitement des Douleurs Chroniques, Sanofi, May 3, 2010, (accessed December 7, 2016): http://www.sanofi.com/Images/13739_20100503_glenmark_agreement_fr.pdf.
- 170. Efficacy and Safety of SAR292833 Administration for 4 Weeks in Patients With Chronic Peripheral Neuropathic Pain, ClinicalTrials.gov, Bethesda MD, (USA): US National Library of Medicine, NLM Identifier: NCT01463397, Sanofi, (accessed December 7, 2016): https://clinicaltrials.gov/ct2/show/NCT01463397.
- 171. GRC-27864 First in Man, Single Ascending Dose Study in Healthy Volunteers, ClinicalTrials.gov, Bethesda MD, (USA): US National Library of Medicine, NLM Identifier: NCTNCT02179645, Glenmark Pharmaceuticals Ltd. India, (accessed December 7, 2016): https://www.clinicaltrials.gov/ct2/show/NCT02179645.
- 172. Tricyclic Compounds as mPGES-1 Inhibitors, Gharat L. A., Kattige V. G., Khairatkar-Joshi N., Muthukaman N., Narayana L., (Glenmark Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), PCT Pat. Appl. WO2012/055995 A1, May 3, 2012.
- 173. Tricyclic Compounds as mPGES-1 Inhibitors, Gharat L. A., Gajera J. M., Narayana L., Khairatkar-Joshi N., Kattige V. G., (Glenmark Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), PCT Pat. Appl. WO2012/110860 A1, August 23, 2012.
- 174. Substituted Pyrimidine Compounds as mPGES-1 Inhibitors, Gharat L. A., Muthukaman N., Tambe M. S., Pisal D., Khairatkar-Joshi N., Kattige V. G., (Glenmark Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), PCT Pat. Appl. WO2015/059618 A1, April 30, 2015.
- 175. Revamilast, WHO Drug Information 2010, 24 (3), 278, (accessed December 7, 2016): http://www.who.int/medicines/publications/druginformation/INN RL64.pdf?ua=1.
- 176. Novel Heterocyclic Compounds Useful for the Treatment of Inflammatory and Allergic Disorders, Gharat L. A., Gopalan B., Khairatkar-Joshi N., (Glenmark Pharmaceuticals S.A., Neuchâtel, Switzerland), PCT Pat. Appl. WO2006/064355 A2, June 22, 2006.
- 177. GRC-10693: A Selective, Orally Active CB2 Agonist for the Treatment of Neuropathic Pain, S. Narayanan, D. Amrutkar, S. Gupta, S. Gullapalli, 37th Annual Meeting of the Society of Neuroscience, Abstract 287.18, San Diego, CA (USA), November 3–7, 2007.
- 178. Tedalinab, WHO Drug Information 2010, 24 (2), 169, (accessed December 7, 2016): http://www.who.int/medicines/publications/druginformation/INN PL103.pdf?ua=1.
- 179. GRC-17536, a Novel, Selective TRPA1 Antagonist for Potential Treatment of Respiratory Disorders, R. Anupindi, I. Mukhopadhyay, A. Thomas, S. Kumar, S. Chaudhari, A. Kulkarni, G. Gudi, N. Joshi, 20th Annual Congress of the European Respiratory Society (ERS), Barcelona, Spain, September 18–22, 2010.
- 180. Glenmark's TRPA1 Antagonist GRC-17536 Shows Positive Data in a Proof-of-Concept Study, Glenmark Pharmaceuticals Ltd., September 17, 2014, (accessed April 23, 2017): http://www.glenmarkpharma.com/sites/default/files/GlenmarksTRPA1antagonistGRC_17536.pdf.
- 181. Annual Reports 2001–2016, Lupin Ltd., (accessed December 13, 2016): http://www.lupin.com/archives-annual-report.php.
- 182. A Purified Arabinogalactan-Protein (AGP) Composition, Arora S. K., Srivastava V., Walunj S. S., (Lupin Ltd., Mumbai, Maharashtra, India), PCT Pat. Appl. WO2006/025068 A1, March 9, 2006.
- 183. Pyrrole Derivatives as Antimycobacterial Compounds, Arora S. K., Sinha N., Jain S., Upadhayaya R. S., Jana G., Ajay S., Sinha R. K., (Lupin Ltd., Mumbai, Maharashtra, India), PCT Pat. Appl. WO2004/026828 A1, April 1, 2004.
- 184. Rivers E. C., Mancera R. L., Drug Discovery Today 2008, 13, 1090–1098. [DOI] [PubMed] [Google Scholar]
- 185. 4-(5-(4-Chlorophenyl)-2-(2-cyclopropylacetyl)-1,4-dimethyl-1H-pyrrol-3-yl)benzenesulfonamide as α7 nAChR Modulator, Sinha N., Karche N. P., Tilekar A. R., Palle V. P., Kamboj R. K., (Lupin Ltd., Santa Cruz East, Maharashtra, India), PCT Pat. Appl. WO2014/195848 A1, December 11, 2014.
- 186. Evaluate 2 Doses of LND101001 in Mild-Moderate Alzheimer's Patients, Eudract number 2013-001851-11, Lupin Ltd., (accessed December 11, 2016): http://www.hra.nhs.uk/news/research-summaries/evaluate-2-doses-of-lnd101001-in-mild-moderate-alzheimers-patients/.
- 187. A Study to Test the Safety/Tolerability of Increasing Doses of LNP1892 versus Placebo in Healthy Male/Female Subjects, ClinicalTrials.gov, Bethesda MD, (USA): US National Library of Medicine, NLM Identifier: NCT02174237, Lupin Ltd., (accessed December 11, 2016): https://clinicaltrials.gov/ct2/show/NCT02174237.
- 188. LNP3794 in patients with Advanced Solid Tumours Having Mutations, Eudract number 2014-001536-10, Lupin Ltd., (accessed December 11, 2016): http://www.hra.nhs.uk/news/research-summaries/lnp3794-in-patients-with-advanced-solid-tumours-having-mutations/.
- 189. A Phase II, Dose Ranging, Exploratory Clinical Study to Assess the Efficacy, Pharmacodynamics, Pharmacokinetics, and Safety of LNP1955 in Patients with Moderate to Severe Rheumatoid Arthritis, Eudract number 2016-001532-35, Lupin Ltd., (accessed December 11, 2016): https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-001532-35/HU.
- 190. Reliance Life Sciences Website, (accessed December 14, 2016): http://www.rellife.com/.
- 191. Compounds for Treatment of Lipase-Mediated Diseases, Upadhyay S., Yadav R., Gangan V., Kanekar Y., (Reliance Life Sciences, Navi Mumbai, Maharashtra, India), PCT Pat. Appl. WO2007/010546 A1, January 25, 2007.
- 192. Novel Chemotherapeutic Agents Against Inflammation and Cancer, Bose J. S., Gangan V., (Reliance Life Sciences Pvt. Ltd., Navi Mumbai, Maharashtra, India), PCT Pat. Appl. WO2008/062466 A2, May 29, 2008.
- 193. Toll-Like Receptor (TLR) Signaling Antagonist, Shakti U., Yogesh K., Rajendra K., Vikram R., Praneel D., Akshaya B., Shiva P. S., (Reliance Life Sciences Pvt. Ltd., Navi Mumbai, Maharashtra, India), PCT Pat. Appl. WO2009/047791 A2, April 16, 2009.
- 194. Kalluri M. D., Datla P., Bellary A., Basha K., Sharma A., Sharma A., Singh S., Upadhyay S., Rajagopal V., FEBS J. 2010, 277, 1639–1652. [DOI] [PubMed] [Google Scholar]
- 195. Datla P., Kalluri M. D., Basha K., Bellary A., Kshirsagar R., Kanekar Y., Upadhyay S., Singh S., Rajagopal V., Br. J. Pharmacol. 2010, 160, 1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Addepalli M. K., Ray K. B., Kumar B., Ramnath R. L., Chile S., Rao H., Gene Ther. 2010, 17, 352–359. [DOI] [PubMed] [Google Scholar]
- 197. Annual Reports 2002–2016, Orchid Chemicals & Pharmaceuticals, (accessed 2010–2016): http://www.orchidpharma.com/.
- 198. Lee W. H., Kim S. G., PPAR Res. 2010, 1–10; (accessed April 13 2017), DOI: <https://doi.org/2010.1155/2010/549101. [Google Scholar]
- 199. Diakron Licenses Anticoagulant Drug Candidate from Merck & Co, Inc; Orchid Pharma Partners with Diakron, FierceBiotech, August 13, 2008, (accessed December 11, 2016): http://www.fiercebiotech.com/biotech/diakron-licenses-anticoagulant-drug-candidate-from-merck-co-inc-mrk-orchid-pharma-partners.
- 200. Drug Profile DP 4088, Adis Insight, (accessed December 11, 2016): http://adisinsight.springer.com/drugs/800028740.
- 201. Narayanan S., Vishwakarma S., Saxena S., Honnegowda S., Ganesan K., Ahamed F. A., Surendran N., Narayanan S., Rajagopal S., Balasubramaniam G., J. Allergy Clin. Immunol. 2010, 125, AB49. [Google Scholar]
- 202. A Phase 1 Dose-Escalation Study of the Safety and Pharmacokinetics of Daily OCID-4681-S-01 Administered Orally to Subjects with Advanced Solid Tumors, Clinical Trials Registry India, CTRI Number CTRI/2011/12/002225, Orchid Research Laboratories Ltd., (accessed December 11, 2016): http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=2976.
- 203. Histone Deacetylase Inhibitors, Rajagopal S., Kachhadia V., Ponpandian T., Keeri A. R., Anandhan K., Rajagopal S., Praveen R., Daivasigamani P., (Orchid Research Laboratories Ltd., Chennai, Tamilnadu, India), PCT Pat. Appl. WO2009/053808 A2, April 30, 2009.
- 204. 2-Substituted Methyl Penam Derivatives, Palanisamy S. U., Gnanaprakasam A., Ganapathy P., Gohain M., Hariharan V., Rajagopal S., Paul-Satyaseela M., Solanki S. S., Devarajan S., (Orchid Chemicals & Pharmaceuticals, Chennai, India), US Pat. US7687488 B2, March 30, 2010.
- 205. New Anti-Infectives Company Allecra Therapeutics Created, April 18, 2013, (accessed December 7, 2016): http://www.allecra.com/news/new-anti-infectives-company-allecra-therapeutics-created-with-15-million-series-a-financing.
- 206. Crystalline β-Lactamase Inhibitor, Lamonica A., Forzatti M., Biondi S., (Allecra Therapeutics SAS, Saint Louis, France), PCT Pat. App. WO2015/067787 A1, March 30, 2010.
- 207. Orchid Chem Out-licenses Molecule to German Firm, S. Sundria, DNA India, April 19 2013, (accessed April 14 2017): http://www.dnaindia.com/money/report-orchid-chem-out-licenses-molecule-to-german-firm-1824296.
- 208. Compounds and Their Use, Palanisamy S. U., Paul-Satyaseela M., Narayanan S., Balasubramania Gopalan, Appu A., Manickam S., Periasamy H., (Allecra Therapeutics GmbH, Weil am Rhein, Germany), US Pat. Appl. US2014/0057888 A1, February 27, 2014.
- 209. Papp-Wallace K. M., Bonomo R. A., Infect. Disease Clin. North Am. 2016, 30, 441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Annual Reports 2003–2016, Suven Life Sciences Ltd., (accessed December 7, 2016): http://www.suven.com/AnnualReports.htm.
- 211. Suven Life in Drug Research Deal with Eli Lilly, Industry Watch India, Asia Pac. Biotech News 2008, 12 (6), 52 (accessed April 23, 2017): http://www.asiabiotech.com/2012/1206/0052_0052.pdf.
- 212. Discovery Pipeline, Suven Life Sciences Ltd., (accessed December 13, 2016): http://www.suven.com/DiscoveryProductPipeline(14-Jul-2016).pdf.
- 213. SUVN-502 With Donepezil and Memantine for the Treatment of Moderate Alzheimer's Disease—Phase 2a Study, ClinicalTrials.gov, Bethesda MD, (USA): US National Library of Medicine, NLM Identifier: NCT02580305 Suven Life Sciences Ltd., (accessed January 18, 2017): https://www.clinicaltrials.gov/ct/show/NCT02580305.
- 214. Nirogi R., Mudigonda K., Penta K. K., Bhyrapuneni G., Benade V., Muddana N., Palacharla V. R. C., Ajjala D. R., Goyal V. K., Pandey S. K., Abraham R., Jayarajan P., Kambhampati R., Shinde A. K., Alzheimer′s Dementia 2016, 12, P434. [Google Scholar]
- 215. Bhyrapuneni G., Mudigonda K., Penta K. K., Palacharla V. R. C., Muddana N., Jayarajan P., Abraham R., Subramanian R., Goyal V. K., Pandey S. K., Boggavarapu R. K., Shinde A. K., Nirogi R., Alzheimer′s Dementia 2016, 12, P618–P619. [Google Scholar]
- 216. Jayarajan P., Bhyrapuneni G., Mudigonda K., Penta K. K., Muddana N., Palacharla V. R. C., Benade V., Abraham R., Subramanian R., Goyal V. K., Pandey S. K., Boggavarapu R. K., Ajjala D. R., Rasheed M. A., Nirogi R., Alzheimer′s Dementia 2016, 12, P823–P824. [Google Scholar]
- 217. Bhyrapuneni G., Muddana N. R., Kandikere V., Benade V., Ponnamaneni R. K., Mudigonda K., Nirogi R., Alzheimer′s Dementia 2010, 6, S563. [Google Scholar]
- 218. Annual Reports 2003–2016, Natco Pharma, (accessed 2010–2016): http://natcopharma.co.in/investors/annual-reports/.
- 219. Amala K., Rao A. K. B., Gorantla B., Gondi C. S., Rao J. S., Int. J. Oncol. 2013, 42, 168–178. [DOI] [PubMed] [Google Scholar]
- 220. 6,7-Dialkoxyquinazoline Derivatives Useful for Treatment of Cancer-Related Disorders, Prasad R. J., Satya B. R. A., Rao B. N., Chowdary N. V., (Natco Pharma Ltd., Andhra Pradesh, India), US Pat. Appl. US2011/0039844 A1, February 17, 2011.
- 221. Annual Reports 2003–2016, Panacea Biotec, (accessed 2010–2016): http://www.panacea-biotec.com/.
- 222. Novel Heterocyclic Compounds, Jain R., Trehan S., Das J., Singh N., Nanda G. K., Magadi S. K., Sharma S. K., (Panacea Biotec Ltd., New Delhi, India), PCT Pat. Appl. WO2009/093269 A1, July 30, 2009.
- 223. Study of Single Ascending Doses of PBL-1427 in Healthy Volunteers, ClinicalTrials.gov, Bethesda MD, (USA): US National Library of Medicine, NLM Identifier: NCT01554293, Piramal Enterprises Ltd., (accessed December 11, 2016): https://clinicaltrials.gov/ct2/show/NCT01554293.
- 224. Annual Reports 2003–2011, Matrix Laboratories.
- 225. Novel Dipeptidyl Peptidase IV Inhibitors and Processes for Their Preparation and Pharmaceutical Compositions Containing Them, Gopalan B., Ravi D., Rasheed M., Sreedhara S. K. H., Ishtiyaque A., (Matrix Laboratories Ltd., Secunderabad, India), PCT Pat. Appl. WO2007/113634 A1, October 11, 2007.
- 226. Dibenzofuran Derivatives as Inhibitors of PDE-4 and PDE-10, Gopalan B., Ravi D., Shrikant H., Sreedhara S. K. H., (Matrix Laboratories Ltd., Secunderabad, India), PCT Pat. Appl. WO2008/032171 A1, March 20, 2008.
- 227. Novel Vanilloid Receptor Modulators, Process for Their Preparation and Pharmaceutical Compositions Containing Them, Gopalan B., Manojit P., Arumugam K., Nidhi D., (Matrix Laboratories Ltd., Secunderabad, India), PCT Pat. Appl. WO2010/023512 A1, March 3, 2010.
- 228.B. Gopalan, ISCBC-2012, Solapur, India, Jan. 21–24, 2012, p57, (accessed December 13, 2016): http://www.iscbindia.com/index.php/downloads/proceedings?download=36 %3A17th-iscbc-interna-tional-conference-iscbc-2012.
- 229. Company Website, Hetero Drugs, (accessed December 7, 2016): http://heteroworld.com/.
- 230. Novel Nucleoside Derivatives, Reddy B. Parthasaradhi, Sharma V. Manohar, Reddy K. Rathnakar, Reddy M. Madhanmohan, Sreenu J., Ratnakar A., (Hetero Research Foundation, Hyderabad, India), PCT Pat. Appl. WO2009/115893 A2, September 24, 2009.
- 231. Novel Carboxamide Derivatives As HIV Inhibitors, Reddy B. Parthasaradhi, Krishna B. Vamsi, Sharma V. Manohar, Reddy K. Rathnakar, Reddy M. Madhanmohan, Lanka S., Kumar M. Prem, (Hetero Research Foundation, Hyderabad, India), PCT Pat. Appl. WO2011/061590 A1, May 26, 2011.
- 232. Novel Betulinic Proline Imidazole Derivatives as HIV Inhibitors, Reddy B. Parthasaradhi, Reddy K. Rathnakar, Reddy A. Panduranga, Levi D. K. Gazula, Bammidi E. R., Kothakapu R. R., (Hetero Research Foundation, Hyderabad, India), PCT Pat. Appl. WO2016/001820 A1, January 7, 2016.
- 233. Interview Bandi Parthasaradhi Reddy, Hetero Drugs, PharmaBoardRoom, July 25, 2012, (accessed April 23, 2017): http://pharmaboardroom.com/interviews/interview-with-d.r.-bandi-parthasaradhi-reddy-hetero-drugs-limited/.
- 234. Annual Reports, Corporate Presentations, Press Releases 2001–2016, Jubilant, (accessed 2008–2016): http://www.jubl.com/.
- 235. Jubilant Biosys and Sanofi Deutschland GmbH Enter Into a Strategic Alliance Focusing on Metabolic Disorders Therapeutic Area, January 7, 2016, (accessed December 11, 2016): http://www.jubilantbiosys.com/media-press-details.aspx?mpgid=391&pgid=392&pressid=268.
- 236. Boyd A. W., Bartlett P. F., Lackmann M., Nat. Rev. Drug Discovery 2014, 13, 39–62. [DOI] [PubMed] [Google Scholar]
- 237. Sivanandhan D., Parikh P. K., Sheshachalam A., Chandregowda V., Bhakthavatchalam R., Gupta M., Giri S., Krishnakumar V., Cancer Res. 2014, 74, LB-232. [Google Scholar]
- 238. Novel Dual Inhibitors of LSD1-HDAC for Treatment of Multiple Myeloma and Other Cancers, D. Sivanandhan, Drug Discovery Chemistry, Cambridge Healthtech Institute, San Diego, April 19–22, 2016, (accessed December 11, 2016): http://www.drugdiscoverychemistry.com/uploadedFiles/Drug_Discovery_Chemistry/Agenda/16/2016-Drug-Discovery-Chemistry-Brochure.pdf.
- 239. Sivanandhan D., Rajagopalan S., Nair S., Dewang P., Kumar D. P., Mulakala C., Mahadevan L., Skariah N., Kavuru V. G., Kuntrapaku D., Sajja S., Zainuddin M., V K., Singh R., V S., Gosu R., Aiyar J., Iyer P., Rajagopal S., Cancer Res. 2015, 75, 3509. [Google Scholar]
- 240. Novel, Small-Molecule BET Inhibitors for Treatment of Cancer, D. Sivanandhan, 15th Annual Discovery on Target: Targeting Epigenetic Readers and Chromatin Remodelers, Boston, MA, USA, September 19–22, 2016, (accessed December 11, 2016): http://www.discoveryontarget.com/epigenetic-readers/15/.
- 241. CK-103 BET Inhibitor, Checkpoint Therapeutics, Pipeline, 2016, (accessed December 11, 2016): http://www.checkpointtx.com/pipeline/ck-103-bet-inhibitor/.
- 242. Anti-Inflammatory Compounds, Sakhardande R., Kulkarni V., Wagh N., Nimbalkar M., Nadkarni S. M., (Elder Pharmaceuticals Ltd., Mumbai, India), PCT Pat. Appl. WO2010/029576 A2, March 18, 2010.
- 243. N-Biphenylacyl Thiazolidine-2,4-dione Derivatives, Their Synthesis and Uses, Sakhardande R., Kulkarni V., Sachan N., Nimbalkar M., (Elder Pharmaceuticals Ltd., Mumbai, India), PCT Pat. Appl. WO2010/082212 A2, July 22, 2010.
- 244. Pharma Firm at Centre of Probe Owes Rs 1500 Cr to Investors, K. Narayan, The Indian Express, July 21, 2016, (accessed December 11, 2016): http://indianexpress.com/article/india/india-news-india/pharma-firm-at-centre-of-probe-owes-rs-1500-cr-to-investors/.
- 245. Annual Reports 2005–2014, Elder Pharmaceuticals, (accessed 2010–2015): http://www.elderindia.com.
- 246. IPCA New Drug Discovery/Development, 2016, (accessed December 12, 2016): http://www.ipcalabs.com/active-pharmaceutical-ingredients-research-development.html.
- 247. IPCA, N. Som, Biospectrum India, April 10, 2012, (accessed December 11, 2016): http://www.biospectrumindia.com/biospecindia/news/158124/ipca.
- 248. Annual Reports 2009–2016, IPCA Laboratories, (accessed 2010–2016): http://www.ipcalabs.com/.
- 249. Mankind Pharma—New Drug Discovery and Research, (accessed December 7, 2016): https://www.mankindpharma.com/rnd/newdrug.
- 250. Alkem Laboratories Website, (accessed December 14, 2016): http://www.alkemlabs.com/.
- 251. Kedari C. K., Choudhury N. R., Sharma S., Kaur P., Guptha S., Panda M., Mukerjee K., Ramachandran V., Bandodkar B., Ramachandran S., Tantry S. J., ACS Med. Chem. Lett. 2014, 5, 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Discovery of Novel Cathepsin K Inhibitors as Leads for Osteoporosis Therapy, B. Bandodkar, K. Mukherejee, H. Nagaraj, T. Banerjee, R. Jayaraman, J. K. Singh, G. Vaidyanathan, H. T. Kiran, S. Yellapu, P. Hanumesh, A. Seebagi, L. Ravilla, M. Goutham, S. Rao, P. Velusamy, S. Ghode, P. Raskar, SelectBio Drug Discovery India 2014, Mumbai, India, September 11–12, 2014, (accessed December 11, 2016): http://www.eposters.net/pdfs/discovery-of-novel-cathepsin-k-inhibitors-as-leads-for-osteoporosis-therapy.pdf.
- 253. Piperidine Ureas as Cathepsin Cysteine Protease Inhibitors, Nagaraj H. K. M., Bandodkar B. S., Yellapu S., Sugandham S. R., Goutham M., Mukherjee K., Thimmappa K. H., Hanumesh P. B., (Alkem Laboratories Ltd., Bangalore, India), PCT Pat. Appl. WO2016/027284 A2, February 25, 2016.
- 254. Bicyclic Heteroaryl Amides as Cathepsin Cysteine Protease Inhibitors, Nagaraj H. K. M., Bandodkar B. S., Ravilla L., Yellapu S., Rudresha A. S., Singh J. K., G Vaidyanathan, (Alkem Laboratories Ltd. Bangalore, India), PCT Pat. Appl. WO2016/027285 A2, February 25, 2016.
- 255. Emcure Pharmaceuticals Website, (accessed December 14, 2016): http://www.emcure.co.in/.
- 256. Pyridone Derivatives as Acid Secretion Inhibitors and Process for Preparation Thereof, Gurjar M. K., Maikap G. S., Tripathy N. K., Mahale R. D., Khaladkar T. P., Chaudhari A. T., Pawar S. S., Kalhapure V. K., Mehta S. S., (Emcure Pharmaceuticals Ltd., Pune, India), PCT Pat. Appl. WO2014/080422 A2, May 30, 2014.
- 257. How India Lost an Iconic Biotech Lab, N. Suresh, S. Chandan, A. Venkatesh, BioSpectrum India February 4, 2015, (accessed April 23, 2017): http://www.biospectrumindia.com/biospecindia/views/220303/how-india-lost-iconic-biotech-lab).
- 258. Annual Reports 1999–2016, AstraZeneca, (accessed April 13, 2017): https://www.astrazeneca.com/investor-relations/annual-reports.html.
- 259. History of the Anti-infectives Research at AstraZeneca—Part II,: Astra/AstraZeneca, Nichols W. W., Franklin T. J., Gravestock M. B., Pring B. G., Ekström B., Balganesh T. S., Kumar S. A., Doig P., Noonan B., SIM News 2010, 60, 4–20, (accessed April 13, 2017): https://www.academia.edu/18641476/History_of_anti-infectives_research_at_AstraZeneca._Part_II_Astra_AstraZeneca. [Google Scholar]
- 260. Status of the TB Drug Pipeline, B. E. Laughon, T. S. Balganesh, 2010, (accessed April 13, 2017): http://www.tballiance.org/events/downloads/of4/presentations/Laughon-Status-of-the-TB-Drug-Pipeline.pdf.
- 261. Balasubramanian V., Solapure S., Iyer H., Ghosh A., Sharma S., Kaur P., Deepthi R., Subbulakshmi V., Ramya V., Ramachandran V., Balganesh M., Wright L., Melnick D., Butler S. L., Sambandamurthy V. K., Antimicrob. Agents Chemother. 2014, 58, 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Hameed S. P., Solapure S., Patil V., Henrich P. P., Magistrado P. A., Bharath S., Murugan K., Viswanath P., Puttur J., Srivastava A., Bellale E., Panduga V., Shanbag G., Awasthy D., Landge S., Morayya S., Koushik K., Saralaya R., Raichurkar A., Rautela N., Choudhury N. R., Ambady A., Nandishaiah R., Reddy J., Prabhakar K. R., Menasinakai S., Rudrapatna S., Chatterji M., Jiménez-Díaz M. B., Martínez M. S., Sanz L. M., Coburn-Flynn O., Fidock D. A., Lukens A. K., Wirth D. F., Bandodkar B., Mukherjee K., McLaughlin R. E., Waterson D., Rosenbrier-Ribeiro L., Hickling K., Balasubramanian V., Warner P., Hosagrahara V., Dudley A., Iyer P. S., Narayanan S., Kavanagh S., Sambandamurthy V. K., Nat. Commun. 2015, 6, 6715–6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. MMV-Supported Projects: MMV253, (accessed April 13 2017): https://www.mmv.org/research-development/mmv-supported-projects.
- 264. TB Alliance Drug Discovery and Development—Harnessing Global Resources to Address a Global Disease, C. B. Cooper, 2016, (accessed April 13, 2017): http://www.optibrium.com/downloads/TBAlliance-Harnessing-Global-Resources.pdf.
- 265. Chatterji M., Shandil R., Manjunatha M. R., Solapure S., Ramachandran V., Kumar N., Saralaya R., Panduga V., Reddy J., Prabhakar K. R., Sharma S., Sadler C., Cooper C. B., Mdluli K., Iyer P. S., Narayanan S., Shirude P. S., Antimicrob. Agents Chemother. 2014, 58, 5325–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Fonteilles-Drabek S., Reddy D., Wells T. N. C., Nat. Rev. Drug Discovery 2017, 16, 223–224. [DOI] [PubMed] [Google Scholar]
- 267. Bristol-Myers Squibb and Syngene International Extend Research Collaboration, 2014, (accessed April 13, 2017): https://news.bms.com/press-release/bristol-myers-squibb-and-syngene-international-extend-research-collaboration.
- 268. Syngene Dedicated Centers, 2017, (accessed April 13, 2017): http://www.syngeneintl.com/services/our-dedicated-centers.
- 269. TCG Lifesciences Company Overview, (accessed April 13, 2017): http://www.tcgls.com/Company_Overview1.pdf.
- 270. Pfizer and TCG Lifesciences Announce a Collaboration to Develop Portfolio of Preclinical Candidate Molecules, 2010, (accessed April 13, 2017): http://www.tcgls.com/news2010_05_jan.php.
- 271. Company Presentations, Website 2005–2016, Sai Life Sciences (accessed 2008–2016): http://www.sailife.com/.
- 272. Annual Report 2008, UCB, (accessed April 26, 2017): http://www.ucb.com/_up/ucb_com_ir/documents/2008_Annual_report_-_ENG.pdf.
- 273. Company Presentations, Website, and Press Releases 2005–2016, Advinus Therapeutics, (accessed 2008–2016): http://www.advinus.com/.
- 274. Press Releases 2006–2016, Advinus Therapeutics, (accessed December 11, 2016): http://www.advinus.com/news-media/press-releases/.
- 275. Liver-Selective Glucokinase Activation for Treating Type 2 Diabetes: Translation from Mouse to Man, K. A. Mookhtiar, Ramanbhai Foundation 7th International Symposium on Current Trends in Pharmaceutical Sciences, Ahmedabad (India), February 2–4, 2015.
- 276.“Glucokinase Activators in Development”: Filipski K. J., Stevens B. D., Pfefferkorn J. A. in New Therapeutic Strategies for Type 2 Diabetes: Small-Molecule Approaches (Ed.: R. M. Jones), RSC Publishing, Cambridge, 2012, pp. 88–108. [Google Scholar]
- 277. Advinus’ GK-Activator Achieves Early POC for Diabetes, November 29, 2011, (accessed December 11, 2016): http://www.advinus.com/wp-content/uploads/2016/04/November-29-2011.pdf.
- 278. Discovery Pipeline, Advinus Therapeutics, (accessed March 21, 2016): http://www.advinus.com/pipeline.asp.
- 279. Business Forum, Advinus Therapeutics, 2015 BIO International Convention, Philadelphia, June 15–18, 2015, (accessed August 31, 2015): http://convention.bio.org/printables/ADVINUS%20THERAPEUTICS%20LTD%20-%20Business%20Forum.html.
- 280. Emerging Significance of Emerging Economies in Creating a New Paradigm for Pharma R&D—Bio-Africa 2014, Pointe aux Piments, Mauritius, R. H. Barbhaiya, April 23–24, 2014, (accessed April 26, 2017): http://www.investmauritius.com/files/bioafrica/session1/1DrRashmiBarbhaiya.pdf.
- 281. Fused Tricyclic Compounds as Adenosine Receptor Antagonists, Barawkar D., Basu S., Ramdas V., Naykodi M., M. Patel, Y. Shejul, S. Thorat, A. Panmand, (Advinus Therapeutics Ltd., Bangalore, India), PCT Pat. Appl. WO2012/038980 A2, March 29, 2012.
- 282. Purine Compounds as Prodrugs of A2B Adenosine Receptor Antagonists, Their Process and Medicinal Applications, Barawkar D., Basu S., Ramdas V., Palle V. P., Waman Y., M. Patel, A. Panmand, (Advinus Therapeutics Private Ltd., Bangalore, India), PCT Pat. Appl. WO2012/035548 A1, March 22, 2012.
- 283. Substituted Fused Tricyclic Compounds, Compositions and Medicinal Applications Thereof, Barawkar D., Bende T., Zahler R., Bandyopadhyay A., Sarangthem R. S., Doshi J., Waman Y., Jadhav R., Singh U. P., (Advinus Therapeutics Ltd., Bangalore, India), PCT Pat. Appl. WO2012/127506 A1, September 27, 2012.
- 284. Substituted Fused Tricyclic Compounds, Compositions and Medicinal Applications Thereof, Barawkar D., Bandyopadhyay A., Zahler R., Sarangthem R., Waman Y., Bonagiri R., Jadhav D., Mukhopadhyay P., (Advinus Therapeutics Ltd.), PCT Pat. Appl. WO2014/045305 A1, March 27, 2014.
- 285. Bicyclic Compounds, Compositions and Medicinal Applications Thereof, Thakkar M., Koul S., Bhuniya D., Singh U., (Advinus Therapeutics Ltd., Bangalore, India), PCT Pat. Appl. WO2013/157021 A1, October 24, 2013.
- 286. Substituted Hetero-Bicyclic Compounds, Compositions and Medicinal Applications Thereof, Thakkar M., Koul S., Bhuniya D., Mookhtiar K., Kurhade S., Munot Y., (Advinus Therapeutics Ltd., Bangalore, India), PCT Pat. Appl. WO2013/157022 A1, October 24, 2013.
- 287. Rashmi Barbhaiya, Cofounder of Advinus Therapeutics Resigns from the Post of Managing Director, V. Dandekar, The Economic Times, May 30, 2016, (accessed December 11, 2016): http://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/rashmi-barbhaiya-co-founder-of-advinus-therapeutics-resigns-from-the-post-of-managing-director/articleshow/52496820.cms?prtpage=1.
- 288. Amidst Crisis, Tata Group's Pharma Unit Advinus Therapeutics Lays Off 50 People, V. Dandekar, The Economic Times, November 15, 2016, (accessed December 11, 2016): http://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/amidst-crisis-tata-groups-pharma-unit-advinus-therapeutics-lays-off-50-people/articleshow/55424515.cms.
- 289. Company Website, Anthem Biosciences, (accessed December 14, 2016): http://www.anthembio.com/.
- 290. India: From Outsourced Work to Outsourced Innovation, September 18, 2013, (accessed December 12, 2016): http://bio2devicegroup.org/content/india-outsourced-work-outsourced-innovation-2013-09-18.
- 291. Histone Deacetylase Inhibitors, Natesan S., Govinda R. G., Annamalai P., Sambasivam G., (Portsmouth Technologies LLC, Upper Saddle River, NJ, USA), PCT Pat. Appl. WO2011/021209 A1, February 24, 2011.
- 292. Hiriyan J., Shivarudraiah P., Gavara G., Annamalai P., Natesan S., Sambasivam G., Sukumaran S. K., Anticancer Res. 2015, 35, 229–237. [PubMed] [Google Scholar]
- 293. Company Presentations, Website, Press Releases and Publications 2003–2016, Aurigene Discovery Technologies, (accessed 2008–2016): http://www.aurigene.com/.
- 294. Dr. Reddy's Laboratories Ltd. 2015 Investor Day, May 18, 2015, (accessed December 7, 2016): http://www.drreddys.com/media/40948/investor-day-2015.pdf.
- 295. Debiopharm and Aurigene Sign Agreement for the Development and Commercialisation of Debio-1142, a Novel Inhibitor of an Undisclosed Oncology Pathway, Debiopharm, April 14, 2011, (accessed December 7, 2016): https://www.debiopharm.com/medias/press-release/item/3113-debiopharm-and-aurigene-sign-agreement-for-the-development-and-commercialisation-of-debio-1142-a-novel-inhibitor-of-an-undisclosed-oncology-pathway.html.
- 296. Immuno-oncology: Licensing Agreement Between Aurigene and Pierre Fabre Pharmaceuticals, February 12, 2014, (accessed December 7, 2016): http://www.pierre-fabre.com/en/news/immuno-oncology-licensing-agreement-between-aurigene-and-pierre-fabre-pharmaceuticals.
- 297. AUNP-12: A Novel Peptide Therapeutic Targeting PD-1 Immune Checkpoint Pathway for Cancer Immunotherapy—Structure Activity Relationships & Peptide/Peptidomimetic Analogues, E. Differding, February 26, 2014, (accessed December 7, 2016): http://www.differding.com/data/AUNP_12_A_novel_peptide_therapeutic_targeting_PD_1_immune_checkpoint_pathway_for_cancer_immunotherapy.pdf.
- 298. Curis and Aurigene Announce Collaboration, License and Option Agreement to Discover, Develop and Commercialize Small-Molecule Antagonists for Immuno-Oncology and Precision Oncology Targets, Aurigene, January 21, 2015, (accessed December 7, 2016): http://aurigene.com/news/curis-and-aurigene-announce-collaboration-license-and-option-agreement-to-discover-develop-and-commercialize-small-molecule-antagonists-for-immuno-oncology-and-precision-oncology-targets/.
- 299. Overview and Path for Growth: Aurigene Strategic Collaboration, Curis Inc, January 21, 2015, (accessed December 7, 2016): http://files.shareholder.com/downloads/ABEA-5QPVEJ/500566877×0x804254/4F1C08B4-25DC-4B17-B512-B006FFA2EB63/Curis%20Aurigene%20Collaboration_21Jan2015_Final.pdf.
- 300. Curis Licenses CA-170, IRAK4 Programs from Aurigene, Genet. Eng. Biotechnol. News, October 19, 2015, (accessed December 7, 2016): http://www.genengnews.com/gen-news-highlights/curis-licenses-ca-170-irak4-programs-from-aurigene/81251868/.
- 301. Aurigene and Orion Corporation Announce Research, Collaboration and Option Agreement for Rights to Aurigene's Epigenetics Program for Pan-BET and Selective BET Bromodomain Inhibitors, June 24, 2014, (accessed December 7, 2016): http://www.aurigene.com/aurigene-and-orion-corporation-announce-research-collaboration-and-option-agreement-for-rights-to-aurigenes-epigenetics-program-for-pan-bet-and-selective-bet-bromodomain-inhibitors/.
- 302. Bicyclic Heterocyclic Derivatives as Bromodomain Inhibitors, Samajdar S., Abbineni C., Sasmal S., Hosahalli S., (Aurigene Discovery Technologies Ltd., Bangalore, India), PCT Pat. Appl. WO2015/104653 A1, July 16, 2015.
- 303. Björkman M., Mattila E., Riikonen R., Abbineni C., Jaleel M., Marappan S., Ikonen T., Nicorici D., Rantala J., Samajdar S., Ramachandra M., Kallio P., Moilanen A., Cancer Res. 2016, 76, 4649. [Google Scholar]
- 304. Chikanna D., Lakshminarasimhan A., Khairnar V., Panigrahi S., Ramanathan A., Rao N., Narayanan K., Gopinath S., Ramachandra R., Chelur S., Pandit C., Ramachandra M., Mol. Cancer Res. 2016, 14, A72. [Google Scholar]
- 305. Satyam L. K., Chikkanna D., Aswani K. G., Khairnar V. V., Reddy S., Durgaprasad V., Radhakrishna K., Panigrahi S. K., Ramanathan A., Mahasweta K., Lakshminarasimhan A., Narasimha R. K., Vinutha R., Gopinath S., Kumar S., Shah M. H., Ramachandra R., Kiran A. B., Pandit C., Ramachandra M., Cancer Res. 2016, 76, 339.26773096 [Google Scholar]
- 306. Poddutoori R., Satyam L. K., Daginakatte G., Mukherjee S., Marappan S., Gopinath S., Ramachandra R., Lakshminarasimhan A., Pothuganti M., Nayak S., Nandish C., Naik C., Ravindra M. V., Dabbeeru M., Antony T., Pandit C., Ramachandra M., Chelur S., Samajdar S., Mol. Cancer Ther. 2016, 14, C190. [Google Scholar]
- 307. Damarla R. K. B., Ujjinamatada R. K., Nellore K., Dodheri S. S., Chelur S., Charamanna K. B., Mukherjee S., Reddy C. S., Balasubramanian W. R., Rathod S. B., Lakshminarasimhan A., Rao K. N., Ramanathan A., Mahalingam N., Girish A. R., Samajdar S., Pandit C., Ramachandra M., J. Immunol. 2016, 196, 139.110. [Google Scholar]
- 308. Takhi M., Sreenivas K., Reddy C. K., Munikumar M., Praveena K., Sudheer P., Rao B. N. V. M., Ramakanth G., Sivaranjani J., Mulik S., Reddy Y. R., Rao K. N., Pallavi R., Lakshminarasimhan A., Panigrahi S. K., Antony T., Abdullah I., Lee Y. K., Ramachandra M., Yusof R., Rahman N. A., Subramanya H., Eur. J. Med. Chem. 2014, 84, 382–394. [DOI] [PubMed] [Google Scholar]
- 309. Company Presentations, Press Releases, Press Articles, Website 2001–2016, GVK Bio, (accessed 2008–2016): http://www.gvkbio.com/.
- 310. Tirunagaru V. G., Nagaswamy K., Mitra S., Maddi S., Adluri R. S., Joshi H., Gupta J. B., Cancer Res. 2016, 76, 2643. [Google Scholar]
- 311. Inhibitors of TrkA Kinase, Nagaswamy K., Tirunagaru V. G., (GVK Biosciences Pvt. Ltd., Hyderabad, India), PCT Pat. Appl. WO2016/116900 A1, July 28, 2016.
- 312. Chowdhury A. R., Athisayamani J. D., Mitra S., Maddi S., Adluri R. S., Bethi S. R., Joshi H., Tirunagaru V. G., Gupta J. B., Cancer Res. 2016, 76, 373. [Google Scholar]
- 313. Kareus Therapeutics Website, (accessed 2012–2016): http://www.kareustherapeutics.com/.
- 314. Compositions for Reducing Aβ42 Production and Their Use in Treating Alzheimer's Disease (AD), Venkateswarlu A., Saxena U., Reddy K. A., (Kareus Therapeutics S.A., La Chaux-de-Fonds, Switzerland), US Pat. Appl. US2012/0322831 A1, December 20, 2012.
- 315. Methods and Compositions for Treatment of Diabetes and Dyslipidemia, Khanna I., Pillarisetti S., (Kareus Therapeutics S.A., La Chaux-de-Fonds, Switzerland), US Pat. US8.623.897 B2, January 7, 2014.
- 316. Connexios Life Sciences Website, (accessed 2010–2016): http://connexios.com/.
- 317. Olefin Substituted Oxindoles Having AMPK Activity, Rao J. M. R., Ranga M. G., Pachiyappan S., (Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany), PCT Pat. Appl. WO2014/202528 A1, December 24, 2014.
- 318. Spiro-substituted Oxindole Derivatives Having AMPK Activity, Rao J. M. R., Ranga M. G., Pachiyappan S., (Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany), PCT Pat. Appl. WO2014/202580 A1, December 24, 2014.
- 319. Agonists of GPR40, Rao J. M. R., Arumugam N., Ansari M. M., Gudla C., Pachiyappan S., Ramalingam M., George J., Arul G. F., Bommegowda Y. K., Angupillai S. K., Kottamalai R., Jidugu P., Rao D. S., (Connexios Life Sciences Pvt. Ltd., Bangalore, India), PCT Pat. Appl. WO2012/011125 A1, January 26, 2012.
- 320. Substituted Bicyclic Heteroaryl Compounds as RXR Agonists, Ranga M. G., Rao J. M. R., Gudla C. S., (Connexios Life Sciences Pvt. Ltd., Bangalore, India), PCT Pat. Appl. WO2015/107549 A1, July 23, 2015.
- 321. Cyclic Amide Derivatives as Inhibitors of 11β-Hydroxysteroid Dehydrogenase and Uses Thereof, Rao J. M. R., Venkatesham U., George J., Fernand G., Doppalapudi S. R., Madhavan G. R., Arumugam N., Ansari M., Murugavel K., Pradeep J., Allavuddeen S., Vijayaramalingam K., Prasad H. S., Raj A. M., Gnanavel S., Kottamalai R., Babu N. M. P. S., Kenchegowda B. Y., (Connexios Life Sciences Pvt. Ltd., Bangalore, India), PCT Pat. Appl. WO2013/128465 A1, September 6, 2013.
- 322. Rhizen Pharmaceuticals Website, (accessed 2010–2016): http://www.rhizen.com/.
- 323. Incozen Therapeutics Website, (accessed 2010–2016): http://www.incozen.com/.
- 324. Novel Kinase Modulators, Muthuppalaniappan M., Viswanadha S., Babu G., Vakkalanka S. K. V. S., (Incozen Therapeutics Pvt. Ltd., Hyderabad, India; Rhizen Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), US Pat. Appl. US2012/0059001 A1, March 8, 2012.
- 325. Burris H. A., Patel M. R., Fenske T. S., O'Connor O. A., Deng C., Brander D. M., Gutierrez M., Jones S. F., Kuhn J. G., Miskin H. P., Sportelli P., Vakkalanka S. V. S., Flinn I., J. Clin. Oncol. 2015, 33, 7069. [Google Scholar]
- 326. Product Pipeline, TG Therapeutics, (accessed December 12, 2016): http://www.tgtherapeutics.com/pipeline/.
- 327. Sneha S., Nivetha R., Viswanadha S., Vakkalanka S., Ganesan T., Cancer Res. 2016, 76, 3331. [Google Scholar]
- 328. Tenalisib, WHO Drug Information, 2015, 29 (4), 569–570, (accessed May 24, 2017): http://www.who.int/medicines/publications/druginformation/issues/PL 114.pdf?ua=1.
- 329. Pipeline, Rhizen Pharmaceuticals S.A., (accessed December 7, 2016): http://www.rhizen.com/research.html.
- 330. Novel 3,5-Disubstituted-3H-imidazo[4,5-b]pyridine and 3,5-Disubstituted-3H-[1,2,3]triazolo[4,5-b]pyridine Compounds as Modulators of c-Met Protein Kinases, Vakkalanka S. K. V. S., Nagarathnam D., Viswanadha S., Muthuppalaniappan M., Babu G., Bhavar P. K., (Rhizen Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland, Incozen Therapeutics Pvt. Ltd., Hyderabad, India), PCT Pat. Appl. WO2013/144737 A2, October 3, 2013.
- 331. Pyrazole Derivatives Modulators of Calcium Release-Activated Calcium Channel and Methods for Treatment of Non-Small Cell Lung Cancer, Muthuppalaniappan M., Viswanadha S., Varanasi K. V., Merikapudi G. S., Vakkalanka S. K. V. S., (Incozen Therapeutics Pvt. Ltd., Hyderabad, India; Rhizen Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), PCT Pat. Appl. WO2011/042798 A1, April 14, 2011.
- 332. Substituted Chromene Derivatives as Selective Dual Inhibitors of PI3δ and γ Protein Kinases, Bhavar P. K., Vakkalanka S. K. V. S., (Rhizen Pharmaceuticals S.A., La Chaux-de-Fonds, Switzerland), PCT Pat. Appl. WO2015/198289 A1, December 30, 2015.
- 333. Sphaera Pharma Website, (accessed 2010–2016): http://www.sphaerapharma.com/.
- 334. Novel Triazine Compounds, Dugar S., Mahajan D., Deokar R. C., Hollinger F. P., Kapoor K. K., (Sphaera Pharma Pvt. Ltd., Manesar, Haryana, India), PCT Pat. Appl. WO2012/101654 A2, August 2, 2012.
- 335. Larsen J. T., Ramakrishnan V., Haug J., Kimlinger T., Sen S., Mahajan D., Dugar S., Rajkumar S. V., Kumar S. K., Cancer Res. 2015, 75, 2653.26071254 [Google Scholar]
- 336. Arora R., Dutta B. K., Goel R., Hollinger F. P., Kulia B., Mahajan D., Mahapatra A. R., Sagar M., Sen S., Sharma A., Dugar S., Cancer Res. 2014, 74, 4515.24848510 [Google Scholar]
- 337. Dugar S., Hollinger F. P., Mahajan D., Sen S., Kuila B., Arora R., Pawar Y., Shinde V., Rahinj M., Kapoor K. K., Bhumkar R., Rai S., Kulkarni R., ACS Med. Chem. Lett. 2015, 6, 1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Sphaera Pharma, THSTI & Wellcome Trust to Jointly Develop Drug for Resistant TB, BioSpectrum India, July 23, 2015, (accessed December 7, 2016): http://www.biospectrumindia.com/biospecindia/news/221936/sphaera-pharma-thsti-wellcome-trust-jointly-develop-drug-resistant-tb.
- 339. SPR-113, (accessed December 12, 2016): http://www.newtbdrugs.org/pipeline/compound/spr-113.
- 340. Novel Compounds as Anti-Tubercular Agents, Dugar S., Mahajan D., Rai S. K., Rao K., Singh V., (Sphaera Pharma Pvt. Ltd., Drug Discovery Research Centre, Gurgaon, Haryana, India), PCT Pat. Appl. WO2015/181837 A2, December 3, 2015.
- 341. Curadev Website, (accessed December 12, 2016): http://curadev.in/.
- 342. Aminonitriles as Kynurenine Pathway Inhibitors, Banerjee M., Middya S., Shrivastava R., Raina S., Surya A., Yadav V. K., Kapoor K. K., (Curadev Pharma Pvt. Ltd., Noida, Uttar Pradesh, India), PCT Pat. Appl. WO2014/141110 A2, September 18, 2014.
- 343. Inhibitors of the Kynurenine Pathway, Banerjee M., Middya S., Shrivastava R., Raina S., Surya A., Yadav D. B., Yadav V. K., Kapoor K. K., Venkatesan A., Smith R. A., Thompson S. K., (Curadev Pharma Pvt. Ltd., Noida, India), PCT Pat. Appl. WO2014/186035 A1, November 20, 2014.
- 344. Curadev Announces Research Collaboration and Licensing Agreement to Develop Cancer Immunotherapeutic, Curadev PR Newswire, April 20, 2015 (accessed December 7, 2016): http://www.prnewswire.com/news-releases/curadev-announces-research-collaboration-and-licensing-agreement-to-develop-cancer-immunotherapeutic-500630711.html.
- 345. Gyulveszi G., Fischer C., Mirolo M., Stern M., Green L., Ceppi M., Wang H., Bürgi B., Staempfli A., Muster W., van Waterschoot R., Gloge A., Sade H., Klaman I., Hoelzlvimmer G., Surya A., Banerjee M., Shrivastava R., Middya S., Yadav D., Basu S., Acuna G., Cancer Res. 2016, 76, LB-085. [Google Scholar]
- 346. Shantani Proteome Analytics Website, (accessed December 12, 2016): http://www.shantani.com/.
- 347. Shantani Company Profile, (accessed December 7, 2016): http://www.shantani.com/docs/1-pager.pdf.
- 348. Shantani Corporate Overview 2014–2015, C. Saxena, 2015.
- 349. A “First-In-Class” Pre-IND Compound for Management of Type-2-Diabetes, C. Saxena, 2015 BIO International Convention, Philadelphia, June 15–18, 2015.
- 350. Novel Indazole Compounds and a Process for the Preparation Thereof, Reddy D. S., Saxena C., Komirishetty K., (Council of Scientific & Industrial Research, New Delhi, India, Shantani Proteome Analytics Pvt. Ltd., Pune, India), PCT Pat. Appl. WO2015/015519 A1, February 5, 2015.
- 351. Vyome Biosciences Website, (accessed December 12, 2016): http://www.vyome.in/.
- 352. Treatments for Resistant Acne, Sengupta S., Chawrai S. R., Ghosh S., Ghosh S., Jain N., Sadhasivam S., Buchta R., Bhattacharyya A., (Vyome Biosciences, New Delhi, India), PCT Pat. Appl. WO2015/114666 A2, August 6, 2015.
- 353. Conjugate-Based Antifungal and Antibacterial Prodrugs, Bapat A. S., Mahesh G., Gokhale R. S., Shah S. S., Sengupta S., Prasad S., Ghosh S., Chawrai S. R., Arora N., Reddy D. S., Mishra M., Bajaj K., (Vyome Biosciences, Delhi, India), PCT Pat. Appl. WO2012/177986 A2, December 27, 2012.
- 354. Vyome Biosciences Administers First In-human Dose of VB 1953 in U.S. Phase 1 Clinical Study in Patients with Facial Acne Vulgaris, BusinessWire, October 31, 2016, (accessed December 12, 2016): http://www.businesswire.com/news/home/20161031005368/en/Vyome-Biosciences-Administers-In-human-Dose-VB-1953.
- 355. Product Pipeline, Vyome Biosciences, (accessed December 12, 2016): http://www.vyome.in/product-pipeline.html.
- 356. Vitas Pharma Website, (accessed December 12, 2016): http://www.vitaspharma.com/.
- 357. Indian Private Investors Extremely Risk Averse, R. Gunashekar, BioSpectrum, May 2014, 12, 5, 64–65.
- 358. Heterocyclic Compounds as Inhibitors of Fatty Acid Biosynthesis for Bacterial Infections, Rangarajan R., Kumar R., Prabhakar B. V., Chandrasekhar P., Mallikarjuna P., Banerjee A., (Vitas Pharma Research Pvt. Ltd., Hyderabad, India), US Pat. Appl. US2014/0249170 A1, September 4, 2014.
- 359. Product Pipeline, Vitas Pharma, (accessed December 7, 2016): http://www.vitaspharma.com/product-pipeline.html.
- 360. Invictus Oncology Website, (accessed December 12, 2016): http://www.invictusoncology.com/.
- 361. Paraskar A. S., Soni S., Chin K. T., Chaudhuri P., Muto K. W., Berkowitz J., Handlogten M. W., Alves N. J., Bilgicer B., Dinulescu D. M., Mashelkar R. A., Sengupta S., Proc. Natl. Acad. Sci. USA 2010, 107, 12435–12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Supramolecular Combinatorial Therapeutics, Sengupta S., Roy M., Hossain S., Sengupta A., Mylavarapu S., (Invictus Oncology Pvt. Ltd., Delhi, India), PCT Pat. Appl. WO2015/153345 A1, October 8, 2015.
- 363. Roy M., Hossain S. S., Sarkar A., Sengupta A., Gupta N., Hussain S., Ansari A., Mylavarapu S., Sengupta S., Cancer Res. 2014, 74, 4483. [Google Scholar]
- 364. Krish Biotech Website, (accessed December 12, 2016): http://www.krishbiotech.com/.
- 365. Discovery of Novel Ursolic Acid Derivatives as ROR-γ Inhibitors, R. Sharma, R. Anupindi, R. Husain, B. Sahu, A. Almeida, N. Chakor, 251st ACS National Meeting, San Diego, CA (USA), March 13–17, 2016.
- 366. KBRPL2001, a Novel GPR120 Agonist, Improves Insulin Sensitivity, Glucose Tolerance and Decreases Hepatic Steatosis in Rodent Models of Type 2 Diabetes, R. Anupindi, R. Sharma, R. Husain, ADA 2016—American Diabetes Association 76th Scientific Sessions, New Orleans, LA (USA), June 10–14, 2016.
- 367. Krish Biotech Research Pipeline, (accessed December 12, 2016): http://www.krishbiotech.com/content.php?id=36&l1id=32.
- 368. WHO Country Profiles, (accessed December 7, 2016): http://www.who.int/countries/en/.
- 369. Eder J., Sedrani R., Wiesmann C., Nat. Rev. Drug Discovery 2014, 13, 577–587. [DOI] [PubMed] [Google Scholar]
- 370. Innovation in the Biopharmaceutical Pipeline: A Multidimensional View G. Long, J. Works, (Analysis Group Inc.) 2013, (accessed December 7, 2016): http://phrma.org/sites/default/files/pdf/2013innovationinthebiopharmaceuticalpipeline-analysisgroupfinal.pdf.
- 371. Filipski K. J., Pfefferkorn J. A., Expert Opin. Ther. Pat. 2014, 24, 875–891. [DOI] [PubMed] [Google Scholar]
- 372. Annual Report 2015, Roche, (accessed December 12, 2016): http://www.roche.com/dam/jcr:9b36e11d-495c-42f5-b757-e80c4e88d793/en/gb15e.pdf.
- 373. Paul S. M., Mytelka D. S., Dunwiddie C. T., Persinger C. C., Munos B. H., Lindborg S. R., Schacht A. L., Nat. Rev. Drug Discovery 2010, 9, 203–214. [DOI] [PubMed] [Google Scholar]
- 374. Cook D., Brown D., Alexander R., March R., Morgan P., Satterthwaite G., Pangalos M. N., Nat. Rev. Drug Discovery 2014, 13, 419–431. [DOI] [PubMed] [Google Scholar]
- 375. Hay M., Thomas D. W., Craighead J. L., Economides C., Rosenthal J., Nat. Biotechnol. 2014, 32, 40–51. [DOI] [PubMed] [Google Scholar]
- 376. World Preview 2016, Outlook to 2022, EvaluatePharma, 2016, (accessed January 17, 2017): http://info.evaluategroup.com/rs/607-YGS-364/images/wp16.pdf.
- 377. Innovation in Life Sciences in India—Current State and Future Imperatives, KPMG, BioAsia 2014, Hyderabad, India, February 17–19, 2014, (accessed December 19, 2016): https://issuu.com/bioasia/docs/innovation_in_ls_11june_bioasiaedit.
- 378. Nature Special: Science in India, Nature May 13, 2015, 521, 125 and 141–155.
- 379. Human Resource and Skill Requirements in the Pharmaceuticals Sector, KPMG, Ministry of Skill Development & Entrepreneurship, Government of India 2013, (accessed January 17, 2017): http://www.nsdcindia.org/sites/default/files/files/Pharmaceuticals.pdf.
- 380. Accelerating Growth: Forging India's Bioeconomy, Burrill Media, 2014, (accessed December 20, 2016): https://www.bio.org/sites/default/files/files/Burrill_AcceleratingGrowth_India-6–9-final.pdf.
- 381. BioAsia 2015 Drug Discovery Conference, February 3, 2015, (accessed December 19, 2016): http://2015.bioasia.in/conference_pages/drug-discovery-conference.html.
- 382. Van Noorden R., Nature 2015, 521, 142–143. [DOI] [PubMed] [Google Scholar]
- 383.Ramanujan Fellowship, Science and Engineering Research Board, Department of Science & Technology, Government of India, (accessed December 12, 2016): http://www.serb.gov.in/rnf.php.
- 384. Ramalingaswami Re-Entry Fellowship, Ministry Of Science & Technology, Department Of Biotechnology, Government of India, (accessed December 12, 2016): http://www.dbtindia.nic.in/wp-content/uploads/Ramalingaswami_fellowship_advt.pdf.
- 385. Wellcome Trust/DBT India Alliance Fellowships, (accessed December 12, 2016): http://wellcomedbt.org/fellowships-at-a-glance.
- 386. Home Calling: India Lures Back 500 NRI Scientists, V. Srivastava Hindustan Times, August 18, 2013, (accessed January 17, 2017): http://www.hindustantimes.com/delhi/home-calling-india-lures-back-500-nri-scientists/story-COscZioDwCc7Jk9jzlsQBL.html.
- 387. Fishburn C. S., Drug Discovery Today 2013, 18, 487–494. [DOI] [PubMed] [Google Scholar]
- 388. Biotechnology Industry Research Assistance Council (BIRAC), (accessed December 7, 2016): http://www.birac.nic.in/.
- 389. IKP Knowledge Park, Hyderabad, (accessed December 7, 2016): http://www.ikpknowledgepark.com/.
- 390. Cover Story: Biotech Parks—Relevance and Untapped Potential, S. Rasoor, Team BioSpectrum, BioSpectrum India 2017, 15, 21–45.
- 391. CEO Conclave at BioAsia 2016, S. Sriram, Biotechin.Asia, 2016, (accessed January 17, 2017): https://biotechin.asia/2016/02/11/ceo-conclave-at-bioasia-2016-make-in-india-and-much-more/.
- 392. Drug Discovery and Development in India, J. Wan BioPharm. Int 2015, 28 (4) (accessed April 25, 2017): http://www.biopharminternational.com/drug-discovery-and-development-india.
- 393. Invest India: Pharmaceuticals, (accessed December 7, 2016): http://www.investindia.gov.in/pharmaceuticals-sector/.
- 394.Federation of Indian Chambers of Commerce and Industry (FICCI) Life Sciences Knowledge Paper (2015)—Indian Life Sciences: Vision 2030, (accessed December 7, 2016): http://www.ficci.com/spdocument/20594/FICCI-Life%20sciences-Knowledge-Paper.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


