Abstract
Aims: Mitochondrial supercomplexes (SCs) are the large supramolecular assembly of individual electron transport chain (ETC) complexes that apparently provide highly efficient ATP synthesis and reduce electron leakage and reactive oxygen species (ROS) production. Oxidative stress during cardiac ischemia–reperfusion (IR) can result in degradation of SCs through oxidation of cardiolipin (CL). Also, IR induces calcium overload and enhances reactive oxygen species (mitROS) in mitochondria that result in the opening of the nonselective permeability transition pores (PTP). The opening of the PTP further compromises cellular energetics and increases mitROS ultimately leading to cell death. Here, we examined the role of PTP-induced mitROS in disintegration of SCs during cardiac IR. The relationship between mitochondrial PTP, ROS, and SCs was investigated using Langendorff-perfused rat hearts subjected to global ischemia (25 min) followed by short-time (5 min) or long-time (60 min) reperfusion in the presence or absence of the PTP inhibitor, sanglifehrin A (SfA), and the mitochondrial targeted ROS and electron scavenger, XJB-5-131. Also, the effects of CL deficiency on SC degradation, PTP, and mitROS were investigated in tafazzin knockdown (TazKD) mice.
Results: Cardiac IR induced PTP opening and mitROS generation, inhibited by SfA. Percent distributions of SCs were significantly affected by IR, and the effects were dependent on the reperfusion time and reversed by SfA and XJB-5-131. TazKD mice demonstrated a 40% lower SC I + III+IV with reduced basal mitochondrial PTP, ROS, and ETC complex activity.
Innovation and Conclusion: Sustained reperfusion after cardiac ischemia induces disintegration of mitochondrial SCs, and PTP-induced ROS presumably play a causal role in SC disassembly. Antioxid. Redox Signal. 27, 57–69.
Keywords: : mitochondria, cardiac ischemia–reperfusion, ETC supercomplexes, permeability transition pore, cardiolipin, cyclophilin D
Introduction
Coronary heart disease is characterized by severe impairment of coronary blood supply, which produces myocardial ischemic injury and is accompanied by a wide range of clinical symptoms. Indeed, timely reperfusion or restoration of coronary perfusion is the only effective therapeutic intervention for protecting the heart against ischemic injury or myocardial infarction (33,44). However, although reperfusion salvages viable myocardium, it can also damage the myocardium up to 50% more than ischemia alone, known as “reperfusion injury” that increases arrhythmia and infarction size, while diminishing myocardial contraction (44,66).
During ischemia, the loss of oxygen compromises mitochondrial ATP production and induces a rise in intracellular [Ca2+]. Reperfusion further increases intracellular [Ca2+], producing a calcium overload in the cytoplasm and mitochondria, and enhancing the generation of reactive oxygen species (ROS) by damaged mitochondrial electron transport chain (ETC) complexes. These factors, along with high [Pi], result in changes in mitochondrial membrane permeability defined as the mitochondrial permeability transition concurrently with opening of the nonselective, high-conductance permeability transition pores (PTP) in the inner mitochondrial membrane (IMM) (8,24). PTP opening further increases mitochondrial Ca2+ and ROS (mitROS) and stimulates protein and lipid oxidation in mitochondria. Oxidation of cardiolipin (CL), a hallmark phospholipid of the IMM, diminishes ETC activity, possibly by degrading ETC supercomplexes (SCs), which ultimately compromises cellular energetics leading to mitochondria-mediated cell death (11,45).
Innovation.
Few studies, if any, have investigated a causal role of permeability transition pores (PTP) in supercomplex (SC) degradation in cardiac ischemia–reperfusion. This study, for the first time, demonstrates that SC disassembly occurs after a long-time (60 min) reperfusion that is prevented by sanglifehrin A (PTP inhibitor) and XJB (a mitochondria-targeted antioxidant), suggesting a causal role of PTP opening in SC degradation. Furthermore, long-time reperfusion induces remarkably higher PTP-dependent mitROS generation compared to short-time (5 min) reperfusion. Notably, PTP opening induced by short-time reperfusion has a greater causal role in mitochondrial swelling than long-time reperfusion. Finally, tafazzin knockdown hearts demonstrate enhanced degradation of the SCs and high basal mitochondrial swelling.
Stress associated with mitochondrial swelling has a strong impact on the proteome biology of cardiac mitochondria (67). Growing evidence indicates that ETC complexes are assembled into large supramolecular complexes, or SCs, under physiological conditions (14). Blue native gel electrophoresis (BNGE) revealed two types of SCs in mammalian mitochondria: I1+III2+IV1 and III2+IV1 (2,56). The main SC, or respirasome I1+III2+IV1, has been found in the mitochondria of rodent (62), dog (54), and bovine (56) hearts. According to the solid-state model, the assembly of SCs can provide high-efficiency electron flux throughout the ETC, increase ATP synthesis, and reduce electron leakage and mitROS production due to short diffusion distances between complexes (2,9,13,35). However, it should be noted that functional and catalytic advantages of SCs are disputed, and it is still not clear whether channeling in SCs is kinetically important to provide highly efficient ATP synthesis (10).
Normal assembly and stability of SCs require CL, a phospholipid that contributes to the function of many proteins and is actively involved in ETC integrity and activity. Electrophoretic (51,68), kinetic (69), and structural (4) studies provide strong evidence that CL is a key to the structural organization, stability, and function of SCs. Indeed, mitochondria lacking CL demonstrate degradation of SCs (51,68). Furthermore, mitROS-induced oxidation and depletion of CL in bovine heart mitochondria were associated with diminished activity of the ETC complexes I, III, and IV (46,47). Studies with artificial membranes indicate that CL peroxidation not only affects the organization and stability of individual ETC complexes but also of entire SCs (21). Likewise, mutation or lack of tafazzin, a mitochondrial transacylase involved in the synthesis of CL, induces inactivation and destabilization of SCs in patients with Barth syndrome (43) and in tafazzin knockdown (TazKD) mice (26).
Few studies, if any, have investigated a link between SC degradation and the PTP in cardiac diseases. Heart failure induced by coronary microembolization decreased the amount of SC I1+III2+IV1 in dogs (54). Ischemic preconditioning had no effect on the level of mitochondrial SCs during global ischemia–reperfusion (IR) in Langendorff-perfused mice hearts (64); however, isoflurane stabilized SCs consisting of complexes III and IV during in vivo cardiac IR (40). Elevated mitROS generation during cardiac IR may significantly destabilize mitochondrial SCs, possibly through CL oxidation, although there are no data confirming this. On the contrary, synthetic oxidized CL (48) and oxidation of endogenous CL (49) sensitized mitochondria to PTP opening only after addition of Ca2+. Although many studies indicate the role of mitochondrial Ca2+ overload and CL oxidation in cardiac IR (37,50), there are no data on the cause-and-effect relationship between CL, SC disintegration, and the PTP.
In this study, we investigated the possible role of PTP opening in the disintegration of SCs, during cardiac IR in rats. We found that SC levels depended on the duration of reperfusion (5 and 60 min) following sustained global ischemia. Degradation of SCs occurs at late reperfusion and can be alleviated by inhibition of the PTP opening and ROS production. In addition, the loss of SC integrity is associated with decreased ETC activity, less ROS production, and mitochondrial swelling in the hearts of TazKD mice.
Results
Postischemic recovery of the heart
The experimental design of the studies is shown in Figure 1. We examined the effects of the PTP inhibitor, sanglifehrin A (SfA), on postischemic recovery of the hearts subjected to IR. SfA improved the recovery of left ventricular developed pressure (LVDP) from 23% in untreated hearts to 42% (p < 0.05, IR60 vs. IR60+SfA) at 60 min of reperfusion (Fig. 2A). In addition, we perfused the hearts with XJB-5-131 (XJB), a recently developed antioxidant (61) that targets mitochondria and provides mitROS and electron scavenging capacity by virtue of its conjugation to a 4-amino-2,2,6,6-tetramethylpiperidinooxy (4-Amino-TEMPO) moiety (20). We have previously shown that XJB protected against global cerebral IR in adult rats (32), and cardiac IR in aged rats (19).
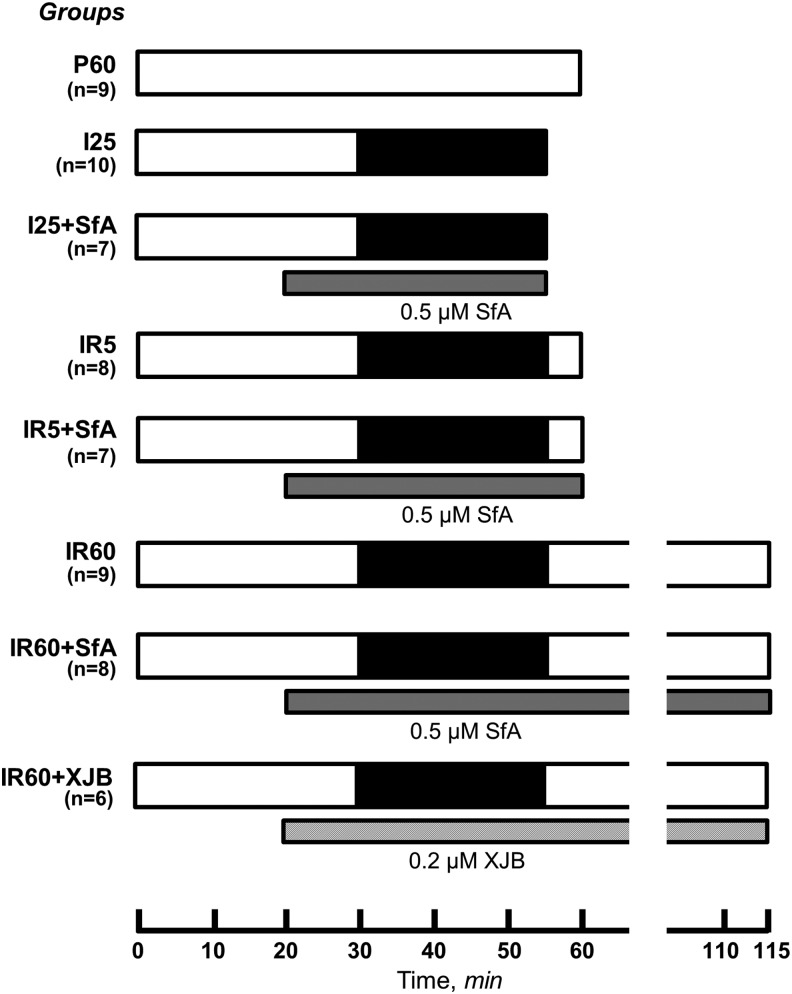
FIG. 1.
Experimental design and groups. Groups: (i) P60, nonischemic hearts perfused for 60 min (n = 9); (ii) I25, hearts subjected to 25-min global ischemia (n = 10); (iii) I25+SfA, hearts subjected to 25-min global ischemia in the presence of 0.5 μM SfA (n = 7); (iv) IR5, hearts subjected to 25-min global ischemia followed by 5-min reperfusion (n = 8); (v) IR5+SfA, hearts subjected to 25-min global ischemia followed by 5-min reperfusion in the presence of 0.5 μM SfA (n = 7); (vi) IR60, hearts subjected to 25-min global ischemia followed by 60-min reperfusion (n = 9); (vii) IR60+SfA, hearts subjected to 25-min global ischemia followed by 60-min reperfusion in the presence of 0.5 μM SfA (n = 8); and (viii) IR60+XJB, hearts subjected to 25-min global ischemia followed by 60-min reperfusion (n = 8) in the presence of 0.2 μM XJB. IR, ischemia–reperfusion; SfA, sanglifehrin A.
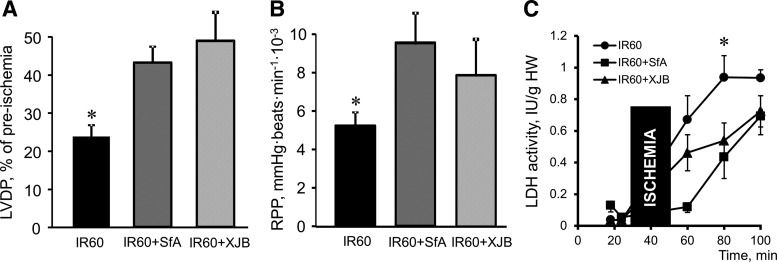
FIG. 2.
Postischemic recovery of Langendorff-perfused hearts at the end of reperfusion (60 min). (A) LVDP. Data are presented as a percentile of preischemic values. (B) RPP. Data are given in mmHg·beats/min. (C) LDH activity in the coronary effluent of the hearts subjected to 25-min ischemia followed by 60-min reperfusion in the presence and absence of SfA or XJB. LDH activity is shown as IU per gram heart. *p < 0.05 IR60 versus IR60+SfA and IR60+XJB. *p < 0.05 versus IR60+SfA and IR60+XJB. LDH, lactate dehydrogenase; LVDP, left ventricular developed pressure; RPP, rate pressure product.
XJB increased the recovery of LVDP from 23% (IR group) to 49% (p < 0.05, IR60 vs. IR60+XJB) at 60 min of reperfusion (Fig. 2A). Likewise, cardiac work expressed as the rate pressure product (RPP) was 79% and 47% (p < 0.05 for both) higher for SfA- and XJB-treated groups, compared to untreated hearts at the end of reperfusion (Fig. 2B). Hearts subjected to IR showed increased lactate dehydrogenase (LDH) activity in the coronary perfusate, indicating increased cell death. Administration of SfA and XJB also decreased the LDH activity at reperfusion (Fig. 2C).
mitROS production and PTP
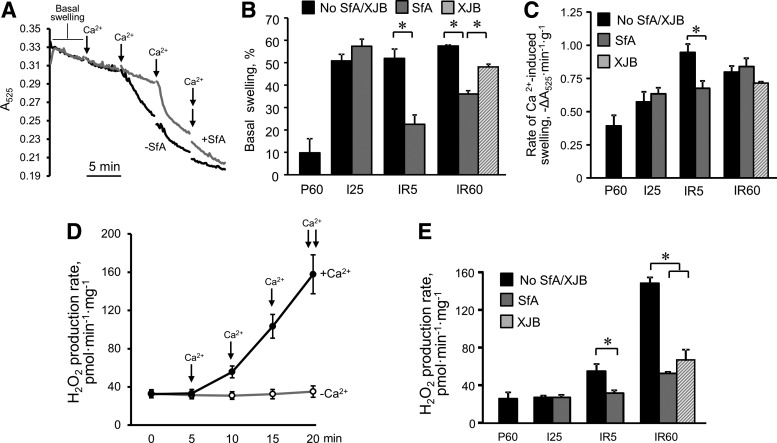
Basal (no Ca2+ added) and Ca2+-induced mitochondrial swelling was determined as a marker of PTP opening (Fig. 3A). Mitochondria isolated from the I25 and IR5 groups showed 29% (p < 0.05) less absorbance, indicating a greater degree of swelling than the control group during ischemia and at the onset (5 min) of reperfusion. The basal swelling was attenuated by 49% (p < 0.05) in the hearts treated with SfA at 5 min, and by 38% (p < 0.05) at 60 min (Fig. 3B). Calcium-induced swelling in the IR5 group was higher than in the IR60 group, and this was relieved by 55% (p < 0.05 IR5 vs. IR5+SfA) in the presence of SfA. Interestingly, SfA failed to prevent Ca2+-induced swelling of mitochondria isolated from heart after 60 min of reperfusion (Fig. 3C). These findings suggest that SfA-sensitive mitochondrial swelling opening occurs at early reperfusion, followed by PTP-independent swelling with progression of reperfusion.
FIG. 3.
PTP opening and ROS production in mitochondria of rat hearts subjected to ischemia followed by 5-min (IR5) or 60-min (IR60) reperfusion in the presence or absence of SfA or XJB. (A) Representative traces of the mitochondrial swelling as the indicator of PTP opening. Swelling of mitochondria in the presence or absence of calcium was determined by monitoring the decrease in light scattering at 525 nm in the presence (gray line) and absence (black line) of 0.5 μM SfA. Each arrow shows the addition of 100 μM CaCl2. (B) Basal (no exogenous Ca2+ added) swelling of mitochondria. (C) Rates of swelling induced by 200 μM CaCl2. (D) Rates of ROS production in the presence (black line, closed circles) and absence (gray line, open circles) of calcium in mitochondria isolated from control (nonischemic) hearts. The values are expressed in pmol of H2O2 produced per minute per mg protein. (E) Rates of ROS production in mitochondria isolated from the hearts in experimental groups. *p < 0.05. PTP, permeability transition pore; ROS, reactive oxygen species.
To determine the role of mitochondrial swelling in mitROS production, we treated isolated mitochondria with gradually increasing concentrations of Ca2+, which increased mitROS generation in a dose-dependent manner (Fig. 3D and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). Analysis of mitROS production in isolated mitochondria revealed remarkable increases of mitROS after both 5 and 60 min of reperfusion that were 2.1 (p < 0.05) and 5.7 times (p < 0.001) higher, respectively, than in control (P60) hearts.
Notably, sustained reperfusion (IR60) dramatically increased mitROS generation compared with short-time reperfusion (IR5) (Fig. 3E). During both periods of reperfusion, mitROS levels were significantly abrogated in the hearts treated with SfA, suggesting that mitROS production was mostly PTP dependent. SfA had no antioxidant effect on isolated mitochondria in vitro in the presence of Ca2+ (Supplementary Fig. S1). Likewise, mitROS production was significantly reduced in the presence of XJB at 60 min of reperfusion (Fig. 3E).
Analysis of the activity of individual ETC complexes revealed no remarkable changes after ischemia and reperfusion (5 and 60 min), except in the activity of complex III that was increased 23% (p < 0.05) at 5 min and decreased 19% (p < 0.05) at 60 min of reperfusion, compared with the control group (Supplementary Fig. S2A–D). Respiration rates (state 3) of mitochondria were lower in all experimental groups than the control group. Ischemia markedly reduced the respiration rate by 63% (p < 0.05) and there were no significant changes at reperfusion in the presence or absence of SfA and XJB (Supplementary Fig. S2E, F). Interestingly, the activity of complex I was not affected in early reperfusion although the respiration rate at complex I was significantly diminished. Our data are consistent with previous studies when the complex I-linked respiration was inhibited after IR with no changes of its enzymatic activity (59).
Degradation of ETC SCs and CL during IR injury
To analyze mitochondrial ETC SCs, isolated mitochondria were treated with the solubilization buffer containing digitonin or dodecyl maltoside (DDM). As reported earlier, only digitonin was able to maintain the structural integrity of SCs in the native electrophoresis condition, whereas DDM strongly induced disintegration of SCs (Fig. 4A). Second-dimension SDS-PAGE with subsequent immunoblotting was used to identify each individual ETC complex as individual bands (Fig. 4B). The results of two-dimensional (2D) BNGE revealed that 58% of complex I was involved in the structure of SCs. Interestingly, the highest molecular weight band, corresponding to the SC I + III+IV, showed a broad range of molecular weight of the SCs (Fig. 4B, C, peak 7).
FIG. 4.
Analysis of ETC SCs in cardiac mitochondria. (A) Representative BN gel image of mitochondria solubilized by DG or DDM demonstrating that DG but not DDM is able to maintain SCs in the native electrophoresis condition. (B) Representative immunoblot of SCs solubilized with DG and separated by BNGE. (C) Densitometry analysis of complex I spots from 2D BNGE-immunoblot. (D) Representative BN gel image with individual bands of SCs identified. S, respiratory SCs containing complexes I, III, and IV; Vm and Vd, monomeric and dimeric complex V; I, Complex I; III, Complex III. (E) Levels of ETC complex I containing SCs (S) normalized by total density (Total) of each lane. *p < 0.05 versus the other groups. (F) The ratio of oxidized CL to intact (nonoxidized) CL in cardiac mitochondria. *p < 0.05 versus I25. BN, blue native; BNGE, blue native gel electrophoresis; DDM, dodecyl maltoside; DG, digitonin; ETC, electron transport chain; SC, supercomplex.
The patterns of the bands were reproducible between different sets of experiments, so we were able to directly identify each SC band on the blue native gel (Fig. 4D). The SC I + III+IV band may contain the individual ETC complexes I, III, and IV in different combinations, including SC I1+III2+IV1. Additional blue native (BN) gel images and 2D Western immunoblots are shown in Supplementary Figures S3 and S4.
Analysis demonstrated that SCs, including complex I (Fig. 4D, marked as “S”), were decreased after 60 min of reperfusion and recovered by SfA or XJB treatment (Fig. 4E). Interestingly, cardiac mitochondria isolated from CyP-D (Ppif) knockout (KO) mice exhibited no changes in SC I + III+IV and ETC individual complex activity between wild-type (WT) and KO mice. Total mitochondrial protein level and citrate synthase activity were 17% and 12% (p < 0.005 for both) less, respectively, in CyP-D KO hearts compared to WT animals (Supplementary Fig. S5).
Analysis of oxidized and intact CL levels in mitochondria demonstrated that ischemia has no effect on CL. However, hearts after reperfusion for 60 min had an apparent trend toward high levels of oxidized CL and low intact CL. The ratio of oxidized CL to intact CL at 60 min of reperfusion (IR60) was 30% (p < 0.11) and 43% (p < 0.05) higher than control (P60) and ischemia (I25), respectively. It should be noted that the reason oxidized CL levels were high in all groups, including control hearts, is to prevent spontaneous lipid oxidation; the antioxidant butylated hydroxytoluene was added to a final pellet of mitochondria at the end of isolation (but not the beginning of isolation). Treatment with SfA or XJB reduced the oxidized CL to intact CL ratio (Fig. 4F). Likewise, mitochondrial protein carbonylation and lipid peroxidation were increased by the end of the 60-min reperfusion (Supplementary Fig. S6).
PTP opening and mitROS generation in cardiac mitochondria of TazKD mice
Mitochondria isolated from TazKD mice demonstrated 27% (p < 0.005) more basal swelling (no Ca2+ added) than mitochondria from WT hearts (Fig. 5A). Furthermore, mitochondria of TazKD hearts responded 49% (p < 0.001) less to Ca2+ compared to WT hearts (Fig. 5B). Notably, SfA significantly reduced mitochondrial swelling in both WT and TazKD hearts, indicating the contribution of PTP opening to the swelling. Genetic ablation of tafazzin may induce basal mitochondrial swelling, independent of CL-related alterations. Therefore, we analyzed the protein level of CyP-D, a key PTP regulator in cardiac mitochondria of TazKD and WT mice.
FIG. 5.
PTP opening and ROS production in cardiac mitochondria isolated from WT and TazKD mice. (A) Basal (no Ca2+ added) swelling of mitochondria measured as the decrease in light scattering at 525 nm. Results are shown as A525. (B) Rates of swelling induced by 200 μM CaCl2. (C) Protein levels of CyP-D. Data were normalized to cytochrome c oxidase IV (COXIV), a mitochondrial housekeeping protein, and expressed as a percent of the WT group. (D) Rates of ROS production in mitochondria in the absence of calcium. (E) Rates of Ca2+-induced ROS production in mitochondria in the presence and absence of SfA. *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001. TazKD, tafazzin knockdown; WT, wild type.
Surprisingly, the CyP-D level in TazKD mice was 13% (p < 0.05) higher than in WT animals (Fig. 5C). Also, although we revealed no differences in basal levels of mitROS between WT and TazKD mice (Fig. 5D), TazKD mice demonstrated 38% (p < 0.05) less Ca2+-induced mitROS generation than WT mice (Fig. 5E). Thus, these findings demonstrate high basal but low Ca2+-induced mitochondrial swelling in TazKD hearts, associated with increased expression of CyP-D.
Tafazzin deficiency results in the disassembly of SCs and reduces activity of individual ETC complexes
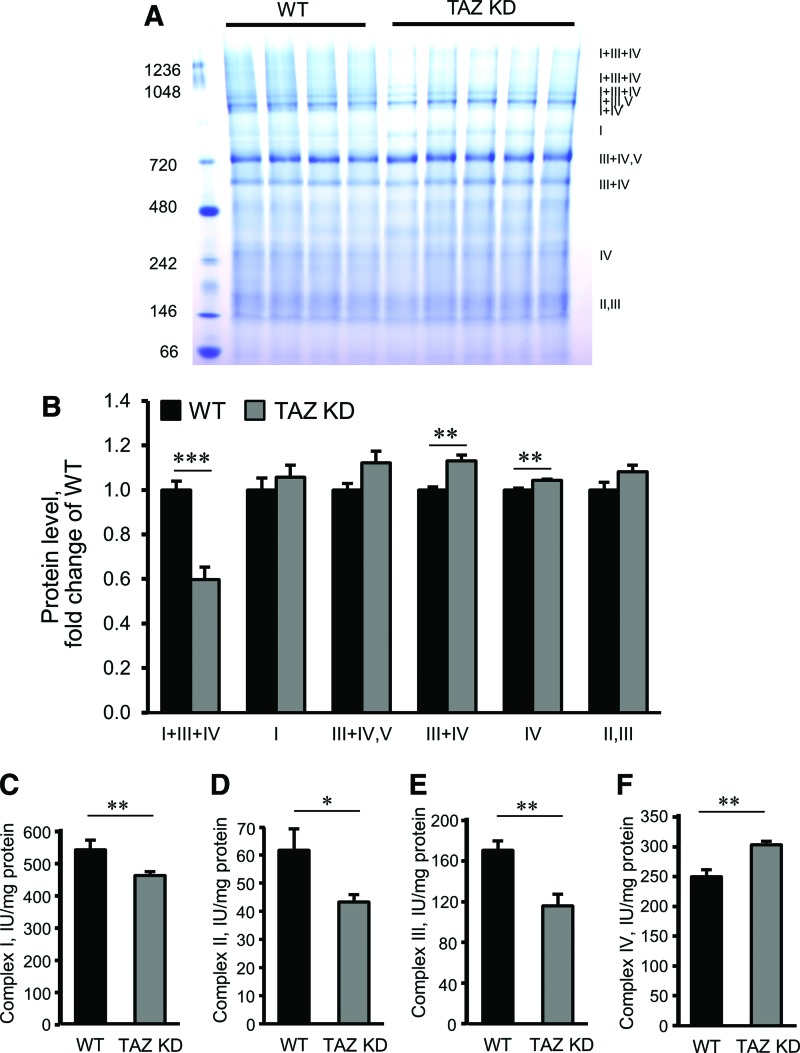
We analyzed mitochondria from WT and TazKD mice to evaluate the effects of CL deficiency on the assembly of ETC SCs. As shown in Figure 6A and B, mitochondria of TazKD mice demonstrated no remarkable changes in complexes I and IV, or SC II+III. However, the levels of SC I + III+IV, also known as the respirasome, in TazKD mitochondria were 40% (p < 0.001) lower than in WT mice. In contrast, the SC III+IV was 13% higher (p < 0.01), compared to mitochondria isolated from WT mice.
FIG. 6.
Analysis of ETC SCs and activity of individual ETC complexes in cardiac mitochondria isolated from WT and TazKD mice. (A) Representative image of Coomassie-stained BNGE of mitochondria. (B) Protein levels of SCs and individual ETC complexes. Data were normalized to a total level of ETC SCs and complexes, and expressed as a fold change compared to the WT group. (C–F) The activity of the ETC complexes I–IV expressed in nmols min−1·mg−1. *p < 0.05, **p < 0.01, ***p < 0.005. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Cardiac mitochondria isolated from TazKD mice showed decreased activities of the individual ETC complexes I, II, and III. Interestingly, the activity of complex IV was higher (22%, p < 0.05) in the hearts of TazKD mice compared to WT animals (Fig. 6C–F).
Discussion
This study demonstrated the following. (i) Both short-time reperfusion and long-time reperfusion (5 and 60 min, respectively) induce the same degree of mitochondrial swelling in the heart. (ii) PTP opening has a greater effect on mitochondrial swelling in short-time reperfusion than in long-time reperfusion, since SfA effectively inhibits the swelling after short-time reperfusion. (iii) Both short-time reperfusion and long-time reperfusion stimulate PTP-dependent mitROS generation; the level of mitROS after long-time reperfusion is significantly higher than that after short-time reperfusion. (iv) Disintegration of SCs occurs only after sustained (60 min) reperfusion, in an SfA-dependent manner. (v) A deficiency of CL synthesis in TazKD mice enhances the degradation of the SC I + III+IV and reduces the activity of complex III. (vi) TazKD mice demonstrate high basal mitochondrial swelling and CyP-D protein expression.
Mitochondrial ETC complexes are believed to dynamically arrange in SCs or respirasomes, although their functional and catalytic advantages remain elusive. Mitochondrial SC association limited production of ROS from complex I (42), and dynamic SC assembly has been shown to determine electron flux from different substrates through the respiratory chain (35) although a robust flux control analysis provided no evidence for substrate channeling between the enzymes in SCs (10).
Although many studies elucidate the structural identity of ETC SCs (34), few, if any, examine the role of SCs in cardiac diseases. Heart failure induced by coronary microembolization for 13 weeks decreased the levels of SC I1+III2+IV1 in canine hearts (54), suggesting that sustained oxidative stress contributes to the disintegration of SCs. In addition, alterations in respiration rates of individual ETC complexes appeared concurrently with SC degradation, indicating that SCs, and not individual ETC complexes, lead to heart failure-induced mitochondrial dysfunction (54). Consistent with these findings, short-term reperfusion had no effect on SC levels; rather, it surprisingly increased the activity of complexes II and IV. Degradation of the SC I + III+IV appeared when the reperfusion period was extended to 60 min, and was associated with reduced activity of complex III.
Degradation of the SC I + III+IV was prevented in hearts treated with SfA, a PTP inhibitor, suggesting the PTP may cause disintegration of SCs. In addition to mitochondrial swelling, PTP-induced ROS presumably contribute to SC disassembly. Previous studies using the [3H]-deoxyglucose method in intact Langendorff-perfused rat hearts demonstrated that the PTP remains closed during cardiac ischemia, but opens upon reperfusion (23,24). However, despite the absence of pore opening, PTP-independent swelling of mitochondria occurs during ischemia (38). A lack of SC degradation at the onset of reperfusion (Fig. 4E) suggests that swelling of the matrix is not sufficient to alter the integrity of SCs. We propose that PTP-induced mitROS play a critical role in the disintegration of SCs via the oxidation of CL. As in previous studies (7), we demonstrated that PTP opening stimulates mitROS production upon reperfusion and is greatly abrogated by SfA treatment.
Notably, despite the same degree of initial pore opening (no exogenous Ca2+) in both periods of reperfusion, long-term reperfusion significantly (2.7-fold) increased mitROS production compared to short-term reperfusion. Indeed, in vitro studies with isolated mitochondria demonstrated that tert-butyl hydroperoxide (t-BuOOH), a lipid-soluble peroxide that promotes lipid peroxidation, induced CL peroxidation in isolated cardiac mitochondria only after the addition of exogenous Ca2+ (49). These data suggest that mitochondrial swelling sensitizes CL to the mitROS attack, leading to degradation of SCs. Simultaneous increases in mitROS and PTP opening appear to be the key players in the promotion of SC disassembly through oxidation of CL.
Cardiac phenotype of TazKD mice mimics biochemical, histological, and clinical findings in humans with Barth syndrome. Tafazzin gene inactivation in mice results in defective CL metabolism associated with a drastic reduction of C18-CL content, and the accumulation of monolysocardiolipins in cardiac mitochondria, increased mitochondrial abnormalities, depressed systolic function, and dilated cardiomyopathy (1,53,57). In addition, fetal cardiomyopathy and embryonic death also have been reported (52).
In our studies, TazKD mice demonstrated a high degree of basal swelling in cardiac mitochondria and were less responsive to exogenous Ca2+ compared to WT mice. Similar to WT hearts, Ca2+-induced mitochondrial swelling was abrogated by SfA in TazKD hearts, further supporting the proposed role of PTP. Nonetheless, TazKD mice have the following limitations for elucidating the cross talk between PTP opening and SC degradation. First, the deficiency in CL remodeling induced by tafazzin knock down is different from CL oxidation induced by oxidative stress. We analyzed mitochondria of TazKD mice to establish if SC disassembling can be detected by BN-PAGE, and whether CL depletion induces any adaptive changes in mitochondria. Second, we have previously shown that the expression of mitochondrial proteins is remarkably affected in TazKD hearts (26), which may compromise interpretation of the results. Notably, the protein level of CyP-D, a main regulator of the PTP, changes in TazKD mice with age; it was 65% less in 3-month-old mice (26) and 13% higher in 8-month-old mice (Fig. 5C). Increased expression of CyP-D in TazKD hearts could explain, at least in part, high basal swelling due to increased PTP opening in these hearts. Hence, PTP-induced mitochondrial swelling, together with deficiency of CL synthesis, could enhance SC disassembly in TazKD mice. Recent studies demonstrated that TazKD does not enhance the vulnerability of the heart to IR injury and has no effect on mitochondrial respiration (58). In contrast to these studies, where TazKD was initiated at an adult age, TazKD in our studies was induced at the early embryo stage (1,53). In addition, no changes in vulnerability can be explained with high basal swelling of mitochondria in TazKD hearts (Fig. 5A) that can protect the mitochondria against IR injury. Interestingly, the extent (40%) of CL reduction found in TazKD hearts (58) was the same for SC degradation found in our studies (Fig. 6B).
The IMM has emerged as an increasingly attractive target for pharmacological and conditional (e.g., ischemic pre/postconditioning) therapies, with the goal of promoting cell survival during cardiac IR [reviewed in Refs. (11,44)]. Although many mitochondria-targeted compounds have been found to limit infarct size in animal models of cardiac IR injury, unfortunately, none has been approved for the treatment of myocardial infarction in humans. Among the many potential mitochondria-targeted therapies, those that inhibit the PTP seem to show the most promise. In particular, the PTP inhibitors, cyclosporine A (CsA) and SfA, have been shown to exert cardioprotective effects against IR in rodents (5,15,39). SfA is a CsA analogue that also potently inhibits the PTP by blocking CyP-D, and, unlike CsA, it does not inhibit calcineurin activity (15).
Intriguingly, CsA and SfA provide protective effects in a narrow concentration range and their effects strongly depend on severity of ischemia and timing of administration of the drugs. The drugs apparently exert protective effects when the heart is subjected to severe IR injury to protect the cells against PTP-induced necrosis. In isolated Langendorff-perfused hearts, severe damage occurs within the first few minutes of reperfusion when LDH release and PTP opening reach their maximum values (23) and this was found to be a critical time window as a cardioprotective strategy through inhibition of the PTP opening. Ca2+-induced mitochondrial swelling was higher at 5 min compared to 60 min of reperfusion, and hence, inhibitory effects of SfA were higher at 5 min of reperfusion (Fig. 3B). Also, we do not exclude the role of PTP-independent swelling of mitochondria at reperfusion since SfA was able to reduce PTP opening up to a certain level for both 5 and 60 min of reperfusion (Fig. 3B).
Dependence of protective effects of CyP-D ablation on duration of ischemia has been shown in previous studies (55). Reperfusion injury at early phase (5 min) caused a moderate accumulation of ROS due to undamaged ETC activity; however, at late phase (60 min), it significantly increased ROS production presumably due to diminished scavenging capacity of mitochondria and dysfunction of ETC complexes, particularly complex III (Supplementary Fig. S2C and Fig. 3E). Accordingly, treatment with SfA or XJB reduced ROS production and preserved the complex III activity at 60 min of reperfusion. These data are in line with a recent study when targeting CyP-D decreased mitochondrial superoxide generation, improved vascular relaxation, and reduced hypertension in CyP-D KO mice (28).
Recent Cyclosporine and Prognosis in Acute Myocardial Infarction Patients (CIRCUS) clinical trial reported that CsA failed to protect reperfusion injury-related outcomes in patients receiving percutaneous coronary intervention for acute anterior ST-segment elevation myocardial infarction (16). Among many possible explanations for the lack of beneficial effects of CsA, the inappropriate time of administration of the drug was the most important, practical issue. CsA and SfA exert cardioprotective effects within a narrow concentration range, early in reperfusion (24,27). In our studies, SfA was present before ischemia and throughout reperfusion, during which it improved heart function and prevented mitROS generation. Although SfA inhibited basal swelling of mitochondria at both early (5 min) and late (60 min) reperfusion, it abrogated Ca2+-induced swelling at only early reperfusion with no effect at late reperfusion (Fig. 3B, C). Likewise, protective effects of CsA against brain ischemia were only observed when the drug was infused immediately upon reperfusion (18).
These data suggest that the PTP contributes less to swelling during late reperfusion, when other PTP-independent mechanisms can enhance the matrix swelling (36). We are tempted to speculate that (i) Ca2+ overload induced by sustained reperfusion exceeds the ability of SfA to inhibit CyP-D, but prevents mitROS production, and (ii) PTP-independent swelling at late reperfusion is possibly related to the structural destabilization of the respiratory SC I + III+IV. The latter is supported by the fact that the mitochondria isolated from TazKD mice exhibited more basal swelling, with no changes in mitROS generation (Fig. 5A, D). In addition, transient inhibition of complex I at the onset of reperfusion showed reduced cardiac injury (12,65). Currently, the lack of understanding of the mechanisms underlying the cross talk between PTP, CL oxidation, and SC degradation limits precise therapeutic targeting of mitochondria.
PTP opening stimulates mitROS generation and leads to dissociation of ETC SCs during sustained reperfusion followed by global ischemia. Inhibition of the PTP by SfA improves postischemic recovery of the heart, and prevents PTP-induced mitROS generation and SC degradation. Cardiac mitochondria of TazKD mice have high basal swelling, associated with increased expression of CyP-D. Deficiency in CL synthesis in TazKD mice leads to SC disintegration and modulates the enzymatic activity of individual ETC complexes.
Materials and Methods
Animals
Male Sprague-Dawley rats weighing 225–275 g were purchased from Charles River (Wilmington, MA). Eight-month-old WT and TazKD mice, mixed males and females, were used. Doxycycline-inducible shRNA-mediated TazKD mice have been described previously (1,53,57). All mice were in C57BL/6J background. TazKD was induced before conception by feeding females doxycycline-containing rodent chow (625 mg/kg) 3 days before mating. During the mating period, doxycycline was temporarily discontinued to avoid reported potential problems with male fertility. After detecting copulatory plugs, females were separated from males and placed back on the doxycycline-containing diet. This approach allows 85%–95% silencing of the tafazzin gene in heart and skeletal muscle (1). One- to 3-month-old WT and CyP-D KO mice, mixed males and females, were also used. All experiments were performed according to protocols approved by the University Animal Care and Use Committee and conformed to the National Research Council Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (2011, Eighth Edition).
Langendorff heart perfusion and animal groups
Rats were deeply anesthetized with anesthetic cocktail (179.2 mg/kg body weight, IP) containing 4.2 mg/kg xylazine, 87.5 mg/kg ketamine, and 87.5 mg/kg acepromazine to avoid any discomfort, distress, and pain in accordance to the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Depth of anesthesia was assessed by lack of response to the withdrawal reflex from a toe pinch, 15 min after the administration of anesthesia.
The hearts were rapidly removed and retrogradely perfused via a Langendorff perfusion system at constant flow as previously described (30). A water-filled latex balloon was inserted into the left ventricle for continuous monitoring of HR and left ventricular systolic (LVSP) and end diastolic (LVEDP) pressures. Measurements were recorded using Labscribe2 (iWorx 308T, Dover, NH). LVDP was calculated as the difference between LVSP and LVEDP (LVDP = LVSP − LVEDP). The RPP, an important marker of cardiac work, was calculated as the product of LVDP and HR (RPP = LVDP × HR) and given in mmHg·beats/min.
Protocols of perfusion are illustrated schematically in Figure 1. Animals were randomly assigned to the following treatment groups: (i) P60, nonischemic hearts perfused for 60 min (n = 9); (ii) I25, hearts subjected to 25-min global ischemia (n = 10); (iii) I25+SfA, hearts subjected to 25-min global ischemia in the presence of 0.5 μM SfA (n = 7); (iv) IR5, hearts subjected to 25-min global ischemia followed by 5-min reperfusion (n = 8); (v) IR5+SfA, hearts subjected to 25-min global ischemia followed by 5-min reperfusion in the presence of 0.5 μM SfA (n = 7); (vi) IR60, hearts subjected to 25-min global ischemia followed by 60-min reperfusion (n = 9); (vii) IR60+SfA, hearts subjected to 25-min global ischemia followed by 60-min reperfusion (n = 8) in the presence of 0.5 μM SfA; (viii) IR60+XJB, hearts subjected to 25-min global ischemia followed by 60-min reperfusion (n = 6) in the presence of 0.2 μM XJB.
Global normothermic ischemia was induced by switching off the pump after a total preischemic period of 25 min, with the heart immersed in buffer maintained at 37°C. In all experiments, an ischemic period of 25 min was followed by 5 or 60 min of reperfusion with flow at preischemic levels. Samples of the coronary effluent were collected before ischemia and during reperfusion at indicated time points to measure the LDH activity. The activity of LDH was assessed by an enzymatic method as previously described (30) with minor modifications. After the corresponding protocols, the hearts were used to isolate mitochondria.
Isolation of mitochondria
To isolate mitochondria, the ventricles were cut, weighed, and homogenized with a Polytron homogenizer at 1500 rpm for 5 s in ice-cold sucrose buffer containing 300 mM sucrose, 10 mM Tris–HCl, and 2 mM EGTA, at pH 7.4 (29). Mitochondria were isolated from the homogenate by centrifugation at 2000 g for 2 min in the sucrose buffer containing 0.5% BSA in a benchtop centrifuge to remove cell debris, followed by centrifugation of the supernatant at 10,000g for 5 min to sediment the mitochondrial suspension. The pellet was then washed two times at 10,000 g for 5 min in 40 ml of sucrose buffer (BSA free). The final pellet was resuspended in 300 μl of the sucrose buffer.
The yield of mitochondria was 12.1 ± 0.3 mg/ml. A 100 μl sample of the suspension was then used for measurement of mitochondrial PTP opening, ROS production, and respiration rate. A 200 μl sample was mixed with protease and phosphatase inhibitor cocktails (Sigma-Aldrich), then immediately frozen in liquid nitrogen, and stored at −80°C for gel analysis.
Measurement of PTP opening
Swelling of mitochondria as an indicator of PTP opening in the presence or absence of Ca2+ was determined by monitoring the decrease in light scattering at 525 nm as described previously (29). Freshly isolated mitochondria (0.4 mg/ml) were incubated at 37°C in 0.1 ml of incubation buffer containing 200 mM sucrose, 10 mM Tris–MOPS, 5 mM α-ketoglutarate, 2 mM malate, 1 mM Pi, 10 μM EGTA–Tris, pH 7.4. The absorbance was monitored for 5 min without Ca2+ (basal swelling) and then with Ca2+ (Ca2+-induced swelling). The rates of swelling were calculated as the change of absorbance induced by Ca2+.
Measurement of mitROS production
Freshly isolated mitochondria were incubated at 37°C in 0.1 ml of the incubation buffer containing 200 mM sucrose, 10 mM Tris–MOPS, 5 mM α-ketoglutarate, 2 mM malate, 1 mM Pi, 10 μM EGTA–Tris, pH 7.4. Production of H2O2 as an indicator of ROS was measured in isolated mitochondria with Amplex Red in the medium containing 0.1 mM Amplex Red (Invitrogen), 50 mM sodium phosphate, pH 7.4, and 0.2 U/ml HRP. ROS production was monitored in the presence or absence of Ca2+ at excitation of 560 nm and emission at 590 nm.
Measurement of mitochondrial respiration rates
Rates of mitochondrial respiration were measured at 37°C using a YSI Oxygraph (Yellow Springs, OH) model 5300 equipped with a Clark-type oxygen electrode (29). Mitochondria were incubated in a buffer containing (in mM) 125 KCl, 20 MOPS, 10 Tris, 0.5 EGTA, and 2 KH2PO4, at pH 7.2, supplemented with 2.5 mM 2-oxoglutarate and 1 mM l-malate to measure the rate of oxygen consumption at complex I. Respiration rates were measured in the absence (state 2) and presence (state 3) of 1 mM ADP. At the end of each run, 0.5 μM antimycin A, followed by 10 mM ascorbate and 0.3 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), was added, and the new rate of respiration was recorded (Supplementary Fig. S2E). Respiration charts were analyzed using Chart5 (PowerLab) and expressed in nmols O2 per min to mg of protein.
Analysis of mitochondrial ETC SCs
Mitochondrial ETC SCs were analyzed by BNGE as previously described with modifications (22,62,63). Briefly, 120 μg of mitochondrial proteins was dissolved with 100 μl of solubilization buffer (50 mM NaCl, 50 mM imidazole-HCl, 2 mM 6-aminohexanoic acid, 1 mM EDTA) supplemented with 4 μl of 20% digitonin (or 1.7 μl of 20% DDM), 1 μl protease and phosphatase inhibitor cocktails (Sigma-Aldrich), and 25 U of Benzonase. Samples were incubated on ice for 20 min and then centrifuged for 20 min at 20,000 g. Supernatants were collected and mixed with 30 μl of sample buffer (50 mM NaCl, 10% glycerol, 0.001% ponceau S, 50 mM Tris–HCl, pH 7.2). BNGE was conducted as per the manufacturer's recommendations (Invitrogen). After electrophoresis, gels were stained by Coomassie brilliant blue G250 and then scanned with the Odyssey CLx Infrared Imaging System (LI-COR Biosciences). The resulting images were analyzed with ImageJ (NIH).
For second-dimension SDS-PAGE, the lanes were cut from native gels after running and incubated with loading buffer (50 mM Tris, pH 8.8, 50 mM DTT, 2% SDS, and 0.01% bromophenol blue) for 5 min. The lanes were loaded into 15% SDS-PAGE gel and sealed with 0.5% agarose. Second-dimension run and subsequent immunoblotting were conducted as per the manufacturer's recommendations (Bio-Rad). The antibody used for the analysis was the Total OXPHOS Rodent WB Antibody Cocktail (Abcam). They were used as per the manufacturer's recommendations. The signals were visualized by VersaDoc 4000 Gel Imaging System (Bio-Rad) and analyzed by ImageJ (NIH).
Analysis of the activity of ETC complexes
Isolated mitochondria were dissolved 0.1 μg/μl in the buffer containing 2 mM EDTA and 0.01% Triton X-100 and then freeze–thawed two times to give the substrate access to the IMM. All assays were performed using SpectraMax M3 at 37°C as previously described (25,31).
Complex I activity was determined by measuring the rotenone-sensitive decrease of the absorbance of NADH at 340 nm. The assay was performed in phosphate buffer containing 25 mM KH2PO4, pH 7.4, 0.5 mM KCN, 5 mM MgCl2, 2 mg/ml BSA, 2.4 μg/mL antimycin, 1 mM NaN3, and 0.24 mM CoQ1. After stabilization, the reaction was started by adding 0.15 mM NADH, and then, rotenone was added to calculate the rotenone-sensitive (complex I) activity. Molar extinction coefficient of NADH (6.22 mM−1 cm−1) was used for calculation.
Complex II activity was measured as the velocity of 2,6-dichlorophenolindophenol (DCPIP) reduction, which corresponded to a decrement of absorbance at 600 nm. The assays were performed in the buffer containing 50 mM KH2PO4, 2 mg/ml BSA, 5 mM KCN, 20 mM succinate, and 4 mM antimycin A. After preincubation with the reaction mixture (17), 50 μM DCPIP was added and the absorbance was measured at 600 nm. Molar extinction coefficient of DCPIP (36 mM−1 cm−1) was used for calculation.
Complex III activity was measured as rotenone-sensitive increment of absorbance at 550 nm (Complex I + III). The assays were performed in the buffer containing 50 mM KH2PO4, 2 mg/ml BSA, 0.5 mM KCN, 1 mM NaN3, and 0.12 mM cytochrome c. After stabilization, the reaction was started by adding 5 mM NADH, and then, rotenone was added to calculate the rotenone-sensitive activity. The molar extinction coefficient of cytochrome c (28 mM−1 cm−1) was used for calculation.
Complex IV activity was measured as oxidation of cytochrome c as the decrease of absorbance at 550 nm. The assays were performed in the buffer containing 10 mM Tris–HCl, pH 7.4, 12 mM KCl. After stabilization, the reaction was started by adding reduced cytochrome c to 0.3 mg/ml. The molar extinction coefficient of cytochrome c (28 mM−1 cm−1) was used for calculation.
Citrate synthase activity was assayed spectrophotometrically by measuring coenzyme A formation at 412 nm as described previously (6). The molar extinction coefficient of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) (14.15 mM−1 cm−1) was used for calculation.
Analysis of protein carbonylation and 4-HNE adducts
Analysis of carbonlyated proteins was conducted as described previously with modifications (41,60). Samples treated with 0.25 U/μl of Benzonase® underwent derivatization using freshly prepared 1 mM 2,4-dinitrophenylhydrazine (DNPH) in 10% trifluoroacetic acid. Resulting mixtures were loaded onto 10% SDS-PAGE gels and underwent standard immunoblot procedure. Anti-DNP antibody (Sigma-Aldrich) was used as per the manufacturer's recommendations. For 4-HNE adduct, Anti-HNE-Michael Adducts antibody (Millipore) was used as per the manufacturer's recommendations.
Analysis of CL content by high-resolution mass spectrometry
The CL content was analyzed (in duplicate) in mitochondria isolated from hearts in five groups (P60, I25, IR60, IR60+SfA, and IR60+XJB; n = 3 per group). After isolation, the mitochondria were kept at −80°C in sucrose buffer containing 300 mM sucrose, 10 mM Tris–HCl, and 2 mM EGTA, pH 7.4. Butylated hydroxytoluene (50 μM) was added to the samples to prevent spontaneous lipid peroxidation. Mitochondrial fraction (7.5 μl), containing 10–20 μg protein/μl, was added to ACN:water (3:1, 475 μl) to give a total volume of 482.5 μl, which was vortexed for 5 min and then centrifuged in an Eppendorf centrifuge 5702 at 4400 rpm and 20°C for 30 min. The supernatant (400 μl) was separated from the protein pellet for further analysis.
Cardiolipin content was quantified on a Thermo Scientific Exactive mass spectrometer using an electrospray ionization source. Samples of the supernatant solution (5 μl injections, five injections per sample) were directly injected into the probe head (−60 V, 350°C). Relative abundances of combined nonoxidized (intact) CL (m/z 723.5 and 1447.9) and combined oxidized CL (m/z 747.5, 760.5, and 790.5) species were determined with Thermo Xcalibur software. Results were averaged across the five injections per each sample and the ratio of oxidized CL to nonoxidized CL was calculated.
Statistical analysis
Values are presented as mean ± SE. Data were analyzed using ANOVA with normality test (Shapiro–Wilk) and pairwise multiple comparison procedures (Holm–Sidak method) in addition to Student's t-test. p < 0.05 was considered statistically significant.
Supplementary Material
Abbreviations Used
- 2D
two dimensional
- BNGE
blue native gel electrophoresis
- CL
cardiolipin
- CsA
cyclosporine A
- CyP-D
cyclophilin D
- DDM
dodecyl maltoside
- ETC
electron transport chain
- IMM
inner mitochondrial membrane
- IR
ischemia–reperfusion
- KO
knockout
- LDH
lactate dehydrogenase
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end diastolic pressure
- LVSP
left ventricular systolic pressure
- mitROS
mitochondrial ROS
- PTP
permeability transition pore
- ROS
reactive oxygen species
- RPP
rate pressure product
- SC
supercomplex
- SfA
sanglifehrin A
- TazKD
tafazzin knockdown
- WT
wild type
- XJB
XJB-5-131
Acknowledgments
The authors thank Desirae L. Crocker for her assistance with lipid extractions. They thank Bryan Agostini for his critical reading of the article. This study was supported by the NHLBI NIH Grants SC1HL118669 (to S.J.) and 1R01HL108867 (Z.K.) and, in part, by the National Center for Research Resources NIH Grants G12RR-003051 and G12MD007600. Publication Charges for the article were covered by the Deanship of the University of Puerto Rico School of Medicine.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, and Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286: 899–908, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, and Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell 32: 529–539, 2008 [DOI] [PubMed] [Google Scholar]
- 3. This reference has been deleted.
- 4.Althoff T, Mills DJ, Popot JL, and Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J 30: 4652–4664, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, and Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 38: 367–374, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Barreto-Torres G, Parodi-Rullan R, and Javadov S. The role of PPARalpha in metformin-induced attenuation of mitochondrial dysfunction in acute cardiac ischemia/reperfusion in rats. Int J Mol Sci 13: 7694–7709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batandier C, Leverve X, and Fontaine E. Opening of the mitochondrial permeability transition pore induces reactive oxygen species production at the level of the respiratory chain complex I. J Biol Chem 279: 17197–17204, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bernardi P. and Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol 78: 100–106, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bianchi C, Genova ML, Parenti Castelli G, and Lenaz G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279: 36562–36569, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Blaza JN, Serreli R, Jones AJ, Mohammed K, and Hirst J. Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc Natl Acad Sci U S A 111: 15735–15740, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown DA, Sabbah HN, and Shaikh SR. Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol Ther 140: 258–266, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Burwell LS, Nadtochiy SM, and Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol 46: 804–810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaban Y, Boekema EJ, and Dudkina NV. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim Biophys Acta 1837: 418–426, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Chance B. and Williams GR. A method for the localization of sites for oxidative phosphorylation. Nature 176: 250–254, 1955 [DOI] [PubMed] [Google Scholar]
- 15.Clarke SJ, McStay GP, and Halestrap AP. Sanglifehrin A acts as a potent inhibitor of the mitochondrial permeability transition and reperfusion injury of the heart by binding to cyclophilin-D at a different site from cyclosporin A. J Biol Chem 277: 34793–34799, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guerin P, Elbaz M, Delarche N, Coste P, Vanzetto G, Metge M, Aupetit JF, Jouve B, Motreff P, Tron C, Labeque JN, Steg PG, Cottin Y, Range G, Clerc J, Claeys MJ, Coussement P, Prunier F, Moulin F, Roth O, Belle L, Dubois P, Barragan P, Gilard M, Piot C, Colin P, De Poli F, Morice MC, Ider O, Dubois-Rande JL, Unterseeh T, Le Breton H, Beard T, Blanchard D, Grollier G, Malquarti V, Staat P, Sudre A, Elmer E, Hansson MJ, Bergerot C, Boussaha I, Jossan C, Derumeaux G, Mewton N, and Ovize M. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 373: 1021–1031, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Degli Esposti M. Assessing functional integrity of mitochondria in vitro and in vivo. Methods Cell Biol 65: 75–96, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Domanska-Janik K, Buzanska L, Dluzniewska J, Kozlowska H, Sarnowska A, and Zablocka B. Neuroprotection by cyclosporin A following transient brain ischemia correlates with the inhibition of the early efflux of cytochrome C to cytoplasm. Brain Res Mol Brain Res 121: 50–59, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Escobales N, Nunez RE, Jang S, Parodi-Rullan R, Ayala-Pena S, Sacher JR, Skoda EM, Wipf P, Frontera W, and Javadov S. Mitochondria-targeted ROS scavenger improves post-ischemic recovery of cardiac function and attenuates mitochondrial abnormalities in aged rats. J Mol Cell Cardiol 77: 136–146, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink MP, Macias CA, Xiao J, Tyurina YY, Jiang J, Belikova N, Delude RL, Greenberger JS, Kagan VE, and Wipf P. Hemigramicidin-TEMPO conjugates: novel mitochondria-targeted anti-oxidants. Biochem Pharmacol 74: 801–809, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Genova ML, Baracca A, Biondi A, Casalena G, Faccioli M, Falasca AI, Formiggini G, Sgarbi G, Solaini G, and Lenaz G. Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Biochim Biophys Acta 1777: 740–746, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gomez LA, Monette JS, Chavez JD, Maier CS, and Hagen TM. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys 490: 30–35, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths EJ. and Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 307 (Pt 1): 93–98, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halestrap AP, Clarke SJ, and Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res 61: 372–385, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hernandez JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, and Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med 18: 709–720, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y, Powers C, Madala SK, Greis KD, Haffey WD, Towbin JA, Purevjav E, Javadov S, Strauss AW, and Khuchua Z. Cardiac metabolic pathways affected in the mouse model of barth syndrome. PLoS One 10: e0128561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husainy MA, Dickenson JM, and Galinanes M. The MPTP status during early reoxygenation is critical for cardioprotection. J Surg Res 174: 62–72, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, and Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension 67: 1218–1227, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang S. and Javadov S. Inhibition of JNK aggravates the recovery of rat hearts after global ischemia: the role of mitochondrial JNK. PLoS One 9: e113526, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javadov S, Choi A, Rajapurohitam V, Zeidan A, Basnakian AG, and Karmazyn M. NHE-1 inhibition-induced cardioprotection against ischaemia/reperfusion is associated with attenuation of the mitochondrial permeability transition. Cardiovasc Res 77: 416–424, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Javadov S, Jang S, Rodriguez-Reyes N, Rodriguez-Zayas AE, Soto Hernandez J, Krainz T, Wipf P, and Frontera W. Mitochondria-targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. Oncotarget 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji J, Baart S, Vikulina AS, Clark RS, Anthonymuthu TS, Tyurin VA, Du L, St Croix CM, Tyurina YY, Lewis J, Skoda EM, Kline AE, Kochanek PM, Wipf P, Kagan VE, and Bayir H. Deciphering of mitochondrial cardiolipin oxidative signaling in cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 35: 319–328, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kloner RA. and Schwartz Longacre L. State of the science of cardioprotection: challenges and opportunities—proceedings of the 2010 NHLBI Workshop on Cardioprotection. J Cardiovasc Pharmacol Ther 16: 223–232, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Kuhlbrandt W. Structure and function of mitochondrial membrane protein complexes. BMC Biol 13: 89, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, Navas P, Cruz R, Carracedo A, Lopez-Otin C, Perez-Martos A, Fernandez-Silva P, Fernandez-Vizarra E, and Enriquez JA. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340: 1567–1570, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Lehninger AL. Reversal of various types of mitochondrial swelling by adenosine triphosphate. J Biol Chem 234: 2465–2471, 1959 [PubMed] [Google Scholar]
- 37.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, and Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol Heart Circ Physiol 280: H2770–H2778, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Lim KH, Javadov SA, Das M, Clarke SJ, Suleiman MS, and Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol 545: 961–974, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim SY, Davidson SM, Hausenloy DJ, and Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res 75: 530–535, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lotz C, Zhang J, Fang C, Liem D, and Ping P. Isoflurane protects the myocardium against ischemic injury via the preservation of mitochondrial respiration and its supramolecular organization. Anesth Analg 120: 265–274, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Luo S. and Wehr NB. Protein carbonylation: avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep 14: 159–166, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Maranzana E, Barbero G, Falasca AI, Lenaz G, and Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal 19: 1469–1480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKenzie M, Lazarou M, Thorburn DR, and Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol 361: 462–469, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Murphy E. and Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paradies G, Paradies V, De Benedictis V, Ruggiero FM, and Petrosillo G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim Biophys Acta 1837: 408–417, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Paradies G, Petrosillo G, Pistolese M, and Ruggiero FM. Reactive oxygen species generated by the mitochondrial respiratory chain affect the complex III activity via cardiolipin peroxidation in beef-heart submitochondrial particles. Mitochondrion 1: 151–159, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Paradies G, Petrosillo G, Pistolese M, and Ruggiero FM. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene 286: 135–141, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Petrosillo G, Casanova G, Matera M, Ruggiero FM, and Paradies G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett 580: 6311–6316, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Petrosillo G, Moro N, Ruggiero FM, and Paradies G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic Biol Med 47: 969–974, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Petrosillo G, Ruggiero FM, Di Venosa N, and Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J 17: 714–716, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, and Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem 278: 52873–52880, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Phoon CK, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, Yu D, Xu Y, Viswanathan N, and Ren M. Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. J Am Heart Assoc 1: e000455, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powers C, Huang Y, Strauss A, and Khuchua Z. Diminished exercise capacity and mitochondrial bc1 complex deficiency in Tafazzin-knockdown mice. Front Physiol 4: 74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, and Hoppel CL. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 80: 30–39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Meana M, Inserte J, Fernandez-Sanz C, Hernando V, Miro-Casas E, Barba I, and Garcia-Dorado D. The role of mitochondrial permeability transition in reperfusion-induced cardiomyocyte death depends on the duration of ischemia. Basic Res Cardiol 106: 1259–1268, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Schagger H. and Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19: 1777–1783, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soustek MS, Falk DJ, Mah CS, Toth MJ, Schlame M, Lewin AS, and Byrne BJ. Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum Gene Ther 22: 865–871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szczepanek K, Allegood J, Aluri H, Hu Y, Chen Q, and Lesnefsky EJ. Acquired deficiency of tafazzin in the adult heart: impact on mitochondrial function and response to cardiac injury. Biochim Biophys Acta 1861: 294–300, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Tompkins AJ, Burwell LS, Digerness SB, Zaragoza C, Holman WL, and Brookes PS. Mitochondrial dysfunction in cardiac ischemia-reperfusion injury: ROS from complex I, without inhibition. Biochim Biophys Acta 1762: 223–231, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Wang P. and Powell SR. Decreased sensitivity associated with an altered formulation of a commercially available kit for detection of protein carbonyls. Free Radic Biol Med 49: 119–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wipf P, Xiao J, Jiang J, Belikova NA, Tyurin VA, Fink MP, and Kagan VE. Mitochondrial targeting of selective electron scavengers: synthesis and biological analysis of hemigramicidin-TEMPO conjugates. J Am Chem Soc 127: 12460–12461, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Wittig I, Carrozzo R, Santorelli FM, and Schagger H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim Biophys Acta 1757: 1066–1072, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Wittig I. and Schagger H. Features and applications of blue-native and clear-native electrophoresis. Proteomics 8: 3974–3990, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Wong R, Aponte AM, Steenbergen C, and Murphy E. Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol 298: H75–H91, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu A, Szczepanek K, Maceyka MW, Ross T, Bowler E, Hu Y, Kenny B, Mehfoud C, Desai PN, Baumgarten CM, Chen Q, and Lesnefsky EJ. Transient complex I inhibition at the onset of reperfusion by extracellular acidification decreases cardiac injury. Am J Physiol Cell Physiol 306: C1142–H1153, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yellon DM. and Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, Liem DA, Mueller M, Wang Y, Zong C, Deng N, Vondriska TM, Korge P, Drews O, Maclellan WR, Honda H, Weiss JN, Apweiler R, and Ping P. Altered proteome biology of cardiac mitochondria under stress conditions. J Proteome Res 7: 2204–2214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang M, Mileykovskaya E, and Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem 277: 43553–43556, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Zhang M, Mileykovskaya E, and Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J Biol Chem 280: 29403–29408, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.