Abstract
Cystic fibrosis (CF) is due to mutations in the CFTR gene, which prevents correct folding, trafficking and function of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) protein. The dysfunctional effect of CFTR mutations, principally the F508del-CFTR mutant, is further manifested by hypersecretion of the pro-inflammatory chemokine interleukin-8 into the airway lumen, which further contributes to morbidity and mortality. We have hypothesized that microRNA (miR)-based therapeutics could rescue the dysfunctional consequences of mutant CFTR. Here we report that a miR-16 mimic can effectively rescue F508del-CFTR protein function in airway cell lines and primary cultures, of differentiated human bronchial epithelia from F508del homozygotes, which express mutant CFTR endogenously. We also identify two other miRs, miR-1 and miR-302a, which are also active. Although miR-16 is expressed at basal comparable levels in CF and control cells, miR-1 and miR-302a are undetectable. When miR mimics are expressed in CF lung or pancreatic cells, the expression of the F508del-CFTR protein is significantly increased. Importantly, miR-16 promotes functional rescue of the cyclic AMP-activated apical F508del-CFTR chloride channel in primary lung epithelial cells from CF patients. We interpret these findings to suggest that these miRs may constitute novel targets for CF therapy.
INTRODUCTION
Cystic fibrosis (CF) is a common, autosomal recessive, life-limiting genetic disease, which is primarily due to the F508del mutation, and infrequently other mutations, in the CFTR gene.1–3 The F508del-CFTR mutation causes the F508del-CFTR protein to misfold, leading to its premature degradation and failure to traffic to the plasma membrane.4 The consequence of this trafficking failure is loss of a cyclic AMP (cAMP)-activated chloride channel,4 and activation of massive pro-inflammatory signaling by expression of interleukin-8 (IL-8) and other cytokines and chemokines.5–8 Functional rescue of the F508del-CFTR-trafficking defect can be achieved, in vitro, by low temperature9 or by small molecules.10–12 One of these, VX-809, has produced relatively promising results in clinical trials.13 However, potential novel therapeutics based on various RNA-based platforms have only recently contributed new, effective, low-toxicity strategies to a variety of disorders.14–16 In the case of the F508del-CFTR mutation, we and others have reported that specific microRNAs (miRs) can reduce pro-inflammatory IL-8 signaling in F508del-CFTR cells17,18 and mediate correction of the trafficking defect in cultured cells expressing F508del-CFTR.17,19 However, the mechanisms by which these potential RNA therapies work remain unknown.
CFPAC6.0 cells, derived from a pancreatic duct adenocarcinoma occurring in a CF patient homozygous for F508del-CFTR, express the protein from its endogenous promoter.20 These cells have the ion transport and single Cl− channel properties characteristic of CF epithelia having this common CF genotype. Our preliminary screening approach was to search for miRs that were aberrantly expressed in CF epithelial cells. We hypothesized that among these reduced miRs, we could identify miRs that could rescue not only the F508del-CFTR-trafficking defect, but also the functional defects in cAMP-activated chloride transport, and reverse hyperexpression of IL-8. Here we report that overexpression of miR-1, miR-16 and miR-302a are able to activate synthesis of F508del-CFTR mRNA in cultured CF cells. Furthermore, in both cultured CF cells and primary cultures of lung epithelial cells from (F508del/F508del) CF patients, we find that the two miRs, miR-16 and miR-302a, are also able to correct not only the F508del-CFTR-trafficking defect, and cAMP-activated chloride channel activity, but also IL-8 hyperexpression. We suggest that these miRs, or others in this class, may constitute the basis of a novel RNA-based approach to CF therapy.
RESULTS
MiRs rescue F508del-CFTR expression/trafficking in native CF pancreatic epithelial cells
To identify candidate miRs with potential to rescue F508del-CFTR, we had recourse to the data from a recent screen of short-hairpin RNAs on cultured CF epithelial cells, in which the end point was functional rescue.21 As listed in Table 1, there were six genes, including HSP90, CASR, HDAC7, SLC26A9, HDAC6 and AHSA1, for which individual short-hairpin RNA-mediated knockdown resulted in functional rescue of F508del-CFTR. We performed in silico analyses of the miRs that were predicted to target these genes, using prediction programs (namely, miRWalk). Included among predicted miRs, we noted miR-1, miR-16 and miR-302a, which we had detected to be aberrantly expressed in CF cells compared with controls. Therefore, we hypothesized that these three miRs might be competent to rescue F508del-CFTR function. As depicted in Table 1, the selected candidate miRs are predicted to target these CFTR-interacting proteins. To test this hypothesis, we examined the ability of identified precursor-miRs (pre-miRs) to support functional rescue of F508del-CFTR mRNA and protein in CF epithelial cells from the lung and pancreas.
Table 1.
In silico identification of miRs predicted to target CFTRinteracting proteins
| Genesa | MiRsb
|
||
|---|---|---|---|
| miR-1 | miR-16 | miR-302a | |
| HSP90 | √ | √ | √ |
| CASR | √ | √ | √ |
| HDAC7 | √ | √ | × |
| HSPA8 | × | √ | √ |
| SLC26A9 | √ | √ | × |
| HDAC6 | √ | × | × |
| AHSA1 | × | √ | × |
Abbreviations: CFTR, cystic fibrosis transmembrane conductance regulator; miR, microRNA; shRNA, short-hairpin RNA; √, predicted target; x, not a predicted target. miRs including miR-1, miR-16 and miR-302a are predicted to target multiple CFTR-interacting proteins, whose individual shRNA-mediated knockdown can rescue F508del-CFTR function.
CFTR-interacting protein whose shRNA-mediated knockdown has been shown to rescue F508del.
MiRs that are predicted to target CFTR-interacting protein as analyzed using miRWalk.
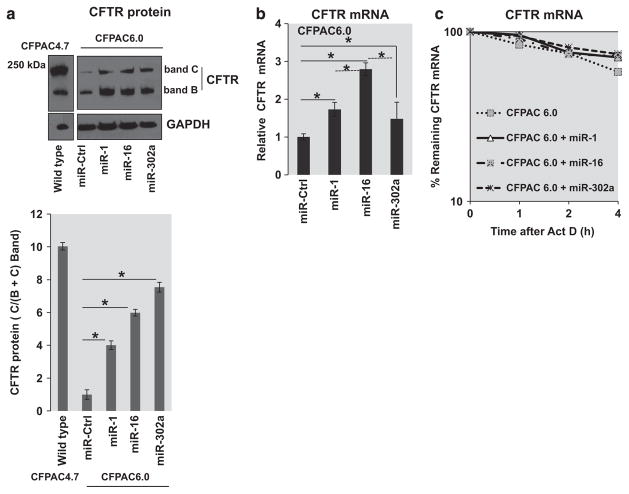
Subsequently, the effect of overexpression of the three miRs, miR-1, miR-16 and miR-302a, that are expressed at a comparable basal level or are undetectable in F508del-CFTR CF cells was analyzed. Figure 1a depicts that when parental CFPAC6.0 cells are incubated with miR-1, miR-16 or miR-302a, substantial amounts of mature band C CFTR are induced compared with controls (miR-Ctrl). In addition, all three miRs induce large increments in immature band B. In contrast, no changes are induced in expression of housekeeping protein glyceraldehyde 3-phosphate dehydrogenase. In the experiment shown, which is representative of three independent experiments, miR-1, miR-16 and miR-302a promote a fourfold, sixfold and eightfold increase in the mature CFTR protein expression (C band/B+C band), respectively (Figure 1a, bottom panel). By contrast, the endogenously expressed F508del-CFTR in parental CFPAC6.0 cells shows low levels of band B and a relatively insignificant band C. However, in the presence of the active miRs, vastly elevated band B and significantly higher levels of band C are produced. This is reflected in the quantitation of the western blot depicted in Figure 1a (bottom panel), indicating the ratio of C band compared with the total of B and C band in comparison with wild-type (WT)-CFTR-expressing CFPAC4.7 cells. This approximately replicates the relative expression levels of immature and mature CFTR in the WT-CFTR-transduced CFPAC4.7 cells, except that much higher levels were observed with miR pre-treatment. In summary, the effect of miR-1, miR-16 and miR-302a on F508del-CFTR is to amplify the pattern of band B and band C expressed by F508del-CFTR by itself.
Figure 1.
MicroRNAs (miR) induce F508del-CFTR expression. (a) CFPAC6.0 cells (2 × 105) were transfected with miR mimics, miR-1 (20 nM), miR-16 (25 nM) or miR-302a (30 nM) and pre-miR-negative control (miR-Ctrl) for 48 h with siPort transfection reagent. The cells were lysed and the isolated protein was analyzed using western blot analysis. CFPAC4.7 and native CFPAC6.0 cells were included as controls. The relative quantitation of the C bands compared with total C+B bands are also indicated. The lane with the WT-CFTR protein is part of the same gel. The data are representative of three or more independent experiments. (b) For analyses of CFTR mRNA, CFPAC6.0 cells (2 × 105) were treated as described before, and total RNA was isolated and analyzed using quantitative real-time PCR (qRT-PCR) specific for CFTR mRNA. The comparison of control versus each of the three miRs is indicated (solid lines, *P<0.05). In addition, comparison of miR-16 with miR-1 or miR-302a is also included (dotted lines, *P<0.05). (c) The stability of CFTR mRNA was also measured in native CFPAC6.0 and miR-treated CFPAC6.0 cells. Subsequent to transfection, the RNA was isolated after treatment with actinomycin D (5 μgml − 1) for the indicated time intervals (0, 1, 2 and 4 h). The remaining RNA was analyzed using qRT-PCR. The data are representative of three or more independent experiments.
Rescue-competent miRs increase F508del-CFTR mRNA expression in native CF pancreatic epithelial cells
CFPAC6.0 parental cells differ from many other available cultured CF cells in that the F508del-CFTR gene is driven by a native CFTR promoter. We therefore tested the effects of rescue-capable miRs on F508del-CFTR mRNA expression. Figure 1b shows that all three rescue-capable miRs are able to elevate F508del-CFTR mRNA over control levels. Here we observe that miR-16 is more efficient than miR-1 and miR-302a (comparisons indicated by dotted lines). Furthermore, the analyses of CFTR mRNA stability in the CFPAC6.0 cells indicate that the overexpression of the three miRs does not have any effect on the CFTR mRNA stability (Figure 1c). Thus, these rescue-capable miRs appear to engineer a functional connection between the process of F508del-CFTR mRNA synthesis, driven by a native promoter, and trafficking correction of the F508del-CFTR protein.
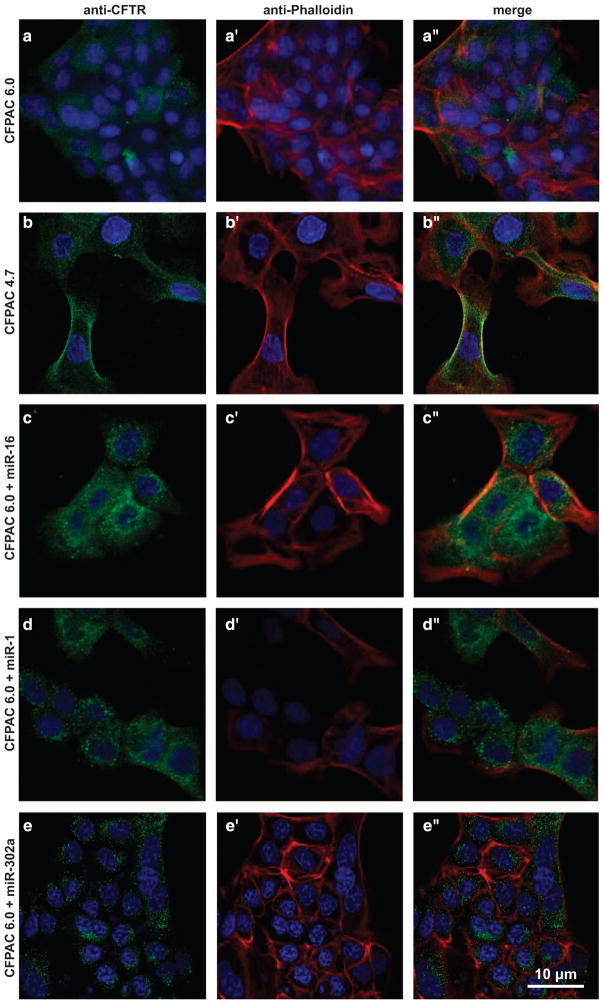
MiR-rescued F508del-CFTR protein distributes to the cytoplasm and the plasma membrane in parental CF pancreatic epithelial cells
Figure 2a shows that CFPAC6.0 cells have low levels of CFTR protein (green) distributed in the cytoplasm and plasma membrane (red). This is consistent with the amount of band B and band C proteins detectable with western blot analysis of parental native CFPAC6.0 cells in Figure 1a. What CFTR label is visible is distributed primarily in a perinuclear pattern, which may represent F508del-CFTR protein in the endoplasmic reticulum. However, Figure 2b shows that when these cells are repaired recombinantly with WT-CFTR (namely, CFPAC4.7 cells), substantial CFTR-specific label (green) can be found distributed in a punctate pattern in the cytosol and in regions close to the plasma membrane (red). Yellow fluorescence in the vicinity of the plasma membrane indicates coincidence of green (CFTR) and red (actin). These data are thus also consistent with western blot data for parental (F508del/F508del) cells and WT-CFTR cells rescued by transduction with WT-CFTR (Figure 1a).
Figure 2.
Expression and localization of CFTR. (a–a″) CFPAC6.0 cells containing the F508del mutation show low levels of CFTR labeling (green) that is faintly perinuclear and punctate. (b–b″) CFPAC4.7 cells that express WT-CFTR at the endogenous locus show robust, punctate CFTR labeling (green, b, b″) throughout the cytoplasm that can overlap with the cell membrane (b′, b″, red). (c–e″) Treating (ΔF508) CFPAC6.0 cells with miR-16 (c–c″), miR-1 (d–d″) or miR-302a (e–e″) cause a large increase in the amount of CFTR labeling (green), with miR-16 causing the greatest increase. Green=a-CFTR (a, a″, b, b″, c, c″, d, d″, e, e″), red=phalloidin (a′, a″, b′, b″, c′, c″, d′, d″, e′, e′), blue=4’,6-diamidino-2-phenylindole (a–e″). The image depicts Z-stacks, and an average of 300 cells in 10 different fields was analyzed.
Figures 2c–e further show subcellular distributions of F508del-CFTR in CFPAC6.0 parental cells treated with miR-16, miR-1 and miR-302a. Nearly every cell has intense punctate perinuclear distributions of cytosolic F508del-CFTR protein. In addition, there is evidence of colocalization of the F508del-CFTR protein with plasmalemmal actin in the region of the plasma membrane. Thus, miR-mediated changes in F508del-CFTR cytoimmunoreactivity appear to mirror the miR-mediated massive changes affecting both band B and band C seen by western blot analysis in Figure 1a. The subcellular character of the distribution of F508del-CFTR protein induced by miRs is thus qualitatively different from that induced by virus-mediated transduction with WT-CFTR. In summary, compared with CFPAC6.0 cells, the rescue-competent miRs, miR-16 being the most effective, induce an increased circum-nuclear expression of F508del-CFTR as well as relatively more membrane localization.
MiR-rescued F508del-CFTR protein distributes to the plasma membrane in native CF lung epithelial cells
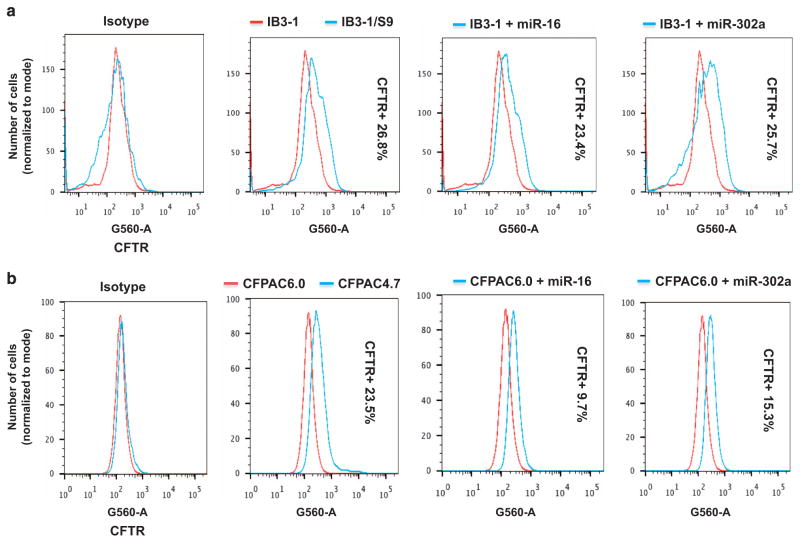
To test whether rescue-capable miRs were also effective in native CF lung epithelial cells, we studied the effects of miR-1, miR-16 and miR-302a on surface expression in human CF lung epithelial IB3-1 cells. As a control, we included IB3-1/S9 cells, which had been corrected with WT-CFTR using the adeno associated virus vector system.22 The IB3-1 cells express low, but measurable, levels of F508del-CFTR protein.23 We used fluorescence-activated cell sorting analyses to quantitatively detect CFTR protein in lung IB3-1 cells and compared the results with parallel experiments on the pancreatic CFPAC-1 cell systems. Equal number of viable cells was acquired on the LSRII cytometer and, presence of antibody was measured on a log scale. Differences in fluorescence are depicted in the histogram as analyzed using the FlowJo software (TreeStar Inc., Ashland, OR, USA).
Figure 3a shows a detectable signal from CFTR protein in IB3-1 cells, and that an additional 26.8% CFTR protein signal can be detected when the IB3-1 cells are corrected with WT-CFTR. Incubation of IB3-1 cells with either miR-16 or miR-302a results in 23.4% and 25.7% increases in the CFTR protein signal, respectively, as compared with the isotype controls. Equivalent studies with the CFPAC6.0 cells show that correction with WT-CFTR (namely, CFPAC4.7) results in a 23.5% increase in CFTR protein signal. Incubation of CFPAC6.0 cells with either miR-16 or miR-302a results in an increase of 9.7% and 15.3% CFTR protein, respectively (Figure 3b). In this case there is a fraction of cells that are virtually ‘null’ in terms of a boost from the rescue-competent miRs. These data thus show that both endogenously expressing CF lung epithelial cells and CF pancreatic epithelial cells can respond to rescue-competent miRs by increased F508del-CFTR expression.
Figure 3.
FACS analyses of CFTR expression. The increased cell surface expression of CFTR induced by overexpression of miRs was analyzed using flow cytometry in two different CF epithelial cells. (a) IB3-1 CF lung epithelial cells (2 × 105) were transfected with miR mimics, and CFTR expression was compared with that in IB3-1 mock-treated cells and and IB3-1/S9 control cells. (b) Similarly, CFPAC6.0 pancreatic epithelial cells (2 × 105) were transfected with miR mimics, and CFTR expression was compared with that in native CFPAC6.0-mock-treated cells and CFPAC4.7 control cells. The data are representative of three or more independent experiments.
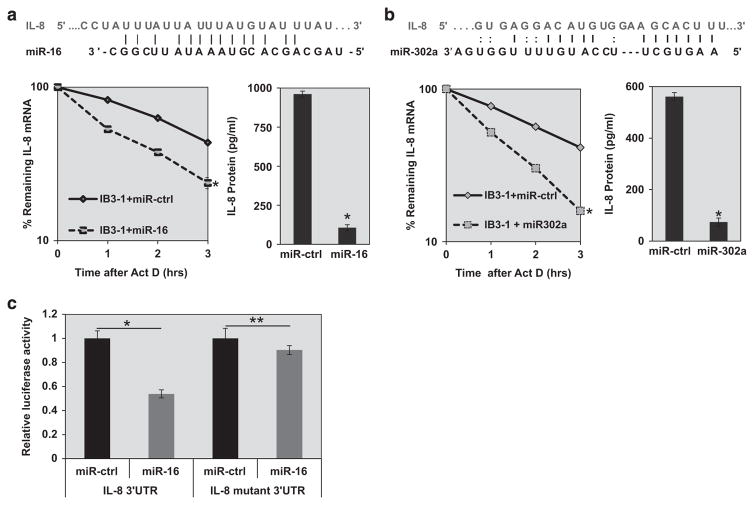
Rescue-competent miRs suppress overexpression of IL-8 by CF epithelial cells
Rescue of F508del-CFTR is associated with reduction of the pro-inflammatory phenotype mediated by enhanced secretion of IL-8. The mechanism has been shown to depend on destabilization of the IL-8 mRNA.24 In previous studies we reported that antisense to the inflammatory miR, miR-155, could suppress IL-8 mRNA and protein expression.18 Therefore, we anticipated that rescue of the F508del-CFTR-trafficking defect with the rescue-competent miRs might also suppress IL-8 secretion. Figure 4a shows that overexpression of miR-16 in CF lung IB3-1 cells results in enhanced degradation of IL-8 mRNA. This effect is accompanied by concomitant reduction in IL-8 protein expression. Similar results for IL-8 mRNA and IL-8 protein expression are also found for miR-302a (Figure 4b). However, it is possible that IL-8 suppression is also due to the fact that sequences of miR-16 and miR-302a also have complementarity to the 3′-untranslated repeat (UTR) of IL-8. Therefore, the effects of miR-16 and miR-302a on IL-8 mRNA may not be solely owing to correction of the F508del-CFTR-trafficking defect. Nonetheless, miR-1 has no such complementarity to the IL-8 3′-UTR, although our earlier studies indicate that miR-1 indeed can regulate IL-8 expression in CF cells through other mechanisms.25 We conclude from these data that rescue-competent miRs have functional consequences for native CF lung epithelial cells in terms of reduced pro-inflammatory IL-8 expression.
Figure 4.
Suppression of IL-8 expression by rescue-competent miRs in CF lung epithelial cells. In silico analyses indicate that both miR-16 and miR-302a have binding sites at the target IL-8 mRNA 3′-UTR sequences. IB3-1 CF lung epithelial cells (2 × 105) were transfected with miR mimics, (a) miR-16 (25 nM) or (b) miR-302a (30 nM) for 48 h with siPort transfection reagent. The RNA was isolated after treatment with actinomycin D (5 μgml − 1) for the indicated time intervals (0, 1, 2 and 3 h). The remaining IL-8 mRNA was analyzed by qRT-PCR (*P<0.05). The corresponding secreted IL-8 protein was measured using ELISA (*P<0.05). (c) Luciferase reporter assays were performed in IB3-1 CF cells transfected with pMIR-report vectors, either controls or those containing IL-8 3′-UTR target sequences of miR-16 (both WT and mutant), in the presence of pre-miR-16 or control pre-miR. The data reflect averages of at least three independent experiments (*P<0.05 and **P>0.05).
To gain more direct evidence that IL-8 might be a target of miR-16 in CF cells, we developed a luciferase reporter assay in which the pMIR-Report vector was constructed with WT or (mutant) IL-8 3′-UTR sequences. These reporter plasmids were then transfected into CF IB3-1 cells, either with pre-miR-16 or a control pre-miR (miR-Ctrl). As shown in Figure 4c, we find that WT IL-8 3′-UTR diminishes luciferase expression in the presence of increased miR-16 in CF cells. Consistently, luciferase expression is not significantly altered by a partially inactivating mutation engineered into the IL-8 3′-UTR sequence. Therefore, miR-16 specifically includes IL-8 mRNA as a target in the CF lung epithelial cells.
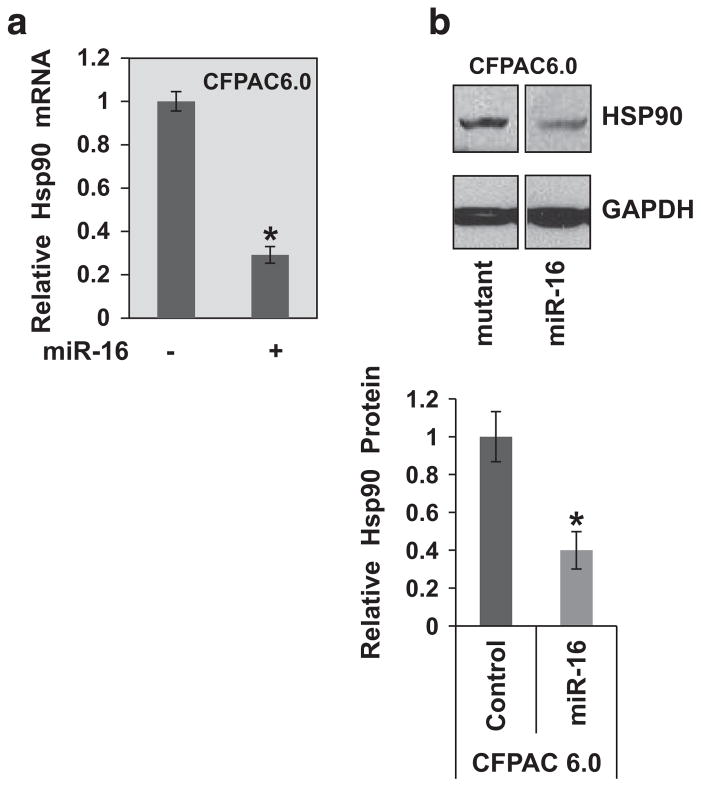
miR-16 suppresses Hsp90 expression in CF epithelial cells to rescue [ΔF508] CFTR
The short-hairpin RNA screen had identified a set of genes, including HSP90, for which reduction led to functional rescue of the F508del-CFTR protein21 (Table 1). We, therefore, analyzed the effect of miR-16 on HSP90 expression. As depicted in Figure 5, we found that indeed increased expression of miR-16 in CFPAC6.0 epithelial cells leads to the significant suppression of Hsp90 mRNA expression (~70%) and a corresponding reduction in the Hsp90 protein (~60%). Thus, a potential mechanism by which miR-16 rescues F508del-CFTR trafficking and activity may include suppression of the chaperone HSP90.
Figure 5.
Suppression of HSP90 expression by miR-16 in CF epithelial cells. CFPAC6.0 pancreatic epithelial cells (2 × 105) were transfected with miR-16 mimic as previously described. To determine the effect of miR-16 overexpression on the expression of HSP90, both (a) Hsp90 mRNA (qPCR) and (b) Hsp90 protein (western blot) levels were analyzed (*P<0.05). The two lanes are part of the same gel. The protein data shown here are representative of three or more independent experiments.
Rescue-competent miRs rescue F508del-CFTR-dependent cAMP-activated chloride conductance in primary cultures of native CF HBE cells
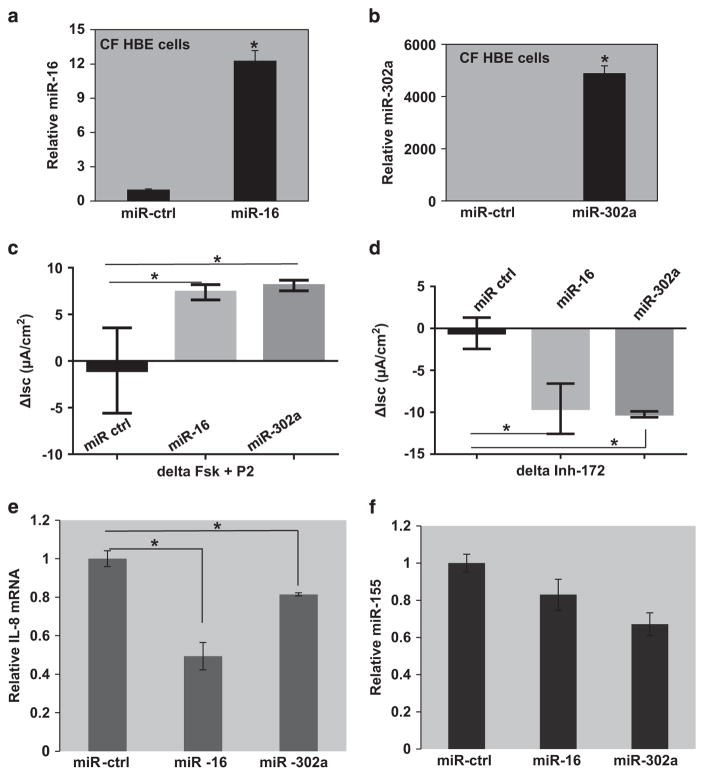
To further test for the efficacy of rescue-competent miRs, primary cultures of human bronchial epithelial (HBE) cells were cultured at the air–liquid interface. After differentiation, they were exposed to either miR-16 or miR-302a for 48 h. The expression of miR-16 and miR-302a was analyzed in the transfected cells using individual Taqman assays. As depicted in Figures 6a, b, transfection of both miR-16 and miR-302a resulted in significantly elevated expression in the CF HBE cells. Figure 6c shows an Ussing chamber experiment in which both miR-16 and miR-302a can induce elevated chloride transport when the cells are exposed to forskolin to elevate cellular cAMP, and then to the CFTR potentiator, P2 (available at: http://cftrfolding.org/CFFTReagents.htm).26 Significant increase in short-circuit current (Isc) was observed between miR-16- and miR-302a-transfected HBEs compared with those transfected with miR-controls (P<0.005 and 0.004, respectively). The increases in current were reduced by addition of the CFTR inhibitor, Inh-172. As depicted in Figure 6d, representative of two independent experiments, miR-16 as well as miR-302a transfection, has a significant effect on Isc compared with those transfected with miR-controls (ΔIsc≈−10 μA cm−2, P<0.04). This is a significant restoration of CFTR activity (the WT-CFTR, average ΔIsc = − 25 μA cm− 2). Furthermore, as shown in Figure 6e, both miR-16 and miR-302a can significantly reduce levels of IL-8 mRNA in these primary cultures, as they do in CF epithelial cell lines, miR-16 being more active. Finally, Figure 6f shows that miR-16 and miR-302a can also slightly reduce miR-155 expression. As miR-155 is itself a functional marker for mutant F508del-CFTR,18 these data suggest that the miRs are also correcting the mutation-dependent functional defect in these primary HBE cultures.
Figure 6.
Rescue of ΔF508-CFTR function and attenuation of inflammation. CF HBE cells transfected with microRNA (miR) mimics, miR-16 (25 nM) and miR-302a (30 nM) for 48 h in air–liquid interface cultures. The expression levels of (a) miR-16 and (b) miR-302a in the CF HBE cells were analyzed using miR-specific Taqman assays. (c) The cAMP-activated short-circuit current was measured after administration of forskolin (10 μM)+P2 (50 μM; *P<0.05). (d) Subsequently, these cells were treated with CFTRinh-172 to inhibit CFTR, followed by measurement of short-circuit current (*P<0.05). The CF HBE cells were lysed and total RNA was isolated and analyzed using the Taqman assays for (e) IL-8 mRNA and (f) miR-155 (*P<0.05).
DISCUSSION
Our data indicate that native CF epithelial cell cultures from both pancreas and lung, as well as primary explants of CF human lung epithelial cells, can respond differentially to rescue-competent miRs by correcting the F508del-CFTR-trafficking defect; by activating cAMP-activated chloride conductance; and by reducing pro-inflammatory IL-8 expression. Collectively, our data indicate that the most potent of these rescue-competent miRs was miR-16. In addition, in the case of parental pancreatic CFPAC6.0 cells, we find that all three of the rescue-competent miRs are able to activate synthesis of F508del-CFTR mRNA. The latter observation can only be determined in native cells and tissues because recombinant CF model systems often have both WT-CFTR and F508del-CFTR driven by a cytomegalovirus or other endogenous promoter.
Our studies on CF pancreatic epithelial cells, which express F508del-CFTR driven by the native promoter, suggest that rescue of the trafficking defect by either of the three rescue-competent miRs may proceed by a qualitatively different mechanism compared with that mediated by recombinant WT-CFTR. The data in Figure 1 clearly show that there is a close relationship between increased CFTR mRNA biosynthesis, and downstream elevation in both band B and band C of F508del-CFTR. In parental CFPAC6.0 cells, which are homozygous for the F508del mutation, the synthesis of F508del-CFTR mRNA depends on a well-orchestrated sequence of activation, initiated at the native promoter and continuing through extensive intron editing. Eventually, this mature mRNA reaches the cytosol, where it binds to ribosomes and is translated into F508del-CFTR band B. The intervening events between activation of CFTR mRNA synthesis and translation to F508del-CFTR band B may not occur if the promoter is non-native, and the F508del-CFTR construct lacks introns and other signaling components. It cannot be ruled out that by continuously driving F508del-CFTR mRNA synthesis, the generation of band B leads to the trafficking and formation of proportionately more band C. In the case of recombinant CFTR, which lacks introns and the native promoter, this kind of ‘start-to-finish’ correction available from the miRs is not possible. Instead, a recognizable pattern of a small amount of band B and a large amount of band C is what is generated. This fundamental difference may be useful in the context of studies with HBEs showing that very small amounts of band B are expressed, and that correctors such as VX-809 do not have an impact on band B levels. The limit on band B thus limits levels of band C that can be achieved. However, it needs to be emphasized here that we still do not know how the rescue-competent miRs accomplish this comprehensive feat of activating F508del-CFTR mRNA, and production of both band B and band C F508del-CFTR protein in CF cells that endogenously express the protein. Our data suggest that suppression of HSP90 expression by miR-16 may be one of the potential mechanisms.
Inflammation mediated by IL-8 in the airway is one of the fundamental problems confronting the CF patient and is very likely a critical contributor to both morbidity and mortality.5–8 The rescue-competent miRs described here have a very complex effect on IL-8 expression, which may ultimately provide a novel anti-inflammatory therapeutic effect on the CF lung. All three of the rescue-competent miRs provide profound rescue of the F508del-CFTR-trafficking defect, and support reduction in IL-8 expression. miR-1 may uniquely provide this effect by acting directly on the F508del-CFTR-trafficking defect alone. Making this correction is sufficient to induce reduction of IL-8 expression, at least through an indirect effect on miR-155.25,27 However, both miR-16 and miR-302a also have complementary seed sequences to the 3′-UTR of the IL-8 mRNA. Thus, both miR-16 and miR-302a could mediate the reduction of airway IL-8 by simultaneously correcting the F508del-CFTR-trafficking defect and by independently reducing IL-8 mRNA stability. The specific connection between correctly trafficking CFTR to the plasma membrane, expressing the cAMP-activated chloride channel, and reducing IL-8 expression, remains unknown. At least one mechanism depends on reduction of miR-155, leading to elevation of the phosphatase SHIP1, activation of protein kinase B,18 and hence to NFκB. However, how the rescue of F508del-CFTR connects to the reduction of miR-155 itself also remains unknown.
We conclude that these data suggest that miR-based therapeutics, based on the three rescue-competent miRs described here, or perhaps others in this class, may find a place in the therapeutic armamentarium for CF. At present, their effect on native CF epithelial cells is comprehensive, starting at mechanisms regulating F508del-CFTR mRNA synthesis and ending with full function at the plasma membrane by rescued F508del-CFTR protein.
MATERIALS AND METHODS
Reagents
LHC-8 media, Dulbecco’s Modified Eagle Media, Trypsin-EDTA (0.05%), puromycin and lipofectamine transfection reagents were purchased from Invitrogen (Life Technologies, Carlsbad, CA, USA). The miRVana kit for RNA isolation, miR assays, miR mimics, pMIR-Report luciferase reporter vector, luciferase assay kits and Taqman quantitative PCR (qPCR) reagents were purchased from Applied Biosystems (Life Technologies, Foster City, CA, USA). The C-terminal-specific CFTR antibody was purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture
IB3-1 CF lung epithelial cells and the control CFTR-repaired IB3-1/S9 cells were maintained in LHC-8 serum-free medium in humidified 5% CO2 as previously described.22 CFPAC-1 cells, derived from a pancreatic duct adenocarcinoma from a CF patient homozygous for F508del-CFTR, have the ion transport properties that are characteristic of CF-affected epithelia. They express mutant CFTR from the endogenous promoter and are designated as CFPAC6.0.20 Repaired cells created by transforming parental CFPAC6.0 cells with the pLJ retrovirus carrying WT-CFTR28 are designated as CFPAC4.7. CFPAC6.0 cells were maintained in high-glucose Dulbecco’s Modified Eagle Media supplemented with 10% fetal bovine serum, 2mM L-glutamine and antibiotics (100 IU ml− 1 penicillin and 100 μgml − 1 streptomycin) in a 5% CO2 environment.
Immunocytochemistry
For immunostaining, CFPAC6.0 cells were seeded onto a cover-glass placed inside a well of a multiwell plate. The coverglass was washed twice with 1 × phosphate-buffered saline and then fixed for 25 min in 4% paraformaldehyde and 20mM formic acid solution (Sigma-Aldrich, St Louis, MO, USA) made in Grace’s media (Lonza, Allendale, NJ, USA). Cells were washed three times for 10 min with antibody wash buffer (1 × phosphate-buffered saline: 0.1% Triton X-100: 0.2% bovine serum albumin) and incubated overnight at 4 °C in primary antibody (mouse anti-CFTR, 1:500, R&D Systems). They were then washed three times for 10 min and incubated for 2 h at room temperature in secondary antibody (anti-mouse IgG2A Alexa 488, Molecular Probes, Invitrogen, Carlsbad, CA, USA). After washing two times for 10 min in antibody wash, rhodamine phalloidin (Molecular Probes, Invitrogen) was added for 15 min. After washing two times for 5 min, 4’,6-diamidino-2-phenylindole was added for 10 min, then removed and then the coverglass was mounted with Vectashield (Vector Laboratories Inc., Burlingame, CA, USA). Samples were imaged using a Zeiss 710 confocal microscope and 63× Plan Apo numerical aperture 1.4 lens. The image depicts Z-stacks, and an average of 300 cells in 10 different fields was analyzed.
Flow cytometry
The F508del-CFTR CF epithelial cells, IB3-1 and CFPAC6.0 were grown in six-well plates (2 × 105 cells) and transfected with miR-1, miR-16 and miR-302a. IB3-1/S9 and CFPAC4.7 cells with WT-CFTR, as well as mock-treated IB3-1 and CFPAC6.0 cells, were included as controls. The media were aspirated and the cells were detached with accutase. Next, the cells were transferred to a 1.5-ml vial, mixed with 1ml of staining buffer (1 × phosphate-buffered saline with 1% fetal bovine serum and 0.2% sodium azide) and harvested at 1400 r.p.m. for 8 min at 4 °C. The pellet containing cells was then resuspended in ice-cold staining buffer at 1400 r.p.m. for 8 min at 4 °C. The cells were then incubated in the primary antibody (C-terminal CFTR antibody, clone 24-1, 1:500, R&D Systems) for 40–60 min at 37 °C on a rocker in the dark, and then washed twice with ice-cold staining buffer. Cells were next incubated with secondary antibody (1:200) for 30 min and again washed twice with ice-cold staining buffer. Subsequently, the cells were fixed with 1% paraformaldehyde and kept at 4 °C in the dark for flow cytometric analyses. Equal numbers of viable cells were acquired on an LSRII cytometer (BD Biosciences, San Jose, CA, USA), and the presence of antibody was measured in log scale. Differences in fluorescence are depicted here in histograms (analyzed using the FlowJo software, TreeStar Inc.), which overlay both the conditions.
Real-time PCR, miR overexpression and assays for mature miRs Transfections with miR mimics (pre-miR-16: ID PM10339, pre-miR-1: ID PM10617, pre-miR-302a: ID PM10936 and pre-miR-negative control: Catalog no. AM17110) were carried out using siPORT NeoFX Transfection Reagent (ABI) for 48 h. Total RNA was isolated from the CF cells using a miRVana isolation kit (ABI). Multiplex reverse transcription was performed with the TaqMan miR Reverse Transcription Kit (ABI). The mRNA expression levels were analyzed with quantitative real-time PCR as described earlier.24 The mature miRs were assayed using the Taqman miR-specific assay (ABI).18
Culture of human primary F508del HBE cells and Ussing chamber assay
Primary HBE cells expressing F508del-CFTR were propagated, differentiated and maintained as described elsewhere.29 F508del-CFTR HBE cells were cultured from excess pathological tissue following lung transplantation and organ donation under a protocol approved by the University of Pittsburgh Institutional Review Board on 6.5-mm Costar Transwell filters (Catalog no. 3470, 0.33 cm2 0.4-mm pore; Lowell, MA, USA) in 0.5% Ultroser G as previously described and used for experimentation after at least 4–6 weeks of culture at an air–liquid interface.30,31 The miR mimics for miR-16 and miR-302a were then added to the basolateral side of the cell monolayer at 37 °C for 48 h using siPORT (6 μl per 2 ml of media). At the end of drug incubation, filters were mounted in Physiologic Instruments Ussing chambers (World Precision Instruments Inc., Sarasota, FL, USA) and allowed to equilibrate for 15 min to permit electrical parameters to stabilize. The basolateral medium consisted of 120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2PO4, 0.8 mM K2HPO4, 1.2 mM CaCl2, 1.2 mM MgCl2 and 10 mM D-glucose. The apical solution NaCl was replaced with sodium gluconate to achieve a 120-mM transepithelial chloride gradient. The bathing solutions were gassed with 95% O2 and 5% CO2 to maintain a pH of 7.4. Isc and transepithelial resistance were continuously measured using a Physiologic Instruments VCC-MC8 and Physiologic Instruments Acquire and Analyze 2.3 data acquisition hardware and software. After the equilibration period, 10 μM amiloride was added to the apical chamber to inhibit epithelial sodium channel-mediated sodium absorption. After 2 min, 10 μM forskolin was added to both the basolateral and apical chambers to activate CFTR-mediated anion excretion. After 2 min, 50 μM P2 (CFTR potentiator; http://cftrfolding.org/CFFTReagents.htm) was added to the basolateral and apical chambers. After another 2 min, CFTR Inhibitor-172 (Sigma-Aldrich) was added to the apical chamber to inhibit CFTR-mediated anion excretion. At these times, currents had achieved steady state. Agonist- or inhibitor-induced changes in Isc (ΔIsc) were calculated from differences in the mean Isc over the 10-s period preceding reagent additions.
Statistical data analyses
Statistical analysis was performed using Excel (Microsoft Office 2010) as well as with GraphPad (GraphPad Software Inc., LaJolla, CA, USA). Significance values (P ≤0.05) were determined by Student’s t-test, for the comparisons between control groups versus respective pre-miR-transfected groups. Analysis of variance was used for multiple group comparisons. Error bars on graphs represent s.e.m.
Acknowledgments
We thank Dr Cara Olsen (Biostatistician, USUHS) for helping with statistical analyses. This study was supported by USU-Intramural Funds (RB); NIH grants DK072506 and DK068196 (RAF); and Cystic Fibrosis Foundation (RB, HBP and RAF).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989;245:1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- 3.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations–correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 4.Welsh MJ, Denning GM, Ostedgaard LS, Anderson MP. Dysfunction of CFTR bearing the delta F508 mutation. J Cell Sci. 1993;17:235–239. [PubMed] [Google Scholar]
- 5.Dean TP, Dai Y, Shute JK, Church MK, Warner JO. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res. 1993;34:159–161. doi: 10.1203/00006450-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutierrez JP, Hull J, et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 7.Dhooghe B, Noel S, Huaux F, Leal T. Lung inflammation in cystic fibrosis: pathogenesis and novel therapies. Clin Biochem. 2014;47:539–546. doi: 10.1016/j.clinbiochem.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 9.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 10.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arispe N, Ma J, Jacobson KA, Pollard HB. Direct activation of cystic fibrosis transmembrane conductance regulator channels by 8-cyclopentyl-1,3-dipropylxanthine (CPX) and 1,3-diallyl-8-cyclohexylxanthine (DAX) J Biol Chem. 1998;273:5727–5734. doi: 10.1074/jbc.273.10.5727. [DOI] [PubMed] [Google Scholar]
- 12.Caohuy H, Yang Q, Eudy Y, Ha TA, Xu AE, Glover M, et al. Activation of 3-phosphoinositide-dependent kinase 1 (PDK1) and serum- and glucocorticoid-induced protein kinase 1 (SGK1) by short-chain sphingolipid C4-ceramide rescues the trafficking defect of DeltaF508-cystic fibrosis transmembrane conductance regulator (DeltaF508-CFTR) J Biol Chem. 2014;289:35953–35968. doi: 10.1074/jbc.M114.598649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rij RP, Andino R. The silent treatment: RNAi as a defense against virus infection in mammals. Trends Biotechnol. 2006;24:186–193. doi: 10.1016/j.tibtech.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Gorman C, Maron DF. The RNA revolution. Sci Am. 2014;310:52–59. doi: 10.1038/scientificamerican0414-52. [DOI] [PubMed] [Google Scholar]
- 16.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oglesby IK, Bray IM, Chotirmall SH, Stallings RL, O’Neill SJ, McElvaney NG, et al. miR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol. 2010;184:1702–1709. doi: 10.4049/jimmunol.0902669. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharyya S, Balakathiresan NS, Dalgard C, Gutti U, Armistead D, Jozwik C, et al. Elevated miR-155 promotes inflammation in cystic fibrosis by driving hyperexpression of interleukin-8. J Biol Chem. 2011;286:11604–11615. doi: 10.1074/jbc.M110.198390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramachandran S, Karp PH, Jiang P, Ostedgaard LS, Walz AE, Fisher JT, et al. A microRNA network regulates expression and biosynthesis of wild-type and DeltaF508 mutant cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 2012;109:13362–13367. doi: 10.1073/pnas.1210906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoumacher RA, Ram J, Iannuzzi MC, Bradbury NA, Wallace RW, Hon CT, et al. A cystic fibrosis pancreatic adenocarcinoma cell line. Proc Natl Acad Sci USA. 1990;87:4012–4016. doi: 10.1073/pnas.87.10.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorscher E, Hong JS, Hutt DM, Chalfunt M, Roth DM, Balch WE, et al. Screening of candidate adenovirus vectors expressing shRNAs for functional recovery of DeltaF508 CFTR. Pediatric Pulmonology; 24th Annual North American Cystic Fibrosis Conference; 21–23 October 2010; Baltimore, MD, USA. [Google Scholar]
- 22.Eidelman O, Srivastava M, Zhang J, Leighton X, Murtie J, Jozwik C, et al. Control of the proinflammatory state in cystic fibrosis lung epithelial cells by genes from the TNF-alphaR/NFkappaB pathway. Mol Med. 2001;7:523–534. [PMC free article] [PubMed] [Google Scholar]
- 23.Caohuy H, Jozwik C, Pollard HB. Rescue of DeltaF508-CFTR by the SGK1/Nedd4-2 signaling pathway. J Biol Chem. 2009;284:25241–25253. doi: 10.1074/jbc.M109.035345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balakathiresan NS, Bhattacharyya S, Gutti U, Long RP, Jozwik C, Huang W, et al. Tristetraprolin regulates IL-8 mRNA stability in cystic fibrosis lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1012–L1018. doi: 10.1152/ajplung.90601.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya S, Kumar P, Tsuchiya M, Bhattacharyya A, Biswas R. Regulation of miR-155 biogenesis in cystic fibrosis lung epithelial cells: antagonistic role of two mRNA-destabilizing proteins, KSRP and TTP. Biochem Biophys Res Commun. 2013;433:484–488. doi: 10.1016/j.bbrc.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Pedemonte N, Sonawane ND, Taddei A, Hu J, Zegarra-Moran O, Suen YF, et al. Phenylglycine and sulfonamide correctors of defective delta F508 and G551D cystic fibrosis transmembrane conductance regulator chloride-channel gating. Mol Pharmacol. 2005;67:1797–1807. doi: 10.1124/mol.105.010959. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharyya S, Gutti U, Mercado J, Moore C, Pollard HB, Biswas R. MAPK signaling pathways regulate IL-8 mRNA stability and IL-8 protein expression in cystic fibrosis lung epithelial cell lines. Am J Physiol Lung Cell Mol Physiol. 2011;300:L81–L87. doi: 10.1152/ajplung.00051.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drumm ML, Pope HA, Cliff WH, Rommens JM, Marvin SA, Tsui LC, et al. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62:1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- 29.Holleran JP, Glover ML, Peters KW, Bertrand CA, Watkins SC, Jarvik JW, et al. Pharmacological rescue of the mutant cystic fibrosis transmembrane conductance regulator (CFTR) detected by use of a novel fluorescence platform. Mol Med. 2012;18:685–696. doi: 10.2119/molmed.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol. 2000;279:C461–C479. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- 31.Myerburg MM, King JD, Jr, Oyster NM, Fitch AC, Magill A, Baty CJ, et al. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:676–684. doi: 10.1165/rcmb.2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]