Abstract
Many phytochemicals show promise in cancer prevention and treatment, but their low aqueous solubility, poor stability, unfavorable bioavailability, and low target specificity make administering them at therapeutic doses unrealistic. This is particularly true for (–)-epigallocatechin gallate, curcumin, quercetin, resveratrol, and genistein. There is an increasing interest in developing novel delivery strategies for these natural products. Liposomes, micelles, nanoemulsions, solid lipid nanoparticles, nanostructured lipid carriers and poly (lactide-co-glycolide) nanoparticles are biocompatible and biodegradable nanoparticles. Those nanoparticles can increase the stability and solubility of phytochemicals, exhibit a sustained release property, enhance their absorption and bioavailability, protect them from premature enzymatic degradation or metabolism, prolong their circulation time, improve their target specificity to cancer cells or tumors via passive or targeted delivery, lower toxicity or side-effects to normal cells or tissues through preventing them from prematurely interacting with the biological environment, and enhance anti-cancer activities. Nanotechnology opens a door for developing phytochemical-loaded nanoparticles for prevention and treatment of cancer.
Keywords: Nanoparticles, Cancer, Biocompatible, Biodegradable, (–)-Epigallocatechin Gallate, Curcumin, Quercetin, Resveratrol, Genistein
Introduction
Cancer has become one of the leading causes of human morbidity and mortality worldwide, accounting for 7.6 million deaths every year [1] There are more than 100 different types of cancer, and they are quite varied and depend on cancer location, metastasis and size [2]. In the United States and many other countries, common types of cancers are skin cancer, breast cancer, colon and rectal cancer, liver cancer, lung cancer, pancreatic cancer and prostate cancer [1, 3].
Phytochemicals are naturally occurring bioactive compounds found in vegetables, fruits, spices, grains, and other plant foods [4]. Many phytochemicals from traditional medicine have been used for the maintenance of health and prevention of diseases, especially cancer [5-6]. Over the past few decades, research evidence from cell culture and some animal studies has supported that many phytochemicals have anti-cancer activities, but inconsistent results are found in some human clinical trials [7-8]. The inconsistence may be due to the infeasibility of high doses of phytochemicals for human studies, the low level of their aqueous solubility, stability, bioavailability and target specificity to cancer cells and tumors, and the high level of degradation and metabolism by enzymes in the gastrointestinal tract, the liver and other tissues and thus short circulation time and low circulation concentrations[7, 9].
Nanotechnology involves the control of matter, generally in the range of 100 nm or smaller [10]. In recent years, the use of nanotechnology to enhance delivery of phytochemicals to tumors or cancer cells for improving therapeutic efficiency has received considerable attention [9, 11]. Many phytochemicals can be loaded into biocompatible and biodegradable nanoparticles, which can enhance their absorption and bioavailability, protect them from degradation by enzymes, enhance their stability, prolong their circulation time, exhibit high differential uptake efficiency in cancer cells (or tumors) over other normal cells (or normal tissues), lower toxicity through preventing them from prematurely interacting with the biological environment [12].
Biocompatible and biodegradable nanoparticles
Liposomes, nanoemulsions, micelles, solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), poly (lactideco-glycolide) (PLGA) nanoparticles are the commonly used biocompatible and biodegradable nanoparticles, and they can be administered via different routes including oral, intravenous, intraperitoneal, transdermal administration [12]. We illustrated the schematic structure of liposomes, emulsions, SLN, micelles, and PLGA nanoparticles in Fig. 1. Poly(ethylene glycerol) (PEG) is incorporated on the surface of most nanoparticles to maintain their integrity and stability via protecting them from degradation and metabolism by enzymes and prolong their circulation by stabilizing them against opsonization [12].
Fig. 1.
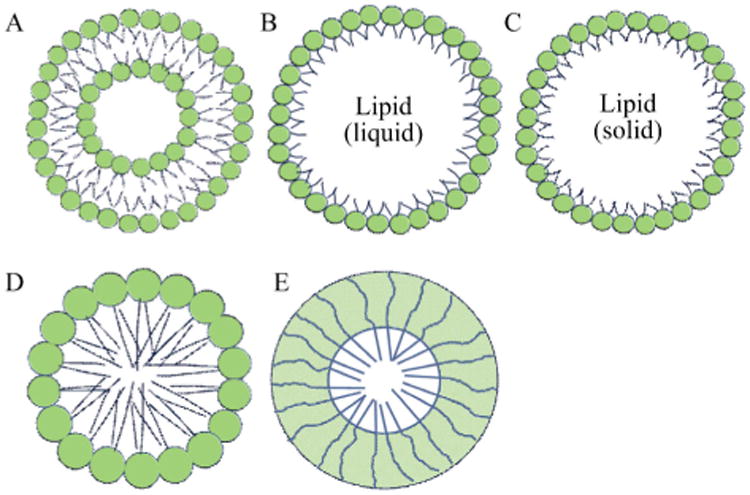
Schematic structure of commonly used biocompatible and biodegradable nanoparticles. A, Liposome; B, Emulsion; C, SLN; D, Micelle; E, Polymeric micelle
Even though those nanoparticles are biocompatible and biodegradable, their toxicity and side effects should be measured. Especially, when the loading capacity and encapsulation efficiency of phytochemicals are low, a large amount of nanoparticles are administered to cells or animals [12]. Cytotoxicity assays are widely used to measure toxicity of nanoparticles to cells. The widely used cytotoxicity experiments include a trypan blue exclusion assay, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) or (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) (MTS) assay, a lactate dehydrogenase (LDH), a sulforhodamine B (SRB) assay and so on. In animal studies, both short- and long-term toxicity and side effects should be measured. The commonly used measurement methods are body weight, blood chemistry test, complete necropsies including full gross and microscopic evaluation of all organ-systems, histological examination and evaluation of all organs and tissues and so on. Currently, an emerging need is to set up standardized in vitro and in vivo models, safety test measures and guidelines to determine toxicity and side effects of nanoparticles [12].
Liposomes
Liposomes are particles having mono- or multi-bilayer of phospholipids structures [13]. Phospholipid is a molecule that has a hydrophilic head and two hydrophobic fatty acid tails. The head group on the surface of liposomes is attracted to water, and the fatty acid tails are repelled by water [12]. Cholesterol is another compound used in liposomes for enhancing liposome physical characteristics [14]. After phytochemicals are loaded into liposomes, their aqueous solubility, stability and circulation time can be enhanced, and their toxicity and side effects can be lowered [15]. Liposomes can entrap hydrophilic phytochemicals in their internal water compartment and hydrophobic phytochemicals into the membrane [16]. Liposomes can be used via oral administration, intravenous injection, subcutaneous administration and topical application [16-17].
Nanoemulsions
Emulsions are prepared by dispersing one liquid dispersed phase into the other continuous phase [12]. Oil is dispersed into water containing a surfactant or emulsifier to form oil-in-water emulsions, which have hydrophilic shells and hydrophobic cores. Nanoemulsions need high-energy input and more surfactants or co-surfactants to lower the surface tension and maintain the size less than 100 nm in diameter [18]. Oil-in-water nanoemulsions are commonly used to deliver hydrophobic phytochemicals, such as curcumin [19], quercetin [20], resveratrol [21] and genistein [22] to tumors and cancer cells. The advantages of nanoemulsions include to increase aqueous solubility of hydrophobic phytochemicals, enhance their stability and circulation time, improve their absorption and bioavailability [18, 23].
SLNs and NLCs
SLNs and NLCs are synthesized lipid particles composed of lipids, surfactants, water, maybe co-surfactants [24]. They have a hydrophilic shell and a hydrophobic lipid core. Phospholipids and surfactants are used to form the hydrophilic shell. Triglycerides, waxes, fatty acids are commonly used to form the hydrophobic lipid core. Different from emulsions, the hydrophobic lipid cores in SLNs and NLCs are solid or semi-solid. SLNs and NLCs have a perfect and imperfect lipid core structure, respectively [12]. The imperfect lipid cores of NLCs increase the loading capacity of phytochemicals [12]. Both SLNs and NLCs are colloidal carriers with an average diameter 100 nm or less [25]. SLNs are developed in the early 1990s, serve as an alternative nanocarrier system to liposomes, nanoemulsions, and polymeric nanoparticles [26-27]. NLCs developed at the end of the 1990s [28]. As a new generation of lipid nanocarriers, NLCs can avoid many limitations of SLNs including low loading capacity, high release potential, and drug expulsion during storage [29].
Micelles
Different from liposomes, micelles have a monolayer of phospholipids (or other amphiphilic monomers or polymers) and a hydrophobic lipid core [30-31]. Many amphiphilic molecules having a polar/hydrophilic group and a nonpolar/ hydrophobic group can be used to synthesize micelles [32]. Surfactants and/or co-surfactants are commonly used in preparing micelles [32]. Micelles can increase the aqueous solubility of hydrophobic phytochemicals, enhance their bioavailability, reduce adverse effects (such as toxicity), enhance permeability across the physiological barriers, and changes their biodistribution in the body [30].
PLGA nanoparticles
Synthetic polymers have many advantages including high purity and reproducibility over natural polymers [33]. The most commonly used synthetic polymer for assembling nanoparticles is PLGA. PLGA is biocompatible and biodegradable, because it yields lactic acid and glycolic acid after it undergoes hydrolysis in the body. PLGA nanoparticles have the properties of increasing drug efficacy and sustained release [34]. In particular, PLGA has been approved by FDA for human therapy [35]. PLGA nanoparticles have been used as carriers to deliver many phytochemicals such as curcumin, resveratrol, and quercetin [36-38].
Phytochemicals-loaded nanoparticles and their anti-cancer activities
In this review, we focus on some commonly consumed phytochemicals, including (–)-epigallocatechin gallate (EGCG) abundant in green tea, curcumin abundant in turmeric, quercetin abundant in red onions, resveratrol abundant in red grapes and genistein abundant in soybeans (Fig. 2), and investigate whether nanoencapsulation can enhance their characteristics and anti-cancer activities.
Fig. 2. Chemical structures of selected phytochemicals discussed in this review.
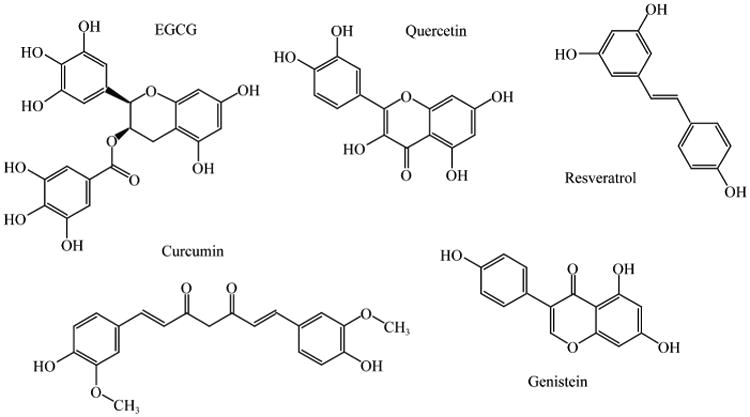
EGCG
Green tea is made from the plant, Camellia sinensis, and has been taken as a healthy drink since ancient times. There are three major types of tea (with consumption rate): fermented black tea (78%–85%), unfermented green tea (14%–20%) and partially fermented oolong tea (less than 2%) [39]. Over the past few decades, scientific studies showed green tea ingestion, not black tea, might prevent many types of cancer such as breast and prostate cancer [40]. There are four major epicatechin derivatives: EGCG, epigallocathechin (EGC) and epicathechin-3 gallate (ECG) and epicatechin (EC) [39]. EGCG accounts for 25%–55% of the total catechins [39]. Green tea contains more catechins than black or oolong tea [39]. One in vitro study screened and determined cancer prevention effects of 10 major polyphenols found in green tea including caffeic acid (CA); gallic acid (GA); EGCG; EGC; ECG; EC; catechin (C); gallocatechin (GC); catechingallate (CG); gallocatechingallate (GCG). EGCG demonstrated the highest chemopreventive potential among them [41]. Many other studies also support these results [40, 42-43]. The underlying mechanisms of anti-cancer activities of EGCG include anti-angiogenesis, apoptosis and cell cycle arrest [37-42]. EGCG significantly inhibited new blood vessels growth and further decreases tumor progression [42]. EGCG also inhibited a crucial enzyme urokinase (uPA) for the growth of a variety of different types of tumors [40]. Moreover, EGCG induced cancer cell apoptosis and cell cycle arrest in the G1 phase, and inhibited cancer cell proliferation [41, 44].
However, EGCG has many limitations. First, EGCG is not stable in both physiological fluids and water [45]. Second, EGCG's bioavailability was extremely low in research animals and humans [46-48]. The oral bioavailability was less than 1% reported by animal and human studies [43, 48]. The peak blood EGCG concentration was 0.15 μmol·L−1 after 2 cups of green tea ingestion [47]. Third, EGCG is quickly degraded or metabolized by enzymes in the liver and other tissues. Fourth, EGCG has a low level of target specificity to cancer cells or tumors. Hence, there is a critical need to overcome those problems. Many studies have demonstrated that nanoparticles can increase EGCG's stability, bioavailability and target specificity to cancer cells, and enhance its anti-cancer activities [43, 48] (Table 1). From our published articles, EGCG's stability in liposomes and NLCs was significantly increased [39, 44, 49]. Nanoparticles can also protect EGCG from premature degradation [39]. EGCG loaded in nanoparticles exhibited a sustained release manner, which lowers treatment frequency, doses and side effects. Moreover, incorporating target ligands on the surface of EGCG nanoparticles can enhance targeted delivery of EGCG to cancer cells [50]. Sanna V et al. incorporated target ligands on the surface of EGCG nanoparticles, which enable the targeted delivery of EGCG to prostate cancer cells expressing the prostate-specific membrane antigen (PSMA) [51]. One study used EGCG derivatives to make micellar nanocomplexes, which carriered and delivered herceptin to breast cancer cells. They found that these nanocomplexes effectively lowered cancer cell viability in vitro and inhibited tumor growth in vivo [52].
Table 1. The characteristics and anti-cancer activities of EGCG nanoparticles.
| Nanoparticle type | Nanoparticle size/nm | Target Yes/No | Experiment model/dose | Cancer/tumor type | Main Outcome | Year & Ref. |
|---|---|---|---|---|---|---|
| Micellar nanocomplexes (comprising EGCG derivatives and a protein drug-Herceptin) | 90 | No | In vitro: BT-474 cells treated with nanocomplexes containing Herceptin: oligomerized EGCG: PEG– EGCG = 0.5: 0.024: 0.26 mg·mL−1; In vivo: Athymic Nude-Foxn1nu female mice inoculated with BT-474 cells; intravenous injection of the nanocomplexes twice per week for five weeks | Breast cancer | ↑ Anti-cancer effects in vitro and in vivo ↑ Better tumor selectivity ↓ Tumor growth ↑ Herceptin circulation time |
2014[52] |
| Chitosan nanoparticles | N/A | No |
In vitro: human melanoma cells treated with 0.5–8 μmol·L−1 of free EGCG or nano-EGCG for 48 h In vivo: Athymic (nu/nu) male nude mice treated with 1XPBS, 1 mg EGCG, 100 μg EGCG, 100 μg nano-EGCG five times a week for 31 days via oral administration. |
Human melanoma | ↑ Dose advantage ↑ Mel 928 cells apoptosis ↓ The growth of Mel 928 tumor xenograft ↓ Proliferation (Ki-67 and PCNA) ↑ Apoptosis in tumors harvested |
2014[53] |
| Nanoliposomes; Chitosan coated nanoliposomes | 55-85 | No | MCF-7 cells treated with 0–10 μmol·L −1 of free EGCG or nano-EGCG | Breast cancer | ↓ Breast cancer MCF-7 cell viability ↑ MCF-7 cell apoptosis |
2013[44] |
| Chitosan nanoparticles | <200 | No | In vivo: 22Rν1 tumor xenografts nude mice treated with void chitosan nanoparticles (control group), 40 mg/kg BW of free EGCG group, 3 mg/kg BW of Chitosan-nanoEGCG or 6 mg/kg BW of chitosan-nanoEGCG via oral intubation five times per week for 60 days | Prostate cancer | ↓ Tumor growth ↑ Apoptosis |
2013[54] |
| PLGA–PEG nanoparticles | Around 80 | Yes (PSMA-targeting ligands) | Prostate cancer (LNCaP) cells treated with 30 μmol·L−1 of free EGCG, targeted or non-targeted nano-EGCG | Prostate cancer | ↓ Viability of LNCaP cells | 2011[51] |
| Gum Arabic and maltodextrin nanoparticles | Around 100 | No | Human prostate carcinoma Du145 cells treated with 0–10 μmol L−1 of free EGCG or nano-EGCG | Prostate cancer | ↑ EGCG anti-cancer activity | 2011[55] |
BW, body weight; N/A, not applicable; PEG, poly(ethylene glycerol); PLGA, poly (lactide-co-glycolide); PMSA, prostate-specific membrane antigen; ↑, increase; ↓, decrease
Hence, nanoparticles can increase EGCG stability and bioavailability, enhance its sustained release and targeted delivery of EGCG to cancer cells, which may open a new door for cancer prevention and treatment.
Curcumin
Curcumin is a hydrophobic polyphenol component abundant in the spice turmeric of ground rhizome of the herb Curcuma longa [56-57]. Curcumin has a potential to inhibit cancer cell proliferation, carcinogenesis, tumorigenic, and angiogenesis, hence it has been used for the prevention and treatment of many chronic diseases, especially cancer [6, 58]. However, a high level of its physical and metabolic instability and a low level of aqueous solubility of free curcumin limit its anti-cancer activities [59].
Many curcumin loaded nanoparticles have been developed to enhance its aqueous solubility, stability, bioavailability, sustained release property, targeted delivery to cancer cells and anti-cancer activities [59] (Table 2). Biocompatible and biodegradable liposomes, PLGA nanoparticles, SLNs, NLCs and micelles have been used to carry and deliver curcumin to cancer cells [66-81]. Curcumin loaded nanoparticles demonstrated a sustained release property and enhanced cellular bioavailability of curcumin, and further decreased cancer cell viability in in vitro studies [60-61]. Nano-curcumin compared to free curcumin decreased cell viability to a fold change of 1.5 in PC3 prostate cancer cells [62], a fold change of 1.5-2.5 in MCF-7 breast cancer cells [63] and a fold change of 1.2 in HepG2 hepatocellular carcinoma cells [64]. In murine models, nanoparticles increased bioavailability, peak blood concentrations and tumor distribution of curcumin, and suppressed tumor/ carcinoma growth and angiogenesis. Nano-curcumin compared to free curcumin resulted in a 50% reduction in prostate tumor growth [65] and a 42% reduction in pancreatic tumor volume [66]. Most of the studies used passive rather than targeted delivery strategies to deliver curcumin to cancer cells or tumors. Tumor development and progression depend on angiogenesis [67]. Curcumin nanoparticles can pass the leaky neovasculature and target to tumorigenic areas by the enhanced permeability and retention effects [68-69]. Targeted delivery of curcumin to cancer cells or tumors requires incorporation of target ligands on the surface of nanoparticles, which consequently maximize the distribution and accumulation of curcumin in cancer cells or tumors [9, 70].
Table 2. The characteristics and anti-cancer activities of curcumin nanoparticles.
| Nanoparticle type | Nanopart icles size /nm | Target Yes/No | Experiment model/dose | Cancer type | Main Outcome | Year & Ref. |
|---|---|---|---|---|---|---|
| Lipid–polymer nanoparticles | Around 172 | No | MDA-MB-231 breast cancer cells treated with 0-40 μmol·L -1 of free curcumin or nano-curcumin | Breast cancer | ↓ Metastasis ↓ Inflammation |
2014[72] |
| Thiolated chitosan nanoparticles | Around 150 | No | HT29 cells treated with 20 μmol·L−1 of free curcumin or nano-curcumin and Swiss Albino mice treated with 25 mg/kg BW of free curcumin or nano-curcumin | Colon cancer | ↑ Sustained release ↑ Cellular uptake ↓ Cell viability ↑ Apoptosis ↑ Anti-cancer activities ↑ Bioavailability in vivo |
2014[73] |
| PLGA nanoparticles | N/A | Yes (PSMA antibody as a target ligand) | Prostate cancer xenograft mice model and prostate cancer cells (C4-2, DU-145, PC-3) treated with free or nano-curcumin (2.5-40 μmol·L−1). PSMA antibody conjugated on the nanoparticles for targeting to cancer cells. | Prostate cancer | ↓ Proliferation of cancer cells ↓ Tumor growth ↓ Key oncogenic proteins ↑ Apoptosis ↓ Oncogenic miR21 ↑ miR-205 |
2014[65] |
| NLCs | Around 108 | No | PC3 cells treated with 20 μmol·L−1 of free or nano-curcumin | Prostate Cancer | ↑ Sustained release ↓ Cell viability ↑ Anti-cancer activities |
2013[62] |
| SLNs | Around 153 | No | MCF-7 cells treated with 5 μg·mL−1 and Male Sprague-Dawley rats (2 mg·kg−1) treated with free or nano-curcumin via intravenous administration | Breast cancer | ↑ Intracellular uptake ↑ Bioavailability of curcumin ↑ Anti-cancer activities |
2013[63] |
| SLNs | 100 | No | SMMC-7721 cells treated with 20–64 μg·mL−1 of free curcumin or nano-curcumin | Liver cancer | ↑ Sustained release ↓ Cell viability ↑ Apoptosis |
2013[74] |
| SLNs | 20–80 | No | A549 cells treated with 4-100 μmol·L−1 of free curcumin or nano-curcumin. Nude mice bearing A549 cells xenografts treated with 200 mg/kg BW of free curcumin or nano-curcumin via intraperitoneal injection | Lung cancer | ↑ Stability of curcumin ↓ Growth of lung cancer cells ↑ Apoptosis of A549 lung cancer cells ↑ Blood plasma of curcumin in vivo ↑ Tumor distribution of curcumin ↓ Tumor volume |
2013[75] |
| Liposomes | Around 100 | No | Human MiaPaCa pancreatic cancer cells treated with 0–30 μmol·L−1 of free curcumin or nano-curcumin and in the pancreatic tumor xenograft study (20 mg/kg BW). | Pancreatic cancer | ↓ Cell viability ↓ Tumor growth ↓ Angiogenesis |
2013[66] |
| PLGA nanoparticles | Around 130 | Yes (APgp as a target ligand) | Multidrug resistant (KB-V1) and drug sensitive (KB-3-1) cervical carcinoma cells treated with 5–30 μmol·L−1 of free curcumin or nano-curcumin | Cervical cancer | ↑ Targeting to cancer cells ↑ Cellular uptake ↓ Cell viability |
2012[76] |
| PLGA nanoparticles | 120–190 | No | HeLa cells treated with 5–25 μmol·L−1 of free curcumin or nano-curcumin | Liver cancer | ↑ Aqueous solubility and sustained release ↑ Cellular uptake ↑ Anti-cancer efficacy |
2012[77] |
| MPEG-PCL micelles | Around 27 | No | BALB/c mice were given curcumin/ MPEG-PCL micelle (25 mg/kg BW curcumin) via intravenously injection | Colon cancer | ↓ Tumor cell-induced angiogenesis ↓ Growth of colon carcinoma |
2011[78] |
Anti-P-glycoprotein, APgp; BW, body weight; MPEG-PCL, monomethoxy poly(ethylene glycol)-poly(3-caprolactone); N/A, not applicable; NLCs, nanostructured lipid carriers; PSMA, prostate specific membrane antigen; PEG, poly(ethylene glycerol); PLGA, (poly lactide-co-glycolide); SLNs, solid lipid nanoparticles; ↑, increase; ↓, decrease.
Taken together, many studies have suggested that nanoparticles (liposomes, SLNs, NLCs and PLGA nanoparticles) can improve characteristics of curcumin including solubility, stability and bioavailability, and its anti-cancer activities, and might be a good strategy for cancer prevention and treatment [18, 59, 71].
Quercetin
Quercetin (3,3′,4′,5′-7-pentahydroxy flavone) is a polyphenolic compound found in onion, apple, berries, tea and brassica vegetables, as well as many nuts, seeds, barks, flowers and leaves [79]. The underlying mechanisms of quercetin as a potential natural anti-cancer agent include apoptosis induction, suppression of proliferation and metastasis [80]. Anti-proliferative activities of quercetin have been demonstrated in breast [81], leukemia [82], colon [83], squamous cell [84], endometrial [85], gastric [86] and non-small cell lung [87] cancer cells. Despite its promising anti-cancer activities, the clinical application of quercetin in cancer treatment is restricted due to its low level of aqueous solubility and tumor-targeting specificity [47].
Many quercetin-loaded nanoparticles have been developed to increase the bioavailability and biopotency of quercetin to improve its anti-cancer activities [95-99] (Table 3). The quercetin loaded nanoliposomes enhanced the cytotoxic effects on C6 glioma cells and induced necrotic death of those cells [88]. Rezaei-Sadabady et al reported that liposomes significantly improved aqueous solubility and bioavailability of quercetin [89]. Its antioxidant capacity and effectiveness for removing reactive oxygen species (ROS) was increased and the cellular uptake by human MCF-7 breast cancer cells was enhanced when encapsulating quercetin in a liposomal delivery system [90]. Nano-quercetin compared to free quercetin significantly decreased the viability of A549 lung cancer cells in vitro. Nano-quercetin and free quercetin at 100 μmol·L−1 decreased the cell viability of A549 lung cancer cells by 60% and 100%, respectively [98]. In vivo anti-cancer efficacy of nanomicellar quercetin was evaluated in human A549 lung tumor xenograft mice received 30 mg/kg body weight of free or nanomicellar quercetin via oral gavage three times per week for three weeks [91]. At the end of this study, nanomicellar quercetin had more than 1.5-fold higher tumor growth inhibition than free quercetin. Importantly, nanomicellar quercetin treatment did not result in weight loss [91]. Chemically modified polymeric nanocapsules as quercetin carriers were described and characterized for the passive and active targeting to cancer cells and tumors [92]. The active targeting to HeLa cells or mice IGROV-1 tumor expressing folate receptors was achieved by conjugating folic acid to PLGA utilizing PEG as a spacer in polymeric nanocapsules [92]. Biocompatible quercetin encapsulated NLCs (Q-NLCs), which consist of natural lipid, vitamin E acetate, surfactant and free quercetin, were successfully synthesized in our group by using a novel phase inversion-based process method [89]. The aqueous solubility of quercetin was improved more than 1 000 times when using NLCs as carriers for quercetin. Compared to free quercetin and void NLCs, Q-NLCs significantly enhanced cytotoxicity and apoptosis of MCF-7 and MDA-MB-231 breast cancer cells, and increased cellular uptake of quercetin by those cells. Importantly, void NLCs and phosphate buffered saline treatments showed similar low cytotoxicity to those cells [89].
Table 3. The characteristics and anti-cancer activities of quercetin nanoparticles.
| Nanoparticle type | Nanoparticles size/nm | Target Yes/No | Experiment model/dose | Cancer type | Main Outcome | Year & Ref. |
|---|---|---|---|---|---|---|
| Folic acid-PEG-PLGA | 150 | Yes (Folic acid as a target ligand) | In vitro: HeLa Cells; In vivo intravenous administration in HeLa or IGROV-1 tumor-bearing mice | Cervical and ovarian cancer | ↑Anti-cancer activities ↑Target specificity to cancer cells and tumors |
2014[92] |
| NLCs | Around 30 | No | MCF-7 and MDA-MB-231 breast cancer cells treated with 0–50 μmol·L−1 of free quercetin or nano-quercetin | Breast cancer | ↑Apoptosis ↑Aqueous solubility improved by 1 000 folds ↓Viability of cancer cells |
2014[90] |
| Liposomes | Around 100 | No | MCF-7 breast cancer cells treated with 0–50 μmol·L−1 of free quercetin or nano-quercetin | Breast cancer | ↑Cellular uptake ↑Antioxidant activities |
2014[89] |
| Nanoliposomes | 62–192 | No | C6 glioma cell treated with 0–400 μmol·L−1 of free quercetin or nano-quercetin | C6 glioma cells | ↓Viability of C6 glioma cells ↑Necrotic cell death |
2012[88] |
| Nanomicelles | Around 16 | No | Lung tumor mice were given 30 mg/kg BW of free quercetin or nano-quercetin via oral gavage 3 times per week for 3 weeks | Lung cancer | ↑Stability ↓Viability of cancer cells ↓Tumor size |
2012[91] |
BW, body weight; NLCs, Nanostructured lipid carriers; PEG, poly(ethylene glycerol); PLGA, poly (lactide-co-glycolide); ↑, increase; ↓, decrease.
In summary, nanotechnology may overcome many characteristic limitations of quercetin and enhance its anti-cancer activities.
Resveratrol
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a natural polyphenolic compound produced by the enzyme stilbene synthase in response to environmental stress like sunlight, heavy metals, fungal infection, injuries or UV irradiation [93], and acts as a natural inhibitor of cell proliferation [94]. Resveratrol is abundant in grapes, red wine, raspberries, mulberries, blueberries and knotweed. Resveratrol has two isomeric forms: cis- and trans-resveratrol, which can convert to each other by yeast during fermentation or UV irradiation. One gram of fresh grape skin contains about 50 to 100 μg of trans-resveratrol, which contributes to high resveratrol concentrations in red wine and grape juice [95].
Many in vitro and animal studies have demonstrated that resveratrol has anti-cancer activities [96-97]. However, the evidence is inconclusive regarding the effectiveness for cancer prevention or treatment in human studies. The major problems are its low level of bioavailability, aqueous solubility and target specificity to cancer cells. In order to overcome those limitations and to enhance anti-cancer activities, scientists have developed many biocompatible and biodegradable nanoparticles including liposomes, albumin nanoparticles, SLNs, NLCs, chitosan nanoparticles and gelatin nanoparticles [98-99] (Table 4). Nano-resveratrol compared to free resveratrol resulted in higher cellular uptake of resveratrol by NCI-H460 lung cancer cells, which was associated with greater DNA damage and apoptotic incidence [100]. The underlying mechanisms for nano-resveratrol included cancer cell apoptosis include the down-regulation of Bcl-2 and NF-κB expression and the up-regulation of Bax, p53, p21 and caspase-3 expression [101]. Other studies also reported that nano-resveratrol activated apoptotic pathways in human lung A549/cDDP cancer cells [99], ovarian carcinoma cells [102], MCF-7 breast cancer cells [103] and PC-3, DU-145, and LNCaP prostate cancer cells [104]. Nano-resveratrol compared to free resveratrol also significantly decreased prostatic cancer incidence [98] and colon cancer growth [105] in animal studies.
Table 4. The characteristics and anti-cancer activities of resveratrol nanoparticles.
| Nanoparticle type | Nanoparticles size/nm | Target Yes/No | Experiment model/dose | Cancer type | Main Outcome | Year & Ref. |
|---|---|---|---|---|---|---|
| Gelatin nanoparticles | 294 | No | NCI-H460 cells treated with 5–10 mg·mL−1 of free resveratrol or nano-resveratrol | Lung cancer | ↑Cell cycle arrest ↑Anti-cancer efficacy |
2015[101] |
| PEG–PLA nanoparticles | 120–260 | No |
In vitro: CT26 colon cancer cells treated with 20 and 40 μmol·L−1 of free resveratrol or nano- resveratrol In vivo: CT26 tumor bearing mice treated with 100 mg/kg BW of void nanoparticles and nano- resveratrol twice per week for 3 weeks |
Colon cancer | ↓CT26 colon cancer cell number ↑Apoptotic cell death ↓Tumor size |
2015[105] |
| SLNs | Around 250 | No | Female Wistar rats treated with 5 mg/kg BW of free resveratrol and nano-resveratrol through intraperitoneal injection | Brain cancer | ↑Brain resveratrol concentrations | 2014[107] |
| Chitosan nanoparticles | 200–300 | Yes (avidin and biotin used as target ligands) |
In vitro: HepG2 cells treated with 0–6 μg·mL−1 of free resveratrol or nano-resveratrol In vivo: Kunming mice treated with 0.25 mg/kg BW of free resveratrol or nano-resveratrol via tail vein injection |
Hepatic carcinoma | ↑Target specificity to liver ↑Anti-cancer activity ↑Cytotoxicity |
2013[106] |
| Bovine serum albumin nanoparticles | N/A | No | The tumor-bearing mice treated with 50-200 mg/kg BW of free resveratrol or nano- resveratrol once a week for 4 weeks | Ovarian cancer | ↓Tumor growth ↑Apoptotic and necrotic characteristics in the tumor tissues |
2010[102] |
BW, body weight; N/A, not applicable; PEG, poly(ethylene glycerol); PLA, poly (D,L-lactide); SLNs, solid lipid nanoparticles; ↑, increase; ↓, decrease.
Bu L et al modified the surface of nanoparticles by two ligands, avidin (A) and biotin (B) to make targeted nanoparticles to enhance target specificity of resveratrol-loaded chitosan nanoparticles to hepatic carcinoma [106]. Targeted compared to non-targeted resveratrol loaded nanoparticles had the higher liver targeting index and more potent cytotoxicity against HepG2 cells.
Overall, biocompatible and biodegradable nanoparticles can enhance aqueous solubility, stability and bioavailability of resveratrol and increase its anti-cancer activities.
Genistein
Genistein (4,5,7-trihydroxyisoflavone, GEN) has been identified as the main isoflavone found in soybeans enriched foods. Genistein intake is high in some Asian countries, especially Japan and China [108]. Some studies have demonstrated that GEN has anti-cancer activities [108-110]. However, only several papers investigated anti-cancer properties of GEN nanoparticles (GEN-NP).
In Table 5, Mendes et al [111] treated Ehrlich Ascites Tumor (EAT) bearing Swiss mice using multicompartimental nanoparticles containing paclitaxel (PTX) and GEN, and found 0.2 mg/kg body weight/day of PTX resulted in 11% of tumor inhibition, but 12 mg/kg body weight/day of GEN caused 44% of tumor inhibition. De Zampieri et al [112] found that GEN-nanoparticles resulted in a higher amount of GEN accumulation in deeper layers of the skin and GEN-nanoparticles might be a promising nanocarrier system for skin delivery of GEN and skin cancer prevention and treatment. Other studies have demonstrated that nano-GEN demonstrated a sustained release manner, increased GEN uptake by cancer cells and enhanced anti-cancer activities of GEN in different cancer cells [113, 114].
Table 5. The characteristics and anti-cancer activities of genistein (GEN) nanoparticles.
| Nanoparticle Type | Nanoparticle size/nm | Target Yes/No | Experiment Model/Dose | Cancer Type | Main Outcome | Year & Ref. |
|---|---|---|---|---|---|---|
| Polymeric nanocapsules | Around 160 | No | Ehrlich Ascites Tumor (EAT)-bearing mice treated with free GEN or nano-GEN | Ehrlich Ascites Tumor | ↑ Tumor inhibition | 2014[111] |
| PLA nanoparticles | Around 140 | No | Franz-type diffusion cells with porcine ear skin treated with free GEN or nano-GEN | Skin Cancer | ↑ Skin delivery of GEN. | 2013[112] |
| NLCs | Around 110 | No | Prostate cancer cells treated with nano-GEN | Prostate Cancer | ↑ Sustained release ↓ Cell viability |
2013[62] |
| Liposomes | Around 161 | No | PC-3 and OVCAR-3 cancer cells treated with 0–80 μmol·L−1 of free GEN or nano-GEN | Ovarian and Prostate Carcinomas | ↑ Cellular delivery ↑ Cytotoxicity ↑ Apoptosis |
2013[113] |
| Lipidic micelles and nanoemulsions | 20-200 | Target | CT26 and HepG2 cells treated with 0-400 μmol·L−1 of free GEN or nano-GEN | Hepatic and Colon Carcinomas | ↑ Cytotoxicity ↑ Anti-cancer activities |
2013[114] |
NLCs, Nanostructured lipid carriers; PLA, poly (D,L-lactide); ↑, increase; ↓, decrease.
Future perspectives
Although nanoparticles can enhance anti-cancer activities of phytochemicals reported in many in vitro and in vivo studies, there are still some concerns regarding their cost, safety, side-effects and long-term toxicity. Hence, a new subdiscipline of nanotechnology called nanotoxicology has emerged [18, 115-116]. Even though the oral administration route is preferred [18], most nanoencapsulated phytochemicals, especially tumor-targeting nanoparticles, are delivered to animals mainly by intravenous, subcutaneous, intraperitoneal administration. Due to gastrointestinal digestion and degradation, developing nano-delivery systems for the oral administration of phytochemicals remains challenging [115]. Improving cancer cell- or tumor-targeting efficiency and specificity of phytochemical nanoparticles is a promising and emerging research area, because they can increase anti-cancer efficacy and effectiveness of phytochemicals, and lower their toxicity and side effects to normal cells and tissues. After finishing cell and animal studies, prospective clinical studies are needed to evaluate their anti-cancer activities and measure toxicity and side effects in humans[14, 117]. Furthermore, there is an urgent need to finalize occupational and environmental safety guidelines for synthesizing and using nanoparticles by the government [118].
Conclusions
In conclusion, liposomes, nanoemulsions, micelles, SLNs, NLCs and PLGA nanoparticles are commonly used biocompatible and biodegradable nanoparticles and used as phytochemical carriers. Nanotechnology has a great potential for improving solubility, stability, bioavailability and anti-cancer activities of EGCG, curcumin, quercetin, resveratrol and genistein. More studies are required to optimize formulations of nanoparticles for enhancing their anti-cancer effectiveness and efficacy and tumor-targeting specificity, and lowering their cost, side-effects, and toxicity.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Integrative Health or the National Institutes of Health. Additional support was provided by the Burleson's Family Foundation and College of Human Sciences, Texas Tech University, Lubbock, TX. LI Chuan received financial support from the Scholarship of Nanchang University, China. CAO Jun acknowledged financial support from the China Scholarship Council.
Research funding: This work was supported by grant from the National Center for Complementary & Integrative Health (Nos. R15AT007013 and R15AT008733).
Footnotes
These authors have no conflict of interest to declare.
References
- 1.Society A. C. American Cancer Society: Cancer Facts and Figures 2015. American Cancer Society; Atlanta, GA: 2015. [Google Scholar]
- 2.Li B, Gao MH, Chu XM, et al. The synergistic antitumor effects of all-trans retinoic acid and C-phycocyanin on the lung cancer A549 cells in vitro and in vivo. Eur J Pharmacol. 2015;749:107–114. doi: 10.1016/j.ejphar.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78(3):517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 5.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 6.Basnet P, Skalko-Basnet N. Curcumin: an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16(6):4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khushnud T, Mousa SA. Potential role of naturally derived polyphenols and their nanotechnology delivery in cancer. Mol Biotechnol. 2013;55(1):78–86. doi: 10.1007/s12033-012-9623-7. [DOI] [PubMed] [Google Scholar]
- 8.Vidya Priyadarsini R, Nagini S. Cancer chemoprevention by dietary phytochemicals: promises and pitfalls. Curr Pharm Biotechnol. 2012;13(1):125–136. doi: 10.2174/138920112798868610. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Zhang J, Chen M, et al. Delivering flavonoids into solid tumors using nanotechnologies. Expert Opin Drug Deliv. 2013;10(10):1411–1428. doi: 10.1517/17425247.2013.807795. [DOI] [PubMed] [Google Scholar]
- 10.Alexis F, Pridgen EM, Langer R, et al. Nanoparticle technologies for cancer therapy. Handb Exp Pharmacol. 2010;197:55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Zeng X, Li P. Nanomaterials in cancer-therapy drug delivery system. J Biomed Nanotech. 2013;9(5):741–750. doi: 10.1166/jbn.2013.1583. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Su R, Nie S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174(1):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 15.Mohanraj V, Chen Y. Nanoparticles-a review. Trop J Pharm Res. 2007;5(1):561–573. [Google Scholar]
- 16.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 17.Torchilin VP. Multifunctional nanocarriers. Adv Drug Del Rev. 2012;64:302–315. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Nair HB, Sung B, Yadav VR, et al. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol. 2010;80(12):1833–1843. doi: 10.1016/j.bcp.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6(3):928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 20.Pool H, Mendoza S, Xiao H, et al. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: impact of physical form on quercetin bioaccessibility. Food Funct. 2013;4(1):162–174. doi: 10.1039/c2fo30042g. [DOI] [PubMed] [Google Scholar]
- 21.Davidov-Pardo G, Mcclements DJ. Nutraceutical delivery systems: Resveratrol encapsulation in grape seed oil nanoemulsions formed by spontaneous emulsification. Food Chem. 2015;167:205–212. doi: 10.1016/j.foodchem.2014.06.082. [DOI] [PubMed] [Google Scholar]
- 22.Argenta DF, De Mattos CB, Misturini FD, et al. Factorial design applied to the optimization of lipid composition of topical antiherpetic nanoemulsions containing isoflavone genistein. Int J Nanomed. 2014;9(1):4737–4747. doi: 10.2147/IJN.S67732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anton N, Benoit JP, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates—a review. J Controlled Release. 2008;128(3):185–199. doi: 10.1016/j.jconrel.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Wissing S, Kayser O, Müller R. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Del Rev. 2004;56(9):1257–1272. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Ekambaram P, Sathali AAH, Priyanka K. Solid lipid nanoparticles: a review. Sci Rev Chem Commun. 2012;2(1):80–102. [Google Scholar]
- 26.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 27.Hou D, Xie C, Huang K, et al. The production and characteristics of solid lipid nanoparticles (SLNs) Biomaterials. 2003;24(10):1781–1785. doi: 10.1016/s0142-9612(02)00578-1. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Ng WK, Tan RB. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur J Pharm Sci. 2012;47(1):139–151. doi: 10.1016/j.ejps.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Fang JY, Fang CL, Liu CH, et al. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur J Pharm Biopharm. 2008;70(2):633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura Y. Poly (amino acid) micelle nanocarriers in preclinical and clinical studies. Adv Drug Del Rev. 2008;60(8):899–914. doi: 10.1016/j.addr.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 2010;5(3):485–505. doi: 10.2217/nnm.10.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed. 2006;17(3):247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 34.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006;27(15):3031–3037. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9(55):1–11. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalil NM, Do Nascimento TCF, Casa DM, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA–PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Sanna V, Roggio AM, Pala N, et al. Effect of chitosan concentration on PLGA microcapsules for controlled release and stability of resveratrol. Int J Biol Macromol. 2015;72:531–536. doi: 10.1016/j.ijbiomac.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Pool H, Quintanar D, De Dios Figueroa J, et al. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater. 2012;2012:86. [Google Scholar]
- 39.Zhang J, Nie S, Wang S. Nanoencapsulation enhances epigallocatechin-3-gallate stability and its antiatherogenic bioactivities in macrophages. J Agric Food Chem. 2013;61(38):9200–9209. doi: 10.1021/jf4023004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankun J, Selman SH, Swiercz R, et al. Why drinking green tea could prevent cancer. Nature. 1997;387(6633):561. doi: 10.1038/42381. [DOI] [PubMed] [Google Scholar]
- 41.Du GJ, Zhang Z, Wen XD, et al. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients. 2012;4(11):1679–1691. doi: 10.3390/nu4111679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Y, Cao R. Angiogenesis inhibited by drinking tea. Nature. 1999;398(6726):381. doi: 10.1038/18793. [DOI] [PubMed] [Google Scholar]
- 43.Lambert JD, Yang CS. Mechanisms of cancer prevention by tea constituents. J Nutr. 2003;133(10):3262S–3267S. doi: 10.1093/jn/133.10.3262S. [DOI] [PubMed] [Google Scholar]
- 44.De Pace RC, Liu X, Sun M, et al. Anticancer activities of (–)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J Liposome Res. 2013;23(3):187–196. doi: 10.3109/08982104.2013.788023. [DOI] [PubMed] [Google Scholar]
- 45.Hong J, Lu H, Meng X, et al. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (–)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62(24):7241–7246. [PubMed] [Google Scholar]
- 46.Chen L, Lee MJ, Li H, et al. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab Dispos. 1997;25(9):1045–1050. [PubMed] [Google Scholar]
- 47.Lee MJ, Maliakal P, Chen L, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (–)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1025–1032. [PubMed] [Google Scholar]
- 48.Warden BA, Smith LS, Beecher GR, et al. Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr. 2001;131(6):1731–1737. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Su R, Nie S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Sci. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 51.Sanna V, Pintus G, Roggio AM, et al. Targeted biocompatible nanoparticles for the delivery of (–)- epigallocatechin 3-gallate to prostate cancer cells. J Med Chem. 2011;54(5):1321–1332. doi: 10.1021/jm1013715. [DOI] [PubMed] [Google Scholar]
- 52.Chung JE, Tan S, Gao SJ, et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat Nanotechnol. 2014;9(11):907–912. doi: 10.1038/nnano.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siddiqui IA, Bharali DJ, Nihal M, et al. Excellent anti-proliferative and pro-apoptotic effects of (–)-epigallo-catechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine. 2014;10(8):1619–1626. doi: 10.1016/j.nano.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Khan N, Bharali DJ, Adhami VM, et al. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis. 2013;35(2):415–423. doi: 10.1093/carcin/bgt321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rocha S, Generalov R, Pereira Mdo C, et al. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine (Lond) 2011;6(1):79–87. doi: 10.2217/nnm.10.101. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Moustaid-Moussa N, Chen L, et al. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014;25(1):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 58.Helson L. Curcumin (diferuloylmethane) delivery methods: a review. BioFactors. 2013;39(1):21–26. doi: 10.1002/biof.1080. [DOI] [PubMed] [Google Scholar]
- 59.Mimeault M, Batra SK. Potential applications of curcumin and its novel synthetic analogs and nanotechnology-based formulations in cancer prevention and therapy. Chin Med. 2011;6(31):8546. doi: 10.1186/1749-8546-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anand P, Nair HB, Sung B, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79(3):330–338. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Bisht S, Feldmann G, Soni S, et al. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J Nanobiotechnol. 2007;5(3):1–18. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aditya N, Shim M, Lee I, et al. Curcumin and genistein coloaded nanostructured lipid carriers: in vitro digestion and antiprostate cancer activity. J Agric Food Chem. 2013;61(8):1878–1883. doi: 10.1021/jf305143k. [DOI] [PubMed] [Google Scholar]
- 63.Sun J, Bi C, Chan HM, et al. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf B Biointerfaces. 2013;111:367–375. doi: 10.1016/j.colsurfb.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 64.Zhou N, Zan X, Wang Z, et al. Galactosylated chitosan–polycaprolactone nanoparticles for hepatocyte-targeted delivery of curcumin. Carbohydr Polym. 2013;94(1):420–429. doi: 10.1016/j.carbpol.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Yallapu MM, Khan S, Maher DM, et al. Anti-cancer activity of curcumin loaded nanoparticles in prostate cancer. Biomaterials. 2014;35(30):8635–8648. doi: 10.1016/j.biomaterials.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranjan AP, Mukerjee A, Helson L, et al. Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: inhibition of tumor growth and angiogenesis. Anticancer Res. 2013;33(9):3603–3609. [PubMed] [Google Scholar]
- 67.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 68.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 69.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanomedicine: a road to cancer therapeutics. Curr Pharm Des. 2013;19(11):1994–2010. doi: 10.2174/138161213805289219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Master AM, Sen Gupta A. EGF receptor-targeted nanocarriers for enhanced cancer treatment. Nanomedicine. 2012;7(12):1895–1906. doi: 10.2217/nnm.12.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17(1):71–80. doi: 10.1016/j.drudis.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palange AL, Di Mascolo D, Carallo C, et al. Lipid–polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomed Nanotechnol Biol Med. 2014;10(5):991–1002. doi: 10.1016/j.nano.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anitha A, Deepa N, Chennazhi K, et al. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim Biophys Acta (BBA)-General Subjects. 2014;1840(9):2730–2743. doi: 10.1016/j.bbagen.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Zhu R, Wu X, Xiao Y, et al. Synergetic effect of SLN-curcumin and LDH-5-Fu on SMMC-7721 liver cancer cell line. Cancer Biother Radiopharm. 2013;28(8):579–587. doi: 10.1089/cbr.2012.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang P, Zhang L, Peng H, et al. The formulation and delivery of curcumin with solid lipid nanoparticles for the treatment of on non-small cell lung cancer both in vitro and in vivo. Mater Sci Eng: C. 2013;33(8):4802–4808. doi: 10.1016/j.msec.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 76.Punfa W, Yodkeeree S, Pitchakarn P, et al. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin. 2012;33(6):823–831. doi: 10.1038/aps.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nair KL, Thulasidasan AKT, Deepa G, et al. Purely aqueous PLGA nanoparticulate formulations of curcumin exhibit enhanced anticancer activity with dependence on the combination of the carrier. Int J Pharm. 2012;425(1):44–52. doi: 10.1016/j.ijpharm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Gou M, Men K, Shi H, et al. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3(4):1558–1567. doi: 10.1039/c0nr00758g. [DOI] [PubMed] [Google Scholar]
- 79.Nathiya S, Durga M, Thiyagarajan D. Quercetin, encapsulated quercetin and its application-a review. Int J Pharmacy Pharm Sci. 2014;6(10):20–26. [Google Scholar]
- 80.Gibellini L, Pinti M, Nasi M, et al. Quercetin and cancer chemoprevention. Evid Based Complement Alternat Med. 2011;591356 doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duo J, Ying GG, Wang GW, et al. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol Med Report. 2012;5(6):1453–1456. doi: 10.3892/mmr.2012.845. [DOI] [PubMed] [Google Scholar]
- 82.Niu G, Yin S, Xie S, et al. Quercetin induces apoptosis by activating caspase-3 and regulating Bcl-2 and cyclooxygenase-2 pathways in human HL-60 cells. AcBBS. 2011;43(1):30–37. doi: 10.1093/abbs/gmq107. [DOI] [PubMed] [Google Scholar]
- 83.Zhang XA, Zhang S, Yin Q, et al. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn Mag. 2015;11(42):404. doi: 10.4103/0973-1296.153096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castillo MH, Perkins E, Campbell JH, et al. The effects of the bioflavonoid quercetin on squamous cell carcinoma of head and neck origin. Am J Surg. 1989;158(4):351–355. doi: 10.1016/0002-9610(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 85.Scambia G, Ranelletti F, Panici PB, et al. Inhibitory effect of quercetin on primary ovarian and endometrial cancers and synergistic activity with cis-diamminedichloroplatinum (II) Gynecol Oncol. 1992;45(1):13–19. doi: 10.1016/0090-8258(92)90484-z. [DOI] [PubMed] [Google Scholar]
- 86.Wang P, Zhang K, Zhang Q, et al. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol In Vitro. 2012;26(2):221–228. doi: 10.1016/j.tiv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 87.Kuhar M, Sen S, Singh N. Role of mitochondria in quercetin-enhanced chemotherapeutic response in human non-small cell lung carcinoma H-520 cells. Anticancer Res. 2006;26(2A):1297–1303. [PubMed] [Google Scholar]
- 88.Wang G, Wang JJ, Yang GY, et al. Effects of quercetin nanoliposomes on C6 glioma cells through induction of type III programmed cell death. Int J Nanomed. 2012;7:271–280. doi: 10.2147/IJN.S26935. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Rezaei-Sadabady R, Eidi A, Zarghami N, et al. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif Cells Nanomed Biotechnol. 2014:1–7. doi: 10.3109/21691401.2014.926456. [DOI] [PubMed] [Google Scholar]
- 90.Sun M, Nie S, Pan X, et al. Quercetin-nanostructured lipid carriers: Characteristics and anti-breast cancer activities in vitro. Colloids Surf B Biointerfaces. 2014;113:15–24. doi: 10.1016/j.colsurfb.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 91.Tan BJ, Liu Y, Chang KL, et al. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int J Nanomed. 2012;7:651–661. doi: 10.2147/IJN.S26538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.El-Gogary RI, Rubio N, Wang JTW, et al. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano. 2014;8(2):1384–1401. doi: 10.1021/nn405155b. [DOI] [PubMed] [Google Scholar]
- 93.Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. The Plant Cell. 1995;7(7):1085. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harikumar KB, Aggarwal BB. Resveratrol: a multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7(8):1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 95.Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res. 2005;11(5):1918–1927. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- 96.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 97.Saiko P, Szakmary A, Jaeger W, et al. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res/Rev Mutat. 2008;658(1):68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Narayanan NK, Nargi D, Randolph C, et al. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer. 2009;125(1):1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 99.Wang XX, Li YB, Yao HJ, et al. The use of mitochondrial targeting resveratrol liposomes modified with a dequalinium polyethylene glycol-distearoylphosphatidyl ethanolamine conjugate to induce apoptosis in resistant lung cancer cells. Biomaterials. 2011;32(24):5673–5687. doi: 10.1016/j.biomaterials.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 100.Karthikeyan S, Prasad NR, Ganamani A, et al. Anticancer activity of resveratrol-loaded gelatin nanoparticles on NCI-H460 non-small cell lung cancer cells. Biomed Prev Nutr. 2013;3(1):64–73. [Google Scholar]
- 101.Karthikeyan S, Hoti SL, Prasad NR. Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed Pharmacother. 2015;70:274–282. doi: 10.1016/j.biopha.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 102.Guo L, Peng Y, Yao J, et al. Anticancer activity and molecular mechanism of resveratrol–Bovine serum albumin nanoparticles on subcutaneously implanted human primary ovarian carcinoma cells in Nude mice. Cancer Biother Radiopharm. 2010;25(4):471–477. doi: 10.1089/cbr.2009.0724. [DOI] [PubMed] [Google Scholar]
- 103.Vergaro V, Lvov YM, Leporatti S. Halloysite clay nanotubes for resveratrol delivery to cancer cells. Macromol Biosci. 2012;12(9):1265–1271. doi: 10.1002/mabi.201200121. [DOI] [PubMed] [Google Scholar]
- 104.Sanna V, Siddiqui IA, Sechi M, et al. Resveratrol-loaded nanoparticles based on poly (epsilon-caprolactone) and poly (d, l-lactic-co-glycolic acid)–poly (ethylene glycol) blend for prostate cancer treatment. Mol Pharm. 2013;10(10):3871–3881. doi: 10.1021/mp400342f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jung KH, Lee JH, Park JW, et al. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int J Pharm. 2015;478(1):251–257. doi: 10.1016/j.ijpharm.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 106.Bu L, Gan LC, Guo XQ, et al. Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma. Int J Pharm. 2013;452(1):355–362. doi: 10.1016/j.ijpharm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Jose S, Anju S, Cinu T, et al. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474(1):6–13. doi: 10.1016/j.ijpharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 108.Banerjee S, Li Y, Wang Z, et al. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andrade LM, De Fátima Reis C, Maione-Silva L, et al. Impact of lipid dynamic behavior on physical stability, in vitro release and skin permeation of genistein-loaded lipid nanoparticles. Eur J Pharm Biopharm. 2014;88(1):40–47. doi: 10.1016/j.ejpb.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 110.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125(3 Suppl):777S–783S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 111.Mendes LP, Gaeti MPN, De vila PHM, et al. Multicompartimental nanoparticles for co-encapsulation and multimodal drug delivery to tumor cells and neovasculature. Pharm Res. 2014;31(5):1106–1119. doi: 10.1007/s11095-013-1234-x. [DOI] [PubMed] [Google Scholar]
- 112.De Zampieri ALTC, Ferreira FS, Resende C, et al. Biodegradable polymeric nanocapsules based on poly (DL-lactide) for genistein topical delivery: obtention, characterization and skin permeation studies. J Biomed Nanotechnol. 2013;9(3):527–534. doi: 10.1166/jbn.2013.1555. [DOI] [PubMed] [Google Scholar]
- 113.Phan V, Walters J, Brownlow B, et al. Enhanced cytotoxicity of optimized liposomal genistein via specific induction of apoptosis in breast, ovarian and prostate carcinomas. J Drug Targeting. 2013;21(10):1001–1011. doi: 10.3109/1061186X.2013.847099. [DOI] [PubMed] [Google Scholar]
- 114.Pham J, Brownlow B, Elbayoumi T. Mitochondria-specific pro-apoptotic activity of genistein lipidic nanocarriers. Mol Pharm. 2013;10(10):3789–3800. doi: 10.1021/mp4004892. [DOI] [PubMed] [Google Scholar]
- 115.Santos IS, Ponte BM, Boonme P, et al. Nanoencapsulation of polyphenols for protective effect against colon–rectal cancer. Biotechnol Adv. 2013;31(5):514–523. doi: 10.1016/j.biotechadv.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 116.Hu CMJ, Zhang L. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem Pharmacol. 2012;83(8):1104–1111. doi: 10.1016/j.bcp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Sanna V, Siddiqui IA, Sechi M, et al. Nanoformulation of natural products for prevention and therapy of prostate cancer. Cancer Lett. 2013;334(1):142–151. doi: 10.1016/j.canlet.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed Nanotechnol Biol Med. 2012;8(2):147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]


