Abstract
Objective
Increase in kidney IGF-I levels due to its increased trapping from the circulation was hypothesized to be a key mediator of compensatory renal enlargement. We tested this hypothesis using genetically engineered mice with extremely low circulating IGF-I levels.
Design
Both IGF-I deficient (ID) and normal (N) mice underwent a uninephrectomy (UNx) and sacrificed 2 or 9 days later.
Results
Initial body weight (BW) and kidney weight (KW) were significantly reduced in ID vs. N mice, while KW/BW ratios were similar. KW increased post-UNx to a comparable extent in ID and N mice (125±4 and 118±6% of pre-UNx KW, p<0.05 vs. C). Kidney IGF-I mRNA levels were similar in the ID and N mice and did not change post-UNx. Kidney IGF-I peptide levels pre-UNx were significantly lower in ID vs. N mice (25±5 vs. 305±39 ng/g) and increased in both groups after UNx, remaining low in ID mice (45±4 in ID vs 561±64 ng/g in N). IGF type 1 receptor phosphorylation was unchanged.
Conclusion
While a severe deficiency of circulating IGF-I impairs body growth, UNx induces a significant and proportional increase in renal mass in ID mice despite markedly decreased kidney IGF-I levels (>90% reduction) and no significant change in receptor phosphorylation. This all suggests that factors other than circulating and locally produced IGF-I are responsible for compensatory renal enlargement.
Keywords: Insulin-like growth factor I, Mice, Gene disruption, Knockout, Renal hypertrophy, Uninephrectomy, Models, Animal
1. Introduction
Human [1] and experimental data show that after the resection of any section of viable kidney tissue, the non-affected kidney will undergo enlargement to compensate for this loss of tissue. This process of original adaptation may lead with time to gradual deterioration in function of the remaining kidney [2]. Several growth factors have been implicated in this process of compensatory renal enlargement (CRE) [3] and depending on the age, gender and species CRE involve cellular hyperplasia, hypertrophy or both [4]. Insulin-like growth factor I (IGF-I), a key mediator of normal growth and regeneration, has been suggested as a main growth factor involved in this process [5]. For example: kidney IGF-I peptide levels increase early after uninephrectomy (UNx) in rats [6]. In addition, administering an IGF-I receptor antagonist prevents CRG [7]. Surprisingly, most studies fail to show an increase in IGF-I mRNA levels to account for the increase in kidney IGF-I peptide in CRG. Perhaps the increase in kidney IGF-I peptide is due to an accumulation of its circulating fraction trapped by local IGF binding proteins. It has been proposed that the trapped IGF-I in turn stimulates renal growth. Recently, genetically engineered mice with a liver specific deletion of the IGF-I gene and global deletion of its main binding protein, the acid labile subunit (ALS) gene have been developed by co-author Yakar et al. [8]. These mice have a major decrease in circulating IGF-I because of the combined lack of liver IGF-I production and a decrease in the stability of the circulating IGF-I derived from nonhepatic sources due to disruption of the Als gene. Body growth of these mice is significantly impaired, as measured; body weight, body length and bone length are reduced. Proximal tibial growth plates are smaller in height and restored with IGF-I treatment. In this study using this mouse strain, our aim was to determine, whether circulating IGF-I is required for CRG. We hypothesized that CRG post Unx will be attenuated in IGF-I deficient mice.
2. Materials and methods
2.1. Experimental animals and protocol
The experimental protocol was approved by the Local Institutional Animal Care and Use Committee. Fifty-four male mice were studied, 6–8 weeks old, of 2 strains: a) Control mice (Normal or “N”) homozygous for the IGF-I floxed allele that have an intact acid labile subunit (ALS) gene but do not have an Alb-Cre transgene; and b) Growth retarded, IGF-I deficient (“ID”) knockout mice generated by liver-specific deletion of IGF-I and global deletion of the acid labile subunit [8]. These mice carry a mixed genetic background, including: FVB/N, C57BL, 129Sv and BALB/c. The 2 strains were divided into 2 groups each, which were subjected to a uninephrectomy (UNx) with the contralateral kidney serving as a baseline control and then studied 2 or 9 days later. The number of mice in each group was as follows; group1, Normal 2 day post-UNx (n=13). Group 2, ID 2 day post-UNx (n =17). Group 3, Normal 9 day post-UNx (n=11). Group 4, ID 9 day post-UNx (N=13). Animals had free access to water and chow (Harlan Teklad Global mice chow #2018, with 18% protein content and <1% salt) and were kept on a 12:12 light: dark cycle. Under anesthesia (an IP ketamine/xylazine cocktail of 120 mg/kg and 12 mg/kg respectively), the surgical site was prepared with 75% alcohol and iodine and a left side UNx was performed. The left kidney was excised, weighed, frozen in liquid nitrogen, and stored at −80 °C to later serve as a control. Mice were then sutured and monitored daily. Two or nine days later, the right kidney was removed and the animals were sacrificed. Blood was collected by capillary tube via the orbital venous sinus, centrifuged immediately, and the serum was stored at −80 °C.
2.2. Biochemistry
Blood serum and kidney peptide IGF-I levels were analyzed using the IDS Mouse IGF-I HS ELISA Kit #AC-42F1 (Immunodiagnostic Systems Limited, Fountain Hills, AZ, USA), following the manufacturer’s instructions.
2.3. Real-time quantitative RT-PCR assay
Kidney tissue was homogenized, processed for total RNA extraction, and used for cDNA synthesis. Real-time quantitative reverse transcription polymerase chain reaction (Q-PCR) was performed by standard methods, using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) and its protocols. Primers for the internal control genes 18S and L7 and target gene IGF-I were designed with Primer Express (Applied Biosystems, Foster City, CA, USA) and synthesized by Operon Biotechnology (Huntsville, AL, USA). The IGF-I primer sequences were: Forward (5′-3′): TGGAYGCTCTTCAGTTCGTGTGTGGA; Reverse (5′-3′): GATCACAGCTCCGGAACGARCACTCA. Results were determined via the relative standard curve method, whereas the target gene is normalized by the endogenous internal control gene standard curve. Samples were analyzed in triplicate and the assay performed on more than one occasion to verify reproducibility.
2.4. Western immunoblotting
The IGF-1R antibody was from Santa Cruz Biotechnology (Santa Cruz, CA) and the antibody that detects both the phosphorylated IGF-type 1 receptor (Tyr1131) and the insulin receptor (Tyr1146) were from Cell Signaling Technology, Inc (Danvers, MA). Lysates were prepared from frozen kidney and used for Western immunoblots analysis as described before [9].
2.5. Data analysis and statistics
The left kidneys served as baseline comparisons for CRG of the right kidney. N left kidney values were often assigned a value of 100 and N right kidney and ID left and right kidneys were expressed relative to this value. Steady state mRNA levels were expressed relative to a housekeeping gene 18S RNA or L7. Results were depicted as mean± SEM. Two-tailed unpaired Student’s t-tests were used to compare N and ID values on the same day, whereas paired t-tests compared data within the strains. A one-way analysis of variance was implemented for comparisons among all days and strains followed by pair wise multiple comparisons using the Tukey post-hoc test. A p-value of <0.05 was considered to be statistically significant. The SPSS statistical software package (SPSS, Inc., Chicago, IL, USA) served for the statistical analyses.
3. Results
3.1. Compensatory renal enlargement is comparable between strains
Initial N and ID mice body weights (21±0.6 vs. 14±0.5 g), kidney weights (KW) (139±6 vs. 96±4 mg), and serum IGF-I levels (465±16 vs. 17±1 ng/ml) were significantly reduced in ID vs. N mice (Fig. 1a–c), while KW/body weight ratios (KW/BW) were similar (6.7% vs. 6.7%). The reduced body and kidney weight of the ID mice are consistent with the growth retarded phenotype as described by Yakar et al. [8]. Two days post-UNx, KW taken to reflect renal mass, increased significantly by an average of 8 mg in ID mice, while kidneys in N mice increased by 19 mg (p<0.05). Though the absolute increase was lower in ID mice, fractional increase did not differ between groups (15±5% for N mice vs. 9±3% for ID; p=0.25) (Fig. 2a). Indeed, post-UNx KW/BW ratios increased significantly vs. basal pre-UNx values but were comparable between N and ID mice (7.7±0.1 and 7.7±0.3% at day 2 post UNX and 0.3±8.2 and 8.9±0.3% at day 9 post UNx in N and ID respectively, vs. 6.7±0.1% in pre-UNx groups) (Fig. 2b). Similar results were seen in the day 9 study for N vs. ID, where initial absolute BW (22+0.7 vs. 14+0.7 g) and KW (149+8 vs. 102+8 mg) were lower in ID mice but the KW/BW ratios remained similar between strains (6.9% vs. 7.4%, p=0.15). Nine days post-UNx, KW had increased by an average of 25 mg in N mice and 23 mg in ID, a much larger extent than in the 2 day study. However, as in the day 2 study, the fractional increase was comparable between N and ID mice (18±6% and 25±4%; p=0.31) (Fig. 2a) and post-UNx KW/BW ratios were similar between the strains as well (8.2+0.3 and 8.9+0.3%) (Fig. 2b) and significantly increased above basal pre-UNX values. Kidney dry/wet ratios obtained by dehydrating kidneys in an oven at 60 °C to constant weight and measured pre-UNx and 9 days post UNx, did not change significantly in the N or ID mice (0.24±0.01 vs. 0.26±0.01 in N and 0.22±0.01 vs. 0.23±0.1 in ID). This excludes an increase in water content as an increase in kidney weight at this time.
Fig. 1.

(a) Body weight, (b) Pre-UNx (left) kidney weight, and (c) serum IGF-I levels are severely reduced in serum IGF-deficient (ID) mice compared to normal control (N) mice. * p<0.001.
Fig. 2.
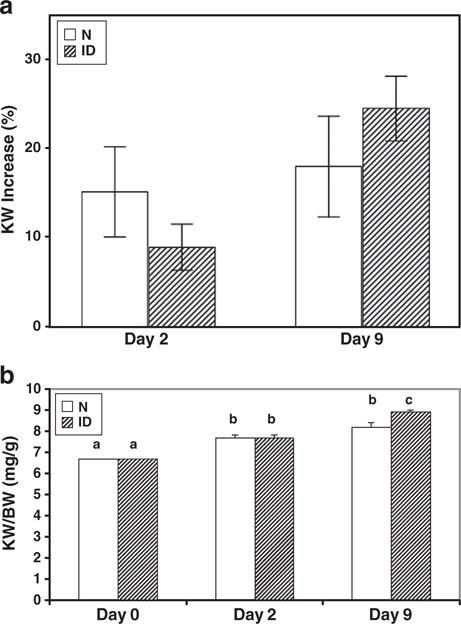
Two days post-UNx, KW increased significantly vs pre-UNx values (2b): there was an average increase of 8 and 19 mg in ID and N mice respectively (p<0.05). However, the percent of KW increase (a) and KW/BW ratio (mg/g) (b) were similar between strains, 2 and 9 days following UNx. Values with a non-identical letters above bars are different (p<0.05). Values with common letters do not differ from each other.
3.2. Kidney IGF-I mRNA levels remain unchanged
Pre-UNx kidney mRNA IGF-I results for the N mice were assigned a value of 100 and N post-UNx, ID pre-UNx, and ID post-UNx IGF-I mRNA kidney data were expressed relative to this value. Pre-UNx, ID mRNA IGF-I levels were 75±8% of N pre-UNx values (p=NS). Two days after UNx, N and ID IGF-I mRNA levels dropped, but the trend was not significant (Fig. 3a). The same trend was seen for the 9 day study (Fig. 3b), with no significant differences between the strains pre-or post-UNx.
Fig. 3.
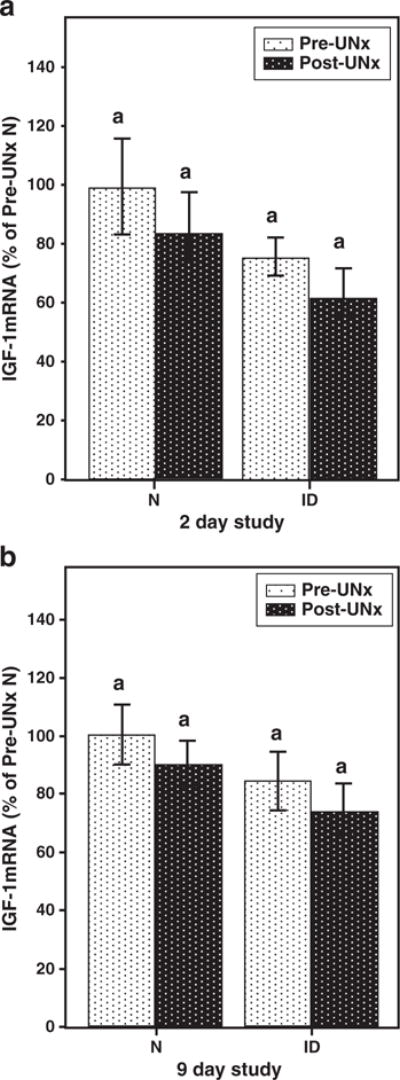
Kidney IGF-I mRNA levels (depicted as percentage of pre-UNx N group) were similar in N and ID mice and did not change following UNx. Values with common letters above bars do not differ from each other.
3.3. Kidney IGF-I peptide levels post-UNX
Initial kidney IGF-I peptide levels were markedly lower in ID vs. N mice (25±5 vs. 305±39 ng/g tissue, respectively) (Fig. 4a). Following 2 days of CRG, the hypertrophied kidney IGF-I peptide levels nearly doubled in both ID and N groups, to 45±4 and 561±64 ng/g, respectively (p<0.01). These results show that even though kidney IGF-I was statistically increased in both groups, the absolute renal IGF-I peptide concentration in the ID was very low. A similar trend was seen on Day 9 (Fig. 4b). In light of the unchanged mRNA levels noted above, the increase in kidney IGF-I peptide levels is consistent with an increase in local trapping of circulating IGF-I. The very small absolute increase in IGF-I suggests that factors other than IGF-I are major mediators of CRG.
Fig. 4.

(a) Two days following UNx, kidney IGF-I peptide concentration increased by 256 ng/g in N mice and by 20 ng/g in ID mice. (b) A similar trend was seen on Day 9. * p<0.05 when Post- vs. pre-UNx are compared. # p<0.05 when N vs. ID are compared.
3.4. Kidney IGF-I receptor levels and phosphorylation are unchanged 2 days post-UNX in ID mice
To assess whether the small increase in IGF-I peptide observed in the ID mice might be playing a role in CRG we measured levels of total and phosphorylated fraction of the IGF-I/insulin receptor. Phosphorylation of both receptors was measured as the only available antibody does not distinguish between the phosphorylated IGF-I and insulin receptor. As shown in Fig. 5, the levels of IGF-I receptor protein and phosphorylated IGF-I/insulin receptor protein in hypertrophying kidneys of the ID mice two days after the removal of the contralateral kidney, did not differ from the levels in the pre-UNx kidneys. Similar results were obtained in the N mice despite the large increase in IGF-I peptide levels noted earlier.
Fig. 5.
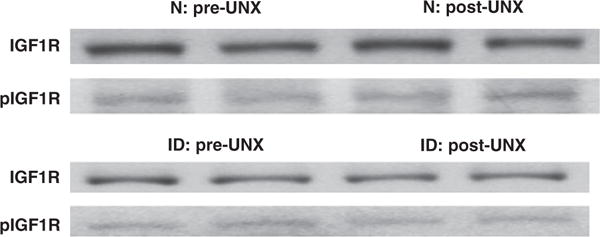
Kidney IGF type 1 receptor (IGF1R) (total and phosphorylated fraction). Representative Western immunoblots of normal (N) (upper panel) and IGF-I deficient (ID) (lower panel) mice, pre-and 2 days post-UNx. Levels of IGF1R protein (MW ~90 KD) and its phosphorylated fraction (pIGF1R) are unaltered. Kidney lysates were subjected directly to Western immunoblot analysis, first with an anti-phosphorylated IGF-I/Insulin receptor antibody, then stripped and reblotted with an anti IGF-I receptor antibody.
4. Discussion
It is now well established that circulating IGF-I plays a key role in mediating linear body growth and skeletal development [8]. Mice with circulating IGF-I levels reduced to less than 10% of normal because of a liver specific deletion of the IGF-I gene and global deletion of the ALS gene are diminished in body weight and body and bone length and density. In this study we not only confirmed that body weight is reduced in these ID mice, but also noted a proportional reduction in kidney mass. Furthermore, in addition to confirming a marked deficiency in circulating IGF-I, we have shown that kidney IGF-I levels are also markedly reduced to less than 10% of normal control values, even though kidney IGF-I mRNA levels are similar to the normal control values. In these ID mice low serum IGF-I levels arise because of the combined lack of liver IGF-I production and a decrease in the stability of the circulating IGF-I derived from nonhepatic sources due to disruption of the Als gene. Measurements by Yakar et al. showed that IGF-I is more rapidly cleared from the serum because of reduced complexing with IGF binding proteins [8]. It is therefore quite conceivable that since the kidney is a major site of low molecular weight protein clearance [5] that renal IGF-I clearance, tubular uptake and metabolism of unbound IGF-I are increased in these mice. Taken together it appears that in the mouse kidney IGF-I peptide is largely serum derived and reflects trapping within the kidney as previously suggested [9].
Interestingly, despite the low serum and kidney IGF-I levels, loss of renal mass was followed by appropriate CRE, the relative increase in kidney weight being similar in ID and N mice. While kidney IGF-I peptide levels did increase significantly in both groups of mice after UNx, the absolute increase in IGF-I in the ID mice kidneys was minimal, ~20 ng/g kidney weight compared to the increase in the N mice, 256 ng/g. Of note despite these increases, kidney IGF-I/insulin receptor phosphorylation was essentially unchanged in both groups of mice. Since CRE appeared to be intact in mice with body growth impairment due to a deficiency in circulating IGF-I and as there is a minimal absolute increase in IGF-I peptide levels in the enlarging kidney following UNx, it is unlikely that serum IGF-I plays a major role in this process and that other factors are operative [3]. In support of this thesis is our finding that IGF-I-insulin receptor phosphorylation is essentially unchanged in the kidney undergoing compensatory enlargement following UNx. Furthermore, taken together the similarities between the N and ID groups in our study suggest that factors other than circulating IGF-I play a role in CRE. Relevant to our observations, in mice with liver IGF-I deletion, but with intact ALs gene expression, low serum IGF-I levels did not prevent compensatory skeletal muscle hypertrophy stimulated by resistance exercise [10]. This positive response may have been in part induced by the observed increase in local IGF-I expression.
The potential for a major role of IGF-I in CRG was based on previous different experimental observations. In a study in rats by Fagin et al. [6], IGF-I mRNA was elevated 5-to 6-fold in the kidneys of UNX rats relative to controls. This induction was present 24 h and continued for at least 7 days after surgery. Kidney radioimmuno-assayable IGF-I content was also increased by 73% in UNx animals, although this was only apparent on the fourth day after surgery. In contrast, liver IGF-I mRNA levels were comparable in both experimental and control animals, suggesting that the IGF-I induction was specific to the tissue undergoing compensatory growth. Serum IGF-I and GH levels were not altered in nephrectomized and control rats for the duration of the experiments [6]. In another study in rats, immunostainable IGF-I was increased markedly in medullary collecting ducts of hypertrophied kidneys 5 days post-UNx compared with kidneys from sham-operated animals. However, no difference in steady-state levels of IGF-I mRNA was detected in whole kidneys or in collecting ducts from UNx or sham-operated rats at any time post-nephrectomy [11]. Others have suggested that there is an age-dependent difference in the effects of UNx on IGF-I gene expression. Mulroney et al. reported that kidney IGF-I mRNA levels were not increased in the adult rat (4 month old) undergoing CRG, either 24 or 48 h post-UNx. In contrast, they found that kidneys from immature (23 days old) rats 24 and 48 h post-UNx, had an average 3–4-fold increase in IGF-I mRNA levels [12]. Changes in IGF-I expression are different in later stages (>1 week) after nephrectomy. In the mature kidney, IGF-I mRNA levels fell without a change in kidney IGF-I peptide content. In the weanling kidneys, IGF-I mRNA and peptide levels and IGF-I receptor binding were unaltered [13]. Thus, the transient increase in kidney IGF-I message following UNx described by Fagin et al. and Mulroney et al. suggested that IGF-I could mediate compensatory renal growth. Later it was shown that this hypertrophy can be prevented by both GH antagonism [14] or IGF-I blockade [7]. On the other hand, earlier studies showed that when nephrectomy was performed on the background of prior hypophysectomy the remaining kidney grew in comparison to the one removed, which indicated that an intact pituitary is not essential to CRG [15]. More recently, mTOR, a major effector of cell growth and proliferation via the regulation of protein synthesis, downstream of many growth factors, including IGF-I, was found to be upregulated in CRG (16 days after UNx). CRG could be inhibited by the use of rapamycin, an mTOR inhibitor [16]. However, other causes (and not just growth factors) including cellular energetics and nutrient availability, may also play a role in regulation of mTOR activity. The regulation mTOR activity by growth factors is mediated by the PI3K/Akt signaling pathway [17], activated by not just IGF-I, but also insulin and PDGF. Other growth factors have shown a similar transient increase in their renal content after UNx, such as epidermal growth factor [18,19], connective tissue growth factor (CTGF), transforming growth factor (TGF)-beta [20], and hepatocyte growth factor (HGF) [21]. In addition, other non-growth factor related stimuli induce renal hypertrophy, such as chronic hypokalemia [22]. Increased workload to which the remaining kidney is subjected may also play a role in mediating CRG. Single-nephron GFR increases and nephron size expands. This could be due to either primary abnormality in vascular control or to a primary augmentation of tubular reabsorption leading to increased filtration. The latter is known as the tubuloglomerular feedback reflex [23], and has been suggested as a mechanism for renal hypertrophy in CRE [24] and diabetes [25].
In summary, while a severe deficiency of circulating IGF-I impairs body growth with a proportional reduction in kidney mass, UNx induces significant and proportionate compensatory renal enlargement in circulating IGF-I deficient mice. This response occurs despite markedly decreased kidney IGF-I levels (>90% lower than in normal mice) and no significant change in receptor phosphorylation. Taken together this suggests that circulating and local IGF-I do not play a significant role in compensatory renal enlargement.
Acknowledgments
This study was supported by a Grant-in-aid of the US-Israel Binational Science Foundation (no. 2003055) to DL, RR and YS and a Merit Review Grant from the Research Service of the USA Department Veterans Affairs to RR.
References
- 1.Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Naito A, Yonou H, Kagawa H. Compensatory renal hypertrophy and changes of renal function following nephrectomy. Hinyokika Kiyo. 2000;46:235–240. [PubMed] [Google Scholar]
- 2.Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. 1995;57:263–278. doi: 10.1146/annurev.ph.57.030195.001403. [DOI] [PubMed] [Google Scholar]
- 3.Sinuani I, Beberashvili I, Averbukh Z, Cohn M, Gitelman I, Weissgarten J. Mesangial cells initiate compensatory tubular cell hypertrophy. Am J Nephrol. 2010;31:326–331. doi: 10.1159/000287229. [DOI] [PubMed] [Google Scholar]
- 4.Mulroney SE, Pesce C. Early hyperplastic renal growth after uninephrectomy in adult female rats. Endocrinology. 2000;141:932–937. doi: 10.1210/endo.141.3.7353. [DOI] [PubMed] [Google Scholar]
- 5.Rabkin R, Schaefer F. New concepts: growth hormone, insulin-like growth factor-I and the kidney. Growth Horm IGF Res. 2004;14:270–276. doi: 10.1016/j.ghir.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Fagin JA, Melmed S. Relative increase in insulin-like growth factor I messenger ribonucleic acid levels in compensatory renal hypertrophy. Endocrinology. 1987;120:718–724. doi: 10.1210/endo-120-2-718. [DOI] [PubMed] [Google Scholar]
- 7.Haylor J, Hickling H, Eter E El, Moir A, Oldroyd S, Hardisty C, El Nahas AM. JB3, an IGF-I receptor antagonist, inhibits early renal growth in diabetic and uninephrectomized rats. J Am Soc Nephrol. 2000;11:2027–2035. doi: 10.1681/ASN.V11112027. [DOI] [PubMed] [Google Scholar]
- 8.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-I directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Sood S, Biada J, Roth R, Rabkin R. Increased workload fully activates the blunted IRS-1/PI3-kinase/Akt signaling pathway in atrophied uremic muscle. Kidney Int. 2008;73:848–855. doi: 10.1038/sj.ki.5002801. [DOI] [PubMed] [Google Scholar]
- 10.Matheny RW, Merritt E, Zannikos SV, Farrar RP, Adamo ML. Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp Biol Med (Maywood) 2009;234:164–170. doi: 10.3181/0808-RM-251. [DOI] [PubMed] [Google Scholar]
- 11.Lajara R, Rotwein P, Bortz JD, Hansen VA, Sadow JL, Betts CR, Rogers SA, Hammerman MR. Dual regulation of insulin-like growth factor I expression during renal hypertrophy. Am J Physiol. 1989;257:F252–261. doi: 10.1152/ajprenal.1989.257.2.F252. [DOI] [PubMed] [Google Scholar]
- 12.Mulroney SE, Haramati A, Roberts CT, Jr, LeRoith D. Renal IGF-I mRNA levels are enhanced following unilateral nephrectomy in immature but not adult rats. Endocrinology. 1991;128:2660–2662. doi: 10.1210/endo-128-5-2660. [DOI] [PubMed] [Google Scholar]
- 13.Fervenza FC, Tsao T, Hsu F, Rabkin R. Intrarenal insulin-like growth factor-1 axis after unilateral nephrectomy in rat. J Am Soc Nephrol. 1999;10:43–50. doi: 10.1681/ASN.V10143. [DOI] [PubMed] [Google Scholar]
- 14.Flyvbjerg A, Bennett WF, Rasch R, van Neck JW, Groffen CA, Kopchick JJ, Scarlett JA. Compensatory renal growth in uninephrectomized adult mice is growth hormone dependent. Kidney Int. 1999;56:2048–2054. doi: 10.1046/j.1523-1755.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 15.Dicker SE, Greenbaum AL, Morris CA. Compensatory renal hypertrophy in hypophysectomized rats. J Physiol. 1977;273:241–253. doi: 10.1113/jphysiol.1977.sp012091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JK, Chen J, Neilson EG, Harris RC. Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy. J Am Soc Nephrol. 2005;16:1384–1391. doi: 10.1681/ASN.2004100894. [DOI] [PubMed] [Google Scholar]
- 17.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 18.Kanda S, Saha PK, Nomata K, Taide M, Nishimura N, Igawa T, Yamada J, Kanetake H, Saito Y. Transient increase in renal epidermal growth factor content after unilateral nephrectomy in the mouse. Acta Endocrinol (Copenh) 1991;124:188–193. doi: 10.1530/acta.0.1240188. [DOI] [PubMed] [Google Scholar]
- 19.Miller SB, Rogers SA, Estes CE, Hammerman MR. Increased distal nephron EGF content and altered distribution of peptide in compensatory renal hypertrophy. Am J Physiol. 1992;262:F1032–1038. doi: 10.1152/ajprenal.1992.262.6.F1032. [DOI] [PubMed] [Google Scholar]
- 20.Rumberger B, Vonend O, Kreutz C, Wilpert J, Donauer J, Amann K, Rohrbach R, Timmer J, Walz G, Gerke P. cDNA microarray analysis of adaptive changes after renal ablation in a sclerosis-resistant mouse strain. Kidney Blood Press Res. 2007;30:377–387. doi: 10.1159/000108624. [DOI] [PubMed] [Google Scholar]
- 21.Tsau YK, Tsai IJ, Chen YM. Transient reciprocal change of renal hepatocyte growth factor and transforming growth factor-beta1 may relate to renal hypertrophy in rats with liver injury or unilateral nephrectomy. Pediatr Res. 2006;59:494–499. doi: 10.1203/01.pdr.0000203101.18174.fe. [DOI] [PubMed] [Google Scholar]
- 22.Ahn KY, Park KY, Kim KK, Kone BC. Chronic hypokalemia enhances expression of the H(+)-K(+)-ATPase alpha 2-subunit gene in renal medulla. Am J Physiol. 1996;271:F314–F321. doi: 10.1152/ajprenal.1996.271.2.F314. [DOI] [PubMed] [Google Scholar]
- 23.Schnermann J, Briggs JP. Tubuloglomerular feedback: mechanistic insights from gene-manipulated mice. Kidney Int. 2008;74:418–426. doi: 10.1038/ki.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blantz RC, Peterson OW, Thomson SC. Tubuloglomerular feedback responses to acute contralateral nephrectomy. Am J Physiol. 1991;260:F749–F756. doi: 10.1152/ajprenal.1991.260.5.F749. [DOI] [PubMed] [Google Scholar]
- 25.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]


