Abstract
Objectives
Emerging data suggests that not all grade 3 (G3) pancreatic neuroendocrine neoplasms (panNENs) behave the same; tumor differentiation may predict outcome.
Methods
Patients with G3 panNENs treated at our institution between 1999 and 2014 were identified. Demographics, response to therapy, and overall survival (OS) were determined.
Results
Forty-five patients were identified, 16 with G3 well differentiated pancreatic neuroendocrine tumors (WD-panNETs) and 29 with poorly differentiated neuroendocrine carcinomas (PDNEC). Median OS in G3 WD-panNET patients was 52.2 months (95% confidence interval (CI) 19.3 to 86.9 months) compared to 10.1 months (95% CI 6.9 to 12.4 months) in PDNEC patients (p-value = 0.0009). Response rate to platinum agents was 10% in G3 WD-panNETs and 37% in PDNEC. Response rate to alkylating agents was 50% in G3 WD-panNETs and 50% in PDNEC.
Conclusions
Both G3 WD-panNETs and PDNEC responded to platinum and alkylating agents. OS was significantly greater in G3 WD-panNETs compared to PDNEC. These findings challenge current classification and suggest that G3 panNENs should be classified by morphology.
Keywords: neuroendocrine tumors, pancreatic neuroendocrine tumors, grade 3 neuroendocrine tumors, morphology, survival
Introduction
Pancreatic neuroendocrine neoplasms (PanNENs) are an uncommon group of tumors that present in diverse manners and exhibit a wide spectrum of clinical behavior with varying degrees of aggressiveness. It has been shown that proliferative rate-based grading, using mitotic rate and Ki-67 proliferation index, stratifies prognostic subgroups of panNENs even with modest differences in tumor proliferative activity.1-3 Based on this understanding, the 2010 World Health Organization (WHO) classification used proliferative activity (both mitotic rate and Ki-67 proliferation index) to separate panNENs into three histologic grades (Table 1): low (grade 1 (G1)), intermediate (grade 2 (G2)), and high (grade 3 (G3)).4 Tumor grade is thought to reflect a given tumor's inherent degree of aggressiveness.
Table 1.
2010 World Health Organization classification of neuroendocrine tumors.
| Grade | Ki-67 proliferation index | Mitotic count / 10 high power fields |
|---|---|---|
| Grade 1 | <3% | <2 |
| Grade 2 | 3-20% | 2-20 |
| Grade 3 | >20% | >20 |
By 2010 WHO criteria, G3 panNENs are defined as a tumor with Ki-67 proliferation index >20% or mitotic rate >20 per 10 high power fields. Traditionally, G3 panNENs have been classified as poorly differentiated neuroendocrine carcinomas (PDNEC), however, some G3 tumors have well differentiated morphology and a distinctly different mutational profile.5,6 In some cases, these G3 well differentiated pancreatic neuroendocrine tumors (G3 WD-panNETs) are thought to develop from progression of low or intermediate grade disease (i.e. neoplastic progression).5 G3 WD-panNETs retain the morphology and cytoarchitectural features of other WD-panNETs, and in comparison to PDNEC, tend to have proliferative rates on the lower end of the high grade range (i.e., Ki-67 proliferation index of 20-50%).7
G3 WD-panNETs do not appear to be as clinically aggressive as PDNECs. Early investigation of G3 WD-panNETs has demonstrated survival times shorter than well differentiated G1 or G2 panNETs, but longer than what is typically described for PDNEC.8 In addition to varying tumor aggressiveness, PDNEC and G3 WD-panNETs differ genetically. Investigation of the genetic changes in PDNEC has identified distinctive alterations in TP53 and RB1.9 In contrast, G3 WD-panNETs harbor changes in genes implicated in chromatin remodeling (MEN1, DAXX, ATRX) and in the PI3K/Akt/mTOR pathway; these genetic alterations are similar to those observed in the less aggressive G1 and G2 WD-panNETs.7,10
Despite these differences, both G3 WD-panNETs and PDNEC are classified in the same G3 category. In addition, current National Comprehensive Cancer Network guidelines recommend platinum based therapy for both G3 WD-panNETs and PDNEC. The only reported study of G3 NENs was a retrospective study of all gastrointestinal G3 NENs (irrespective of morphology and site of origin). The authors reported that tumors with a Ki-67 proliferation index <55% were less responsive to first-line platinum-based chemotherapy, though this subgroup did achieve longer survival when compared to G3 NENs with a Ki-67 proliferation index >55%.11
The differences in pathology, clinical behavior, and genetic features suggest that G3 WD-panNETs and PDNEC represent two distinct entities with distinct disease-specific outcomes, and may have varying responses to available therapies. In an effort to answer these questions, we retrospectively reviewed the clinical treatments of G3 panNENs in a single institution. The goals of this study were to evaluate best response to therapy and overall survival (OS). This review includes only pancreatic primary G3 NENs and excludes G3 NENs of other origin to maintain a homogeneous patient population.
Materials and Methods
Study Patients
We identified patients with G3 panNENs (local or metastatic) treated at Memorial Sloan Kettering Cancer Center (MSKCC) between 1999 and 2014 from our institutional database. The inclusion criteria were: histopathological confirmed diagnosis of G3 NEN (defined as having Ki-67 proliferation index > 20%) with a pancreas primary. Morphologically the tumor was described as either well differentiated, small cell carcinoma, or large cell neuroendocrine carcinoma. Mixed tumors based on pathologic architecture (i.e., with an adenocarcinoma or acinar cell carcinoma component) were excluded. Electronic medical records were retrospectively reviewed for data on patient demographics and characteristics, pathology reports (including morphology, mitotic count, and Ki-67 proliferation index), systemic treatments (including chemotherapy, targeted therapy, and octreotide-based therapy), and outcomes (overall survival (OS)). When characterizing chemotherapy regimens, alkylating agents included either temozolomide or dacarbazine-based treatments and targeted agents included either sunitinib or everolimus therapy. The first three lines of therapy were retrospectively reviewed for all patients.
Patients were followed from the date of cancer diagnosis until date of death or date of last known follow-up. Dedicated MSKCC gastrointestinal pathologists experienced in the diagnosis of NENs reviewed all pathology; the distinction between G3 WD-panNET and PDNEC was determined by review of pathology. Approval for data collection and analysis for this retrospective chart review was obtained from the MSKCC Institutional Review Board. When tissue was available, Ki-67 proliferation index was retested; otherwise grade was determined by information provided in the pathology reports.
Evaluation of response
Best therapy response (complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD)) to platinum agents, alkylating agents, and targeted agents was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 parameters based on radiology reports.
Statistical analysis
Patient characteristics were compared between G3 WD-panNETs and PDNEC using Fisher's exact test for categorical variables and Wilcoxon rank sum test for continuous predictors. OS, defined as time from initiation of first-line systemic therapy until death or last known follow-up, was estimated using the Kaplan-Meier method and survival curves compared by the log-rank test. To examine the difference in survival between G3 WD-panNETs and PDNEC after adjusting for potential confounders, Cox regression models were employed. Platinum, alkylating, and targeted agents were included as time-dependent variables in the Cox regression models.
Results
Patient characteristics
A total of 45 patients (19 females and 26 males) were identified during the study period. Sixteen patients (36%) had G3 WD-panNETs and 29 patients (64%) had PDNEC. Seven patients (16%) were thought to have localized disease at presentation and 38 patients (84%) had known metastatic disease. Four patients (9%, all G3 WD-panNETs) had functional tumors. Octreoscan™ imaging was performed on 27/45 patients (60%). Of those 27 patients, 19 patients (70%, 13 G3-WD PanNETs and 6 PDNEC) had Octreoscan™ positive disease. Median follow-up was 17.8 months in G3 WD-panNETs and 9.1 months in PDNEC. Patient characteristics are summarized in Table 2.
Table 2.
Baseline patient characteristics (blank cells indicate nonsignificant p-values at 0.05 level).
| G3 panNENs | All (n=45) | G3 WD-panNETs (n=16) | PDNEC (n=29) | Significant differences between G3 WD- panNETs and PDNEC |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | p-value | |
|
| ||||
| Mean age, years | 54 | 47 | 58 | 0.016 |
| Range | (26-78) | (26-73) | (32-78) | |
|
| ||||
| Sex | ||||
| Female | 19 (42%) | 9 (56%) | 10 (34%) | |
| Male | 26 (58%) | 7 (44%) | 19 (66%) | |
|
| ||||
| Performance Status (ECOG) | 0.04 | |||
| 0 | 6 (13%) | 5 (31%) | 1 (3%) | |
| 1 | 31 (69%) | 10 (63%) | 21 (72%) | |
| 2 | 6 (13%) | 1 (6%) | 5 (17%) | |
| 3-4 | 2 (4%) | 0 (0%) | 2 (7%) | |
|
| ||||
| Smoker/prior smoker | 21 (47%) | 4 (25%) | 17 (59%) | 0.0307 |
|
| ||||
| History of second malignancy | 4 (9%) | 1 (6%) | 3 (10%) | |
|
| ||||
| Symptomatic at presentation | 43 (96%) | 15 (94%) | 28 (97%) | |
| Pain | 29 (64%) | 10 (63%) | 18 (62%) | |
| Weight loss | 25 (56%) | 5 (31%) | 20 (69%) | 0.0271 |
| Jaundice | 10 (22%) | 0 (0%) | 10 (34%) | 0.0080 |
| Hyperglycemia | 3 (7%) | 0 (0%) | 3 (10%) | |
|
| ||||
| Primary tumor location | ||||
| Uncinate process | 2 (4.4%) | 2 (13%) | 0 (0%) | |
| Head | 16 (36%) | 3 (19%) | 13 (45%) | |
| Body | 7(16%) | 0 (0%) | 6 (21%) | |
| Tail | 18 (40%) | 11 (69%) | 8 (28%) | 0.0118 |
| Peripancreatic soft tissue | 2 (4.4%) | 0 (0%) | 2 (7%) | |
|
| ||||
| Mean primary tumor size (cm) | 4.2 | 4.3 | 4.1 | |
| Range | (1-13.5) | (1-13.5) | (1.4-9) | |
|
| ||||
| Primary tumor resected | 8 (18%) | 6 (38%) | 2 (7%) | |
|
| ||||
| Location of metastases at presentation | ||||
| Liver | 39 (87%) | 14 (88%) | 25 (86%) | |
| Lymph nodes | 20 (44%) | 4 (25%) | 16 (55%) | |
| Lung | 1 (2%) | 0 (0%) | 1 (3%) | |
| Bone | 3 (7%) | 2 (13%) | 1 (3%) | |
| Brain | 0 (0%) | 0 (0%) | 0 (0%) | |
| Ovary | 3 (7%) | 3 (19%) | 0 (0%) | 0.0395 |
| Other | 3 (7%) | 0 (0%) | 3 (10%) | |
|
| ||||
| Octreoscan positive | 19/27 patients tested (70%) | 13/15 patients tested (87%) | 6/12 patients tested (50%) | |
|
| ||||
| Functional | 4 (8.9%) | 4 (25%) | 0 (0%) | 0.0122 |
| Insulinoma | 1 (2.2%) | 1 (6%) | ||
| Gastrinoma | 3 (6.7%) | 3 (19%) | ||
|
| ||||
| Paraneoplastic syndrome at presentation | 1 (2.2%) | 0 (0%) | 1 (3%) | |
| Histology | ||||
| Small cell | 5 (11%) | 0 (0%) | 5 (17%) | |
| Large cell | 40 (89%) | 16 (100%) | 24 (83%) | |
|
| ||||
| Average Ki-67 proliferation index % (range) | 47 (25-80) | 73 (20-95) | ||
Abbreviations: G3 panNENs, grade 3 pancreatic neuroendocrine neoplasms; G3 WD-panNETs, grade 3 well differentiated pancreatic neuroendocrine tumors; PDNEC, poorly differentiated neuroendocrine carcinomas.
Five patients with G3 WD-panNETs (5/16, 31%) were believed to have localized disease at presentation and went to the operating room for curative intent. After resection of the primary, 4/5 patients (80%) developed recurrent disease, and the fifth patient had biopsy-proven metastatic disease to the liver identified intraoperatively. Among the 4 patients who developed recurrent disease after resection of the primary lesion, average time to tumor recurrence was 19.4 months (range 2.3 to 39.6 months). Two patients with PDNEC (2/29, 7%) were believed to have localized disease at presentation and went to the operating room for removal of the primary pancreas tumor. Both of these patients were found to have metastatic disease intraoperatively.
Among the entire cohort, 4/45 patients (9%) had a history of a second malignancy. One G3 WD-panNET patient had previously been treated for a desmoid tumor. Of the patients with PDNEC, one patient had a history of triple negative breast cancer and acute promyelocytic leukemia, one patient had a history of prostate adenocarcinoma, and one patient had a history of basal cell carcinoma of the skin. No underlying genetic syndromes were uncovered on further evaluation of these four patients.
The majority of patients with PDNEC (13/29 patients, 45%) presented with primary tumors in the pancreatic head; as expected, many of these patients presented with jaundice. In contrast, G3 WD-panNETs were more commonly in the pancreatic tail (11/16 patients, 69%). In both G3 WD-panNETs and PDNEC, the liver was the most common site of metastatic disease, however, only G3 WD-panNETs demonstrated evidence of metastatic disease in the ovaries, identified in 3/16 patients (19%). In addition, while no PDNEC were functional tumors, there were 4 functional G3 WD-panNETs (1 insulinoma and 3 gastrinomas). A paraneoplastic syndrome was identified in one patient with PDNEC, who presented with the syndrome of inappropriate antidiuretic hormone secretion.
Pathologic features
Among the 45 G3 panNENs, 16 tumors (36%) were G3 WD-panNETs and 29 (64%) PDNEC; among the 29 PDNEC, 5 were small cell carcinomas (17%) and 24 were large cell neuroendocrine carcinomas (83%). In evaluable tumor tissue, the average Ki-67 proliferation index was 47% (range, 25% to 80%) in G3 WD-panNETs and 73% (range, 20% to 95%) in PDNEC.
Treatment Regimens
For first-line therapy of G3 WD-panNET patients, 5/16 patients (31%) received platinum agents, 9/16 patients (56%) received alkylating agents, 1/16 patients (6%) received targeted therapy with sunitinib, and 1/16 patients (6%) received octreotide long-acting-release (LAR).
For first-line therapy of PDNEC patients, 25/29 patients (86%) received platinum agents, 0/29 patients (0%) received alkylating agents, and 1/29 patients (3%) received octreotide LAR; of the remaining 3 patients, 2/29 patients (7%) received gemcitabine-based regimens and 1/29 patients (3%) received capecitabine alone.
Second-line therapy was administered to 11/16 (69%) G3 WD-panNET patients and 18/29 (62%) PDNEC patients. Third-line therapy was administered to 9/16 (56%) G3 WD- panNET patients and 9/29 (31%) PDNEC patients.
Best therapy response
Platinum agents
Among all lines of therapy, 10/16 (63%) G3 WD-panNET patients received platinum agents (which included platinum/etoposide or platinum/oxaliplatin based regimens); 1/10 patients (10%) achieved a PR, 5/10 patients (50%) had SD, and 4/10 patients (40%) had PD. Of the PDNEC patients, 27/29 (93%) received platinum agents; 1/27 patients (4%) achieved a CR, 9/27 patients (33%) had PR (CR + PR = 37%), 10/27 patients (37%) had SD, and 7/27 patients (26%) had PD.
Alkylating agents
Among all lines of therapy, 12/16 (75%) G3 WD-panNET patients received alkylating agents; of those, 6/12 patients (50%) achieved a PR, 3/12 patients (25%) had SD, and 3/12 patients (25%) had PD. Six PDNEC patients (6/29, 21%) received alkylating agents, 3/6 patients (50%) had PR, 1/6 patients (17%) had SD, 2/6 patients (33%) had PD.
Targeted agents
Among all lines of therapy, 6/16 (38%) G3 WD-panNET patients received targeted agents, 1/6 patients (17%) had SD and 5/6 patients (83%) had PD. One patient (1/29, 3%) with PDNEC received a targeted agent; this patient achieved a PR.
Somatostatin analog therapy
Among all lines of therapy, 3/16 (19%) G3 WD-panNET patients received octreotide LAR; 1/3 (33%) had SD, 2/3 (67%) had PD. One (3%) PDNEC patient received octreotide LAR, which was given first-line; this patient had PD.
Survival
Median OS in patients with G3 WD-panNETs was 52.2 months (95% CI 19.3 to 86.9 months) compared to a median OS in patients with PDNEC of 10.1 months (95% CI 6.9 to 12.4 months). This OS difference was statistically significant (Figure 1, p-value = 0.0009). One-year OS in G3 WD-panNET patients was 92%, compared to 33% in PDNEC patients. After adjusting for potential confounders such as ECOG and the different therapies received (platinum, alkylating, or targeted agents), OS remained worse in PDNEC group (p = 0.02, HR 3 with 95% CI 1.2-7.5).
Figure 1.
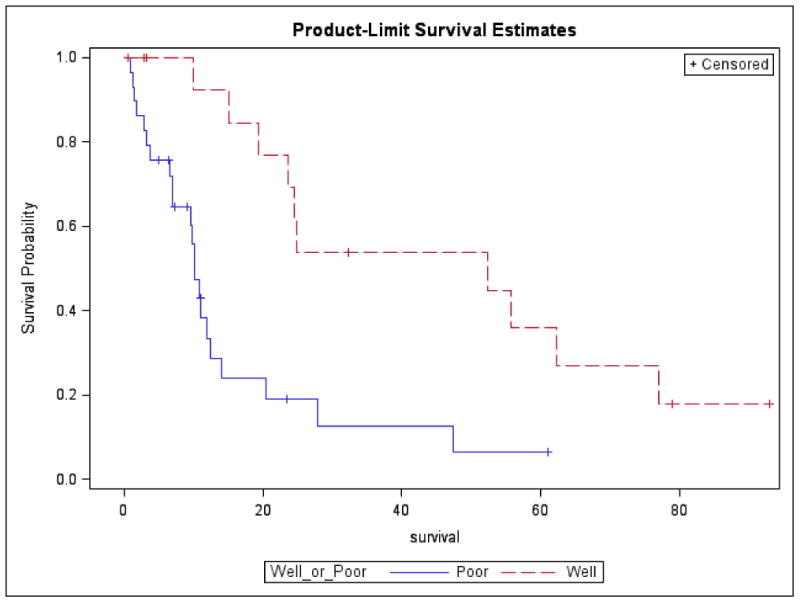
Kaplan-Meier overall survival by tumor differentiation, G3 WD-panNETs versus PDNEC. Abbreviations: G3 WD-panNETs, grade 3 well differentiated pancreatic neuroendocrine tumors; PDNEC, poorly differentiated neuroendocrine carcinomas.
Discussion
To our knowledge, this is the largest series (45 patients) reported of clinical outcomes of G3 panNENs, an uncommon and aggressive neoplasm with limited treatment options. The rarity of this disease makes it challenging to conduct prospective studies. Our results, albeit retrospective, allow us to better understand the heterogeneity that exists within G3 panNENs in an effort to define and improve our treatment options.
Almost all patients (43 patients, 96%), irrespective of morphology, were symptomatic at presentation, and most commonly presented with metastases to the liver. Primary tumor location varied between G3 WD-panNETs and PDNEC; G3 WD-panNETs presented more commonly with tail masses, and PDNEC presented more commonly with head masses. Given an increased number of pancreatic head masses, PDNEC patients initially presented with jaundice; jaundice was not observed in patients with G3 WD-panNETs group even when presenting with a head of pancreas mass. Such a clinical finding may be important as pancreaticoduodenectomy should be generally avoided in PDNEC patients as metastatic disease is almost universal, and median survival is expected to be < 1 year.
In the neuroendocrine community, it is widely accepted that pathologic differentiation is associated with the presence of somatostatin receptors; the majority of WD NETs are somatostatin scintigraphy positive. In contrast, PDNEC are typically not somatostatin scintigraphy positive and instead are pet avid.12 Based on this understanding, we expected that within G3 panNENs, somatostatin scintigraphy could help differentiate G3 WD-panNETs from PDNEC. The results from our series, however, were not consistent with this hypothesis: although 13/15 (87%) G3 WD-panNETs were somatostatin avid, 6/12 (50%) PDNEC were also somatostatin avid. Notably, only one patient with PDNEC was treated with somatostatin analog therapy and experienced PD, and no patients with PDNEC received peptide receptor radiotherapy. At this time, the therapeutic implications of having an octreotide avid PDNEC remains unknown.
In our investigation of best response to treatment (CR + PR), we observed that among G3 panNENs, treatment responses varied based on differentiation status. Although the numbers are small, 37% of PDNECs responded to platinum based therapy versus 10% of G3 WD-panNETs; of note, 37% of PDNEC and 50% of G3 WD-panNETs had SD. Alkylating agents achieved a PR in 50% of PDNEC and 50% of G3 WD-panNETs; 25% of G3 WD-panNETs had SD, in comparison to 17% of PDNEC. Response to targeted therapies was more divergent, with only 1 patient with PDNEC receiving a targeted agent (sunitinib, given in the second line) and experiencing a PR, in contrast to 6 patients with G3 WD-panNETs receiving targeted agents, 1 with SD and 5 with PD.
What can we learn from these treatment responses? The NORDIC study included G3 NENs of many gastrointestinal sites of origin and the results suggested that platinum agents should be considered only for patients whose tumors have a Ki-67 >55% (RR 42% in Ki-67 >55% and RR 15% in Ki-67<55%, P<0.001), which presumably represented predominantly PDNECs (differentiation status was not stated in that study); in our series however, platinum agents did exhibit activity in patients with G3 WD-panNETs, many of whom (13/16 patients, 81%) had Ki-67 < 55%.11 Indeed, other small phase II studies have observed response to platinum agents in advanced WD NETs irrespective of grade (including G1 to G3). A study with gemcitabine and oxaliplatin reported a response rate of 17% and treatment duration of 7 months.13 More recently, two reports from the San Francisco area showed activity of fluorouracil, oxaliplatin, and bevacizumab combination.14,15 Different platinum-based regimens (i.e. platinum/etoposide, oxaliplatin/fluorouracil, oxaliplatin/gemcitabine) may have different efficacies. Our numbers are too small, however, to draw any definitive conclusions.
In our series, both G3 WD-panNETs and PDNEC responded to the alkylating agents dacarbazine and temozolomide; this is in line with prior studies independent of tumor grade and differentiation status. In the seminal ECOG (E6282) phase II study of dacarbazine, in 50 patients with advanced panNETs with documented clinical or radiographic disease progression, a response rate of 34% was observed,; median OS was 19.3 months.16 In a subsequent study using modern response criteria, the combination of temozolomide and thalidomide was tested in 29 patients with metastatic NETs (including carcinoid tumors, pheochromocytomas, and panNETs); the investigators reported a 45% radiologic response rate in panNETs, and 7% radiologic response rate in carcinoid tumors.17 The most recent study to evaluate combination capecitabine and temozolomide was retrospectively conducted in 30 patients with metastatic panNETs, and 21/30 (70%) patients demonstrated an objective radiographic response, with median PFS 18 months, and 2-year OS 92%.18 Currently, there is an ECOG study actively accruing, which randomizes patients to single agent temozolomide versus capecitabine plus temozolomide to ask the question of the benefit of single versus combination therapy (NCT01824875). At this time, it is difficult to know if there is a meaningful difference between the activity of temozolomide and dacarbazine in panNETs, as no trials have directly compared these two agents.
Finally, looking specifically at the use of targeted agents in our cohort of G3 panNENs, targeted agents were rarely used, with signal of activity noted in few patients (1 patient with PDNEC who experienced a PR). This observation speaks to the aggressive clinical course of this disease irrespective of pathologic differentiation. Indeed, targeted agents such as everolimus and sunitinib are not considered a feasible treatment option in this setting, nor are they FDA approved for the management of G3 disease. In our series, treatment with targeted agents was more frequently attempted in patients with G3 WD-panNETs, likely reflective of the more indolent clinical course exhibited in this cohort, although no responses were seen.
In summary, the treatment of panNENs poses a significant challenge, both because of tumor heterogeneity and varying degree of aggressiveness. In recent years, an understanding of tumor signaling mechanisms has led to promising agents that target clinically significant pathways and such agents are now FDA-approved for well or moderately differentiated, G1 and G2 tumors. Cytotoxic chemotherapy is traditionally reserved for G3 tumors, although all the reported cytotoxic chemotherapy trials are nonrandomized, in small patient populations, and comprise a heterogeneous cohort in terms tumor grade, making the results difficult to interpret.
In this study, we looked specifically at G3 panNENs, and attempted to better characterize this patient population, investigating differences in survival and treatment response based on pathologic differentiation (G3 WD-panNETs and PDNEC). Our data demonstrate that while G3 WD-panNETs and PDNEC both respond to cytotoxic agents (platinum and alkylating agents), the outcomes were dramatically different. These findings call into question the current 2010 WHO classification of G3 tumors, which are regarded to be poorly differentiated by definition. Future investigation should focus on the identification of biomarkers that will allow us better individualize therapy within G3 panNENs, and such evaluations are ongoing. In addition, future studies in G3 panNENs should include differentiation status and mutational profiles (i.e. Rb protein expression) when defining the patient population.
Acknowledgments
Source of Funding: For the remaining authors none were declared. This work had no specific funding.
Footnotes
Conflicts of Interest: Dr. Diane Reidy-Lagunes has received honoraria and research funding from Novartis. In addition, Dr. Reidy-Lagunes does consulting/advising for Novartis, Ipsen, and Pfizer. Dr. Peter Allen does consulting/advising for Sanofi. In addition, Dr. Allen has received research funding from Novartis.
Contributor Information
Nitya Raj, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York.
Emily Valentino, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, New York.
Marinela Capanu, Department of Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York.
Laura H. Tang, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York.
Olca Basturk, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York.
Brian R. Untch, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
Peter J. Allen, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, New York.
David S. Klimstra, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York.
Diane Reidy-Lagunes, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY.
References
- 1.Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 2.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Liu TC, Hamilton N, Hawkins W, et al. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2013;37:853–859. doi: 10.1097/PAS.0b013e31827fcc18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosman FT, World Health Organization, International Agency for Research on Cancer . WHO classification of tumours of the digestive system. 4. Lyon: International Agency for Research on Cancer; 2010. [Google Scholar]
- 5.Basturk O, Tang L, Hruban RH, et al. Poorly differentiated neuroendocrine carcinomas of the pancreas: a clinicopathologic analysis of 44 cases. Am J Surg Pathol. 2014;38:437–447. doi: 10.1097/PAS.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang LH, Untch BR, Reidy DL, et al. Well-Differentiated Neuroendocrine Tumors with a Morphologically Apparent High-Grade Component: A Pathway Distinct from Poorly Differentiated Neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011–1017. doi: 10.1158/1078-0432.CCR-15-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi C, Klimstra DS. Pancreatic neuroendocrine tumors: pathologic and molecular characteristics. Semin Diagn Pathol. 2014;31:498–511. doi: 10.1053/j.semdp.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Basturk O, Yang Z, Tang LH, et al. Increased (> 20%) Ki67 Proliferation Index in Morphologically Well Differentiated Pancreatic Neuroendocrine Tumors (PanNETs) Correlates with Decreased Overall Survival. Modern Pathol. 2013;26:423a–423a. [Google Scholar]
- 9.Yachida S, Vakiani E, White CM, et al. Small Cell and Large Cell Neuroendocrine Carcinomas of the Pancreas are Genetically Similar and Distinct From Well-differentiated Pancreatic Neuroendocrine Tumors. Am J Surg Pathol. 2012;36:173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiao YC, Shi CJ, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR Pathway Genes Are Frequently Altered in Pancreatic Neuroendocrine Tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorbye H, Welin S, Langer SW, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]
- 12.Adams S, Baum R, Rink T, et al. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83. doi: 10.1007/s002590050197. [DOI] [PubMed] [Google Scholar]
- 13.Cassier PA, Walter T, Eymard B, et al. Gemcitabine and Oxaliplatin Combination Chemotherapy for Metastatic Well-differentiated Neuroendocrine Carcinomas A Single-Center Experience. Cancer. 2009;115:3392–3399. doi: 10.1002/cncr.24384. [DOI] [PubMed] [Google Scholar]
- 14.Venook AP, Ko AH, Tempero MA, et al. Phase II trial of FOLFOX plus bevacizumab in advanced, progressive neuroendocrine tumors. J Clin Oncol (Meeting Abstracts) 2008;26 15_suppl 15545. [Google Scholar]
- 15.Kunz PL, Kuo T, Zahn JM, et al. A phase II study of capecitabine, oxaliplatin, and bevacizumab for metastatic or unresectable neuroendocrine tumors. J Clin Oncol (Meeting Abstracts) 2010;28 15_suppl 4104. [Google Scholar]
- 16.Ramanathan R, Cnaan A, Hahn R, et al. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma. Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol. 2001;12:1139–1143. doi: 10.1023/a:1011632713360. [DOI] [PubMed] [Google Scholar]
- 17.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 18.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]


