Abstract
The contribution of the gonadotropic axis to skeletal sexual dimorphism (SSD) was clarified in recent years. Studies with animal models of estrogen receptor (ER) or androgen receptor (AR) null mice, as well as mice with bone cell-specific ablation of ER or AR, revealed that both hormones play major roles in skeletal acquisition, and that estrogen regulates skeletal accrual in both sexes. The growth hormone (GH) and its downstream effector, the insulin-like growth factor-1 (IGF-1) are also major determinants of peak bone mass during puberty and young adulthood, and play important roles in maintaining bone integrity during aging. A few studies in both humans and animal models suggests that in addition to the differences in sex steroid actions on bone, sex-specific effects of GH and IGF-1, play essential roles in SSD. However, the contributions of the somatotropic (GH/IGF-1) axis to SSD are controversial and data is difficult interpret. GH/IGF-1 are pleotropic hormones that act in an endocrine and autocrine/paracrine fashion on multiple tissues, affecting body composition as well as metabolism. Thus, understanding the contribution of the somatotropic axis to SSD requires the use of mouse models that will differentiate between these two modes of action. Elucidation of the relative contribution of GH/IGF-1 axis to SSD is significant because GH is approved for the treatment of normal children with short stature and children with congenital growth disorders. Thus, if the GH/IGF-1 axis determines SSD, treatment with GH may be tailored according to sex. In the following review, we give an overview of the roles of sex steroids in determining SSD and how they may interact with the GH/IGF-1 axis in bone. We summarize several mouse models with impaired somatotropic axis and speculate on the possible contribution of that axis to SSD.
Keywords: Skeletal sexual dimorphism (SSD), Growth hormone receptor (GHR), insulin-like growth factor-1 (IGF-1), parathyroid hormone (PTH), bone, osteocyte, micro-computed tomography
Introduction
What are the characteristics of skeletal sexual dimorphism (SSD)? The short answer is that during growth males show greater gains in bone strength than females, and during aging males show lesser declines in bone strength than females. Males grow longer and wider bones that are stronger than female bones. The increases in linear growth in males depend largely on increases in the height of the long bones, while the increase in the height of the vertebrae is smaller, and its contribution to the sex differences in height is modest. The sex differences in bone strength are established during puberty. In humans, pubertal growth in boys starts approximately 1 year later than girls, lasts a little longer, and growth velocity is greater than in girls (1, 2). These temporal differences in the males contribute to their longer and wider bones.
Bone strength is largely determined by bone size, geometry, and quality. The amount of mineral per unit volume of bone, referred as volumetric bone mineral density (vBMD), is not different between the two sexes either for appendicular or axial skeletons (3, 4). Thus, differences in bone size are the major contributor to the sex differences in bone strength (5). The contribution of bone quality to sex differences in bone strength is less well known. We note that changes in bone-size, -geometry, and – quality, continue to occur during growth, puberty and adulthood, and are largely determined by genetic and hormonal factors.
Bone size is regulated by the activity of osteoblasts (bone building cells) and osteoclasts (bone resorbing cells) on the periosteal and endosteal surfaces of the bone, processes collectively referred as bone modeling (during growth). Periosteal bone apposition in both sexes results in increased bone diameter, an important factor determining bone strength. Pubertal-growth in males associates with enhanced periosteal bone apposition with increased endocortical bone resorption and little endosteal bone expansion, processes that contribute to wider bones with proportionally thicker cortex. In females, however, periosteal bone apposition during pubertal growth slows down, while endosteal bone expands as a consequence of reduced resorption at the endosteal surface resulting in smaller marrow cavity (6). During aging periosteal apposition in males continues at a slow rate, while in females the periosteal surface is inactive (7). Both males and females show increases in endosteal bone resorption (bone remodeling). However, since males have continuous periosteal bone apposition (though at a slower rate), the net cortical bone loss is smaller than females. Sex differences during aging are also apparent in the trabecular bone compartment. Males loose trabecular bone during aging via thinning of the trabeculae (3, 4). Females, however, remodel trabecular bone earlier in life (midlife) leading to thinning of trabeculae and reduced remodeling surfaces with time. During aging, remodeling of the trabecular bone compartment in females leads to loss of trabeculae connectivity (3, 4).
Elucidation of SSD requires understanding of the hormonal and tissue factors network that operates during growth and aging. There are some experimental evidence from humans and animal models suggesting that in addition to the differences in sex steroid actions on bone, sex-specific effects of the growth hormone (GH) and its downstream effector, insulin-like growth factor-1 (IGF-1), play roles in skeletal sexual dimorphism (SSD). Both humans (8, 9) and rodents (10) show sexual dimorphism in the GH secretory patterns, which consequently result in pulsatile activation of the signal transducer and activator of transcription (STAT) 5b, a mediator of the GHR. Loss of STAT5b in male mice results in a feminized liver gene expression profile, while females are not affected (11). Likewise, studies of the molecular basis for GH-mediated sexual dimorphism show that female livers are less responsive to GH than male livers (12–14). Similarly, in adults, it has been established that women who are taking oral estrogens require higher GH dose to achieve equivalent serum IGF-1 levels (15–20). Understanding how GH/IGF-1 axis affects SSD is significant because GH is approved for the treatment of normal children with short stature, children who are born small for gestational age, children with idiopathic short stature, and children with congenital GH deficiency, Noonan syndrome, Turner syndrome, and Prader Willi syndrome (21). Additionally, it was lately suggested that using individually tailored doses of GH for treatment of short stature provides a more optimal skeletal outcomes (22). Therefore, should the GH/IGF-1 axis determine SSD, GH treatment may also be tailored according to sex. This review will summarize briefly the contribution of sex steroids to SSD and focus specifically on the roles of GH/IGF-1 in determining SSD based on studies done in mice.
The contribution of sex steroids to SSD
Ovaries, in females, and testis, in males, are the main source of sex steroids, while the adrenals contribute about 5% to circulating sex steroids. In men, most of the circulating sex steroids are bound to the sex hormone binding globulins (SHBGs), while in rodents, which lack SHBGs, sex steroids circulate in a free form. In males the predominant sex steroid, testosterone (T), is produced from C19 by the testis and from dehydroepiandrosterone (DHEA) by the adrenals. DHEA can be further converted in tissues to dehydrotestosterone (DHT), a more potent androgen, by the 5a-reductase. Testosterone can also be converted to 17b-estradiol (E2) by aromatase (CYP19A1), thus leading to E2-like effects. Testosterone and E2 bind to the androgen receptor (AR) or the estrogen receptor (ER), respectively, which are found in almost all tissues, including bone. Bone tissue not only expresses the ER and AR but also the 5a-reductase and aromatase enzymes, suggesting that sex steroids also provoke autocrine/paracrine actions in bone.
Understanding the contributions of sex steroids to SSD was extensively studied using gonadectomized animals (Table 1A). In general, ovariectomy (OVX) or orchidectomy (ORX) resulted in decreases in bone mass, largely via decreases in trabecular bone, but also via decreases in bone mineral content (BMC) in the cortical bone envelope. Notably, however, gonadectomy has temporal effects. Therefore, the age at which removal of gonads is performed determines the skeletal outcomes. Growth rate accelerates during early puberty (in rodents at 3–5 weeks of age) and decreases later on (at 5–8 weeks of age). ORX results in increased body weight and decreased cross-sectional bone area only if done at the end of pubertal growth (23). ORX done in aged animals does not affect total cross-sectional bone area but results in significant thinning of the bone cortices, suggesting an enhanced endosteal bone resorption (24). On the other hand, OVX done during prepuberty results in significant increases in bone length and bone cross-sectional area (23, 25), and associates with increases in serum IGF-1 levels in one study (25). When performed in adults, OVX results in significant increases in body adiposity and significant reductions in BMC in both cortical and trabecular bone compartments due to increased bone resorption. In this context, it is important to note that gonadectomy not only removes estrogen and androgen, but also removes other ovarian and testicular hormones. Nonetheless, gonadectomy studies support the notion that in female mice, estrogen inhibits periosteal bone expansion during early puberty, while in male mice androgen stimulates periosteal bone apposition during late puberty. This concept was also supported by studies with mice overexpressing aromatase globally (aromatase-Tg) via the human ubiquitin C promoter (Table 1B) (26, 27). As expected, aromatase-Tg male mice exhibited increases in serum E2, which associated with decreased total cross-sectional area and increased endocortical bone apposition. Female aromatase-Tg mice also showed decreases in total cross-sectional area without alterations in endocortical bone apposition. When aromatase was overexpressed specifically in osteoblasts (OB-aromatase-Tg), serum E2 levels were normal and thus, total cross-sectional area in male mice remained normal (Table 1B) (28). In both sexes of OB-Aromatase-Tg endocortical bone apposition increased, and in females it resulted in increased cortical bone thickness.
Table 1.
Skeletal characterization of mouse models with impaired gonadotropic axis.
| A. Gonadectomy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse model (ref) |
Males ORX | Females OVX | ||||||||
| Linear growt h |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Serum IGF-1 |
Linear growth |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Serum IGF-1 |
|
| ORX (23, 24, 41) OVX (23, 25) | Normal | Decreased femoral cross- sectional area at the mid-diaphysis (only if done at beginning of puberty), reduced cortical area, cortical thickness, and periosteal circumference | Significant reductions in trabecular bone volume | Reduced T | Normal | Linear growth increased if ovx done during prepuberty or beginning of puberty. | Increased femoral cross- sectional area at the mid- diaphysis (only if done during prepuberty), reduced cortical bone area, cortical thickness, | Reduced trabecular bone volume in adult mouse | E2 not detectable | Normal, occasionally increased |
| B. Global and cell-specific overexpression; Transgenic mice | ||||||||||
|
Mouse model (ref) |
Males | Females | ||||||||
|
Linear growt h |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Serum IGF-1 |
Linear growth |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Serum IGF-1 |
|
| Aromatase -Tg (human ubiqtin C promoter) (26, 27) | Reduced bone length | Bone area at the femoral shaft decreased, while cortical area and cortical BMD increased (via endocortical bone apposition) | Increased trabecular bone density | Increased E2 and decreased | Not reported | Reduced bone length | Bone area at the shaft decreased. Normal cortical BMD | Normal trabecular bone density | Normal | Not reported |
| OB- Aromatase -Tg (Coll- 1a1 promoter) (28) | Normal | Normal cross- sectional area with increased cortical bone thickness and BMD due to increased endosteal apposition. | Increased trabecular bone density | Normal | Normal | Normal | Increased cortical bone thickness | Normal | Normal | Normal |
| OB-AR-Tg (Col1a13.6 promoter) (43) | Reduced bone length | Reduced cortical bone area, enhanced periosteal bone expantion with inhibited endosteal bone apposition | Increased trabecular bone volume | Normal | Not reported | Normal | Normal | Normal | Normal | Not reported |
| C. Global gene knockout | ||||||||||
|
Mouse model (ref) |
Males | Females | ||||||||
|
Linear growt h |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Serum IGF-1 |
Linear growth |
Radial growth |
Trabecula r bone |
Serum sex steroids |
Seru m IGF-1 |
|
| ERa−/− (29, 32) | Normal | Decreased periosteal circumference, decreased cortical bone thickness and BMC | Increased trabecular bone volume, number, and BMD | Increased E2 and T | Decreased levels | Reduced bone length | Increased cortical bone area and BMD. Normal periosteal circumference with reduced endosteal circumference | Increased trabecular bone volume | Elevated E2 and T | Decreased levels |
| ERb−/− (29, 49, 79) | Normal | Normal | Normal | Not reported | Not reported | Increased femoral length | Increased periosteal circumference, increased cortical bone area, increased cortical BMC | Normal BMD, increased trabecular volume | Not reported | Increased levels |
| ERa/ERb− /− (29–31, 80) | Reduced bone length | Decreased cortical vBMC, decreased cross- sectional area and cortical bone thickness | Slight increase in trabecular bone volume in the adult mouse | Not reported | Decreased levels | Intermediate length between Era−/− and WT. | Increased cortical bone area and BMD. Normal periosteal circumference with reduced endosteal circumference | Decreased trabecular volume and BMD | Elevated E2 and T | Normal |
| Aromatase −/− (81) | Reduced bone length | Total-cross- sectional area was not reported, cortical bone thickness reduced. | Reduced trabecular bone volume | Normal T, Normal Estrone | Not reported | Normal | Total-cross- sectional area was not reported, cortical bone thickness reduced. | Reduced trabecular bone volume | Increased T, normal serum Estrone, but decreased uterin weight, suggesting estrogen deficiency | Not reported |
| 5a- reductase 1−/− (82) | Normal | Decreased periosteal circumference, decreased cortical bone area | Reduced BMD/BMC | Normal E2, T, DHT | Normal | Normal | Normal periosteal circumference, increased cortical bone area | Normal | Normal E2, elevated T and DHT | Normal levels |
| AR−/− (32, 41, 42) | Normal | Decreased periosteal circumference, decreased cortical bone thickness and BMC | Diminished trabecular bone volume and number | Increased E2 and decreased T | Normal | Normal | Normal | Normal | Normal E2 and decreased T | Not reported |
| CMV- mediated Cre AR−/− (42) | Normal | Decreased cortical bone thickness and BMC | Diminished trabecular bone volume and number | Decreased T, normal E2 | Not reported | Normal | Normal | Normal | Decreased T, normal E2 | Not reporte d |
| AR/ERa−/− (32) | Normal | Decreased periosteal and endosteal circumference, decreased cortical bone thickness and BMC | Diminished trabecular bone volume and number | Normal E2 and decreased T | Decreased levels | Not reported | Not reported | Not reported | Not reported | Not reported |
| D. Cell specific gene knockout | ||||||||||
|
Mouse model (ref) |
Males | Females | ||||||||
|
Linear growt h |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Seru m IGF-1 |
Linear growt h |
Radial growth |
Trabecula r bone |
Serum sex steroid s |
Seru m IGF-1 |
|
| OB progenitors ERa−/− (Prx promoter) (33) | Not reported | Decreased cortical bone thickness | Normal | Not reported | Not reported | Normal | Decreased cortical bone thickness | Normal | Not reported | Not reported |
| OB progenitors ERa−/− Osx-1 promoter) (33) | Not reported | Not reported | Not reported | Not reported | Not reported | Reduced bone length | Decreased cortical bone thickness, decreased periosteal and endosteal circumferences | Normal | Not reported | Not reported |
| OB-Era−/− (Col1a1 promoter) (33) | Not reported | Normal femur BMD | Not reported | Not reported | Not reported | Normal | Normal | Normal | Not reported | Not reported |
| OB-Era−/− (osteocalcin promoter) (34, 35) | Normal | Normal, a small reduction in endosteal circumference at one age point | Reduced BMD/BMC in adult mice, reduced trabecular number | Normal | Not reported | Normal | Reduced cortical BMC, reduced cortical bone thickness and periosteal circumference in adult mice | reduced BMD/BMC in adult mice, reduced trabecular number and thickness | Normal | Not reported |
| Osteocyte- ERa−/− (DMP-1 promoter) (36) | Normal | Normal | Reduced trabecular bone volume | Normal | Not reported | Normal | Normal | Normal | Normal | Not reported |
| Chondrocyte -ERa−/− (Col2a1 promoter) (39) | Normal | Normal | Normal | Normal | Normal | Normal during growth, show increased length during adulthood | Normal | Normal | Normal | Normal |
| OC-ERa−/− (Cathepsin K promoter) (37) | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Reductions in trabecular bone volume | Normal | Normal |
| OC-ERa−/− (Lysm promoter) (38, 44) | Normal | Normal | Normal | Not reported | Not reported | Not reported | Normal femur BMD during growth, show slight decreases in femoral BMD during adulthood, decrease in cortical bone thickness | Decreased trabecular bone volume | Not reported | Not reported |
| neurone- ERa−/− (nestin promoter) (40) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Increased cortical vBMD | Increased trabecular bone volume | Normal | Not reported |
| OB progenitors AR−/− (Prx promoter) (44) | Normal | Normal | Reduced trabecular bone volume | Not reported | Not reported | Not reported | Not reported | Decreased trabecular BMD | Not reported | Not reported |
| OB-AR−/− (col2.3 promoter) (45) | Normal | Normal | Reduced trabecular bone volume after 32 weeks of age | Normal | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| OB-AR−/− (osteocalcin promoter) (46) | Normal | Normal | Reduced trabecular bone volume at 6 months of age | Not reported | Not reported | Normal | Normal | Reduced trabecular bone volume at 6 months of age | Not reported | Not reported |
| Osteocyte- AR−/− (DMP- 1 promoter) (47) | Normal | Normal | Reduced trabecular bone volume mainly after sexual maturation (in the adult) | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| OC-AR−/− (Lysm promoter) (44) | Not reported | Normal | Normal | Not reported | Not reported | Not reported | Normal | Normal | Not reported | Not reported |
The estrogen receptor
To better understand the roles of sex steroids in skeletal acquisition and growth, animal models of global or tissue-specific ablation of the estrogen and androgen receptors were generated. There are two known E2 receptors; ERa and ERb, which are expressed in bone (including the growth plate) and regulate skeletal homeostasis. ERa and ERb have different actions on bone. ERa null females show decreased bone length, while ERb null females exhibit increases in bone length (29). In female mice, ablation of ERa results in increased body adiposity and increase in trabecular bone volume, while ablation of the ERb does not affect body composition but results also in increased trabecular bone traits (Table 1C). With respect to the cortical bone compartment, both ERa and ERb female null mice showed increases in total cross-sectional bone area, which associated with increased cortical bone thickness, suggesting that ERa/b inhibit radial bone growth. In males, ablation of ERa did not affect bone length, however resulted in significantly decreased total cross-sectional area, decreased cortical bone thickness, and thus decreased cortical BMC, suggesting that ERa plays essential roles in establishing bone size in males. Interestingly, total ablation of both Era and ERb in mice resulted in a bone phenotype similar to that observed for the ERa single gene ablation in both sexes. In males, double knockout of ERa/ERb resulted in decreased linear growth, decreased cortical bone area and thickness, which associated with decreases in serum IGF-1 levels (29–31). Trabecular bone, however, increased in the ERa/ERb double knockout male mice. In contrast, in females double knockout of ERa/ERb resulted in normal periosteal circumference but increased cortical thickness, while trabecular bone volume and BMD decreased (29–31). It should be noted that ablation of both AR and the ERa in male mice resulted in decreased cortical bone traits and diminished trabecular bone, which associated with decreased levels of serum IGF-1 (32). Overall these studies suggest that estrogen plays a role in skeletal growth and integrity in both sexes and that the predominant sex steroid receptor affecting bone morphology and BMD is the ERa.
Notwithstanding the valuable data from animal models with global ER inactivation, interpretation of the data is complicated by the fact that these mice show elevated serum sex steroid levels. To overcome this caveat a strategy of cell-specific ER ablation was developed, in which systemic regulation of sex steroid is normal (Table 1D). Ablation of the ERa in osteoblast-progenitors (using the paired related homeobox 1 (Prx) or osterix (Osx) promoter-driven Cre) resulted in decreased cortical vBMD in both sexes with no effect on trabecular bone (33), suggesting that in contrast to the inhibitory effects of E2 on periosteal bone expansion (seen with the global gene ablation approach), ERa in osteoblast-precursors is required for periosteal bone formation and optimal bone accrual during growth in males and females. On the other hand, ablation of ERa in mature osteoblasts (using the collagen 1 alpha 1 (Col1a1) promoter) did not affect bone mass or bone morphology in female mice (33). However, an independent study where inactivation of ERa in osteoblasts (OB-ERa−/−) was achieved via the osteocalcin promoter-driven Cre, bone accrual reduced during growth in female mice but not in male mice (34, 35). This was reflected by decreased cortical bone area and cortical BMC as well as by diminished trabecular bone volume in female OB-ERa−/− mice. Male OB-ERa−/− mice showed decreases in trabecular bone volume only during adulthood, suggesting that OB-ERa may play roles in trabecular bone maintenance in males (34, 35). Ablation of the ERa in osteocytes, the bone resident cells, using the DMP-1 promoter-driven Cre, did not affect cortical bone in both sexes, but resulted in decreased trabecular bone volume in male mice, suggesting that trabecular bone sparing in males is mediated by ERa in osteocytes (36). In females, however, trabecular bone sparing is mediated via ERa on osteoclasts. In two independent studies where the ERa was ablated in osteoclasts using the cathepsin K promoter-driven Cre (37) or the Lysm promoter-driven Cre (38), female mice showed significant decreases in trabecular bone volume, while no effects on bone reported for male mice. Sex differences in linear bone growth were studied in mice lacking the ERa in chondrocytes, using the Col2a1 promoter-driven Cre (39). Ablation of ERa in chondrocytes did not affect linear growth during sexual maturation, however, resulted in continuous growth in female mice, suggesting an inhibitory effect of ERa in the female growth plate. In addition to bone cells, ERa was also ablated in the central nervous system using the nestin promoter-driven Cre (40). This study showed that inactivation of central ERa resulted in increased bone mass in female mice (males were not reported). The authors reported secondary increases in circulating leptin levels that may have contributed to increases in bone formation. The relative contribution of central ERa in contributing to E2 effects on the skeleton remain to be established.
The androgen receptor
Global (Table 1C) and tissue-specific (Table 1D) ablations of the androgen receptor have advanced our understanding of SSD. Mouse models with total ablation of the AR exhibit a phenotype similar to that obtained after ORX, with decreased cortical bone area and circumference owing to inhibited periosteal bone apposition, as well as diminished trabecular bone volume (32, 41, 42). On the other hand, overexpression of the AR under the Col1a1-3.6 promoter, specifically in osteoblasts, resulted in shorter bones with enhanced periosteal apposition, but significantly decreased endosteal apposition, producing thinner cortices (43). Despite decreases in cortical bone volume, trabecular bone volume in the osteoblast-specific AR transgenic mice increased (43). Neither total AR ablation nor osteoblast-specific AR overexpression resulted in a skeletal phenotype in female mice, suggesting that the AR plays minor roles in skeletal acquisition and morphology in the female mice.
The cre/loxP system was also used to ablate the AR in a cell-specific manner to better understand how AR actions in osteogenic cells regulate bone accrual and SSD. Ablation of AR in osteoblast progenitors using the Prx promoter-driven Cre resulted in decreased trabecular volume in male mice and trabecular BMD in female mice, suggesting that the anti-resorptive actions of AR on trabecular bone are mediated via cells of the osteoblastic lineage (44). Likewise, AR inactivation in mature osteoblasts using the Col2.3 (45) or the osteocalcin (46) promoter-driven Cre, resulted in decreased trabecular bone volume in males only during advanced adulthood (24–32 weeks of age), while in females this was observed only using the osteocalcin promoter-driven Cre, also in late adulthood (46). These studies suggest that during adulthood, AR in osteoblasts plays a role in maintaining trabecular bone integrity. Similarly, ablation of AR in osteocytes, using the DMP-1 promoter-driven Cre, did not affect cortical bone morphology, but resulted in decreased trabecular bone volume in male mice (47). In contrast to the effects of AR ablation in the osteoblasts lineage on trabecular bone, inactivation of AR in the myeloid lineage did not affect bone morphology in either sex (44), suggesting that AR does not exert its effects on the skeleton via osteoclasts.
In short, AR stimulates periosteal bone growth in male mice and is important in maintaining trabecular bone integrity via its actions on the osteoblastic lineage. AR dose not exert any effects on bone via cells of the myeloid lineage. In female mice, AR has minimal effects mainly on trabecular bone, and these are mediated via its actions on the osteoblastic cell lineage. On the other hand, the ER has effects on bone in both sexes. ERa is the main regulator of skeletal acquisition in both sexes. In females, ERa exerts its effects on bone via its actions in cells of the osteoblast lineage as well as via inhibition of osteoclast activity and induction of osteoclasts apoptosis. ERa inhibits periosteal bone apposition in female mice but enhances peak bone mass via its actions on pre- and mature osteoblasts. In male mice, ERa actions in cells of the osteoblast lineage are important for maintaining trabecular bone volume. On the other hand, ERb does not appear to play a role in skeletal acquisition in the male mice, while in females it is important for enhancement of bone length and periosteal bone acquisition (29, 48, 49).
Experimental evidence for the contribution of GH/IGF-1 to SSD
GH is secreted from the pituitary in a sex dependent manner in humans and many animal models. In both humans and rodents, female pituitaries secrete GH in a continuous manner with frequent peaks and short GH-free intervals. In contrast, in males GH peaks are higher, less frequent, and the GH-free intervals between the peaks are longer. The sex differences in GH secretion pattern translate into sex differences in liver gene expression that largely depend on STAT5b, the GH receptor (GHR) downstream mediator (11). Sex differences in GH-regulated genes were also found in heart and kidney (50), however, were not studied in details at the molecular level in bone or in the growth plates. GH production and release are significantly affected by sex hormones. In fact, GH and estrogen levels show positive correlations in prepubertal girls and boys (51, 52). GH levels in prepubertal boys are lower than those of pubertal boys, and when prepubertal boys are given a bolus of testosterone, GH levels increase (53). It has been shown that the stimulatory effect of testosterone on GH secretion is partially mediated via its aromatization into estrogen, since treatment with nonaromatizable androgens (oxandrolone or DHT) failed to increase GH secretion (54–56). Interestingly, a cross-sectional study with North American males showed that GH peaks during puberty correlate with peak height velocity. However, after reaching final height, GH levels decline to prepubertal levels, despite sustained increases in sex steroids, suggesting that the impact of sex steroids on GH secretion happen largely during the years of puberty (57). Likewise, estrogen given orally increases GH secretion in prepubertal girls (58). Further, children with precocious puberty are often treated with GnRH analog, leading to inhibition of sex steroid secretion and consequent reductions in serum GH/IGF-1 levels (59–61). While normal linear growth and skeletal acquisition in both sexes require cross talk between the somatotropic (GH/IGF-1) and gonadotropic (E2/T) axes, there are still important unanswered questions: 1) What is the contribution of the somatotropic axis to SSD? 2) What are the relevance of the findings in animal models to humans? When answering these questions we should also differentiate between the contribution of the circulating pool of GH/IGF-1 and the contributions of the tissue levels/actions of these two pleotropic factors to SSD.
As mentioned above, in mice, radial bone growth, which depends on periosteal and endosteal bone apposition, contributes to SSD. Studies have established that in males absolute periosteal bone apposition is higher than females, while endosteal bone apposition is higher in females during pubertal growth (23). Skeletal characterizations of several mouse models with impaired GH/IGF-1 axis have established a link between this axis and radial bone growth; GHRKO mice exhibit ~95% decreases in serum IGF-1 levels along with significant reductions in tissue IGF-1 gene expression. Body weight of GHRKO male and female mice were significantly decreased as compared to controls, but did not show significant sex differences (23). GHRKO mice show impaired skeletal growth, evident by reductions in bone length and bone diameter with severely decreased periosteal bone apposition, but without gender-genotype interactions (23). However, E2 treatment of gonadectomized male (ORX) GHRKO mice resulted in increased serum IGF-1 levels, enhanced radial bone growth, evident by increased total cross-sectional area, increased endocortical and periosteal bone perimeter, and thickening of the cortex (62), suggesting that serum IGF-1 may contribute to SSD via its interactions with E2 (independent of GH). Likewise, IGF-1KO male and female mice exhibit severe decreases in longitudinal bone growth, radial bone growth, mineral apposition and bone formation rates, as compared to controls (63), but do not show SSD. Therefore, the two detailed skeletal characterizations of both sexes of the global GHRKO and the IGF-1KO mice demonstrate that the actions of GH/IGF-1 axis on bone are sexually dimorphic and likely involve interactions with sex steroids. Nonetheless, we should keep in mind that both hormones affect the reproduction systems and that the GHRKO and the IGF-1KO male mice exhibit compromised or blunted fertility, respectively, associated with significant reductions in testicular and seminal vesicle weights. GHRKO mice, for example, show low levels of serum luteinizing hormone (LH) and reduced LH-induced testosterone release (64). Thus, interpretation of the data is complicated since in GHRKO or the IGF-1KO mice both the somatotropic and gonadotropic axes are compromised.
Over the last 10 years several mouse models with decreased/elevated serum levels of IGF-1 as well as mouse models with bone-specific disruption of the GH/IGF-1 axis were characterized. We summarize herein data on longitudinal and radial bone growth of the femur in mice on two genetic backgrounds (FVB/N (Figure 1) and C57Bl/6 (Figure 2)) in both sexes after peak bone mass acquisition, at 16 weeks of age. Undoubtedly, there are differences between sexes also during aging, however data is not yet available for all the models.
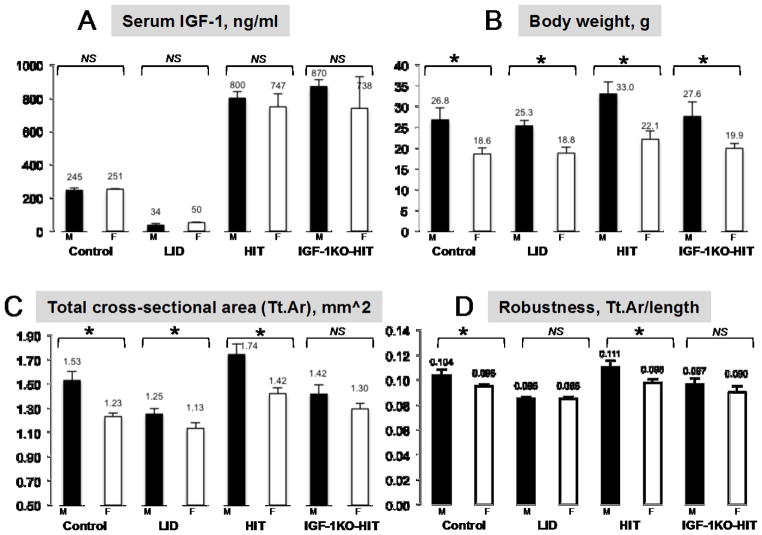
Figure 1. Manipulations of endocrine IGF-1 do not contribute to SSD.
A. Serum IGF-1 levels of mice at 16 weeks of age, determined by RIA in control (n=12 per sex), liver IGF-1 deficient, LID mice (n=12 per sex), hepatic IGF-1 transgenic, HIT mice (n=9 per sex), and IGF-1 null mice expressing the HIT, IGF-1KO-HIT mice (n=9 per sex). B. Body weight of mice at 16 weeks of age; Controls, LID, HIT, and IGF-1KO-HIT mice (n>15 per sex in each genotype). C–D. Micro-CT data of femurs from 16 weeks old mice on FVB/N genetic background, acquired using the eXplore Locus SP Pre-Clinical Specimen MicroComputed Tomography system (GE Healthcare, London, Ontario) at 8.7micron voxel resolution; Controls (female n=15, male n=9), LID (female n=8, male n=9), HIT (female n=14, male n=11), and IGF-1KO-HIT (female n=13, male n=10) mice. Data presented as mean±SEM for serum IGF-1 levels and body weight, while for skeletal traits by mCT we report mean±SD. Statistical difference between sexes was determined by t-test, where significance accepted at p<0.05.
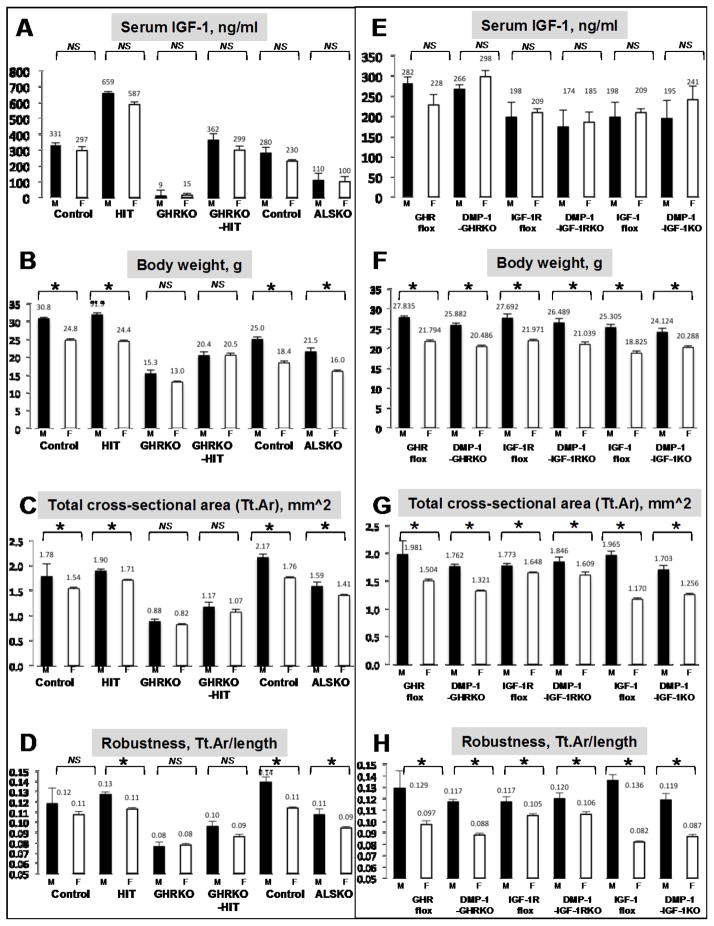
Figure 2. Reductions/elevations in serum IGF-1 or inactivation of the GH/IGF-1 axis in osteocytes do not contribute to SSD.
A., E. Serum IGF-1 levels of mice at 16 weeks of age, determined by RIA in control, hepatic IGF-1 transgenic, HIT mice, GHRKO, GHRKO mice expressing the HIT, GHRKO-HIT mice, GHR floxed mice, DMP-1-GHRKO mice, IGF-1R floxed mice, DMP-1-IGF-1RKO mice, IGF-1 floxed mice, and DMP-1-IGF-1KO mice (n>8 per sex in each genotype). B., F. Body weight of mice at 16 weeks of age; Controls, hepatic IGF-1 transgenic, HIT mice, GHRKO, GHRKO mice expressing the HIT, GHRKO-HIT mice, GHR floxed mice, DMP-1-GHRKO mice, IGF-1R floxed mice, DMP-1-IGF-1RKO mice, IGF-1 floxed mice, and DMP-1-IGF-1KO mice (n>15 per sex in each genotype). C-H. Micro-CT data of femurs from 16 weeks old mice on C57Bl/6 genetic background, acquired using the SkyScan 1172 (Microphotonics) system at 9.7micron voxel resolution. Controls (female n=14, male n=14), hepatic IGF-1 transgenic, HIT mice (female n=12, male n=12), GHRKO (female n=12, male n=12), GHRKO mice expressing the HIT, GHRKO-HIT mice (female n=8, male n=15), GHR floxed mice (female n=10, male n=13), DMP-1-GHRKO mice (female n=13, male n=16), IGF-1R floxed mice (female n=7, male n=10), DMP-1-IGF-1RKO mice (female n=7, male n=7), IGF-1 floxed mice (female n=11, male n=10), and DMP-1-IGF-1KO mice (female n=12, male n=11). Data presented as mean±SEM for serum IGF-1 levels and body weight, while for skeletal traits by mCT we report mean±SD. Statistical difference between sexes was determined by t-test, where significance accepted at p<0.05.
Generation of the liver IGF-1 deficient (LID) mice enabled dissection of the roles of serum IGF-1 in SSD. Initial studies with the LID mice revealed sexual dimorphism in GH-induced gains in body weight (65). While LID female mice, treated daily with GH, showed increases in body weight as compared to vehicle treated mice, treatment of male LID mice with GH did not result in increases in body weight. This sexual dimorphic response to GH was also seen when gonads were removed from the LID mice, such that OVX LID females increased body weight in response to GH, while ORX LID males did not (65), suggesting that sex differences may have been imprinted early in development, or that the gonadal steroids environment does not play a role in sexual dimorphic response to GH. Unfortunately, bone morphology was not assessed in that study. Later on, detailed skeletal characterization of LID male (66) and female (67) mice (on FVB/N genetic background) has taught us that reductions in serum IGF-1 levels minimally affect linear growth but cause significant reductions in radial bone growth in both sexes. Nonetheless, despite 75% reductions in serum IGF-1 levels, LID mice displayed sexual dimorphism, such that males LID were heavier than female LID mice and total cross-sectional area (Tt.Ar) at the femoral mid-diaphysis of male LID mice was larger than that of LID females (Figure 1). However, robustness, a measure of radial versus longitudinal bone growth (Tt.Ar/length), which is normally higher in males than females, did not differ between LID males and females, suggesting a possible contribution of serum IGF-1 to SSD likely via affecting longitudinal bone growth. On the other hand, 2fold increase in serum IGF-1 levels in hepatic IGF-1 transgenic (HIT) mice (on FVB/N genetic background) (68, 69) resulted in increased body weight and skeletal properties in the HIT mice, such that males HIT were heavier than female HIT mice and total cross-sectional area at the femoral mid-diaphysis of male HIT mice was larger than that of HIT females (Figure 1). Lastly, robustness of bones from HIT males was significantly higher than HIT females (on FVB/N genetic background). Together, these studies suggest that although serum IGF-1 pool regulates diaphysial radial bone growth, its contribution to SSD is minimal. As mentioned above, total ablation of IGF-1 caused decreases in body weight, bone length and bone diameter, and loss of SSD (63), suggesting that tissue/bone IGF-1 action is important in determining SSD. Indeed, even when serum IGF-1 was restored in the IGF-1 null mice by expressing the hepatic IGF-1 transgene (IGF-1KO-HIT) (68–70), it was not able to restore SSD, and despite significant differences in body weight between male and female IGF-1KO-HIT mice, total cross-sectional area at the femoral mid-diaphysis and bone robustness did not differ between sexes (Figure 1).
Mice were also characterized on C57Bl/6 genetic background (Figure 2). SSD was evaluated in a mouse model where the acid labile subunit (ALS), an IGF-1 carrier protein, was ablated in mice (ALSKO). Like the LID, ALSKO mice exhibit ~65% reductions in serum IGF-1 (Figure 2A) associated with minor decreases in bone length but significant reductions in bone width in both sexes. Despite the significant differences in skeletal properties between controls and ALSKO mice, we found SSD such that ALSKO males were heavier than female ALSKO mice (Figure 2B) and total cross-sectional area at the femoral mid-diaphysis of ALSKO male mice was larger than that of ALSKO females (Figure 2C). Once again, the ALSKO model revealed that although serum IGF-1 levels regulate transversal bone growth, they do not determine SSD. In contrast, GHRKO mice with diminished serum IGF-1 levels (Figure 2A) and decreased skeletal IGF-1 gene expression, exhibit decreases in body weight, bone length and bone diameter (63), and do not show SSD. Our findings with GHRKO mice on C57Bl/6 genetic background are similar (Figure 2A–D). Furthermore, even when serum IGF-1 was restored in the GHRKO mice by expressing the hepatic IGF-1 transgene (GHRKO-HIT) (71), it was not able to restore SSD, such that body weight and total cross-sectional area at the femoral mid-diaphysis did not differ between sexes of the GHRKO-HIT mice (Figure 2A–D). Once again, this model suggests that tissue/bone GH/IGF-1 actions, and not serum IGF-1, are more important in determining SSD and may interact with sex steroids indirectly or directly at the bone level.
The role of the IGF binding proteins (IGFBPs), which control the levels of circulating IGF-1 and the bound/free IGF-1 ratio in tissues, in regulathion of SSD was not explored in details. Studies with IGFBP-2 null mice revealed sex-, age-, and compartment-specific differences in skeletal parameters as compared to controls (72). In particular, male IGFBP-2 null mice exhibited reduced trabecular bone density at 8 and 16 weeks of age, whereas female IGFBP-2 null mice had increased cortical bone acquisition such that at 8 weeks radial bone growth did not differ between sexes (of IGFBP-2 null mice) but, several weeks after the release of pubertal hormones sex differences were detected. In a study that explored the role of several binding proteins on body size and metabolism, the authors have shown that ablation of IGFBP-3 or IGFBP-5, the main carriers of IGF-1 in serum, and the only binding proteins that form ternary complex with ALS, did not alter growth as measured by body weight and body length (73). On the other hand, IGFBP-4 null mice showed growth retardation when compared to controls however, as seen for the IGFBP-3 or IGFBP-5 null mice, they also retained sex differences in body weight and length (73). Unfortunately, SSD was not explored in that study. Skeletal morphology was also studied in IGFBP-4 or IGFBP-5 transgenic mouse models. overexpression of IGFBP-4 in osteoblasts decreases bone turnover and causes global growth retardation (74). Morphometric analyses of skeletal growth assessed at 6 weeks of age of both males and females, showed reduced femur lengths and significant reductions in cortical bone volume as compared to WT mice. This was attributed to sequestration of IGF-1 from binding to its receptor. Interestingly, the authors reported no significant differences in morphometric parameters between males and females, suggesting that IGFBP-4 plays a role in SSD. However, we note that the sample size was small (4 males, 6 females) and analyses were done during the rapid growing phase in mice. Thus, conclusions regarding SSD in that case are hard to make. Overexpression of IGFBP-5 in mice revealed sex-dependent effects where males affected more significantly than females (75). Body weight and body/tibial lengths differed significantly between sexes in IGFBP-5 transgenic mice. However, with respect to the absolute values of BMD the authors did not find sex differences, suggesting that Igfbp5 overexpression has a sex-neutralizing effect. The differences in BMD between sexes were attributed to a significantly greater rate of bone formation at the endosteum in females, and reduced bone formation at the periosteum in both sexes. Nonetheless, several weeks after the release of pubertal hormones sex differences were noted in the IGFBP-5 transgenic mice.
All osteogenic cells express the IGF-1 receptor and the GHR and may contribute to SSD via interactions with the AR or ER. However, despite extensive studies on the effects of cell-specific inactivation of IGF-1, IGF-1R, and the GHR, sex differences were not always reported. IGF-1 was ablated in osteoblasts using the Col1a2 promoter-driven Cre and resulted in significant reductions in cortical and trabecular bone volumes, significantly inhibited periosteal and endosteal bone apposition (76, 77), however, sex differences were not reported. Ablation of the IGF-1R in osteoblasts using the osteocalcin promoter-driven Cre resulted in mineralization defect (that was tested in young adult mice at 6–8 weeks of age), affecting mostly trabecular bone volume (78), but once again sex differences were not reported. Likewise, inactivation of the IGF-1R in chondrocytes (using the Col2a1 promoter-driven Cre) resulted in “collapse” of the growth plates and severe growth retardation (79, 80), but SSD was not studied. IGF-1 gene inactivation in chondrocytes (using the Col2a1 promoter-driven Cre) resulted also in reduced bone length, bone diameter, and BMC (81). However, all growth and skeletal parameters showed sex differences with males being greater than females, suggesting that although chondrocyte-derived IGF-1 plays significant roles in long bone growth, it does not contribute to SSD.
Recently, the GH/IGF-1 axis was studied in osteocytes. Members of the GH/IGF-1 axis were ablated in mature osteoblasts/osteocytes using the DMP-1 promoter-derived Cre (Figure 2E–H). Disruption of IGF-1 in osteocytes resulted in minor reduction in bone length (4–7%), but significant reductions in radial bone growth in female mice (82). Using a similar strategy, we found differences in radial bone growth in both sexes (83). DMP-mediated IGF-1KO mice exhibited SSD, as was evident by sex differences in body weight (Figure 2F), total cross-sectional area of the femur (Figure 2G), and bone robustness (Figure 2H), suggesting that the lack of local/bone IGF-1 production does not contribute to SSD and may be compensated for by the normal serum IGF-1 levels in the DMP-1-IGF-1KO mice. When the IGF-1R was ablated in mature osteoblasts/osteocytes we found significant reductions in cortical bone area in both sexes, as well as significant reductions in cortical bone thickness as compared to controls (83), likely due to increased endosteal bone resorption in the DMP-1-IGF-1R. However, also here, despite ablation of the IGF-1R in mature osteoblasts/osteocytes the mice exhibited SSD. Inactivation of the GHR in osteocytes also compromised skeletal integrity and resulted in reduced radial bone growth, producing slender bones in both sexes (83). However, as for IGF-1 and the IGF-1R, we found that DMP-mediated GHRKO mice exhibit SSD (Figure 2E–H).
Overall, we are certain that the somatotropic axis affects bone morphology and strength. Total inactivation of the GHR or the IGF-1 results in growth retardation, compromised skeletal growth in both sexes, and blunted SSD. The lack of SSD in GHRKO or the IGF-1KO mice is likely due to indirect interactions of GH/IGF-1 and sex steroids in bone, or alternatively, may due to secondary effect on other tissues that participate in regulation of bone metabolism. Mice with genetic manipulations of serum IGF-1 levels (LID, ALSKO, and HIT mice) retained their SSD, and even inactivation of members of the GH/IGF-1 axis in mature osteoblast/osteocytes (DMP-1-mediated gene recombination) did not affect SSD. Data from mouse models where the GH/IGF-1 axis was impaired in a bone-cell specific manner are not facile to interpret for several reasons; 1) not all studies addressed sex effects, 2) not all animals were studied at the same sexual maturation state, 3) not all studies use the same methodology to assess skeletal morphology, and 4) not all mice were on a similar genetic background.
Supplementary Material
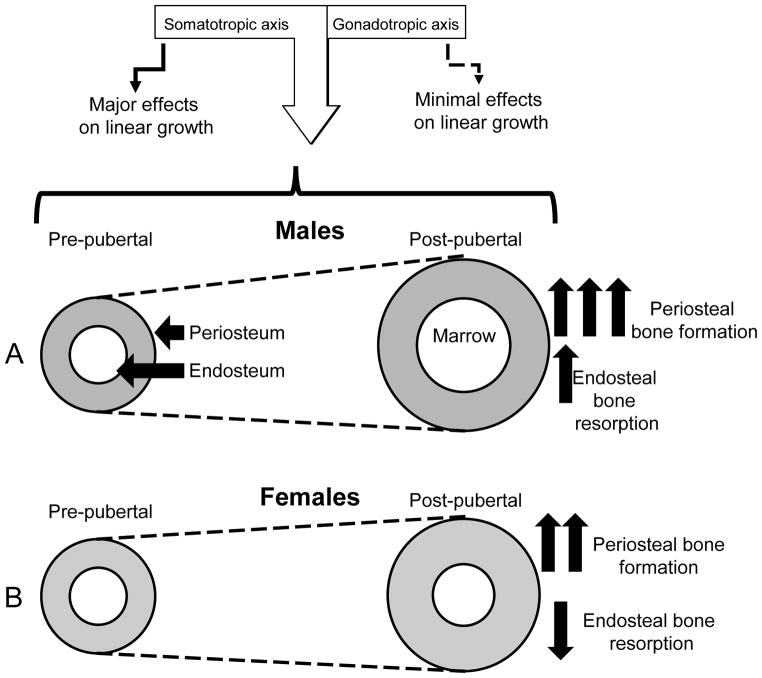
Figure 3. Integrations of signals from the gonadotropic and somatotropic axes regulate radial bone growth in males and females.
Pubertal growth associates with increases in serum sex steroids as well as increases in the levels of GH and IGF-1. During that period both males and females increase linear and radial bone growth and accumulate bone mineral that reaches its peak when growth is ceased. A. During puberty males increase periosteal bone apposition and endosteal bone resorption leading to overall increase in bone diameter and a more robust skeleton. During that period both the somatotropic and the gonadotropic axes activate periosteal bone apposition in males. B. In contrast, during pubertal growth in females, periosteal bone apposition is slower than in males, while endosteal bone resorption decreases leading to preservation of the amount of cortical bone. During that period the somatotropic axis enhances endosteal bone apposition in females, while the gonadotropic axis (estrogen) inhibits periosteal bone apposition.
Acknowledgments
Funding: Financial support received from funding agencies in the United States. NIH Grant DK100246 to SY, NIH Grant AR048139 to SM, Endocrine Scholars Award (by Genentech) in Growth Hormone Research to ZL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol. 1976;3:109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 2.Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 3.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–1850. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 4.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–4584. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 5.Schoenau E, Neu CM, Rauch F, Manz F. The development of bone strength at the proximal radius during childhood and adolescence. J Clin Endocrinol Metab. 2001;86:613–618. doi: 10.1210/jcem.86.2.7186. [DOI] [PubMed] [Google Scholar]
- 6.Libanati C, Baylink DJ, Lois-Wenzel E, Srinvasan N, Mohan S. Studies on the potential mediators of skeletal changes occurring during puberty in girls. J Clin Endocrinol Metab. 1999;84:2807–2814. doi: 10.1210/jcem.84.8.5905. [DOI] [PubMed] [Google Scholar]
- 7.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 8.Veldhuis JD, Anderson SM, Shah N, Bray M, Vick T, Gentili A, Mulligan T, Johnson ML, Weltman A, Evans WS, Iranmanesh A. Neurophysiological regulation and target-tissue impact of the pulsatile mode of growth hormone secretion in the human. Growth Horm IGF Res. 2001;11(Suppl A):S25–37. doi: 10.1016/s1096-6374(01)80005-8. [DOI] [PubMed] [Google Scholar]
- 9.Jansson JO, Eden S, Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985;6:128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- 10.MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131:395–399. doi: 10.1677/joe.0.1310395. [DOI] [PubMed] [Google Scholar]
- 11.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 12.Cohen P, Bright GM, Rogol AD, Kappelgaard AM, Rosenfeld RG. Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002;87:90–98. doi: 10.1210/jcem.87.1.8150. [DOI] [PubMed] [Google Scholar]
- 13.Mauras N. Growth hormone and sex steroids. Interactions in puberty. Endocrinol Metab Clin North Am. 2001;30:529–544. doi: 10.1016/s0889-8529(05)70200-0. [DOI] [PubMed] [Google Scholar]
- 14.Span JP, Pieters GF, Sweep CG, Hermus AR, Smals AG. Gender difference in insulin-like growth factor I response to growth hormone (GH) treatment in GH-deficient adults: role of sex hormone replacement. J Clin Endocrinol Metab. 2000;85:1121–1125. doi: 10.1210/jcem.85.3.6463. [DOI] [PubMed] [Google Scholar]
- 15.Bex M, Abs R, Maiter D, Beckers A, Lamberigts G, Bouillon R. The effects of growth hormone replacement therapy on bone metabolism in adult-onset growth hormone deficiency: a 2-year open randomized controlled multicenter trial. J Bone Miner Res. 2002;17:1081–1094. doi: 10.1359/jbmr.2002.17.6.1081. [DOI] [PubMed] [Google Scholar]
- 16.Gotherstrom G, Svensson J, Koranyi J, Alpsten M, Bosaeus I, Bengtsson B, Johannsson G. A prospective study of 5 years of GH replacement therapy in GH-deficient adults: sustained effects on body composition, bone mass, and metabolic indices. J Clin Endocrinol Metab. 2001;86:4657–4665. doi: 10.1210/jcem.86.10.7887. [DOI] [PubMed] [Google Scholar]
- 17.Bengtsson BA, Abs R, Bennmarker H, Monson JP, Feldt-Rasmussen U, Hernberg-Stahl E, Westberg B, Wilton P, Wuster C. The effects of treatment and the individual responsiveness to growth hormone (GH) replacement therapy in 665 GH-deficient adults. KIMS Study Group and the KIMS International Board. J Clin Endocrinol Metab. 1999;84:3929–3935. doi: 10.1210/jcem.84.11.6088. [DOI] [PubMed] [Google Scholar]
- 18.Drake WM, Coyte D, Camacho-Hubner C, Jivanji NM, Kaltsas G, Wood DF, Trainer PJ, Grossman AB, Besser GM, Monson JP. Optimizing growth hormone replacement therapy by dose titration in hypopituitary adults. J Clin Endocrinol Metab. 1998;83:3913–3919. doi: 10.1210/jcem.83.11.5223. [DOI] [PubMed] [Google Scholar]
- 19.Johannsson G, Rosen T, Bengtsson BA. Individualized dose titration of growth hormone (GH) during GH replacement in hypopituitary adults. Clin Endocrinol (Oxf) 1997;47:571–581. doi: 10.1046/j.1365-2265.1997.3271123.x. [DOI] [PubMed] [Google Scholar]
- 20.Johannsson G, Bjarnason R, Bramnert M, Carlsson LM, Degerblad M, Manhem P, Rosen T, Thoren M, Bengtsson BA. The individual responsiveness to growth hormone (GH) treatment in GH-deficient adults is dependent on the level of GH-binding protein, body mass index, age, and gender. J Clin Endocrinol Metab. 1996;81:1575–1581. doi: 10.1210/jcem.81.4.8636370. [DOI] [PubMed] [Google Scholar]
- 21.Kappelgaard AM, Laursen T. The benefits of growth hormone therapy in patients with Turner syndrome, Noonan syndrome and children born small for gestational age. Growth Horm IGF Res. 2011;21:305–313. doi: 10.1016/j.ghir.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Jung H, Land C, Nicolay C, De Schepper J, Blum WF, Schonau E. Growth response to an individualized versus fixed dose GH treatment in short children born small for gestational age: the OPTIMA study. Eur J Endocrinol. 2009;160:149–156. doi: 10.1530/EJE-08-0301. [DOI] [PubMed] [Google Scholar]
- 23.Callewaert F, Venken K, Kopchick JJ, Torcasio A, van Lenthe GH, Boonen S, Vanderschueren D. Sexual dimorphism in cortical bone size and strength but not density is determined by independent and time-specific actions of sex steroids and IGF-1: evidence from pubertal mouse models. J Bone Miner Res. 2010;25:617–626. doi: 10.1359/jbmr.090828. [DOI] [PubMed] [Google Scholar]
- 24.Vandenput L, Boonen S, Van Herck E, Swinnen JV, Bouillon R, Vanderschueren D. Evidence from the aged orchidectomized male rat model that 17beta-estradiol is a more effective bone-sparing and anabolic agent than 5alpha-dihydrotestosterone. J Bone Miner Res. 2002;17:2080–2086. doi: 10.1359/jbmr.2002.17.11.2080. [DOI] [PubMed] [Google Scholar]
- 25.Govoni KE, Wergedal JE, Chadwick RB, Srivastava AK, Mohan S. Prepubertal OVX increases IGF-I expression and bone accretion in C57BL/6J mice. Am J Physiol Endocrinol Metab. 2008;295:E1172–1180. doi: 10.1152/ajpendo.90507.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng Z, Li X, Makela S, Vaananen HK, Poutanen M. Skeletal changes in transgenic male mice expressing human cytochrome p450 aromatase. J Bone Miner Res. 2004;19:1320–1328. doi: 10.1359/JBMR.040510. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Nokkala E, Yan W, Streng T, Saarinen N, Warri A, Huhtaniemi I, Santti R, Makela S, Poutanen M. Altered structure and function of reproductive organs in transgenic male mice overexpressing human aromatase. Endocrinology. 2001;142:2435–2442. doi: 10.1210/endo.142.6.8211. [DOI] [PubMed] [Google Scholar]
- 28.Sjogren K, Lagerquist M, Moverare-Skrtic S, Andersson N, Windahl SH, Swanson C, Mohan S, Poutanen M, Ohlsson C. Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J Bone Miner Res. 2009;24:1263–1270. doi: 10.1359/jbmr.090208. [DOI] [PubMed] [Google Scholar]
- 29.Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. 2001;171:229–236. doi: 10.1677/joe.0.1710229. [DOI] [PubMed] [Google Scholar]
- 30.Moverare S, Venken K, Eriksson AL, Andersson N, Skrtic S, Wergedal J, Mohan S, Salmon P, Bouillon R, Gustafsson JA, Vanderschueren D, Ohlsson C. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci U S A. 2003;100:13573–13578. doi: 10.1073/pnas.2233084100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindberg MK, Moverare S, Skrtic S, Alatalo S, Halleen J, Mohan S, Gustafsson JA, Ohlsson C. Two different pathways for the maintenance of trabecular bone in adult male mice. J Bone Miner Res. 2002;17:555–562. doi: 10.1359/jbmr.2002.17.4.555. [DOI] [PubMed] [Google Scholar]
- 32.Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, Van Oosterwyck H, Boonen S, Bouillon R, Verhoeven G, Vanderschueren D. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. Faseb J. 2009;23:232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 33.Almeida M, Iyer S, Martin-Millan M, Bartell SM, Han L, Ambrogini E, Onal M, Xiong J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. Estrogen receptor-alpha signaling in osteoblast progenitors stimulates cortical bone accrual. J Clin Invest. 2013;123:394–404. doi: 10.1172/JCI65910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melville KM, Kelly NH, Khan SA, Schimenti JC, Ross FP, Main RP, van der Meulen MC. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res. 2014;29:370–379. doi: 10.1002/jbmr.2082. [DOI] [PubMed] [Google Scholar]
- 35.Maatta JA, Buki KG, Gu G, Alanne MH, Vaaraniemi J, Liljenback H, Poutanen M, Harkonen P, Vaananen K. Inactivation of estrogen receptor alpha in bone-forming cells induces bone loss in female mice. Faseb J. 2013;27:478–488. doi: 10.1096/fj.12-213587. [DOI] [PubMed] [Google Scholar]
- 36.Windahl SH, Borjesson AE, Farman HH, Engdahl C, Moverare-Skrtic S, Sjogren K, Lagerquist MK, Kindblom JM, Koskela A, Tuukkanen J, Divieti Pajevic P, Feng JQ, Dahlman-Wright K, Antonson P, Gustafsson JA, Ohlsson C. Estrogen receptor-alpha in osteocytes is important for trabecular bone formation in male mice. Proc Natl Acad Sci U S A. 2013;110:2294–2299. doi: 10.1073/pnas.1220811110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borjesson AE, Lagerquist MK, Liu C, Shao R, Windahl SH, Karlsson C, Sjogren K, Moverare-Skrtic S, Antal MC, Krust A, Mohan S, Chambon P, Savendahl L, Ohlsson C. The role of estrogen receptor alpha in growth plate cartilage for longitudinal bone growth. J Bone Miner Res. 2010;25:2690–2700. doi: 10.1002/jbmr.156. [DOI] [PubMed] [Google Scholar]
- 40.Ohlsson C, Engdahl C, Borjesson AE, Windahl SH, Studer E, Westberg L, Eriksson E, Koskela A, Tuukkanen J, Krust A, Chambon P, Carlsten H, Lagerquist MK. Estrogen receptor-alpha expression in neuronal cells affects bone mass. Proc Natl Acad Sci U S A. 2012;109:983–988. doi: 10.1073/pnas.1111436109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venken K, De Gendt K, Boonen S, Ophoff J, Bouillon R, Swinnen JV, Verhoeven G, Vanderschueren D. Relative impact of androgen and estrogen receptor activation in the effects of androgens on trabecular and cortical bone in growing male mice: a study in the androgen receptor knockout mouse model. J Bone Miner Res. 2006;21:576–585. doi: 10.1359/jbmr.060103. [DOI] [PubMed] [Google Scholar]
- 42.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci U S A. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiren KM, Zhang XW, Toombs AR, Kasparcova V, Gentile MA, Harada S, Jepsen KJ. Targeted overexpression of androgen receptor in osteoblasts: unexpected complex bone phenotype in growing animals. Endocrinology. 2004;145:3507–3522. doi: 10.1210/en.2003-1016. [DOI] [PubMed] [Google Scholar]
- 44.Ucer S, Iyer S, Bartell SM, Martin-Millan M, Han L, Kim HN, Weinstein RS, Jilka RL, O'Brien CA, Almeida M, Manolagas SC. The Effects of Androgens on Murine Cortical Bone Do Not Require AR or ERalpha Signaling in Osteoblasts and Osteoclasts. J Bone Miner Res. 2015;30:1138–1149. doi: 10.1002/jbmr.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WS, Glatt V, Kream BE, Handelsman DJ, Morris HA, Zajac JD, Davey RA. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22:347–356. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 46.Maatta JA, Buki KG, Ivaska KK, Nieminen-Pihala V, Elo TD, Kahkonen T, Poutanen M, Harkonen P, Vaananen K. Inactivation of the androgen receptor in bone–forming cells leads to trabecular bone loss in adult female mice. Bonekey Rep. 2013;2:440. doi: 10.1038/bonekey.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinnesael M, Claessens F, Laurent M, Dubois V, Boonen S, Deboel L, Vanderschueren D. Androgen receptor (AR) in osteocytes is important for the maintenance of male skeletal integrity: evidence from targeted AR disruption in mouse osteocytes. J Bone Miner Res. 2012;27:2535–2543. doi: 10.1002/jbmr.1713. [DOI] [PubMed] [Google Scholar]
- 48.Linton K, Howarth C, Wappett M, Newton G, Lachel C, Iqbal J, Pepper S, Byers R, Chan WJ, Radford J. Microarray gene expression analysis of fixed archival tissue permits molecular classification and identification of potential therapeutic targets in diffuse large B-cell lymphoma. J Mol Diagn. 2012;14:223–232. doi: 10.1016/j.jmoldx.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flores-Morales A, Stahlberg N, Tollet-Egnell P, Lundeberg J, Malek RL, Quackenbush J, Lee NH, Norstedt G. Microarray analysis of the in vivo effects of hypophysectomy and growth hormone treatment on gene expression in the rat. Endocrinology. 2001;142:3163–3176. doi: 10.1210/endo.142.7.8235. [DOI] [PubMed] [Google Scholar]
- 51.Veldhuis JD, Roemmich JN, Rogol AD. Gender and sexual maturation-dependent contrasts in the neuroregulation of growth hormone secretion in prepubertal and late adolescent males and females--a general clinical research center-based study. J Clin Endocrinol Metab. 2000;85:2385–2394. doi: 10.1210/jcem.85.7.6697. [DOI] [PubMed] [Google Scholar]
- 52.Kerrigan JR, Rogol AD. The impact of gonadal steroid hormone action on growth hormone secretion during childhood and adolescence. Endocr Rev. 1992;13:281–298. doi: 10.1210/edrv-13-2-281. [DOI] [PubMed] [Google Scholar]
- 53.Mauras N, Blizzard RM, Link K, Johnson ML, Rogol AD, Veldhuis JD. Augmentation of growth hormone secretion during puberty: evidence for a pulse amplitude-modulated phenomenon. J Clin Endocrinol Metab. 1987;64:596–601. doi: 10.1210/jcem-64-3-596. [DOI] [PubMed] [Google Scholar]
- 54.Veldhuis JD, Metzger DL, Martha PM, Jr, Mauras N, Kerrigan JR, Keenan B, Rogol AD, Pincus SM. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth hormone)-insulin-like growth factor I axis in the human: evidence from pubertal pathophysiology and sex-steroid hormone replacement. J Clin Endocrinol Metab. 1997;82:3414–3420. doi: 10.1210/jcem.82.10.4317. [DOI] [PubMed] [Google Scholar]
- 55.Eakman GD, Dallas JS, Ponder SW, Keenan BS. The effects of testosterone and dihydrotestosterone on hypothalamic regulation of growth hormone secretion. J Clin Endocrinol Metab. 1996;81:1217–1223. doi: 10.1210/jcem.81.3.8772602. [DOI] [PubMed] [Google Scholar]
- 56.Keenan BS, Richards GE, Ponder SW, Dallas JS, Nagamani M, Smith ER. Androgen-stimulated pubertal growth: the effects of testosterone and dihydrotestosterone on growth hormone and insulin-like growth factor-I in the treatment of short stature and delayed puberty. J Clin Endocrinol Metab. 1993;76:996–1001. doi: 10.1210/jcem.76.4.8473416. [DOI] [PubMed] [Google Scholar]
- 57.Martha PM, Jr, Rogol AD, Veldhuis JD, Kerrigan JR, Goodman DW, Blizzard RM. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J Clin Endocrinol Metab. 1989;69:563–570. doi: 10.1210/jcem-69-3-563. [DOI] [PubMed] [Google Scholar]
- 58.Mauras N, Rogol AD, Veldhuis JD. Specific, time-dependent actions of low-dose ethinyl estradiol administration on the episodic release of growth hormone, follicle-stimulating hormone, and luteinizing hormone in prepubertal girls with Turner's syndrome. J Clin Endocrinol Metab. 1989;69:1053–1058. doi: 10.1210/jcem-69-5-1053. [DOI] [PubMed] [Google Scholar]
- 59.Mericq MV, Eggers M, Avila A, Cutler GB, Jr, Cassorla F. Near final height in pubertal growth hormone (GH)-deficient patients treated with GH alone or in combination with luteinizing hormone-releasing hormone analog: results of a prospective, randomized trial. J Clin Endocrinol Metab. 2000;85:569–573. doi: 10.1210/jcem.85.2.6343. [DOI] [PubMed] [Google Scholar]
- 60.Neely EK, Bachrach LK, Hintz RL, Habiby RL, Slemenda CW, Feezle L, Pescovitz OH. Bone mineral density during treatment of central precocious puberty. J Pediatr. 1995;127:819–822. doi: 10.1016/s0022-3476(95)70182-6. [DOI] [PubMed] [Google Scholar]
- 61.Attie KM, Ramirez NR, Conte FA, Kaplan SL, Grumbach MM. The pubertal growth spurt in eight patients with true precocious puberty and growth hormone deficiency: evidence for a direct role of sex steroids. J Clin Endocrinol Metab. 1990;71:975–983. doi: 10.1210/jcem-71-4-975. [DOI] [PubMed] [Google Scholar]
- 62.Venken K, Schuit F, Van Lommel L, Tsukamoto K, Kopchick JJ, Coschigano K, Ohlsson C, Moverare S, Boonen S, Bouillon R, Vanderschueren D. Growth without growth hormone receptor: estradiol is a major growth hormone-independent regulator of hepatic IGF-I synthesis. J Bone Miner Res. 2005;20:2138–2149. doi: 10.1359/JBMR.050811. [DOI] [PubMed] [Google Scholar]
- 63.Bikle D, Majumdar S, Laib A, Powell-Braxton L, Rosen C, Beamer W, Nauman E, Leary C, Halloran B. The skeletal structure of insulin-like growth factor I-deficient mice. J Bone Miner Res. 2001;16:2320–2329. doi: 10.1359/jbmr.2001.16.12.2320. [DOI] [PubMed] [Google Scholar]
- 64.Chandrashekar V, Dawson CR, Martin ER, Rocha JS, Bartke A, Kopchick JJ. Age-related alterations in pituitary and testicular functions in long-lived growth hormone receptor gene-disrupted mice. Endocrinology. 2007;148:6019–6025. doi: 10.1210/en.2007-0837. [DOI] [PubMed] [Google Scholar]
- 65.Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141:4436–4441. doi: 10.1210/endo.141.12.7825. [DOI] [PubMed] [Google Scholar]
- 66.Yakar S, Canalis E, Sun H, Mejia W, Kawashima Y, Nasser P, Courtland HW, Williams V, Bouxsein M, Rosen C, Jepsen KJ. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24:1481–1492. doi: 10.1359/JBMR.090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritton JC, Emerton KB, Sun H, Kawashima Y, Mejia W, Wu Y, Rosen CJ, Panus D, Bouxsein M, Majeska RJ, Schaffler MB, Yakar S. Growth hormone protects against ovariectomy-induced bone loss in states of low circulating insulin-like growth factor (IGF-1) J Bone Miner Res. 2010;25:235–246. doi: 10.1359/jbmr.090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elis S, Courtland HW, Wu Y, Fritton JC, Sun H, Rosen CJ, Yakar S. Elevated serum IGF-1 levels synergize PTH action on the skeleton only when the tissue IGF-1 axis is intact. J Bone Miner Res. 2010;25:2051–2058. doi: 10.1002/jbmr.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elis S, Courtland HW, Wu Y, Rosen CJ, Sun H, Jepsen KJ, Majeska RJ, Yakar S. Elevated serum levels of IGF-1 are sufficient to establish normal body size and skeletal properties even in the absence of tissue IGF-1. J Bone Miner Res. 2010;25:1257–1266. doi: 10.1002/jbmr.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elis S, Wu Y, Courtland HW, Sun H, Rosen CJ, Adamo ML, Yakar S. Increased serum IGF-1 levels protect the musculoskeletal system but are associated with elevated oxidative stress markers and increased mortality independent of tissue igf1 gene expression. Aging Cell. 2011;10:547–550. doi: 10.1111/j.1474-9726.2011.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y, Sun H, Basta-Pljakic J, Cardoso L, Kennedy OD, Jasper H, Domene H, Karabatas L, Guida C, Schaffler MB, Rosen CJ, Yakar S. Serum IGF-1 is insufficient to restore skeletal size in the total absence of the growth hormone receptor. J Bone Miner Res. 2013;28:1575–1586. doi: 10.1002/jbmr.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeMambro VE, Clemmons DR, Horton LG, Bouxsein ML, Wood TL, Beamer WG, Canalis E, Rosen CJ. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology. 2008;149:2051–2061. doi: 10.1210/en.2007-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ning Y, Schuller AG, Bradshaw S, Rotwein P, Ludwig T, Frystyk J, Pintar JE. Diminished growth and enhanced glucose metabolism in triple knockout mice containing mutations of insulin-like growth factor binding protein-3, -4, and -5. Mol Endocrinol. 2006;20:2173–2186. doi: 10.1210/me.2005-0196. [DOI] [PubMed] [Google Scholar]
- 74.Zhang M, Faugere MC, Malluche H, Rosen CJ, Chernausek SD, Clemens TL. Paracrine overexpression of IGFBP-4 in osteoblasts of transgenic mice decreases bone turnover and causes global growth retardation. J Bone Miner Res. 2003;18:836–843. doi: 10.1359/jbmr.2003.18.5.836. [DOI] [PubMed] [Google Scholar]
- 75.Salih DA, Mohan S, Kasukawa Y, Tripathi G, Lovett FA, Anderson NF, Carter EJ, Wergedal JE, Baylink DJ, Pell JM. Insulin-like growth factor-binding protein-5 induces a gender-related decrease in bone mineral density in transgenic mice. Endocrinology. 2005;146:931–940. doi: 10.1210/en.2004-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kesavan C, Wergedal JE, Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1alpha collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301:E1191–1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Govoni KE, Wergedal JE, Florin L, Angel P, Baylink DJ, Mohan S. Conditional deletion of insulin-like growth factor-I in collagen type 1alpha2-expressing cells results in postnatal lethality and a dramatic reduction in bone accretion. Endocrinology. 2007;148:5706–5715. doi: 10.1210/en.2007-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 79.Wu S, Yang W, De Luca F. Insulin-Like Growth Factor-Independent Effects of Growth Hormone on Growth Plate Chondrogenesis and Longitudinal Bone Growth. Endocrinology. 2015;156:2541–2551. doi: 10.1210/en.2014-1983. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen MT, Mackem S, Clemens TL, Bikle DD, Chang W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J Bone Miner Res. 2011;26:1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Govoni KE, Lee SK, Chung YS, Behringer RR, Wergedal JE, Baylink DJ, Mohan S. Disruption of insulin-like growth factor-I expression in type IIalphaI collagen-expressing cells reduces bone length and width in mice. Physiol Genomics. 2007;30:354–362. doi: 10.1152/physiolgenomics.00022.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheng MH, Zhou XD, Bonewald LF, Baylink DJ, Lau KH. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52:133–144. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 83.Liu Z, Kennedy OD, Cardoso L, Basta-Pljakic J, Partridge NC, Schaffler MB, Rosen CJ, Yakar S. DMP-1-mediated Ghr gene recombination compromises skeletal development and impairs skeletal response to intermittent PTH. Faseb J. 2015 doi: 10.1096/fj.15-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.