Abstract
Objectives
Pre-exposure prophylaxis (PrEP) is recommended for preventing HIV infection among individuals at high risk, including men who have sex with men (MSM). Although its individual-level efficacy is proven, questions remain regarding population-level impact of PrEP implementation.
Design
We developed an agent-based simulation of HIV transmission among MSM, accounting for demographics, sexual contact network, HIV disease stage and use of antiretroviral therapy. We use this framework to compare PrEP delivery strategies in terms of impact on HIV incidence and prevalence.
Results
The projected reduction in HIV incidence achievable with PrEP reflects both population-level coverage and individual-level adherence (as a proportion of days protected against HIV transmission). For example, provision of PrEP to 40% of HIV-negative MSM reporting more than one sexual partner in the last 12 months, taken with sufficient adherence to provide protection on 40% of days, can reduce HIV incidence by 9.5% (95% uncertainty range: 8–11%) within five years. However, if this could be increased to 80% coverage on 80% of days (e.g., through mass campaigns with a long-acting injectable formulation), a 43% (42–44%) reduction in HIV incidence could be achieved. Delivering PrEP to MSM at high risk for HIV acquisition can augment population-level impact up to 1.8-fold.
Conclusions
If highly ambitious targets for coverage and adherence can be achieved, PrEP can substantially reduce HIV incidence in the short-term. While the reduction in HIV incidence largely reflects the proportion of person-years protected, the efficiency of PrEP delivery can be enhanced by targeting high-risk populations.
Keywords: HIV, Pre-exposure prophylaxis, Prevention, Computer simulation, Homosexuality, Male
BACKGROUND
Pre-exposure prophylaxis (PrEP) is increasingly recognized as an important component of any comprehensive strategy to end local HIV epidemics. Several clinical trials have established the effectiveness and safety of PrEP [1–3], and the United States Food and Drug Administration (FDA) has approved the provision of tenofovir/emtricitabine for prevention of HIV among high-risk populations [4]. Since then, many cities have begun implementation of PrEP, using a variety of venues and strategies [5]. Despite these promising developments, questions remain regarding the likely population-level impact of PrEP, as implemented in various settings and with varying degrees of targeting to populations at highest risk of HIV infection [2,6,7].
In the absence of cluster-randomized trials (which would encounter ethical problems given the known efficacy of PrEP), mathematical models can be a useful way to project the potential impact of PrEP on HIV incidence and prevalence at the community level. In particular, agent-based simulations have the ability to incorporate several sources of individual-level heterogeneity, such as sexual mixing patterns, behavioral rules, and treatment adherence, as relevant to transmission and control of the HIV epidemic [8–12]. In this work, we present an agent-based simulation model of PrEP delivery among Men who have Sex with Men (MSM) to assess the population-level impact on HIV incidence and prevalence. Using Baltimore City as an illustrative case, we study various PrEP strategies targeting subpopulations at high-risk of HIV infection and compare the epidemiological impact of these strategies under various assumptions regarding the population-level coverage of PrEP and the degree of protection afforded by PrEP at the individual level (for example, reflecting different levels of adherence).
METHODS
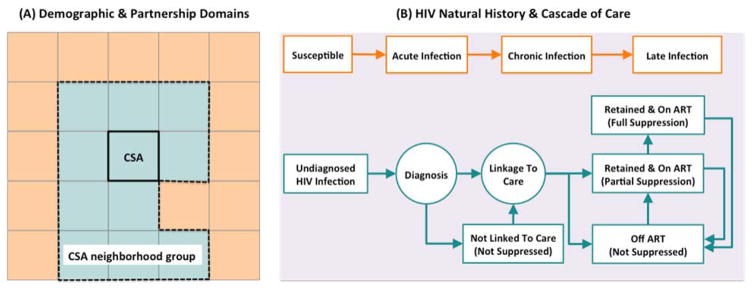
Our agent-based simulation model of the HIV epidemic among MSM in Baltimore City is structured as a collection of different modules that govern various aspects of population demographics, partnerships, HIV natural history and the cascade of care (Figure 1). Each “agent” represents a single MSM, characterized by age, race, and place of residence. The simulation modules are programmed at the individual agent level but calibrated against available population-level epidemiological data. A natural history module characterizes the progression of HIV among infected individuals according to disease stage (acute, early, and late). Each stage is associated with a different per-act risk of HIV transmission, and progression from early to late disease can be prevented (and/or reversed) by provision of ART. A continuum of care module estimates individuals’ probabilities of HIV testing, linkage to care, disengagement/re-engagement, and ART provision/viral suppression. A sexual network and transmission module creates and modifies each individual’s sexual network as a series of stable (long term) and casual (short term) partnerships, modeling HIV transmission as a per-act probability among serodiscordant partners according to frequency of unprotected sex acts, HIV stage of the infected partner, and ART/PrEP use. The probability of engaging in new partnerships reflects an individual’s age, level of sexual activity, and current partnership status. New partnerships are modeled as assortative according to age, race, and location of residence (within Community Statistical Areas [CSAs], and within contiguous “CSA groups” that are similar according to race and socioeconomic status). Finally, the demographic module accounts for aging, death, and birth. The model is updated using a time step of one week and followed for 5 years. Further details regarding the structure of simulation modules are provided in section 1 of the Appendices.
Figure 1. Overview of simulation modules.
This figure illustrates the schematic simulation logic for modeling the demographic and partnership networks among MSM (panel A), as well as HIV natural history and the cascade of care (panel B). Panel A: The population is structured into a collection of Community Statistical Areas (CSAs) and CSA neighborhood groups, which are in turn based on geographical proximity, and the level of similarity in income and racial structure (represented schematically by same colors). Partnership domains are determined via discrete choice within an individual’s own CSA of residence, a random neighboring CSA, or a non-neighbor CSA. Once the partnership domain is established, individuals follow a search mechanism based on a combination of race- and age-dependent mixing patterns to select their future partner from the pool of eligible people in that domain. Panel B: HIV infection is modeled as a gradual decline in CD4 count and via three main states in the absence of HIV treatment (acute/chronic/late infection). The cascade of care models the processes for screening infected individuals, linking to care, retaining in care and starting ART.
Parameterization/Calibration
Simulation parameters are categorized as either fixed (known) or variable (unknown). Fixed parameters are those for which strong individual-level evidence from the literature exists (Table 1); these parameters are set to specific values throughout the modeling process (though varied in sensitivity analysis). Variable parameters are those for which an individual-level estimate is unknown or unavailable. These parameters are calibrated in an iterative stepwise procedure designed to create a simulated population that matches selected population-level targets. Using Baltimore City as an illustrative case, demographic parameters (such as the birth rates within CSAs) are calibrated to replicate the average MSM population size and demographic structure available from census data [13]; partnership parameters (such as the individual likelihood of forming new partnerships) are tuned to replicate the reported frequency and type of sexual partnerships available from the National HIV Behavioral Surveillance study in Baltimore (BESURE) [14]; the parameters used for modeling HIV care (such as probability of HIV testing) are tuned to replicate the proportion of the infected population estimated to be aware of their HIV status, linked and virally suppressed in this population [15]; and the per-week probability of HIV transmission over each active contact was tuned to replicate the reported level of HIV prevalence among Baltimore City’s MSM in year 2012 [15]. For more details regarding the calibration procedure and a list of model parameters and values see section 2 of the Appendices.
Table 1.
List of fixed model parameters1
| Model Parameters | Value/[Range]2 | Reference |
|---|---|---|
|
PARTNERSHIPS
|
||
| Stable partnership duration (mean) | 4 years3 | [9] |
| Casual partnership duration | 1 week time-step | Assumed |
|
HIV MEASURES
|
||
| Disease state duration | ||
| Acute infection (CD4 >500 cells/μL) | [6, 9] weeks | [9,26,27] |
| Chronic infection (CD4 200–499 cells/μL) | [8, 10] years | [9,10] |
| Late infection (CD4 <200 cells/μL) 4 | [1, 3] years | [9,10,26] |
| Time from ART initiation to full viral suppression | [3, 6] months | [28] |
| Time from ART discontinuation to pre-ART CD4 nadir5 | [3, 9] months | [29–32] |
| Mortality rate 3 | [33–35] | |
| Acute and chronic, no ART | 5 per 1000 person years | |
| Reduction in mortality due to ART | 0.58% * Mortality rate in chronic state | |
| Probability of ART discontinuation | (24%,50%,90%) by the end of (1st,2nd,8th) year6 50% per year afterward |
[36] |
| Gap in care after ART discontinuation | 26 weeks | [36] |
| Average viral load (log10 copies/mL) | [9] | |
| Acute, no ART | 6.5 | |
| Chronic, no ART | 4.5 | |
| Late, no ART | 5 | |
| On ART, partially suppressed | 3.5 | |
| On ART, fully suppressed | 1.5 | |
| Infectiousness per sexual contact | 2.45(log(VL)−4.5) | [9] |
For a complete list of all fixed and variable parameters (including the probability of testing, linkage to care and ART initiation/re-initiation), see Table 2 in the Appendices.
Values generated via Uniform distribution over the specified range unless stated otherwise
Values generated via a Geometric distribution with specified mean
Mortality rate in late infection is defined as 1/(duration in the late infection disease state.
Infectiousness assumed equal to that of the chronic state
Values are adjusted via a simulation coefficient (p=0.7) for calibration to Baltimore’s setting
Simulated Population and Experiments
Baltimore City is a moderate-sized city (2015 population 620,961 [13]) whose HIV epidemic encapsulates some of the dynamics of HIV transmission seen in many U.S. cities, including heterogeneity in transmission, disparity in prevalence by age and race, and a relatively weak continuum of care. In 2012, Baltimore City had 469 reported new HIV diagnoses (89.9 infections per 100,000), 85.7% of which occurred among non-Hispanic blacks (referred to as Black) and 55.6% in MSM [15]. Among young (<30) MSM in Baltimore in 1996–2000, estimated annual HIV incidence was 11.0% (95% CI: 5.5–19.7%) in Blacks, versus 0.6% in non-Hispanic whites [16]. Rates by ZIP code likewise vary by a factor of ten [15]. Only 31% of all PLHIV in Baltimore are estimated to have a suppressed viral load, with prominent disparities by age, race, and HIV risk factors [15]. Using the current estimate of Baltimore City’s male population who are 15 year or older in age and the estimated percentage of adult MSM in each racial group (7.5% of white males and 5.8% of black males [17]), we estimate the size of Baltimore’s MSM population at approximately ~15,000 men in Baltimore City (See section 1 of the Appendices).
Corresponding to known risk factors for increased risk of HIV infection in this population, we further consider a set of hypothetical scenarios for annual provision of PrEP to eligible MSM, where eligibility criteria are based on the frequency of reported partnerships, age, and race as follows:
We define emblematic scenarios of PrEP administration based on eligibility criteria as follows:
S1: Universal administration of PrEP: All HIV-negative MSM are eligible for PrEP.
S2: Focused administration for MSM with >1 partner: All HIV-negative MSM with more than 1 partner in the last year are eligible.
S3: Focused administration for MSM with >5 partners: All HIV-negative MSM with more than 5 partners in the last year are eligible.
S4: Focused administration for young MSM: All HIV-negative MSM younger than 30 years old are eligible.
S5: Focused administration for young Black MSM: All HIV-negative Black MSM younger than 30 years old are eligible.
While these scenarios do not precisely reflect the CDC guidelines for implementation of PrEP, they may more closely describe the likely implementation of these guidelines in actual clinical or public health practice (S2). Scenarios S3–S5 were designed using categories in available data to target sufficiently large subpopulations at increased risk of HIV infection (e.g., those with a large number of partnerships [18] or young age [19]).
In each scenario, we assume that a proportion of eligible individuals (population coverage) receive PrEP at the beginning of each year and continue to take PrEP at a level of adherence capable of providing protection against HIV infection on a specified percentage of days (protection), for at least one year. At the end of each year, those currently on PrEP who are no longer eligible to receive PrEP (e.g., due to new HIV infection or reduction in condomless sex) are discontinued. Those discontinuing PrEP can re-start PrEP in subsequent years, if still eligible. We model PrEP delivery for five consecutive years (year 1 to 5), and map the percent reduction in HIV incidence and prevalence compared to a baseline scenario without PrEP, at various levels of individual-level HIV protection and population coverage. Given the variation in the size of the PrEP-eligible population under each scenario (for example, about 11,000 MSM are eligible for PrEP in scenario 1, versus 3,300 in scenario 5), we control the total number of people on treatment during each year and use this number as an alternative measure of PrEP coverage across all scenarios.
Simulation procedure
Each simulation run was first carried out over a long “burn-in” period of 200 years until equilibrium is reached (baseline), targeting Baltimore’s estimate of 3,300 MSM living with HIV in 2014 [15]. At baseline, we then carried each simulation forward for an additional 5 years under each PrEP scenario described above, repeating the analysis for different levels of individual-level HIV protection provided by PrEP (range [0%–100%]) and population coverage (in terms of the number of people receiving PrEP, as well as the proportion of the eligible population receiving PrEP, range [0%–100%]) in each PrEP scenario. We estimated the required number of independent simulations to provide precision of at most 1% around the main output (HIV prevalence) and repeated the analysis across all (~100) independent models. For more information, see section 2.1 of the Appendices.
Sensitivity analysis
One-way sensitivity analysis was performed by varying all simulation parameters (fixed and variable) to +/−25% of their original values. Since small variations in parameters governing transmission had a disproportionate impact on HIV incidence, we evaluated those parameters in a way that changed baseline HIV incidence by <+/−25%. In each sensitivity analysis, we compared the annual impact of PrEP on HIV incidence after parameter variation relative to its impact at baseline (original parameter setting), using scenario 1 and maintaining 2000 randomly-selected-MSM with more than 1 partner on PrEP each year, assuming adherence sufficient to provide protection on 60% of days.
RESULTS
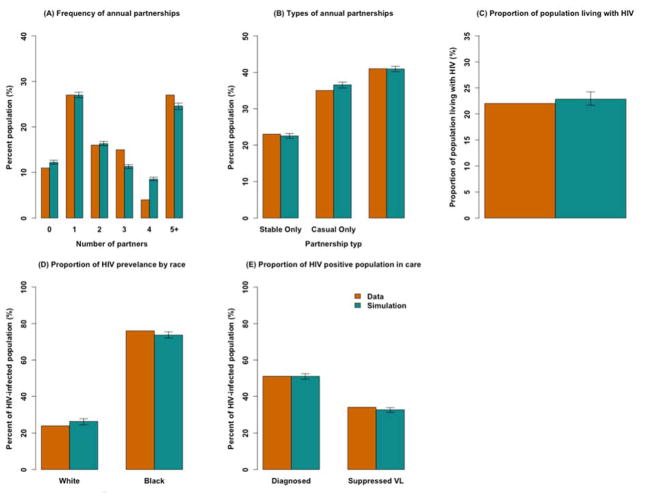
At baseline, the median age in the simulated population was 39.04 [95% uncertainty range1: 39–39.08] years, and 54.4% [54.3–54.5%] of MSM were Black. Figure 2 provides a summary of the model’s primary calibration targets, illustrating that the model population is similar to that of available data. In any given year, 22.5% [22.4–22.6%] of MSM in our model are only engaged in stable partnerships, while 40.9% [40.8–41%] have both stable and casual partnerships. The simulated median number of annual partners, by age group, was 5.2 (ages 15–24), 4.2 (ages 25–34), 3.6 (ages 35–44) and 3.0 (ages 45 and above).
Figure 2. Model Calibration to Epidemiological Data.
Shown are the mean values of 200 simulations (in green) compared against empirical data (in orange). The black arrows represent the range of observations around each simulated measure. The data bars represent available point estimates on each measure including measures of partnership frequency (Panels A and B) reported through unpublished data from the latest survey of Baltimore’s MSM in 2014 [14], as well as available estimates of HIV prevalence, racial disparities and the HIV continuum of care among Baltimore’s MSM in year 2012 (Figure 2-Panels C through E) [15].
After calibrating to an HIV prevalence of 22% among MSM [15] (Figure 2-Panel C), our model projects an HIV incidence of 1390 cases per 100,000 person year [1374–1406] in the absence of PrEP. Young Black MSM between ages of 15 to 29 account for 18.5% [17.9–19.1%] of the population but 36% [35.5–36.6%] of incident HIV infections. Black MSM account for 80% [75–85%] of HIV incidence in this model, and they have 30–50% lower levels of being diagnosed, in care on ART, and virally suppressed, relative to white MSM. For more information, see section 3.1 in the Appendices.
Impact of PrEP as a function of coverage and individual-level protection
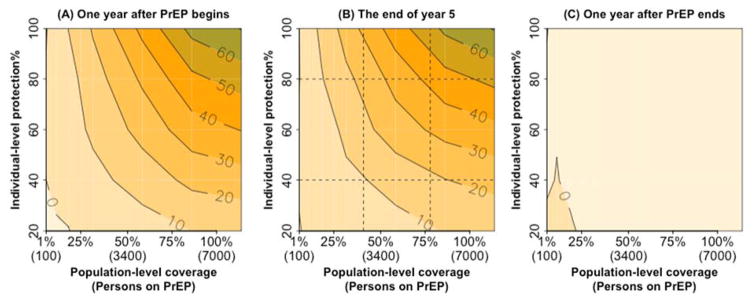
The impact of PrEP largely reflected the number of individuals in the population who were protected at any given time and was thus highly sensitive to both population-level coverage and the proportion of days protected at the individual level (a proxy for adherence). At 40% coverage and 40% individual-level protection, community-wide provision of PrEP to individuals with more than 1 partner in the last 12 months (Scenario 2) is estimated to reduce HIV incidence by 9.5% [8–11%] after one year (Figure 3, panel A, bottom center), and by 10.6% [9–12%] after five years (Figure 3 panel B, bottom center). If, however, coverage and individual-level protection could both be increased to 80% (for example, through a mass community campaign using an injectable formulation), five-year impact on HIV transmission could be increased to 43% [42–44%]. The impact of PrEP on HIV incidence is achieved rapidly upon PrEP scale-up (Figure 3, left panel) and lost with similar speed when PrEP protection ends, as PrEP has little effect on HIV prevalence over five years (Figure 3, right panel). The impact of improving coverage (as a proportion of the eligible population covered) is similar to that of improving individual-level HIV protection. For example, starting from a baseline of 40% coverage and 40% protection, achieving a 2-fold increase in coverage would reduce incidence by 19.4% [18–21%] whereas a 2-fold increase in individual-level protection would reduce incidence by 24% [23% – 25%]. Simultaneous reductions in both coverage and individual-level HIV protection generate approximately additive reductions in population-level impact (Figure 3, left and center panels). For more information, see section 3.2 in the Appendices.
Figure 3. Percent reduction in HIV incidence after delivering PrEP to MSM reporting more than one partner in a year (scenario 2) at different individual-level HIV protection and population-level coverage.
Contour lines show the percent reduction in HIV incidence at various levels of population coverage (on the x-axis, with the percent of the eligible population covered on top and the numbers of MSM on PrEP in parentheses) and individual-level protection (on the y-axis). Panel A shows impact after one year of PrEP delivery, Panel B shows the same impact after sustaining the same level of PrEP for five years, and Panel C shows the impact one year after stopping PrEP (at the end of year five). All impact estimates are shown relative to a baseline of no PrEP delivery.
Comparing the impact of PrEP targeted at various risk groups
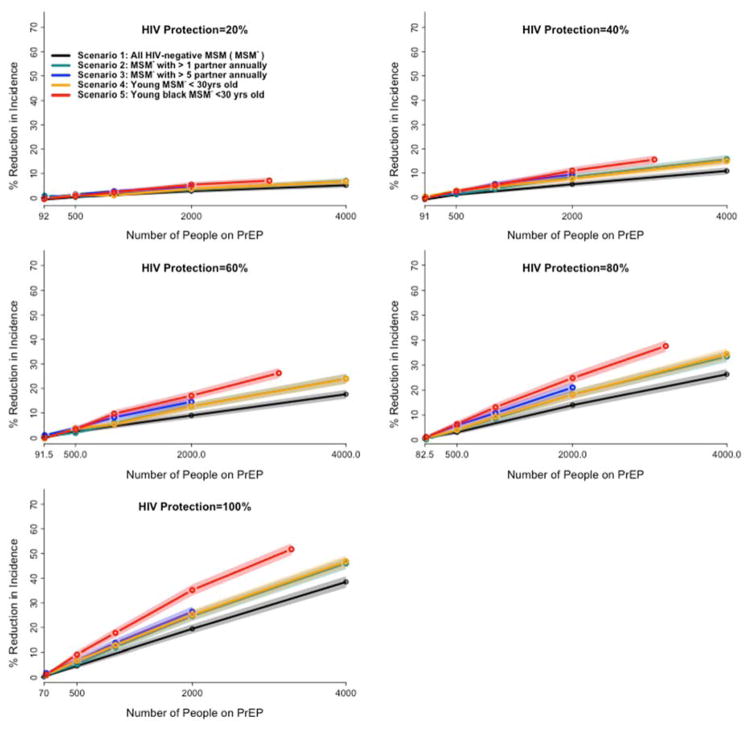
Given that some scenarios have large eligible pools with relatively less targeting (e.g., scenario 1) while others have smaller but more targeted eligible pools (e.g., scenario 5), we compared the scenarios as a function of the number of people on PrEP in each year and individual-level protection (Figure 4). Per person prescribed PrEP, targeting PrEP to a demographic group at high risk for HIV infection (young Black MSM, scenario 5) results in the greatest reduction in HIV incidence. For example, under the assumption of full individual-level protection, providing PrEP to 1,500 young Black MSM has equivalent projected impact (27% [25.5%–28%] reduction in incidence) to covering 3,000 randomly selected eligible MSM in the general population (30% [29%–31%] reduction in incidence). While targeting PrEP to those at higher sexual risk (>1 partner in the previous 12 months: Scenario 2, green line in Figure 4) modestly improved impact over untargeted delivery (Scenario 1, black line), additional targeting to those at very high sexual risk (>5 partners per year: Scenario 3, blue line) or young age alone (Scenario 4, yellow line) did not further increase PrEP impact. For example, maintaining 2000 randomly selected uninfected MSM on PrEP was projected to reduce HIV incidence at the end of five years by 20% [18.3%–21.4%] in Scenario 1 (middle of x-axis, Figure 4). A similar campaign targeting MSM with >1 annual partner augmented PrEP impact 1.2-fold, versus 1.3-fold if targeted to those with >5 partners or to those below age 30. By contrast, a campaign delivering PrEP to 2000 young Black MSM (Scenario 5, red line) achieved 1.8 times greater reduction in community-wide HIV incidence compared to the baseline scenario. While the absolute impact of targeted delivery declined proportionally with decreasing individual-level protection, the relative impact of targeting was preserved, regardless of individual-level protection levels.
Figure 4. Comparative impact of targeted PrEP scenarios on HIV incidence at various level of HIV protection.
Each panel maps the comparative impact of PrEP scenarios on HIV incidence at a different level of achievable protection under PrEP, ranging from 20% (top left) to 100% (bottom left). The y-axis of each panel shows the percent reduction in incidence under each PrEP scenario at the end of the 5th year of implementation, relative to a baseline with no PrEP. The x-axis represents the number of MSM receiving PrEP in each of five scenarios (described in the inset and the manuscript text). Shaded areas represent the 95% confidence interval around the simulated means. The end of each line represents the total eligible population in each scenario except for scenario 1 (e.g., S3 is continued until the estimated 2000 MSM in Baltimore with more than 5 partners are all covered). For a given number of MSM on PrEP, all targeted scenarios (Scenarios 2–4) show modest improvements over random selection (Scenario 1), and Scenario 5 shows the greatest impact of all strategies evaluated.
In contrast to the rapid impact of PrEP on HIV incidence that quickly plateaus (as shown in Figure 3), impact on HIV prevalence is slower but builds over time. For example, delivering PrEP to MSM with more than 1 partner in a year (scenario 2) was projected to reduce HIV prevalence by only 15% [12%–17%] after 5 years, but if this program could be sustained over 20 years, the corresponding reduction in HIV prevalence was projected to reach 54% [51% – 56%]. For more information, see section 3.3 in the Appendices.
Sensitivity Analysis
The relative impact of PrEP was most sensitive to increased frequency of stable and casual partnerships, HIV viral load during the acute and chronic states as well the overall probability of HIV transmission upon contact (Appendix Figure 16), but there was no parameter for which a 25% variation (or variation sufficient to change HIV incidence by 25%) resulted in a sustainable change (>25%) in the projected impact of PrEP after 10 years. The results suggest that the efficacy of PrEP for prevention of HIV is likely to be similar in a variety of other epidemiological settings. A complete description of sensitivity analysis and the results are provided in Section 4 of the Appendices.
DISCUSSION
This agent-based simulation of HIV transmission among MSM illustrates that the impact of PrEP depends both on population-level coverage and individual-level protection (as a proxy for adherence). If delivered with exceptionally high population-level coverage and individual-level protection, PrEP can have a major and immediate impact on the rate of HIV incidence in this population. For example, we estimated that a delivery strategy that covers 80% of the HIV-negative MSM population with >1 sex partner in the past year, taken with sufficient adherence to provide protection on 80% of days, can reduce city-wide HIV incidence among MSM by almost half within one year. However, under more realistic assumptions regarding coverage and adherence, the impact of PrEP alone is much more modest – emphasizing the importance of including PrEP with other interventions if local HIV epidemics are to be brought under control. The impact of PrEP can be augmented (on a per-person basis) by targeting demographic groups at high risk of HIV infection (for example, young Black MSM in Baltimore). The scope of this impact, however, is limited to the size of the high-risk population in question and the ability to provide PrEP to that population with high coverage and adherence using available resources. Providing PrEP to populations with lower risk (but still eligible) does further enhance impact on incidence – and may be logistically more feasible (for example, providing PrEP to eligible individuals already connected to healthcare). Decisions about targeting PrEP delivery must therefore balance expected increases in efficiency from targeting demographic groups at high transmission risk, achievable levels of uptake and adherence in these groups, and logistical/resource considerations that may favor other strategies.
Our results are consistent with those of other studies of the population-level impact of PrEP. For example, these results agree with the argument of van der Straten et al that low population uptake and suboptimal adherence can undermine the impact of PrEP at the population level [20]. Other modeling studies have forecasted the possibility of substantial reductions in HIV incidence in the United States, South Africa, and India [21–24], and have suggested higher cost-effectiveness ratios for programs targeting younger and higher-risk populations [21,22]. Our results extend these earlier findings by incorporating heterogeneities in the transmission and control of HIV that can serve as the basis for targeted PrEP delivery. As a result, we are able to quantify, for example, the additional impact that might be achieved by focusing PrEP interventions among young Black MSM in an urban U.S. city characterized by racial disparities in HIV. Another modeling study incorporating CDC guidelines for implementation of PrEP among MSM in US estimated that a PrEP campaign with coverage of 40% and adherence of 62% could reduce HIV incidence by 33% over a decade [25], almost identical to our corresponding Scenario 1 estimate of a 32% [UR: 12.2 – 44.8%] reduction under similar coverage and adherence.
Our results have a number of practical implications for the implementation of PrEP programs at the local level. First, if PrEP is taken with relatively low adherence (for example, sufficient to provide protection against HIV on only 20–40% of days), very high levels of population coverage must be achieved to attain substantive reductions in HIV incidence. Similarly, if population coverage is capped at a given threshold (because of drug supply or inability to reach certain sub-populations), then adherence must be exceptionally high. Our results suggest that at low level of coverage and adherence, a small number of settings may still experience an increase in HIV incidence after implementation of PrEP due to random variation and the low number of HIV infections that occur on a population level in any given year (i.e., 95%UR being less than zero). This model also suggests that targeting of race/age-based demographic groups at high risk for HIV infection may substantially increase the efficiency of PrEP delivery and may – at least in this simulated population – have greater impact than targeting groups with high sexual frequency or low age alone. These findings highlight that HIV transmission largely occurs in specific assortative sexual networks (which may correlate with certain demographic characteristics such as race), rather than simply occurring among those who have the largest number of sex partners.
As with any modeling study, our results are limited by certain simplifying assumptions used in design and calibration of the simulation model. In the absence of spatial sexual-network data, we adopted a simplified approach in modeling geographical mixing based on location of residence. The simplified definition of stable/casual contacts in this model further limits our ability to represent MSM with very high rate of partner turnover such as in sex work or group sex settings. Additional simplifying assumptions that may also impact our ability to precisely project HIV transmission dynamics include homogeneous sexual position preference, frequency of sexual acts in each type of partnerships, and the likelihood of condom use (conditional on partnership type). For purposes of maintaining an appropriately simple model structure, we limited our analysis to MSM; future studies could extend this work to other population groups including high-risk heterosexuals, injection drug users and female sex workers. Additional efforts could also seek to characterize migration patterns across cities, behavioral changes while on PrEP, differences in PrEP uptake by race and age, interplay between PrEP and ART, and the role of other preventive interventions (e.g., condoms and male circumcision) in modulating the impact of PrEP.
In summary, the advent of PrEP presents a major opportunity to make substantial progress toward ending HIV epidemics at the local level. This simulation shows that, although the projected epidemic impact of PrEP at currently feasible levels of implementation is modest, PrEP can rapidly and substantially reduce HIV transmission if ambitious targets for coverage and individual-level protection can be achieved through additional innovation in delivery and/or formulation. This impact is largely proportional to the number of individuals at high risk for transmission who successfully take PrEP, and it can be enhanced by targeting demographic groups at high risk for HIV infection. While our results are based on a single setting (Baltimore), we feel they may be generalizable to epidemics among MSM in many settings. In order to maximize the impact of PrEP as an individual- and population-level HIV prevention strategy, it is ultimately necessary to consider PrEP as part of a comprehensive package that also includes ART for HIV-positive individuals and other behavioral intervention options. Nonetheless, our model illustrates that this novel biomedical intervention can play an important role in altering the face of the HIV epidemic in high-risk populations at the city level.
Supplementary Material
Acknowledgments
Funding:
This work was supported by B. Frank and Kathleen Polk Assistant Professorship in Epidemiology, Johns Hopkins Bloomberg School of Public Health [DWD].
This research was funded in part by a 2015 developmental grant from the Johns Hopkins University Center for AIDS Research, an NIH funded program (P30AI094189), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIGMS, NIDDK, and OAR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH [DWD, JP, PK].
Footnotes
This is the 95% uncertainty range around the mean of 100 simulations ( see section 2.2.1 of the appendices for a description of methods).
Contributions: Designed the study [PK and DWD]; Wrote the model code [PK and JP]; Provided data [DG, CF], Analyzed the data [PK]; supervised the analyses [DWD]; wrote the first draft of the manuscript [PK]; revised the manuscript and contributed intellectual content [PK, JF, SB, MS, DG, CF, CB, DWD]; All authors saw and approved the final manuscript.
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration. FDA Approves First Medication to Reduce HIV Risk. Consum Updat. 2016 [Google Scholar]
- 5.Mayer KH, Hosek S, Cohen S, Liu A, Pickett J, Warren M, et al. Antiretroviral pre-exposure prophylaxis implementation in the United States: a work in progress. J Int AIDS Soc. 2015;18 doi: 10.7448/IAS.18.4.19980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Underhill K, Morrow KM, Colleran CM, Holcomb R, Operario D, Calabrese SK, et al. Access to healthcare, HIV/STI testing, and preferred pre-exposure prophylaxis providers among men who have sex with men and men who engage in street-based sex work in the US. 2014:e112425. doi: 10.1371/journal.pone.0112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdool Karim SS, Baxter C. Antiretroviral prophylaxis for the prevention of HIV infection: future implementation challenges. HIV Ther. 2009;3:3–6. [Google Scholar]
- 8.Marshall BD, Paczkowski MM, Seemann L, Tempalski B, Pouget ER, Galea S, et al. A complex systems approach to evaluate HIV prevention in metropolitan areas: preliminary implications for combination intervention strategies. PLoS One. 2012;7:e44833. doi: 10.1371/journal.pone.0044833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380:367–77. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alam SJ, Meyer R, Norling E. A model for HIV spread in a South African village. Multi-Agent-Based Simul IX. 2009:33–45. [Google Scholar]
- 11.Goodreau SM, Carnegie NB, Vittinghoff E, Lama JR, Fuchs JD, Sanchez J, et al. Can Male Circumcision Have an Impact on the HIV Epidemic in Men Who Have Sex with Men? 2014:e102960. doi: 10.1371/journal.pone.0102960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallett TB, Gregson S, Dube S, Garnett GP. The impact of monitoring HIV patients prior to treatment in resource-poor settings: insights from mathematical modelling. PLoS Med. 2008;5:e53. doi: 10.1371/journal.pmed.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alliance BNI. Vital Signs. 2012;12 http://bniajfi.org/vital_signs/ [Google Scholar]
- 14.Maryland Department of Health and Mental Hygiene. BESURE Study 2004–2014: Baltimore site of National HIV Behavioral Surveillance (NHBS) 2015. [Google Scholar]
- 15.Maryland Department of Health and Mental Hygiene, MDHMH. 2012 Baltimore City Annual HIV Epidemiological Profile. Baltimore: 2012. [Google Scholar]
- 16.Sifakis F, Hylton JB, Flynn C, Solomon L, Mackellar DA, Valleroy LA, et al. Racial disparities in HIV incidence among young men who have sex with men: the Baltimore Young Men’s Survey. J Acquir Immune Defic Syndr. 2007;46:343–8. doi: 10.1097/QAI.0b013e31815724cc. [DOI] [PubMed] [Google Scholar]
- 17.Lieb S, Fallon SJ, Friedman SR, Thompson DR, Gates GJ, Liberti TM, et al. Statewide Estimation of Racial/Ethnic Populations of Men Who Have Sex with Men in the U. S. Public Health Rep. 2011;126:60–72. doi: 10.1177/003335491112600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maulsby Cathy, Sifakis Frangiscos, German Danielle, Flynn Colin P, DH Partner characteristics and undiagnosed HIV seropositivity among men who have sex with men only (MSMO) and men who have sex with men and women (MSMW) in Baltimore. AIDS Behav. 2012;16:543–553. doi: 10.1007/s10461-011-0046-4. [DOI] [PubMed] [Google Scholar]
- 19.Maulsby C, Jain K, Sifakis F, German D, Flynn CP, Holtgrave D. Individual-Level and Partner-Level Predictors of Newly Diagnosed HIV Infection Among Black and White Men Who Have Sex with Men in Baltimore, MD. AIDS Behav. 2014:1–9. doi: 10.1007/s10461-014-0861-5. [DOI] [PubMed] [Google Scholar]
- 20.van der Straten A, Van Damme L, Haberer JE, Bangsberg DR. Unraveling the divergent results of pre-exposure prophylaxis trials for HIV prevention. AIDS. 2012;26:F13–9. doi: 10.1097/QAD.0b013e3283522272. [DOI] [PubMed] [Google Scholar]
- 21.Paltiel AD, Freedberg KA, Scott CA, Schackman BR, Losina E, Wang B, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas UL, Anderson RM, Mellors JW. Potential Impact of Antiretroviral Chemoprophylaxis on HIV-1 Transmission in Resource-Limited Settings. PLoS One. 2007;2:e875. doi: 10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vissers DCJ, Voeten HACM, Nagelkerke NJD, Habbema JDF, de Vlas SJ. The Impact of Pre-Exposure Prophylaxis (PrEP) on HIV Epidemics in Africa and India: A Simulation Study. PLoS One. 2008;3:e2077. doi: 10.1371/journal.pone.0002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai K, Sansom SL, Ackers ML, Stewart SR, Hall HI, Hu DJ, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. Aids. 2008;22:1829–1839. doi: 10.1097/QAD.0b013e32830e00f5. [DOI] [PubMed] [Google Scholar]
- 25.Jenness S, Goodreau S. Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States. [accessed 6 Dec 2016];J Infect. doi: 10.1093/infdis/jiw223. Published Online First: 2016. http://jid.oxfordjournals.org/content/early/2016/07/12/infdis.jiw223.abstract. [DOI] [PMC free article] [PubMed]
- 26.Baggaley RF, Ferguson NM, Garnett GP. The epidemiological impact of antiretroviral use predicted by mathematical models: a review. Emerg Themes Epidemiol. 2005;2:9. doi: 10.1186/1742-7622-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salomon JA, Hogan DR, Stover J, Stanecki KA, Walker N, Ghys PD, et al. Integrating HIV prevention and treatment: from slogans to impact. PLoS Med. 2005;2:e16. doi: 10.1371/journal.pmed.0020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currie SKE, Rogstad AP, Herman S. Time taken to undetectable viral load, following the initiation of HAART. Int J STD AIDS. 2009;20:265–266. doi: 10.1258/ijsa.2008.008268. [DOI] [PubMed] [Google Scholar]
- 29.Wit FW, Blanckenberg DH, Brinkman K, Prins JM, van der Ende ME, Schneider MM, et al. Safety of long-term interruption of successful antiretroviral therapy: the ATHENA cohort study. Aids. 2005;19:345–348. [PubMed] [Google Scholar]
- 30.Maggiolo F, Ripamonti D, Gregis G, Quinzan G, Callegaro A, Suter F. Effect of prolonged discontinuation of successful antiretroviral therapy on CD4 T cells: a controlled, prospective trial. Aids. 2004;18:439–446. doi: 10.1097/00002030-200402200-00010. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz GM, Wellons M, Brancato J, Vo HT, Zinn RL, Clarkson DE, et al. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc Natl Acad Sci. 2001;98:3288–13293. doi: 10.1073/pnas.221452198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. New Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 33.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 36.Rana AI, Liu T, Gillani FS, Reece R, Kojic EM, Zlotnick C, et al. Multiple gaps in care common among newly diagnosed HIV patients. AIDS Care. 2015;27:697–687. doi: 10.1080/09540121.2015.1005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.