Abstract
Background: Knowledge of the mechanisms by which aging affects contracting lymphatic vessels remains incomplete; therefore, the functional role of histamine in the reaction of aged lymphatic vessels to increases in flow remains unknown.
Methods and Results: We measured and analyzed parameters of lymphatic contractility in isolated and pressurized rat mesenteric lymphatic vessels (MLVs) obtained from 9- and 24-month Fischer-344 rats under control conditions and after pharmacological blockade of nitric oxide (NO) by Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME, 100 μM) or/and blockade of histamine production by α-methyl-DL-histidine dihydrochloride (α-MHD, 10 μM). We also quantitatively compared results of immunohistochemical labeling of the histamine-producing enzyme, histidine decarboxylase (HDC) in adult and aged MLVs. Our data provide the first demonstration of an increased functional role of histamine as an endothelial-derived relaxing factor in aged MLVs, which appears in parallel with the abolished role of NO in the reactions of these lymph vessels to increases in flow. In addition, we found an increased expression of HDC in endothelium of aged MLVs.
Conclusions: Our findings provide the basis for better understanding of the processes of aging in lymphatic vessels and for setting new important directions for investigations of the aging-associated disturbances in lymph flow and the immune response.
Keywords: : mesenteric lymphatic vessels, lymphatic endothelium, histamine, EDRF (endothelium-derived relaxing factor)
Introduction
Lymph flow is generated by lymph formation in initial lymphatics and maintained by tonic and phasic contractions of muscle-containing lymphatic vessels as well as by passive lymph-driving forces that periodically support lymph flow.1–8 Lymph flow is necessary for vital body functions, such as fluid and macromolecular homeostasis, transportation of lipids, and transport of immune cells. Aging significantly alters all of these functions. However, the understanding of the mechanisms of aging-associated changes in lymphatic functions still remains far from complete despite the findings of recent years.9–14 As a result, the lymphatic-related components in the pathogenesis of many diseases of the elderly remain to a large degree unknown. There is still much more to be done in terms of discovery-driven lymphatic research due to the general lack/limitations of knowledge on processes of basic lymphatic biology.
In particular, we recently discovered that histamine plays a role as an endothelium-derived relaxing factor (EDRF) in mesenteric lymphatic vessels (MLVs)15 in adult rats. At the same time, there is no literature data indicating how aging affects such role of histamine in MLVs. Our previous detailed comparisons of contractility in adult and aged MLVs revealed intriguing features of aged MLVs. The aging-diminished sensitivity of aged MLVs to the imposed flow gradient-generated wall shear stress cannot be eliminated by blockade of nitric oxide synthases (NOS).10 Based on these findings and analogous studies, we performed on MLVs obtained from adult rats15; in this study, we evaluated the potential functional roles of histamine as an EDRF in aged MLVs. Furthermore, we performed a comparative analysis of the obtained data pooled together with normalized data obtained in similar experiments with adult MLVs and published earlier.15 Finally, we also quantitatively compared results of immunohistochemical labeling of the histamine-producing enzyme, histidine decarboxylase (HDC), in adult and aged MLVs.
Materials and Methods
Animal procedures
For the current studies, we used Fischer-344 (F-344) male rats (obtained from the aged rat colony maintained by the National Institute of Aging at the NIH). The animals represented two age groups: adult (9-month-old [9-mo]) and aged (24-month-old [24-mo]). All animal procedures were reviewed and approved by our Institutional Animal Care and Use Committee and were in accordance with federal and local regulations. The average body weight of the rats used in this study was 429 ± 14 g in 9-mo rats and 420 ± 19 g in 24-mo rats. It should be noted that for the F-344 rat strain, the majority of the weight gain occurs before 7 months of age.16 Therefore, the weights of both 9-mo and 24-mo rats are within ranges slightly above 400 g and are not significantly different.
On the experimental day, all rats were anesthetized with a solution containing a combination of fentanyl/droperidol (0.3 mL/kg IM) and diazepam (2.5 mg/kg IM). A 4-cm-long midline abdominal incision was made through the skin, underlying fascia, and muscle layers. A small loop of intestine, 6–7 cm in length, was exteriorized through the incision. A section of the mesentery containing lymphatic vessels was positioned in a dissection chamber within the field of view of a dissecting microscope and continuously suffused with standard Dulbecco's phosphate-buffered saline (PBS; Invitrogen Corp., Carlsbad, CA; catalog no. 14040-133). Suitable MLVs were identified and cleared of all surrounding tissue. Sections of MLVs 1.4–1.5 cm in length (equal for all preparations) were carefully dissected and used for experiments. (When pressurized to a transmural pressure of 5 cm H2O in isolated vessel chambers for analysis of lymphatic contractility, the outer diastolic diameters of the MLV segments averaged 136 ± 8 μm (for 9-mo rats) and 129 ± 12 μm (for 24-mo rats), and were not statistically different [p < 0.05].) After isolating MLVs, the chest was opened as a terminal procedure.
Pharmacological blockade of NO and histamine production in isolated MLVs
Once exteriorized, the isolated MLVs were transferred to standard 35-mm Petri dishes (one vessel/dish) completely filled with ∼38°C Dulbecco's modified Eagle's medium/F12 (DMEM/F12) solution (Invitrogen Corp.; catalog no. 11039) supplemented with antibiotic mixture (Invitrogen Corp.; catalog no. 15140) to achieve a concentration of 100 IU/mL of penicillin and 100 μg/mL of streptomycin per milliliter of DMEM/F12. We have demonstrated that lymphatic contractility is not affected by overnight incubation in this solution.15,17 Care was taken to ensure that the mesenteric segment was completely submerged under the medium in the dish. MLVs were separated into two groups—control and α-methyl-DL-histidine dihydrochloride (α-MHD)-treated groups. On each experimental day, we used both control and α-MHD-treated MLVs obtained from the same animal. The α-MHD (Sigma-Aldrich, St. Louis, MO; catalog no. M8628) was used at 10 μM overnight (18–20 hours) to effectively block HDC, the histamine-producing enzyme in MLVs, and effectively deplete MLVs of internally stored histamine produced earlier.15
After overnight culture, isolated control or α-MHD-treated MLVs were transferred to an isolated vessel chamber (single-vessel chamber model CH/1; modified Living Systems Instrumentation, Inc., St. Albans City, VT) filled with prewarmed 38°C DMEM/F12 (pH 7.36). The isolated MLV segments were cannulated and tied onto two carefully matched glass pipettes (100–110 μm). Great care was taken to prepare and select pairs of resistance-matched pipettes for these experiments, as described in our previous studies.8,10,18,19 The vessels were positioned just above the glass coverslip comprising the chamber bottom. The chamber was transferred to the stage of a microscope.
The inflow and outflow pipettes were connected to independently adjustable pressure reservoirs filled with DMEM/F12 with equal length of tubing for input and output systems. The vessels were set to an equilibration transmural pressure of 3 cm H2O at 38°C for 15–20 minutes. Once tone and spontaneous contractions were observed, the vessels were allowed to equilibrate at 3 cm H2O for another 30 minutes before beginning the experiment. During all experiments, MLV segments were constantly superfused with 38°C ± 0.1°C DMEM/F12 (with or without pharmacological blockers as described below). A CCD video camera, monitor, Windows-operated computer supplied with a National Instruments data acquisition card (NI PCI-1410), and DVD/HDD recorder were used to observe and record the lymphatic segments and to track their diameter continuously in all experiments.
In every experiment, we evaluated the contractile responses of MLV segments at transmural pressures of 1, 3, and 5 cm H2O for 5 minutes at each pressure, the common pressure range used to evaluate stretch sensitivity of contracting MLVs.8,10,18,20 To determine the imposed flow-generated wall shear stress-induced responses of MLVs, vessel segments were exposed to imposed flow gradients, which were generated using previously established techniques.8,10,18,20
This was accomplished by raising the pressure on the inflow end of the isolated vessel segment and lowering the pressure on the outflow end of the isolated vessel segment by identical amounts. Thus, we created axial intraluminal pressure gradients (imposed flow gradient) of 1 and 5 cm H2O in MLV segments for 5 minutes at each gradient at transmural pressure of 5 cm H2O. At this transmural pressure, the isolated MLVs display maximal active pumping at 5 cm H2O.8,10,18,20 The measurements of the lymphatic contractile activity were performed while superfusing with DMEM/F12 for control vessels, and with DMEM/F12 supplemented with 10 μM α-MHD for the α-MHD-treated MLV segments.
After completion of measurements of contractile activity of MLVs at the consequent parts of experiments with the transmural pressure and imposed flow gradient ranges in DMEM/F12 (control) or in DMEM/F12 with α-MHD (α-MHD-treated group), the solutions in the chamber were additionally supplemented for 15 minutes with the NOS inhibitor Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) (Sigma-Aldrich; catalog no. N5751) at 100 μM.21–24 The effectiveness of NOS blockade in rat lymphatic vessels induced by application of L-NAME at this concentration has been demonstrated by us in numerous previous reports.10,19,25,26
After the initial 15 minutes of incubation in the L-NAME-containing solution, measurements of contractile activity of MLVs during consequent transmural pressure and imposed flow gradient ranges were repeated in the presence of L-NAME in vessels from control (L-NAME-treated group) and α-MHD-treated groups (α-MHD+L-NAME-treated group). At the end of each experiment, the passive (relaxed) diameter was measured at each pressure (1, 3, and 5 cm H2O for 3 minutes at each pressure) after the MLVs were exposed to a nominally calcium-free, ethylenediaminetetraacetic acid (EDTA)- supplemented physiological saline solution (PSS) (in mM: 145.0 NaCl, 4.7 KCl, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 dextrose, 2.0 sodium pyruvate, 3.0 EDTA, 3.0 MOPS [3-(N-morpholino)propanesulfonic acid]) for 15 minutes.
Data analysis and statistics for isolated vessel experiments
Lymphatic diameters were tracked continuously during experiments using “Vessel Track” software developed previously.27 The end-diastolic and end-systolic points in the diameter tracings were determined for each 5-minute interval for a transmural pressure of 5 cm H2O and for imposed flow gradients of 1, 3, and 5 cm H2O. From the lymphatic end-diastolic diameter (EDD) and end-systolic diameter (ESD), we calculated lymphatic tone index (LTI) (the difference between the passive lymphatic diameter in Ca++-free PSS and EDD, expressed as a percentage of the passive lymphatic diameter in Ca++-free PSS), lymphatic contraction amplitude (AMP) (the difference between EDD and ESD), lymphatic contraction frequency (FREQ), ejection fraction (EF, the fraction of end-diastolic volume ejected during the single lymphatic contraction, calculated using the formula EF = (EDD2 − ESD2)/EDD2,28 and fractional pump flow (FPF, an index of lymph pump flow, calculated as EF*FREQ). To compare the changes in diameter during the lymphatic contractile cycle, the EDD and ESD were normalized to the passive lymphatic diameters in Ca++-free PSS at the corresponding transmural pressure because of the anatomical variations between lymphatic vessels.
Statistical differences were determined by paired one-way analysis of variance (ANOVA) followed by a Dunnett's multiple-comparison correction, regression analysis, and Student's t-test (JMP software version 9.0.2. for Windows; SAS Institute, Inc., Cary, NC) and considered significant at p < 0.05. Data in this article are presented as mean ± SEM. (A similar approach for statistical analysis was used for analysis of data obtained in the experiments described below in the Immunohistochemical analysis of HDC inside endothelium of MLVs section.) To investigate solely the comparative roles of NO and histamine in flow-dependent adaptive reactions of the adult and aged MLVs, we normalized all data in each data set with imposed flow gradient ranges to the values of corresponding parameters at zero imposed flow gradient conditions. Only one vessel segment was used from one animal for control and one for the α-MHD-treated group.
We used data from seven 9-mo animals and from eight 24-mo animals for the functional analysis (the 9-mo MLV data set consists of two parts—pressure-induced responses and imposed flow-induced responses). The latter data set was presented earlier in a different format,15 so in this study, we used some of these data (normalized) for comparison with imposed flow-induced responses in 24-mo MLVs. We pooled data obtained from both ages for statistical analysis and for subsequent comparative presentation of parts of the previously used 9-mo data set formatted differently than published earlier.15
Immunohistochemical analysis of HDC inside endothelium of MLVs
For immunohistochemical labeling, the isolated MLV segments of both age groups (obtained from animals other than those used for functional analysis) were cannulated on glass pipettes in a custom-made single-vessel chamber, fixed for 15 minutes at room temperature in 4% paraformaldehyde, and washed with PBS containing 0.05% Tween-20 (PBST). The vessels were blocked for 1 hour at room temperature in PBST containing 5% normal goat serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA; catalog no. 005-000-121), washed three times for 15 minutes each in PBST, and then incubated overnight at 4°C in PBST containing primary antibodies against HDC (Abcam, Cambridge, MA; catalog no. ab37291; 2.6 μg/mL).
The next day, after washing three times for 15 minutes each with PBST, the vessels were stained for CD31 (PECAM-1) (to visualize endothelial cell junctions to delineate the position of lymphatic endothelium and lymphatic valves), using clone TLD-3A12, IgG1 isotype, (BD Pharmingen/BD Biosciences, San Jose, CA; catalog no. 555025; 2 μg/mL) for 1 hour. After washing three times for 15 minutes each with PBST, the vessels were incubated 1 hour in PBST containing goat anti-mouse IgG1 (catalog no. A21121) conjugated with Alexa Fluor 488 and anti-rabbit IgG (catalog no. A21245) conjugated with Alexa Fluor 647 (both from Invitrogen Corp.). After further washing in PBST, the MLVs were transferred to an isolated vessel chamber (CH/1), cannulated, slightly pressurized (∼1 cm H2O), and were finally placed on the surface of the bottom of the chamber, so each vessel touched the bottom as much as possible.
To obtain the greatest possible detail concerning the levels of expression of HDC in adult and aged MLVs, with reference to position of lymphatic endothelium, we imaged these vessels using two different microscope setups with various imaging settings. The group of fluorescently labeled MLVs (n = 4 for both age groups with one vessel used from each animal) was imaged on a Leica AOBS SP2 confocal multiphoton microscope system (Leica Microsystems, Wetzlar, Germany) using an Olympus UAPO 340/cc 40 × water-immersion objective (1.15 N.A.) (Olympus America, Inc., Center Valley, PA). Other groups of fluorescently labeled MLVs were imaged on an Olympus Fluoview300 confocal microscope using a PLAPON 60 × oil-immersion objective (1.42 N.A.) or UPLSAPO 100 × oil-immersion objective (1.4 N.A.) (Olympus America, Inc.) (n = 5 for both age groups with one vessel used from each animal for 60 × objective imaging and n = 4 for both age groups with one vessel used from each animal for 100 × objective imaging).
For all imaging, the two fluorescence channels were captured sequentially using 488 and 633 nm lasers for excitation with 2 × frame averaging. The confocal aperture diameter was set to one airy disk and Z-sections (axial vessel sections) were captured per region with a 0.3 μm Z-axis step size. We imaged two to four zones of interest per vessel. The same parameters of image capturing were utilized for all MLVs imaged on a given microscope under a given magnification. Subsequently, from each zone of interest, we produced average intensity projections of Z-axis sections in the middle of the vessel corresponding to 15 μm of vessel thickness using Leica confocal software (for Leica microscope-generated images) or NIH ImageJ software29 (for Olympus confocal microscope-generated images). These projections were used for quantitative analysis and displayed in this article.
Captured sets of images were analyzed using a Windows-operating computer. The intensity of HDC staining was assessed by measuring pixel intensity of its red signal, using NIH ImageJ software.29 The mean pixel intensity was measured in multiple regions of interest (ROIs) within the MLV wall, which were purposely selected to be equal within each image of each MLV, and then recalculated per 1 μm2 of the entire MLV wall. The mean pixel intensity in all ROIs within any one particular image of a given vessel was averaged (being careful not to include more than once those areas in images that depicted overlap between subsequent images from that vessel), and then the average numbers obtained from all images of the vessel were grouped and averaged again.
In addition, to visually evaluate and to display the localization of the HDC signal in reference to the position of lymphatic endothelial cells, we overlapped each image containing the HDC signal (red) with a corresponding image containing the CD31 signal (green) with a subsequent setting of 50% opacity of the green signal-based image layer using Adobe Photoshop ver. 12.1 x64 (Adobe Systems, Inc., San Jose, CA).
Results
Internally derived histamine does not affect the pressure-dependent reactions of MLVs
We performed a comparative evaluation of contractile activity of adult and aged MLVs during increases in transmural pressure from 1 to 5 cm H2O, before and after overnight HDC blockade induced by α-MHD. In control conditions, responses of 9-mo and 24-mo MLVs were identical to those described in our previous publication,10 demonstrating weakening of the lymphatic pump in aged MLVs. It is notable that HDC blockade was not able to induce any significant changes in any of the evaluated parameters (LTI, AMP, FREQ, FPF) when no flow was imposed in either adult or aged MLVs (data not shown), demonstrating that histamine released from MLVs does not affect their stretch-related sensitivity.
Aging increases the involvement of histamine as an EDRF in imposed flow/wall shear stress-dependent reactions of MLVs while it abolishes NO-dependent sensitivity of aged MLVs to imposed flow
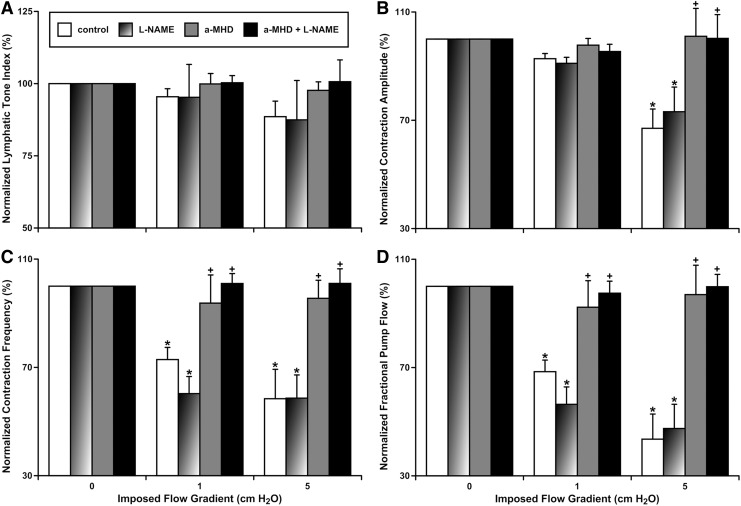
In our past studies, we established that the imposed flow gradient-generated wall shear stress-dependent tonic relaxation does not exist in aged MLVs, while the changes in phasic contractions induced by such shear stress cannot be eliminated by NOS blockade.10 In this study, we performed NOS blockade overnight in cultured aged MLVs. Our new data strengthen these conclusions, demonstrating the absence of statistically significant differences for any measured parameters of phasic contractile activity of aged MLVs at any value of imposed flow gradient between control conditions and after application of L-NAME (Fig. 1A–D).
FIG. 1.
Effects of pharmacological blockade of NO and histamine production on imposed flow-dependent relaxation and contractile inhibition of aged MLVs. (A) Lymphatic tone index; (B) contraction amplitude; (C) contraction frequency; (D) fractional pump flow. Control, control MLVs after overnight culture; L-NAME, same control vessels after subsequent acute pharmacological blockade of NO production by the NOS nonspecific blocker, L-NAME (100 μM); a-MHD, MLVs after overnight pharmacological blockade of histamine production by the HDC-specific blocker, α-MHD at 10 μM; a-MHD+L-NAME, same α-MHD-treated vessels after subsequent acute pharmacological blockade of NO production by NOS nonspecific blocker, L-NAME in 100 μM. “*” indicates significant difference (p < 0.05) between lymphatic contractile parameters within each treatment group at no-flow and at any imposed flow conditions. “+” indicates significant difference (p < 0.05) between lymphatic contractile parameters in any treatment group and control group within any given imposed flow condition. α-MHD, α-methyl-DL-histidine dihydrochloride; HDC, histidine decarboxylase; L-NAME, Nω-nitro-L-arginine methyl ester hydrochloride; MLV, mesenteric lymphatic vessel; NOS, nitric oxide synthase.
A remarkable difference in sensitivity of aged MLVs to imposed flow was observed in these isolated lymphatic vessels after overnight α-MHD blockade of HDC, the histamine-producing enzyme. Even for lymphatic tone, which was nonsignificantly diminished by the high imposed flow gradient of 5 cm H2O (89% ± 5% of LTI at zero imposed flow gradient conditions, Fig. 1A), we observed a trend toward complete elimination of these minute effects of imposed flow gradient on tone of aged MLVs after HDC blockade (98% ± 3%) (Fig. 1A). Other parameters of contractility of aged MLVs were significantly diminished in the presence of the high imposed flow gradient of 5 cm H2O: AMP (67% ± 7%) (Fig. 1B), FREQ (58% ± 11%) (Fig. 1C), and FPF (44% ± 9%) (Fig. 1D). Notably, even the low imposed flow gradient of 1 cm H2O was able to induce a significant drop in FREQ (73% ± 4%) (Fig. 1C) and in FPF (44% ± 9%) (Fig. 1D) in aged MLVs.
Overnight blockade of HDC, and therefore elimination of histamine from aged MLVs, completely eliminated all imposed flow/wall shear stress-dependent changes of their AMP, FREQ, and FPF (these parameters at the high imposed flow gradient of 5 cm H2O were not significantly different from those at the zero imposed flow gradient conditions). AMP was restored to 101% ± 10% (Fig. 1B), FREQ was 96% ± 7% (Fig. 1C), and FPF was 97% ± 11% (Fig. 1D). Moreover, overnight incubation of aged MLVs with α-MHD, even at low imposed flow gradient of 1 cm H2O, returned FREQ and FPF to levels not significantly different from those observed at zero imposed flow gradient conditions: FREQ (94% ± 10%) (Fig. 1C) and FPF (93% ± 10%) (Fig. 1D). Subsequent additional blockade of NOS by L-NAME in α-MHD-treated aged MLVs was not able to induce any further changes of any parameters of contractility of these vessels (Fig. 1).
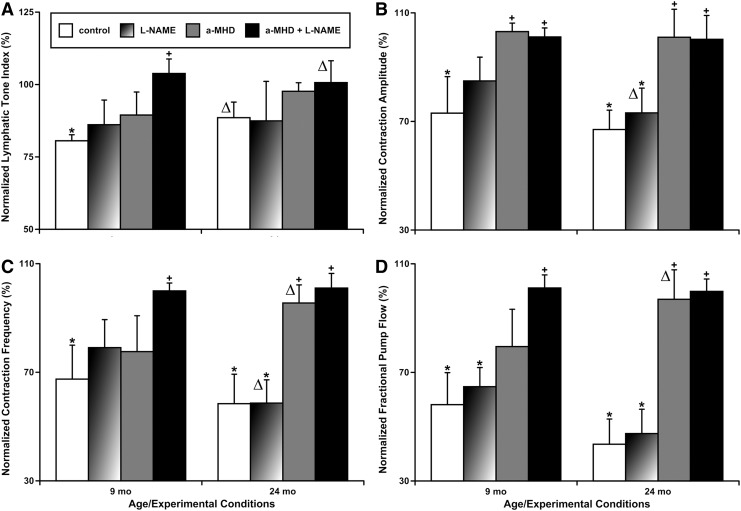
Comparative evaluation of the importance of NO and histamine for imposed flow gradient-dependent wall shear stress-induced relaxation and contractile inhibition in adult and aged MLVs
Earlier we demonstrated that only combined pharmacological blockade of NO and histamine production in adult MLVs completely eliminated their imposed flow-dependent relaxation and contractile inhibition.15 In this study, with the goal to compare imposed flow-induced responses of 9-mo and 24-mo MLVs and to evaluate the relative importance of NO and histamine in their generation, we compared part of the data obtained in experiments with adult MLVs (from our earlier study15) and data obtained in experiments with aged MLVs described above. This new comparative analysis revealed important significant differences in reactions of adult and aged MLVs in response to imposed flow.
Lymphatic tone of adult MLVs was significantly diminished by the high imposed flow gradient of 5 cm H2O, while the imposed flow-induced relaxation does not exist in aged MLVs, as we mentioned above (Fig. 2A). Only combined blockade of both NO and histamine synthesis in adult MLVs was able to completely eliminate, confirming the importance of both molecules in this effect (Fig. 2A). Phasic contractions AMP was reduced by the high imposed flow gradient of 5 cm H2O in both adult and aged MLVs (Fig. 2B). HDC blockade alone was able to completely eliminate this effect in MLVs of both ages, however, in adult MLVs, NOS blockade made this inhibition of AMP nonsignificant from zero imposed flow gradient conditions. In aged MLVs, the NOS blockade had no effect on AMP under similar experimental settings (Fig. 2B).
FIG. 2.
Comparison of the effects of pharmacological blockade of NO and histamine production on high flow-induced (5 cm H2O of imposed flow gradient) relaxation and contractile inhibition in adult (9-mo) and aged (24-mo) MLVs. (A) Lymphatic tone index; (B) contraction amplitude; (C) contraction frequency; (D) fractional pump flow. Control, control MLVs after overnight culture; L-NAME, same control vessels after subsequent acute pharmacological blockade of NO production by the NOS nonspecific blocker, L-NAME (100 μM); a-MHD, MLVs after overnight pharmacological blockade of histamine production by the HDC-specific blocker, α-MHD at 10 μM; a-MHD+L-NAME, same α-MHD-treated vessels after subsequent acute pharmacological blockade of NO production by the NOS nonspecific blocker, L-NAME at 100 μM. “*” indicates significant difference (p < 0.05) between lymphatic contractile parameters within each treatment group at no-flow and at conditions of 5 cm H2O of imposed flow gradient. “+” indicates significant difference (p < 0.05) between lymphatic contractile parameters in any treatment group and the control group. “Δ” indicates significant changes (loss or gain of statistical significance, p < 0.05) of lymphatic contractile parameters in any pair of values in treatment/flow combinations within the aged MLV group when compared to similar pairs of values in the adult MLV group. 9-mo, 9-month-old; 24-mo, 24-month-old.
Similarly, FREQ was reduced by the high imposed flow gradient of 5 cm H2O in MLVs of both ages (Fig. 2C). In adult MLVs, only combined blockade of both NO and histamine synthesis in adult MLVs was able to completely eliminate the imposed flow-induced decrease in FREQ, confirming the importance of both molecules in this effect (Fig. 2C). In aged MLVs, NOS blockade had no effect on FREQ, while HDC blockade alone was able to completely eliminate this effect in similar experimental settings (Fig. 2C).
Cumulatively, pumping of MLVs (FPF) from rats of both ages was reduced by the high imposed flow gradient of 5 cm H2O (Fig. 2D). In adult MLVs, only combined blockade of both NO and histamine synthesis was able to completely eliminate the imposed flow-induced decrease in FPF, confirming the cumulative role of both molecules in this effect, while this did not occur when NOS blockade was performed alone (Fig. 2D). In aged MLVs, NOS blockade performed alone had no effect on FPF, while HDC blockade alone was able to completely eliminate this effect in similar experimental settings (Fig. 2D).
Increased expression of HDC in endothelial cells of aged MLVs
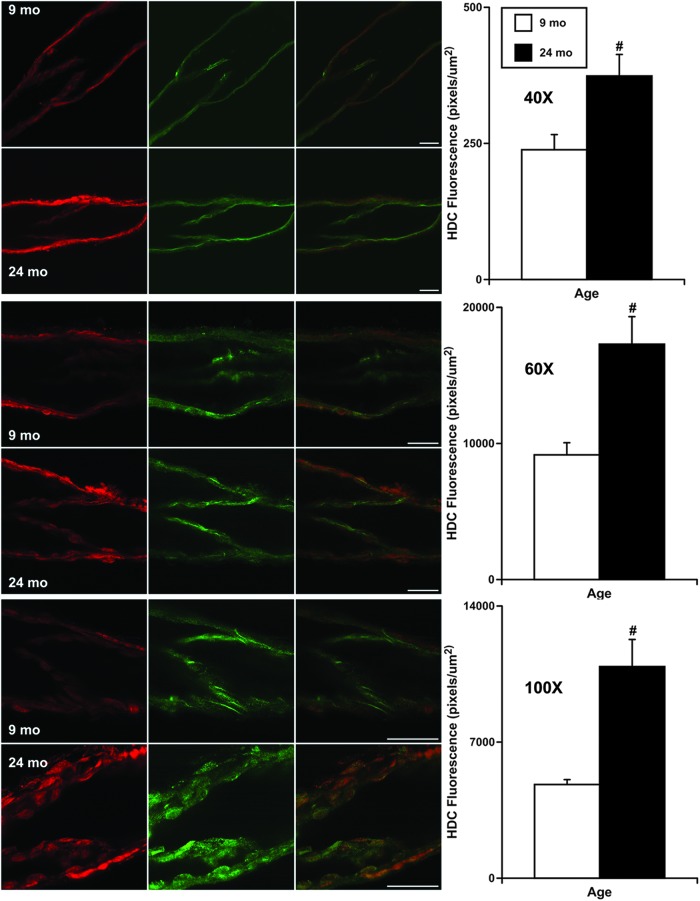
To determine if HDC exists only inside the lymphatic endothelium and if its expression is potentially increased in aged MLVs, we performed fluorescence imaging of these vessels using two different microscope setups with various imaging settings. This approach allowed us to obtain the greatest possible detail about the expression levels of HDC in aged MLVs in comparison with their adult counterparts with reference to the position of lymphatic endothelial cells. Immunohistochemical labeling of MLVs by antibodies specific for CD31 (PECAM-1) permitted us to visualize lymphatic endothelial cell junctions and therefore to delineate the position of the lymphatic endothelial cells.
At the same time, we used antibodies specific for HDC to detect and localize this enzyme within MLVs. After we overlapped all pairs of CD31 and HDC images taken at the same MLV zones, we found that in 100% of cases, for vessels of both ages, the HDC signal was always localized inside of lymphatic endothelial cells delineated by CD31 (red signal occurred only inside of cells labeled by green; representative overlapped images shown in Fig. 3). As a consistent finding through all of these experiments, we found that intensity of the fluorescent signal of HDC in endothelial cells of aged MLVs was always higher than in adult ones: ∼1.6 times for 40 × objective-based settings; ∼1.9 times for 60 × objective-based settings; and ∼2.3 times for 100 × objective-based settings (Fig. 3).
FIG. 3.
Representative confocal images of adult (9-mo) and aged (24-mo) isolated MLVs and results of quantitative analysis of the intensity of fluorescence of the HDC signal (in red) in images taken at various settings for microscopy (40 × , 60 × , 100 × , see the Materials and Methods section for details). CD31 (PECAM-1) staining, shown in green, indicates lymphatic endothelial cell junctions and therefore delineates the location of lymphatic endothelial cells. Merged images in the third column demonstrate localization of HDC inside the lymphatic endothelial cells. A scale bar within each row of images represents 50 μm and applies to all images in that row. “#” indicates significant difference (p < 0.05) between adult and aged MLVs.
Conclusions
Functional roles of NO and histamine in the regulation of lymphatic contractility
In 2002, we demonstrated the existence of an imposed flow gradient-generated relaxation and inhibition in lymphatic vessels, partially driven by NO.8 We concluded that “at high levels of lymph formation, passive lymph flow could become a greater driving force to move lymph than the lymph pump. Flow-dependent inhibition of the active lymph pump in such situations could be a reasonable physiological mechanism to save metabolic energy by temporarily decreasing or stopping contractions during the time when the lymphatic does not need it.”8
Later, these basic conclusions were supported by computational analysis and were termed the “lymphatic pump-conduit duality of lymphangions.”30,31 At approximately the same time, in 2006, we found that in conditions of an imposed flow gradient, “the reduction of tone in lymphatic segments generated by the phasic contractions improves their diastolic filling (enhanced lusitropy…), makes lymphatic contractions stronger (enhanced inotropy…) and propels more fluid forward during each contraction (elevated ejection fraction) while decreasing contraction frequency (reduced chronotropy).”19 We determined that this fast phasic lymphatic contraction-generated regulatory mechanism is mediated solely by NO, a quickly released and fast-acting molecule.19
We concluded that when discussing the flow conditions in a single lymphangion, it is reasonable to divide the flow pattern into two components: “intrinsic flow” (meaning the flow that is a result of the contractions of that lymphangion) and “extrinsic flow” (meaning the flow that is a result of all influences from outside that single lymphangion, predominantly from upstream of that single lymphangion but including some downstream influences as well).7 From the isolated lymphatic vessel experiments with an imposed flow, we know that “as the imposed flow was increased, the degree of inhibition of lymphatic pumping increased.”3,8,18 Further studies confirmed the details of these phenomena based on direct measurements of NO in the lymphatic wall.25,32 Fast phasic spike-like releases of NO25,32 have been linked to the transient increases in wall shear stress in contracting and pumping lymphangions.33
Cumulatively, all of these data, previously published by us and by others, allow us to conclude that “there is a complex interplay between the influences of phasic and tonic components of the stretch-dependent myogenic responses and the influences of phasic contraction-generated NO release in mesenteric lymphatic vessel.”7
More recently, in 2015, another group34 hypothesized that “Ca2+ and NO cooperate to control lymphatic transport via mechanobiological feedback loops: during a lymphatic contraction cycle, increased shear causes local endothelial NOS activation, and the subsequent production of NO results in blunting and/or reversal of the Ca2+-dependent contraction. As the vessel relaxes, NO degrades rapidly and its production drops due to the reduced fluid velocity in the now larger-diameter vessel.” Using their computational model and some experimental evidence obtained in lymphatic vessels in mice, these authors stated that their model “suggests that shear-driven NO feedback enables lymph transport over a range of transwall and axial pressure gradient conditions not possible in the absence of NO signaling.”34
Based on these experiments and their corresponding analysis, the authors concluded that “this mechanism is self-regulating and robust over a range of fluid pressure environments, allowing the lymphatic vessels to provide pumping when needed but remain open when flow can be driven by tissue pressure or gravity.”34 Thus, despite serious limitations35 in the authors' approach to interpreting their data, this group34 simplistically recapitulated the summary of findings of the experiments performed during the last 15 years by us and our collaborators, therefore demonstrating the general significance and wide applicability of our previous conclusions.
In relation to the subject of this study, these authors stated that “other endothelial-derived relaxation factors, such as histamine,15 generally have much longer lifetimes and would not be able to drive the oscillations,”34 pointing to the oscillatory nature of spontaneous phasic contractions and wall shear stress generated by them in lymphatic vessels. This conclusion parallels our earlier published data demonstrating that contraction-initiated NO-dependent lymphatic diastolic relaxation is solely an NO-dependent self-regulatory mechanism.19
Current data clearly support these findings—overnight HDC blockade in MLVs, which allows enough time for slow, but complete depletion of all intracellular stores of premade intralymphatic-generated histamine, was not able to affect the stretch-dependent reactivity of contracting MLVs in either age group. This provides important confirmation of our previous conclusions described above and new knowledge that the aging-associated increase of lymphatic endothelium-derived histamine in MLVs (our experiments on isolated MLVs dealt with only histamine generated by lymphatic endothelium) is not involved, at least directly, in fast phasic contraction-generated regulation of the lymphatic contractile cycle in aged MLVs.
Increased role of histamine as an EDRF in aged MLVs
Our current data, for the first time, demonstrate that aging differentially affects the roles of NO and histamine in imposed flow gradient-generated steady wall shear stress-dependent regulation of contractility of MLVs. Steady flow-induced negative inotropy (lowering of contraction amplitude), negative chronotropy (decrease in contraction frequency), and diminished lymphatic pumping (smaller FPF) are completely mediated by histamine in aged MLVs, while the involvement of NO in this regulation is abolished. In terms of the altered lymph dynamics in the elderly, this may reflect potentially stronger intralymphatic-borne histamine-induced contractile inhibition of aged MLVs during acute inflammatory events in aged mesentery and in the gut.
These conditions are likely to maintain peripheral edema and increased passive lymph flow in aged MLVs for comparatively long periods of time. However, the question of whether it would be beneficial for decreasing the immune response in the aged body or whether it will worsen it remains to be carefully studied.
Histamine in aged MLVs and perilymphatic tissues
In this study we, for the first time, demonstrated that aging introduces significant changes in wall shear stress-dependent modulation of lymphatic contractility related to the increased functional role of histamine as an EDRF in aged MLVs and simultaneous elimination of the NO as a molecular player in such regulatory reactions. These findings correlate with the increased expression of HDC, the histamine-producing enzyme, in aged MLVs. At the same time, we confirmed that transmural pressure/stretch-dependent regulatory reactions of contracting MLVs are not affected by intralymphatic-borne histamine, neither in adult nor in aged MLVs. Such is not true for effects of histamine delivered from sources external to lymphatic vessel on the lymphatic contractility.
In particular, data from the literature suggest that histamine is a potent dose-dependent modulator of lymphatic contractility.36–44 At lower concentrations, histamine stimulates lymphatic contractility, while at higher concentrations, histamine induces relaxation and contractile inhibition.36–49 Such findings illustrate the clear necessity of further in-depth discovery-driven research efforts to establish complicated interrelations between histamine-producing lymphatic vessels and histamine-producing cells of perilymphatic tissues.
The findings of the current study arose from our discovery-driven experiments, which clearly illustrate the continued necessity of describing the many unknown features of lymphatic vessels to gain insight into the origin and pathogenesis of diseases of the lymphatic system, for example, lymphedema. As we mentioned earlier,15 histamine, as a known chemoattractant of mast cells,50 may be responsible for increased density of mast cells observed near MLVs.12 In light of our current findings, an observed further increase of mast cell density in aged perilymphatic mesenteric tissues (compared to adult tissues)12 directly correlates with the increased expression of HDC in aged MLVs demonstrated in this study.
Increased levels of histamine in aged MLVs, with corresponding increased diffusion of this molecule out of lymphatic vessels, are very likely important for increased recruitment, and potentially for chronic basal activation of perilymphatic mast cells in the elderly.12,14 Taking into account the known role of mast cell-released mediators necessary for modulation of the immune response,51–53 the chronically increased production of histamine in aged MLVs could be linked to consequences of aging-associated basal mast cell activation in aged perilymphatic mesenteric tissues. The aging-associated activation of mast cells maintains chronic histamine-dependent activation of NF-κB signaling in aged perilymphatic mesenteric tissues and limits NF-κB activation in aged mesentery in response to acute inflammation.54 These findings correlate with our previously published findings that link together chronic activation of aged mast cells with diminished recruitment/activation of MHC class II+ cells and eosinophils toward/near aged MLVs during acute inflammation.14
In conclusion, we believe that further in-depth studies to establish all functional links in these complex intercellular interactions within and between the MLVs and surrounding tissues are necessary to move this field forward.
Acknowledgments
This work was supported, in parts, by the National Institutes of Health (NIH RO1 grants AG030578 and DK099161) and by the Texas A&M University Health Science Center College of Medicine and Department of Medical Physiology. The authors thank Anna Webb and the Texas A&M University Health Science Center Integrated Microscopy and Imaging Laboratory for help in the confocal imaging studies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mislin H. Active contractility of the lymphangion and coordination of lymphangion chains. Experientia 1976; 32:820–822 [DOI] [PubMed] [Google Scholar]
- 2.Zawieja DC. Lymphatic microcirculation. Microcirculation 1996; 3:241–243 [DOI] [PubMed] [Google Scholar]
- 3.Gashev AA. Physiologic aspects of lymphatic contractile function: Current perspectives. Ann N Y Acad Sci 2002; 979:178–187; discussion 188–196 [DOI] [PubMed] [Google Scholar]
- 4.Ohhashi T, Mizuno R, Ikomi F, Kawai Y. Current topics of physiology and pharmacology in the lymphatic system. Pharmacol Ther 2005; 105:165–188 [DOI] [PubMed] [Google Scholar]
- 5.Gashev AA. Lymphatic vessels: Pressure- and flow-dependent regulatory reactions. Ann N Y Acad Sci 2008; 1131:100–109 [DOI] [PubMed] [Google Scholar]
- 6.Zawieja DC. Contractile physiology of lymphatics. Lymphat Res Biol 2009; 7:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gashev AA. Basic mechanisms controlling lymph transport in the mesenteric lymphatic net. Ann N Y Acad Sci 2010; 1207 Suppl 1:E16–E20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 2002; 540(Pt 3):1023–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 2011; 301:H1828–H1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 2011; 18:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thangaswamy S, Bridenbaugh EA, Gashev AA. Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol 2012; 10:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 2012; 303:H693–H702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridenbaugh EA, Nizamutdinova IT, Jupiter D, Nagai T, Thangaswamy S, Chatterjee V, Gashev AA. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol 2013; 11:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee V, Gashev AA. Mast cell-directed recruitment of MHC class II positive cells and eosinophils towards mesenteric lymphatic vessels in adulthood and elderly. Lymphat Res Biol 2014; 12:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nizamutdinova IT, Maejima D, Nagai T, Bridenbaugh E, Thangaswamy S, Chatterjee V, Meininger CJ, Gashev AA. Involvement of histamine in endothelium-dependent relaxation of mesenteric lymphatic vessels. Microcirculation 2014; 21:640–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 1999; 54:B492–B501 [DOI] [PubMed] [Google Scholar]
- 17.Gashev AA, Davis MJ, Gasheva OY, Nepiushchikh ZV, Wang W, Dougherty P, Kelly KA, Cai S, Von Der Weid PY, Muthuchamy M, Meininger CJ, Zawieja D.C. Methods for lymphatic vessel culture and gene transfection. Microcirculation 2009; 16:615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 2004; 11:477–492 [DOI] [PubMed] [Google Scholar]
- 19.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: A self-regulatory mechanism in rat thoracic duct. J Physiol 2006; 575(Pt 3):821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gashev AA, Delp MD, Zawieja DC. Inhibition of active lymph pump by simulated microgravity in rats. Am J Physiol Heart Circ Physiol 2006; 290:H2295–H2308 [DOI] [PubMed] [Google Scholar]
- 21.Rees DD, Palmer RM, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 1990; 101:746–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol 1998; 274(3 Pt 2):R790–R796 [DOI] [PubMed] [Google Scholar]
- 23.Watanabe S, Yashiro Y, Mizuno R, Ohhashi T. Involvement of NO and EDHF in flow-induced vasodilation in isolated hamster cremasteric arterioles. J Vasc Res 2005; 42:137–147 [DOI] [PubMed] [Google Scholar]
- 24.Arenas IA, Xu Y, Davidge ST. Age-associated impairment in vasorelaxation to fluid shear stress in the female vasculature is improved by TNF-{alpha} antagonism. Am J Physiol Heart Circ Physiol 2006; 290:H1259–H1263 [DOI] [PubMed] [Google Scholar]
- 25.Bohlen HG, Wang W, Gashev A, Gasheva O, Zawieja D. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. Am J Physiol Heart Circ Physiol 2009; 297:H1319–H1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gashev AA, Zawieja DC. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology 2010; 17:277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis MJ, Zawieja DC, Gashev AA. Automated measurement of diameter and contraction waves of cannulated lymphatic microvessels. Lymphat Res Biol 2006; 4:3–10 [DOI] [PubMed] [Google Scholar]
- 28.Benoit JN, Zawieja DC, Goodman AH, Granger HJ. Characterization of intact mesenteric lymphatic pump and its responsiveness to acute edemagenic stress. Am J Physiol 1989; 257(6 Pt 2):H2059–2069 [DOI] [PubMed] [Google Scholar]
- 29.Rasband WS, Image J. 1997–2016, U.S. National Institutes of Health, Bethesda, MD: http://rsb.info.nih.gov/ij/ [Google Scholar]
- 30.Quick CM, Venugopal AM, Gashev AA, Zawieja DC, Stewart RH. Intrinsic pump-conduit behavior of lymphangions. Am J Physiol Regul Integr Comp Physiol 2007; 292:R1510–R1518 [DOI] [PubMed] [Google Scholar]
- 31.Quick CM, Ngo BL, Venugopal AM, Stewart RH. Lymphatic pump-conduit duality: Contraction of postnodal lymphatic vessels inhibits passive flow. Am J Physiol Heart Circ Physiol 2009; 296:H662–H668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohlen HG, Gasheva OY, Zawieja DC. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. Am J Physiol Heart Circ Physiol 2011; 301:H1897–H1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixon JB, Greiner ST, Gashev AA, Cote GL, Moore JE, Zawieja DC. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 2006; 13:597–610 [DOI] [PubMed] [Google Scholar]
- 34.Kunert C, Baish JW, Liao S, Padera TP, Munn LL. Mechanobiological oscillators control lymph flow. Proc Natl Acad Sci U S A 2015; 112:10938–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis MJ. Is nitric oxide important for the diastolic phase of the lymphatic contraction/relaxation cycle? Proc Natl Acad Sci U S A 2016; 113:E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohhashi T, Kawai Y, Azuma T. The response of lymphatic smooth muscles to vasoactive substances. Pflugers Arch 1978; 375:183–188 [DOI] [PubMed] [Google Scholar]
- 37.Johnston MG, Kanalec A, Gordon JL. Effects of arachidonic acid and its cyclo-oxygenase and lipoxygenase products on lymphatic vessel contractility in vitro. Prostaglandins 1983; 25:85–98 [DOI] [PubMed] [Google Scholar]
- 38.Unthank JL, Hogan RD. The effect of vasoactive agents on the contractions of the initial lymphatics of the Bat's wing. Blood Vessels 1987; 24:31–44 [DOI] [PubMed] [Google Scholar]
- 39.Dobbins DE, Buehn MJ, Dabney JM. Constriction of perfused lymphatics by acetylcholine, bradykinin and histamine. Microcirc Endothelium Lymphatics 1990; 6:409–425 [PubMed] [Google Scholar]
- 40.Fox JLR, von der Weid P-Y. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 2002; 136:1210–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaku KJ, von der Weid PY. Mast cell degranulation alters lymphatic contractile activity through action of histamine. Microcirculation 2006; 13:219–227 [DOI] [PubMed] [Google Scholar]
- 42.Petunov SG, Egorova AA, Orlov RS, Nikitina ER. Effect of histamine on spontaneous contractions of mesenteric lymphatic vessels and lymph nodes of white rats: Endothelium-dependent responses. Dokl Biol Sci 2010; 432:176–180 [DOI] [PubMed] [Google Scholar]
- 43.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation 2014; 21:593–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurtz KM, Souza-Smith FM, Breslin JW. Involvement of NO/sGC, but not ROCK, in histamine-induced collecting lymphatic relaxation. FASEB J 2013; 27:681.12 [Google Scholar]
- 45.Mislin H. [The contractile properties of lymphatic vessels]. Angiologica 1971; 8:207–211 [PubMed] [Google Scholar]
- 46.Orlov R, Lobov G. [Ionic mechanisms of the electrical activity of the smooth-muscle cells of the lymphatic vessels]. Fiziol Zh SSSR Im I M Sechenova 1984; 70:712–721 [PubMed] [Google Scholar]
- 47.Ferguson MK, Shahinian HK, Michelassi F. Lymphatic smooth muscle responses to leukotrienes, histamine and platelet activating factor. J Surg Res 1988; 44:172–177 [DOI] [PubMed] [Google Scholar]
- 48.Watanabe N, Kawai Y, Ohhashi T. Dual effects of histamine on spontaneous activity in isolated bovine mesenteric lymphatics. Microvasc Res 1988; 36:239–249 [DOI] [PubMed] [Google Scholar]
- 49.Pan'kova MN, Lobov GI, Chikhman VN, Solnyshkin SD. [Effects of histamine on contractile activity of lymphatic node capsules. The NO role]. Ross Fiziol Zh Im I M Sechenova 2011; 97:633–640 [PubMed] [Google Scholar]
- 50.Keles N, Yavuz Arican R, Coskun M, Elpek GO. Histamine induces the neuronal hypertrophy and increases the mast cell density in gastrointestinal tract. Exp Toxicol Pathol 2012; 64:713–716 [DOI] [PubMed] [Google Scholar]
- 51.Amin K. The role of mast cells in allergic inflammation. Respir Med 2012; 106:9–14 [DOI] [PubMed] [Google Scholar]
- 52.Harvima IT, Nilsson G. Mast cells as regulators of skin inflammation and immunity. Acta Derm Venereol 2011; 91:644–650 [DOI] [PubMed] [Google Scholar]
- 53.St. John AL, Abraham SN. Innate immunity and its regulation by mast cells. J Immunol 2013; 190:4458–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nizamutdinova IT, Dusio GF, Gasheva OY, Skoog H, Tobin R, Peddaboina C, Meininger CJ, Zawieja DC, Newell-Rogers MK, Gashev AA. Mast cells and histamine are triggering the NF-kappaB-mediated reactions of adult and aged perilymphatic mesenteric tissues to acute inflammation. Aging 2016; 8:3065–3090 [DOI] [PMC free article] [PubMed] [Google Scholar]