Abstract
BACKGROUND
Abundant cross-sectional evidence links eveningness (a preference for later sleep-wake timing) and increased alcohol and drug use among adolescents and young adults. However, longitudinal studies are needed to examine whether eveningness is a risk factor for subsequent alcohol and drug use, particularly during adolescence, which is marked by parallel peaks in eveningness and risk for the onset of alcohol use disorders. The present study examined whether eveningness and other sleep characteristics were associated with concurrent or subsequent substance involvement in a longitudinal study of adolescents.
METHODS
Participants were 729 adolescents (368 females; age 12–21 years) in the National Consortium on Adolescent Neurodevelopment and Alcohol [NCANDA] study. Associations between the sleep variables (circadian preference, sleep quality, daytime sleepiness, sleep timing, and sleep duration) and three categorical substance variables (at-risk alcohol use, alcohol bingeing, and past year marijuana use (y/n)) were examined using ordinal and logistic regression with baseline age, sex, race, ethnicity, socioeconomic status, and psychiatric problems as covariates.
RESULTS
At baseline, greater eveningness was associated with greater at-risk alcohol use, greater bingeing, and past-year use of marijuana. Later weekday and weekend bedtimes, but not weekday or weekend sleep duration, showed similar associations across the three substance outcomes at baseline. Greater baseline eveningness was also prospectively associated with greater bingeing and past-year use of marijuana at the 1-year follow-up, after covarying for baseline bingeing and marijuana use. Later baseline weekday and weekend bedtimes, and shorter baseline weekday sleep duration, were similarly associated with greater bingeing and past-year use of marijuana at the 1-year follow-up after covarying for baseline values.
CONCLUSIONS
Findings suggest that eveningness and sleep timing may be under-recognized risk factors and future areas of intervention for adolescent involvement in alcohol and marijuana that should be considered along with other previously-identified sleep factors such as insomnia and insufficient sleep.
Keywords: sleep, circadian preference, adolescence, alcohol, marijuana
INTRODUCTION
A link between sleep and involvement in alcohol and other drugs has long been recognized (Johnson and Breslau, 2001, Conroy and Arnedt, 2014, Blank et al., 2014). Not only do sleep disturbance and chronic alcohol and drug use frequently co-occur, but pre-existing sleep factors such as insomnia are a risk factor for the onset of alcohol and drug problems across child, adolescent, and adult samples (Breslau et al., 1996, Wong et al., 2004, Mike et al., 2016, Hasler et al., 2014a, Wong et al., 2015a, Wong et al., 2010, Hasler et al., 2016, Pieters et al., 2015). Notably, although a burgeoning literature demonstrates consistent cross-sectional associations between a tendency towards an evening circadian preference (i.e., eveningness, or preferred later sleep-wake timing) and the use of alcohol and other drugs (Pieters et al., 2010, Giannotti et al., 2002, Negriff et al., 2011, Urban et al., 2011, Gau et al., 2007), few studies have examined whether eveningness is a prospective risk factor for future alcohol and other drug involvement, including both use and problems. A better understanding of the longitudinal associations between eveningness and alcohol involvement are particularly needed during adolescence, when there are parallel increases in eveningness (Randler, 2011, Roenneberg et al., 2004) and risk for the onset of alcohol use disorders (Brown et al., 2008).
Most longitudinal studies have focused on sleep problems such as insomnia, hypersomnia, or insufficient sleep (based on short sleep duration or daytime sleepiness) and alcohol- and marijuana-related substance outcomes. Other sleep measures have included sleep quality (tapping the construct of sleep disturbance more broadly) and variability in sleep timing or duration. Specific substance outcomes have varied, most often focusing on use (frequency and outcome over varying time frames), and less frequently focusing on substance-related problems (including symptoms or diagnoses) or the age of onset of use, intoxication, and/or diagnosis. In adolescent samples, insomnia-type complaints (broadly-defined) and shorter sleep duration most consistently predict alcohol and/or marijuana involvement at follow-up (Mike et al., 2016, Hasler et al., 2014a, Wong et al., 2015a), although at least one large (n=4,494) longitudinal study reported null findings based on an insomnia diagnosis (Roane and Taylor, 2008).
Longitudinal evidence of associations between eveningness preference or actual sleep timing and alcohol/drug-related outcomes remain limited, although abundant cross-sectional findings exist (Pieters et al., 2010, Giannotti et al., 2002, Gau et al., 2007, Negriff et al., 2011, Urban et al., 2011). Indeed, to our knowledge, only one published study has examined whether eveningness predicts later alcohol and drug involvement. Of 942 undergraduates (age 17–25 years) examined, greater eveningness predicted higher substance use 1–2 years later (Tavernier et al., 2015). Eveningness was assessed with two yes/no questions, and substance use was a composite measure of alcohol and marijuana use. Although that study suggests that eveningness may be a risk factor for alcohol and other drug involvement, evidence is lacking on earlier adolescence when eveningness tendencies start increasing (Hagenauer et al., 2009). Furthermore, the use of a composite measure of substance use precludes examining specific and potentially differential associations between eveningness (or other sleep variables) and alcohol or marijuana involvement.
In the present study, we tested links between sleep and adolescent initiation and use of alcohol and marijuana in three key ways. First, we examined both cross-sectional and longitudinal associations between eveningness and substance involvement outcomes, making ours the first such longitudinal examination to include early and middle adolescence when eveningness is increasing. Second, in addition to considering eveningness, a preference measure that may or may not reflect actual sleep behavior, we examined self-reported actual sleep timing across both weekdays and weekends, including whether weekday-weekend delays in sleep timing are associated with substance involvement. Third, although our primary focus was on eveningness and sleep timing, our study included the most comprehensive range of sleep factors to date in a study of adolescent substance involvement, additionally assessing sleep quality, daytime sleepiness, and weekday/weekend sleep duration. Furthermore, we selected three distinct substance outcomes to elucidate potentially disparate associations between selected sleep characteristics and at-risk alcohol use, binge alcohol use, and/or marijuana use. To address these questions, we used data collected from the National Consortium on Adolescent Neurodevelopment and Alcohol [NCANDA] study, an ongoing accelerated longitudinal study of adolescents, age 12–21 years, designed to disentangle associations between alcohol use and neurobiological and neurocognitive development. We hypothesized that greater eveningness, later sleep timing, greater weekday-weekend delays in sleep timing, shorter sleep duration, worse sleep quality, and greater daytime sleepiness would show concurrent and prospective associations with alcohol and marijuana involvement.
METHODS
Participants
A total of 831 participants, aged 12.0–21.9 years, were recruited across the five NCANDA data collection sites: Duke University, University of Pittsburgh School of Medicine, Oregon Health & Science University, University of California San Diego, and SRI International. The Institutional Review Board at each site approved the study. All adult participants provided written informed consent and all children provided written assent along with consent from a parent/legal guardian.
For details regarding recruitment, the full assessment protocol, study design, and data management of NCANDA, see Brown and colleagues (Brown et al., 2015). The present data are based on the baseline and 1-year assessments of the longitudinal study. Each site endeavored to represent local racial/ethnic distributions, include equal sex proportions in each age group, and to preferentially recruit youth at greater risk for heavy drinking in order to comprise approximately 50% of the sample. The protocol preferentially recruited participants with limited exposure to alcohol or other drugs at baseline; 83% of the full sample had no-to-low exposure (as described in (Brown et al., 2015). The present analyses included 729 participants with complete baseline data on circadian preference, alcohol use, and the relevant covariates, including age, sex, race, ethnicity, and parental socioeconomic status (SES). Parental SES was determined based on a combination of parent education and income. Participants were excluded from the original sample of 831 for missing SES data (n=91), with 11 additional participants excluded for missing substance use (n=9) or sleep data (n=6). These excluded participants reported higher at-risk and binge alcohol use, were more likely to use marijuana, and had later weekday and weekend bedtimes and later weekday rise times. In the present sample (n=729), 85% (n=623) at baseline met alcohol and drug use criteria for no-to-low exposure. Given the extremely low prevalence of nicotine use [weekly use at baseline: n=16 (2.2%); follow-up: n=29 (3.8%)] or drugs other than alcohol or marijuana [lifetime use of other drugs at baseline: n=27 (3.2%; follow-up: n=45 (5.9%)], we did not include these variables in the present analyses.
Measures
Substance use
Past and recent alcohol and other substance use were determined by the Customary Drinking and Drug Use Record [CDDR: (Brown et al., 1998)]. The measure includes items on alcohol and marijuana use, including use frequency in the past year, and the maximum number of drinks in a drinking episode during the past year.
At-Risk Alcohol Use
Alcohol use frequency, defined by days in the past year, has been found to useful for identifying at-risk alcohol use patterns among adolescents [e.g., (Chung et al., 2012, Clark et al., 2016)]. Here, using data from the CDDR, we defined participants as having high, moderate, or low risk levels for alcohol use patterns according to the recommendations of The National Institute on Alcohol Abuse and Alcoholism (NIAAA) Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide (Alcoholism, 2011): High Risk were age 12–15 y: 3+ days per year; age 16 y: 12 + days; age 17 y: 24+ days; age 18+ y: 52+ days; Moderate Risk were age 12–15 y: 1+ days per year; age 16–17 y: 3+ days; age 18+ y: 12+ days. Subjects below these thresholds were classified as Low Risk.
Binge Alcohol Use
While the traditional definition of a drinking binge has typically been applied across development, binge definitions based on estimated blood alcohol concentrations (eBAC) have been developed that are more appropriate for younger teens (Donovan, 2009). Using the “lifetime greatest number of drinks” response, the eBAC binge thresholds were calculated as follows: ages ≤13 years: ≥3 drinks; 14 or 15 years: ≥4 for male, ≥3 for female participants; 16 or 17 years: ≥5 for male, ≥3 for female participants. Heavy binge levels were defined as double and extreme binge levels were triple the initial age-based binge levels (Patrick, 2016).
Past-year Marijuana Use
Given the highly-skewed distribution of the marijuana use data, a dichotomous (yes/no) variable was created from the relevant CDDR item based on any endorsement of marijuana use in the past year.
Sleep
Sleep characteristics were assessed through several measures, as follows. Circadian preference was assessed using an abbreviated 4-item version (CSM-4) of the Composite Scale of Morningness (CSM; (Smith et al., 1989)). These items were selected to capture theoretically-important constructs, including preferred rise and bedtimes (#1 and 2 from the original scale), difficulty rising in the morning (#3), and self-identification of a morning-versus evening-type (#9). The possible CSM-4 score ranges from 4 to 18, with higher scores indicating greater morningness. This abbreviated scale correlated strongly (r=0.89) with the full CSM in a sub-sample (n=284) from two sites (Pittsburgh and SRI) that completed both. Internal consistency of the CSM-4 was adequate (α=0.64) given the expected deflation due to the small number of items (on the full CSM, α=0.83). Sleep quality was assessed using a single item (“During the past month, how would you rate your sleep quality overall?”) drawn from the Pittsburgh Sleep Quality Index (Buysse et al., 1989). Response options ranged from 1 to 4 in this order: “very good,” “fairly good,” “fairly bad,” and “very bad.” The Sleep Quality item correlated strongly (r=0.71) with the full PSQI in the Pittsburgh-SRI subsample. Daytime sleepiness was assessed using an abbreviated 5-item version (CASQ-5) of the Cleveland Adolescent Sleepiness Questionnaire (CASQ; (Spilsbury et al., 2007)). The items assess whether participants ever fall asleep during different parts of the school day (e.g., morning classes, afternoon classes, etc.; items #1, 3, 6, 15 from the full CASQ) or feel tired during the school day (#2), with response options ranging from “never” to “almost every day”. The possible CASQ-5 score ranges from 5 to 23, with higher scores indicating greater daytime sleepiness. The CASQ-5 correlated strongly (r=0.75) with the full CASQ in the Pittsburgh-SRI subsample. Internal consistency on the CASQ-5 was acceptable (α=0.71). Finally, habitual sleep timing (bedtime and rise time) and sleep duration were assessed separately for weekdays and weekends, and weekday-weekend shifts in sleep timing (weekend minus weekday) were calculated separately for bedtime and rise time. Specifically, participants were asked a series of four questions drawn from the Sleep Timing Questionnaire (Monk et al., 2003) about when they “normally sleep,” assessing “good night time” (defined as the time when they are finally in bed and trying to fall asleep) and “good morning time” (defined as the time at which they finally get out of bed and start their day) on “school or work days” and “days off (e.g., weekends)”. Sleep duration was calculated as the difference between good night time and good morning time.
Psychiatric symptoms
Psychiatric symptoms were assessed using the broad-band Internalizing and Externalizing scales from the baseline administration of the Achenbach System of Empirically Based Assessments (ASEBA; (Achenbach and Rescorla, 2001, Achenbach and Rescorla, 2003)), which were completed by each participant and a parent. Participants aged 12–17 completed the Youth Self-Report and participants over age 18 completed the Adult Self-Report; parents of participants aged 12–17 also completed the Child Behavior Checklist. Normalized t-scores were calculated based on age and sex, and the maximum t-score among the measures was used for each participant.
Data analyses
Test results were uploaded into the software platform Scalable Informatics for Biomedical Imaging Studies (SIBIS) at SRI International. The longitudinal data used in this manuscript were organized via a formal, locked data release (NCANDA_RELEASE_00001_V01), which was previously described in (Sullivan et al., 2017). Additional information about the NCANDA Data Management System has been published elsewhere (Rohlfing et al., 2014, Nichols and Pohl, 2015). Associations between baseline sleep variables and the three categorical substance measures were examined using ordinal regression (At-risk Alcohol Use, Binge Alcohol Use) or logistic regression (Past-Year Marijuana Use). We initially included baseline age, sex, race, and parental socioeconomic status (SES) as covariates1. We added psychiatric problems as an additional covariate in a second set of analyses. Ordinal and logistic regression analyses were conducted in SAS 9.4 (2014); all other analyses were conducted in SPSS v24 (2016). When examining associations between baseline sleep and the substance outcomes at the 1-year follow-up, the baseline values for the substance variables were additionally included as covariates. Prior to performing the ordinal regression analyses, assessment of the proportional odds assumption was performed using the Score test and graphical assessment of proportional odds. Given the number of analyses, the Holm-Sidak method was applied to correct for multiple comparisons.
RESULTS
Sample characteristics (Table 1)
Table 1.
Demographic and baseline substance use and sleep characteristics of NCANDA sample (n=729)
| Measure | Mean/median | SD | range |
|---|---|---|---|
| Age at baseline visit (years) | 15.9 | 2.4 | 12–21 |
| Sex | 368 female (51%), 361 male (49%) | ||
| Race | 549 white (75%), 89 African-American (12%), 50 Asian-American (7%), 41 other (6%) | ||
| Ethnicity | 641 non-Hispanic (88%), 88 Hispanic (12%) | ||
| Parental SES | 224 low (31%), 232 medium (32%), 272 high (37%) | ||
| Internalizing (ASEBA t-score) | 48.4 | 9.0 | 27–85 |
| Externalizing (ASEBA t-score) | 46.7 | 8.4 | 30–74 |
| Alcohol use, days in past year | 0.0a | 11.0 | 0–137 |
| Alcohol binges, days in past year | 0.0a | 7.8 | 0–137 |
| Marijuana use, days in past year | 0.0a | 17.0 | 0–363 |
| Sleep quality (single-item) | 1.8 | 0.7 | 1–4 |
| Circadian preference (CSM-4) | 10.2 | 2.4 | 4–18 |
| Daytime sleepiness (CASQ-5) | 8.4 | 2.9 | 5–23 |
| Weekday sleep | |||
| Bedtime | 22:30 | 1:10 | 20:00-3:00 |
| Wake time | 6:48 | 1:04 | 5:00–12:30 |
| Duration (hours) | 8.2 | 1.3 | 4–13.5 |
| Weekend sleep | |||
| Bedtime | 23:54 | 1:26 | 20:30-4:30 |
| Waketime | 9:30 | 1:30 | 5:00–14:30 |
| Duration (hours) | 9.6 | 1.5 | 4.5–15.5 |
| Weekday-weekend delays | |||
| Bedtime (hours) | 1.3 | 1 | −4.5 – 6.0 |
| Waketime (hours) | 2.7 | 1.6 | −3.5 – 9.5 |
Notes:
Median listed instead of mean due to skewed distribution of data.
Participants ranged in age between 12 and 21 years old at baseline, with nearly equal distribution of the sexes. Participants were predominantly white and non-Hispanic, and were relatively evenly divided among low, medium, and high categories of parental SES. Internalizing and externalizing symptoms were in the non-clinical range (t-score < 60) on average. Frequency of alcohol use, alcohol binges, and marijuana use were all low on average (<3 days in the past year), but ranged upwards of 137 days of alcohol use/binges and nearly daily marijuana use. On average, participants reported circadian preferences in the direction of eveningness, sleep quality in the direction of better sleep quality, and relatively low daytime sleepiness. Compared with weekdays, participants reported weekend bed and rise times that were later (delayed), and sleep durations that were longer.
Sleep and demographic characteristics
Male and female participants did not differ on any of the baseline sleep variables, although there was a trend (p=0.079) towards better sleep quality among the girls and young women. Age correlated with nearly all of the sleep variables, with older age associated with worse sleep quality (r=0.13, p<0.001), greater eveningness (r=−0.16, p<0.001), greater daytime sleepiness (r=0.21, p<0.001), later weekday and weekend bedtimes (r=0.54 and r=0.42, both p<0.001), later weekday and weekend rise times (r=0.19, p<0.001; r=0.09; p=0.01), and shorter weekday and weekend sleep durations (both r=−0.32, p<0.001). Several sleep variables differed across SES categories, including daytime sleepiness (F(2,726)=7.47, p=0.001), weekend bedtime (F(2,726)=6.39), weekend rise time (F(2,726)=3.14, p=0.044), weekend bedtime delays (F(2,726)=14.91, p<0.001), and weekend rise time delays (F(3,726)=4.55, p=0.011). Based on post-hoc comparisons (Tukey HSD), low SES was associated with greater daytime sleepiness, later weekend bedtime, and larger weekend delays (bedtime and rise time) than either medium or high SES. Low SES was also associated with later weekend rise times than high SES. Racial differences were observed only for weekend bedtimes (F(3,725)=9.80, p<0.001), weekday rise times (F(3,725)=4.64, p=0.003), weekend rise times (F(3,725)=5.80, p=0.001), weekend bedtime delays (F(3,725)=17.81, p<0.001), and weekend rise time delays (F(3,725)=11.99, p<0.001).
Based on post-hoc comparisons, African-Americans had later weekend bedtimes, earlier weekday rise times, later weekend rise times, and larger weekend delays in bedtime and rise time relative to Caucasian participants, as well as earlier weekday rise times and larger weekend delays in bedtime rise time relative to Asian-American participants. Hispanic participants reported worse sleep quality (t=2.21, p=0.027) and greater daytime sleepiness (t=2.81, p=0.005) than Non-Hispanic participants.
A few baseline sleep differences emerged across sites, including weekend bedtimes (F(4,724)=4.79, p=0.001) as well as weekend delays in bedtime (F(4, 724)=4.71, p=0.001) and rise time (F(4,724)=3.53, p=0.007). Based on post-hoc comparisons, participants at the Pittsburgh site had later weekend bedtimes than those at SRI, OHSU, and UCSD, participants at both Pittsburgh and Duke had larger weekend delays in bedtime than participants at SRI, and participants at Duke had larger weekend delays in rise time than participants at OHSU.
Baseline sleep and substance associations (Tables 2A–C; Figure 1A–C)
Table 2.
Baseline sleep and substance involvement associations
| A | At-Risk Alcohol Use - baseline | Including demographic covariatesa | Including demographic and psychiatric covariatesb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline sleep | Low (n=616) | Moderate (n=82) | High (n=31) | OR | 95% CI | p | OR | 95% CI | p |
| Circadian preference | 10.42 ± 2.40 | 9.40 ± 2.28 | 8.74 ± 2.02 | 0.83 | [0.75, 0.90] | <0.001* | 0.86 | [0.78, 0.94] | 0.002* |
| Sleep quality | 1.80 ± 0.64 | 1.94 ± 0.69 | 2.19 ± 0.75 | 1.56 | [1.15, 2.12] | 0.005* | 1.49 | [1.06, 2.10] | 0.023 |
| Daytime sleepiness | 8.26 ± 2.82 | 9.50 ± 3.46 | 8.81 ± 2.70 | 1.08 | [1.01, 1.15] | 0.024 | 1.03 | [0.96, 1.11] | 0.383 |
| Weekday | |||||||||
| Bedtime | 22:26 ± 1:08 | 23:03 ± 1:13 | 23:13 ± 1:15 | 1.31 | [1.08, 1.60] | 0.006* | 1.25 | [1.02, 1.53] | 0.029 |
| Rise time | 6:42 ± 1:00 | 7:03 ± 1:15 | 7:04 ± 1:27 | 1.16 | [0.97, 1.39] | 0.100 | 1.14 | [0.94, 1.37] | 0.180 |
| Duration | 8.27 ± 1.28 | 7.99 ± 1.39 | 7.85 ± 1.30 | 0.93 | [0.78, 1.10] | 0.412 | 0.95 | [0.80, 1.13] | 0.566 |
| Weekend | |||||||||
| Bedtime | 23:44 ± 1:25 | 0:34 ± 1:19 | 0:31 ± 1:15 | 1.33 | [1.14, 1.57] | <0.001* | 1.22 | [1.03, 1.44] | 0.022 |
| Rise time | 9:25 ± 1:32 | 9:55 ± 1:23 | 9:55 ± 1:18 | 1.23 | [1.07, 1.42] | 0.004* | 1.20 | [1.03, 1.38] | 0.016 |
| Duration | 9.69 ± 1.45 | 9.35 ± 1.41 | 9.40 ± 1.49 | 0.99 | [0.85, 1.15] | 0.842 | 1.04 | [0.89, 1.21] | 0.642 |
| Weekend delay | |||||||||
| Bedtime | 1.30 ± 1.05 | 1.51 ± 1.01 | 1.31 ± 0.96 | 1.20 | [0.97, 1.47] | 0.091 | 1.08 | [0.87, 1.33] | 0.514 |
| Rise time | 2.73 ± 1.63 | 2.87 ± 1.64 | 2.85 ± 1.26 | 1.12 | [0.97, 1.28] | 0.116 | 1.10 | [0.95, 1.26] | 0.196 |
| B | Binge Alcohol Use - baseline | Including demographic covariatesa | Including demographic and psychiatric covariatesb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline sleep | None (n=629) | Binge (n=63 ) | Heavy (n= 24) | Extreme (n= 13) | OR | 95% CI | p | OR | 95% CI | p |
| Circadian preference | 10.38 ± 2.39 | 9.71 ± 2.28 | 8.08 ± 2.21 | 9.38 ± 2.22 | 0.82 | [0.74, 0.90] | <0.001* | 0.84 | [0.76, 0.93] | 0.001* |
| Sleep quality | 1.80 ± 0.65 | 1.97 ± 0.60 | 2.00 ± 0.59 | 2.31 ± 0.95 | 1.72 | [1.23, 2.41] | 0.002* | 1.71 | [1.17, 2.49] | 0.005* |
| Daytime sleepiness | 8.34 ± 2.88 | 8.78 ± 3.15 | 8.92 ± 3.23 | 9.69 ± 2.46 | 1.03 | [0.95, 1.11] | 0.478 | 1.00 | [0.92, 1.08] | 0.954 |
| Weekday | ||||||||||
| Bedtime | 22:25 ± 1:07 | 23:20 ± 1:02 | 23:20 ± 1:17 | 23:09 ± 1:56 | 1.38 | [1.12, 1.70] | 0.003* | 1.30 | [1.04, 1.61] | 0.019 |
| Rise time | 6:42 ± 1:00 | 7:01 ± 1:15 | 7:31 ± 1:26 | 7:19 ± 1:48 | 1.25 | [1.03, 1.51] | 0.024 | 1.23 | [1.01, 1.50] | 0.039 |
| Duration | 8.27 ± 1.28 | 7.69 ± 1.31 | 8.19 ± 1.59 | 8.15 ± 1.30 | 0.95 | [0.79, 1.14] | 0.592 | 1.00 | [0.83, 1.20] | 0.975 |
| Weekend | ||||||||||
| Bedtime | 23:43 ± 1:25 | 24:37 ± 1:04 | 0:44 ± 1:27 | 1:02 ± 1:44 | 1.46 | [1.21, 1.75] | <0.001* | 1.34 | [1.11, 1.63] | 0.003* |
| Rise time | 9:27 ± 1:32 | 9:40 ± 1:21 | 10:12 ± 1:07 | 9:55 ± 1:43 | 1.23 | [1.05, 1.45] | 0.010 | 1.21 | [1.02, 1.42] | 0.026 |
| Duration | 9.72 ± 1.44 | 9.06 ± 1.49 | 9.46 ± 1.03 | 8.88 ± 1.80 | 0.92 | [0.78, 1.09] | 0.354 | 0.97 | [0.82, 1.15] | 0.729 |
| Weekend delay | ||||||||||
| Bedtime | 1.31 ± 1.06 | 1.29 ± 0.88 | 1.40 ± 1.14 | 1.88 ± 0.74 | 1.26 | [0.99, 1.60] | 0.056 | 1.19 | [0.93, 1.52] | 0.176 |
| Rise time | 2.76 ± 1.65 | 2.65 ± 1.36 | 2.67 ± 1.59 | 2.62 ± 1.54 | 1.07 | [0.91, 1.26] | 0.388 | 1.05 | [0.89, 1.24] | 0.560 |
| C | MJ use (Y/N) - baseline | Including demographic covariatesa | Including demographic and psychiatric covariatesb | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline sleep | No (n=624) | Yes (n= 104) | OR | 95% CI | p | OR | 95% CI | p |
| Circadian preference | 10.43 ± 2.38 | 9.08 ± 2.21 | 0.80 | [0.73, 0.88] | <0.001* | 0.82 | [0.74, 0.91] | <0.001* |
| Sleep quality | 1.80 ± 0.65 | 2.01 ± 0.63 | 1.47 | [1.06, 2.04] | 0.021 | 1.28 | [0.89, 1.85] | 0.189 |
| Daytime sleepiness | 8.25 ± 2.81 | 9.44 ± 3.30 | 1.09 | [1.01, 1.17] | 0.019 | 1.04 | [0.97, 1.13] | 0.275 |
| Weekday | ||||||||
| Bedtime | 22:26 ± 1:08 | 23:08 ± 1:14 | 1.22 | [0.99, 1.50] | 0.061 | 1.15 | [0.93, 1.43] | 0.194 |
| Rise time | 6:42 ± 1:01 | 7:04 ± 1:18 | 1.12 | [0.92, 1.35] | 0.253 | 1.12 | [0.92, 1.36] | 0.276 |
| Duration | 8.27 ± 1.28 | 7.94 ± 1.36 | 0.95 | [0.80, 1.14] | 0.603 | 0.99 | [0.83, 1.19] | 0.945 |
| Weekend | ||||||||
| Bedtime | 23:43 ± 1:24 | 0:38 ± 1:20 | 1.33 | [1.12, 1.57] | 0.001* | 1.21 | [1.01, 1.44] | 0.039 |
| Rise time | 9:27 ± 1:32 | 9:49 ± 1:18 | 1.16 | [1.00, 1.34] | 0.053 | 1.14 | [0.98, 1.33] | 0.090 |
| Duration | 9.72 ± 1.45 | 9.17 ± 1.38 | 0.92 | [0.79, 1.08] | 0.317 | 0.99 | [0.84, 1.17] | 0.938 |
| Weekend delay | ||||||||
| Bedtime | 1.29 ± 1.03 | 1.51 ± 1.11 | 1.29 | [1.04, 1.60] | 0.023 | 1.16 | [0.92, 1.45] | 0.208 |
| Rise time | 2.75 ± 1.65 | 2.75 ± 1.62 | 1.08 | [0.93, 1.25] | 0.299 | 1.06 | [0.92, 1.24] | 0.421 |
Notes:
Covariates = baseline age, race, ethnicity, and parental SES
Covariates = above demographic variables as well as internalizing and externalizing (T-scores from the ASEBA)
meets Holm-Sidak corrected threshold at p<0.05
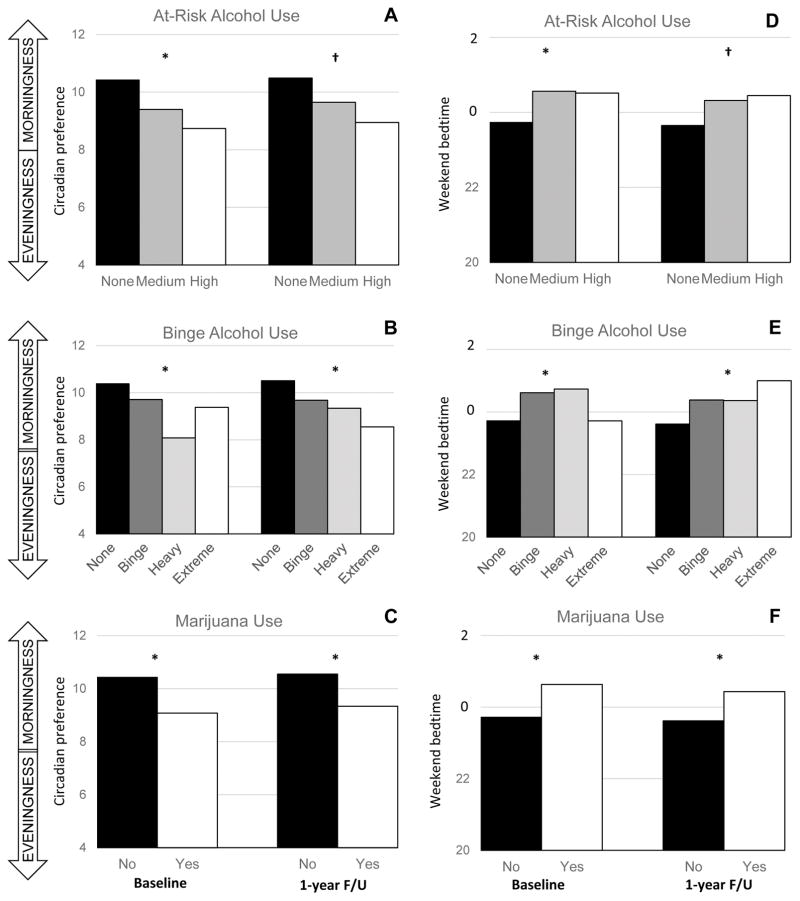
Figure 1.
Column graphs displaying associations between baseline circadian preference (A–C) or weekend bedtime (D–F) and substance involvement at baseline and the 1-year follow-up. Substance involvement measures included at-risk alcohol use (A, D), binge alcohol use (B, E), and marijuana use (C, F). Results based on ordinal and logistic regressions that included baseline age, race, ethnicity, and parental SES as covariates, as well as the relevant baseline substance involvement measure when predicting the 1-year follow-up. * = p <0.05 after Holm-Sidak correction for multiple comparisons; † = p < 0.05 before Holm-Sidak correction
After adjusting for socio-demographic variables, greater eveningness was associated with greater baseline substance involvement on all three categorical substance measures. Weekday and weekend bedtimes showed a similar pattern, with later times associated with greater substance involvement, although only weekend bedtimes were associated with marijuana use. Weekend rise times were also associated with at-risk alcohol use. Worse sleep quality was associated with higher at-risk alcohol use and heavier binge alcohol use, but did not show a statistically significant association with marijuana use. Daytime sleepiness, weekday/weekend sleep duration, and weekend bedtime/rise time delays were all unrelated to baseline substance involvement. Regarding covariates in the cross-sectional models, increasing age was consistently associated with greater substance involvement across all models, male sex was associated with a greater likelihood of marijuana use, and higher parental SES was associated with heavier binge alcohol use.
Including internalizing and externalizing symptoms as additional covariates tended to have only marginal effects on the odds ratios, but also tended to increase p-values above the statistical threshold corrected for multiple comparisons. Eveningness maintained a positive association with greater baseline substance involvement on all three categorical substance measures. In addition, lower sleep quality and later weekend bedtimes were both associated with heavier binge alcohol use. No other associations met criteria for the corrected statistical threshold. Across all models, greater internalizing and externalizing symptoms were consistently associated with worse substance outcomes.
Baseline sleep predicting substance involvement at the 1-year follow-up (Tables 3A–C; Figure 1A–C)
Table 3.
Baseline sleep predicting substance involvement at the 1-year follow-up
| A | At-Risk Alcohol Use – 1-year f/u | Including demographic covariatesa | Including demographic and psychiatric covariatesb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline sleep | Low (n=530) | Moderate (n=112) | High (n=40) | OR | 95% CI | p | OR | 95% CI | p |
| Circadian preference | 10.49 ± 2.40 | 9.65 ± 2.38 | 8.95 ± 2.14 | 0.90 | [0.82, 0.98] | 0.021 | 0.90 | [0.82, 0.99] | 0.034 |
| Sleep quality | 1.79 ± 0.66 | 1.97 ± 0.65 | 1.93 ± 0.62 | 1.17 | [0.84, 1.61] | 0.360 | 1.16 | [0.83, 1.63] | 0.391 |
| Daytime sleepiness | 8.18 ± 2.81 | 9.04 ± 2.96 | 8.95 ± 3.26 | 1.06 | [0.99, 1.14] | 0.085 | 1.06 | [0.99, 1.15] | 0.107 |
| Weekday | |||||||||
| Bedtime | 22:21 ± 1:07 | 23:00 ± 1:08 | 23:06 ± 1:13 | 1.25 | [1.02, 1.53] | 0.034 | 1.26 | [1.03, 1.55] | 0.028 |
| Rise time | 6:40 ± 1:00 | 6:49 ± 1:07 | 7:14 ± 1:15 | 1.02 | [0.83, 1.26] | 0.847 | 1.03 | [0.83, 1.27] | 0.791 |
| Duration | 8.33 ± 1.28 | 7.83 ± 1.38 | 8.15 ± 0.98 | 0.88 | [0.74, 1.04] | 0.124 | 0.87 | [0.73, 1.04] | 0.118 |
| Weekend | |||||||||
| Bedtime | 23:39 ± 1:25 | 0:19 ± 1:15 | 0:27 ± 1:25 | 1.25 | [1.05, 1.49] | 0.011 | 1.26 | [1.05, 1.50] | 0.012 |
| Rise time | 9:24 ± 1:32 | 9:37 ± 1:26 | 9:57 ± 1:16 | 1.10 | [0.95, 1.28] | 0.197 | 1.10 | [0.95, 1.28] | 0.199 |
| Duration | 9.75 ± 1.46 | 9.29 ± 1.50 | 9.51 ± 1.18 | 0.94 | [0.80, 1.09] | 0.387 | 0.94 | [0.80, 1.09] | 0.411 |
| Weekend delay | |||||||||
| Bedtime | 1.30 ± 1.04 | 1.33 ± 1.05 | 1.36 ± 1.07 | 1.11 | [0.89, 1.38] | 0.354 | 1.10 | [0.88, 1.37] | 0.416 |
| Rise time | 2.73 ± 1.65 | 2.80 ± 1.49 | 2.73 ± 1.34 | 1.09 | [0.94, 1.26] | 0.255 | 1.08 | [0.94, 1.25] | 0.282 |
| B | Binge Alcohol Use – 1-year f/u | Including demographic covariatesa | Including demographic and psychiatric covariatesb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline sleep | None (n=524) | Binge (n=88) | Heavy (n=50) | Extreme (n=20) | OR | 95% CI | p | OR | 95% CI | p |
| Circadian preference | 10.51 ± 2.41 | 9.68 ± 2.25 | 9.34 ± 2.26 | 8.55 ± 2.33 | 0.87 | [0.79, 0.95] | 0.001* | 0.86 | [0.78, 0.94] | 0.001* |
| Sleep quality | 1.79 ± 0.66 | 1.88 ± 0.60 | 1.92 ± 0.53 | 2.35 ± 0.75 | 1.34 | [0.98, 1.83] | 0.071 | 1.34 | [0.96, 1.87] | 0.086 |
| Daytime sleepiness | 8.21 ± 2.87 | 8.91 ± 2.85 | 8.62 ± 2.90 | 9.35 ± 2.94 | 1.02 | [0.95, 1.10] | 0.524 | 1.01 | [0.94, 1.09] | 0.737 |
| Weekday | ||||||||||
| Bedtime | 22:16 ± 1:04 | 23:13 ± 1:16 | 23:10 ± 1:02 | 23:24 ± 1:03 | 1.43 | [1.18, 1.74] | <0.001* | 1.46 | [1.20, 1.78] | <0.001* |
| Rise time | 6:40 ± 0:58 | 6:58 ± 1:12 | 6:59 ± 1:13 | 6:55 ± 1:09 | 0.97 | [0.80, 1.19] | 0.790 | 1.01 | [0.82, 1.23] | 0.954 |
| Duration | 8.38 ± 1.25 | 7.77 ± 1.37 | 7.82 ± 1.25 | 7.55 ± 1.34 | 0.76 | [0.64, 0.90] | 0.001* | 0.77 | [0.65, 0.91] | 0.003* |
| Weekend | ||||||||||
| Bedtime | 23:37 ± 1:25 | 0:23 ± 1:14 | 0:22 ± 1:16 | 1:00 ± 1:14 | 1.26 | [1.07, 1.50] | 0.006* | 1.25 | [1.05, 1.48] | 0.011 |
| Rise time | 9:24 ± 1:32 | 9:48 ± 1:23 | 9:27 ± 1:18 | 9:51 ± 1:18 | 1.07 | [0.93, 1.24] | 0.355 | 1.10 | [0.95, 1.27] | 0.225 |
| Duration | 9.79 ± 1.42 | 9.41 ± 1.53 | 9.09 ± 1.40 | 8.85 ± 1.75 | 0.89 | [0.77, 1.04] | 0.131 | 0.93 | [0.80, 1.08] | 0.313 |
| Weekend delay | ||||||||||
| Bedtime | 1.33 ± 1.05 | 1.17 ± 1.06 | 1.20 ± 0.83 | 1.60 ± 1.27 | 0.95 | [0.77, 1.17] | 0.630 | 0.90 | [0.72, 1.12] | 0.346 |
| Rise time | 2.75 ± 1.66 | 2.82 ± 1.44 | 2.47 ± 1.43 | 2.95 ± 1.00 | 1.08 | [0.94, 1.25] | 0.283 | 1.08 | [0.94, 1.25] | 0.271 |
| C | MJ use (Y/N) – 1-year f/u | Including demographic covariatesa | Including demographic and psychiatric covariatesb | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline sleep | No (n=517) | Yes (n=165) | OR | 95% CI | p | OR | 95% CI | p |
| Circadian preference | 10.55 ± 2.41 | 9.34 ± 2.23 | 0.86 | [0.78, 0.94] | 0.001* | 0.87 | [0.79, 0.96] | 0.007 |
| Sleep quality | 1.77 ± 0.65 | 2.02 ± 0.63 | 1.72 | [1.24, 2.39] | 0.001* | 1.64 | [1.16, 2.33] | 0.005* |
| Daytime sleepiness | 8.13 ± 2.77 | 9.10 ± 3.11 | 1.07 | [0.99, 1.15] | 0.083 | 1.05 | [0.97, 1.13] | 0.244 |
| Weekday | ||||||||
| Bedtime | 22:21 ± 1:08 | 22:57 ± 1:08 | 1.34 | [1.08, 1.66] | 0.008 | 1.31 | [1.05, 1.63] | 0.016 |
| Rise time | 6:43 ± 1:01 | 6:47 ± 1:07 | 0.87 | [0.70, 1.10] | 0.234 | 0.89 | [0.71, 1.12] | 0.317 |
| Duration | 8.37 ± 1.28 | 7.82 ± 1.25 | 0.74 | [0.62, 0.89] | 0.002* | 0.76 | [0.63, 0.92] | 0.005* |
| Weekend | ||||||||
| Bedtime | 23:37 ± 1:24 | 24:26 ± 1:18 | 1.33 | [1.12, 1.57] | 0.001* | 1.27 | [1.07, 1.51] | 0.007 |
| Rise time | 9:23 ± 1:30 | 9:46 ± 1:29 | 1.16 | [1.00, 1.34] | 0.048 | 1.14 | [0.95, 1.32] | 0.085 |
| Duration | 9.77 ± 1.43 | 9.33 ± 1.51 | 0.93 | [0.80, 1.09] | 0.366 | 0.95 | [0.82, 1.12] | 0.553 |
| Weekend delay | ||||||||
| Bedtime | 1.26 ± 1.00 | 1.47 ± 1.16 | 1.17 | [0.95, 1.43] | 0.138 | 1.11 | [0.90, 1.43] | 0.314 |
| Rise time | 2.66 ± 1.62 | 2.99 ± 1.52 | 1.21 | [1.05, 1.39] | 0.007 | 1.18 | [1.03, 1.36] | 0.021 |
Notes:
Covariates = baseline age, race, ethnicity, parental SES, and baseline substance variable (At-risk alcohol use, binge alcohol use, or past-year marijuana use)
Covariates = above demographic and substance variables as well as internalizing and externalizing (T-scores from the ASEBA)
meets Holm-Sidak corrected threshold at p<0.05
After adjusting for socio-demographic variables and baseline substance involvement, greater baseline eveningness predicted heavier binge alcohol use and a greater likelihood of using marijuana, but not at-risk alcohol use, at the 1-year follow-up assessment. As they did at baseline, weekday and weekend bedtimes showed a parallel pattern to eveningness, with later bedtimes at baseline associated with heavier binge alcohol use and a greater likelihood of using marijuana at 1-year follow-up. Shorter weekday (but not weekend) sleep durations at baseline predicted both heavier binge alcohol use and a greater likelihood of using marijuana at 1-year follow-up. Worse baseline sleep quality predicted a greater likelihood of using marijuana at the 1-year follow-up, but was not predictive of at-risk alcohol use or binge alcohol use. Baseline daytime sleepiness, rise times, weekend sleep duration, and weekend delays were all unrelated to substance involvement at the 1-year follow-up. Regarding covariates in the longitudinal models, increasing age and baseline substance involvement were both associated with greater substance involvement across all models, male sex was associated with a greater likelihood of marijuana use, and higher parental SES was associated with greater at-risk alcohol use and heavier binge alcohol use.
Paralleling the baseline analyses, including internalizing and externalizing symptoms as additional covariates tended to have only marginal effects on the odds ratios for the 1-year outcomes, but also tended to increase p-values above the statistical threshold corrected for multiple comparisons. Greater eveningness, later weekday bedtimes, and shorter weekend sleep duration at baseline all predicted heavier binge alcohol use at the 1-year follow-up assessment. Furthermore, poorer sleep quality and shorter weekday sleep durations at baseline each predicted a greater likelihood of using marijuana at the 1-year follow-up. No other associations met criteria for the corrected statistical threshold. In these models, greater internalizing and externalizing symptoms predicted heavier binge alcohol use and a greater likelihood of marijuana at the 1-year follow-up but not greater at-risk alcohol use.
DISCUSSION
The present findings extend a burgeoning literature on circadian preference and substance use, showing that eveningness during adolescence is not only cross-sectionally associated with greater involvement in alcohol and marijuana, but also predicts escalations in substance involvement a year later. Notably, eveningness predicted both greater binge alcohol use, a risk factor for alcohol problems, and a higher likelihood of marijuana use. In parallel to the eveningness preference findings, later actual sleep timing (weekday and weekend bedtimes) also showed both cross-sectional and longitudinal associations with binge alcohol use and marijuana use. Somewhat in contrast to expectations and the prior literature, sleep duration showed more circumscribed associations with substance involvement; no cross-sectional associations emerged, and only shorter weekday sleep duration predicted binge alcohol use and marijuana use. Finally, worse sleep quality showed a limited span of associations with substance involvement, including predicting marijuana use at follow-up. The associations between eveningness and substance involvement persisted after accounting for baseline psychiatric symptoms. Taken together, our findings suggest that both preferred and actual sleep timing may be more consistently linked to substance involvement than are other sleep characteristics, and are thus due greater consideration when assessing risk and planning treatment. These results are particularly salient during adolescence, when eveningness peaks (Roenneberg et al., 2004, Randler, 2011).
Among an array of sleep characteristics, eveningness and later weekday/weekend bedtimes showed the most consistent links to greater alcohol and marijuana use, both cross-sectionally and longitudinally. These findings extend a literature on eveningness that was primarily based on cross-sectional data, with only one prior published paper reporting a longitudinal association between eveningness and substance use (alcohol and marijuana) over a 1–2 year span in a sample of 17–25 year-old undergraduates (Tavernier et al., 2015). The present data indicates that eveningness and later sleep timing are predictors of substance involvement among a wider age range including young adolescents. Our findings have several other implications. First, the longitudinal associations suggest that the link between later sleep timing and greater substance involvement is not simply a consequence of staying up late to engage in substance use. Indeed, shifts towards an eveningness preference and delayed sleep/circadian begin during puberty, and typically prior to regular substance use (Hagenauer et al., 2009). Next, the fact that we observed a parallel pattern for “actual” later bedtimes, not just eveningness preference, suggests that association is not simply a reflection of the non-sleep/circadian constructs tapped by the CSM questionnaire. That is, a “morning affect” factor has been identified in the CSM that is based on items devoid of specific time frames (Caci et al., 2005), and thus could arguably drive some previously reported associations between eveningness and depression or substance use. Another implication can be drawn from our finding is that it was bedtimes, not waketimes, that were consistently associated with the substance outcomes, which could be explained by the fact that adolescents tend to have less volitional control over rise times due to other obligations, particularly on weekdays when school or work is in session, and thus rise times in this context may be less likely to represent individual tendencies.
The mechanism by which eveningness and later sleep timing leads to greater substance involvement remains an open question and an area of ongoing research (Hasler and Clark, 2013). One popular explanation is so-called “social jetlag,” in which a mismatch between a tendency for later sleep timing and the early schedules imposed by work or school obligations leads to chronic circadian misalignment and sleep loss. Social jet lag is typically characterized by delays in weekend versus weekday sleep timing, and indeed, others have linked it to wide-ranging negative outcomes such as smoking, alcohol use, depression, and cardiometabolic risk (O’Brien and Mindell, 2005, Pasch et al., 2010, Wittmann et al., 2006, Wong et al., 2015b), as well as to potential mechanistic indicators such as an altered neural response to monetary rewards (Hasler et al., 2012). Interestingly, we did not see any statistically significant associations between weekend delays in bedtime or rise time and our substance outcomes. This finding is consistent with the other study reporting a prospective eveningness-substance involvement association; they found no such association using their social jetlag variable (Tavernier et al., 2015). However, others have asserted that social jetlag can only be properly assessed by comparing timing on work/school days to timing on free days, when individuals can wake spontaneously without the use of an alarm or other means, and thus the weekday-weekend comparison is an imperfect one. Other putative mechanisms are plausible. Eveningness has also been linked to increased impulsivity, sensation-seeking, and altered reward-related brain function (Kang et al., 2015, Russo et al., 2012, Hasler et al., 2013), all of which have been linked in turn to alcohol and marijuana involvement (Hasler et al., 2013, Littlefield et al., 2016, MacPherson et al., 2010). As more follow-up assessments are completed in the NCANDA study, it will be useful to examine whether changes in impulsivity, risk-taking, or brain function mediate the sleep-substance associations.
We found more limited associations between sleep duration and the substance outcomes, and none with daytime sleepiness. Specifically, shorter weekday, not weekend, sleep durations predicted greater alcohol binge use and a higher likelihood of marijuana use at follow-up. We speculate that constrained sleep during the school week may take a toll, leading to impulsivity and/or increased reward sensitivity over time. The lack of further associations between sleep duration and substance outcomes is somewhat surprising given prior studies in adolescent samples, which have reported both concurrent and prospective associations between shorter sleep duration and increased alcohol and marijuana involvement (Mike et al., 2016, Pasch et al., 2010, Pasch et al., 2012). Notably, at least one study (in middle age adult women) found a curvilinear association with alcohol use, such that both abstainers and heavy drinkers reported long sleep (Patel et al., 2006). However, we did not observe any evidence of a U-shaped curve; instead, sleep durations consistently trended towards shorter with increasing degrees of substance involvement but with substantial between-participant variability that precluded a statistically significant association. With regard to the lack of associations with daytime sleepiness, we speculate that adolescents are more likely to turn to stimulants when suffering from sleep debt and excessive daytime sleepiness, rather than consuming alcohol, which has been shown to exacerbate daytime sleepiness (Roehrs and Roth, 2001). Future studies in this area should also consider energy drink use, which is associated with later sleep timing and various sleep problems among adolescents (Troxel et al., 2016), and which not only is used in conjunction with alcohol but may be a risk factor for increasing alcohol use (Choi et al., 2016).
Sleep quality was also cross-sectionally associated with risky alcohol use and alcohol binges, as well as longitudinally with marijuana use. This extends prior work linking sleep quality to concurrent binge alcohol use and alcohol problems in undergraduates (Kenney et al., 2012). Although the literature on sleep and marijuana among adolescents remains limited, the adult literature suggests that sleep quality is worse among adult marijuana users (Conroy et al., 2016) and that poor sleep quality is a risk factor among adult military veterans for lapses during attempts to abstain from marijuana use (Babson et al., 2013). Although the present links with sleep quality were less pervasive than for eveningness and sleep timing, our findings suggest that sleep quality remains an important construct to assess in the context of prevention of adolescent substance involvement. Notably, we assessed sleep quality with a single item drawn from the PSQI, and thus it should be highly feasible to include during brief risk assessments in clinical settings.
Including psychiatric symptoms (internalizing and externalizing) in the model had small, but notable impact on the pattern of results. Many of the p-values for our sleep characteristics remained significant at a conventional level, but some did increase above our strict statistical threshold corrected for multiple comparisons. While the odd-ratios of eveningness predicting alcohol and marijuana use remained essentially the same, other odds-ratios (e.g., baseline sleep quality predicting marijuana use at the 1-year follow-up) showed small attenuations. Such attenuations suggest that psychiatric problems may partly account for some sleep-substance associations, which is not surprising given evidence that sleep and circadian rhythms impact affective and motivational processes, such as impulsivity, reward function, and negative affect (reviewed in (Hasler et al., 2014b), which have been linked in turn to substance involvement (e.g., (Littlefield et al., 2014, Sher and Grekin, 2007)). Further research, including experimental designs, is needed to elucidate the causal mechanisms linking various sleep characteristics to specific alcohol and drug outcomes.
We should note several limitations in the present analyses. First, we relied on self-reports of circadian preference and other sleep characteristics, which may be subject to issues of bias or shared method variance, rather than objective measures of sleep or circadian timing, such as wrist actigraphy or salivary melatonin. Polysomnographic (PSG) sleep measures are included in a laboratory study of a subset of NCANDA participants (Baker et al., 2016), and follow-up studies should examine the correspondence between circadian preference and PSG-based sleep measures. Also, our abbreviated scales were drawn from well-validated questionnaires, but have not undergone the same degree of psychometric testing (although, as noted, the abbreviated measures correlated strongly with the full measures in a subsample that completed both). Another limitation is that substance use was limited in both scope and degree in the NCANDA sample by design at baseline. It will be important to reexamine these associations at future follow-up assessments as a larger portion of the sample initiates or escalates substance involvement. Further, the participants excluded from the present analyses (due to missing data) tended to use more substances and have later timing, consequently, the restricted range on these variables may have limited our ability to detect associations, thereby biasing the sample and increasing the likelihood of Type II errors. Finally, our study did not assess patterns of caffeine use, which may be an important consideration in future studies given increasing use of energy drinks and evidence that caffeine use is associated with both sleep disturbances (Bonnar and Gradisar, 2015) and use of alcohol and other drugs (Choi et al., 2016, Arria et al., 2010).
In conclusion, we report that eveningness and later bedtimes in a representative sample of adolescents are both concurrently and prospectively associated with heavier binge alcohol use and a greater likelihood of marijuana use. In conjunction with the sole other study reporting a longitudinal association between eveningness and substance involvement, these findings underscore the need for greater attention to sleep timing as a potentially modifiable risk factor for adolescent alcohol and marijuana problems. Data in adults samples suggest that eveningness is modifiable with both behavioral and pharmacological treatment (Corruble et al., 2014, Hasler et al., 2015, Natale et al., 2008). Sleep and endogenous circadian timing respond to chronotherapeutic interventions such as sleep scheduling, bright light, and melatonin (e.g., (Emens and Burgess, 2015). Such treatments have demonstrated efficacy for adolescents with delayed sleep phase [e.g., (Gradisar et al., 2014)] and thus may be useful in the context of preventing or treating adolescent substance problems. Although sleep quality and sleep duration showed more limited associations with the substance outcomes in the present sample, the larger literature supports continued attention to these sleep characteristics as well. In particular, that shorter weekday sleep duration predicted later substance involvement is consistent with a host of data suggesting that early school start times are detrimental to the adolescent health (Minges and Redeker, 2016). As reviewed in detail elsewhere (Hasler et al., 2014b), we speculate that circadian misalignment (i.e., social jet lag) may underlie a range of sleep disturbances, including lower sleep quality and shorter weekday sleep duration, suggesting that policy-level interventions to delay school start times are worth consideration. In summary, our findings indicate that eveningness and sleep timing, along with other sleep characteristics, are under-recognized risk factors and potential future areas of intervention for adolescent substance involvement.
Acknowledgments
This work was supported by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Child Health and Human Development [NCANDA grant numbers: AA021695 (SAB+SFT), AA021692 (SAB+SFT), AA021697 (AP+KMP), AA021696 (IMC+FCB), AA021681 (MDDB), AA021690 (DBC), AA021691 (BN); also K01DA032557 (BPH)].
Footnotes
Given site differences in weekend bedtimes as well as bedtime and rise time delays, we also considered site as an additional covariate in the respective analyses. However, including site had no perceptible effect on the findings, and thus we left it out of the final models for the sake of parsimony.
References
- ACHENBACH TM, RESCORLA LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- ACHENBACH TM, RESCORLA LA. Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- ALCOHOLISM, N. I. O. A. A. A. Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2011. [Google Scholar]
- ARRIA AM, CALDEIRA KM, KASPERSKI SJ, O’GRADY KE, VINCENT KB, GRIFFITHS RR, WISH ED. Increased alcohol consumption, nonmedical prescription drug use, and illicit drug use are associated with energy drink consumption among college students. Journal of addiction medicine. 2010;4:74. doi: 10.1097/ADM.0b013e3181aa8dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABSON KA, BODEN MT, HARRIS AH, STICKLE TR, BONN-MILLER MO. Poor sleep quality as a risk factor for lapse following a cannabis quit attempt. J Subst Abuse Treat. 2013;44:438–43. doi: 10.1016/j.jsat.2012.08.224. [DOI] [PubMed] [Google Scholar]
- BAKER F, WILLOUGHBY A, DE ZAMBOTTI M, FRANZEN P, PROUTY D, JAVITZ H, HASLER B, CLARK D, COLRAIN I. Age-Related Differences in Sleep Architecture and Electroencephalogram in Adolescents in the National Consortium on Alcohol and NeuroDevelopment in Adolescence Sample. Sleep. 2016 doi: 10.5665/sleep.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANK M, ZHANG J, LAMERS F, TAYLOR A, HICKIE I, MERIKANGAS K. Health Correlates of Insomnia Symptoms and Comorbid Mental Disorders in a Nationally Representative Sample of US Adolescents. Sleep. 2014 doi: 10.5665/sleep.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONNAR D, GRADISAR M. Caffeine and sleep in adolescents: a systematic review. Journal of Caffeine Research. 2015;5:105–114. [Google Scholar]
- BRESLAU N, ROTH T, ROSENTHAL L, ANDRESKI P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biological Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- BROWN SA, BRUMBACK T, TOMLINSON K, CUMMINS K, THOMPSON WK, NAGEL BJ, DE BELLIS MD, HOOPER SR, CLARK DB, CHUNG T. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A multisite study of adolescent development and substance use. Journal of Studies on Alcohol and Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN SA, MCGUE M, MAGGS J, SCHULENBERG J, HINGSON R, SWARTZWELDER S, MARTIN C, CHUNG T, TAPERT SF, SHER K. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121:S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN SA, MYERS MG, LIPPKE L, TAPERT SF, STEWART DG, VIK PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- BUYSSE DJ, REYNOLDS CF, MONK TH, BERMAN SR, KUPFER DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- CACI H, ADAN A, BOHLE P, NATALE V, PORNPITAKPAN C, TILLEY A. Transcultural properties of the composite scale of morningness: the relevance of the “morning affect” factor. Chronobiology international. 2005;22:523–40. doi: 10.1081/CBI-200062401. [DOI] [PubMed] [Google Scholar]
- CHOI HJ, WOLFORD-CLEVENGER C, BREM MJ, ELMQUIST J, STUART GL, PASCH KE, TEMPLE JR. The temporal association between energy drink and alcohol use among adolescents: a short communication. Drug and alcohol dependence. 2016;158:164–166. doi: 10.1016/j.drugalcdep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG T, SMITH GT, DONOVAN JE, WINDLE M, FADEN VB, CHEN CM, MARTIN CS. Drinking frequency as a brief screen for adolescent alcohol problems. Pediatrics, peds. 2012:2011–1828. doi: 10.1542/peds.2011-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK DB, MARTIN CS, CHUNG T, GORDON AJ, FIORENTINO L, TOOTELL M, RUBIO DM. Screening for Underage Drinking and Diagnostic and Statistical Manual of Mental Disorders, Alcohol Use Disorder in Rural Primary Care Practice. The Journal of pediatrics. 2016;173:214–220. doi: 10.1016/j.jpeds.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONROY DA, ARNEDT JT. Sleep and substance use disorders: An update. Current Psychiatry Reports. 2014;16:1–9. doi: 10.1007/s11920-014-0487-3. [DOI] [PubMed] [Google Scholar]
- CONROY DA, KURTH ME, STRONG DR, BROWER KJ, STEIN MD. Marijuana use patterns and sleep among community-based young adults. Journal of addictive diseases. 2016;35:135–143. doi: 10.1080/10550887.2015.1132986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORP I. 24.0. Armonk, NY: IBM Corp; 2016. [Google Scholar]
- CORRUBLE E, FRANK E, GRESSIER F, COURTET P, BAYLE F, LLORCA PM, VAIVA G, GORWOOD P. Morningness-eveningness and treatment response in major depressive disorder. Chronobiology International. 2014;31:283–9. doi: 10.3109/07420528.2013.834924. [DOI] [PubMed] [Google Scholar]
- DONOVAN JE. Estimated blood alcohol concentrations for child and adolescent drinking and their implications for screening instruments. Pediatrics. 2009;123:e975–e981. doi: 10.1542/peds.2008-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMENS JS, BURGESS HJ. Effect of Light and Melatonin and Other Melatonin Receptor Agonists on Human Circadian Physiology. Sleep Med Clin. 2015;10:435–53. doi: 10.1016/j.jsmc.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAU SS, SHANG CY, MERIKANGAS KR, CHIU YN, SOONG WT, CHENG AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22:268–74. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- GIANNOTTI F, CORTESI F, SEBASTIANI T, OTTAVIANO S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- GRADISAR M, SMITS MG, BJORVATN B. Assessment and treatment of delayed sleep phase disorder in adolescents: recent innovations and cautions. Sleep Medicine Clinics. 2014;9:199–210. [Google Scholar]
- HAGENAUER MH, PERRYMAN JI, LEE TM, CARSKADON MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Developmental Neuroscience. 2009;31:276–84. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, BUYSSE DJ, GERMAIN A. Shifts Toward Morningness During Behavioral Sleep Interventions Are Associated With Improvements in Depression, Positive Affect, and Sleep Quality. Behavioral sleep medicine. 2015:1–12. doi: 10.1080/15402002.2015.1048452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, CLARK DB. Circadian misalignment, reward-related brain function, and adolescent alcohol involvement. Alcoholism: Clinical and Experimental Research. 2013;37:558–565. doi: 10.1111/acer.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, DAHL RE, HOLM SM, JAKUBCAK JL, RYAN ND, SILK JS, PHILLIPS ML, FORBES EE. Weekend-weekday advances in sleep timing are associated with altered reward-related brain function in healthy adolescents. Biological Psychology. 2012;91:334–41. doi: 10.1016/j.biopsycho.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, KIRISCI L, CLARK DB. Restless sleep and variable sleep timing during late childhood accelerate the onset of alcohol and drug involvement. Journal of Studies on Alcohol and Drugs. 2016;77:649–655. doi: 10.15288/jsad.2016.77.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, MARTIN CS, WOOD DS, ROSARIO B, CLARK DB. A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2014a;38:2225–33. doi: 10.1111/acer.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, SITNICK SL, SHAW DS, FORBES EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging. 2013;214:357–64. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASLER BP, SOEHNER AM, CLARK DB. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2014b;49:377–387. doi: 10.1016/j.alcohol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSTITUTE, S. Base SAS 9.4 Procedures Guide. SAS Institute; 2014. [Google Scholar]
- JOHNSON EO, BRESLAU N. Sleep problems and substance use in adolescence. Drug and Alcohol Dependence. 2001;64:1–7. doi: 10.1016/s0376-8716(00)00222-2. [DOI] [PubMed] [Google Scholar]
- KANG JI, PARK CI, SOHN SY, KIM HW, NAMKOONG K, KIM SJ. Circadian preference and trait impulsivity, sensation-seeking and response inhibition in healthy young adults. Chronobiol Int. 2015;32:235–41. doi: 10.3109/07420528.2014.965313. [DOI] [PubMed] [Google Scholar]
- KENNEY SR, LABRIE JW, HUMMER JF, PHAM AT. Global sleep quality as a moderator of alcohol consumption and consequences in college students. Addictive Behaviors. 2012;37:507–12. doi: 10.1016/j.addbeh.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD AK, STEVENS AK, ELLINGSON JM, KING KM, JACKSON KM. Changes in negative urgency, positive urgency, and sensation seeking across adolescence. Personality and individual differences. 2016;90:332–337. doi: 10.1016/j.paid.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD AK, STEVENS AK, SHER KJ. Impulsivity and alcohol involvement: Multiple, distinct constructs and processes. Current Addiction Reports. 2014;1:33–40. doi: 10.1007/s40429-013-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON L, MAGIDSON JF, REYNOLDS EK, KAHLER CW, LEJUEZ C. Changes in sensation seeking and risk-taking propensity predict increases in alcohol Use among early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKE T, SITNICK S, SHAW D, FORBES E, HASLER B. The hazards of bad sleep: Sleep duration and quality as predictors of adolescent alcohol and cannabis use. Drug and Alcohol Dependence. 2016;168:335–339. doi: 10.1016/j.drugalcdep.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINGES KE, REDEKER NS. Delayed school start times and adolescent sleep: A systematic review of the experimental evidence. Sleep Medicine Reviews. 2016;28:82–91. doi: 10.1016/j.smrv.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONK T, BUYSSE D, KENNEDY K, POTTS J, DEGRAZIA J, MIEWALD J. Measuring sleep habits without using a diary: The sleep timing questionnaire (STQ) Sleep. 2003;26:208–12. doi: 10.1093/sleep/26.2.208. [DOI] [PubMed] [Google Scholar]
- NATALE V, BALLARDINI D, SCHUMANN R, MENCARELLI C, MAGELLI V. Morningness-eveningness preference and eating disorders. Personality and Individual Differences. 2008;45:549–553. [Google Scholar]
- NEGRIFF S, DORN LD, PABST SR, SUSMAN EJ. Morningness/eveningness, pubertal timing, and substance use in adolescent girls. Psychiatry Research. 2011;185:408–413. doi: 10.1016/j.psychres.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLS BN, POHL KM. Neuroinformatics Software Applications Supporting Electronic Data Capture, Management, and Sharing for the Neuroimaging Community. Neuropsychol Rev. 2015;25:356–68. doi: 10.1007/s11065-015-9293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’BRIEN EM, MINDELL JA. Sleep and risk-taking behavior in adolescents. Behavioral Sleep Medicine. 2005;3:113–33. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- PASCH KE, LASKA MN, LYTLE LA, MOE SG. Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? American Journal of Health Behavior. 2010;34:237–48. doi: 10.5993/ajhb.34.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASCH KE, LATIMER LA, CANCE JD, MOE SG, LYTLE LA. Longitudinal bi-directional relationships between sleep and youth substance use. Journal of Youth and Adolescence. 2012;41:1184–1196. doi: 10.1007/s10964-012-9784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATEL SR, MALHOTRA A, GOTTLIEB DJ, WHITE DP, HU FB. Correlates of long sleep duration. Sleep. 2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATRICK ME. A Call for Research on High-Intensity Alcohol Use. Alcoholism: Clinical and Experimental Research. 2016;40:256–259. doi: 10.1111/acer.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIETERS S, BURK WJ, VAN DER VORST H, DAHL RE, WIERS RW, ENGELS RC. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J Youth Adolesc. 2015;44:379–88. doi: 10.1007/s10964-014-0213-9. [DOI] [PubMed] [Google Scholar]
- PIETERS S, VAN DER VORST H, BURK WJ, WIERS RW, ENGELS RC. Puberty-dependent sleep regulation and alcohol use in early adolescents. Alcoholism: Clinical and Experimental Research. 2010;34:1512–8. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- RANDLER C. Age and gender differences in morningness-eveningness during adolescence. The Journal of genetic psychology. 2011;172:302–8. doi: 10.1080/00221325.2010.535225. [DOI] [PubMed] [Google Scholar]
- ROANE BM, TAYLOR DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31:1351–6. [PMC free article] [PubMed] [Google Scholar]
- ROEHRS T, ROTH T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Medicine Reviews. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- ROENNEBERG T, KUEHNLE T, PRAMSTALLER PP, RICKEN J, HAVEL M, GUTH A, MERROW M. A marker for the end of adolescence. Current Biology. 2004;14:R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- ROHLFING T, CUMMINS K, HENTHORN T, CHU W, NICHOLS BN. N-CANDA data integration: anatomy of an asynchronous infrastructure for multi-site, multi-instrument longitudinal data capture. J Am Med Inform Assoc. 2014;21:758–62. doi: 10.1136/amiajnl-2013-002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSO PM, LEONE L, PENOLAZZI B, NATALE V. Circadian preference and the big five: The role of impulsivity and sensation seeking. Chronobiology international. 2012;29:1121–1126. doi: 10.3109/07420528.2012.706768. [DOI] [PubMed] [Google Scholar]
- SHER KJ, GREKIN ER. Alcohol and affect regulation. Guilford Press; New York, NY: 2007. [Google Scholar]
- SIBIS. sibis.sri.com. [Online]. [Accessed] [Google Scholar]
- SMITH CS, REILLY C, MIDKIFF K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74:728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- SPILSBURY JC, DROTAR D, ROSEN CL, REDLINE S. The Cleveland adolescent sleepiness questionnaire: a new measure to assess excessive daytime sleepiness in adolescents. Journal of Clinical Sleep Medicine. 2007;3:603–612. [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN EV, BRUMBACK T, TAPERT SF, PROUTY D, FAMA R, THOMPSON WK, BROWN SA, CUMMINS K, COLRAIN IM, BAKER FC. Effects of prior testing lasting a full year in NCANDA adolescents: contributions from age, sex, socioeconomic status, ethnicity, site, family history of alcohol or drug abuse, and baseline performance. Developmental cognitive neuroscience. 2017;24:72–83. doi: 10.1016/j.dcn.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVERNIER R, MUNROE M, WILLOUGHBY T. Perceived morningness–eveningness predicts academic adjustment and substance use across university, but social jetlag is not to blame. Chronobiology international. 2015;32:1233–1245. doi: 10.3109/07420528.2015.1085062. [DOI] [PubMed] [Google Scholar]
- TROXEL WM, TUCKER JS, EWING B, MILES JN, D’AMICO EJ. Sleepy Teens and Energy Drink Use: Results From an Ethnically Diverse Sample of Youth. Behavioral Sleep Medicine. 2016:1–14. doi: 10.1080/15402002.2016.1188390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URBAN R, MAGYARODI T, RIGO A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiology International. 2011;28:238–47. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITTMANN M, DINICH J, MERROW M, ROENNEBERG T. Social jetlag: Misalignment of biological and social time. Chronobiology International. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- WONG MM, BROWER KJ, FITZGERALD HE, ZUCKER RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clinical and Experimental Research. 2004;28:578–87. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]
- WONG MM, BROWER KJ, NIGG JT, ZUCKER RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcoholism: Clinical and Experimental Research. 2010;34:1033–44. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG MM, ROBERTSON GC, DYSON RB. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcoholism, clinical and experimental research. 2015a;39:355–62. doi: 10.1111/acer.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG PM, HASLER BP, KAMARCK TW, MULDOON MF, MANUCK SB. Social jetlag, chronotype, and cardiometabolic risk. The Journal of Clinical Endocrinology & Metabolism. 2015b;100:4612–4620. doi: 10.1210/jc.2015-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]