Abstract
We describe a system of inducible insertional mutagenesis based on the Ac-Ds family of transposons for targeted tagging in Arabidopsis (Arabidopsis thaliana). In this system, the Ac and Ds elements are carried within the same T-DNA and a heat shock-inducible transposase fusion is utilized to control the levels of transposase gene expression, generating transpositions that can be subsequently stabilized without requiring crossing or segregation. We have mapped 40 single-copy lines by thermal asymmetric interlaced-PCR, which can be used as potential launch pads for heat shock mutagenesis. Using a starter line selected for detailed analysis, the efficiency of tagging over a 50-kb region in the genome was examined. Hits were obtained in the targeted genes with multiple alleles for most genes, with approximately equal numbers of hits detected in genes on either side of the T-DNA. These results establish the feasibility of our approach for localized saturation mutagenesis in Arabidopsis. This system is very efficient and much less laborious as compared to conventional crossing schemes and may be generally applicable to other plant species for which large-scale T-DNA tagging is not currently feasible.
The biological functions of most of the 25,000 genes in the Arabidopsis (Arabidopsis thaliana) genome have not yet been established (Arabidopsis Genome Initiative, 2000). While several large-scale reverse genetics resources for analysis of gene function are now publicly available from stock centers, like the sequenced insertion lines from the SALK (Alonso et al., 2003; http://signal.salk.edu/cgi-bin/tdnaexpress), SAIL (Sessions et al., 2002; www.syngenta.com), and other collections, to obtain insertional knockouts of all the smaller genes (e.g. the CLV3-like genes or micro-RNA genes), an even larger number of lines may be required (Krysan et al., 1999). A targeted disruption strategy for specific genes could circumvent the necessity for generating this very large number of insertion lines.
Currently, T-DNA and transposons are the two main insertional mutagens used widely for gene disruption in Arabidopsis (Fedoroff, 1989; Errampalli et al., 1991; Bancroft and Dean, 1993a; Fedoroff and Smith, 1993; Apiroz-Lenehan and Feldmann, 1997; Martienssen, 1998; Krysan et al., 1999; Parinov and Sundaresan, 2000). While T-DNA insertions are easily generated in Arabidopsis, it is difficult to generate a large collection of independent T-DNA lines in plant species for which transformation methods are more laborious. In contrast, transposon mutagenesis can be accomplished using a limited number of starter lines generated by transformation (for review, see Ramachandran and Sundaresan, 2001). Transposons integrate in the genome as single intact elements, as compared to the complex integration patterns that are frequently generated by T-DNA, and can be used for reversion analysis by remobilizing the transposon insertion. It is well established that transposons of the Ac-Ds family preferentially jump to linked sites (e.g. Bancroft and Dean, 1993b; Machida et al., 1997; for review, see Ramachandran and Sundaresan, 2001). This property of transposable elements can be exploited for targeted (i.e. localized) transposon mutagenesis of a chromosomal region, which has been used in endogenous systems (for review, see Sundaresan, 1996) as well as in heterologous systems (Smith et al., 1996; Muskett et al., 2003; Zhang et al., 2003). Localized transposon mutagenesis can also be used to generate double knockouts of tandemly duplicated genes through reinsertion of transposed elements (Tantikanjana et al., 2004), which would otherwise require a tedious screen for recombinants of two closely linked insertions within the duplicated genes.
We describe an approach of localized saturation mutagenesis, which minimizes the effort involved. The Ac and Ds elements are placed in the same T-DNA construct, reducing the labor-intensive crosses needed to generate large numbers of independent insertion lines. Controlled transposition is obtained by employing an inducible system where the transposase gene is under the control of a heat shock promoter. The induction of the transposase results in transposition of Ds elements from the T-DNA into adjacent genomic regions, generating new transposon lines that will be stable. These insertion lines are used for forward and reverse genetic screens, and can also serve as launch pads for further mutagenesis by resubjecting the plants to heat shock treatment.
RESULTS
Design of the T-DNA Vector
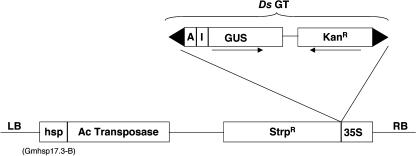
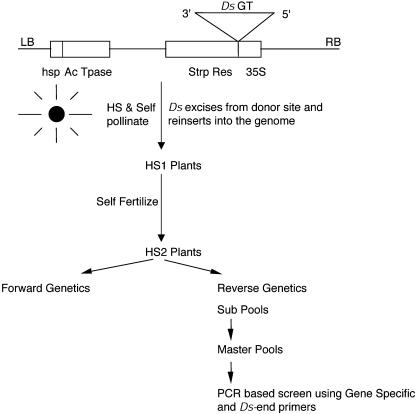
A schematic representation of the construct pYS11 used for transformation is shown in Figure 1. In this construct, both Ac and Ds elements are placed on the same T-DNA, similar to the En/Spm transposase in cis-systems (Tissier et al., 1999). The transposase gene is under the control of a heat shock promoter from Glycine max, Gmhsp 17.3B, which allows the transposase gene to be expressed only when the plants are subjected to high temperatures (Baumann et al., 1987). The heat shock promoter has previously been shown to be active in developing embryos and to have a good potential for transposon tagging (Balcells et al., 1994). The Ds element used in our study is a gene trap element containing a β-glucuronidase (GUS) reporter gene, which can be histochemically stained for GUS activity, and an NPTII gene, which confers resistance to the antibiotic kanamycin, as described before (Sundaresan et al., 1995), and is inserted into a streptomycin gene driven by the 35S promoter. Kanamycin serves as a reinsertion marker, whereas streptomycin serves as an excision marker of Ds from the original donor site when the plants are subjected to heat shock treatment.
Figure 1.
A schematic representation of the T-DNA vector harboring the plasmid pYS11 used for transforming wild-type Arabidopsis Ws-0. LB, Left border sequence of T-DNA; hsp, heat shock promoter from Glycine max; Ac, activator element; Ds, dissociation element; GT, gene trap; StrpR, streptomycin resistance gene; A, triple-splice acceptor; I, Arabidopsis intron; GUS, β-glucuronidase gene; KanR, kanamycin resistance gene; 35S, 35S cauliflower mosaic virus promoter; and RB, right border sequence of T-DNA.
Generation of Starter Lines
It has been reported previously that there is a higher probability of obtaining single copy T-DNA insertions when root transformation is the chosen method of transformation (Grevelding et al., 1993). The construct was transformed to wild-type Arabidopsis of ecotype Wassilewskija-0 (Ws-0), following a protocol of root transformation (Valvekens et al., 1988) with incorporation of minor modifications. We generated 107 independent transgenic starter lines, which were characterized for determining the copy number of the T-DNA. Since the nonautonomous Ds element contains the kanamycin resistance gene, seeds were germinated on Murashige and Skoog (MS) medium supplemented with kanamycin to determine the segregation ratio. Transgenic lines segregating as a single Mendelian locus for the kanamycin resistance gene in the T2 seedlings were further characterized by Southern-blot analysis to eliminate lines with multiple integrations of T-DNA at the same locus. Among the transgenic lines obtained, 40 lines were found to contain a single copy of the transgene.
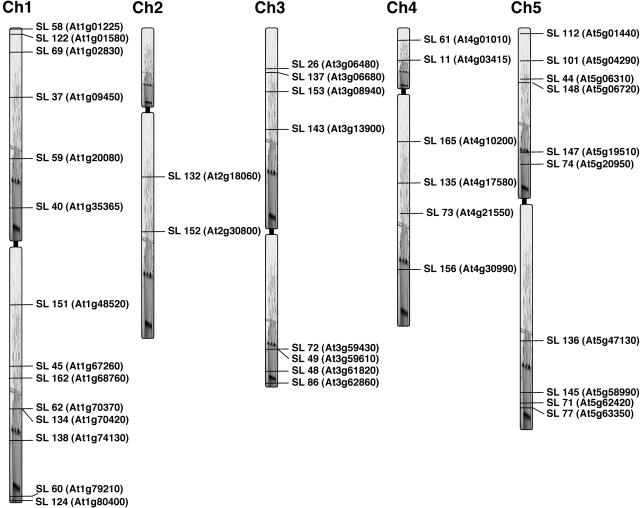
To determine the flanking sequences for the T-DNA integration sites, a thermal asymmetric interlaced (TAIL)-PCR-based approach was used on 40 starter lines utilizing primers from ends of T-DNA and arbitrary degenerate (AD) primers with minor modifications from the original protocol (Liu et al., 1995; Parinov et al., 1999). The sequences obtained were subjected to the National Center for Biotechnology Information (NCBI) BLAST searches. Gene-specific primers were designed and used in combination with T-DNA end primers to verify the insertion sites in the Arabidopsis genome. All the lines have been mapped (Fig. 2) and will be provided to the research community to identify launch pads surrounding their genes of interest. See Supplemental Table I for sequences obtained from TAIL-PCR.
Figure 2.
A schematic representation of the distribution of T-DNA loci for 40 independent starter lines on the 5 chromosomes of Arabidopsis. Flanking sequences obtained from TAIL-PCR were subjected to BLAST searches and the sequences obtained were used to assign genomic locations to the starter lines. SL, Starter line (the gene identified by At no. in parenthesis indicates that the T-DNA is inserted within or very close to that gene).
Induction of Transposase
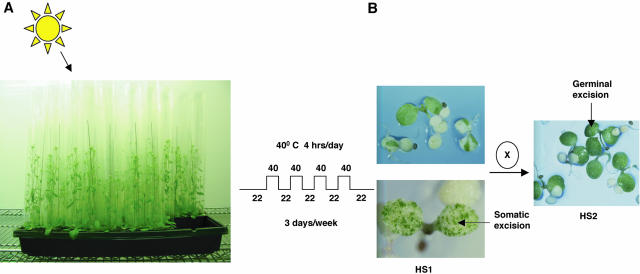
Nineteen starter lines containing a single copy of the transgene were chosen to check the efficacy of the system, particularly the activation of the Ds element by heat shock treatment. Homozygous plants for these lines were subjected to heat shock treatment for induction of transposase and initiating mutagenesis. Subjecting the plants to heat shock treatment during reproductive growth leads to efficient transposon excision in the developing embryos (Balcells et al., 1994). We followed the protocol reported previously and treated flowering plants to heat shock treatment, as described in “Materials and Methods” (Fig. 3A). The plants could easily tolerate this periodic high-temperature treatment and each heat-shocked plant gave rise to approximately 10,000 to 15,000 HS1 seeds. Seeds were collected from heat-shocked plants (HS1 seeds) and germinated on MS medium containing the antibiotics kanamycin and streptomycin. After 2 to 3 weeks postgermination on antibiotic-containing media, the control plants grown under normal conditions gave rise to only sensitive seedlings, indicating that the Ds elements were stable under noninductive conditions, whereas up to 80% of variegated seedlings with green sectors were observed from the seeds of the heat-shocked plants. As described previously (Balcells et al., 1994), these sectors are derived from independent excision events occurring in the embryos during heat shock, and clonally propagated in the developing seedlings. Very few completely green seedlings were observed at this stage (less than 1%), consistent with the transposition events occurring predominantly during the postzygotic stages of embryo development. When HS1 plants are allowed to self-fertilize without further heat shock treatment, the resulting generation, termed HS2, showed a high percentage of germinal excisions indicated by the occurrence of completely green seedlings when germinated on kanamycin- and streptomycin-containing MS media (Fig. 3B). Germinal excisions are defined here as excisions that are transmitted through the male or female gametes so that the progeny is completely revertant. HS2 seedlings are either completely green (resistant) or white (sensitive) and never variegated, showing that variegation is not an ongoing process, but occurs only in the embryos of the heat-shocked plants. Conversely, mostly variegated seedlings were observed in the HS1 generation and the relatively rare cases mentioned above, where completely green seedlings were observed in the HS1 generation might be due to Ds excision events that may have occurred during the gametophytic or zygotic stages. From observations of sectored seedlings on streptomycin plates, a wide spectrum of frequencies of somatic excision could be observed among HS1 seedlings (7% to 67%; data not shown). We observed that starter lines that showed an apparently low somatic excision frequency when grown on streptomycin selection in the HS1 generation were nonetheless capable of yielding a reasonable frequency of germinal transpositions in the HS2 generation.
Figure 3.
A, Plants were subjected to heat shock treatment under high humidity for 1 h at 40°C followed by a rest period of 1 h at 22°C. This was repeated 4 times a day for 3 weeks until the plants started to senesce and turned yellow. B, HS1 seeds derived from heat-shocked plants when germinated on medium containing streptomycin have green sectors conferring streptomycin resistance and are indicative of somatic excision events. HS2 plants, derived from self-fertilized HS1 plants when grown on streptomycin-containing medium, are completely green, indicative of germline excision events.
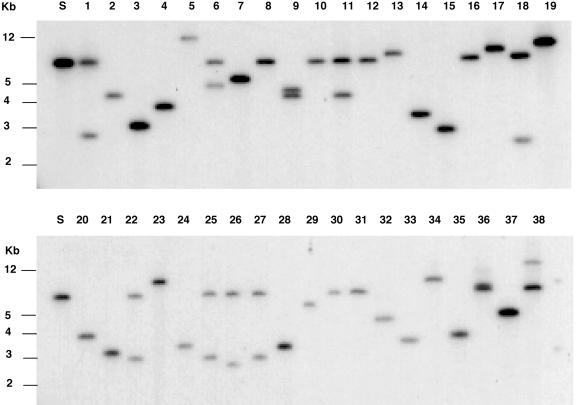
DNA gel-blot hybridization was performed on randomly selected HS2 plants using the GUS gene as a probe to look for transposed Ds after the plants were subjected to heat shock treatment. Border fragments of variable sizes were observed indicative of Ds transposition from its original site of T-DNA insertion and its reinsertion randomly into the genome (Fig. 4).
Figure 4.
Southern-blot analysis of streptomycin- and kanamycin-resistant HS2 plants from a single starter line using the GUS gene as a probe. S, Starter line (1–38 represent selected plants from 38 independent HS2 families). Size markers after ethidium bromide staining are indicated on the left of each blot. When the filter was washed under low-stringency conditions, the bands could be observed on the film directly.
Strategy for Pooled PCR Screen
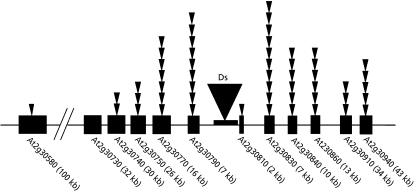
A single starter line, SL152.2, was chosen for exhaustive heat shock mutagenesis and reverse genetic screening to identify insertions in the surrounding genes and further experimental analysis by pool PCRs. The selected starter line has the T-DNA inserted in the first exon of the gene At2g30800 that lies toward the end of the long arm of chromosome 2. The gene encodes for a DEIH-BOX RNA/DNA helicase and has no visible phenotype because of the T-DNA insertion. Twelve neighboring genes that were targeted for detecting insertions have been described in Table I. A total of 1,100 HS2 families were used to generate insertion lines by germination on media containing kanamycin and streptomycin, out of which 953 families gave rise to one or more completely dark green double-resistant seedlings. These numbers are indicative of germline transposition events of as high as 86.6%.
Table I.
Details of the genes analyzed
| Gene Locusa | Distanceb | Size |
|---|---|---|
| kb | bpc | |
| At2g30810 | 2.2 | 703 |
| At2g30790 | 6.5 | 1,044 |
| At2g30830 | 7.0 | 1,578 |
| At2g30840 | 10.0 | 1,420 |
| At2g30860 | 13.3 | 1,249 |
| At2g30770 | 15.8 | 2,198 |
| At2g30750 | 26.2 | 2,016 |
| At2g30740 | 29.2 | 2,238 |
| At2g30730 | 32.4 | 1,533 |
| At2g30910 | 33.5 | 3,884 |
| At2g30940 | 42.9 | 1,655 |
| At2g30580 | 100.0 | 4,662 |
AGI gene locus.
Distance from the original site of T-DNA integration.
Size of the AGI genomic locus sequence according to annotations by the TAIR database.
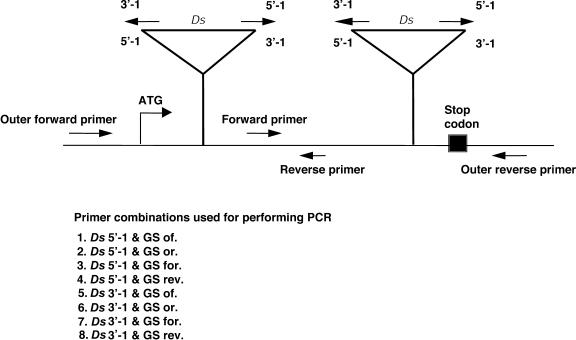
Tissue material was collected from 953 families in subpools that were later added to constitute master pools. A subpool contained tissue samples from five independent HS2 families and 10 such subpools were combined to create one master pool. Therefore, for 953 HS2 families, 19 master pools were generated and PCR was performed on these master pools. Four gene-specific primers (outer forward, forward, reverse, and outer reverse) were designed for each targeted gene in such a way that they roughly covered the entire gene, including the 5′ and 3′untranslated regions, with the exception of one gene, At2g30580 (a putative C3HC4 zinc finger protein), which was relatively large and required 8 primers for complete coverage. These primers, in combination with Ds end-specific primers, were used to identify insertions in the genes flanking the original site of the T-DNA insertion site. The position of primers with respect to the gene and a complete strategy of heat shock mutagenesis can be referred to in Figures 5 and 6, respectively.
Figure 5.
A diagrammatic representation of location and orientation of gene-specific and Ds end-specific primers to identify knockouts in the HS2 population. The primer combinations listed below were used to identify hits in the targeted genes by performing PCR. ATG, translation start site; of., outer forward primer; for., forward primer; or., outer reverse primer; rev., reverse primer; Ds, dissociation transposable element; 5′, Ds 5′ end; and 3′, Ds 3′ end.
Figure 6.
A strategy for generating Ds transpositions by heat shocking single-copy starter lines harboring the plasmid pYS11. A heat shock promoter from Glycine max drives the transposase gene. HS1 seeds are collected, germinated on medium containing the antibiotics streptomycin and kanamycin, and allowed to self-pollinate. The resulting HS2 plants can be used both for forward and reverse genetic screens. Ds, Dissociation element; GT, gene trap; hsp, heat shock promoter; LB, left border; RB, right border; and HS, heat shock.
Analysis of Gene Disruptions in Targeted Regions
A 50-kb region was examined for knockouts for targeted genes on either side of the original site of the T-DNA insertion, and 12 genes within that region were chosen for designing primers to detect Ds insertions. As noted earlier, Ac-Ds transposons preferentially jump to linked sites. However, the size of the gene also determines the probability of identifying hits in that gene, i.e. the larger the gene size, the higher the probability of obtaining knockouts. A schematic representation shown in Figure 7 can be referred to for the number of hits detected in each gene as a function of increasing distance from the original site of T-DNA integration. We obtained a total of 53 independent insertions for 11 of the 12 genes that were examined within this region. PCR was performed on master pools followed by verification in the corresponding subpools. Plant families from the positive subpools were grown and tissue PCR performed in the HS3 generation (Klimyuk et al., 1993). Insertions were detected by running the PCR product on a 1.5% agarose gel. Multiple insertions obtained within the same gene were inferred to be independent based both on independence of descent (i.e. originating from different master pools or, if from the same master pool, then from different subpools) and on fragment sizes (different PCR product sizes).
Figure 7.
Ds insertion distribution for 12 genes within a 50-kb region on both sides of the original site of T-DNA integration for SL152.2 from 953 families for which primers were designed and PCR performed. Each black arrowhead represents an independent insertion in each of these genes. The sizes of the genes are not to scale and only approximate. Ds, Dissociation element (the black box with a triangle in the middle is indicative of the original T-DNA insertion site carrying the Ds element in the starter line).
GUS Expression Patterns
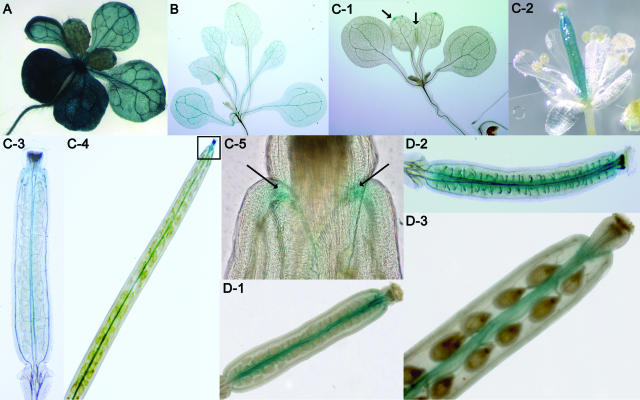
The Ds element contains a GUS reporter gene that acts as a gene trap (Sundaresan et al., 1995). A GUS staining screen was set up for the Ds insertion lines at three different stages of plant development to identify patterns of gene expressions (“Materials and Methods”). Out of the 53 gene disruptions identified, the GUS gene was inserted in the same orientation as the gene for 22 lines out of which 9 lines displayed GUS activity. Staining patterns were also observed in 11 lines where the GUS gene was inserted in the opposite orientation to the direction of transcription. Therefore, a total of 20 out of 53 (37%) lines identified displayed GUS activity at different stages of plant development (Fig. 8). These numbers are comparable to our previously published findings, where approximately 25% of the total gene trap lines displayed GUS activity (Sundaresan et al., 1995). The disparity in numbers may be due to the relatively small number of insertion lines used in our study. GUS staining patterns were also observed in several cases where the Ds containing the GUS gene was in the opposite orientation with respect to the gene, suggesting that the gene trap element can occasionally act as an enhancer trap, which is consistent with unpublished observations from our and other laboratories.
Figure 8.
GUS expression patterns for four independent representative insertion lines. A, L267. Insertion in At2g30740 (Ser/Thr protein kinase). Intense GUS expression is observed in the entire seedling at 19 d postgermination. B, L441. Insertion in At2g30790 (PSII oxygen-evolving complex 23), in the opposite orientation to the direction of transcription. GUS is expressed in the leaves including the vasculature of the entire seedling. C subsection 1, L479. Insertion in At2g30830 (2-oxoglutarate-dependent dioxygenase). GUS staining at the serration points of leaf margins and apices of newly emerging leaves, but not in the cotyledons. Arrows indicate sites of GUS activity. C subsection 2, GUS activity is diffuse throughout the gynoecium shortly after fertilization. C subsections 3 and 4, GUS staining becomes restricted to the valve margins after fertilization and persists until the silique matures (stage 18). C subsection 5, The apex of the valve (boxed area in C subsection 4) shows persistent GUS expression even at the time of dehiscence. Arrows indicate sites of GUS activity. D subsection 1, L360. Insertion in At2g30810 (gibberellin-regulated family protein similar to GASA5), in the opposite orientation to the direction of transcription. GUS staining is observed in the transmitting tract and placenta of the ovary. D subsection 2, GUS activity in the placenta of the carpel at a later stage of development than D subsection 1. D subsection 3, A mature silique displays maintenance of GUS activity in the placenta just before dehiscence (approximately stage 18). The stages of carpel development mentioned above are according to Smyth et al. (1990).
DISCUSSION
We describe a new strategy for insertional mutagenesis in Arabidopsis, a system with potential for use in other plant systems also. In this approach, both Ac, the autonomous, and Ds, the nonautonomous, elements are placed on the same T-DNA vector and the transposase is under the control of a heat shock promoter. This circumvents the need for time-consuming and labor-intensive crosses that are required for initiating mutagenesis, and the necessary subsequent segregation of the transposase source, which have been employed in previous schemes for general as well as targeted transposon mutagenesis using Ac-Ds (Sundaresan et al., 1995; Zhang et al., 2003). In this inducible system, the levels of transposase can be regulated by heat shock. One heat-shocked plant has the potential of producing 10,000 to 15,000 HS1 seeds from which we can recover approximately 10,000 independent transpositions in the HS2 generation, assuming the efficiency of approximately 80% that we observed in this study. Therefore, heat shock of a single flat of 30 plants can easily generate approximately 200,000 independent transpositions, which should be sufficient to saturate a genomic region of 1 to 2 Mb. In this study, we have provided 40 starter lines that can be used for saturation coverage of localized genomic regions totaling 40 to 80 Mb, although several of these regions are overlapping due to the uneven distribution of the starter T-DNAs. Saturation coverage of the whole genome would, in theory, be possible by using 60 to 120 evenly distributed launch pads, which could be achieved by generating and mapping additional transformants using the T-DNA vector described here.
Within a relatively small population of 953 insertion lines, we have identified insertions in 11 out of 12 genes that were tested for identifying targeted knockouts. Twenty-eight out of the 53 insertions identified are around the 5′ end of the genes, i.e. insertions within the first exon, including within the 5′ untranslated region. This bias for insertion at the 5′ ends of the genes corroborates previously published results from our lab (Parinov et al., 1999).
The number of insertions identified per gene not only depends on the distance from the original site of T-DNA integration, but also on the size of the gene. At2g30810, a gibberellin-regulated gene related to the GASA gene family, is a good example of a small gene of only 703 bp that is linked to the original site of T-DNA integration. We have identified a single insertion in this gene for which there is currently no knockout available in the SALK and SAIL databases. This system therefore provides a very useful approach for inactivating small genes, which might include small genes coding for peptide ligands or micro-RNAs using a starter T-DNA close to the gene of interest. Further, the system is convenient for establishing whether a specific mutant phenotype is caused by an insertion. Because the hsp-Ac transposase fusion is linked to the SPT gene and therefore present in all the insertion lines, heat shock of the plants can be used to remobilize the nonautonomous Ds insertion to generate revertants and also to generate double knockouts of tandemly duplicated genes (Tantikanjana et al., 2004).
The Ac-Ds family of transposable elements has been shown to be active in other heterologous systems besides Arabidopsis. Therefore, this system of inducible gene tagging could be very useful for plant systems for which transformation is not routine, since coverage of the whole genome can be achieved through a relatively small number of transformants that act as starter lines. As discussed above, using this system, labor-intensive crosses between parent plants to initiate mutagenesis are also avoided. In conclusion, our systematic analysis of this new system of inducible targeted tagging demonstrates that it can be an efficient, useful, and advantageous approach toward insertional mutagenesis in Arabidopsis to saturate localized regions of the genome, with the potential for application in other plant species as well.
MATERIALS AND METHODS
Plant Growth and Transformation
All plants were Arabidopsis (Arabidopsis thaliana) ecotype Ws-0 grown in Premier Pro-Mix potting soil (http://www.living-learning.com/store/containers/premier%20soil.htm) and watered with nutrient-containing solution. The plants were grown in growth chambers at 22°C with 16 h of continuous daylight followed by 8 h of continuous darkness.
For transformation, Ws-0 seeds were stratified for 2 d at 4°C and germinated on MS medium (0.25% phytagel) for 10 d. They were transferred to flasks containing liquid B5 medium for rooting and placed on a shaker incubator maintained at 200 rpm for 10 d. The seedlings were taken out and the roots chopped and transferred to 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin-containing media for preincubation. Three days after preincubation, they were transformed with Agrobacterium strain LBA4404 harboring the plasmid pYS11. The chopped roots were allowed to cocultivate for 3 d, after which they were washed, first with sterile water, followed by 3 rounds of washes with a liquid B5 salt-containing medium supplemented with 2,4-D, kinetin, carbenicillin, and kanamycin. The roots were blotted on sterile Whatman filter paper (Whatman, Clifton, NJ) for removal of excessive liquid medium, mixed with 0.6% agarose containing 2-ip, indole-3-acetic acid (IAA), kanamycin, and carbenicillin, and plated on media containing 2-ip, IAA, kanamycin, and carbenicillin. Resistant calluses were observed as green callus growing on yellow root explants, which eventually gave rise to shoots. These shoots were cut and transferred to MS plates containing the antibiotics kanamycin and carbenicillin. After a few days, resistant shoots were transferred to phytocons (Sigma, St. Louis) containing MS medium and the antibiotics kanamycin and carbenicillin. Seeds were carefully collected when the plants senesced, germinated on kanamycin-containing medium, and analyzed for copy number of the transgene. All the hormones and antibiotics were dissolved in dimethyl sulfoxide and water, respectively. The working concentrations used for the hormones 2,4-D, kinetin, 2-ip, and IAA, and antibiotics carbenicillin and kanamycin were 0.5 mg/L, 0.05 mg/L, 5.0 mg/L, 0.15 mg/L, 100.0 mg/L, and 50.0 mg/L, respectively.
DNA Gel-Blot Hybridization
To determine the copy number of starter lines, DNA was extracted from buds and leaves of starter lines and digested with EcoRI for 4 h, followed by running the digested product on a 0.8% agarose gel. The 2.2-kb EcoRI fragment from the Ds-T-DNA vector pYS11 was used as a probe to identify the number of T-DNA copies integrated in the genome. Single-copy insertions result in 2 bands, an internal positive control of 1.8 kb from the left border (LB) of the T-DNA and a band of variable size, depending on where the next EcoRI site was present in the genome.
To determine the transposition of Ds from its original site after the plants were subjected to heat shock treatment, Southern blot was performed on HS2 families using the GUS gene as a probe. DNA was extracted and digested with EcoRI. There is one EcoRI site in the GUS gene and, depending on where the next EcoRI site was in the genome, a band of variable size was obtained in the blot once the Ds element had transposed to a new site. Standard protocol was followed for performing DNA gel-blot hybridization (Sambrook et al., 1989).
Mapping of Insertion Lines
TAIL-PCR with minor modifications was performed on 40 independent insertion lines that were identified as containing a single copy of the T-DNA (Liu et al., 1995), utilizing AD and T-DNA end primers. The AD primers and the PCR program were similar, as reported previously (Parinov et al., 1999), and the T-DNA end primers have been described below. The flanking sequences obtained were subjected to BLAST searches of the NCBI and the genomic sequences obtained were put in the sequence viewer of the the Arabidopsis Information Resource (TAIR) Web site (www.arabidopsis.org) to identify the exact location of the T-DNA insertion site. Later, gene-specific forward and reverse primers were designed and, in combination with T-DNA end primers, used to determine and confirm the insertion sites. The LB primers used for TAIL-PCR were D14LB (5′-AGGTAATGGGCTACACTGAATTGG-3′), LBNew2 (5′-GTAGCTCAAACTGTCAGTAT-3′), and LBNew3A (5′-TCAAGGCATCGATCGTGAAGT-3′); the RB primers used were B50 (5′-TCTAATTTCAAACTATTCGGGCCTAAC-3′), C30RBT (5′-CTTATCGACCATGTACGTAAGCGC-3′), and C32RBN (5′-CCTGTGGTCTCAAGATGGATCAT-3′).
Heat Shock and Ds Transposition
The three different heat shock regimes have been discussed before (Balcells et al., 1994). We followed their protocol of heat shocking plants at the flowering stage since the heat shock promoter has been shown to be active in embryos developing inside the growing flower. The plants were heat shocked for 1 h at 40°C to 42°C, followed by a rest period of 1 h. This was repeated 4 times a day for 3 alternate days of the week and for 3 to 4 weeks until the plants started to turn yellow and senesce. High-humidity conditions were maintained by placing a tray of water at the bottom of the heat shock incubator throughout the heat shock treatment. The water in the tray at the bottom of the chamber was checked frequently. Placing a thermometer on the inside of the incubator regularly monitored the temperature of the incubator.
Selection Procedure for Detection of Somatic and Germinal Excision Events
Seeds were collected from the plants that were heat shocked (HS1 seeds), sterilized, and germinated on MS medium supplemented with kanamycin and streptomycin. Seedlings were allowed to grow on selection plates for 10 to 14 d, after which they were transferred for 7 to 10 d to plain MS plates for the plants to recover from the double antibiotic shock. Non-heat-shock seeds were always plated simultaneously on an antibiotic-containing medium as negative controls. This procedure was followed each time seeds from heat-shocked parents were germinated. A similar protocol was followed to identify germinal excision events where progeny from HS1 generation were sown on MS-, kanamycin-, and streptomycin-containing media. Streptomycin sulfate from two different companies (Sigma; http://www.sigmaaldrich.com and Gibco-BRL, Cleveland; http://www.invitrogen.com) was examined and streptomycin sulfate from Sigma (catalog no. S–6501) worked better than that from Gibco-BRL (catalog no. 11860–038). Seedlings grown on media containing streptomycin from Gibco-BRL were pale green and the distinction between resistant and sensitive seedlings was very ambiguous. The streptomycin selection step was found to be important to increase frequency of germinal transpositions. Streptomycin acts in a cell-autonomous manner in cotyledons by binding to the S12 protein of the 30S ribosomal subunit causing inhibition of initiation of chloroplast protein synthesis. On streptomycin selection media, sensitive cells do not die, but bleach out if they are provided with a carbon source, therefore making it an efficient visible selection mechanism for our study (Dean et al., 1992). It is possible that, during seedling growth and development on streptomycin selection media, streptomycin-sensitive cells in the shoot apex are replaced by neighboring active growing cells that are streptomycin resistant, and that these ultimately give rise to the germline. Streptomycin sulfate was dissolved in water and the working concentration used was 200 mg L−1.
Identification of Insertion Lines Carrying the Ds Element
Four gene-specific primers were designed (two primers each in the forward and reverse orientations; Fig. 5) to cover the entire targeted gene. Ds insertions were identified by performing PCR utilizing Ds 5′-1 and Ds 3′-1 primers (Parinov et al., 1999), in combination with the gene-specific primers first in the master pools followed by subpools. The gene-specific primers were designed such that PCR products of sizes not more than 2 kb were expected, which could be amplified relatively easily in the master pools (consisting of approximately 50 HS2 families each), followed by reproducible results in the subpools (consisting of 5 HS2 families each) that constituted that master pool. Eight PCR reactions were set up for each master pool per gene as shown in Figure 5 to identify Ds insertion in both orientations covering the entire gene. Therefore, insertions within a single target gene in the set of 953 HS2 families were identified using a total of 152 PCR reactions. Tissue PCR was performed on individual members of the families constituting the positive subpools in the HS3 generation utilizing the same primer combinations that yielded positive bands for the master and subpools from which the insertion was originally identified (Klimyuk et al., 1993). The PCR products from reciprocal primer combinations were run side by side on a 1.5% agarose gel, and an insertion was considered to be true when fragments of expected sizes were obtained. Individual plants giving the right fragment sizes of PCR products within a single HS3 family were identified, and one individual plant was selected from the family to establish an insertion line by self-fertilization.
Staining for GUS Activity
The Ds-gene trap (GT) insertion lines were histochemically stained for GUS activity at three different stages of plant development: 11 and 19 d postgermination and staining of the inflorescence after the plants had bolted. The GUS screen was performed under 2 conditions, 1 with 1 mm potassium ferrocyanide and 1 mm potassium ferricyanide in the GUS staining solution, and the other with none. Seedlings and inflorescence were stained overnight in GUS staining solution at 37°C. The GUS stain was removed the next day and replaced with 70% ethanol or with clearing solution (chloral hydrate, 80 g; glycerol, 10 mL; water, 30 mL). The tissue was observed under Nikon SMZ-10A and Nikon SMZ-800 dissecting microscopes (Nikon, Tokyo) and digital pictures were taken using softwares RT-Color Spot (Diagnostic Instruments, Sterling Heights, MI) and Auto-Montage version 4.00.0413. Pictures were modified using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining such permission will be the responsibility of the requester. Seed stocks for all the starter lines will subsequently be made available from the Arabidopsis Biological Resource Center (ABRC).
Supplementary Material
Acknowledgments
We thank Sevugan Mayalagu for advice and assistance with TAIL-PCR on some of the starter lines, Zhuang Yuan for technical assistance with the constructs and heat shock conditions, Mumtaz Hussain for assistance with Southern blotting, Dr. Cameron S. Johnson for useful discussions, Dr. Megan E. Griffith for critically reading the manuscript, and Dr. George Coupland for the hsp-transposase fusion.
This work was supported by research funds from Temasek holdings, Singapore, and by grants from the National Science Foundation and the Davis Agricultural Experiment Station.
The online version of this article contains Web-only data.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Apiroz-Lenehan R, Feldmann KA (1997) T-DNA insertional mutagenesis in Arabidopsis: going back and forth. Trends Genet 13: 152–156 [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815 [DOI] [PubMed] [Google Scholar]
- Balcells L, Sundberg E, Coupland G (1994) A heat-shock promoter fusion to the Ac transposase gene drives inducible transposition of a Ds element during Arabidopsis embryo development. Plant J 5: 755–764 [Google Scholar]
- Bancroft I, Dean C (1993. a) Factors affecting the excision frequency of the maize transposable element Ds in Arabidopsis thaliana. Mol Gen Genet 240: 65–72 [DOI] [PubMed] [Google Scholar]
- Bancroft I, Dean C (1993. b) Transposition pattern of the maize element Ds in Arabidopsis thaliana. Genetics 134: 1221–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann G, Raschke E, Bevan M, Schoffl F (1987) Functional analysis of sequences required for transcriptional activation of a soyabean heat shock gene in transgenic tobacco plants. EMBO J 6: 1161–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Sjodin C, Page T, Jones J, Lister C (1992) Behaviour of the maize transposable element Ac in Arabidopsis thaliana. Plant J 2: 69–81 [Google Scholar]
- Errampalli D, Patton D, Castle L, Mickelson L, Hansen K, Schnall J, Feldmann K, Meinke D (1991) Embryonic lethals and T-DNA insertional mutagenesis in Arabidopsis. Plant Cell 3: 149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff NV (1989) About maize transposable elements and development. Cell 56: 181–191 [DOI] [PubMed] [Google Scholar]
- Fedoroff NV, Smith DL (1993) A versatile system for detecting transposition in Arabidopsis. Plant J 3: 273–289 [DOI] [PubMed] [Google Scholar]
- Grevelding C, Fantes V, Kemper E, Schell J, Masterson R (1993) Single-copy T-DNA insertions in Arabidopsis are the predominant form of integration in root-derived transgenics, whereas multiple insertions are found in leaf discs. Plant Mol Biol 23: 847–860 [DOI] [PubMed] [Google Scholar]
- Klimyuk VI, Carroll BJ, Thomas CM, Jones JD (1993) Alkali treatment for rapid preparation of plant material for reliable PCR analysis. Plant J 3: 493–494 [DOI] [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Sussman MR (1999) T-DNA as an insertional mutagen in Arabidopsis. Plant Cell 11: 2283–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8: 457–463 [DOI] [PubMed] [Google Scholar]
- Machida C, Onouchi H, Koizumi J, Hamada S, Semiarti E, Torikai S, Machida Y (1997) Characterization of the transposition pattern of the Ac element in Arabidopsis thaliana using endonuclease I-SceI. Proc Natl Acad Sci USA 94: 8675–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martienssen RA (1998) Functional genomics: probing plant gene function and expression with transposons. Proc Natl Acad Sci USA 95: 2021–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskett PR, Clissold L, Marocco A, Springer PS, Martienssen R, Dean C (2003) A resource of mapped dissociation launch pads for targeted insertional mutagenesis in the Arabidopsis genome. Plant Physiol 132: 506–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinov S, Sevugan M, Ye D, Yang WC, Kumaran M, Sundaresan V (1999) Analysis of flanking sequences from dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell 11: 2263–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinov S, Sundaresan V (2000) Functional genomics in Arabidopsis: large-scale insertional mutagenesis complements the genome sequencing project. Curr Opin Biotechnol 11: 157–161 [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Sundaresan V (2001) Transposons as tools for functional genomics. Plant Physiol Biochem 39: 243–252 [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sessions A, Burke E, Presting G, Aux G, McElver J, Patton D, Dietrich B, Ho P, Bacwaden J, Ko C, et al (2002) A high-throughput Arabidopsis reverse genetics system. Plant Cell 14: 2985–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Yanai Y, Liu YG, Ishiguro S, Okada K, Shibata D, Whittier RF, Fedoroff NV (1996) Characterization and mapping of Ds-GUS-T-DNA lines for targeted insertional mutagenesis. Plant J 10: 721–732 [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V (1996) Horizontal spread of transposon mutagenesis: new use of old elements. Trends Plant Sci 1: 184–190 [Google Scholar]
- Sundaresan V, Springer P, Volpe T, Haward S, Jones JD, Dean C, Ma H, Martienssen R (1995) Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev 9: 1797–1810 [DOI] [PubMed] [Google Scholar]
- Tantikanjana T, Mikkelsen MD, Hussain M, Halkier BA, Sundaresan V (2004) Functional analysis of the tandem-duplicated P450 genes SPS/BUS/CYP79F1 and CYP79F2 in glucosinolate biosynthesis and plant development by Ds transposition-generated double mutants. Plant Physiol 135: 840–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier AF, Marillonnet S, Klimyuk V, Patel K, Torres MA, Murphy G, Jones JD (1999) Multiple independent defective suppressor-mutator transposon insertions in Arabidopsis: a tool for functional genomics. Plant Cell 11: 1841–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D, Montagu MV, Lijsebettens MV (1988) Agrobacterium-mediated transformation of Arabidopsis root explants using kanamycin selection. Proc Natl Acad Sci USA 85: 5536–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Raina S, Li H, Li J, Dec E, Ma H, Huang H, Fedoroff NV (2003) Resources for targeted insertional and deletional mutagenesis in Arabidopsis. Plant Mol Biol 53: 133–150 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.