Abstract
Background
Percutaneous nephrolithotomy (PCNL) is performed to treat relatively large renal stones. Recent publications indicate that tubeless and total tubeless (stentless) PCNL is safe in selected patients. We performed a systematic review and network meta-analysis to evaluate the feasibility and safety of different PCNL procedures, including total tubeless, tubeless with stent, small-bore tube, and large-bore tube PCNLs.
Methods
PubMed, Cochrane Central Register of Controlled Trials, and EMBASE™ databases were searched to identify randomized controlled trials published before December 30, 2013. One researcher examined all titles and abstracts found by the searches. Two investigators independently evaluated the full-text articles to determine whether those met the inclusion criteria. Qualities of included studies were rated with Cochrane’s risk-of-bias assessment tool.
Results
Sixteen studies were included in the final syntheses including pairwise and network meta-analyses. Operation time, pain scores, and transfusion rates were not significantly different between PCNL procedures. Network meta-analyses demonstrated that for hemoglobin changes, total tubeless PCNL may be superior to standard PCNL (mean difference [MD] 0.65, 95% CI 0.14–1.13) and tubeless PCNLs with stent (MD -1.14, 95% CI -1.65–-0.62), and small-bore PCNL may be superior to tubeless PCNL with stent (MD 1.30, 95% CI 0.27–2.26). Network meta-analyses also showed that for length of hospital stay, total tubeless (MD 1.33, 95% CI 0.23–2.43) and tubeless PCNLs with stent (MD 0.99, 95% CI 0.19–1.79) may be superior to standard PCNL. In rank probability tests, small-bore tube and total tubeless PCNLs were superior for operation time, pain scores, and hemoglobin changes.
Conclusions
For hemoglobin changes, total tubeless and small-bore PCNLs may be superior to other methods. For hospital stay, total tubeless and tubeless PCNLs with stent may be superior to other procedures.
Keywords: Calculi, Lithotripsy, Nephrostomy, Percutaneous, Meta-analysis, Bayes theorem
Background
Urinary stone is one of the most prevalent urological disorders. Reports suggest that up to 12% of people will suffer from urinary tract calculi during their lifetime, and the rates of recurrence is close to 50% [1]. There are several treatment modalities for renal stones, including observation expecting spontaneous passage, extracorporeal shock wave lithotripsy (ESWL), percutaneous nephrolithotomy (PCNL), and retrograde intrarenal surgery (RIRS) using flexible ureterorenoscope [2]. PCNL is currently the standard treatment for large renal stones considered too large for or refractory to shock wave lithotripsy [3, 4]. Conventionally, a 20-24 French nephrostomy catheter is placed routinely after PCNL to provide urine drainage, prevent extravasation of urine, and make tamponade against bleeding [5, 6]. In addition, it can be used as a tract for a second-look PCNL [7]. The need for placing a conventional large-bore nephrostomy catheter has been questioned because of its accompanying increase in postoperative discomfort and other morbidity, and the low incidence of second-look operations [8, 9]. In recent years, tubeless or small-bore PCNL has been widely used, and previously reported systematic reviews have demonstrated the safety and efficacy in these techniques.
The recently introduced network meta-analysis is a meta-analysis in which multiple treatments are compared using both direct comparisons of interventions within randomized controlled trials (RCTs), and indirect comparisons across trials based on a common comparator [10–14]. Thus, we performed a systematic review and network meta-analysis based on published relevant studies to evaluate the feasibility and safety of each PCNL procedure, including total tubeless, tubeless with stent, small-bore tube, and large-bore tube PCNLs, for the treatment of renal stones.
Methods
Inclusion and exclusion criteria
Reported RCTs that fitted the following criteria were selected: (i) a design of each study that involved comparing the feasibility and safety for least two PCNL procedures, including total tubeless, tubeless with stent, small-bore tube, and large-bore tube PCNLs; (ii) the study groups were matched for baseline characteristics, including the total number of subjects and the values of each variable; (iii) at least one of the following outcomes was assessed: operation time, hospital stay length, hemoglobin decrease, return to normal activity, and complication rate; and (iv) the full text of each study was accessible and written in English.
The exclusion criteria were as follows: (i) noncomparative studies; (ii) the trial included children; and (iii) the trial did not exclude patients who underwent bilateral simultaneous PCNL or had complete or partial staghorn stones, more than two nephrostomy tracts, anatomical anomalies, or urinary infection. This report was prepared in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (accessible at http://www.prisma-statement.org/) [15].
Search strategy
A literature search was performed to identify RCTS published prior to December 30, 2013 in PubMed, the Cochrane Central Register of Controlled Trials, and EMBASE™ online databases. A cross-reference search of eligible articles was performed to identify additional studies not found by the computerized search. Combinations of the following MeSH and key words were used: percutaneous nephrolithotomy or nephrostomy or percutaneous nephrostomy or nephrolithiasis or PCNL or PCN or PNL, and total tubeless or tubeless or nephrostomy free.
Data extraction
One researcher (J.Y.L.) screened the title and abstract of all articles retrieved using the search strategy. The other two investigators (D.H.K. and H.L.) independently assessed the full text of the articles to determine whether they met the inclusion criteria. For each included study, the following data were extracted independently as follows; authors, date, demographics of included patients, PCNL methods, feasibility, efficacy outcomes, complications, and inclusion of a reference standard. Disagreements arising in the study selection and data extraction processes were resolved by discussion until a consensus was reached or by arbitration employing another researcher (K.S.C.).
Study quality assessment
Once the final group of articles was agreed upon, two researchers (J.Y.L. and D.H.K.) independently examined the quality of each article using the Cochrane’s risk-of-bias as a quality assessment tool for RCTs. The assessment involves the assignment of a “yes,” “no,” or “unclear” rating for each domain, designating a low, high, or unclear risk of bias, respectively. If ≤1 domain was rated “unclear” or “no,” the study was classified as having a low risk of bias. If ≥4 domains were rated “unclear” or “no,” the study was classified as having a high risk of bias. If 2 or 3 domains were rated “unclear” or “no,” the study was classified as having a moderate risk of bias. [16]. Quality assessment was performed using Review Manager 5.2 (RevMan 5.2.11, Cochrane Collaboration, Oxford, UK).
Statistical analyses
Each outcome variable at specific time-points was compared by network meta-analysis using the odds ratio (OR) or mean difference (MD) with 95% confidence interval (CI). A random-effect model was used. Each analysis was based on non-informative priors for effect size and precision. Convergence and lack of auto-correlation were checked and confirmed after four chains and a 50,000-simulation burn-in phase, and direct probability statements were based on an additional 100,000-simulation phase. Calculation of the probability that each group had the lowest rate of clinical events was performed using Bayesian Markov Chain Monte Carlo modeling. Sensitivity analyses were performed by repeating the main computations using a fixed-effect method. Model fit was appraised by computing and comparing estimates for deviance and deviance information criterion. Pairwise inconsistency and inconsistency between direct and indirect effect estimates were assessed with the I2-statistic, with values <25%, 25% to 50%, and >50% representing mild, moderate, and severe inconsistency, respectively. The extent of small study effects/publication bias was assessed by visual inspection of funnel plots for the pairwise meta-analyses. All statistical analyses were performed using Review Manager 5 and R (R version 3.0.3, R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) [17], and its meta, forestplot, gemtc, and R2WinBUGS packages for pairwise and network meta-analyses using Bayesian Markov Chain Monte Carlo modeling.
Results
Eligible studies
Our database search identified 43 studies that could be potentially included in the meta-analysis. Based on the inclusion and exclusion criteria, 18 articles were excluded during screening of the titles and abstracts because they were retrospective studies (11 articles) or case series (7 articles). This left 25 RCTS that evaluated various types of PCNL procedures for renal stones. After reviewing the full-text articles for these studies, 9 were excluded because they reported irrelevant results. Therefore, 16 RCTs were ultimately included in the qualitative analysis, as well as the quantitative synthesis using pairwise and network meta-analyses (Fig. 1).
Fig. 1.
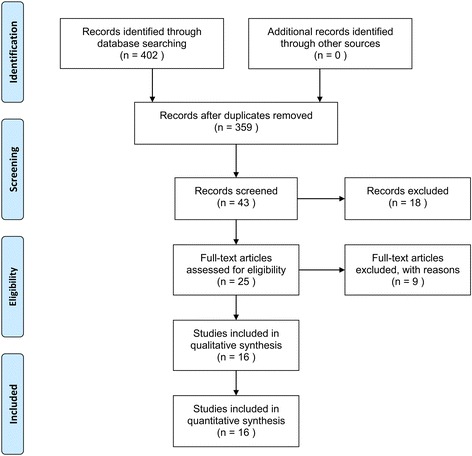
Search strategy for a systematic review and meta-analysis to compare the feasibility and safety of different PCNL procedures, including total tubeless, tubeless with stent, small-bore tube, and large-bore tube PCNLs
There were differences in procedures among the included studies. Five studies included comparisons between standard and total tubeless PCNLs, and five RCTs also compared standard and tubeless PCNLs. Four trials reported on various factors in small-bore and tubeless PCNLs. In two studies, the results of three arms—standard, small-bore, and tubeless PCNLs—were published (Table 1). Finally, the included studies covered four different PCNL procedures: total tubeless, tubeless, standard and small-bore PCNLs (Fig. 2).
Table 1.
Characteristics of included trials
| Study | Year | Design | Procedures | Sample size | Age (year) | Stone burden | Tube | Stone-free rate | Quality assessment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Size | P-value | (%) | P-value | ||||||||
| Chang et al. [41]. | 2011 | RCT | Standard | 63 | 58.7 | 24.86 ± 2.78 mm | 0.722 | 20 Fr (7 Fr) | 75% | 0.51 | Low |
| Total tubeless | 68 | 59.2 | 24.74 ± 2.69 mm | None | 74% | ||||||
| Aghamir et al. [42]. | 2011 | RCT | Standard | 35 | 40 | 2.87 ± 0.62 cm2 | 0.66 | NA | 83% | NA | Low |
| Total tubeless | 35 | 38.4 | 2.81 ± 0.59 cm2 | None | 86% | ||||||
| Kara et al. [43]. | 2010 | RCT | Standard | 30 | 66.5 | 25.6 mm | NA | 18 Fr | 90% | >0.05 | Low |
| Total tubeless | 30 | 67.7 | 22.3 mm | None | 96% | ||||||
| Mishra et al. [44]. | 2010 | RCT | Standard | 11 | 42.5 | 2737 μL | 0.18 | 20 Fr | 81.8% | 0.14 | Low |
| Tubeless | 11 | 42.3 | 2934.2 μL | None (6 Fr) | 72.7% | ||||||
| Istanbulluoglu et al. [45]. | 2009 | RCT | Standard | 45 | 43.9 | 432.35 ± 195.97 mm2 | 0.46 | 14 Fr | NA | NA | Low |
| Total tubeless | 45 | 47.5 | 448.93 ± 249.13 mm2 | None | NA | ||||||
| Crook et al. [46]. | 2008 | RCT | Standard | 25 | 53 | 17.5 mm | NA | 26 Fr | 84% | NA | Low |
| Total tubeless | 25 | 52 | 21.6 mm | None | 96% | ||||||
| Agrawal et al. [47]. | 2008 | RCT | Standard | 101 | 31 | NA | NA | 16 Fr | 100% | – | Low |
| Tubeless | 101 | 33 | NA | None (6 Fr) | 100% | ||||||
| Singh et al. [48]. | 2008 | RCT | Standard | 30 | 34 | 800 mm2 | >0.05 | 22 Fr | 93.3% | 0.64 | Moderate |
| Tubeless | 30 | 31 | 750 mm2 | None (NA) | 90% | ||||||
| Shah et al. [18]. | 2008 | RCT | Small-bore tube | 32 | 46.7 | 495.92 mm2 | 0.88 | 8Fr | 87.5% | 0.96 | Low |
| Tubeless | 33 | 44.1 | 535.36 mm2 | None (6 Fr) | 87.9% | ||||||
| Sofikerim et al. [35]. | 2007 | RCT | Standard | 24 | 54.1 | 425 mm2 | NA | 24 Fr or 18 Fr | 85% (24 Fr), | 0.71 | Moderate |
| Tubeless | 24 | 47.8 | 428 mm2 | None (6 Fr) | 83% (18 Fr), 79% | ||||||
| Tefekli et al. [49]. | 2007 | RCT | Standard | 18 | 41.32 | 3.1 cm | NA | 14 Fr | 89% | >0.05 | Moderate |
| Tubeless | 17 | 38.4 | 2.8 cm | None (NA) | 94% | ||||||
| Weiland et al. [50]. | 2007 | RCT | Small-bore tube | 9 | 65 | 6.7 cm2 | 0.15 | 8.3 Fr | Moderate | ||
| Tubeless | 9 | 54 | 3.2 cm2 | None (8.2 Fr) | |||||||
| Choi et al. [32]. | 2006 | RCT | Small-bore tube | 12 | 47 | 32.41 mm | 0.77 | 8.2 Fr | 91.7% | 0.64 | High |
| Tubeless | 12 | 52.9 | 28.5 mm | None (6 Fr) | 100% | ||||||
| Desai et al. [51]. | 2004 | RCT | Standard | 10 | 43.4 | 263.7 mm2 | >0.05 | 20 Fr | 100% | – | Moderate |
| Small-bore tube | 10 | 44.8 | 243 mm2 | 9 Fr | 100% | ||||||
| Tubeless | 10 | 41.1 | 249.1 mm2 | None (6 Fr) | 100% | ||||||
| Marcovich et al. [52]. | 2004 | RCT | Standard | 20 | 58 | 3.6 cm | 0.64 | 24 Fr | 0.63 | Moderate | |
| Small-bore tube | 20 | 61 | 3 cm | 8 Fr | |||||||
| Tubeless | 20 | 57 | 3.4 cm | None (NA) | |||||||
| Feng et al. [53]. | 2001 | RCT | Standard | 10 | 53 | 8.4 cm3 | 0.75 | 22 Fr | 31.5% | NA | Moderate |
| Tubeless | 8 | 62 | 4.4 cm3 | None (NA) | 71.4% | ||||||
NA not applicable, RCT randomized controlled trial
Fig. 2.
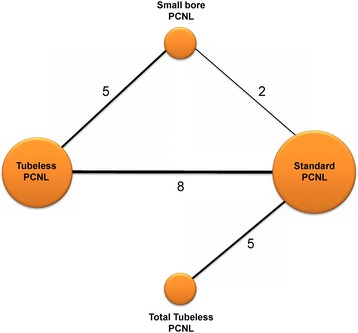
Comparison network of included randomized controlled trials. Five studies included comparisons between standard and total tubeless PCNLs, and five RCTs also compared standard and tubeless PCNLs. Four trials reported on various factors in small-bore and tubeless PCNLs. In two studies, the results of three arms—standard, small-bore, and tubeless PCNLs—were published
Quality assessment and publication bias
Figures 3 and 4 present the details of quality assessment, as measured by the Cochrane Collaboration risk-of-bias tool. Seven trials exhibited a moderate risk of bias for all quality criteria and only one study was classified as having a high risk of bias (Table 1). For operation time, hemoglobin change, and transfusion rate, little evidence of publication bias was demonstrated on funnel plots; however, for the visual analogue scale (VAS) pain score and hospital stay, moderate evidence of publication bias was demonstrated on these plots (Fig. 5).
Fig. 3.
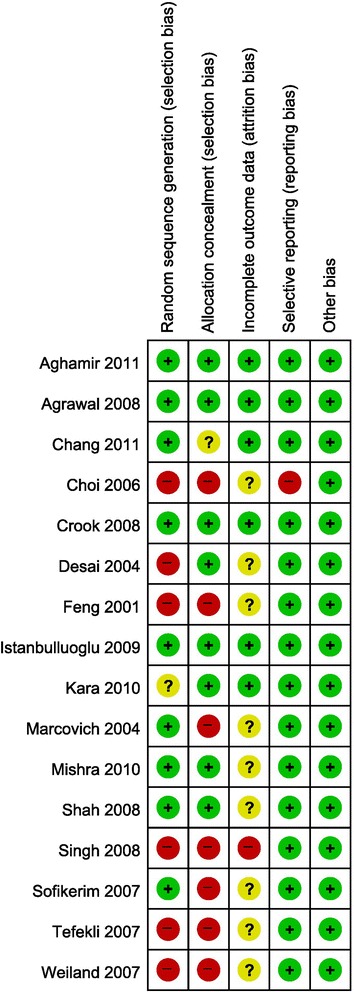
Risk-of-bias summary: review of the authors’ judgments on each risk-of-bias item for each included study
Fig. 4.
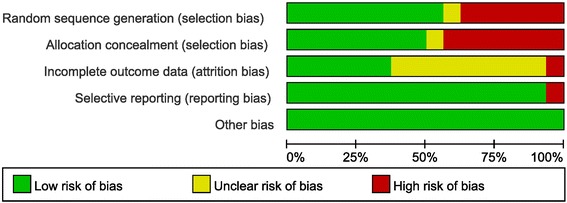
Risk-of-bias graph: review of the authors’ judgments on each risk-of-bias item presented as percentages across all included studies
Fig. 5.
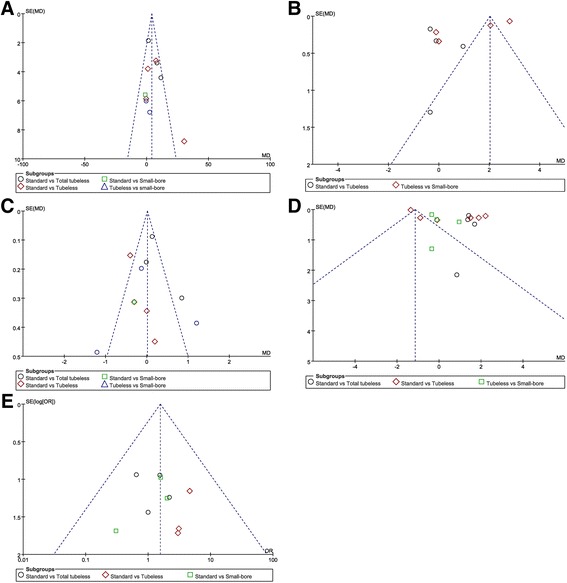
Funnel plots of each variable. a Operation time, b visual analogue scale (VAS) pain score, c hemoglobin change, d length of stay, and e transfusion rate. For operation time, hemoglobin change, and transfusion rate, little evidence of publication bias was demonstrated on visual or statistical examination of the funnel plots; however, for VAS scores and hospital stay, moderate evidence of publication bias was demonstrated on visual or statistical examination of the plots
Operation time
During the pairwise meta-analysis of operation time between standard and total tubeless PCNLs, there was a significant degree of heterogeneity among these studies, and data were pooled with a random effects model (P = 0.04, I2 = 69%). There was no statistically significant difference in operation time between standard and total tubeless PCNLs, although the MD was 6.19 (95% CI -0.14 to 12.52) (Fig. 6a). Between standard and tubeless PCNLs with stent, the MD also demonstrated no statistical difference (MD 7.43, 95% CI -1.70 to 16.57) (Fig. 6b). Likewise, the MDs did not exhibit statistically significant differences for standard versus small-bore PCNLs (MD -1.0, 95% CI -11.93 to 9.93) or tubeless versus small-bore PCNLs (MD 0.86, 95% CI -7.95 to 9.68) (Fig. 6c). Using network meta-analysis, there were no significant differences among all procedures (Fig. 7a) (Table 2), although total tubeless and small-bore PCNLs had higher rank probabilities than the other procedures (Fig. 8a).
Fig. 6.
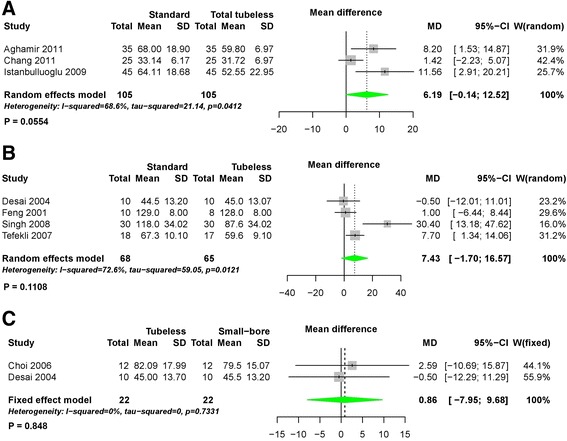
Forest plots for operation time using pairwise meta-analysis. a Standard versus total tubeless PCNLs, b standard versus tubeless PCNLs, and c tubeless versus small-bore PCNLs. SD, standard deviation; MD, Mean difference; CI, confidence interval; W, Weight
Fig. 7.
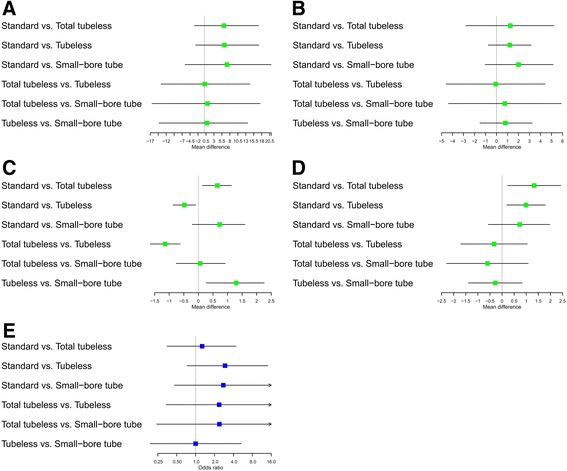
Forest plots for (a) operation time, b visual analogue scale, c hemoglobin change, d hospital stay, and e transfusion rate using network meta-analysis
Table 2.
Results of network and pairwise meta-analyses comparing procedures for operation time, visual analogue scale pain score, hemoglobin change, and hospital stay
| Procedures | Network meta-analysis | Pairwise meta-analysis | ||
|---|---|---|---|---|
| Mean difference | 95% CI | Mean difference | 95% CI | |
| Operation time | ||||
| Standard | ||||
| Total tubeless | 6.11 | −3.14 – 17.02 | 6.19a | −0.14 – 12.52 |
| Tubeless | 6.28 | −2.71 – 17.06 | 7.43a | −1.70 – 16.57 |
| Small-bore tube | 7.09 | −6.03 – 20.95 | NA | |
| Total tubeless | ||||
| Tubeless | 0.08 | −13.60 – 14.27 | NA | |
| Small-bore tube | 0.95 | −16.46 – 17.52 | NA | |
| Tubeless | ||||
| Small-bore tube | 0.80 | −14.27 – 13.60 | 0.86b | −7.95 – 9.68 |
| Visual analogue scale pain score | ||||
| Standard | ||||
| Total tubeless | 1.25 | −2.80 – 5.22 | NA | |
| Tubeless | 1.20 | −0.75 – 3.14 | 0.06a | −0.56 – 0.69 |
| Small-bore tube | 2.00 | −1.03 – 5.14 | NA | |
| Total tubeless | ||||
| Tubeless | −0.07 | −4.58 – 4.41 | NA | |
| Small-bore tube | 0.75 | −4.37 – 5.89 | NA | |
| Tubeless | ||||
| Small-bore tube | 0.80 | −1.51 – 3.24 | 1.21a | −0.02 – 2.44 |
| Hemoglobin change | ||||
| Standard | ||||
| Total tubeless | 0.65 | 0.14 – 1.13 | 0.23a | −0.12 – 0.58 |
| Tubeless | −0.48 | −0.87 – −0.09 | -0.29a | −0.53 – −0.05 |
| Small-bore tube | 0.73 | −0.21 – 1.60 | NA | |
| Total tubeless | ||||
| Tubeless | −1.14 | −1.65 – −0.62 | NA | |
| Small-bore tube | 0.06 | −0.76 – 0.92 | NA | |
| Tubeless | ||||
| Small-bore tube | 1.30 | 0.27 – 2.26 | −0.02a | −1.13 – 1.10 |
| Hospital stay | ||||
| Standard | ||||
| Total tubeless | 1.33 | 0.23 – 2.43 | 1.42b | 1.10 – 1.75 |
| Tubeless | 0.99 | 0.19 – 1.79 | 0.54a | −1.03 – 2.11 |
| Small-bore tube | 0.73 | −0.57 – 1.98 | NA | |
| Total tubeless | ||||
| Tubeless | −0.33 | −1.71 – 1.04 | NA | |
| Small-bore tube | −0.60 | −2.29 – 1.08 | NA | |
| Tubeless | ||||
| Small-bore tube | −0.28 | −1.39 – 0.83 | 0.06a | −0.56 – 0.69 |
CI confidence interval, NA not applicable
aRandom-effect model with inverse variance method
bFixed-effect model with inverse variance method
Fig. 8.
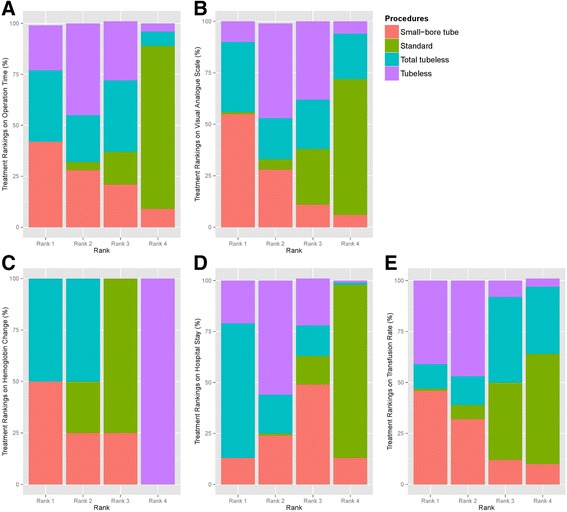
Rank probability test. a Operation time, b visual analogue scale, c hemoglobin change, d hospital stay, and e transfusion rate
Visual analogue scale pain score
In the pairwise meta-analysis of VAS pain scores, there was a significant degree of heterogeneity among studies and the data were pooled with a random effects model. There were no statistically significant differences comparing standard versus total tubeless PCNLs with stent (MD 0.06, 95% CI -0.56 to 0.69, P = 0.84) (Fig. 9a) or tubeless versus small-bore PCNLs (MD 1.21, 95% CI -0.02 to 2.44, P = 0.05) (Fig. 9b). In the network meta-analysis, there were no statistically significant differences among all procedures for VAS pain scores (Fig. 7b) (Table 2), although the rank probabilities demonstrated that small-bore and total tubeless PCNLs may be superior to the other procedures (Fig. 8b).
Fig. 9.
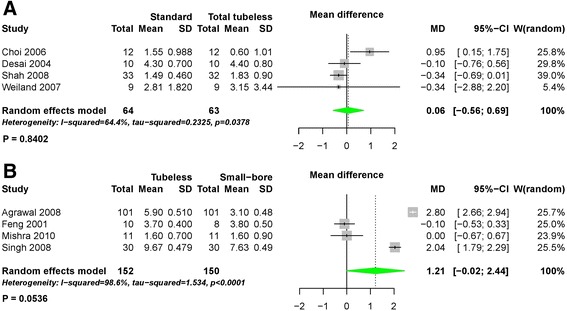
Forest plots for visual analogue scale pain score using pairwise meta-analysis. a Standard versus total tubeless PCNLs, and b standard versus tubeless PCNLs, SD, standard deviation; MD, Mean difference; CI, confidence interval; W, Weight
Hemoglobin change
Using pairwise meta-analysis for hemoglobin change, three comparisons, including standard versus total tubeless PCNLs, standard versus tubeless PCNLs with stent, and tubeless versus small-bore PCNLs, were examined (Fig. 10). Only one comparison for standard versus tubeless PCNLs with stent showed a statistically significant difference (MD -0.29, 95% CI -0.53 to −0.05, P = 0.02) (Fig. 10b). Network meta-analysis demonstrated that total tubeless PCNL may be superior to standard PCNL (MD 0.65, 95% CI 0.14 to 1.13). Total tubeless (MD -1.14, 95% CI -1.65 to −0.62), and small-bore PCNLs (MD 1.30, 95% CI 0.27 to 2.26) were also superior to tubeless PCNL with stent for hemoglobin change (Fig. 7c) (Table 2). In rank probabilities, total tubeless and small-bore PCNLs were ranked higher than the other procedures (Fig. 8c).
Fig. 10.

Forest plots for hemoglobin change using pairwise meta-analysis. a Standard versus total tubeless PCNLs, b standard versus tubeless PCNLs, and c tubeless versus small-bore PCNLs. SD, standard deviation; MD, Mean difference; CI, confidence interval; W, Weight
Hospital stay
The length of hospital stay in patients who underwent total tubeless PCNL was shorter than for those who underwent standard PCNL (MD 1.42, 95% CI 1.10 to 1.75, P < 0.01) during pairwise meta-analysis (Fig. 11). Network meta-analysis also demonstrated that total tubeless (MD 1.33, 95% CI 0.23 to 2.43) and tubeless PCNLs with stent (MD 0.99, 95% CI 0.19 to 1.79) may be superior to standard PCNL, producing a shorter hospital stay (Fig. 7d). However, there was no significant difference between total tubeless and tubeless PCNLs with stent (MD -0.33, 95% CI -1.71 to 1.04) (Table 2), although total tubeless PCNL showed the highest rank probability of all procedures (Fig. 8d).
Fig. 11.

Forest plots for hospital stay using pairwise meta-analysis. a Standard versus total tubeless PCNLs, b standard versus tubeless PCNLs, and c tubeless versus small-bore PCNLs. SD, standard deviation; MD, Mean difference; CI, confidence interval; W, Weight
Transfusion rate
The transfusion rate did not exhibit significant differences between any of the procedures during both pairwise analysis (Fig. 12) and network meta-analysis (Fig. 7e) (Table 3). Rank probabilities demonstrated that small-bore and tubeless PCNLs with stent may be superior to the other procedures (Fig. 8e).
Fig. 12.
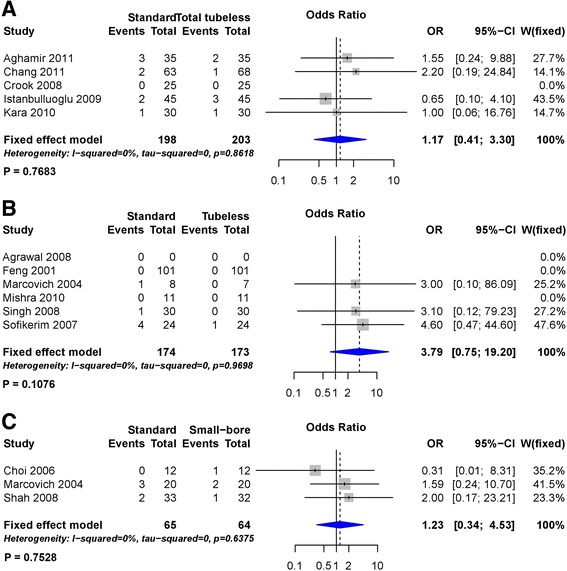
Forest plots for transfusion rate using pairwise meta-analysis. a Standard versus total tubeless PCNLs, b standard versus tubeless PCNLs, and c tubeless versus small-bore PCNLs. OR, Odds ratio; CI, confidence interval; W, Weight
Table 3.
Results of network and pairwise meta-analyses comparing procedures for transfusion rate
| Procedures | Network meta-analysis | Pairwise meta-analysisa | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Standard | ||||
| Total tubeless | 1.27 | 0.35–4.40 | 1.17 | 0.41–3.30 |
| Tubeless | 2.94 | 0.73–14.06 | 3.79 | 0.75–19.20 |
| Small-bore tube | 2.76 | 0.46–24.52 | NA | |
| Total tubeless | ||||
| Tubeless | 2.37 | 0.34–18.19 | NA | |
| Small-bore tube | 2.39 | 0.24–22.21 | NA | |
| Tubeless | ||||
| Small-bore tube | 1.00 | 0.19–5.30 | 1.23 | 0.34–4.53 |
CI confidence interval, OR odds ratio
aFixed-effect model with Mantel-Haenszel method
Discussion
Conventionally, the placement of a nephrostomy tube after PCNL was considered a necessary safety option. However, the use of a nephrostomy tube has been associated with a prolonged hospital stay and more postoperative pain [18]. In 1997, Bellman et al. first reported the use of tubeless PCNL using a double-J ureteral stent and Council catheter [19]. They demonstrated that hospital length of stay, analgesia requirements, time to return to normal activities, and cost were significantly less with this procedure. Although the procedure gained popularity, tubeless PCNL with stent had two important problems: ureteral stent discomfort and loss of the advantages of a nephrostomy tube. Thus, some urologists used the approach of placing the smallest possible nephrostomy tube to minimize patient discomfort while maintaining access to the renal collecting system [20]. With the recent development of a high-density telescope, high-quality lithotripters, and radiological interventional techniques to embolize blood vessels, several investigators reported that tubeless and total tubeless (stentless) PCNL in selected patients was safe and associated with a reduced hospital length of stay and analgesic requirements.
The results of RCTs for each PCNL procedure have been reported, and previous systematic reviews and meta-analyses have been published. However, most of the studies reported in the previous meta-analyses compared standard PCNL versus tubeless PCNL with stent or standard PCNL versus total tubeless PCNL [21–25]. Therefore, an integrated analysis of standard, small-bore tube, tubeless with stent, and total tubeless PCNLs has not yet been published.
In our study, using network meta-analysis, there were no significant differences in operation time for the four procedures. It is known that large stones increase operation time and complication rates [26, 27], and operation times vary depending on the size and characteristics of the stone.
We also detected no statistically significant differences between methods for the VAS pain scores. No significant differences were observed between standard versus total tubeless PCNLs and tubeless versus small-bore tube PCNLs not only during the network meta-analysis, but even during pairwise meta-analyses. Operation-related factors that may prolong pain after PCNL include the nephrostomy tube size [28] and stent discomfort caused by a double-J stent [29], but statistically significant differences between procedures were not observed. This finding is presumably due to the relatively small sample size (only eight studies reported the VAS pain scores), and the possibility of publication bias, as suggested by the asymmetric funnel plot (Fig. 5b). However, in the rank probability test of pain scores using Bayesian Markov Chain Monte Carlo modeling, small-bore tube PCNL was ranked highest, followed by the total tubeless PCNL and then tubeless PCNL with stent (Fig. 8b). Additional RCTs are necessary in the future to more definitively address this issue.
With regard to the hemoglobin changes, network meta-analysis showed that total tubeless and small-bore tube PCNLs were superior, and tubeless with stent PCNL was the worst. In addition, total tubeless and small-bore PCNLs showed similar superiority in the network meta-analysis and rank probability test (Fig. 8c). Considering that all enrolled studies were RCTs, the possibility of selection bias between patients who had total tubeless or small-bore tube PCNLs and other procedures should be relatively low. For tubeless PCNLs, the possibility of bleeding caused by ureteral stenting should be considered. In previous studies, hematuria accounted for 13.6% of early complications and 18.1% of late complications after tubeless PCNL with stent [29]. In contrast to the hemoglobin changes, transfusion rates were not different between the four procedures. This lack of difference is likely due to the development of high-quality surgical skills and patient monitoring approaches because of the popularity of PCNL procedures.
For the length of hospital stay, the total tubeless and tubeless PCNLs showed superiority. We assumed that this is because these methods do not require additional procedures, such as nephrostomy tube removal or tract revision.
During the rank probability for each variable, small-bore and tubeless PCNLs were ranked higher for operation time, VAS pain scores, and hemoglobin change. In addition, total tubeless PCNL was ranked highest for hospital stay and transfusion rate. Notably, total tubeless PCNL was ranked highest for each item. However, total tubeless PCNL has not been in widespread use, even considering the potential benefits of this approach, because of concerns that potentially fatal complications, such as massive bleeding without a nephrostomy tube in place, may occur [30]. Because omitting a nephrostomy catheter may potentially increase the risk of bleeding and serious complications, various methods have been used in an attempt to seal the tract. Milkahi and colleagues were the first to describe the instillation of the hemostatic agent Tiseel® into the nephrostomy tract [31]. However, they were unable to determine whether this diminished postoperative bleeding or urinary extravasation following tubeless PCNL. Choi et al. instilled gel matrix thrombin (Floseal®) into the tract whenever persistent bleeding was observed after omitting the nephrostomy catheter [32]. Okeke et al. explored cryoablation of the nephrostomy tract after tubeless PCNL, where they inserted a cryoprobe into the access tract and performed a 10-min freeze-thaw cycle at a temperature -20 °C. This method did not significantly affect the rate of delayed bleeding or urinary extravasation [33]. Recently, a randomized study by Cormio et al. showed that TachoSil® provided better tract control and a shorter hospital stay than nephrostomy tube placement, although it did not reduce pain or analgesic requirements [34].
Total tubeless PCNL is advocated by leading surgeons in the field of endourology. The future role of tubed PCNL will likely reside primarily in cases of severe intraoperative bleeding or major damage to the collecting system, and when there is the possibility of a second-look operation. However, some controversies remain about the feasibility and efficacy of tubeless PCNLs in certain clinical settings. In their prospective randomized study, Shoma et al. suggested that the tubeless approach might not be suitable for patients with chronic kidney disease or those who require a supracostal approach [30]. However, Shah et al. reported the successful use of a tubeless technique in a patient with chronic kidney disease. Likewise, Sofikerim et al. reported that tubeless PCNL is a safe and effective technique, even for supracostal access, and is associated with less postoperative pain and shorter hospital stay [35]. Resorlu et al. maintained that single or no nephrostomy drainage following multitract PCNL offered the potential advantages of decreased postoperative analgesic requirements and shorter hospital stay, without increasing the rate of complications [36].
A limitation of our study was that we did not perform subgroup analyses based on the size of the stone. We also did not compare success rates because the success rates were high in each study. In addition, there was some degree of publication bias. However, in the review of 48 articles from the Cochrane Database of Systematic Reviews performed by Sutton et al., publication or related biases were noted to be common within the sample of assessed meta-analyses, but did not affect the conclusions in most cases [37]. Additionally, the position of the patient during PCNL (prone or supine position) can influence the outcomes of a tubeless or not tubeless procedure. Anesthesiologists prefer the supine position because of better airway control during procedures. Another advantage of the supine position is that there is no need for position changes when performing additional endoscopic procedures, such as cystoscopic or ureteroscopic operations [38]. Endoscopic combined intrarenal surgery is also a novel way of performing PCNL in the supine position [39]. Better visualization with the procedure allows for correct puncture of the kidney, and thus, can improve the safety and feasibility of a tubeless or total tubeless procedure.
Despite these limitations and shortcomings, our study has the substantial advantage of including larger samples from each study than the previously conducted pairwise meta-analyses [40]. Moreover, this is the first study to use network meta-analysis to compare PCNL methods, which enhances the statistical confidence and overcomes the limitations of pairwise meta-analyses.
Conclusions
In comparing each procedure through network meta-analysis, total tubeless and small-bore PCNLs were superior in terms of hemoglobin change, and total tubeless and tubeless PCNLs were superior with regard to the length of hospital stay. These findings indicate that conventional PCNL can be replaced with other techniques, especially total tubeless PCNL, in selected patients.
Acknowledgments
Funding
This study was supported by a faculty research grant from the Yonsei University College of Medicine for 2014 (6-2014-0156).
Availability of data and materials
All the data supporting our findings is contained within the manuscript.
Abbreviations
- PCNL
Percutaneous nephrolithotomy
- RCT
Randomized controlled trial
- VAS
Visual analogue scale
Authors’ contributions
Systematic review and meta-analysis JYL, SUJ, MDK, DHK, JKK, WSH, YDC, KSC. Identification of studies, critical evaluation and discussion. JYL, KSC, DHK, WSH, YDC. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was exempted by the institutional review boards because of meta-analysis.
Consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joo Yong Lee, Email: joouro@yuhs.ac.
Seong Uk Jeh, Email: judies99@gmail.com.
Man Deuk Kim, Email: mdkim@yuhs.ac.
Dong Hyuk Kang, Email: dhkang0424@inhauh.com.
Jong Kyou Kwon, Email: jkstorm@yuhs.ac.
Won Sik Ham, Email: uroham@yuhs.ac.
Young Deuk Choi, Email: youngd74@yuhs.ac.
Kang Su Cho, Phone: +82-02-2019-3471, Email: kscho99@yuhs.ac.
References
- 1.Teichman JM. Clinical practice. Acute renal colic from ureteral calculus. N Engl J Med. 2004;350:684–693. doi: 10.1056/NEJMcp030813. [DOI] [PubMed] [Google Scholar]
- 2.Lee JW, Park J, Lee SB, Son H, Cho SY, Jeong H. Mini-percutaneous Nephrolithotomy vs Retrograde Intrarenal Surgery for Renal Stones Larger Than 10 mm: A Prospective Randomized Controlled Trial. Urology. 2015;86:873–877. doi: 10.1016/j.urology.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Sivalingam S, Al-Essawi T, Hosking D. Percutaneous nephrolithotomy with retrograde nephrostomy access: a forgotten technique revisited. J Urol. 2013;189:1753–1756. doi: 10.1016/j.juro.2012.11.169. [DOI] [PubMed] [Google Scholar]
- 4.Jung GH, Jung JH, Ahn TS, Lee JS, Cho SY, Jeong CW, et al. Comparison of retrograde intrarenal surgery versus a single-session percutaneous nephrolithotomy for lower-pole stones with a diameter of 15 to 30 mm: A propensity score-matching study. Korean J Urol. 2015;56:525–532. doi: 10.4111/kju.2015.56.7.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Istanbulluoglu MO, Cicek T, Ozturk B, Gonen M, Ozkardes H. Percutaneous nephrolithotomy: nephrostomy or tubeless or totally tubeless? Urology. 2010;75:1043–1046. doi: 10.1016/j.urology.2009.06.104. [DOI] [PubMed] [Google Scholar]
- 6.Paul EM, Marcovich R, Lee BR, Smith AD. Choosing the ideal nephrostomy tube. BJU Int. 2003;92:672–677. doi: 10.1046/j.1464-410X.2003.04454.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah HN, Kausik VB, Hegde SS, Shah JN, Bansal MB. Tubeless percutaneous nephrolithotomy: a prospective feasibility study and review of previous reports. BJU Int. 2005;96:879–883. doi: 10.1111/j.1464-410X.2005.05730.x. [DOI] [PubMed] [Google Scholar]
- 8.Akman T, Binbay M, Yuruk E, Sari E, Seyrek M, Kaba M, et al. Tubeless Procedure is Most Important Factor in Reducing Length of Hospitalization After Percutaneous Nephrolithotomy: Results of Univariable and Multivariable Models. Urology. 2011;77:299–304. doi: 10.1016/j.urology.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Zhang Z, Li H, Xing Y, Zhang G, Kong X. Ultrasonography-guided percutaneous nephrolithotomy for the treatment of urolithiasis in patients with scoliosis. Int Surg. 2012;97:182–188. doi: 10.9738/CC93.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ. 2013;346:f2914. doi: 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 12.Yuan J, Zhang R, Yang Z, Lee J, Liu Y, Tian J, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63:902–912. doi: 10.1016/j.eururo.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Kwon JK, Cho KS, Oh CK, Kang DH, Lee H, Ham WS, et al. The beneficial effect of alpha-blockers for ureteral stent-related discomfort: systematic review and network meta-analysis for alfuzosin versus tamsulosin versus placebo. BMC Urol. 2015;15:55. doi: 10.1186/s12894-015-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JY, Cho KS, Kang DH, Jung HD, Kwon JK, Oh CK, et al. A network meta-analysis of therapeutic outcomes after new image technology-assisted transurethral resection for non-muscle invasive bladder cancer: 5-aminolaevulinic acid fluorescence vs hexylaminolevulinate fluorescence vs narrow band imaging. BMC Cancer. 2015;15:566. doi: 10.1186/s12885-015-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung JH, Lee SW. Assessing the quality of randomized controlled urological trials conducted by korean medical institutions. Korean J Urol. 2013;54:289–296. doi: 10.4111/kju.2013.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Development Core Team . R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 18.Shah HN, Sodha HS, Khandkar AA, Kharodawala S, Hegde SS, Bansal MB. A randomized trial evaluating type of nephrostomy drainage after percutaneous nephrolithotomy: small bore v tubeless. J Endourol. 2008;22:1433–1439. doi: 10.1089/end.2007.0350. [DOI] [PubMed] [Google Scholar]
- 19.Bellman GC, Davidoff R, Candela J, Gerspach J, Kurtz S, Stout L. Tubeless percutaneous renal surgery. J Urol. 1997;157:1578–1582. doi: 10.1016/S0022-5347(01)64799-2. [DOI] [PubMed] [Google Scholar]
- 20.Kim SC, Tinmouth WW, Kuo RL, Paterson RF, Lingeman JE. Using and choosing a nephrostomy tube after percutaneous nephrolithotomy for large or complex stone disease: a treatment strategy. J Endourol. 2005;19:348–352. doi: 10.1089/end.2005.19.348. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Q, Zheng C, Mo J, Piao Y, Zhou Y, Jiang Q. Total tubeless versus standard percutaneous nephrolithotomy: a meta-analysis. J Endourol. 2013;27:420–426. doi: 10.1089/end.2012.0421. [DOI] [PubMed] [Google Scholar]
- 22.Yuan H, Zheng S, Liu L, Han P, Wang J, Wei Q. The efficacy and safety of tubeless percutaneous nephrolithotomy: a systematic review and meta-analysis. Urol Res. 2011;39:401–410. doi: 10.1007/s00240-010-0355-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Zhao C, Zhang C, Fan X, Lin Y, Jiang Q. Tubeless vs standard percutaneous nephrolithotomy: a meta-analysis. BJU Int. 2012;109:918–924. doi: 10.1111/j.1464-410X.2011.10463.x. [DOI] [PubMed] [Google Scholar]
- 24.Shen P, Liu Y, Wang J. Nephrostomy tube-free versus nephrostomy tube for renal drainage after percutaneous nephrolithotomy: a systematic review and meta-analysis. Urol Int. 2012;88:298–306. doi: 10.1159/000332151. [DOI] [PubMed] [Google Scholar]
- 25.Borges CF, Fregonesi A, Silva DC, Sasse AD. Systematic Review and Meta-Analysis of Nephrostomy Placement Versus Tubeless Percutaneous Nephrolithotomy. J Endourol. 2010;24:1739–46. [DOI] [PubMed]
- 26.Michel MS, Trojan L, Rassweiler JJ. Complications in percutaneous nephrolithotomy. Eur Urol. 2007;51:899–906. doi: 10.1016/j.eururo.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Lee JK, Kim BS, Park YK. Predictive factors for bleeding during percutaneous nephrolithotomy. Korean J Urol. 2013;54:448–453. doi: 10.4111/kju.2013.54.7.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrow PK, Auge BK, Lallas CD, Santa-Cruz RW, Newman GE, Albala DM, et al. Pain after percutaneous nephrolithotomy: impact of nephrostomy tube size. J Endourol. 2003;17:411–414. doi: 10.1089/089277903767923218. [DOI] [PubMed] [Google Scholar]
- 29.Damiano R, Oliva A, Esposito C, De Sio M, Autorino R, D'Armiento M. Early and late complications of double pigtail ureteral stent. Urol Int. 2002;69:136–140. doi: 10.1159/000065563. [DOI] [PubMed] [Google Scholar]
- 30.Shoma AM, Elshal AM. Nephrostomy tube placement after percutaneous nephrolithotomy: critical evaluation through a prospective randomized study. Urology. 2012;79:771–776. doi: 10.1016/j.urology.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Mikhail AA, Kaptein JS, Bellman GC. Use of fibrin glue in percutaneous nephrolithotomy. Urology. 2003;61:910–914. doi: 10.1016/S0090-4295(03)00112-2. [DOI] [PubMed] [Google Scholar]
- 32.Choi M, Brusky J, Weaver J, Amantia M, Bellman GC. Randomized trial comparing modified tubeless percutaneous nephrolithotomy with tailed stent with percutaneous nephrostomy with small-bore tube. J Endourol. 2006;20:766–770. doi: 10.1089/end.2006.20.766. [DOI] [PubMed] [Google Scholar]
- 33.Okeke Z, Lee BR. Small renal masses: the case for cryoablation. J Endourol. 2008;22:1921–1923. doi: 10.1089/end.2008.9776. [DOI] [PubMed] [Google Scholar]
- 34.Cormio L, Perrone A, Di Fino G, Ruocco N, De Siati M, de la Rosette J, et al. TachoSil((R)) sealed tubeless percutaneous nephrolithotomy to reduce urine leakage and bleeding: outcome of a randomized controlled study. J Urol. 2012;188:145–150. doi: 10.1016/j.juro.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Sofikerim M, Demirci D, Huri E, Ersekerci E, Karacagil M. Tubeless percutaneous nephrolithotomy: safe even in supracostal access. J Endourol. 2007;21:967–972. doi: 10.1089/end.2006.0216. [DOI] [PubMed] [Google Scholar]
- 36.Resorlu B, Kara C, Sahin E, Unsal A. Comparison of nephrostomy drainage types following percutaneous nephrolithotomy requiring multiple tracts: single tube versus multiple tubes versus tubeless. Urol Int. 2011;87:23–27. doi: 10.1159/000324264. [DOI] [PubMed] [Google Scholar]
- 37.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–1577. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung DY, Lee JY, Kim KH, Choi JH, Cho KS. Feasibility and efficacy of intermediate-supine percutaneous nephrolithotomy: initial experience. Chonnam Med J. 2014;50:52–57. doi: 10.4068/cmj.2014.50.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cracco CM, Scoffone CM. ECIRS (Endoscopic Combined Intrarenal Surgery) in the Galdakao-modified supine Valdivia position: a new life for percutaneous surgery? World J Urol. 2011;29:821–827. doi: 10.1007/s00345-011-0790-0. [DOI] [PubMed] [Google Scholar]
- 40.Li K, Lin T, Zhang C, Fan X, Xu K, Bi L, et al. Optimal frequency of shock wave lithotripsy in urolithiasis treatment: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2013;190:1260–1267. doi: 10.1016/j.juro.2013.03.075. [DOI] [PubMed] [Google Scholar]
- 41.Chang CH, Wang CJ, Huang SW. Totally tubeless percutaneous nephrolithotomy: a prospective randomized controlled study. Urol Res. 2011;39:459–465. doi: 10.1007/s00240-011-0363-0. [DOI] [PubMed] [Google Scholar]
- 42.Aghamir SM, Modaresi SS, Aloosh M, Tajik A. Totally tubeless percutaneous nephrolithotomy for upper pole renal stone using subcostal access. J Endourol. 2011;25:583–586. doi: 10.1089/end.2010.0064. [DOI] [PubMed] [Google Scholar]
- 43.Kara C, Resorlu B, Bayindir M, Unsal A. A randomized comparison of totally tubeless and standard percutaneous nephrolithotomy in elderly patients. Urology. 2010;76:289–293. doi: 10.1016/j.urology.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 44.Mishra S, Sabnis RB, Kurien A, Ganpule A, Muthu V, Desai M. Questioning the wisdom of tubeless percutaneous nephrolithotomy (PCNL): a prospective randomized controlled study of early tube removal vs tubeless PCNL. BJU Int. 2010;106:1045–1048. doi: 10.1111/j.1464-410X.2010.09223.x. [DOI] [PubMed] [Google Scholar]
- 45.Istanbulluoglu MO, Ozturk B, Gonen M, Cicek T, Ozkardes H. Effectiveness of totally tubeless percutaneous nephrolithotomy in selected patients: a prospective randomized study. Int Urol Nephrol. 2009;41:541–545. doi: 10.1007/s11255-008-9517-6. [DOI] [PubMed] [Google Scholar]
- 46.Crook TJ, Lockyer CR, Keoghane SR, Walmsley BH. A randomized controlled trial of nephrostomy placement versus tubeless percutaneous nephrolithotomy. J Urol. 2008;180:612–614. doi: 10.1016/j.juro.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal MS, Agrawal M, Gupta A, Bansal S, Yadav A, Goyal J. A randomized comparison of tubeless and standard percutaneous nephrolithotomy. J Endourol. 2008;22:439–442. doi: 10.1089/end.2007.0118. [DOI] [PubMed] [Google Scholar]
- 48.Singh I, Singh A, Mittal G. Tubeless percutaneous nephrolithotomy: is it really less morbid? J Endourol. 2008;22:427–434. doi: 10.1089/end.2007.0269. [DOI] [PubMed] [Google Scholar]
- 49.Tefekli A, Altunrende F, Tepeler K, Tas A, Aydin S, Muslumanoglu AY. Tubeless percutaneous nephrolithotomy in selected patients: a prospective randomized comparison. Int Urol Nephrol. 2007;39:57–63. doi: 10.1007/s11255-006-9040-6. [DOI] [PubMed] [Google Scholar]
- 50.Weiland D, Pedro RN, Anderson JK, Best SL, Lee C, Hendlin K, et al. Randomized prospective evaluation of nephrostomy tube configuration: impact on postoperative pain. Int Braz J Urol. 2007;33:313–318. doi: 10.1590/S1677-55382007000300003. [DOI] [PubMed] [Google Scholar]
- 51.Desai MR, Kukreja RA, Desai MM, Mhaskar SS, Wani KA, Patel SH, et al. A prospective randomized comparison of type of nephrostomy drainage following percutaneous nephrostolithotomy: large bore versus small bore versus tubeless. J Urol. 2004;172:565–567. doi: 10.1097/01.ju.0000130752.97414.c8. [DOI] [PubMed] [Google Scholar]
- 52.Marcovich R, Jacobson AI, Singh J, Shah D, El-Hakim A, Lee BR, et al. No panacea for drainage after percutaneous nephrolithotomy. J Endourol. 2004;18:743–747. doi: 10.1089/end.2004.18.743. [DOI] [PubMed] [Google Scholar]
- 53.Feng MI, Tamaddon K, Mikhail A, Kaptein JS, Bellman GC. Prospective randomized study of various techniques of percutaneous nephrolithotomy. Urology. 2001;58:345–350. doi: 10.1016/S0090-4295(01)01225-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting our findings is contained within the manuscript.


