Abstract
Significance: Breast cancer is the second leading cause of cancer-related deaths among women in the United States. Development and progression of malignancy are associated with diverse cell signaling pathways that control cell proliferation, survival, motility, invasion, and metastasis.
Recent Advances: An increasing number of clinical studies have implicated a strong relationship between elevated tumor nitric oxide synthase-2 (NOS2) expression and poor patient survival.
Critical Issues: Herein, we review what we believe to be key mechanisms in the role(s) of NOS2-derived nitric oxide (NO) as a driver of breast cancer disease progression. High NO increases cyclooxygenase-2 activity, hypoxia inducible factor-1 alpha protein stabilization, and activation of important cell signaling pathways, including phosphoinositide 3-kinase/protein kinase B, mitogen-activated protein kinase, epidermal growth factor receptor, and Ras, through post-translational protein modifications. Moreover, dysregulated NO flux within the tumor microenvironment has other important roles, including the promotion of angiogenesis and modulation of matrix metalloproteinase/tissue inhibitor matrix metalloproteinase associated with tumor progression.
Future Directions: The elucidation of these and other NO-driven pathways implicates NOS2 as a key driver of breast cancer disease progression and provides a new perspective in the identification of novel targets that may be therapeutically beneficial in the treatment of estrogen receptor-negative disease. Antioxid. Redox Signal. 26, 1044–1058.
Keywords: : nitric oxide, NOS2, cancer progression, metastasis, biomarker
Introduction
Breast cancer is a heterogeneous disease defined by distinct tumor phenotypes that vary in prognosis and therapeutic response and is the second leading cause of cancer-related deaths among women in the United States (26). While disease management has improved prognosis and quality of life, 16% of women with regional lesions and 76% of women with metastatic lesions continue to succumb to disease within 5 years of diagnosis.
Clinical management distinguishes disease subtypes according to estrogen (ER) and progesterone (PR) hormone receptor status, as well as human epidermal growth factor receptor-2 (HER2) status. ER status is defined by the presence (ER+) or absence (ER−) of the alpha form of the receptor. Approximately 70% of breast cancer patients are diagnosed with having ER+ status, while 30% present with the more aggressive ER− subtype. ER+ tumors can be successfully treated with hormone-based therapies, including antiestrogens and aromatase inhibitors, while patients with triple-negative (ER−/PR−/HER2−) breast cancer (TNBC) have fewer options. Toward this end, the identification of novel molecular targets can improve therapeutic response and survival in TNBC patients.
Nitric oxide (NO) is released intracellularly during the oxidation of l-arginine by nitric oxide synthase (NOS) enzymes and has numerous physiologic and pathologic roles (117). Three isoforms of NOS have been identified; neuronal (NOS1) and endothelial (NOS3) are constitutive Ca+-dependent forms of the enzyme that are regulated by negative feedback mechanisms and release low-flux NO over a short period to regulate neural and vascular function, respectively (35), and the Ca+-independent inducible (nitric oxide synthase-2 [NOS2]) form can produce higher levels of NO, depending upon the stimulant, and is known classically as a mediator of immune surveillance (32, 35, 87).
Recently, NOS2 was identified as a biomarker of breast cancer disease progression and patient survival (16, 36, 44, 75). Moreover, NOS2-derived NO can alter the redox state of cells, induce DNA, lipid, and protein modifications, promote an immunosuppressive microenvironment, and mediate angiogenesis and wound response, which are all key events in cancer disease progression (1). Previous work from our laboratory has elucidated mechanisms of feed-forward tumor NOS2 regulation by components of the tumor microenvironment, including nutrient deprivation, inflammatory cytokines, and hypoxia (44, 76). In this review, we will discuss our current understanding of NOS2-derived NO mechanisms associated with breast cancer disease progression as well as therapeutic implications.
NOS2 and P53 Mutation
The activation of p53 is a critical component of cell cycle arrest, DNA repair, senescence, and apoptosis (69, 70). While P53 is not required for cell viability, the loss of its functions allows the accumulation of genetically damaged cells, which precedes the development of neoplastic lesions (69). Toward this end, mutations in p53 are among the most common changes found in human cancers (69, 70). The more aggressive TNBC accounts for a high percentage of death among breast cancer patients and p53 mutations are reported in 60–80% of these cases (149). Our recent breast cancer study found a significant correlation between increased p53 mutation and high tumor NOS2 expression (odds ratio [OR] 3.02; 95% confidence interval 1.19–7.66; p-value 0.020) (36).
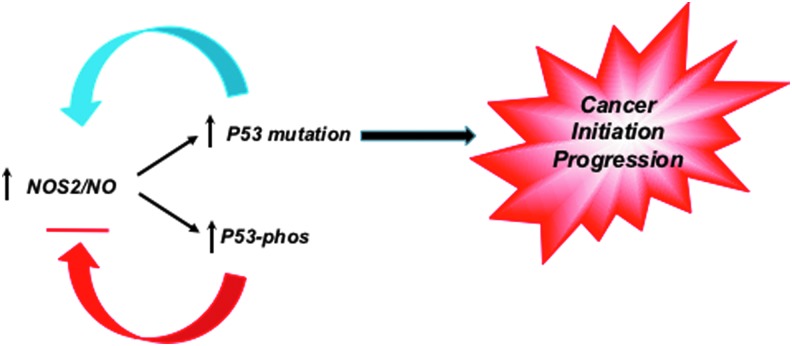
Nitric oxide is a key bioactive modulator of several processes, including angiogenesis and host defense, which are dysregulated in cancer (52). Given that p53 is a transrepressor of NOS2 gene expression, these observations suggest that the loss of this negative feedback loop may provide selection pressure for tumor initiation and progression (Fig. 1) (2, 3, 34). Inflammatory factors and components of the tumor microenvironment increase NOS2 expression, which include hypoxia, nutrient deprivation, prostaglandin E2 (PGE2), interleukin-6 (IL-6), and interferon gamma (44, 129, 144). PGE2 can increase NOS2, while IL-6 induction of signal transducer and activator of transcription 3 leads to further activation of NOS2 (129, 144). These pathways conspire to form protumorigenic, feed-forward autocrine loops leading to increased metastasis (44). Furthermore, numerous factors from immune cells and stroma lead to acceleration of these loops, suggesting that NOS2 feed-forward signaling can perpetuate these mutation pathways (60, 81).
FIG. 1.
NOS2/NO increases P53 mutation and loss of negative feedback regulation, which may provide selection pressure for cancer initiation and progression. NO, nitric oxide; NOS2, nitric oxide synthase-2. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Tumor Hypoxia
Hypoxia is a common characteristic of the tumor microenvironment that drives disease progression and is associated with oxygen deficit in avascular tumors leading to metastasis (48). Multiple studies have identified associations between reduced intratumoral pO2 and decreased disease-free survival in cancer patients (153). Uncontrolled tumor cell proliferation leads to nutrient depletion and hypoxia, which are also major contributors of chronic inflammation within the tumor microenvironment. Hypoxic/necrotic regions within the tumor induce proinflammatory immune mediators that culminate in a local immunosuppressive microenvironment, which induces angiogenesis, tumor cell proliferation, migration, and invasion (79, 91). These events are mediated by hypoxia-inducible factor-1 alpha (HIF-1α) adaptive signaling that promotes chemoresistance, metastasis, and poor patient survival (5, 21, 77).
HIF-1α can be upregulated by other factors, including insulin, insulin-like growth factor (IGF-1 or IGF-2), v-src proto-oncogene, nonreceptor tyrosine kinase (Src), lactate, pyruvate, and tumor inflammation, as well as genetic alterations, including activation of oncogenes or inactivation of tumor suppressor genes (5). In addition, under normoxia, NO mediates HIF-1α protein stabilization through nitrosative mechanisms that block its proteasomal degradation (14, 72, 82, 139). NOS2 has emerged as a biomarker of poor survival in patients with aggressive tumors, suggesting that nitrosative/nitrosylative mechanisms that promote HIF-1α protein stability may be important in protumorigenic signaling associated with high NOS2 tumors (139).
Another impact of altered oxygen gradient and tumor hypoxia involves the generation of reactive oxygen species (ROS) and altered redox status. In addition to ROS, redox status is influenced by other small reactive molecules, including NO, and other nitrogen oxides, as well as the eicosanoids (i.e., cyclooxygenase-2 [COX2] and lipoxygenase) (140). Molecules, including carbon monoxide (CO) derived from heme oxygenase and hydrogen sulfide (H2S), a product of thiol metabolism, are components of redox inflammation. These molecules whose metabolism is either directly related to O2 or arise in response play critical roles in oxidative stress. Toward this end, O2 tension is a major determinant of NOS2-derived NO and downstream signaling because in addition to arginine, O2 is also a substrate of NOS.
The O2 availability within a tissue bed is a function of the rate of arterial delivery versus that of mitochondrial O2 consumption. Michaelis-Menten enzyme kinetics is a mathematical model that predicts the amount of product formed upon the binding interaction of an enzyme with its substrate (83). The equation employs the Michaelis constant (KM) substrate concentration where the reaction rate is at half-maximal and is an inverse measure of the substrates’ affinity to the enzyme (83). Based upon the NOS2 KM for O2 (135 μM), the work of Hickok et al. has shown a requirement of 3–5% O2 for maximum NO flux derived from the NOS2 enzyme (46). Moreover, NO consumption is also O2 dependent; therefore, steady-state NO flux and downstream signaling depend on the relative rates of these variables (46).
Chronic Inflammation, NOS2, and the Tumor Microenvironment
Current statistics estimate that chronic inflammation associated with inflammatory diseases contributes to a 25% increased risk of cancer occurrence (6, 80). Beyond the increased risk of occurrence, chronic inflammation within the tumor microenvironment has long been proposed as a contributing factor in tumor promotion and disease progression (6, 101, 113). These findings are supported, in part, by observations that modest intake of nonsteroidal anti-inflammatory drugs (NSAIDs) reduces cancer growth and recurrence (12, 23, 121, 122). The inflammatory composition of the tumor microenvironment has long been compared with nonhealing wounds (6). Dvorak observed a striking resemblance between the tumor stroma and tissue granulation of healing wounds, which implicated a role of host wound response in the formation of tumor stroma and disease progression (28).
Cancer inflammation involves the subtle coordination between tumor cells, activated stromal cells, including endothelial cells, fibroblasts, stem cells, and immune cell mediators. Together, this network provides an immunosuppressive environment rich in growth factors and cytokines that promote uncontrolled, sustained tumor cell proliferation and survival with proangiogenic and metastatic capabilities (40, 41). Importantly, this process is not self-limiting in the tumor microenvironment, thus implicating a key role of the presence of unresolved chronic inflammation in the promotion of metastatic disease progression and therapeutic resistance.
Cancerous tissue overexpresses COX2, NOS2, and ROS, which are associated with disease progression and reduced patient survival. In lung and gastric cancer, COX2 inhibition has positive therapeutic effects, while elevated COX2 expression is a characteristic of aggressive tumors (8, 37, 121, 122). Similarly, nicotinamide adenine dinucleotide phosphate oxidase and Duox expression in ovarian and pancreatic cancer drives mechanisms associated with disease progression (123). CO produced from heme oxygenase-1 (HO-1) has been shown to suppress T-cell proliferation by inhibiting IL-2 production (100). Recently, the involvement of the H2S/persulfide-producing enzymes, cystathionine β-synthase and cystathionine γ-lyase, in disease progression of colon cancer was shown (18, 137). These and other reports demonstrate the utility of the redox inflammation profile for elucidation of pathways that drive cancer progression, which can be therapeutically exploited.
While many cancer studies have focused on COX2 and ROS, NOS2 has recently emerged as a predictive biomarker in many solid tumors. Several reports have correlated high tumor NOS2 expression with reduced patient survival (29, 30, 33, 36, 65, 73, 75, 125, 166). Traditionally, NOS2 has been associated with immune activation (78, 158). NOS2 has an important role in murine biology and murine leukocytes can produce high concentrations of NO (upto 0.15 μM for 4 h) for prolonged periods of time (32). However, human NOS2 does not seem to have the same role and is expressed in a surprising number of epithelial cells (1).
An earlier study revealed moderate to high NOS2 expression within tumor epithelium in 73% of all patients with breast cancer regardless of ER status (36, 108). The same study identified a positive correlation between tumor NOS2 expression and protein kinase B (Akt) pathway activation, suggesting a mechanistic link with prosurvival signaling within the tumor (108). Exposure of breast cancer cells to NO donors further supported NO activation of Akt (106, 108). This study also revealed a positive association between NOS2 and p53 mutation frequency (36). Moreover, both NOS2 and COX2 predicted poor breast cancer survival in ER−, but not ER+, patients (36, 37). Together, these studies implicate a key role for NOS2 in breast cancer disease progression, which is supported by the finding that 92% of deceased patients in this cohort exhibited elevated tumor NOS2 expression (36).
Importantly, only 4 of 247 patients presented with lymph node-positive disease, which suggests that elevated tumor NOS2 expression may predict clinically undetected metastasis. Other studies have shown upregulation of NOS3 and NOS2 by mutated myeloid leukemia factor 2 (MLF2) and ribosomal protein L39 (RPL39), which correlated with poor disease-specific survival in patients with ER– breast cancer (22, 24, 38).
The mechanistic role of NOS2 in cancer progression has been examined using cell culture models exposed to NONOate donor agents. NONOates, or diazenium diolates, release NO in a defined manner at neutral pH and provide powerful tools for controlling the flux of NO in biological experiments (59, 142). The variety of different structures having pH- and time-dependent rates of NO release allows the generation of specific flux profiles, which can be compared with NOS2-derived NO.
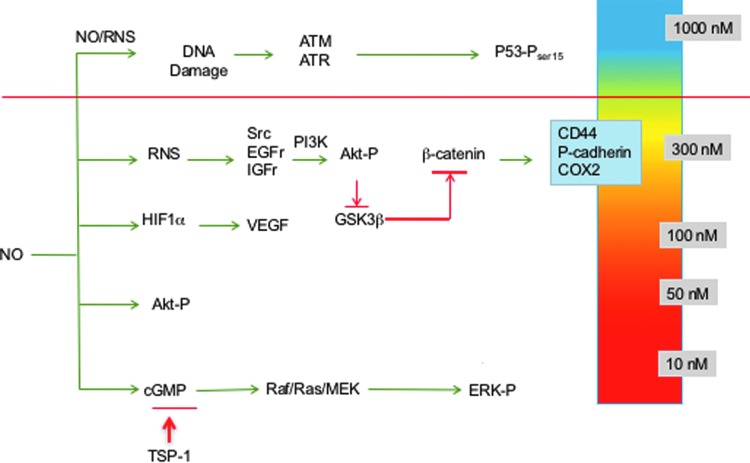
Using this strategy, NO flux-dependent activation of specific signaling cascades has been identified in breast cancer cells (36, 108, 112, 134, 135, 139). The concentration- and temporally dependent NO activation of extracellular signal-regulated kinase (ERK) and Akt, as well as HIF-1α stabilization, occurs at levels ranging from 200 to 500 nM steady-state NO, while phosphorylation of p53 occurs at higher levels of 700–800 nM NO (139). Inhibition of enzymes involved in DNA repair also occurred at higher NO flux (71), while modification of transforming growth factor-beta (TGF-β) (154) and matrix metalloproteinase (MMP) (11, 119) occurred at lower levels. Moreover, low NO donor concentrations that produce pM NO flux mediate cyclic guanosine monophosphate (cGMP)-dependent downregulation of the antiangiogenic molecule, thrombospondin-1 (50, 116).
These NO concentrations and downstream signaling effects can be achieved using activated murine macrophages as well as NONOate donors (108, 135, 139). Thus, NONOate donors can be effectively and reproducibly employed to examine NO mechanisms in cancer (139).
Three distinct NO flux ranges define NO-mediated signaling; low NO <100 nM cGMP-dependent signaling, higher levels ranging from 200 to 600 nM NO involve nitrosative signaling that is cGMP independent, and >600 nM generally involves stress response as well as antiproliferation mechanisms (Fig. 2) (46). Observations of elevated tumor NOS2 expression in breast cancer patients suggest that targeted pathway activations can affect patient outcome (16, 36, 75, 108). The role of NO in promoting or inhibiting cancer progression has been controversial. However, clarification of various roles of NO can arise by discussing phenotypes in the context of these different levels of NO and the respective signaling effects. We will begin with a discussion of the role of NOS2-derived NO in poor outcome of ER− breast cancer and the range of NO flux that upregulates predictive biomarkers identified in high NOS2-expressing breast tumors (36). In addition, the influence of cGMP-dependent processes will be discussed as well as higher levels of NO that affect p53-dependent signaling and other cell growth inhibitory pathways.
FIG. 2.
Steady-state NO-mediated signaling. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
ER− Breast Cancer, NOS2, and Nitrosative Signaling
Elevated tumor NOS2 expression predicts poor outcome in ER− breast cancer patients (36). In addition, high NOS2 tumors exhibited elevated expression of predictive basal-like and stem cell biomarkers, including P-cadherin, IL-8, and cluster of differentiation 44; hyaluronic acid receptor (CD44) (36). Protein levels of these biomarkers were significantly enhanced in ER− breast cancer cells exposed to the NO donor Diethylenetriamine NONOate (DETA/NO) (∼600 nM steady-state NO flux), which further supports a mechanistic role for NO as a driver of breast cancer disease progression (36). Importantly, phenotypic and predictive biomarker analyses of patient tumors combined with the assessment of NO-induced protein regulation of these predictive biomarkers in breast cancer cells provide an invaluable tool for estimating the steady-state NO flux generated in high NOS2 tumors.
Pathway activation of phosphoinositide 3-kinase (PI3k)/Akt, mitogen-activated protein kinase (MAPK), epidermal growth factor receptor (EGFR), and protein c-ets-1 (Ets-1) signaling cascades promotes breast cancer disease progression and they are targets of NO (36, 108, 112, 134, 135, 139). Earlier findings of Prueitt et al. identified a strong correlation between tumor NOS2 expression and phosphorylated Akt, which suggested the increased occurrence of Akt pathway activation in high NOS2-expressing breast tumors (108). This report was further supported by observations of NO-induced Akt phosphorylation in breast cancer cells treated with NO donors (106, 108, 112, 135).
Similarly, a strong correlation between EGFR tyrosine phosphorylation and elevated tumor NOS2 expression was also identified in breast tumors as well as NO-induced EGFR phosphorylation in cells grown in culture (36). A subsequent study demonstrated nitrosation of EGFR, which mediated ligand-independent activation of its kinase receptor (86, 105). Earlier studies have also identified S-nitrosylation as a mediator of EGFR and Src activation (86, 105, 109). Moreover, high-flux NO decreased kinase activity of these membrane proteins, indicating concentration-dependent biphasic effects of NO (86), which was supported by Switzer et al. who showed that peak activation of these signaling pathways occurred at ∼400 nM steady-state NO (135). This intermediate level of NO also upregulates COX2 expression in breast cancer cells (135). Similarly, COX2 correlates with Akt pathway activation and predicts poor outcome in breast cancer patients (37).
Nitrosative signaling is mediated by reactive nitrogen species (RNS) such as N2O3 (118). Further analysis of nitrosative EGFR and Src activation implicated the requirement of a nitrosative species such as N2O3 (135). Addition of superoxide dismutase (SOD) to scavenge O2− and prevent peroxynitrite formation actually increased Akt phosphorylation (unpublished results). Moreover, Thomas et al. demonstrated an antagonistic relationship between O2− and NO during nitrosative signaling in breast cancer cells (141). Thus, NO and O2− are mutual antagonists of their respective signaling pathways, which may be exploited by tumor cells for maintenance of optimal redox signaling conditions that promote their survival and growth.
Interestingly, during inflammatory response, ROS, NO, and even CO seem to be temporally distinct. There are additional mechanisms of NO beyond nitrosation involving N2O3; however, antioxidants and inhibitors of nitrosation such as azide, reduced glutathione, urate, and ascorbate abate NO signaling (135). While we have long proposed that NO/O2− can lead to N2O3 and other RNS, in our hands, the addition of SOD enhanced Spermine NONOate (SPER/NO)-induced cGMP output by 10-fold in MCF-7 cells (141). Importantly, the titration of SPER/NO in the presence of hypoxanthine/xanthine oxidase did not mediate cytotoxicity, suggesting that ROS such as superoxide controls NO bioavailability (141).
NO signaling influences nonheme metal chemistry, including HIF-1α stabilization and prolyl hydroxylase activity. Moreover, NO signaling through PI3K and RAS appears to involve a nitrosating species such as N2O3. These and other observations suggest that targeting specific redox species may be therapeutically beneficial.
Prosurvival signaling mediated by MAPK is also involved in cancer progression. Pathway activation of ERK initiates many pathways associated with cancer such as c-Myc and activator protein-1. RAS and raf-1 proto-oncogene serine/threonine protein kinase (RAF-1) are key mediators upstream of ERK. Toward this end, cGMP-dependent signaling through RAF-1 increased ERK phosphorylation in MCF-7 breast cancer cells (106). Moreover, RAS inhibitors prevented ERK-dependent Ets-1 activation in NO-treated MB-231 and MB-468 cells (134). Many mutations are found in HRAS and KRAS that are thought to be important drivers in cancer. However, NO activates p21ras through S-nitrosation of a key cysteine residue (67). In addition, S-NO and sulfonic acid post-translational modifications have been shown, which regulate enzymatic activities (84). Ets activation can also occur through B-Raf and Raf-1, cAMP, or via PGE2 (51, 155). Thus, ERK pathway activation can go through several routes that circumvent direct inhibition of RAF.
NO and cGMP Signaling
The guanylyl cyclase (GC) enzymes catalyze the conversion of guanosine triphosphate to the second messenger cGMP. The soluble (sGC) and particulate isoforms are ligand activated by NO and hormones/natriuretic peptides, respectively (160). Downstream cGMP effectors include cyclic nucleotide-gated ion channels and cGMP-dependent protein kinases, as well as phosphodiesterase (PDE) enzymes, which promote cGMP degradation to control its intracellular levels. In addition, multidrug-resistant proteins (MRP4, MRP5, and MRP8) also regulate intracellular cGMP levels (54, 61, 120, 159). Early studies have implicated aberrant cGMP regulation in breast and other cancers where low cGMP levels were identified in neoplastic tissue when compared with normal tissue regions. These observations coincided with increased expression and altered compartmentalization of MRPs and PDE enzymes, which correlated with tumor grade, stage, and lymph node metastasis (47, 58, 85, 92, 132).
Mechanistically, cGMP-mediated activation of protein kinase G (PKG) leads to the phosphorylation and subsequent degradation of the oncogenic transactivator β-catenin, which culminates in the downregulation of growth-promoting and apoptosis-inhibiting proteins, including cyclin D1, c-myc, and survivin (143, 145, 146). Toward this end, PDE inhibition by sulindac sulfide elevated cGMP levels, inhibited growth, abated Wnt/β-catenin prosurvival signaling, and induced apoptosis in breast and colon cancer cells (145–147). Interestingly, PDE inhibition is a secondary COX-independent target of clinically available NSAIDs, which have demonstrated chemopreventive and chemotherapeutic activities (145, 146). While COX inhibition is generally thought to be the primary antitumor mechanism of NSAIDs, other studies have shown (i) that the growth inhibitory activity of NSAIDs is not reversed by exogenous prostaglandins (ii) discrepancy between NSAID IC50 concentrations associated with COX inhibition and abated tumor cell proliferation, and (iii) cGMP activation (39, 42, 143, 145, 146).
These results implicate other targets, including PDEs in the antitumor effects of NSAIDs. Toward this end, the PDE5-selective inhibitors, sildenafil, tadalafil, and MY5445, enhanced intracellular cGMP/PKG signaling, which correlated with abated cancer cell proliferation and increased apoptosis (145). Interestingly, cytokine-induced NOS2 led to S-nitrosylation and inhibition of sGC activity, as well as reduced formation of cGMP and increased PDE1 in smooth muscle cells (110). These results suggest that high-flux NO derived from NOS2 abates cGMP signaling through S-nitrosylation and PDE mechanisms.
NO Regulation of HIF-1α
The HIF-1 transcriptional pathway is activated under conditions of reduced O2 bioavailability, which initiates physiological processes that when dysregulated can become pathological. These responses include angiogenesis, erythropoiesis, and vasomotor control, as well as modulation of energy metabolism and cell survival. The regulation of O2 gradients by HIF-1 is precisely controlled for ATP synthesis as well as prevention of excess O2 toxicity (56, 128). Under normoxic conditions, HIF-1 levels are regulated by the turnover of HIF-1α subunit by E3 ligase where hydroxylation of two proline residues (Pro402 and Pro564) by prolyl hydroxylase (PHD) targets the protein for ubiquitination and proteasomal degradation (57, 124).
In addition, normoxic conditions mediate the hydroxylation of Asn803 and Asn851 on HIF-1α and HIF-2α, respectively, which silences their COOH-terminal transactivation domains by abating HIF-1 interactions with coactivator protein p300/CREB-binding protein during transcriptional activation of target genes (68). Under hypoxic conditions, these protein modifications are attenuated, thus allowing HIF-1α protein stabilization and HIF-1 pathway activation.
In addition to hypoxia, NO also stabilizes HIF-1α by abated PHD-mediated HIF-1α ubiquitination and turnover (15, 57, 124). PHD is a nonheme Fe2+ enzyme that utilizes oxygen, α-ketoglutarate, and ascorbic acid to hydroxylate HIF-1α and is part of a larger family of oxygenases that includes ten-eleven translocation and Jumonji family that regulates DNA and histone demethylation (45, 64, 88). NO directly interacts with the nonheme Fe2+ site to inhibit PHD activity (82). In addition, S-nitrosylation of Cys533 within the oxygen-dependent degradation domain has been shown to stabilize HIF-1α protein levels in a manner independent of PHD activity (72). HIF-1α stabilization by NO activates a number of signaling pathways mediated by the promoter hypoxia response element (HRE) to confer survival and growth advantage, angiogenesis, wound repair, and tumor development (130, 163).
Moreover, HIF-1α stabilization promotes self-renewal of bone marrow-derived mesenchymal stromal cells, which involves the induction of pluripotent genes, including octamer-binding transcription factor 4 and kruppel-like factor-4, to abate terminal differentiation pathways (102). HIF-1α can activate multiple genes associated with such diverse functions as cell proliferation, cell survival, apoptosis, motility, invasion, cytoskeletal structure, cell adhesion, erythropoiesis, angiogenesis, vascular tone, transcriptional regulation, drug resistance, and metabolism (127).
HIF-1α also plays an important role in the expression of proteins associated with tumor development and progression, including erythropoietin (EPO), glucose transporter 1 (GLUT1), epithelial–mesenchymal transition (EMT), and vascular endothelial growth factor (VEGF) (148). EPO is a glycoprotein hormone and main regulator of red blood cell production and is associated with hematological malignancies. The EPO receptor is expressed in many organs and may function as an antiapoptotic factor; it is overexpressed in multiple cancers, including breast cancer, and mediates cell proliferation and angiogenesis (99, 161). Increased VEGF promotes angiogenesis, while elevated GLUT1 modulates metabolism to favor glycolysis. HIF-1α also modulates the EMT markers class A basic helix-loop-helix transcription factor (TWIST), vimentin, and E-cadherin in clinical samples and nonsmall cell lung cancer cells, implicating a role of TWIST in hypoxia-induced invasion and metastasis (157). The immune checkpoint inhibitor, programmed death ligand 1 (PD-L1), contains an HRE in its promoter and is a direct target of HIF-1α (89, 90). Importantly, blockade of PD-L1 enhances myeloid-derived suppressor cell (MDSC)-mediated T-cell activation and potentiates radiation therapeutic efficacy (90, 152). Thus, HIF-1α stabilization promotes EMT (metastasis), angiogenesis through VEGF, and PD-L1-mediated immunosuppression, indicating that HIF-1α is a key mediator of processes common to the most aggressive tumors.
NO and Mitochondrial Targets
Several metabolic enzymes are targeted by NO and S-nitrosation, including complex I and complex IV of the mitochondrial electron transport chain (13, 17, 20, 27). Targeted S-nitrosation of complex I has been shown to limit electron flux and minimize oxidative damage during reperfusion injury (107). NO regulation of O2 consumption by direct binding and reversible inhibition at the ferrous heme site of complex IV has been well documented (20). In addition, inhibition of the glycolytic enzyme, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), by NO has been shown (13, 53). Other studies have employed extracellular flux technology to show distinct mechanisms of NO and S-nitrosation in the regulation of glycolysis and oxidative phosphorylation (25).
The NO donor, DETA/NO, stimulated glycolysis while impairing mitochondrial reserve capacity with no impact on basal respiration, which was reversed by the NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO), thus suggesting direct NO-heme interaction (25). Similar to the effects of DETA/NO, low (50 μM) concentrations of l-cysteine nitric oxide (CysNO) also stimulated glycolysis, which was abated by PTIO; however, higher (200 μM) concentrations inhibited glycolysis, which required amino acid transport and suggested S-nitrosation-dependent GAPDH inhibition (13, 25, 43). In addition, l-CysNO dose-dependently inhibited basal respiration, ATP-linked O2 consumption rate, and reserve capacity (25). Together, this work elegantly demonstrated a temporal relationship between free NO and S-nitrosation mechanisms where NO caused early rapid responses in mitochondrial function, which were later abated by slower, S-nitrosothiol-mediated inhibitory mechanisms (25).
Angiogenesis Versus Immunosuppression
The endothelial barrier maintains vascular and tissue homeostasis and is a key modulator of processes, including angiogenesis and immune response. In cancer, the endothelial barrier is disorganized, which leads to permeable or leaky vasculature that drives tumor-induced angiogenesis, altered blood flow, leukocyte infiltration, and tumor extravasation (4).
The angiogenic cytokine, VEGF, produced within the tumor microenvironment is a key driver of tumor angiogenesis and vascular permeability by Src-dependent VE-cadherin adhesion destabilization (31, 66). In addition, VEGF promotes immune suppression by disrupting the maturation of dendritic precursor cells and tumor-activated CD8+ T-cell function, thus limiting the efficacy of immunotherapies (49, 133, 138). Moreover, the VEGF antibody Avastin can reverse VEGF-mediated disruption of dendritic cell maturation and T-cell proliferation, recruitment, and infiltration at the tumor site (95, 97).
Similarly, the CD40 antitumor immune response was also potentiated by a neutralizing anti-VEGF antibody (126). Toward this end, an emerging paradigm suggests that improved tumor response to therapy requires normalized vasculature, a responsive endothelium, and correctly polarized immune mediators. In fact, some articles have shown that immune modulation is far more important for improved tumor response to radiation therapy (114, 152).
Increased tumor angiogenesis and increased cluster of differentiation 31; platelet endothelial cell adhesion molecule (CD31) leads to bidirectional flow where tumor vasculature lacks the ability to produce intracellular adhesion molecule and vascular cell adhesion molecule, which are both critical for the recruitment of cytotoxic leukocytes. VEGF can activate MDSCs as well as suppress T-cell expansion. Elevated IL-8 promotes tumor angiogenesis and vascular permeability, as well as the expansion of MDSCs. IL-10 and nuclear factor (erythroid-derived 2)-like 2 increased HO-1 and CO, which promotes angiogenesis and inhibits T-cell expansion. Thus, the promotion of tumor angiogenesis and immune suppression go hand-in-hand as it occurs during wound response, which may, at least in part, explain why putative antiangiogenic agents have multiple beneficial effects in cancer therapy.
Extracellular Matrix MMPs and Tissue Inhibitor of Matrix Metalloproteinases
The importance of the tumor microenvironment during cancer progression has become increasingly evident. The tumor microenvironment comprises immune cells, fibroblasts, endothelial cells, adipocytes, and extracellular matrix (ECM). Importantly, the ECM encompasses a complex network, which transmits biochemical and biomechanical cues to tumor cells that are actively involved in disease progression and metastasis. Moreover, the ECM in breast cancer is similar to that of mammary gland involution and wound response, which is characterized by the upregulation of fibrillar collagens, fibronectin, and matricellular proteins. In addition, ECM remodeling enzymes are aberrantly upregulated in advanced tumors (98). ECM remodeling enzymes that contribute to breast cancer progression include MMPs as well endogenous tissue inhibitor of matrix metalloproteinases (TIMPs) (7, 74, 111, 112, 162).
MMPs comprise a family of structurally similar endopeptidases with zinc ions at the active site. MMPs process components of ECM, which impacts the biological and functional properties of the targeted proteins, including cytokines. For example, MMP-9 truncates IL-8, which potentiates its biological activity and binding of its cell surface receptor in neutrophils (150, 151). In addition, MMP-9 has been shown to process IL-1β, leading to its activation and feed-forward regulation of MMP-9 expression (96). Similarly, MMP-9 processes IL-2 receptor α (CD25), which abates the function of tumor-reactive T cells and cytotoxic lymphocytes (131). Active MMP-9 has been shown to augment the release of VEGF from ECM stores as well as activate latent TGF-β via degradation of the latency-associated peptide to facilitate tumor angiogenesis and invasion (9, 165). Collectively, these observations provide evidence that MMP-9 modulates immune function to promote immunosuppression, tumor angiogenesis, and invasion within the tumor microenvironment (96, 131).
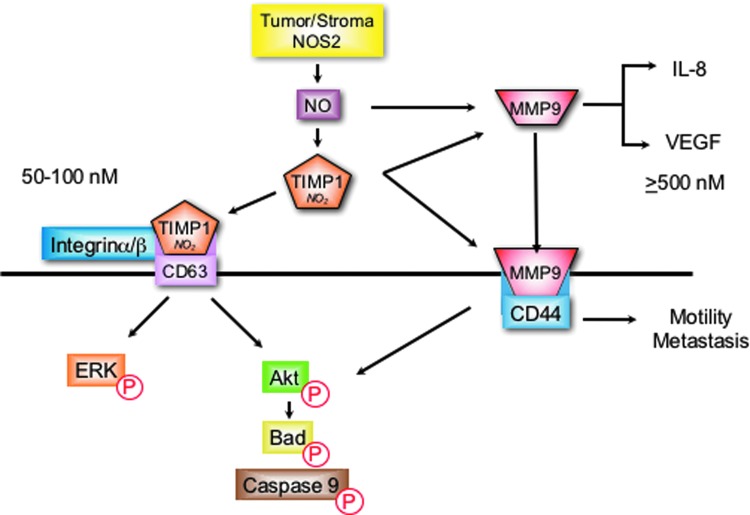
NO has multiple roles in the regulation of MMP-9 (94). NO/RNS and other electrophiles can activate MMPs through attacking the Zn thiolate bond of the latent protein (62, 63, 167). This activation has been shown at NO flux between 300 and 500 nM (119). In macrophages and microglia cells, NO increased MMP-9 activity via cGMP-dependent suppression of TIMP-1 (115, 119). Interestingly, as NO flux increased to higher levels that activate p53, MMP-9 activity diminished (119). These results demonstrate biphasic regulation of MMP-9 by NO, which is consistent with other progrowth signaling pathways that are similarly regulated by 300–500 steady-state nM NO flux (136). Interestingly, colocalization of NOS2 with MMP-9 was observed at the leading edge of migrating cells (93). Moreover, tyrosine nitration of MMP-9 resulted in enzyme activation in migrating astrocytes and reduced MMP-9 activity was observed in NOS2−/− mice (115, 156).
Protein localization through receptor binding provides an additional mechanism of MMP-9 regulation that mediates cell migration and invasion. Toward this end, the cell surface hyaluronan receptor, CD44, has been identified as a receptor for MMP-9 (96). This MMP-9/CD44 complex has been identified as a mechanism of localizing or concentrating MMP-9 at the leading edge of invasive breast cancer cells to facilitate enhanced metastasis during disease progression (10, 104, 164). NOS2 upregulates CD44 as well as IL-8 and correlates with MMP-9, TIMP-1, and enhanced tumor vascularization in ER− breast tumors (36, 112).
A recent mechanism for NO modulation of MMP-9 activity involves TIMP-1 protein nitration of key tyrosine residues that interfere with TIMP-1/MMP-9 binding, which abates TIMP-1 inhibition of active MMP-9 (103). Molecular modeling predicted two key tyrosine residues (Y95 and Y143) in loop structures that are critical for TIMP-1 inhibition of active MMP-9 (103). Interestingly, tyrosine nitration of these specific residues was later identified by mass spectrometry in recombinant human TIMP-1 protein following overnight exposure to the NO donor, DETA/NO (112).
In addition to its MMP inhibitory function, TIMP-1 also facilitates MMP-independent prosurvival PI3k/Akt/BAD and ERK pathway activation via interaction with the cell surface protein cluster of differentiation 63 (CD63) (19, 55). Toward this end, TIMP-1/CD63 colocalization and PI3k/Akt/BAD prosurvival signaling were enhanced in MB-231 breast cancer cells by NO concentrations that were optimal for TIMP-1 nitration (112). Importantly, TIMP-1 predicted poor breast cancer disease-specific survival, which was restricted to patients with high NOS2 tumor expression (112). Moreover, a direct correlation between NOS2 and pAkt (OR 4.5) was dramatically augmented (OR 12.7) in breast tumors expressing elevated TIMP-1, but reduced (OR 2.5) in tumors with low TIMP-1 expression (112).
Together, these results suggest a plausible mechanism for NO during breast cancer progression, where TIMP-1 nitration abates it MMP inhibitory function, which may favor TIMP-1/CD63 interaction and downstream PI3k/Akt/BAD prosurvival signaling while preserving MMP-9 activity to facilitate tumor angiogenesis, migration, and invasion, as summarized in Figure 3 (36, 112).
FIG. 3.
TIMP-1 nitration favors CD63 binding, which promotes the induction of prosurvival signaling while preserving MMP-9 activity for maintenance of angiogenesis, cell migration, and invasion. CD63, cluster of differentiation 63; MMP, matrix metalloproteinase; TIMP, tissue inhibitor matrix metalloproteinase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Conclusion
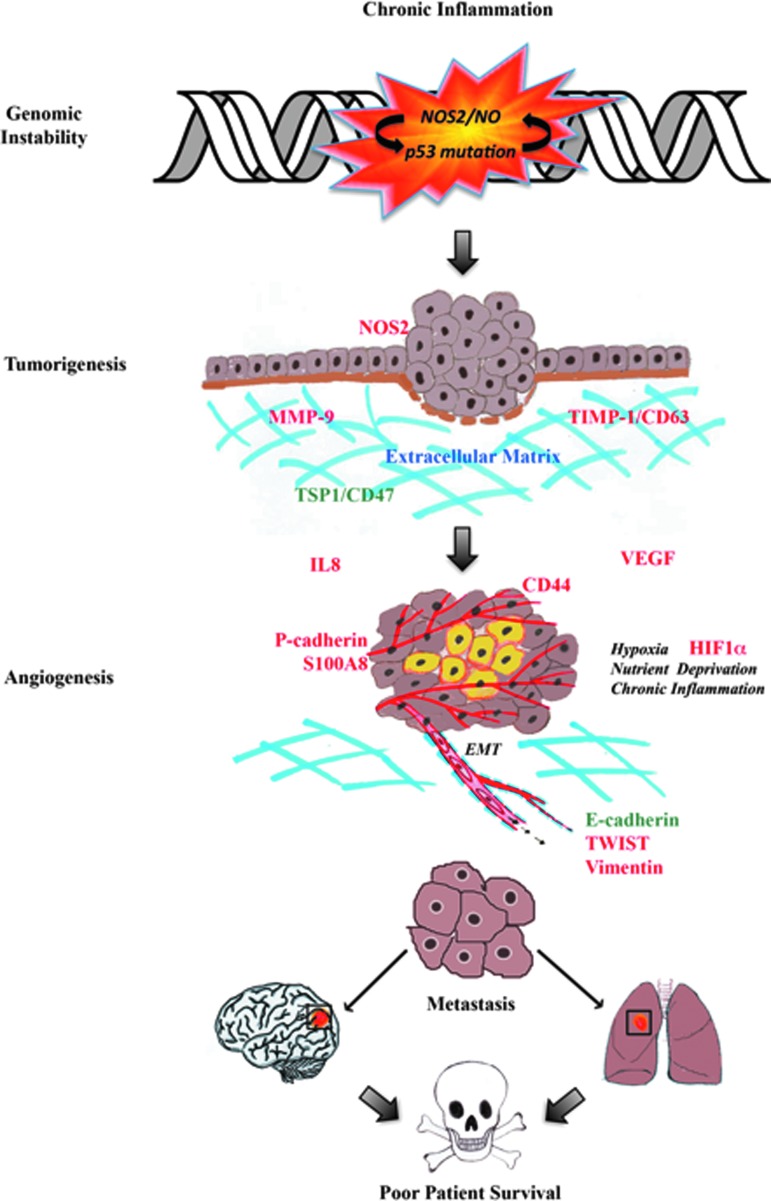
NO performs distinct and vastly different functions, which are concentration, spatially, and temporally dependent. Low nM NO produced by NOS1 and NOS3 regulates neuronal and vascular processes. In contrast, inflammatory processes generate NOS2-derived NO (ranging between 100 and 600 nM) that promotes nitrosative signaling. High tumor NOS2 expression has significantly correlated with increased p53 mutations, the vascular marker, CD31, and poor survival among breast cancer patients with the more aggressive ER− phenotype. Regarding disease progression, NOS2-derived NO upregulates prosurvival signaling pathways, including PI3k/Akt and ERK, promotes HIF1α protein stabilization, and induces NOS2 and COX2 (summarized in Fig. 4). NO-stabilized HIF1α helps the tumor as well as stromal cells cope with hypoxic stress by inducing angiogenesis, immunosuppression, chemoresistance, proliferation, and metastasis, which implicates the targeted inhibition of tumor NOS2 as a novel therapeutic strategy.
FIG. 4.
A summary of the roles of NO in breast cancer disease progression. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Toward this end, two novel cancer genes (RPL39 and MLF2) were recently identified in breast tumors that are regulated by hypoxia and NOS2 signaling (24). Mutational analysis identified gain-of-function effects in RPL39 (A14V and G50S) and MLF2 (D12H and R158 W) in a wound assay and significantly shorter time to relapse (p = 0.0259, χ2 test) in patients (24). Selective NOS2 inhibition abated RPL39 and MLF2 protein expression in breast cancer cells and siRNA targeting reduced tumor growth and improved median survival of mice treated with docetaxel (24). These promising findings warrant further clinical investigation of therapeutic applications of NOS inhibitors in breast cancer treatment.
Abbreviations Used
- Akt
protein kinase B
- CD31
cluster of differentiation 31; platelet endothelial cell adhesion molecule
- CD44
cluster of differentiation 44; hyaluronic acid receptor
- CD63
cluster of differentiation 63
- cGMP
cyclic guanosine monophosphate
- CO
carbon monoxide
- COX2
cyclooxygenase-2
- CysNO
cysteine nitric oxide
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- EMT
epithelial–mesenchymal transition
- EPO
erythropoietin
- ER
estrogen receptor
- ERK
extracellular signal-regulated kinase
- Ets-1
protein c-ets-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GC
guanylyl cyclase
- GLUT1
glucose transporter 1
- H2S
hydrogen sulfide
- HER2
human epidermal growth factor receptor-2
- HIF-1α
hypoxia-inducible factor-1 alpha
- HO-1
heme oxygenase-1
- HRE
hypoxia response element
- IGF
insulin-like growth factor
- IL
interleukin
- MAPK
mitogen-activated protein kinase
- MDSC
myeloid-derived suppressor cell
- MLF2
myeloid leukemia factor 2
- MMP
matrix metalloproteinase
- MRP
multidrug-resistant protein
- NO
nitric oxide
- NOS2
nitric oxide synthase-2
- OR
odds ratio
- PD-L1
programmed death ligand 1
- PGE2
prostaglandin E2
- PHD
prolyl hydroxylase
- PI3k
phosphoinositide 3-kinase
- PKG
protein kinase G
- PR
progesterone receptor
- PTIO
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide
- RAF-1
raf-1 proto-oncogene serine/threonine protein kinase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RPL39
ribosomal protein L39
- sGC
soluble guanylyl cyclase
- SOD
superoxide dismutase
- Src
src proto-oncogene, nonreceptor tyrosine kinase
- TGF-β
transforming growth factor-beta
- TIMP
tissue inhibitor matrix metalloproteinase
- TNBC
triple-negative breast cancer
- TWIST
class A basic helix-loop-helix transcription factor
- VEGF
vascular endothelial growth factor
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the NIH, Cancer and Inflammation Program. G.A.de.O. is supported by the program Science Without Borders—CNPq process number 205342/2014-0.
References
- 1.Ambs S. and Glynn SA. Candidate pathways linking inducible nitric oxide synthase to a basal-like transcription pattern and tumor progression in human breast cancer. Cell Cycle 10: 619–624, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, and Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res 58: 334–341, 1998 [PubMed] [Google Scholar]
- 3.Ambs S, Ogunfusika MO, Merriam WG, Bennett WP, Billiar TR, and Harris CC. Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc Natl Acad Sci U S A 95: 8823–8828, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzi S, Hebda JK, and Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol 3: 211, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer 138: 1058–1066, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkwill F. and Mantovani A. Inflammation and cancer: back to virchow? Lancet 357: 539–545, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Banerjee K. and Resat H. Constitutive activation of STAT3 in breast cancer cells: a review. Int J Cancer 138: 2570–2578, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron TI, Flahavan EM, Sharp L, Bennett K, and Visvanathan K. Recent prediagnostic aspirin use, lymph node involvement, and 5-year mortality in women with stage I–III breast cancer: a nationwide population-based cohort study. Cancer Res 74: 4065–4077, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, and Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2: 737–744, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourguignon LY, Gunja-Smith Z, Iida N, Zhu HB, Young LJ, Muller WJ, and Cardiff RD. CD44v(3,8–10) is involved in cytoskeleton-mediated tumor cell migration and matrix metalloproteinase (MMP-9) association in metastatic breast cancer cells. J Cell Physiol 176: 206–215, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, and van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol 36: 138–146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers LW, Maximo IX, Brenner AJ, Beeram M, Hursting SD, Price RS, Tekmal RR, Jolly CA, and deGraffenried LA. NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactions. Cancer Res 74: 4446–4457, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Broniowska KA. and Hogg N. Differential mechanisms of inhibition of glyceraldehyde-3-phosphate dehydrogenase by S-nitrosothiols and NO in cellular and cell-free conditions. Am J Physiol Heart Circ Physiol 299: H1212–H1219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brune B. and Zhou J. The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1alpha (HIF-1alpha). Curr Med Chem 10: 845–855, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Brune B. and Zhou J. Hypoxia-inducible factor-1alpha under the control of nitric oxide. Methods Enzymol 435: 463–478, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bulut AS, Erden E, Sak SD, Doruk H, Kursun N, and Dincol D. Significance of inducible nitric oxide synthase expression in benign and malignant breast epithelium: an immunohistochemical study of 151 cases. Virchows Arch 447: 24–30, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, and Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J 394: 627–634, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai WJ, Wang MJ, Ju LH, Wang C, and Zhu YC. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int 34: 565–572, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Chirco R, Liu XW, Jung KK, and Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev 25: 99–113, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Cooper CE. Competitive, reversible, physiological? Inhibition of mitochondrial cytochrome oxidase by nitric oxide. IUBMB Life 55: 591–597, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Cosse JP. and Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem 8: 790–797, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, and Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 106: 13820–13825, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ, and Thun M. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 10: 501–507, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Dave B, Granados-Principal S, Zhu R, Benz S, Rabizadeh S, Soon-Shiong P, Yu KD, Shao Z, Li X, Gilcrease M, Lai Z, Chen Y, Huang TH, Shen H, Liu X, Ferrari M, Zhan M, Wong ST, Kumaraswami M, Mittal V, Chen X, Gross SS, and Chang JC. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc Natl Acad Sci U S A 111: 8838–8843, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diers AR, Broniowska KA, Darley-Usmar VM, and Hogg N. Differential regulation of metabolism by nitric oxide and S-nitrosothiols in endothelial cells. Am J Physiol Heart Circ Physiol 301: H803–H812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donepudi MS, Kondapalli K, Amos SJ, and Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther 10: 506–511, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Dranka BP, Hill BG, and Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: the impact of nitric oxide and reactive oxygen species. Free Radic Biol Med 48: 905–914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Ekmekcioglu S, Ellerhorst J, Smid CM, Prieto VG, Munsell M, Buzaid AC, and Grimm EA. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res 6: 4768–4775, 2000 [PubMed] [Google Scholar]
- 30.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, and Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer 119: 861–866, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, and Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell 4: 915–924, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Espey MG, Miranda KM, Pluta RM, and Wink DA. Nitrosative capacity of macrophages is dependent on nitric-oxide synthase induction signals. J Biol Chem 275: 11341–11347, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, Goldman SA, Bredel M, McLendon RE, Sloan AE, Hjelmeland AB, and Rich JN. Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146: 53–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, Felley-Bosco E, Wang XW, Geller DA, Tzeng E, Billiar TR, and Harris CC. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci U S A 93: 2442–2447, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forstermann U, Schmidt HH, Pollock JS, Sheng H, Mitchell JA, Warner TD, Nakane M, and Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol 42: 1849–1857, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, Martin DN, Switzer CH, Hudson RS, Wink DA, Lee DH, Stephens RM, and Ambs S. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest 120: 3843–3854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glynn SA, Prueitt RL, Ridnour LA, Boersma BJ, Dorsey TM, Wink DA, Goodman JE, Yfantis HG, Lee DH, and Ambs S. COX-2 activation is associated with Akt phosphorylation and poor survival in ER-negative, HER2-positive breast cancer. BMC Cancer 10: 626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granados-Principal S, Liu Y, Guevara ML, Blanco E, Choi DS, Qian W, Patel T, Rodriguez AA, Cusimano J, Weiss HL, Zhao H, Landis MD, Dave B, Gross SS, and Chang JC. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res 17: 25, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurpinar E, Grizzle WE, and Piazza GA. NSAIDs inhibit tumorigenesis, but how? Clin Cancer Res 20: 1104–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D. and Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21: 309–322, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D. and Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, and Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol 52: 237–245, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, and Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol 7: 665–674, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Heinecke JL, Ridnour LA, Cheng RY, Switzer CH, Lizardo MM, Khanna C, Glynn SA, Hussain SP, Young HA, Ambs S, and Wink DA. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc Natl Acad Sci U S A 111: 6323–6328, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hickok JR, Vasudevan D, Antholine WE, and Thomas DD. Nitric oxide modifies global histone methylation by inhibiting Jumonji C domain-containing demethylases. J Biol Chem 288: 16004–16015, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickok JR, Vasudevan D, Jablonski K, and Thomas DD. Oxygen dependence of nitric oxide-mediated signaling. Redox Biol 1: 203–209, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honorat M, Mesnier A, Vendrell J, Guitton J, Bieche I, Lidereau R, Kruh GD, Dumontet C, Cohen P, and Payen L. ABCC11 expression is regulated by estrogen in MCF7 cells, correlated with estrogen receptor alpha expression in postmenopausal breast tumors and overexpressed in tamoxifen-resistant breast cancer cells. Endocr Relat Cancer 15: 125–138, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Hoos MD, Vitek MP, Ridnour LA, Wilson J, Jansen M, Everhart A, Wink DA, and Colton CA. The impact of human and mouse differences in NOS2 gene expression on the brain's redox and immune environment. Mol Neurodegener 9: 50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Goel S, Duda DG, Fukumura D, and Jain RK. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 73: 2943–2948, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, and Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A 102: 13141–13146, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ito H, Duxbury M, Benoit E, Clancy TE, Zinner MJ, Ashley SW, and Whang EE. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res 64: 7439–7446, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Ito K, Scott SA, Cutler S, Dong LF, Neuzil J, Blanchard H, and Ralph SJ. Thiodigalactoside inhibits murine cancers by concurrently blocking effects of galectin-1 on immune dysregulation, angiogenesis and protection against oxidative stress. Angiogenesis 14: 293–307, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izumi Y. and Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci Lett 478: 131–135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jedlitschky G, Burchell B, and Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275: 30069–30074, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Jung KK, Liu XW, Chirco R, Fridman R, and Kim HR. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J 25: 3934–3942, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaelin WG., Jr. How oxygen makes its presence felt. Genes Dev 16: 1441–1445, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Kallio PJ, Wilson WJ, O'Brien S, Makino Y, and Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J Biol Chem 274: 6519–6525, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Karami-Tehrani F, Moeinifard M, Aghaei M, and Atri M. Evaluation of PDE5 and PDE9 expression in benign and malignant breast tumors. Arch Med Res 43: 470–475, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Keefer LK. Fifty years of diazeniumdiolate research. From laboratory curiosity to broad-spectrum biomedical advances. ACS Chem Biol 6: 1147–1155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keibel A, Singh V, and Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des 15: 1949–1955, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, and Borst P. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 57: 3537–3547, 1997 [PubMed] [Google Scholar]
- 62.Krishnatry AS, Fung SM, Brazeau DA, Soda D, and Fung HL. Nitroglycerin alters matrix remodeling proteins in THP-1 human macrophages and plasma metalloproteinase activity in rats. Nitric Oxide 24: 66–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnatry AS, Kamei T, Wang H, Qu J, and Fung HL. Identification of nitroglycerin-induced cysteine modifications of pro-matrix metalloproteinase-9. Rapid Commun Mass Spectrom 25: 2291–2298, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Kuttan R. Characterization of activatable form of prolyl hydroxylase in L929 fibroblasts. Biochim Biophys Acta 660: 243–250, 1981 [DOI] [PubMed] [Google Scholar]
- 65.Lagares-Garcia JA, Moore RA, Collier B, Heggere M, Diaz F, and Qian F. Nitric oxide synthase as a marker in colorectal carcinoma. Am Surg 67: 709–713, 2001 [PubMed] [Google Scholar]
- 66.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, and Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 174: 593–604, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lander HM, Ogiste JS, Pearce SF, Levi R, and Novogrodsky A. Nitric oxide-stimulated guanine nucleotide exchange on p21ras. J Biol Chem 270: 7017–7020, 1995 [DOI] [PubMed] [Google Scholar]
- 68.Lando D, Peet DJ, Whelan DA, Gorman JJ, and Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295: 858–861, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Lane DP. Cancer. p53, guardian of the genome. Nature 358: 15–16, 1992 [DOI] [PubMed] [Google Scholar]
- 70.Lane DP. p53 and human cancers. Br Med Bull 50: 582–599, 1994 [DOI] [PubMed] [Google Scholar]
- 71.Laval F, Wink DA, and Laval J. A discussion of mechanisms of NO genotoxicity: implication of inhibition of DNA repair proteins. Rev Physiol Biochem Pharmacol 131: 175–191, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, and Li CY. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26: 63–74, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li LG. and Xu HM. Inducible nitric oxide synthase, nitrotyrosine and apoptosis in gastric adenocarcinomas and their correlation with a poor survival. World J Gastroenterol 11: 2539–2544, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu D, Guo H, Li Y, Xu X, Yang K, and Bai Y. Association between polymorphisms in the promoter regions of matrix metalloproteinases (MMPs) and risk of cancer metastasis: a meta-analysis. PLoS One 7: e31251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loibl S, Buck A, Strank C, von Minckwitz G, Roller M, Sinn HP, Schini-Kerth V, Solbach C, Strebhardt K, and Kaufmann M. The role of early expression of inducible nitric oxide synthase in human breast cancer. Eur J Cancer 41: 265–271, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Loibl S, Bratengeier J, Farines V, von Minckwitz G, Spänkuch B, Schini-Kerth V, Nepveu F, Strebhardt K, and Kaufmann M. Investigations on the inducible and endothelial nitric oxide synthases in human breast cancer cell line MCF-7 - estrogen has an influence on e-NOS, but not on i-NOS. Pathol Res Pract 202: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Lunt SJ, Chaudary N, and Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis 26: 19–34, 2009 [DOI] [PubMed] [Google Scholar]
- 78.MacMicking J, Xie QW, and Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol 15: 323–350, 1997 [DOI] [PubMed] [Google Scholar]
- 79.Mamlouk S. and Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. Int J Cancer 132: 2721–2729, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Mantovani A, Allavena P, Sica A, and Balkwill F. Cancer-related inflammation. Nature 454: 436–444, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Maturu P, Overwijk WW, Hicks J, Ekmekcioglu S, Grimm EA, and Huff V. Characterization of the inflammatory microenvironment and identification of potential therapeutic targets in wilms tumors. Transl Oncol 7: 484–492, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Metzen E, Zhou J, Jelkmann W, Fandrey J, and Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell 14: 3470–3481, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michaelis L, Menten ML, Johnson KA, and Goody RS. The original Michaelis constant: translation of the 1913 Michaelis-Menten paper. Biochemistry 50: 8264–8269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mirza UA, Chait BT, and Lander HM. Monitoring reactions of nitric oxide with peptides and proteins by electrospray ionization-mass spectrometry. J Biol Chem 270: 17185–17188, 1995 [DOI] [PubMed] [Google Scholar]
- 85.Mourskaia AA, Amir E, Dong Z, Tiedemann K, Cory S, Omeroglu A, Bertos N, Ouellet V, Clemons M, Scheffer GL, Park M, Hallett M, Komarova SV, and Siegel PM. ABCC5 supports osteoclast formation and promotes breast cancer metastasis to bone. Breast Cancer Res 14: R149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murillo-Carretero M, Torroglosa A, Castro C, Villalobo A, and Estrada C. S-Nitrosylation of the epidermal growth factor receptor: a regulatory mechanism of receptor tyrosine kinase activity. Free Radic Biol Med 46: 471–479, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Nathan CF. and Hibbs JB., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 3: 65–70, 1991 [DOI] [PubMed] [Google Scholar]
- 88.Niu Y, DesMarais TL, Tong Z, Yao Y, and Costa M. Oxidative stress alters global histone modification and DNA methylation. Free Radic Biol Med 82: 22–28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noman MZ. and Chouaib S. Targeting hypoxia at the forefront of anticancer immune responses. Oncoimmunology 3: e954463, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, and Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 211: 781–790, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noman MZ, Messai Y, Carre T, Akalay I, Meron M, Janji B, Hasmim M, and Chouaib S. Microenvironmental hypoxia orchestrating the cell stroma cross talk, tumor progression and antitumor response. Crit Rev Immunol 31: 357–377, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Nooter K, Brutel de la Riviere G, Look MP, van Wingerden KE, Henzen-Logmans SC, Scheper RJ, Flens MJ, Klijn JG, Stoter G, and Foekens JA. The prognostic significance of expression of the multidrug resistance-associated protein (MRP) in primary breast cancer. Br J Cancer 76: 486–493, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Novaro V, Colman-Lerner A, Ortega FV, Jawerbaum A, Paz D, Lo Nostro F, Pustovrh C, Gimeno MF, and Gonzalez E. Regulation of metalloproteinases by nitric oxide in human trophoblast cells in culture. Reprod Fertil Dev 13: 411–420, 2001 [DOI] [PubMed] [Google Scholar]
- 94.O'Sullivan S, Medina C, Ledwidge M, Radomski MW, and Gilmer JF. Nitric oxide-matrix metaloproteinase-9 interactions: biological and pharmacological significance—NO and MMP-9 interactions. Biochim Biophys Acta 1843: 603–617, 2014 [DOI] [PubMed] [Google Scholar]
- 95.Oelkrug C. and Ramage JM. Enhancement of T cell recruitment and infiltration into tumours. Clin Exp Immunol 178: 1–8, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Opdenakker G, Van den Steen PE, Van Damme J. and Gelatinase B. a tuner and amplifier of immune functions. Trends Immunol 22: 571–579, 2001 [DOI] [PubMed] [Google Scholar]
- 97.Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, Clay T, and Morse MA. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother 57: 1115–1124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast 22 Suppl 2: S66–S72, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Osterborg A, Aapro M, Cornes P, Haselbeck A, Hayward CR, and Jelkmann W. Preclinical studies of erythropoietin receptor expression in tumour cells: impact on clinical use of erythropoietic proteins to correct cancer-related anaemia. Eur J Cancer 43: 510–519, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, and Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol 172: 4744–4751, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Pandey S, Singh S, Anang V, Bhatt AN, Natarajan K, and Dwarakanath BS. Pattern recognition receptors in cancer progression and metastasis. Cancer Growth Metastasis 8: 25–34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park IH, Kim KH, Choi HK, Shim JS, Whang SY, Hahn SJ, Kwon OJ, and Oh IH. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Exp Mol Med 45: e44, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patruno A, Pesce M, Marrone A, Speranza L, Grilli A, De Lutiis MA, Felaco M, and Reale M. Activity of matrix metallo proteinases (MMPs) and the tissue inhibitor of MMP (TIMP)-1 in electromagnetic field-exposed THP-1 cells. J Cell Physiol 227: 2767–2774, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Peng ST, Su CH, Kuo CC, Shaw CF, and Wang HS. CD44 crosslinking-mediated matrix metalloproteinase-9 relocation in breast tumor cells leads to enhanced metastasis. Int J Oncol 31: 1119–1126, 2007 [PubMed] [Google Scholar]
- 105.Peranovich TM, da Silva AM, Fries DM, Stern A, and Monteiro HP. Nitric oxide stimulates tyrosine phosphorylation in murine fibroblasts in the absence and presence of epidermal growth factor. Biochem J 305 (Pt 2): 613–619, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pervin S, Singh R, Hernandez E, Wu G, and Chaudhuri G. Nitric oxide in physiologic concentrations targets the translational machinery to increase the proliferation of human breast cancer cells: involvement of mammalian target of rapamycin/eIF4E pathway. Cancer Res 67: 289–299, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, and Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A 106: 10764–10769, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prueitt RL, Boersma BJ, Howe TM, Goodman JE, Thomas DD, Ying L, Pfiester CM, Yfantis HG, Cottrell JR, Lee DH, Remaley AT, Hofseth LJ, Wink DA, and Ambs S. Inflammation and IGF-I activate the Akt pathway in breast cancer. Int J Cancer 120: 796–805, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Rahman MA, Senga T, Ito S, Hyodo T, Hasegawa H, and Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J Biol Chem 285: 3806–3814, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rajagopal S, Nalli AD, Kumar DP, Bhattacharya S, Hu W, Mahavadi S, Grider JR, and Murthy KS. Cytokine-induced S-nitrosylation of soluble guanylyl cyclase and expression of phosphodiesterase 1A contribute to dysfunction of longitudinal smooth muscle relaxation. J Pharmacol Exp Ther 352: 509–518, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ren F, Tang R, Zhang X, Madushi WM, Luo D, Dang Y, Li Z, Wei K, and Chen G. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: a systematic review and meta-analysis. PLoS One 10: e0135544, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ridnour LA, Barasch KM, Windhausen AN, Dorsey TH, Lizardo MM, Yfantis HG, Lee DH, Switzer CH, Cheng RY, Heinecke JL, Brueggemann E, Hines HB, Khanna C, Glynn SA, Ambs S, and Wink DA. Nitric oxide synthase and breast cancer: role of TIMP-1 in NO-mediated Akt activation. PloS One 7: e44081, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ridnour LA, Cheng RY, Switzer CH, Heinecke JL, Ambs S, Glynn SA, Young HA, Trinchieri G, and Wink DA. Molecular pathways: toll-like receptors in the tumor microenvironment: poor prognosis or new therapeutic opportunity. Clin Cancer Res 19: 1340–1346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ridnour LA, Cheng RY, Weiss JM, Kaur S, Soto-Pantoja DR, Basudhar D, Heinecke JL, Stewart CA, DeGraff W, Sowers AL, Thetford A, Kesarwala AH, Roberts DD, Young HA, Mitchell JB, Trinchieri G, Wiltrout RH, and Wink DA. NOS inhibition modulates immune polarization and improves radiation-induced tumor growth delay. Cancer Res 75: 2788–2799, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ridnour LA, Dhanapal S, Hoos M, Wilson J, Lee J, Cheng RY, Brueggemann EE, Hines HB, Wilcock DM, Vitek MP, Wink DA, and Colton CA. Nitric oxide-mediated regulation of beta-amyloid clearance via alterations of MMP-9/TIMP-1. J Neurochem 123: 736–749, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, and Wink DA. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci U S A 102: 13147–13152, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA, and Isenberg JS. The biphasic nature of nitric oxide responses in tumor biology. Antioxid Redox Signal 8: 1329–1337, 2006 [DOI] [PubMed] [Google Scholar]
- 118.Ridnour LA, Thomas DD, Mancardi D, Espey MG, Miranda KM, Paolocci N, Feelisch M, Fukuto J, and Wink DA. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol Chem 385: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- 119.Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP, Roberts DD, and Wink DA. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci U S A 104: 16898–16903, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Kock K, and Kroemer HK. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev 37: 253–278, 2005 [DOI] [PubMed] [Google Scholar]
- 121.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, and Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377: 31–41, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, and Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379: 1591–1601, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H, Jiang G, Lu J, Antony S, and Doroshow JH. NADPH oxidases and cancer. Clin Sci (Lond) 128: 863–875, 2015 [DOI] [PubMed] [Google Scholar]
- 124.Salceda S. and Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272: 22642–22647, 1997 [DOI] [PubMed] [Google Scholar]
- 125.Sandes EO, Faletti AG, Riveros MD, Vidal Mdel C, Gimenez L, Casabe AR, and Eijan AM. Expression of inducible nitric oxide synthase in tumoral and non-tumoral epithelia from bladder cancer patients. Nitric Oxide 12: 39–45, 2005 [DOI] [PubMed] [Google Scholar]
- 126.Selvaraj S, Raundhal M, Patidar A, and Saha B. Anti-VEGF antibody enhances the antitumor effect of CD40. Int J Cancer 135: 1983–1988, 2014 [DOI] [PubMed] [Google Scholar]
- 127.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3: 721–732, 2003 [DOI] [PubMed] [Google Scholar]
- 128.Semenza GL. and Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Senggunprai L, Kukongviriyapan V, Prawan A, and Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res 28: 841–848, 2014 [DOI] [PubMed] [Google Scholar]
- 130.Sheta EA, Trout H, Gildea JJ, Harding MA, and Theodorescu D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene 20: 7624–7634, 2001 [DOI] [PubMed] [Google Scholar]
- 131.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, and Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res 61: 237–242, 2001 [PubMed] [Google Scholar]
- 132.Singer AL, Sherwin RP, Dunn AS, and Appleman MM. Cyclic nucleotide phosphodiesterases in neoplastic and nonneoplastic human mammary tissues. Cancer Res 36: 60–66, 1976 [PubMed] [Google Scholar]
- 133.Soto-Ortiz L. A cancer treatment based on synergy between anti-angiogenic and immune cell therapies. J Theor Biol 394: 197–211, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Switzer CH, Cheng RY, Ridnour LA, Glynn SA, Ambs S, and Wink DA. Ets-1 is a transcriptional mediator of oncogenic nitric oxide signaling in estrogen receptor-negative breast cancer. Breast Cancer Res 14: R125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Switzer CH, Glynn SA, Cheng RY, Ridnour LA, Green JE, Ambs S, and Wink DA. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res 10: 1203–1215, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Switzer CH, Ridnour LA, Cheng R, Heinecke J, Burke A, Glynn S, Ambs S, and Wink DA. S-nitrosation mediates multiple pathways that lead to tumor progression in estrogen receptor-negative breast cancer. For Immunopathol Dis Therap 3: 117–124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, Yanagi K, and Bouillaud F. Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Pharmacol 171: 2099–2122, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E, and Taieb J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 73: 539–549, 2013 [DOI] [PubMed] [Google Scholar]
- 139.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, and Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A 101: 8894–8899, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas DD, Heinecke JL, Ridnour LA, Cheng RY, Kesarwala AH, Switzer CH, McVicar DW, Roberts DD, Glynn S, Fukuto JM, Wink DA, and Miranda KM. Signaling and stress: the redox landscape in NOS2 biology. Free Radic Biol Med 87: 204–225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thomas DD, Ridnour LA, Espey MG, Donzelli S, Ambs S, Hussain SP, Harris CC, DeGraff W, Roberts DD, Mitchell JB, and Wink DA. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem 281: 25984–25993, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, and Wink DA. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 45: 18–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, and Pamukcu R. Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res 60: 3338–3342, 2000 [PubMed] [Google Scholar]
- 144.Timoshenko AV, Lala PK, and Chakraborty C. PGE2-mediated upregulation of iNOS in murine breast cancer cells through the activation of EP4 receptors. Int J Cancer 108: 384–389, 2004 [DOI] [PubMed] [Google Scholar]
- 145.Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, and Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/beta-catenin-mediated transcription in human breast tumor cells. Cancer Prev Res (Phila) 4: 1275–1284, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, and Piazza GA. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther 8: 3331–3340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, Maxuitenko YY, Keeton AB, and Piazza GA. Colon tumor cell growth-inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res (Phila) 3: 1303–1313, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tsui AK, Marsden PA, Mazer CD, Adamson SL, Henkelman RM, Ho JJ, Wilson DF, Heximer SP, Connelly KA, Bolz SS, Lidington D, El-Beheiry MH, Dattani ND, Chen KM, and Hare GM. Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia. Proc Natl Acad Sci U S A 108: 17544–17549, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D'Incalci M, Piccolo S, Veronesi A, Zambelli A, Del Sal G, and Di Leo A. Targeting triple negative breast cancer: is p53 the answer? Cancer Treat Rev 39: 541–550, 2013 [DOI] [PubMed] [Google Scholar]
- 150.Van den Steen PE, Proost P, Wuyts A, Van Damme J, and Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood 96: 2673–2681, 2000 [PubMed] [Google Scholar]
- 151.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, and Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem 270: 3739–3749, 2003 [DOI] [PubMed] [Google Scholar]
- 152.Vanpouille-Box C, Diamond JM, Pilones KA, Zavadil J, Babb JS, Formenti SC, Barcellos-Hoff MH, and Demaria S. TGFbeta is a master regulator of radiation therapy-induced antitumor immunity. Cancer Res 75: 2232–2242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vaupel P, Hockel M, and Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 9: 1221–1235, 2007 [DOI] [PubMed] [Google Scholar]
- 154.Vodovotz Y, Chesler L, Chong H, Kim SJ, Simpson JT, DeGraff W, Cox GW, Roberts AB, Wink DA, and Barcellos-Hoff MH. Regulation of transforming growth factor beta1 by nitric oxide. Cancer Res 59: 2142–2149, 1999 [PubMed] [Google Scholar]
- 155.Vossler MR, Yao H, York RD, Pan MG, Rim CS, and Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89: 73–82, 1997 [DOI] [PubMed] [Google Scholar]
- 156.Wang HH, Hsieh HL, and Yang CM. Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J Cell Physiol 226: 2244–2256, 2011 [DOI] [PubMed] [Google Scholar]
- 157.Wei L, Sun JJ, Cui YC, Jiang SL, Wang XW, Lv LY, Xie L, and Song XR. Twist may be associated with invasion and metastasis of hypoxic NSCLC cells. Tumour Biol 37: 9979–9987, 2016 [DOI] [PubMed] [Google Scholar]
- 158.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 86: 1184–1195, 1995 [PubMed] [Google Scholar]
- 159.Wielinga PR, van der Heijden I, Reid G, Beijnen JH, Wijnholds J, and Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem 278: 17664–17671, 2003 [DOI] [PubMed] [Google Scholar]
- 160.Windham PF. and Tinsley HN. cGMP signaling as a target for the prevention and treatment of breast cancer. Semin Cancer Biol 31: 106–110, 2015 [DOI] [PubMed] [Google Scholar]
- 161.Wojtukiewicz MZ, Hempel D, Kruszewska J, Zimnoch L, Kisiel W, and Sierko E. Erythropoietin receptor and tissue factor are coexpressed in human breast cancer cells. J BUON 20: 1426–1431, 2015 [PubMed] [Google Scholar]
- 162.Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD, Yang F, and Xu XC. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int J Cancer 122: 2050–2056, 2008 [DOI] [PubMed] [Google Scholar]
- 163.Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, and Lee JM. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem 281: 24566–24574, 2006 [DOI] [PubMed] [Google Scholar]
- 164.Yu Q. and Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev 13: 35–48, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu Q. and Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev 14: 163–176, 2000 [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang W, He XJ, Ma YY, Wang HJ, Xia YJ, Zhao ZS, Ye ZY, and Tao HQ. Inducible nitric oxide synthase expression correlates with angiogenesis, lymphangiogenesis, and poor prognosis in gastric cancer patients. Human Pathol 42: 1275–1282, 2011 [DOI] [PubMed] [Google Scholar]
- 167.Zhang Z, Kolls JK, Oliver P, Good D, Schwarzenberger PO, Joshi MS, Ponthier JL, and Lancaster JR., Jr. Activation of tumor necrosis factor-alpha-converting enzyme-mediated ectodomain shedding by nitric oxide. J Biol Chem 275: 15839–15844, 2000 [DOI] [PubMed] [Google Scholar]