Abstract
Many people avidly consume foods and drinks containing caffeine, despite its bitter taste. Here, we review what is known about caffeine as a bitter taste stimulus. Topics include caffeine's action on the canonical bitter taste receptor pathway and caffeine's action on noncanonical receptor-dependent and -independent pathways in taste cells. Two conclusions are that (1) caffeine is a poor prototypical bitter taste stimulus because it acts on bitter taste receptor-independent pathways, and (2) caffeinated products most likely stimulate “taste” receptors in nongustatory cells. This review is relevant for taste researchers, manufacturers of caffeinated products, and caffeine consumers.
Keywords: : caffeine, bitterness, taste, taste receptors, TAS2R43
Introduction
The sense of taste helps to determine whether or not a food or beverage will be ingested. Typically, sweet tastes motivate intake and bitter tastes discourage intake. However, two of the most widely ingested beverages—coffee and tea1—are bitter, which contradicts this general rule. One factor that likely contributes to the popularity of coffee and tea is that they contain the psychoactive alkaloid, trimethylxanthine (caffeine).
Many reviews describe the mechanisms supporting the behavioral, cognitive, and emotional effects of caffeine.2–10 However, none have evaluated caffeine as a bitter taste, or covered what is known about caffeine action in cells between the tongue and the brain. These topics deserve attention because (1) caffeine is sometimes considered a prototypical bitter;11–17 (2) there are currently more caffeinated products on the market than there have been in the past and many contain higher concentrations of caffeine than do coffee and tea18; (3) new mechanisms may be exposed by recent discoveries of bitter taste receptors lining the digestive tract, which must come into contact with caffeine; and (4) new mechanisms may be exposed by recent discoveries that caffeine targets (e.g., adenosine receptors [ARs], GABA receptors, intracellular receptors, and so on) in nontaste cells (e.g., neurons) can modulate taste in the mouth. Thus, this review will place a particular emphasis on caffeine's effects on taste cells and other caffeine-responsive cells that reside outside of the central nervous system.
What Is a Bitter Taste?
Before discussing caffeine's bitterness, we will briefly review bitter taste in general. Bitter chemicals are structurally diverse and include alkaloids (e.g., caffeine, quinine, nicotine, and morphine),19 some L-amino acids,20–23 urea,24 phenylthiocarbamide,24 6-n-propylthiouracil,24 and some divalent salts25 (for a more comprehensive list of bitter chemicals see Beckett et al. 26). It has been suggested that the more bitter a compound, the more toxic it is, although there are many exceptions.27 Like sweet tastes, most bitter tastes, regardless of their structure, are detected by G-protein-coupled receptors (GPCRs) in type 2 taste cells (taste receptor cells [TRCs]).
When a taste (i.e., a chemical that elicits a taste percept) binds to a GPCR expressed by a TRC, it activates an intracellular signaling cascade that can result in the release of adenosine triphosphate (ATP) and stimulation of peripheral nerve fibers. Whether or not a TRC is activated by a taste depends on the receptor it expresses. TRCs that express T1Rs are activated by sweet or umami tastes, and cells that express taste 2 receptors (T2Rs) are activated by bitter tastes.
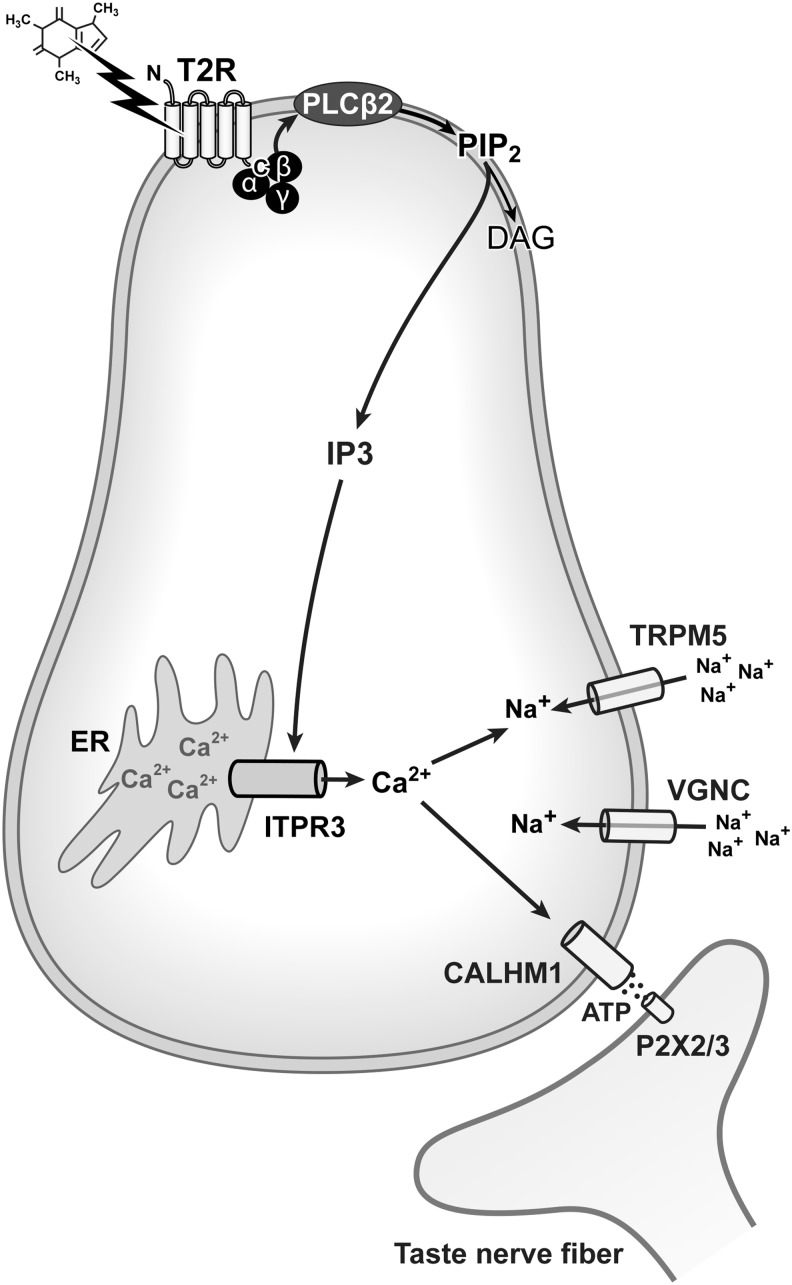
In the canonical bitter taste transduction cascade (Fig. 1), intracellular signaling starts with activation of G-proteins such as α-gustducin. This results in the dissociation of βγ subunits, which activate phospholipase Cβ2 (PLCβ2). PLCβ2 then cleaves phosphatidylinositol 4,5-biphosphate (PIP2) into inositol (1,4,5) triphosphate (IP3). The IP3 triggers release of calcium from the endoplasmic reticulum by binding to type 3 IP3 receptors (ITPR3s). This calcium release activates and opens the nonselective cation channel, transient receptor potential cation channel subfamily M member 5 (TRPM5), leading to cation influx and depolarization of the taste cell (see Kinnamon28 for a review). This depolarization activates voltage-gated sodium channels, which trigger the release of ATP through CALHM1 channels.29–31 The signal, transmitted by ATP release, is then conveyed to the brain through peripheral nerve fibers that express purinergic receptors.32
FIG. 1.
The canonical taste transduction pathway. Caffeine, and other chemicals that elicit bitter taste sensations, activate T2R-type GPCRs. GPCRs have seven domains that span the plasma membrane. When bitter tasting chemicals bind to T2Rs, this elicits an intracellular signaling cascade that starts with activation of G-proteins (e.g., α-gustducin). Activation of Gα causes the dissociation of βγ subunits, which then activate the enzyme PLCβ2. PLCβ2 then cleaves PIP2 into IP3. The IP3 triggers release of calcium from the endoplasmic reticulum by binding to ITPR3s. This calcium release activates and opens TRPM5 leading to sodium influx and depolarization of the taste cell. This depolarization activates voltage-gated sodium channels (VGNC)182 boosting the depolarization triggered by TRPM5, which triggers the release of ATP through CALHM1 channels. The signal, transmitted by ATP release, is then conveyed to the brain through peripheral nerve fibers that express purinergic receptors. ATP, adenosine triphosphate; T2R, taste 2 receptor; TRPM5, transient receptor potential cation channel subfamily M member 5.
Taste information from the peripheral nerve fibers is first sent to a relay station in the brainstem, the nucleus of the solitary tract, which controls swallowing, salivation, and automatic behaviors related to taste rejection or acceptance.33–35 Thereafter, taste information is sent through multiple synaptic connections to higher brain areas where taste quality perceptions are generated. Although there is some debate about the exact route that taste information follows (see de Brito Sanchez and Giurfa36 for a discussion), qualities such as bitterness and sweetness can be generated by stimulating bitter and sweet cortical fields in the gustatory cortex alone,37 which provides strong support that the gustatory cortex harbors taste percepts.
Taste compounds activate taste receptors found not only in the tongue38,39 but also in the alimentary tract,38–44 pancreas,45 other endocrine glands,46,47 the airway epithelium,38,48–52 and brain.38,53–57 The ubiquitous expression of taste receptors raises the possibility that chemicals that are tasted also exert a variety of other functional consequences. There is already evidence that taste stimuli influence the function of cells involved in nutrient absorption,40,53,58–61 satiety,62 fertility,47,63 respiration,64 and metabolism.65 Bitter taste receptors specifically have been implicated in several nontaste functions ranging from innate immunity52 to neurite outgrowth.54
Caffeine's Action on the Canonical Bitter Taste Pathway
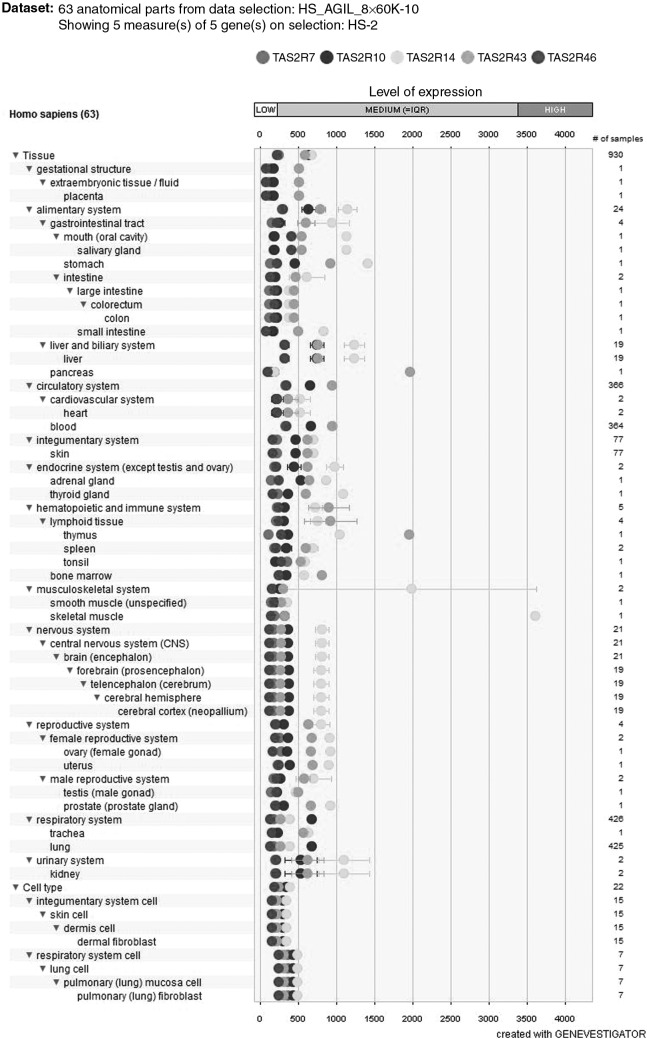
Caffeine has a bitter taste, and bitter tasting chemicals are detected by a large family of GPCRs (T2Rs). Humans have at least 25 functional T2R subtypes, and rodents have at least 29. Meyerhof et al. demonstrated that caffeine is a ligand of five human bitter taste receptors as follows: hTAS2R7, TAS2R10, TAS2R14, TAS2R43, and TAS2R4666 (see Fig. 2 for tissue-specific expression of caffeine-responsive T2Rs). In their study, the level of intracellular calcium induced by caffeine was analyzed in HEK293T cells transfected with TAS2R genes.66 Although the effects of caffeine on taste cells that endogenously express T2Rs need to be confirmed, there is good reason to believe that the receptors identified by Meyerhof et al. are important targets: Two studies show that polymorphisms in TAS2R43 (a gene encoding one of the receptors that Meyerhof et al. found to be responsive to caffeine) are associated with coffee liking67 and the perceived bitterness of caffeine.68 This suggests that TAS2R43 might be particularly important for caffeine taste detection in humans. Interestingly, ratings of caffeine bitterness in humans are also positively correlated with levels of TAS2R43 mRNA.69 Thus, polymorphisms in TAS2R43 associated with the perceived bitterness of caffeine may indirectly control the expression level of the T2R43 receptor. Alternatively, caffeine drinking could increase expression of T2R43. However, given that the TAS2R43 gene is absent from the genome of ∼18–30% of people,69–72 yet subjects with this deletion report they can taste caffeine's bitterness,69 non-T2R43 mechanisms must be partly responsible for the bitter taste of caffeine. A genome-wide meta-analysis identified polymorphisms in TAS2R7 and TAS2R14 as being associated with coffee liking,73 again supporting the conclusion that Meyerhof et al. identified important targets for caffeine detection in their in vitro study. Although coffee contains multiple bitter chemicals, and caffeine is responsible for only a fraction of coffee's bitterness,74 the fact that TAS2R7 and TAS2R14 were identified as caffeine-responsive in a heterologous expression system suggests that these receptors might be especially important for caffeine taste. However, there are no reports that TAS2R46 is associated with coffee liking or the perceived bitterness of caffeine. With that said, a recently published study found that the odorant citronellal attenuates caffeine bitterness by blocking T2R43 and T2R46, further evidence that these receptors are important for caffeine taste.75
FIG. 2.
Tissue-specific expression of caffeine-responsive T2Rs. The gene IDs for TAS2R7, TAS2R10, TAS2R14, TAS2R43, and TAS2R46 were entered in GENEVESTIGATOR® and the Agilent Human Gene Expression 8x60K Microarray dataset was selected. In this dataset, TAS2R14 showed the highest expression in most tissues, including the oral cavity. However, TAS2R43 expression was high relative to TAS2R7, 10, 14, and 46 in the pancreas and thymus. Whether or not caffeine modulates the function of tissues in the alimentary, circulatory, integumentary, endocrine, immune, musculoskeletal, nervous, reproductive, respiratory, and urinary systems by acting on caffeine-responsive T2Rs is a relatively unexplored area of research.
Caffeine taste is aversive to both vertebrates and invertebrates, and bitter-responsive GPCRs are at least partly responsible for aversive responses to caffeine.76 However, bitter taste receptors are not conserved across species77 and, therefore, neither are the mechanisms responsible for caffeine taste. For example, a recent study found that caffeine activated only one mouse T2R: Tas2R121, which is encoded by the gene, Tas2r121,14 an ortholog of human TAS2r13. Polymorphisms in hTAS2R13 have been associated with ethanol preference78 and intake in humans,79 but hTAS2R13 is not responsive to caffeine.66
Caffeine's action on endogenously expressed T2Rs has not yet been confirmed in taste cells (the ability to knockdown or block T2Rs expressed by taste cells in culture or to conditionally knockout [KO] T2Rs in type II TRCs would be required for confirmation), but there is evidence that caffeine is a T2R ligand in other cells that endogenously express T2Rs. For example, Xu et al. demonstrated that caffeine increases calcium release in haploid germ cells (e.g., spermatids and spermatozoa) from mouse seminiferous tubules. Caffeine-induced calcium release was most likely mediated by T2Rs because a T2R antagonist blocked the effect.63 Although the T2R antagonist used by Xu et al., probenecid, has other pharmacological targets, we30 (and others31) found that knocking out one of the main alternative targets (Panx1)80 has no effect on taste responses. Moreover, α-gustducin (also known as Gnat-3) KO mice do not avoid caffeine in brief-access taste tests,81 which suggest that α-gustducin, another protein important for signaling through T2Rs,82–87 is necessary for detecting the aversive (presumably bitter) taste of caffeine. It is noteworthy that in a previously published study we found no difference between Itpr3 WT and KO mice in intake of caffeine during 48-hour tests88 (Itpr3 is necessary for T2R-mediated signaling). Because caffeine is a psychoactive drug with postingestive effects, it is possible that the Itpr3 KO mice used cues other than bitterness to avoid drinking the caffeine solutions in the study.
Caffeine is a toxic deterrent to honeybees89 and other insects at higher concentrations (e.g., the concentration found in vegetative and seed tissues90), but at lower concentrations (e.g., the concentration found in pollen, which is below the bitter detection threshold for bees) it can enhance a pollinator's memory of reward, which could increase the likelihood of the plant's reproductive success.91 Caffeine appears to be the product of selective pressure on plants to protect their leaves and seeds, while encouraging pollination. Like in mammals, caffeine activates canonical bitter taste receptors in insects. In drosophila, at least Gr33, Gr66, and Gr93 are important for aversive responses to caffeine.92 Knocking out Gr66 diminishes aversive responses to the drug93,94 and activation of neurons that are responsive to bitter receptor cells causes insensitivity to caffeine.95 Interestingly, inducing apoptosis in gustatory receptor neurons that express Gr93 completely abolishes caffeine sensing in drosophila.13 Either Gr93 is sufficient for caffeine sensing or the cells that express Gr93 are necessary for caffeine sensing. Caterpillars also display aversive behavioral responses to caffeine, which are mediated by their epipharyngeal and lateral styloconi taste sensillum—sensilla that are also sensitive to other chemicals that humans describe as bitter.96,97 However, it is not clear if bitter taste receptors mediate these responses.
Because bitter taste receptors are responsible for rejection of bitter food across (nearly) all species, it is likely that caffeine binds to these receptors because the taste of caffeine is universally avoided. Goldfish reject caffeine.98 Guinea pigs, hamsters, and mice also avoid the taste of caffeine, suggesting that it is bitter (or elicits a negative taste quality) to these species as well.17,99 Rhesus macaques generalize between quinine and caffeine (i.e., they do not discriminate between the taste of quinine and the taste of caffeine), again demonstrating its similarity to other bitter tastes, at least in primates.100 In rodents, discrimination between caffeine and other chemicals that taste bitter to humans has not received enough attention. As would be expected based on their aversive responses to the taste of caffeine, rats easily discriminate between sweet taste (which elicits appetitive responses) and caffeine taste.101 However, somewhat surprisingly, one study found that golden hamsters do not cross-generalize a conditioned taste aversion to bitter tastes and caffeine, suggesting that caffeine possesses qualities beyond bitterness that this species can detect.17 The authors proposed that caffeine may elicit aversive reflexes in hamsters using a nontaste route. This seems possible given that caffeine elicits little to no chorda tympani17 or glossopharyngeal nerve responses102 in rodents. With that said, a separate study using single neuron recordings found that caffeine both inhibits and activates chorda tympani and glossopharyngeal neurons in rats.103 Although whole-nerve recordings show small responses to caffeine, individual gustatory nerve fibers in rodents likely respond to caffeine. Overall, responses to the aversive properties of caffeine are well-conserved, but whether or not these properties always include bitterness as humans perceive it or some other quality remains to be determined.
In summary, bitter taste receptors are likely the main mechanism responsible for caffeine's bitterness across species. However, there is good reason to believe that caffeine acts on pathways that do not involve bitter taste receptors. There are several lines of evidence that support this claim: First, even after total ablation of bitter responsive neurons in drosophila larvae, some avoidance to caffeine remains intact.13 Second, some species do not generalize between caffeine and other bitter tastes, suggesting that caffeine may not elicit a purely bitter taste. Finally, as will be discussed in the following section, caffeine is known to act on a number of molecular pathways that do not involve bitter taste receptors.
T2R-Independent Mechanisms of Caffeine Taste
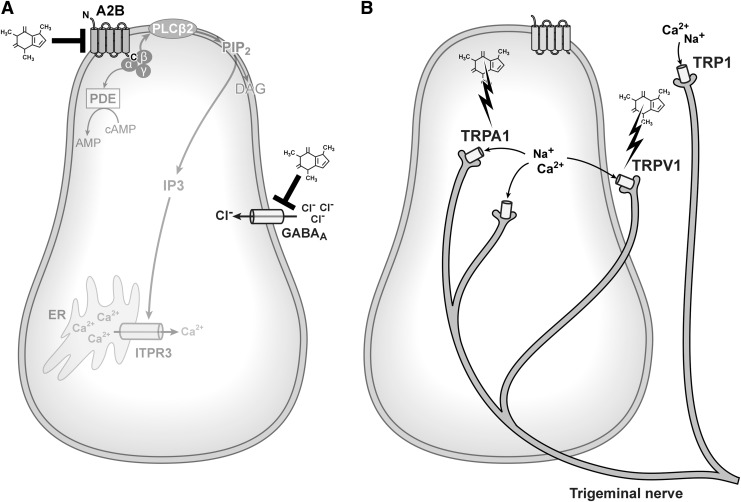
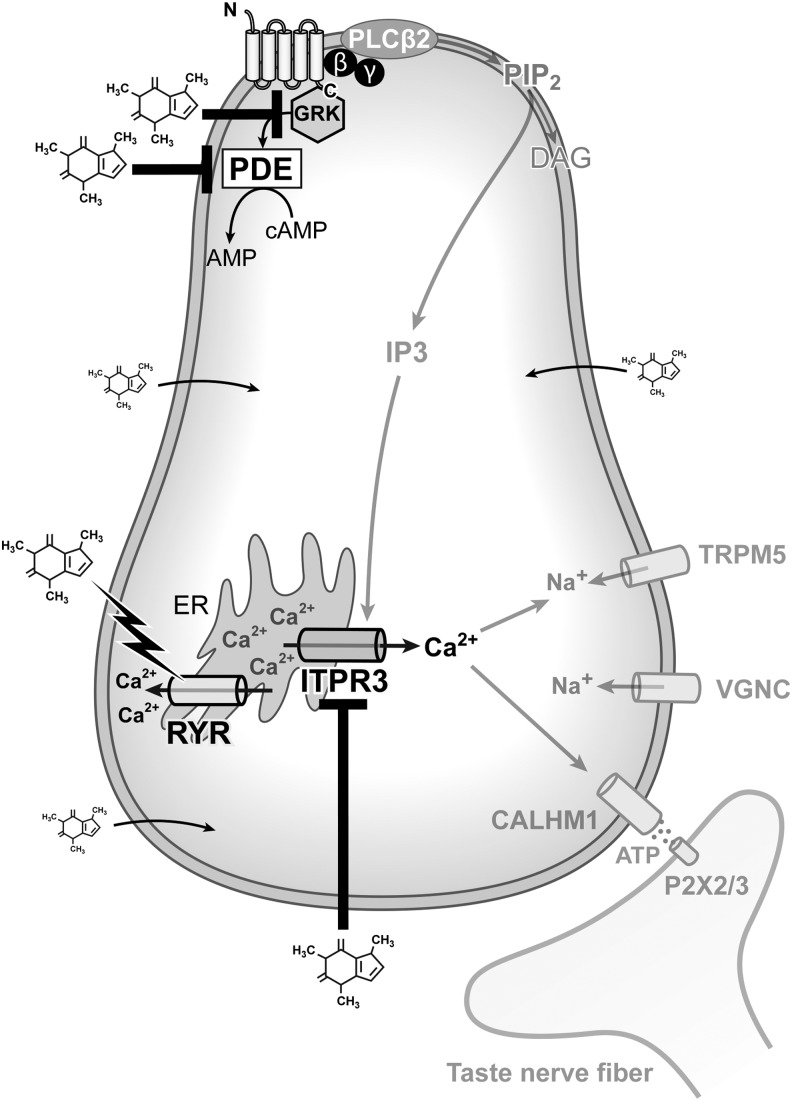
T2R-independent mechanisms of caffeine action on taste cells are mediated by three types of mechanism: (1) non-T2R receptors on TRCs (Fig. 3A), (2) plasma membrane ion channels on nerve fibers (Fig. 3B), and (3) intracellular (both receptor-dependent and independent) (Fig. 4). We discuss each in turn.
FIG. 3.
T2R-independent mechanisms of caffeine taste. Caffeine can inhibit the A2B GPCR and the GABAA channel on TRCs (A). Caffeine can activate Trpa1 and TRPV1 channels, which are expressed by trigeminal nerve fibers (B). GPCRs, G-protein-coupled receptors; TRCs, taste receptor cells.
FIG. 4.
Intracellular mechanisms of caffeine taste. Caffeine can directly block PDEs, GRKS, and ITPR3 by entering TRCs and other cells. Caffeine can activate RYRs. GRKs, GPCR kinases; PDE, phosphodiesterase; RYRs, ryanodine receptors.
Non-T2R receptors on TRCs
Adenosine receptors
Caffeine is a nonspecific AR antagonist.4 In fact, its action as an AR antagonist in the brain is the primary mechanism responsible for its psychoactive effects.2 Like T2Rs, ARs are GPCRs that modulate activity of effector molecules such as adenylate cyclase and PLC.104 However, in contrast to T2Rs, which are always linked to inhibitory G proteins (i.e., G proteins that decrease levels of cAMP), AR antagonism can both increase and decrease cAMP levels depending on whether or not the AR is linked to a stimulatory G protein or an inhibitory G protein. The ultimate effect of an AR antagonist such as caffeine depends on the relative expression of the AR subtype in the tissue. Four ARs have been cloned as follows: A1, A2A, A2B, and A3.105 A1 and A3 are coupled to inhibitory G proteins. This means that their activation inhibits adenylate cyclase and decreases the production of cAMP. In contrast, A2A and A2B are coupled to stimulatory G proteins.106 Caffeine displays similar micromolar (μM) affinity at A1, A2A, and A2B and lower affinity for A3.107
Taste cells show prominent expression of A2B (stimulatory), moderate expression of A1 (inhibitory), and no expression of A2A and A3.108 Thus, because caffeine is a nonspecific AR antagonist with micromolar affinities for all ARs, caffeine most likely antagonizes A2B (Fig. 3A) and A1, the receptors expressed by taste cells, at the concentration typically found in coffee (coffee contains ∼0.75 mg/mL [∼4 mM] caffeine); a typical 8-oz cup and provides a dose of 2.5 mg/kg and a peak plasma concentration of 10 μM.4 Because salivary concentrations are 65–85% of plasma concentrations if caffeine is taken in pill form, the concentration of caffeine in the oral cavity likely ranges between 8.5 μM and 4 mM (depending on whether or not coffee is in the mouth). Although no studies have explored whether or not caffeine antagonism of A2B or A1 is responsible for aspects of caffeine taste, several studies have indirectly examined the interplay between adenosine and caffeine on taste with contradictory results. One study found that adenosine reverses caffeine-induced enhancement of NaCl and quinine taste in humans109 (the ability of caffeine to enhance flavors, at least the flavor of soda, remains controversial110–112). In contrast, a later study found that neither caffeine nor caffeine paired with adenosine influenced taste responsivity or taste intensity ratings in humans.113 Adenosine can enhance sweet taste through A2B receptors in mouse taste bud cells that coexpress the sweet sensing receptor subunit Tas1r2.28,108 Therefore, it is possible that adenosine has a general bitter-masking effect, at least in rodents (and maybe in humans, too109). However, this seems unlikely because adenosine does not mask responses to other bitter stimuli in mice.108 Therefore, caffeine may elicit bitterness or reduce sweetness by blocking the action of endogenous adenosine at A2B. Interestingly, one study found that preadministration of an adenosine agonist increases caffeine consumption114 suggesting that adenosine decreases caffeine's aversive taste qualities. Furthermore, a genome-wide meta-analysis identified 15 polymorphisms in ADORA2B, the gene that encodes A2B, as being associated with coffee drinking.73 Whether or not caffeine's bitterness can be partly attributed to its ability to antagonize A2B receptors remains unknown.
GABA receptors
Caffeine (and other methylxanthine stimulants) is thought to be an antagonist at the benzodiazepine-positive modulatory site on GABAA receptors (Fig. 3A). The first evidence for this was provided by Marangos et al.,115 who found that caffeine competitively inhibited H3 diazepam binding. Although it remains somewhat unclear if caffeine directly binds to GABAA receptors, or indirectly decreases binding of GABAA ligands (i.e., if caffeine's effect on GABAA ligand binding or currents are primary or secondary),4 the effects of caffeine on GABAA signaling have been demonstrated using several different methods and cell types (mainly neuronal subtypes116–119 and caffeine seem to either effect GABAA ligand binding directly or to affect the stability or function of GABAA receptors). Interestingly, GABA (the endogenous amino acid ligand for GABAA receptors) masks the bitter taste of caffeine (in addition to the bitter taste of quinine, coca, and chocolate).120 It was reported that GABA is a bitter taste receptor antagonist, but it is also possible that GABA prevents caffeine blockade of GABAA receptors. In taste buds, like in the mature brain, GABA is an inhibitory transmitter. In TRCs during taste stimulation, GABA acts on both GABAA and GABAB receptors to suppress ATP secretion.121 It has been postulated that GABA not only suppresses ATP secretion in taste cells but also regulates the growth and differentiation of taste buds.121 In support of the role of GABA in development of oral tissue, GABAA receptor KO mice show abnormal palate development.122 Because GABAA receptors are also expressed in other cells in the gastrointestinal tract, including the duodenum where they appear to regulate motility,123 it is possible that caffeine can also modulate gastric motility by acting on GABA transmission in duodenal cells. In fact, increased gut motility is a well-documented side effect of caffeine. Although this effect of caffeine can be partly attributed to its blockade of ARs,124 caffeine action at GABA receptors is a possibility as well.
Plasma membrane ion channels
TRP channels
There is evidence that caffeine is not only a bitter taste but also has other chemosensory qualities such as oral irritation (e.g., burning and tingling). However, the mechanisms supporting these sensations are not yet clear. Published anecdotal evidence led to recommendations that patients who have facial pain caused by trigeminal neuralgia should reduce or avoid food and drink that contains caffeine.125 There is experimental evidence to support this. For example, cultured rat trigeminal neurons respond to caffeine as measured by changes in intracellular calcium.126 However, other alkaloids, such as nicotine, seem to have a much stronger effect on trigeminal neurons than does caffeine. The principal machinery responsible for trigeminal neuron activation in response to oral stimulation with taste stimuli is TRP channels. TRP channels are expressed in taste buds and trigeminal nerve fibers, and are important for transducing signals that give rise to the sensations of taste, irritation, warmth, coolness, and pungency.127 Several TRP channels could be responsible for aspects of caffeine taste (e.g., TRPM5, TRPV1, and TRPA1). TRPM5 is downstream of all signaling through taste receptors and triggers ATP release necessary for the detection of taste qualities, including sweetness and bitterness (Fig. 1). Caffeine does not appear to act directly on Trpm5.128 In contrast, caffeine does act on TRP channels expressed by sensory fibers (Fig. 3B). For example, Trpv1, a receptor for irritants such as capsaicin—the chemical responsible for the spiciness and burning sensation caused by hot chili peppers—is present in sensory fibers, but not TRCs. Trpv1 is activated by acidic (and basic) pH.129 Caffeine solutions are neutral (∼6.9) and there are no published reports of caffeine activating Trpv1 channels expressed by trigeminal fibers. However, caffeine activates Trpv1 channels in rat nodose ganglion neurons.130 Furthermore, other bitter alkaloids (with the exception of quinine) activate Trpv1.127,131,132 Therefore, it is reasonable to conclude that caffeine activates Trpv1 channels in trigeminal fibers as well. Caffeine activates mouse Trpa1 channels in a heterologous expression system, but inhibits human TRPA1 channels.133 Why caffeine would have different effects on Trpa1 signaling in rodents and humans is not clear (however, the aversive quality of caffeine does seem to be more than bitterness in rodents. As mentioned previously, rodents do not generalize between caffeine and other bitter tastes, but primates do). Caffeine does not act on Trpa1 channels in moths either, again highlighting the species-specific responses to caffeine.134
Potassium, sodium, and chloride channels
Caffeine modulates voltage-activated ionic currents in taste cells.135 Using patch clamp and ratiometric imaging techniques on dissociated rat TRCs, Zhao et al. found that caffeine inhibits outwardly and inwardly rectifying potassium currents.135 In the same study, caffeine inhibited sodium and calcium currents, yet had no effect on chloride currents.135 These results are interesting because Breslin et al. found that NaCl suppresses the bitterness of caffeine in humans.136 Together, these studies raise the possibility that the ability of NaCl to suppress caffeine bitterness is due to NaCl-induced reversal of the inhibitory effect of caffeine on sodium currents.
Intracellular targets
Caffeine is lipophilic (i.e., fat soluble). As a result, it readily crosses all biological membranes and, thus, has the potential to exert direct intracellular effects, independently of cell surface receptors or ion channels (Fig. 4). This is an important point for understanding caffeine taste because taste quality is determined by the cell, not by the receptor expressed by the cell. For example, sweet taste cells that are genetically engineered to express bitter taste receptors confer appetitive qualities to aversive chemicals like caffeine.86 Therefore, if caffeine unselectively enters all types of taste cells, it could produce an unpredictable taste quality (i.e., a combination of all tastes).
ITPR3 and other intracellular calcium channels
Caffeine elicits distinct effects in cells that express many of the effector proteins in the canonical taste pathway (e.g., ITPR3 and TRPM5), but do not express T2Rs. Caffeine has no effect on TRPM5 currents; however, it can modestly inhibit ITPR3-mediated calcium flux (Fig. 4).128 Given that Itpr3s have a caffeine binding domain,137 caffeine could modulate intracellular calcium release independently of T2Rs by directly binding to ITPR3. Much evidence suggests that caffeine also increases intracellular calcium levels by acting on ryanodine receptors (RYRs)138,139 (Fig. 4). The importance of intracellular receptors in the effect of caffeine on calcium levels is strengthened by the finding that caffeine-induced changes in calcium levels are dependent on intracellular stores, not extracellular calcium levels in TRCs.135 In addition, our unpublished work showing that Itpr3 KO mice do not detect the aversive taste of caffeine during brief-access taste tests suggests that a direct interaction between ITPR3 and caffeine could modulate taste.
Intracellular enzymes
Phosphodiesterases: Although much is known about caffeine's action as a phosphodiesterase (PDE) inhibitor in insects, little is known about how caffeine acts on mammalian PDEs. As was mentioned earlier (What Is a Bitter Taste section), when taste receptors are activated, the βγ subunit of the GPCR activates PLCβ and IP3-mediated release of calcium from intracellular stores. Another part of the taste transduction pathway involves the α subunit, α-gustducin, which likely activates PDEs to decrease intracellular cAMP levels140–144 (Fig. 4). If caffeine is able to directly interfere with PDE activity in taste cells, this could prolong stimulus-induced increases in intracellular cAMP or cGMP. In fact, Rosenzweig et al. found that caffeine induces rapid, transient, and gustatory tissue-specific increases in cGMP levels (cGMP is a second messenger like cAMP; however, cGMP is a more specialized messenger than cAMP) and hypothesized that this rise in cGMP levels was responsible for caffeine's bitter taste.145 The authors proposed that the increase in cGMP was due to caffeine's action as a PDE inhibitor (PDEs inhibit the degradation of cGMP). However, this hypothesis was not investigated further.146,147
GPCR kinases: In intestinal cells, caffeine activates GPCR kinases (GRKs) and increases calcium in a PLC-dependent manner.148 In contrast, caffeine and other amphipathic taste compounds have been shown to inhibit GRKs in taste cells (Fig. 4).149 Because GRKs are important for phosphorylation and desensitization of GPCRs (including bitter taste receptors),150 caffeine may be able to modulate the responsiveness of T2Rs, ARs, and GABARs to their ligands. In other words, caffeine may have the ability to modulate its own receptors by directly activating GRKs.
Other Factors Contributing to Caffeine's Bitterness
Over the past 15 years twin studies, double-blind/placebo-controlled trials, and genome-wide association studies have identified associations between more than 20 genes and caffeine taste, responses to caffeine, and/or drinking of caffeinated beverages.73,151–162 This work sheds light on mechanisms responsible for caffeine detection and voluntary caffeine consumption. Genes encoding bitter taste receptors, ARs, and intracellular enzymes have all been implicated in responses to caffeine and caffeinated beverages, as described in What Is a Bitter Taste, Caffeine's Action on the Canonical Bitter Taste Pathway, and T2R-Independent Mechanisms of Caffeine Taste sections. However, there are many variables to take into consideration when analyzing these genetic studies, including the caffeine vehicle (e.g., water, coffee, or soda), whether or not the subject or animal has had prior experience with caffeine, and age, to name a few. Next, we will discuss these other factors.
Vehicle
Caffeine is a natural compound found in coffee and tea and is an additive found in sodas and energy drinks. Because of its reinforcing properties, it has been challenging to determine how the taste of caffeine per se is influenced by other compounds in coffee and tea or by sugars or other chemicals in sodas and energy drinks (i.e., how caffeine taste is influenced by the vehicle). It also remains unclear how the temperature or pH of caffeinated solutions influences caffeine action and caffeine taste. There is evidence both supporting109 and refuting111,112 caffeine's purported role as a flavor enhancer. However, there is evidence that fatty vehicles interfere with caffeine taste. One study demonstrated that caffeine can be sequestered by biophenols in olive oil, which could prevent it from reaching its targets and decrease its bitterness.163 Another study demonstrated that lipoproteins can inhibit nerve responses to caffeine.164 Although beverage manufacturers have described caffeine as a “flavor enhancer,” evidence does not support this claim. For example, most people cannot distinguish between caffeinated and noncaffeinated beverages.111 Only after repeated pairings of caffeine and various flavors do preferences begin to shift, suggesting that caffeine's purported flavor enhancing effects are actually the result of its action in the central nervous system.111,165–167 Similarly, caffeine increases soda liking in adolescents, but only after repeated exposures,168 suggesting that immediate taste responses are not involved. In contrast, as mentioned previously, Breslin et al. found that NaCl reduces the bitterness of caffeine.136 Therefore, the salt content of a beverage may affect caffeine taste. More work is needed to understand under what circumstances caffeine modulates taste and flavor perception.
Saliva composition
Saliva composition may also determine the taste of caffeine. Dsamou et al. demonstrated that subjects who are hypersensitive to the bitterness of caffeine had higher levels of amylase fragments, immunoglobulins, and serum albumin in their saliva.169 The saliva of the same hypersensitive subjects contained lower levels of cystatin SN (a protease inhibitor). Therefore, proteolysis within the oral cavity may, in part, determine sensitivity to the bitter taste of caffeine.
Prior exposure to caffeine
Substances that are perceived as bitter are typically avoided by animals and this may be an adaptation that protects them from consuming foods that will produce adverse physiological effects.170–173 Consumption of bitter substances such as caffeine may include a learned component. For instance, Newland et al. found that when caffeine-naive rats were presented with caffeine or water, they consumed very little caffeine.114 However, rats with a history of forced caffeine consumption consumed caffeine more readily. Therefore, repeated exposure to caffeine likely results in positive associations between the bitterness of caffeine and its psychoactive effects.
There may be other effects of prior exposure to caffeine that increase its palatability, but that do not involve learning. For example, Lipchock et al. recently found that caffeine intake is correlated with PAV-TAS2R38 expression.174 Therefore, caffeine intake may alter expression of taste receptor genes and influence bitter detection as a consequence.
Psychiatric conditions
Differential reactivity to sweet and bitter tastes is associated with psychiatric disorders such as depression,175 alcoholism,176,177 and posttraumatic stress disorder.178 Taste preferences may therefore be a marker for drug abuse vulnerability and other psychiatric disturbances. Interestingly, caffeine-enhanced sensitivity to the bitterness of quinine may be a characteristic of panic disorder.178
The focus of this review is on caffeine action in taste cells, not the brain, but the line between taste and nontaste receptors has become blurry. Neurons are able to respond to various taste compounds and caffeine may act directly on bitter taste receptors in the brain to modulate bitter perception. Singh et al. found that T2Rs are expressed in multiple regions of the rat brain. Tas2r4, Tas2r107, and Tas2r38 transcripts were present in the brain stem, cerebellum, cortex, and nucleus accumbens.54 In the same study the authors demonstrated that quinine could activate these T2Rs. Caffeine likely activates T2Rs in the brain as well because caffeine-responsive T2Rs are expressed there (Fig. 2).56 Whether or not caffeine taste perception is mediated by both peripheral and central bitter taste receptors remains uncertain.
Age-related responses to caffeine
Children generally dislike bitter tastes more than adults do.179 Not surprisingly, children also display different responses to bitter-masking compounds. Although sodium gluconate decreases caffeine bitter perception in adults, it has no effect on caffeine taste responses in children.180 Interestingly, both children and adults respond similarly to the bitter masking effect of sucrose on caffeine.180 Age-related differences in caffeine taste deserve more attention because caffeinated beverages are marketed to children and adolescents.
Conclusion
Caffeine directly activates T2Rs,14,66 but there are other taste transduction mechanisms as well, including ARs, GABA receptors, TRP channels, and intracellular receptors and enzymes. These targets, including T2Rs, are expressed not only in taste tissue but also in diverse cell types throughout the digestive, endocrine, and reproductive systems (Fig. 2). In summary, caffeine should not be considered a prototypical bitter taste—not only can it act on many T2R-independent pathways in taste cells but also it can activate the trigeminal system, and it acts directly on the central nervous system.
Public health message: At low concentrations the effects of caffeine are likely benign, which is why it has been labeled a GRAS substance by the FDA. However, exposure to caffeine is increasing due to an increase in the number of products containing caffeine18,112 and the concentration of caffeine added to them.181 Furthermore, caffeinated products are being marketed to, and consumed by, children, whose taste systems and preferences are developing. Therefore, it is important to understand how caffeine might influence taste sensation and perception and the function of the digestive system. Specifically, more research is needed to better understand how higher concentrations of caffeine might influence the function and development of the taste system and digestive system.
Acknowledgments
The authors thank Dr. Danielle Reed for her helpful comments on an earlier draft of this article. Financial support was provided by Monell Institutional Funds and T32 DC000014.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Weinberg BA, Bealer BK. The World of Caffeine: The Science and Culture of the World's Most Popular Drug. New York, NY: Routledge; 2001 [Google Scholar]
- 2.Ribeiro JA, Sebastiao AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20 Suppl 1, S3–S15 [DOI] [PubMed] [Google Scholar]
- 3.Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20 Suppl 1:S85–S94 [DOI] [PubMed] [Google Scholar]
- 4.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133 [PubMed] [Google Scholar]
- 5.Fredholm BB. Notes on the history of caffeine use. Handb Exp Pharmacol. 2011;1–9 [DOI] [PubMed] [Google Scholar]
- 6.Fredholm BB. On the mechanism of action of theophylline and caffeine. Acta Med Scand. 1985;217:149–153 [DOI] [PubMed] [Google Scholar]
- 7.Ferre S. Caffeine and substance use disorders. J Caffeine Res. 2013;3:57–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferre S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis. 2010;20 Suppl 1:S35–S49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferre S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079 [DOI] [PubMed] [Google Scholar]
- 10.Daly JW, Fredholm BB. Caffeine—An atypical drug of dependence. Drug Alcohol Depend. 1998;51:199–206 [DOI] [PubMed] [Google Scholar]
- 11.Gautam SH, Rebello MR, Verhagen JV. Taste quality and intensity of 100 stimuli as reported by rats: The taste-location association task. Front Behav Neurosci. 2012;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sammons JD, Weiss MS, Victor JD, Di Lorenzo PM. Taste coding of complex naturalistic taste stimuli and traditional taste stimuli in the parabrachial pons of the awake, freely licking rat. J Neurophysiol. 2016;116:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apostolopoulou AA, Kohn S, Stehle B, et al. Caffeine taste signaling in Drosophila larvae. Front Cell Neurosci. 2016;10:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lossow K, Hubner S, Roudnitzky N, et al. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem. 2016;291:15358–15377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laska M, Rivas Bautista RM, Hernandez Salazar LT. Gustatory responsiveness to six bitter tastants in three species of nonhuman primates. J Chem Ecol. 2009;35:560–571 [DOI] [PubMed] [Google Scholar]
- 16.Mattes RD. Effects of linoleic acid on sweet, sour, salty, and bitter taste thresholds and intensity ratings of adults. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1243–G1248 [DOI] [PubMed] [Google Scholar]
- 17.Frank ME, Wada Y, Makino J, et al. Variation in intake of sweet and bitter solutions by inbred strains of golden hamsters. Behav Genet. 2004;34:465–476 [DOI] [PubMed] [Google Scholar]
- 18.Harris JL, Munsell CR. Energy drinks and adolescents: What's the harm? Nutr Rev. 2015;73:247–257 [DOI] [PubMed] [Google Scholar]
- 19.Fattorusso E, Taglialatela-Scafati O. Modern Alkaloids: Structure, Isolation, Synthesis and Biology. Weinheim, Germany: Wiley-VCH; 2008 [Google Scholar]
- 20.Birch GG, Kemp SE. Apparent specific volumes and tastes of amino acids. Chem Senses. 1989;14:249–258 [Google Scholar]
- 21.Fuke S. Taste-active components of seafoods with special reference to umami substances. In: Seafoods: Chemistry, Processing Technology and Quality. Shahidi F. and Botta J.R. (Eds). London: Chapman & Hall; 1994: pp. 115–139 [Google Scholar]
- 22.Linden GLD. Amino acids and peptides. In: New Ingredients in Food Processing. Biochemistry and Agrigulture. Linden G. and Lorient D. (Eds). Boca Raton, FL: CRC Press; 1999: pp. 315–336 [Google Scholar]
- 23.Bassoli A, Borgonovo G, Caremoli F, Mancuso G. The taste of D- and L-amino acids: In vitro binding assays with cloned human bitter (TAS2Rs) and sweet (TAS1R2/TAS1R3) receptors. Food Chem. 2014;150:27–33 [DOI] [PubMed] [Google Scholar]
- 24.Drewnowski A. The science and complexity of bitter taste. Nutr Rev. 2001;59:163–169 [DOI] [PubMed] [Google Scholar]
- 25.Lim J, Lawless HT. Qualitative differences of divalent salts: Multidimensional scaling and cluster analysis. Chem Senses. 2005;30:719–726 [DOI] [PubMed] [Google Scholar]
- 26.Beckett EL, Martin C, Yates Z, Veysey M, Duesing K, Lucock M. Bitter taste genetics—The relationship to tasting, liking, consumption and health. Food Funct. 2014;5:3040–3054 [DOI] [PubMed] [Google Scholar]
- 27.Scott TR, Mark GP. The taste system encodes stimulus toxicity. Brain Res. 1987;414:197–203 [DOI] [PubMed] [Google Scholar]
- 28.Kinnamon SC. Taste receptor signalling—From tongues to lungs. Acta Physiol (Oxf). 2012;204:158–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taruno A, Matsumoto I, Ma Z, Marambaud P, Foskett JK. How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays. 2013;35:1111–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tordoff MG, Aleman TR, Ellis HT, et al. Normal taste acceptance and preference of PANX1 knockout mice. Chem Senses. 2015;40:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenbeuch A, Anderson CB, Kinnamon SC. Mice lacking pannexin 1 release ATP and respond normally to all taste qualities. Chem Senses. 2015;40:461–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandenbeuch A, Larson ED, Anderson CB, et al. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol. 2015;593:1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Lorenzo PM, Lemon CH. The neural code for taste in the nucleus of the solitary tract of the rat: Effects of adaptation. Brain Res. 2000;852:383–397 [DOI] [PubMed] [Google Scholar]
- 34.Travers JB, Grill HJ, Norgren R. The effects of glossopharyngeal and chorda tympani nerve cuts on the ingestion and rejection of sapid stimuli: An electromyographic analysis in the rat. Behav Brain Res. 1987;25:233–246 [DOI] [PubMed] [Google Scholar]
- 35.Norgren R, Nishijo H, Travers SP. Taste responses from the entire gustatory apparatus. Ann N Y Acad Sci. 1989;575:246–263; discussion 263–244 [DOI] [PubMed] [Google Scholar]
- 36.de Brito Sanchez G, Giurfa M. A comparative analysis of neural taste processing in animals. Philos Trans R Soc Lond B Biol Sci. 2011;366:2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Y, Gillis-Smith S, Jin H, Trankner D, Ryba NJ, Zuker CS. Sweet and bitter taste in the brain of awake behaving animals. Nature. 2015;527:512–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwatsuki K, Torii K. Peripheral chemosensing system for tastants and nutrients. Curr Opin Endocrinol Diabetes Obes. 2012;19:19–25 [DOI] [PubMed] [Google Scholar]
- 39.Behrens M, Meyerhof W. Oral and extraoral bitter taste receptors. Results Probl Cell Differ. 2010;52:87–99 [DOI] [PubMed] [Google Scholar]
- 40.Iwatsuki K, Ichikawa R, Uematsu A, Kitamura A, Uneyama H. Torii K. Detecting sweet and umami tastes in the gastrointestinal tract. Acta Physiol (Oxf). 2012;204:169–177 [DOI] [PubMed] [Google Scholar]
- 41.Iwatsuki K, Uneyama H. Sense of taste in the gastrointestinal tract. J Pharmacol Sci. 2012;118:123–128 [DOI] [PubMed] [Google Scholar]
- 42.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. 2009;1170:91–94 [DOI] [PubMed] [Google Scholar]
- 43.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822S–825S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakagawa Y, Nagasawa M, Yamada S, et al. Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS One. 2009;4:e5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokrashvili Z, Yee KK, Ilegems E, et al. Endocrine taste cells. Br J Nutr. 2014;111 Suppl 1:S23–S29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F. Taste perception: From the tongue to the testis. Mol Hum Reprod. 2013;19:349–360 [DOI] [PubMed] [Google Scholar]
- 48.Finger TE, Bottger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100:8981–8986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tizzano M, Finger TE. Chemosensors in the nose: Guardians of the airways. Physiology (Bethesda). 2013;28:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dehkordi O, Rose JE, Balan KV, Millis RM, Bhatti B, Jayam-Trouth A. Co-expression of nAChRs and molecules of the bitter taste transduction pathway by epithelial cells of intrapulmonary airways. Life Sci. 2010;86:281–288 [DOI] [PubMed] [Google Scholar]
- 51.Robinett KS, Koziol-White CJ, Akoluk A, An SS, Panettieri RA., Jr. Liggett SB. Bitter taste receptor function in asthmatic and nonasthmatic human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2014;50:678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci. 2009;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh N, Vrontakis M, Parkinson F, Chelikani P. Functional bitter taste receptors are expressed in brain cells. Biochem Biophys Res Commun. 2011;406:146–151 [DOI] [PubMed] [Google Scholar]
- 55.Dehkordi O, Rose JE, Fatemi M, et al. Neuronal expression of bitter taste receptors and downstream signaling molecules in the rat brainstem. Brain Res. 2012;1475:1–10 [DOI] [PubMed] [Google Scholar]
- 56.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176 [DOI] [PubMed] [Google Scholar]
- 57.Voigt A, Bojahr J, Narukawa M, Hubner S, Boehm U, Meyerhof W. Transsynaptic Tracing from Taste Receptor Cells Reveals Local Taste Receptor Gene Expression in Gustatory Ganglia and Brain. J Neurosci. 2015;35:9717–9729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renwick AG, Molinary SV. Sweet-taste receptors, low-energy sweeteners, glucose absorption and insulin release. Br J Nutr. 2010;104:1415–1420 [DOI] [PubMed] [Google Scholar]
- 59.Shirazi-Beechey SP, Moran AW, Batchelor DJ, Daly K, Al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. Proc Nutr Soc. 2011;70:185–193 [DOI] [PubMed] [Google Scholar]
- 60.Kaji I, Karaki S, Kuwahara A. Taste sensing in the colon. Curr Pharm Des. 2014;20:2766–2774 [DOI] [PubMed] [Google Scholar]
- 61.Daniel H, Zietek T. Taste and move: Glucose and peptide transporters in the gastrointestinal tract. Exp Physiol. 2015;100:1441–1450 [DOI] [PubMed] [Google Scholar]
- 62.Laffitte A, Neiers F, Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. 2014;17:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu J, Cao J, Iguchi N, Riethmacher D, Huang L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod. 2013;19:17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douglas JE, Saunders CJ, Reed DR, Cohen NA. A role for airway taste receptor modulation in the treatment of upper respiratory infections. Expert Rev Respir Med. 2016;10:157–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith KR, Hussain T, Karimian Azari E, et al. Disruption of the sugar sensing receptor T1R2 attenuates metabolic derangements associated with diet-induced obesity. Am J Physiol Endocrinol Metab. 2016;310:E688–E699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyerhof W, Batram C, Kuhn C, et al. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170 [DOI] [PubMed] [Google Scholar]
- 67.Pirastu N, Kooyman M, Traglia M, et al. Association analysis of bitter receptor genes in five isolated populations identifies a significant correlation between TAS2R43 variants and coffee liking. PLoS One. 2014;9:e92065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ledda M, Kutalik Z, Souza Destito MC, et al. GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum Mol Genet. 2014;23:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lipchock SV, Spielman Al, Mennella JA, Mansfield CJ, Hwang LD, Douglas JE, Reed DR. Caffeine bitterness is related to daily caffeine intake and bitter receptor mRNA abundance in human taste tissue. Perception, 2017, pp. 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pronin AN, Xu H, Tang H, Zhang L, Li Q, Li X. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Curr Biol. 2007;17:1403–1408 [DOI] [PubMed] [Google Scholar]
- 71.Roudnitzky N, Risso D, Drayna D, Behrens M, Meyerhof W, Wooding SP. Copy number variation in TAS2R bitter taste receptor genes: Structure, origin, and population genetics. Chem Senses. 2016;41:649–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roudnitzky N, Bufe B, Thalmann S, et al. Genomic, genetic and functional dissection of bitter taste responses to artificial sweeteners. Hum Mol Genet. 2011;20:3437–3449 [DOI] [PubMed] [Google Scholar]
- 73.Cornelis MC, Monda KL, Yu K, et al. Genome-wide meta-analysis identifies regions on 7p21 (AHR) and 15q24 (CYP1A2) as determinants of habitual caffeine consumption. PLoS Genet. 2011;7:e1002033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofmann T. Identification of the key bitter compounds in our daily diet is a prerequisite for the understanding of the hTAS2R gene polymorphisms affecting food choice. Ann N Y Acad Sci. 2009;1170:116–125 [DOI] [PubMed] [Google Scholar]
- 75.Suess B, Brockhoff A, Meyerhof W, Hofmann T. The Odorant (R)-Citronellal attenuates caffeine bitterness by inhibiting the bitter receptors TAS2R43 and TAS2R46. J Agric Food Chem. 2016. [Epub ahead of print]. DOI: 10.1021/acs.jafc.6b03554 [DOI] [PubMed] [Google Scholar]
- 76.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: From mammals to insects. Cell. 2009;139:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi P, Zhang J, Yang H, Zhang YP. Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol Biol Evol. 2003;20:805–814 [DOI] [PubMed] [Google Scholar]
- 78.Allen AL, McGeary JE, Hayes JE. Polymorphisms in TRPV1 and TAS2Rs associate with sensations from sampled ethanol. Alcohol Clin Exp Res. 2014;38:2550–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dotson CD, Wallace MR, Bartoshuk LM, Logan HL. Variation in the gene TAS2R13 is associated with differences in alcohol consumption in patients with head and neck cancer. Chem Senses. 2012;37:737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glendinning JI, Bloom LD, Onishi M, et al. Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem Senses. 2005;30:299–316 [DOI] [PubMed] [Google Scholar]
- 82.Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800 [DOI] [PubMed] [Google Scholar]
- 83.Wong GT, Ruiz-Avila L, Ming D, Gannon KS, Margolskee RF. Biochemical and transgenic analysis of gustducin's role in bitter and sweet transduction. Cold Spring Harb Symp Quant Biol. 1996;61:173–184 [PubMed] [Google Scholar]
- 84.Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604 [DOI] [PubMed] [Google Scholar]
- 85.Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266 [DOI] [PubMed] [Google Scholar]
- 86.Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229 [DOI] [PubMed] [Google Scholar]
- 87.Ishimaru Y, Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J Dent Res. 2009;88:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tordoff MG, Ellis HT. Taste dysfunction in BTBR mice due to a mutation of Itpr3, the inositol triphosphate receptor 3 gene. Physiol Genomics. 2013;45:834–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Detzel A, Wink M. Attraction, deterrance, or intoxication of bees (apis mellifera) by plant allelochemicals. Chemoecology. 1993;4:8–18 [Google Scholar]
- 90.Ashihara H, Crozier A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001;6:407–413 [DOI] [PubMed] [Google Scholar]
- 91.Wright GA, Baker DD, Palmer MJ, et al. Caffeine in floral nectar enhances a pollinator's memory of reward. Science. 2013;339:1202–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:4495–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817 [DOI] [PubMed] [Google Scholar]
- 94.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079 [DOI] [PubMed] [Google Scholar]
- 95.Huckesfeld S, Peters M, Pankratz MJ. Central relay of bitter taste to the protocerebrum by peptidergic interneurons in the Drosophila brain. Nat Commun. 2016;7:12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glendinning JI, Tarre M, Asaoka K. Contribution of different bitter-sensitive taste cells to feeding inhibition in a caterpillar (Manduca sexta). Behav Neurosci. 1999;113:840–854 [PubMed] [Google Scholar]
- 97.Glendinning JI, Brown H, Capoor M, Davis A, Gbedemah A, Long E. A peripheral mechanism for behavioral adaptation to specific “bitter” taste stimuli in an insect. J Neurosci. 2001;21:3688–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamb CF, Finger TE. Gustatory control of feeding behavior in goldfish. Physiol Behav. 1995;57:483–488 [DOI] [PubMed] [Google Scholar]
- 99.Field KL, Beauchamp GK, Kimball BA, Mennella JA, Bachmanov AA. Bitter avoidance in guinea pigs (Cavia porcellus) and mice (Mus musculus and Peromyscus leucopus). J Comp Psychol. 2010;124:455–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aspen J, Gatch MB, Woods JH. Training and characterization of a quinine taste discrimination in rhesus monkeys. Psychopharmacology (Berl). 1999;141:251–257 [DOI] [PubMed] [Google Scholar]
- 101.Lal H, Shearman GT, Cafaro MP. Discrimination of sucrose-taste by the rat as a potential bioassay for sweeteners. Drug Nutr Interact. 1982;1:119–124 [PubMed] [Google Scholar]
- 102.Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, et al. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 2007;282:37225–37231 [DOI] [PubMed] [Google Scholar]
- 103.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34 [DOI] [PubMed] [Google Scholar]
- 104.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fredholm BB.IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552 [PMC free article] [PubMed] [Google Scholar]
- 106.Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastiao AM. Adenosine: Setting the stage for plasticity. Trends Neurosci. 2013;36:248–257 [DOI] [PubMed] [Google Scholar]
- 107.Muller CE, Jacobson KA. Xanthines as adenosine receptor antagonists. Handb Exp Pharmacol. 2011;200:151–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 2012;32:322–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schiffman SS, Gill JM, Diaz C. Methyl xanthines enhance taste: Evidence for modulation of taste by adenosine receptor. Pharmacol Biochem Behav. 1985;22:195–203 [DOI] [PubMed] [Google Scholar]
- 110.Keast RS, Riddell LJ. Caffeine as a flavor additive in soft-drinks. Appetite. 2007;49:255–259 [DOI] [PubMed] [Google Scholar]
- 111.Griffiths RR, Vernotica EM. Is caffeine a flavoring agent in cola soft drinks? Arch Fam Med. 2000;9:727–734 [DOI] [PubMed] [Google Scholar]
- 112.Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—A growing problem. Drug Alcohol Depend. 2009;99:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brosvic GM, Rowe MM. Methyl xanthine, adenosine, and human taste responsivity. Physiol Behav. 1992;52:559–563 [DOI] [PubMed] [Google Scholar]
- 114.Newland MC, Brown K. Oral caffeine consumption by rats: The role of flavor history, concentration, concurrent food, and an adenosine agonist. Pharmacol Biochem Behav. 1992;42:651–659 [DOI] [PubMed] [Google Scholar]
- 115.Marangos PJ, Paul SM, Parma AM, Goodwin FK, Syapin P, Skolnick P. Purinergic inhibition of diazepam binding to rat brain (in vitro). Life Sci. 1979;24:851–857 [DOI] [PubMed] [Google Scholar]
- 116.Shi D, Padgett WL, Daly JW. Caffeine analogs: Effects on ryanodine-sensitive calcium-release channels and GABAA receptors. Cell Mol Neurobiol. 2003;23:331–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taketo M, Matsuda H, Yoshioka T. Calcium-independent inhibition of GABA(A) current by caffeine in hippocampal slices. Brain Res. 2004;1016:229–239 [DOI] [PubMed] [Google Scholar]
- 118.Lopez F, Miller LG, Greenblatt DJ, Kaplan GB, Shader RI. Interaction of caffeine with the GABAA receptor complex: Alterations in receptor function but not ligand binding. Eur J Pharmacol. 1989;172:453–459 [DOI] [PubMed] [Google Scholar]
- 119.Roseti C, Martinello K, Fucile S, et al. Adenosine receptor antagonists alter the stability of human epileptic GABAA receptors. Proc Natl Acad Sci U S A. 2008;105:15118–15123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ley JP. Masking bitter taste by molecules. Chem Percept. 2008;1:58–77 [Google Scholar]
- 121.Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci. 2011;31:5782–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hagiwara N, Katarova Z, Siracusa LD, Brilliant MH. Nonneuronal expression of the GABA(A) beta3 subunit gene is required for normal palate development in mice. Dev Biol. 2003;254:93–101 [DOI] [PubMed] [Google Scholar]
- 123.Krantis A, Mattar K, Glasgow I. Rat gastroduodenal motility in vivo: Interaction of GABA and VIP in control of spontaneous relaxations. Am J Physiol. 1998;275:G897–G903 [DOI] [PubMed] [Google Scholar]
- 124.Antonioli L, Fornai M, Colucci R, et al. Regulation of enteric functions by adenosine: Pathophysiological and pharmacological implications. Pharmacol Ther. 2008;120:233–253 [DOI] [PubMed] [Google Scholar]
- 125.Glore S, Ricker A. Trigeminal neuralgia: Case study of pain cessation with a low-caffeine diet. J Am Diet Assoc. 1991;91:1120–1121 [PubMed] [Google Scholar]
- 126.Liu L, Simon SA. Responses of cultured rat trigeminal ganglion neurons to bitter tastants. Chem Senses. 1998;23:125–130 [DOI] [PubMed] [Google Scholar]
- 127.Roper SD. TRPs in taste and chemesthesis. Handb Exp Pharmacol. 2014;223:827–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gees M, Alpizar YA, Luyten T, et al. Differential effects of bitter compounds on the taste transduction channels TRPM5 and IP3 receptor type 3. Chem Senses. 2014;39:295–311 [DOI] [PubMed] [Google Scholar]
- 129.Dhaka A, Uzzell V, Dubin AE, et al. TRPV1 is activated by both acidic and basic pH. J Neurosci. 2009;29:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Daher JP, Gover TD, Moreira TH, Lopes VG, Weinreich D. The identification of a caffeine-induced Ca2+ influx pathway in rat primary sensory neurons. Mol Cell Biochem. 2009;327:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kichko TI, Lennerz J, Eberhardt M, et al. Bimodal concentration-response of nicotine involves the nicotinic acetylcholine receptor, transient receptor potential vanilloid type 1, and transient receptor potential ankyrin 1 channels in mouse trachea and sensory neurons. J Pharmacol Exp Ther. 2013;347:529–539 [DOI] [PubMed] [Google Scholar]
- 132.Liu L, Zhu W, Zhang ZS, et al. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. J Neurophysiol. 2004;91:1482–1491 [DOI] [PubMed] [Google Scholar]
- 133.Nagatomo K, Kubo Y. Caffeine activates mouse TRPA1 channels but suppresses human TRPA1 channels. Proc Natl Acad Sci U S A. 2008;105:17373–17378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Afroz A, Howlett N, Shukla A, et al. Gustatory receptor neurons in Manduca sexta contain a TrpA1-dependent signaling pathway that integrates taste and temperature. Chem Senses. 2013;38:605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao FL, Lu SG, Herness S. Dual actions of caffeine on voltage-dependent currents and intracellular calcium in taste receptor cells. Am J Physiol Regul Integr Comp Physiol. 2002;283:R115–R129 [DOI] [PubMed] [Google Scholar]
- 136.Breslin PA, Beauchamp GK. Suppression of bitterness by sodium: Variation among bitter taste stimuli. Chem Senses. 1995;20:609–623 [DOI] [PubMed] [Google Scholar]
- 137.Maes K, Missiaen L, Parys JB, et al. Adenine-nucleotide binding sites on the inositol 1,4,5-trisphosphate receptor bind caffeine, but not adenophostin A or cyclic ADP-ribose. Cell Calcium. 1999;25:143–152 [DOI] [PubMed] [Google Scholar]
- 138.Guerreiro S, Marien M, Michel PP. Methylxanthines and ryanodine receptor channels. Handb Exp Pharmacol. 2011;200:135–150 [DOI] [PubMed] [Google Scholar]
- 139.Mustard JA. The buzz on caffeine in invertebrates: Effects on behavior and molecular mechanisms. Cell Mol Life Sci. 2014;71:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clapp TR, Trubey KR, Vandenbeuch A, et al. Tonic activity of Galpha-gustducin regulates taste cell responsivity. FEBS Lett. 2008;582:3783–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hoon MA, Northup JK, Margolskee RF, Ryba NJ. Functional expression of the taste specific G-protein, alpha-gustducin. Biochem J. 1995;309:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kolesnikov SS, Margolskee RF. A cyclic-nucleotide-suppressible conductance activated by transducin in taste cells. Nature. 1995;376:85–88 [DOI] [PubMed] [Google Scholar]
- 143.Ruiz-Avila L, McLaughlin SK, Wildman D, et al. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature. 1995;376:80–85 [DOI] [PubMed] [Google Scholar]
- 144.Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23:9947–9952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosenzweig S, Yan W, Dasso M, Spielman AI. Possible novel mechanism for bitter taste mediated through cGMP. J Neurophysiol. 1999;81:1661–1665 [DOI] [PubMed] [Google Scholar]
- 146.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702 [DOI] [PubMed] [Google Scholar]
- 147.Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711 [DOI] [PubMed] [Google Scholar]
- 148.Masuho I, Tateyama M, Saitoh O. Characterization of bitter taste responses of intestinal STC-1 cells. Chem Senses. 2005;30:281–290 [DOI] [PubMed] [Google Scholar]
- 149.Malach E, Shaul ME, Peri I, et al. Membrane-permeable tastants amplify beta2-adrenergic receptor signaling and delay receptor desensitization via intracellular inhibition of GRK2's kinase activity. Biochim Biophys Acta. 2015;1850:1375–1388 [DOI] [PubMed] [Google Scholar]
- 150.Zubare-Samuelov M, Shaul ME, Peri I, Aliluiko A, Tirosh O, Naim M. Inhibition of signal termination-related kinases by membrane-permeant bitter and sweet tastants: Potential role in taste signal termination. Am J Physiol Cell Physiol. 2005;289:C483–C492 [DOI] [PubMed] [Google Scholar]
- 151.Amin N, Byrne E, Johnson J, et al. Genome-wide association analysis of coffee drinking suggests association with CYP1A1/CYP1A2 and NRCAM. Mol Psychiatry. 2012;17:1116–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sulem P, Gudbjartsson DF, Geller F, et al. Sequence variants at CYP1A1-CYP1A2 and AHR associate with coffee consumption. Hum Mol Genet. 2011;20:2071–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matthaei J, Tzvetkov MV, Strube J, et al. Heritability of caffeine metabolism: Environmental effects masking genetic effects on CYP1A2 activity but not on NAT2. Clin Pharmacol Ther. 2016;100:606–616 [DOI] [PubMed] [Google Scholar]
- 154.Denden S, Bouden B, Haj Khelil A, Ben Chibani J, Hamdaoui MH. Gender and ethnicity modify the association between the CYP1A2 rs762551 polymorphism and habitual coffee intake: Evidence from a meta-analysis. Genet Mol Res. 2016;15; DOI: 10.4238/gmr.15027487 [DOI] [PubMed] [Google Scholar]
- 155.Platt DE, Ghassibe-Sabbagh M, Salameh P, et al. Caffeine impact on metabolic syndrome components is modulated by a CYP1A2 variant. Ann Nutr Metab. 2016;68:1–11 [DOI] [PubMed] [Google Scholar]
- 156.Renda G, Committeri G, Zimarino M, et al. Genetic determinants of cognitive responses to caffeine drinking identified from a double-blind, randomized, controlled trial. Eur Neuropsychopharmacol. 2015;25:798–807 [DOI] [PubMed] [Google Scholar]
- 157.Cornelis MC, El-Sohemy A, Campos H. Genetic polymorphism of the adenosine A2A receptor is associated with habitual caffeine consumption. Am J Clin Nutr. 2007;86:240–244 [DOI] [PubMed] [Google Scholar]
- 158.Cornelis MC, Kacprowski T, Menni C, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet. 2016;9:1. [DOI] [PubMed] [Google Scholar]
- 159.Hansen JL, Reed DR, Wright MJ, Martin NG, Breslin PA. Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem Senses. 2006;31:403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rogers PJ, Hohoff C, Heatherley SV, et al. Association of the anxiogenic and alerting effects of caffeine with ADORA2A and ADORA1 polymorphisms and habitual level of caffeine consumption. Neuropsychopharmacology. 2010;35:1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Childs E, Hohoff C, Deckert J, Xu K, Badner J, de Wit H. Association between ADORA2A and DRD2 polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2008;33:2791–2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702 [DOI] [PubMed] [Google Scholar]
- 163.Rescifina A, Chiacchio U, Iannazzo D, Piperno A, Romeo G. beta-cyclodextrin and caffeine complexes with natural polyphenols from olive and olive oils: NMR, thermodynamic, and molecular modeling studies. J Agric Food Chem. 2010;58:11876–11882 [DOI] [PubMed] [Google Scholar]
- 164.Katsuragi Y, Yasumasu T, Kurihara K. Lipoprotein that selectively inhibits taste nerve responses to bitter substances. Brain Res. 1996;713:240–245 [DOI] [PubMed] [Google Scholar]
- 165.Yeomans MR, Spetch H, Rogers PJ. Conditioned flavour preference negatively reinforced by caffeine in human volunteers. Psychopharmacology (Berl). 1998;137:401–409 [DOI] [PubMed] [Google Scholar]
- 166.Yeomans MR, Jackson A, Lee MD, Nesic J, Durlach PJ. Expression of flavour preferences conditioned by caffeine is dependent on caffeine deprivation state. Psychopharmacology (Berl). 2000;150:208–215 [DOI] [PubMed] [Google Scholar]
- 167.Yeomans MR, Durlach PJ, Tinley EM. Flavour liking and preference conditioned by caffeine in humans. Q J Exp Psychol B. 2005;58:47–58 [DOI] [PubMed] [Google Scholar]
- 168.Temple JL, Ziegler AM, Graczyk A, Bendlin A, O'Leary S, Schnittker YS. Influence of caffeine on the liking of novel-flavored soda in adolescents. Psychopharmacology (Berl). 2012;223:37–45 [DOI] [PubMed] [Google Scholar]
- 169.Dsamou M, Palicki O, Septier C, et al. Salivary protein profiles and sensitivity to the bitter taste of caffeine. Chem Senses. 2012;37:87–95 [DOI] [PubMed] [Google Scholar]
- 170.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294 [DOI] [PubMed] [Google Scholar]
- 172.Garcia J, Hankins WG, Rusiniak KW. Letter: Flavor aversion studies. Science. 1976;192:265–267 [DOI] [PubMed] [Google Scholar]
- 173.Meyerhof W, Behrens M, Brockhoff A, Bufe B, Kuhn C. Human bitter taste perception. Chem Senses. 2005;30 Suppl 1:i14–i15 [DOI] [PubMed] [Google Scholar]
- 174.Lipchock SV, Mennella JA, Spielman AI, Reed DR. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am J Clin Nutr. 2013;98:1136–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22:1481–1485 [DOI] [PubMed] [Google Scholar]
- 176.Pelchat ML, Danowski S. A possible genetic association between PROP-tasting and alcoholism. Physiol Behav. 1992;51:261–1266 [DOI] [PubMed] [Google Scholar]
- 177.Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: A review of animal and human studies. Alcohol Alcohol. 1999;34:386–395 [DOI] [PubMed] [Google Scholar]
- 178.DeMet E, Stein MK, Tran C, Chicz-DeMet A, Sangdahl C, Nelson J. Caffeine taste test for panic disorder: Adenosine receptor supersensitivity. Psychiatry Res. 1989;30:231–242 [DOI] [PubMed] [Google Scholar]
- 179.Mennella JA, Bobowski NK. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol Behav. 2015;152:502–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mennella JA, Reed DR, Roberts KM, Mathew PS, Mansfield CJ. Age-related differences in bitter taste and efficacy of bitter blockers. PLoS One. 2014;9:e103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Seifert SM, Schaechter JL, Hershorin ER, Lipshultz SE. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011;127:511–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vandenbeuch A, Kinnamon SC. Why do taste cells generate action potentials? J Biol 8 2009;42 DOI: 10.1186/jbiol38 [DOI] [PMC free article] [PubMed] [Google Scholar]