Abstract
To examine the role of isoamylase1 (ISA1) in amylopectin biosynthesis in plants, a genomic DNA fragment from Aegilops tauschii was introduced into the ISA1-deficient rice (Oryza sativa) sugary-1 mutant line EM914, in which endosperm starch is completely replaced by phytoglycogen. A. tauschii is the D genome donor of wheat (Triticum aestivum), and the introduced fragment effectively included the gene for ISA1 for wheat (TaISA1) that was encoded on the D genome. In TaISA1-expressing rice endosperm, phytoglycogen synthesis was substantially replaced by starch synthesis, leaving only residual levels of phytoglycogen. The levels of residual phytoglycogen present were inversely proportional to the expression level of the TaISA1 protein, although the level of pullulanase that had been reduced in EM914 was restored to the same level as that in the wild type. Small but significant differences were found in the amylopectin chain-length distribution, gelatinization temperatures, and A-type x-ray diffraction patterns of the starches from lines expressing TaISA1 when compared with wild-type rice starch, although in the first two parameters, the effect was proportional to the expression level of TaISA. The impact of expression levels of ISA1 on starch structure and properties provides support for the view that ISA1 is directly involved in the synthesis of amylopectin.
Amylopectin is generally the major constituent of starch, accounting for about 65% to 85% of storage starch. The remainder is amylose, which is essentially linear. Amylopectin has a defined structure composed of tandem linked clusters (approximately 9–10 nm each in length), where linear α-1,4-glucan chains are regularly branched via α-1,6-glucosidic linkages, whereas the glycogens of bacteria and animals have a more randomly branched structure (Thompson, 2000). The distinct structure of amylopectin (referred to as a tandem-cluster structure) contributes to the crystalline organization of the starch granule (Gallant et al., 1997). Variation of the cluster fine structure is considered to cause variations in starch functional properties between species (e.g. maize [Zea mays] starch versus potato [Solanum tuberosum] starch), tissues (e.g. storage starch versus assimilatory starch), and genetic backgrounds (e.g. japonica rice [Oryza sativa] starch versus indica rice starch). However, genetic engineering could remove such species-specific limitations of starch functional properties by modifying the fine structure of amylopectin in a variety of ways.
According to our current understanding, the structure of amylopectin is determined by four classes of enzymes: ADP-Glc pyrophosphorylase (AGPase), soluble starch synthase (SS), starch-branching enzyme (BE), and starch-debranching enzyme (DBE; Van den Koornhuyse et al., 1996; Smith et al., 1997; Kossmann and Lloyd, 2000; Myers et al., 2000; Nakamura, 2002; Ball and Morell, 2003; James et al., 2003). A current focus of research in starch biosynthesis is to evaluate the metabolic functions of individual enzymes involved in amylopectin biosynthesis and examine how manipulation of the enzymes can produce novel starches with distinct physicochemical properties. This will help to clarify the contribution of each enzyme to the structure of amylopectin and the impact of expression level on the structure and functionality of the starch.
Higher plants are known to have two types of DBE, isoamylase (ISA) and pullulanase (Lee and Whelan, 1971; Nakamura, 1996; Beatty et al., 1999; Fujita et al., 1999; Hussain et al., 2003). Several investigations have revealed that mutation in the ISA1 gene causes a dramatic change of the structure of amylopectin into a randomly and more highly branched structure of α-1,6-/α-1-4-polyglucans referred to as phytoglycogen (James et al., 1995; Mouille et al., 1996; Zeeman et al., 1998; Kubo et al., 1999; Burton et al., 2002). The reduction of ISA1 activity to about only 6% by using antisense technology results in alteration of normal amylopectin into modified amylopectin with more short side chains and soluble polyglucans (Fujita et al., 2003). Recently, Bustos et al. (2004) hypothesized that starch granule initiation is hampered in transgenic potato tuber with a reduced RNA for ISA genes. These observations show that ISA plays an essential role in amylopectin biosynthesis. However, the mechanism(s) through which ISA and/or pullulanase plays a part in the synthesis of amylopectin still remains to be resolved, although several ideas or models have been proposed (Ball et al., 1996; Zeeman et al., 1998; Myers et al., 2000; Burton et al., 2002; Nakamura, 2002; Ball and Morell, 2003, James et al., 2003).
Recently, Hussain et al. (2003) characterized three isoforms of ISA from potato tuber, designated as Stisa1, Stisa2, and Stisa3, and proposed that all higher plants produce these distinct isoforms encoded by three ISA gene families. The Sugary1 (Su1) protein of maize, the Sugary-1 protein of rice, and the Stisa1 protein of potato are the products of the same gene family that can be referred to as the ISA1 gene, whereas the Stisa2 gene together with the DBE1 locus in Arabidopsis (Arabidopsis thaliana; the Atisa2 gene) form the second ISA gene family (ISA2).
Detailed biochemical studies with ISA1-deficient mutants strongly suggest that there are phenotypic differences among plant species and tissues. For example, starch-type polyglucans and phytoglycogen-type water-soluble polysaccharides (WSP) coexist in the same cells even when ISA1 activity is completely lacking in maize endosperm (Dinges et al., 2001), barley (Hordeum vulgare) endosperm (Burton et al., 2002), and Arabidopsis leaf (Zeeman et al., 1998), whereas starch-type polyglucans are completely replaced by phytoglycogen in Chlamydomonas cell (Mouille et al., 1996) and rice endosperm (Nakamura et al., 1997). The basis for these differences in partitioning between starch and phytoglycogen in different species has yet to be understood.
Little is known about the exact roles of the three ISA isoforms in amylopectin biosynthesis. This study was conducted to clarify the role of ISA1 in amylopectin biosynthesis by introducing the wheat (Triticum aestivum) ISA1 gene (TaISA1; from the D genome donor Aegilops tauschii) into a rice sugary-1 mutant line EM914 in which starch is completely replaced by phytoglycogen in the endosperm (Nakamura et al., 1997; Kubo et al., 1999). Both TaISA1 and rice ISA1 gene (OsISA1) belong to the same gene family, and their predicted amino acid sequences show 83% identity (Rahman et al., 2003). We demonstrate that there are consistent relationships between the expression level of the wheat TaISA1 gene and the structure and properties of the starch present in the endosperm of the transgenic lines, indicating that ISA1 plays a direct role in amylopectin synthesis in plants.
RESULTS
Screening Strategy and Transformant Isolation
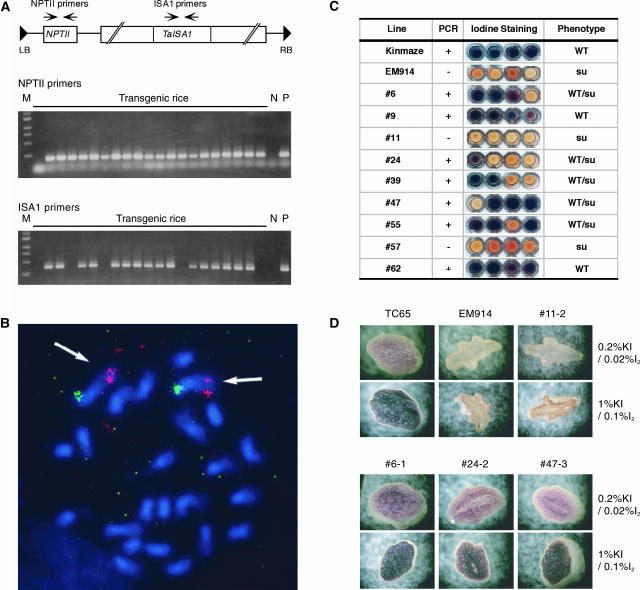
Figure 1 shows the screening strategy for rice (japonica-type cultivar Taichung 65 [TC65]) sugary-1 transformants containing the TaISA1 gene. A bacterial artificial chromosome (BAC) clone including the TaISA1 gene was isolated from a wheat D genome donor BAC library (Moullet et al., 1999) by hybridization to a known wheat ISA1 sequence (Rahman et al., 2003) and used directly for transformation as described in “Materials and Methods.” The BAC clone contained an approximately 75-kb DNA insert that also contained the gene for the selective marker neomycin phosphotransferase II (NPTII). The presence of this DNA fragment in the sugary-1 mutant line EM914 was detected by PCR using NPTII and TaISA1 primers (Fig. 1A). To evaluate the presence of T-DNA in the regenerated plants, total DNA was extracted from 130 transformed plants for PCR analysis, using primers amplifying regions of NPTII and TaISA1. The expected TaISA band was present in about 65% of the 130 transformants, whereas NPTII was detected in all plants examined. Fluorescence in situ hybridization experiments with the BAC clone including the TaISA1 gene showed hybridization signals on the chromosomes of rice. The chromosomes could be identified by double labeling using chromosome marker probes. One pair of signals for the TaISA1 gene was located at the distal tip of the long arm of chromosome 1 of transgenic rice line 47-3 (Fig. 1B). The location of TaISA1 gene was also determined to be at the distal tip of the short arm of chromosome 3 in line 6-1 and at the middle part of the short arm of chromosome 11 in line 24-2 (data not shown).
Figure 1.
Complementation of sugary-1 phenotype of EM914 in rice endosperm transformed by introduction of the TaISA1 gene. A, Representation of the BAC construct insert containing the TaISA1 gene. The introduced TaISA1 gene was detected by PCR method as described in “Materials and Methods.” NPT, Neomycin phosphotransferase; LB, left border; RB, right border; M, DNA size markers; N, nontreated control line; P, pCLD04541. B, Fluorescence in situ hybridization of a probe for the wheat DNA including the TaISA1 gene (red as indicated by arrows) and the 1S marker probe (green) with the transgenic rice (line 47-3) chromosomes. C, Screening strategy. The complementation of sugary-1 phenotype was shown by the accumulation of iodine-stained starch granules in endosperm from the TaISA1 transformant seeds at the nonsegragated T1 generation since phytoglycogen in the host sugary-1 mutant line, EM914, did not react with iodine while wild-type cv Kinmaze contained iodine-stained starch granules. D, Staining of cross sections of rice kernel with iodine solution. Mature sugary-1 (EM914) seeds were shrunken and did not stain with iodine solution due to a complete replacement of starch by phytoglycogen, whereas wild-type seeds contained dark-brown starch granules. Note that the homozygous seed phenotypes in some transformed lines (6-1, 24-2, and 47-3) at the T2 generation containing starch-like polyglucans were similar to the wild type.
T1 generation seeds from the TaISA1 transformants that produced starch-like polysaccharides in endosperm could be easily distinguished from those from sugary-1 mutant EM914 by iodine staining (Fig. 1C). The degree of recovery of the normal seed morphology in the transformants was directly related to the starch production in endosperm (Fig. 1D). The endosperm of a homozygous line 24-2 at the T2 generation had a central region that did not stain positive with iodine while the outer region of the endosperm was filled with starch (Fig. 1D), as observed in some alleles of sugary-1 mutant lines of rice (Nakamura et al., 1997).
Four homozygous lines were selected on the criteria that all of their seeds in the T2 and the following generation had starch (6-1, 24-2, and 47-3) or phytoglycogen (11-2). Seeds from the T3 and T4 generations were used for further analyses since all of their distinct phenotypes examined were stably inherited.
The Expression and Activities of OsISA Isoforms
Hussain et al. (2003) reported that higher plants have three isoforms of ISA, and that in potato tuber Stisa1 (GenBank accession no. AY132996) and Stisa2 (AY132997) are associated as a multimeric enzyme. The draft sequence of the rice genome (Yu et al., 2002) provides us with nucleotide sequences encoding OsISA2 (AC132483) and OsISA3 (AP005574). The cDNA clones of OsISA2 and OsISA3 were isolated from developing endosperm of rice cv TC65, and their nucleotide sequences were determined (data not shown). There were no introns on OsISA2 as reported in maize ISA2 (ZmISO2; AY172633), potato Stisa2, and Arabidopsis Atisa2 (AY139980). The predicted amino acid sequence of OsISA2 was most similar to ZmISO2 (78.5% identity), while the similarities with Stisa2 and Atisa2 were 51.5% and 30.8%, respectively. Homologies of the entire amino acid sequence of OsISA3 with maize ISA3 (ZmISO3; AY172634), Stisa3 (AY132998), and Atisa3 (AY091058) were 86.9%, 73.4%, and 72.9%, respectively.
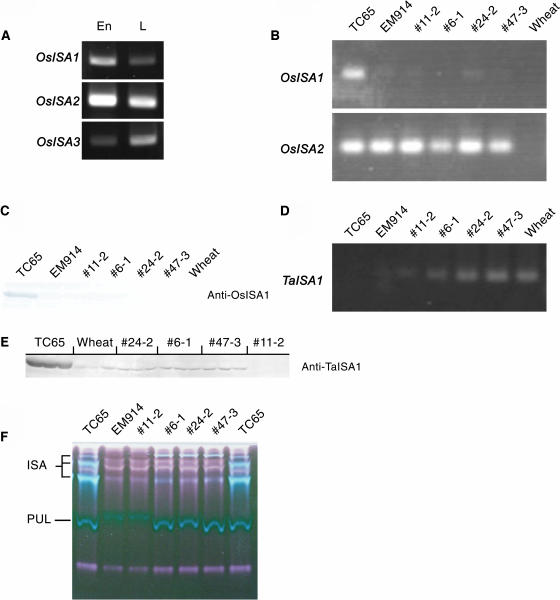
Figure 2A illustrates that OsISA1 (AB093426) was strongly expressed in developing endosperm at the early milking stage, whereas it was present at lower expression levels in green leaves. To understand the state of the TaISA1 protein (AF548379), it is necessary to know which of the other native ISA genes are expressed in rice endosperm. The OsISA2 was highly expressed in both endosperm and leaves. The level of OsISA3 transcript was extremely low in endosperm, whereas it was expressed in leaves.
Figure 2.
Expression and activities of ISA isoforms. A, Expression of OsISA1, OsISA2, and OsISA3 in developing endosperm and young green leaves of wild-type rice cv TC65 as measured by the RT-PCR method. B, Expression of OsISA1 and OsISA2 in developing endosperm at the early milking stage of the TaISA1 transgenic lines, TC65, and EM914 as measured by the RT-PCR method. C, Western-blot analysis of OsISA1 in developing endosperm at the late-milking stage of the TaISA1 transgenic lines, TC65, EM914, and wild-type wheat using polyclonal antibodies raised against purified OsISA1 (Fujita et al., 1999). Note that the anti-OsISA1 did not react to TaISA1. D, Expression of TaISA1 in developing endosperm at the early milking stage of the TaISA1 transgenic lines, TC65, EM914, and wild-type wheat as measured by the RT-PCR method. E, Western-blot analysis of ISA1 in developing endosperm at the late-milking stage of the TaISA1 transgenic lines, TC65, and wild-type wheat using polyclonal antibodies raised against purified TaISA1. Note that the anti-TaISA1 reacted not only to TaISA1 but also to OsISA1, and that the molecular size of the cross-reacted OsISA1 band was slightly larger than those of TaISA1 bands in wheat and the TaISA1 transgenic lines of rice. The data are the results of three separate measurements using separate single seeds. F, Native-PAGE activity staining of starch debranching enzymes in developing endosperm at the late-milking stage of transgenic lines (11-2, 26-1, 24-2, and 47-3), TC65, and EM914. The soluble protein extract was separated on a polyacrylamide gel containing 0.3% (w/v) amylopectin. After electrophoresis, the gel was incubated at 30°C for 2 h and stained with iodine solution. The positions of the ISA and pullulanase activity bands stained blue.
The reverse transcription (RT)-PCR analysis showed that the transcript of OsISA2 in endosperm of sugary-1 mutant and transformants was at the same level as that of the wild type, whereas the OsISA1 transcript was lacking in both the mutant and transformants (Fig. 2B). The absence of the OsISA1 protein in all the transformants as well as the mutant was shown by western-blot analysis (Fig. 2C).
Expression of the TaISA1 Gene in Endosperm from Transformed sugary-1 Mutants of Rice
Significant amounts of the TaISA1 transcript were detected in the endosperm of three transformed lines (6-1, 24-2, and 47-3) possessing starch, whereas a small amount of the transcript was detected in 11-2 (Fig. 2D). The TaISA1 antibodies cross-reacted not only with the TaISA1 protein expressed in rice and wheat endosperm, but also the OsISA1 protein in wild-type rice endosperm (Fig. 2E). The transformed lines 24-2, 6-1, and 47-3 had significant amounts of the TaISA1 protein (15.0%, 17.3%, and 22.5%, respectively) of the levels of rice ISA1 in wild-type endosperm if calculated on the assumption that the polyclonal TaISA1 antibodies cross-react equally with TaISA1 and OsISA1. Otherwise, the relative amounts of TaISA1 would possibly be lower than those values. Rice antibody against OsISA1 protein did not detect any ISA in these lines (but did detect ISA1 in wild-type rice), demonstrating that the protein detected was not derived from rice.
The ISA activity could be visualized by native-PAGE/activity staining analysis (Fig. 2F). No ISA activity was found in nontransformed sugary-1 mutant EM914 and the transgenic line 11-2, whereas the three transformed lines (6-1, 24-2, and 47-3) gave blue-staining ISA bands. The TaISA1 amount was quantified in these three lines as well as the line 11-2 (see Fig. 8 later).
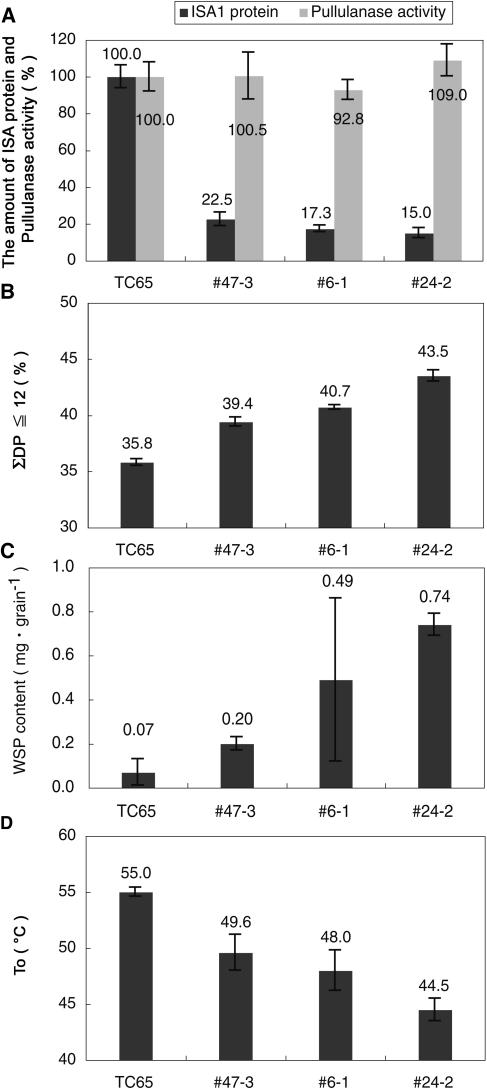
Figure 8.
Relationship between levels of DBE and the structure of amylopectin, the WSP content, and onset gelatinization temperature (T0). A, The ISA protein content and the activity of pullulanase. The values are means ± sd of nine separate measurements using nine separate single seeds, respectively. The data include the results shown in Figure 2E. B, The percentage of short chains with DP ≤ 12 of polyglucans. The data include the results shown in Table III. C, The content of WSP. The data are obtained from the results shown in Table II. D, The T0 values. The data are obtained from the results shown in Table III.
Effects of TaISA1 Expression on the Activities and Amounts of Enzymes Involved in Starch Biosynthesis
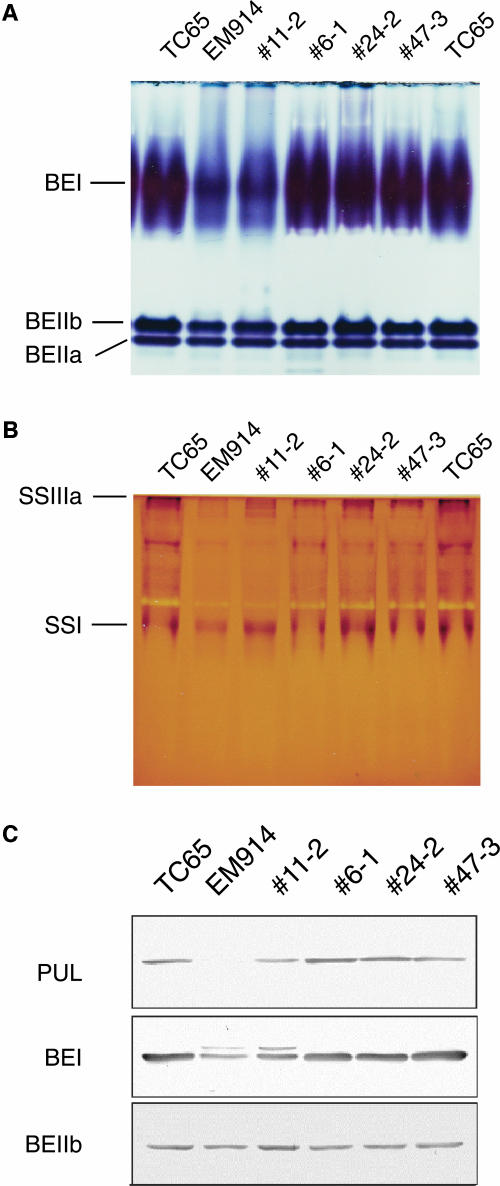
To examine the pleiotropic effects of the expression of TaISA1 gene in sugary-1 mutant on starch-metabolizing enzymes, the activities and amounts of these enzymes were quantified. The reduction of activity and amount of pullulanase found in EM914 (mutant) and typical of rice sugary-1 mutants (Nakamura et al., 1992b, 1996b, 1997) recovered to wild-type levels in the transformed lines 6-1, 24-2, and 47-1 (Figs. 2F and 3C). It is interesting that in line 11-2 the amount and activity of pullulanase was partially recovered (Fig. 3C; Table I), even with a low level of TaISA1 expression (Fig. 2D). Figure 3A indicates that the activities of both BEIIa and BEIIb isoforms were not affected in the sugary-1 mutation or by subsequent complementation by TaISA1. The level of BEI was significantly reduced in EM914 compared with the wild type. BEI activity was restored to wild-type levels in lines 6-1, 24-2, and 47-1; however, in line 11-2 the level was not markedly increased (Fig. 3, A and C). Figure 3B shows that the activities of SSI and SSIII isoforms were similar among transformants, EM914 (mutant), and TC65 (wild type). No marked differences in the total activities of AGPase or SS were observed among transformants, TC65, and EM914 (Table I).
Figure 3.
Detection of enzymes involved in starch biosynthesis in developing endosperm at the early milking stage of the TaISA1 transgenic lines, TC65, and EM914. A, Native-PAGE staining of BE isoforms BEI, BEIIa, and BEIIb. Soluble proteins were separated in native-PAGE gel. The gel was incubated in a solution containing phosphorylase a for 5 h at 30°C and then added by an iodine solution. These three BE activity bands stained purple. B, Native-PAGE staining of SS isoforms. Soluble proteins were separated in native-PAGE gels containing 0.8% (w/v) of oyster glycogen, incubated in the reaction solution containing 0. 5 m sodium citrate, and added by an iodine solution. The SSI and SSIIa activity bands stained dark blue. C, Western-blot analysis of pullulanase (PUL), BEI, and BEIIb with polyclonal antibodies raised against the respective enzymes purified from developing rice endosperm.
Table I.
The activities of enzymes in crude extracts of developing endosperms of wild-type TC65, sugary-1 mutant EM914, and transgenic lines
Developing endosperms from grains of 20 mg fresh weight were extracted as described in “Materials and Methods” and assayed for enzyme activities. Values are means ± se of measurements made on a number of independent experiments (shown in brackets).
| Activity (mmol min−1 g−1 Fresh Weight)
|
|||
|---|---|---|---|
| AGPase | SS | Pullulanase | |
| TC65 | 7.15 ± 1.11 [6] | 0.876 ± 0.144 [4] | 32.83 ± 2.61 [4] |
| 47-3 | 5.18 ± 1.07 [6] | 0.704 ± 0.113 [4] | 35.77 ± 3.12 [3] |
| 6-1 | 4.62 ± 1.07 [5] | 0.574 ± 0.041 [4] | 30.47 ± 1.65 [3] |
| 24-2 | 3.57 ± 0.50 [5] | 0.762 ± 0.155 [4] | 32.98 ± 4.21 [3] |
| 11-2 | 5.81 ± 1.14 [5] | 0.460 ± 0.090 [4] | 10.44 ± 3.26 [4] |
| EM914 | 4.58 ± 0.30 [6] | 0.540 ± 0.161 [4] | 5.86 ± 2.67 [4] |
Effects of TaISA1 Expression on the Morphology of the Seed
The mature dry seed morphology of the sugary-1 mutant was dramatically changed in terms of the size (Fig. 1D) and the weight (Table II). The TaISA1 transformant lines 47-3 and 6-1 had both grain weight and starch content comparable to those of TC65 (wild type); however, in line 24-2, which accumulated starch, and in line 11-2, which accumulated phytoglycogen, the seed size remained comparable to EM914 (mutant). It is not clear why the small difference in the amount of TaISA1 accumulated between lines 6-1 and 24-2 was associated with such a large difference in seed weight.
Table II.
Carbohydrate content in endosperms of wild-type TC65, sugary-1 mutant EM914, and transgenic lines
Values are means ± se of measurements made on a number of replications (shown in brackets).
| Grain Weight | Starch | WSP | WSP | |
|---|---|---|---|---|
| mg | mg/grain | mg/grain | %a | |
| TC65 | 23.6 ± 1.4 [5] | 13.9 ± 0.8 [3] | 0.07 ± 0.06 [3] | 0.5 |
| 47-3 | 20.3 ± 0.5 [5] | 15.4 ± 0.5 [3] | 0.20 ± 0.03 [3] | 1.3 |
| 6-1 | 22.2 ± 0.7 [5] | 13.5 ± 0.3 [3] | 0.49 ± 0.37 [3] | 3.5 |
| 24-2 | 14.9 ± 0.9 [5] | 10.6 ± 0.6 [3] | 0.74 ± 0.05 [3] | 6.5 |
| 11-2 | 14.1 ± 0.5 [5] | 0.39 ± 0.14 [3] | 4.40 ± 0.29 [3] | 91.8 |
| EM914 | 11.6 ± 0.6 [5] | 0.37 ± 0.05 [3] | 4.92 ± 0.30 [3] | 93.1 |
The amounts of WSP were expressed as percentages of total polyglucans.
Effects of TaISA1 Expression on the Structure of Starch and Amylopectin
Table II shows that in transformed lines 6-1, 24-2, and 47-3, in which TaISA1 was being expressed, the amount of phytoglycogen was dramatically reduced, whereas amount of starch synthesized was comparable to wild type. Therefore, these three transformants had a high proportion of starch to phytoglycogen in their endosperm as compared with EM914 (mutant) and line 11-2. The starch content per grain of these transformants was decreased in the order of 47-3, 6-1, and 24-2, respectively. The transgenic line 11-2 was devoid of starch and only produced phytoglycogen in the endosperm.
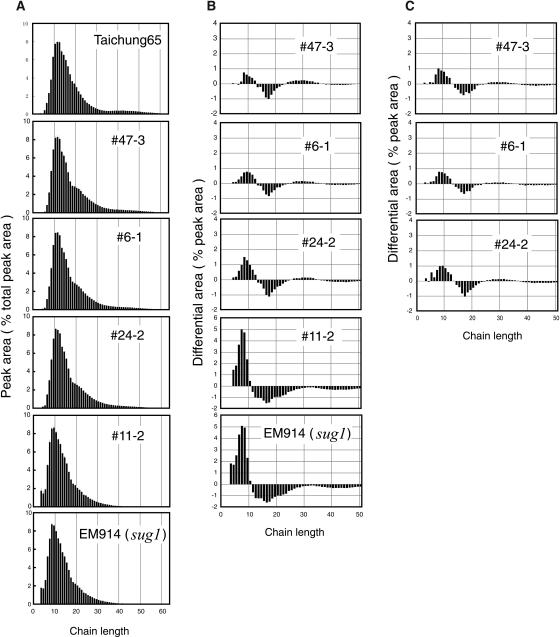
The differences in the fine structure of amylopectin in endosperm among transformants, the sugary-1 mutant, and the wild type were determined by measuring the chain-length distribution of their isoamylolysates on molar basis by capillary electrophoresis (O'Shea and Morell, 1996). The chain-length distribution of phytoglycogen in EM914 (mutant) was characterized by enrichment of short chains with degree of polymerization (DP) ≤ 10 and depletion of long chains with DP > 40 (Fig. 4A). The chain-length distributions of amylopectin as well as total polyglucans of transformants 6-1, 24-2, and 47-3 were similar to that of wild-type amylopectin, but there were small reproducible and distinct differences between each of their profiles and the wild type (Fig. 4, B and C). The chain profile of 11-2 polyglucans could not be distinguished from that of EM914 phytoglycogen.
Figure 4.
Chain-length distribution of amylopectin and total polyglucans in mature endosperm of the TaISA1 transgenic lines, TC65, and EM914. A, Normalized chain-length distribution of total polyglucans as percentage for each peak area of the total areas of all peaks with 3 ≤ DP ≤ 60. B, Differential plot derived by subtracting molar percentages of wild-type TC65 polyglucans from those of transformants and sugary-1 mutant EM914 (shown in A). C, Differential plot similar to B, except that amylopectin separated from soluble polyglucans by centrifugation was used instead of total polyglucans, as described by Fujita et al. (2003). Note that the data are similar between B and C, although amounts of very short chains with DP ≤ 8 in total polyglucans of line 24-2 (B) were higher to some extent than those in purified amylopectin (C), possibly due to the WSP included in total polyglucan preparation.
Effects of TaISA1 Expression on Physicochemical Properties of Starch Granules
The thermal properties of the starch granules in the endosperm of transformants were measured by differential scanning calorimeter (DSC). No DSC parameters were obtained from polyglucans in sugary-1 mutant and the line 11-2 because of the absence of granular starch. Onset temperatures for gelatinization (T0) of transformed polyglucans were 49.6°C, 48.0°C, and 44.5°C in 47-3, 6-1, and 24-2, respectively, these values being significantly lower than that (55.5°C) of wild-type starch (Table III). Gelatinization enthalpy changes (ΔH) for wild type, 47-3, 6-1, and 24-2 were 6.46, 5.39, 5.25, and 3.94 J/g, respectively.
Table III.
Gelatinization and properties of rice starches from wild-type TC65 and transgenic lines
T0, TP, and TC, Onset, peak, and conclusion temperatures (°C) of endotherm. ΔH, Enthalpy change of gelatinization and melting. Values are mean ± sd. ND, No detection.
| T0 | TP | TC | ΔH | |
|---|---|---|---|---|
| °C | °C | °C | J/g | |
| TC65 | 55.5 ± 0.4 | 63.2 ± 0.1 | 70.5 ± 0.3 | 6.46 ± 0.59 |
| 47-3 | 49.6 ± 1.6 | 61.6 ± 0.6 | 72.4 ± 1.6 | 5.39 ± 0.70 |
| 6-1 | 48.0 ± 1.8 | 56.3 ± 0.9 | 65.7 ± 1.0 | 5.25 ± 1.40 |
| 24-2 | 44.5 ± 1.0 | 53.7 ± 0.8 | 63.8 ± 1.0 | 3.94 ± 0.32 |
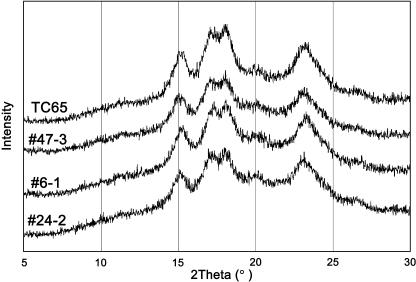
Polyglucans in transformed (6-1, 24-2, and 47-3) and wild-type endosperm all showed A-type x-ray diffraction patterns (Fig. 5). The sugary-1 mutant and the line 11-2 did not exhibit any diffraction pattern. It is noted that the major peak heights were significantly lower in the three transformants than in wild type, although the degree of crystallinity of starch among transformants was higher in the order of 6-1, 24-2, and 47-3. The result suggests that these transformants have lower crystallinity of starch as compared with wild-type starch, but the degree of crystallinity of the starch was not related with the onset gelatinization temperature. It is interesting that the degree of crystallinity of line 24-2 (with lower starch content and amount of TaISA1) should be higher than that of line 6-1, although the reason is unknown.
Figure 5.
X-ray diffraction pattern of starch in endosperm of the TaISA1 transgenic lines and TC65.
Effects of TaISA1 Expression on the Structure of Starch Granules
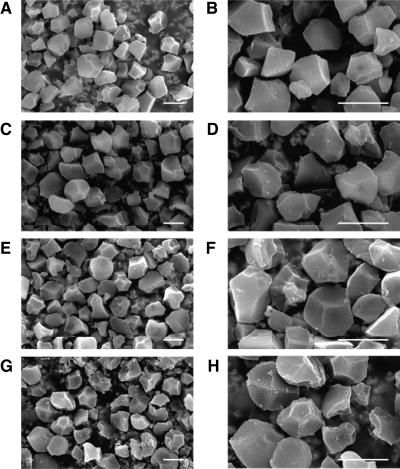
Scanning electron microscopy observations showed that the wild-type endosperm starch formed similar sizes of polygonal granules with sharp edges (Fig. 6). Although transformed endosperm of lines 6-1, 24-2, and 47-3 contained the large polygonal starch granules with the same sizes as those of wild-type starch granules, they had less distinct contour. In addition, these transformants contained more small granules with irregular shapes than the wild type. The proportion of these small granules was not necessarily high in the transformants, but the value increased in the order of 47-3, 6-1, and 24-2.
Figure 6.
Scanning electron micrographs of starch granules in endosperm of the TaISA1 transgenic lines and TC65. Starches were purified as described in “Materials and Methods.” Labels are TC65 (A and B), 47-3 (C and D), 6-1 (E and F), and 24-2 (G and H). The bars in A to H = 5 μm.
Measurements of TaISA1 Expressed in Transformed sugary-1 Rice Endosperm
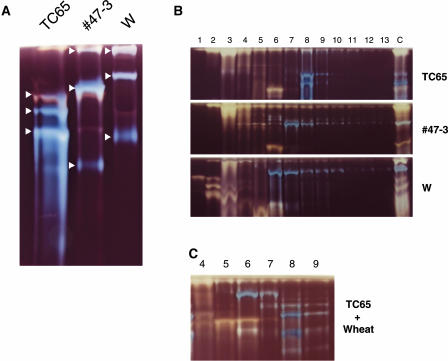
To ascertain whether there are any differences in the properties of the ISA1 expressed in rice endosperm from that of wheat endosperm, ISA extracts from the wild-type rice and wheat and from a TaISA1-transformed rice mutant were analyzed by native PAGE and anion-exchange chromatography. Figure 7A shows that both the patterns and migration rates of ISA bands on native gel differed between rice and wheat endosperm. It is clear that ISA activity bands in line 47-3 migrated differently from those of the wild-type rice, suggesting that the ISA activity of this line was derived from the TaISA1 product and not from OsISA1. Figure 7B demonstrates that TaISA1 eluted earlier than OsISA1 in Hitrap Q anion-exchange column (Amersham Pharmacia Biotech, Uppsala) chromatography. When the enzyme extract from endosperm of the transformed line 6-1 was applied onto the Hitrap Q column, the ISA activity was detected earlier than the wild-type rice ISA (Fig. 7C). These results indicate that the ISA activity detected in the transformed lines was the product of the TaISA1 gene.
Figure 7.
Comparison of chromatographic behavior of ISA between rice and wheat. Electrophoretic mobility of ISA activities on native gel containing 0.3% amylopectin was detected by staining of polyglucans with iodine solution. A, The ISA preparations were partially purified from 20 developing endosperm of TC65 and the TaISA1 transformant line 47-3 and 40 endosperm of wild-type wheat (W). The ISAI activity bands are indicated by arrows. B, The Hitrap-Q column chromatography of the ISA activity from developing endosperm of TC65, line 47-3, and wheat. The number on top of each lane indicates fraction number. c, The crude enzyme extract from TC65 endosperm. C, The Hitrap-Q column chromatography of the ISA activity from a mixture of developing endosperm of TC65 and wheat with the same volume. The number on top of each lane means fraction number.
Both rice and wheat endosperm ISA1 had the same retention time in TSKgel G3000SWXL (Tosoh, Tokyo) gel filtration chromatography, their molecular sizes being equivalent to about 400 to 500 kD (data not shown). This result suggests that both rice and wheat ISA1 form a multimeric structure, presumably a heptamer or a hexamer.
DISCUSSION
Complementation of sugary-1 Phenotype of Rice Endosperm by the TaISA1 Gene Expression
The current concept that starch DBE plays a crucial part in amylopectin biosynthesis is based on the observation that ISA-deficient mutants isolated from various plant species, including maize (James et al., 1995), rice (Kubo et al., 1999), barley (Burton et al., 2002), Arabidopsis (Zeeman et al., 1998), and Chlamydomonas (Mouille et al., 1996), are defective in amylopectin synthesis. In these mutants, amylopectin is largely or completely replaced by phytoglycogen. Phytoglycogen lacks the distinct tandem-cluster structure of amylopectin, but is very similar to glycogen in animals and bacteria in terms of their chain-length distribution and molecular size (Wong et al., 2003). Unlike starch, phytoglycogen does not form an insoluble complex, shows no crystallinity, and undergoes no thermal transition on heating in water (Wong et al., 2003).
This investigation proves that the expression of the normal TaISA1 gene could restore the capacity for synthesizing amylopectin and granular starch instead of phytoglycogen in endosperm of rice sugary-1 mutant line EM914. The transgenic lines 47-3, 6-1, and 24-2 that had significant TaISA1 activities produced amylopectin, as evidenced by production of polyglucans with amylopectin-like chain-length distribution (Fig. 4) as well as by accumulation of iodine-stained polyglucans like starch (Fig. 1D). The polyglucans in these lines formed polygonal granular structure (Fig. 5), and their morphological feature was similar to that of the wild-type rice starch granules (Fig. 6). On the contrary, the transformed line 11-2 with little expression of the TaISA1 did not exhibit such phenotypic changes in terms of polyglucan structure and properties (Figs. 1D and 4). The ISA activities (Fig. 2F) and amounts (Fig. 2E) detected in rice transgenic lines were derived from the introduced TaISA1 gene product since the TaISA1 transcript was measured in the TaISA1-transformed lines (Fig. 2D) and could be clearly differentiated from products of the rice OsISA1 in terms of their mobility patterns in native polyacrylamide electrophoresis (Fig. 7A) and their elution rates in anion-exchange column chromatography (Fig. 7, B and C). These results clearly indicate that TaISA1 can complement the metabolic role of endogenous OsISA1 in amylopectin biosynthesis of rice endosperm, confirming the genetic data that demonstrate that ISA1 is essential for amylopectin synthesis.
The Pleiotropic Effect of the TaISA1 Expression on Starch-Synthesizing Enzymes
A marked reduction of pullulanase activity was reported in the sugary-1 mutant endosperm of maize (Pan and Nelson, 1986; Dinges et al., 2001, 2003) and rice (Nakamura et al., 1992b, 1996b, 1997), whereas no changes in pullulanase activity were observed in mutants having a defective ISA gene in Chlamydomonas cell (Mouille et al., 1996), Arabidopsis leaf (Zeeman et al., 1998), and barley endosperm (Burton et al., 2002). Although the reason is unknown as to why there are such differences in the effects of the lesion of the ISA gene on pullulanase activity among plant species, it is interesting that pullulanase activity reverted to the wild-type level when the introduced TaISA1 gene was expressed in the endosperm of rice sugary-1 mutant (Fig. 3A; Table I). We found that in some mutant lines such as EM914, in which starch is completely converted to phytoglycogen, BEI activity is significantly lower than that in the wild type, whereas BEII isoforms are not significantly affected (Nakamura et al., 1992b; Fig. 3A). However, rice transgenic lines with ISA activity derived from the TaISA1 gene had the same level of BEI activities as the wild type (Fig. 4A). These results suggest that defect of the ISA1 gene has a pleiotropic effect on activities of pullulanase and BEI, but these activities are recovered when the ISA activity is rescued, although the mechanism of such phenomenon is unresolved.
Differing TaISA1 Gene Expression Levels Illustrate Relationships between the Structure of Amylopectin and Physicochemical Properties of Starch, and Indicate a Direct Role for Isoamylase in Amylopectin and Granular Starch Synthesis
The expression level of TaISA1 in sugary-1 rice varied from undetectable levels through to 22.5% of the level of expression of OsISA1 in wild-type rice, based on the assumption that the cross-reaction of polyclonal antibodies raised against TaISA1 is equivalent between TaISA1 and OsISA1. Among three lines (47-3, 6-1, and 24-2) in which there was significant conversion of phytoglycogen to starch as a result of TaISA1 expression, there were small but reproducible differences in structure and properties between the transgenic lines that correlated with the gene expression level. These are highly informative in terms of the role of ISA1 in amylopectin biosynthesis and the relationships between starch structure and granule properties. The fact that in these three lines the levels of pullulanase and BEI recovered to the same levels as in the wild type (Figs. 2F and 3A; Table I) indicates that the variations among lines were caused by the differences in the levels of TaISA1. Figure 8 compares the extents of these phenotypes with the levels of TaISA1 protein in transformed lines and wild-type TC65. There are three lines of evidence that support the hypothesis that ISA1 is directly involved in amylopectin synthesis, which in turn leads to starch granule assembly and properties.
First, there was a consistent relationship between TaISA1 expression level and starch and WSP production (Table II; Fig. 8C), such that increased expression of TaISA1 leads to an increase in starch content and a decrease in WSP content, consistent with the overall conclusion of this work that ISA activity is the essential factor for starch granule, as opposed to phytoglycogen, production.
Second, there was a consistent relationship between TaISA1 expression and the percentage of amylopectin chains of DP ≤ 12 (Figs. 4 and 8B). With increasing expression of TaISA1, the polyglucan produced moved from 58.3% DP ≤ 12 in line 11-2, which is typical of phytoglycogen (58.8%), to 39.4% DP ≤ 12 (47-3), which is close to wild-type starch (35.8%). The results obtained are consistent with the observations of Dinges et al. (2001), who demonstrated there are more short chains in the su1-ref and su1-r4582::Mu1 alleles of the maize sugary-1 locus compared to the wild type.
Third, there was a consistent relationship between TaISA1 expression and granule properties and crystallinity, as measured by DSC (Table II; Fig. 8D) and x-ray diffraction (Fig. 5). The sugary-1 mutant and the transformed line 11-2, which have no detectable levels of TaISA1, contained phytoglycogen only and therefore did not show either a gelatinization enthalpy peak in DSC or an A-type pattern in x-ray diffraction analysis (data not shown). These results also support the conclusion that there is a direct relationship between amylopectin chain-length distribution and starch granule properties, as shown in this example in a common genetic background. There was a direct correlation between the proportion of amylopectin short chains with DP ≤ 12, gelatinization onset temperature, and gelatinization enthalpy of the starch granules, suggesting that the chain-length distribution of the amylopectin underpins the strength of the interactions between amylopectin molecules that in turn determines granule properties. These results are consistent with data from other comparisons of different species, and mutants within species, that suggest that there is a causal relationship between amylopectin chain-length distribution and gelatinization and crystallinity parameters (Jane et al., 1999). Given that ISA has also been suggested to play a role in ordering the structure of amylopectin such that crystallization occurs more readily (Myers et al., 2000), it would be interesting to continue the analysis of these transgenic lines to investigate if there is a higher degree of clustering of branch points and a more ordered alignment of external chains, as exemplified by chains in structures such as Naegeli dextrins.
As TaISA1 expression level increased, both the onset temperature of gelatinization and the gelatinization enthalpy increased (Table III), indicating increased crystallinity in the granule. However, the data for line 24-2 suggest that the relationship between gelatinization properties and intensity of diffraction is complex (Fig. 5). The TaISA1 gene was located in chromosomes 1, 3, and 11 in the transformed lines 47-3, 6-1, and 24-2, respectively (Fig. 1B; data not shown), and, therefore, the expression pattern of the TaISA1 gene could possibly be altered temporally and spatially in rice endosperm, depending on the position of the chromosome where the gene was introduced. Anyhow, the fact that all complemented lines were still apparently less crystalline than the wild-type control is suggestive of an average significant difference between the complemented mutants and the wild type.
It is noted that the chain profiles of amylopectin and physicochemical properties of starches in transformants were not identical with those in the wild type (Figs. 4 and 5; Table III). These differences might have been caused by lower levels of TaISA in rice transformants (Fig. 2E). There may be an overall difference in strength of the promoter of the TaISA1 gene compared with the OsISA1 gene in the rice endosperm. It is also possible that the enzymatic properties of ISA1 differ between rice and wheat, although we reported that the TaISA1 gene shows 83.5% similarity to OsISA1 gene (Rahman et al., 2003). Figure 7 demonstrates that TaISA1 behaved differently from OsISA1 in nondenaturing electrophoresis, suggesting some differences in properties of ISA1 between these two plant species. It is also conceivable that there are differences between the activity of the wheat gene as expressed in wheat compared to the wheat gene expressed in rice due to differences in either intrinsic kinetic properties or due to the ability of the wheat gene to form appropriate protein-protein interactions in the rice endosperm. A number of starch biosynthetic enzymes have recently been shown to form protein-protein complexes in wheat endosperm (Tetlow et al., 2004), and it is possible that ISA1 participates in, or has interactions with, such complexes.
The fact that the introduction of the TaISA1 gene alone could restore the synthesis of amylopectin in the sugary-1 mutant of rice suggests the specific role of ISA1 in amylopectin biosynthesis in rice endosperm despite a significant expression of the OsISA2 gene (Fig. 2B). This may be the reason why the whole starch is replaced by phytoglycogen when the OsISA1 is deficient in rice endosperm of some sugary-1 mutant lines, such as EM914 (Nakamura et al., 1997; Kubo et al., 1999).
Two types of models have been proposed to explain the consistent observation from genetic studies that ISAs are required for wild-type levels of starch granule synthesis. One model proposes that the role of ISAs is to remove phytoglycogen from the stroma of the plastid, thus eliminating a source of competition for the growing starch granule. Associated with this model is the suggestion that ISAs are involved in controlling starch granule initiation (Burton et al., 2002; Bustos et al., 2004). The second model proposes that the ISAs are required to edit or trim the emerging amylopectin structure by eliminating excessively branched chains (Ball et al., 1996) or sparsely but ill-positioned branches (Nakamura, 2002), such that amylopectin is competent to crystallize within the starch granule matrix (Myers et al., 2000). This study provides support for the latter hypothesis, invoking a direct role of ISA1 in amylopectin and starch granule synthesis through the observation that there is a strong correlation between ISA expression level and starch structure and crystallinity. This is shown by the observation that as ISA activity increases, there are concomitant increases in chain-length distribution. These increases in chain-length distribution are in turn associated with an increase in crystallinity, as revealed by increased gelatinization temperatures and increases in crystallinity measured by x-ray diffraction. The results presented here do not preclude ISAs having additional roles in starch synthesis, such as in the removal of phytoglycogen from the stromal fraction. Further research to unambiguously define the role(s) of ISAs in plant starch synthesis is required.
MATERIALS AND METHODS
Plant Materials
A sugary-1 rice (Oryza sativa) mutant line, EM914, and its parent cv TC65 were used in this study. The mutant was induced by treatment of fertilized egg cells of the parent cultivar with 1.0 mm N-methyl-N-nitrosourea, as described previously (Satoh and Omura, 1979). EM914 is a homozygous sugary-1 progeny of BC2F3 line derived from backcrossings with TC65. All rice plants were grown in a greenhouse under daylight at 28°C and darkness at 22°C, respectively. As an enzyme source, the developing grains at the late-milking stage were collected and stored at −30°C until use. For preparation of starch samples, the mature grains were harvested before complete dryness and stored at −30°C.
Transformation of sugary-1 of Rice
The pCLD04541 containing the wheat (Triticum aestivum) ISA gene was introduced into EM914 by Agrobacterium tumefaciens EHA105 (Hood et al., 1993)-mediated transformation. Procedures for rice tissue culture and transformation with A. tumefaciens were as described by Toki (1997). For selection, transformed calli were cultured on the medium containing 35 mg G418 L−1 and 500 mg carbenicillin L−1.
Screening of the TaISA1 Transgenic Lines of Rice
About 150 plantlets were regenerated from different callus backgrounds. The confirmation of the insertion of wheat DNA was performed by PCR method using calli. Rice DNA was isolated from leaves by the method of Edwards et al. (1991). The DNA fragment encoding part of NPTII was amplified with the sense primer 5′-GGTGGTCGAATGGGCAGGTAGC-3′ and the antisense primer 5′-CACGACGGGCGTTCCTTGC-3′. The DNA fragment encoding part of TaISA1 was amplified with the sense primer 5′-TGGAGAGAACATGAGAGAGC-3′ and the antisense primer 5′-CTCGCTTTGCTGACAGTGCC-3′.
For the color screening of endosperm polyglucans, a cross section of a dry seed was gelatinized for 1 h in 100 μL of 1 n sodium hydroxide and homogenized. After neutralization, 50 μL of the polyglucan solution was transformed to a microplate containing 50 μL of distilled water and 50 μL of iodine solution (0.1% I2, 1% KI).
Isolation of RNA and cDNA Synthesis
Total RNA was extracted from 0.7 g of rice developing seeds and leaves, as described by Chang et al. (1993). cDNA was prepared from 2 μg of total RNA with Superscript II RT (Invitrogen, Paisley, UK) and an oligo(dT)12-18 primer at a temperature of 42°C according the manufacturer's instruction.
The cDNA Cloning and Sequencing of OsISA2 and OsISA3
To clone OsISA2 and OsISA3, we used RT-PCR on RNA prepared from developing endosperm by the method described above. The PCR reactions were carried out using the Takara LA Taq kit (Takara, Shiga, Japan). Cycling parameters were 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 2 min. The cDNA fragment encoding the OsISA2 open reading frame was amplified with the sense primer 5′-ACGGTGTCCTTCAAGGTCC-3′ and the antisense primer 5′-ATCATAGGTTTGTGATCC-3′. The cDNA fragment encoding the OsISA3 open reading frame was amplified with the sense primer 5′- GCCCTATAACTAGGCACC-3′ and the antisense primer 5′-AACCAAGCCAGCATTGGTGG-3′. PCR products were cloned into the pGEM-T Easy vector (Promega, Madison, WI) and were completely sequenced using the Applied Biosystems (Foster City, CA) ABI PRISM 3100 genetic analyzer.
Measurements of the Transcripts of OsISA1, OsISA2, OsISA3, and TaISA3 by the PCR Methods
For quantitative measurements of ISA transcripts by the real-time RT-PCR method, 100 ng of cDNA was used as a template for the PCR amplification of each product using standard PCR procedures. Cycling parameters were 50 cycles of 94°C for 15 s, 50°C for 15 s, and 72°C for 30 s. The cDNA fragment encoding part of OsISA1 was amplified with the sense primer 5′-TGTCCCCGGTCCTGGTGGCG-3′ and the antisense primer 5′-CTTGGAAGAGCAGCTGA-3′. The cDNA fragment encoding part of OsISA2 was amplified with the sense primer 5′-TAGAGGTCCTCTTGGAGG-3′ and the antisense primer 5′-AATCAGCTTCTGAGTCACCG-3′. The cDNA fragment encoding part of OsISA3 was amplified with the sense primer 5′-CTTTGTCGTGGACCAGATGG-3′ and the antisense primer 5′-ACCATGGTAAGGCTTCCG-3′. The cDNA fragment encoding part of TaISA1 was amplified with the sense primer 5′-TTCCGGCGCGTGGTAACA-3′ and the antisense primer 5′-CTTGGAAGAAGAGGTTG-3′. PCR products after 35 cycles by iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, CA) were taken and separated on a 1.2% (w/v) agarose gel.
In Situ Hybridization
The genomic clone DBEI was used in the fluorescent in situ hybridization analysis. The chromosome-specific BAC clones and short arm of rice chromosome 1-specific probe (1S marker) were used for identification of the rice chromosomes. DBEI clone and chromosome marker probes were labeled with digoxigenin-11-dUTP and biotin-16-dUTP, respectively, by nick translocation according to Mukai et al. (1993). Fluorescence in situ hybridization analysis was carried out as described by Turner et al. (1999).
Gel Electrophoresis, Activity Staining, and Immunoblotting
Separation of proteins on native amylopectin-containing gels for detection of starch-hydrolyzing enzymes in developing endosperm was performed as described by Nakamura et al. (1997). Detection of the BEs and SSs were performed as described by Yamanouchi and Nakamura (1992) and Nishi et al. (2001), respectively.
SDS-PAGE and immunoblotting were carried out as described previously (Fujita et al., 1999). Antisera were raised against the pullulanase (Nakamura et al., 1996a), BEI, BEIIb (Nakamura et al., 1992a), and ISA (Fujita et al., 1999) purified from rice endosperm. Synthetic peptide (C)GTFDWEGDLPLRYPQK was used to produce polyclonal antibodies for TaISA1 in rabbit.
Enzyme Assays
For the assay of endosperm enzymes, dehulled grains with their embryos removed were homogenized with 0.4 mL of ice-cold buffer solution of 50 mm HEPES-NaOH, pH 7.4, 2 mm MgCl2, 50 mm 2-mercaptoethanol, and 12.5% (v/v) glycerol. The homogenate was centrifuged at 10,000g for 10 min, and the resulting supernatant was used as the enzyme preparation for the measurement of AGPase, SS, and pullulanase activities, as described by Nishi et al. (2001).
Purification of ISA from Rice Endosperms
Fifty grams of developing endosperm of japonica-type rice (cv Fujihikari) at the late-milking stage were homogenized in 300 mL of medium A (50 mm imidazol, pH 7.4, 8 mm MgCl2, 500 mm 2-mercaptoethanol, 12.5% (v/v) glycerol) using prechilled mortar and pestle. The homogenate was squeezed through gauze, and the filtrate was twice centrifuged at 10,000g at 4°C for 20 min. The resulting supernatant was used as a crude extract. The purification procedures were the same as described by Fujita et al. (1999). The finally purified preparation was concentrated to 100 μL using Microcon 50 (Millipore, Billerica, MA).
Partial Purification of ISA
The soluble extracts of 20 endosperms of TC65 and line 47-3 and 40 endosperms of wheat homogenized with medium A containing 12.5% glycerol were applied to a 5-mL Hitrap Q column equilibrated with medium A, as described above. The fractions with the highest ISA activity were combined and concentrated to 40 μL by Microcon 50 and used for their chromatographic behavior, as shown in Figure 7.
Extraction and Measurement of Carbohydrates
To measure both soluble and insoluble glucans in the same samples of rice endosperms, starch granules and soluble glucans were separated as described by Fujita et al. (2003). The polyglucan contents of both soluble and insoluble fractions were assayed as the amounts of Glc released after digestion with glucoamylase, as described by Wong et al. (2003).
Branch Chain-Length Distribution of α-Polyglucans
The methods of polyglucan preparations and analysis of their debranched samples by capillary electrophoresis were performed as described by Fujita et al. (2003) and Wong et al. (2003), respectively. The 8-amino-1,3,6-pyrenetrisulfonic acid-labeled α-1,4-glucan were analyzed by using an eCAP N-linked oligosaccharide profiling kit and a P/ACE System 5000 high-resolution capillary electrophoresis equipped with a laser-induced fluorescence detector (Beckman Instruments, Coulter, CA) according to the method by O'Shea and Morell (1996).
Physicochemical and Morphological Characteristics of Starches
DSC of starches was measured as described by Nakamura et al. (2002).
X-ray diffraction pattern of starches was obtained with copper, nickel foil-filtered, Kα radiation using a diffractometer RINT2000 (RIGAKU, Tokyo) at 50 kV and 27 mA. The scanning region of the two-theta angle was to 40.0 from 4.0 with a scan speed of 0.3 s−1.
Purified starch granules were sputter coated with gold and examined with a scanning electron microscope (JSM-56000LV; JEOL, Tokyo) at 20 kV.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AB093426, AC132483, AF548379, AP005574, AY091058, AY132996, AY132997, AY132998, AY139980, AY172633, and AY172634.
Acknowledgments
We thank Dr. P.B. Francisco Jr. for reading the manuscript. We also thank Dr. Kazuko Ono for instruction to prepare transgenic rice plants.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.051359.
References
- Ball SG, Guan H-P, James M, Myers A, Keeling P, Mouille G, Buleon A, Colonna P, Morell MK (1996) From glycogen to amylopectin: a model explaining the biosynthesis of the plant starch granule. Cell 86: 349–352 [DOI] [PubMed] [Google Scholar]
- Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54: 207–233 [DOI] [PubMed] [Google Scholar]
- Beatty MK, Rahman A, Cao H, Woodman W, Lee M, Myers AM, James MG (1999) Purification and molecular genetic characterization of ZPU1, a pullulanase-type starch-debranching enzyme from maize. Plant Physiol 119: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Jenner H, Carrangis L, Fahy B, Fincher GB, Hylton C, Laurie DA, Parker M, Waite D, van Wegen S, et al (2002) Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J 31: 97–112 [DOI] [PubMed] [Google Scholar]
- Bustos R, Fahy B, Hylton CM, Seale R, Nebane NM, Edwards A, Martin C, Smith AM (2004) Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. Proc Natl Acad Sci USA 101: 2215–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolation RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Dinges JR, Colleoni C, James MG, Myers AM (2003) Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 15: 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges JR, Colleoni C, Myers AM, James MG (2001) Molecular structure of three mutations at the maize sugary1 locus and their allele-specific phenotypic effects. Plant Physiol 125: 1406–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Francisco PB Jr, Nakakita M, Harada K, Minaka N, Nakamura Y (1999) Purification, characterization, and cDNA structure of isoamylase from developing endosperm of rice. Planta 208: 283–293 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kubo A, Suh D-S, Wong K-S, Jane J-L, Ozawa K, Takaiwa F, Inaba Y, Nakamura Y (2003) Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm. Plant Cell Physiol 44: 607–618 [DOI] [PubMed] [Google Scholar]
- Gallant DJ, Bouchet B, Baldwin PM (1997) Microscopy of starch: evidence of a new level of granule organization. Carbohydr Polym 32: 177–191 [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2: 208–218 [Google Scholar]
- Hussain H, Mant A, Seale R, Zeeman S, Hinchliffe E, Edwards A, Hylton C, Bornemann S, Smith AM, Martin C, et al (2003) Three isoforms of isoamylase contribute different catalytic properties for the debranching of potato glucans. Plant Cell 15: 133–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6: 215–222 [DOI] [PubMed] [Google Scholar]
- James MG, Robertson DS, Myers AM (1995) Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 7: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T (1999) Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem 76: 629–637 [Google Scholar]
- Kossmann J, Lloyd J (2000) Understanding and influencing starch biochemistry. Crit Rev Biochem Mol Biol 35: 141–196 [PubMed] [Google Scholar]
- Kubo A, Fujita N, Harada K, Matsuda T, Satoh H, Nakamura Y (1999) The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol 121: 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EYC, Whelan WJ (1971) Glycogen and starch-debranching enzymes. In PD Boyer, ed, The Enzymes, Vol 5. Academic Press, New York, pp 191–234
- Mouille G, Maddelein ML, Libessart N, Talaga P, Decq A, Delrue B, Ball S (1996) Preamylopectin processing: a mandatory step for starch biosynthesis in plants. Plant Cell 8: 1353–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moullet O, Zhang HB, Lagudah ES (1999) Construction and characterization of a large DNA insert library from the D genome of wheat. Theor Appl Genet 99: 305–313 [Google Scholar]
- Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36: 489–495 [DOI] [PubMed] [Google Scholar]
- Myers AM, Morell MK, James MG, Ball SG (2000) Recent progress toward understanding the amylopectin crystal. Plant Physiol 122: 989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y (1996) Some properties of starch debranching enzymes and their possible role in amylopectin biosynthesis. Plant Sci 121: 1–18 [Google Scholar]
- Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43: 718–725 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kubo A, Shimamune T, Matsuda T, Harada K, Satoh H (1997) Correlation between activities of starch debranching enzyme and a-polyglucan structure in endosperms of sugary-1 mutants of rice. Plant J 12: 143–153 [Google Scholar]
- Nakamura Y, Sakurai A, Inaba Y, Kimura K, Iwasawa N, Nagamine T (2002) The fine structure of amylopectin in endosperm from Asian cultivated rice can be largely classified into two classes. Starch 54: 117–131 [Google Scholar]
- Nakamura Y, Takeichi T, Kawaguchi K, Yamanouchi H (1992. a) Purification of two forms of starch branching enzyme (Q-enzyme) from developing rice endosperm. Physiol Plant 84: 336–342 [Google Scholar]
- Nakamura Y, Umemoto T, Ogata N, Kuboki Y, Yano M, Sasaki T (1996. a) Starch debranching enzyme (R-enzyme or pullulanase) from developing rice endosperm: purification, cDNA and chromosomal localization of the gene. Planta 199: 209–218 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Umemoto T, Takahata Y, Amano E (1992. b) Characteristics and roles of key enzymes associated with starch biosynthesis in rice endosperm. Gamma Field Symposia 31: 25–44 [Google Scholar]
- Nakamura Y, Umemoto T, Takahata Y, Komae K, Amano E, Satoh H (1996. b) Changes in structure of starch and enzyme activities affected by sugary mutations in developing rice endosperm. Physiol Plant 97: 491–498 [Google Scholar]
- Nishi A, Nakamura Y, Tanaka N, Satoh H (2001) Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127: 459–472 [PMC free article] [PubMed] [Google Scholar]
- O'Shea MG, Morell MK (1996) High resolution slab gel electrophoresis of 8-amino-1,3, 6-pyrenetrisulfonic acid (APTS) tagged oligosaccharides using a DNA sequencer. Electrophoresis 17: 681–686 [DOI] [PubMed] [Google Scholar]
- Pan D, Nelson OE (1986) A debranching enzyme deficiency in endosperms of the sugary-1 mutants of maize. Plant Physiol 74: 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Nakamura Y, Li Z, Clarke B, Fujita N, Mukai Y, Yamamoto M, Regina A, Tan Z, Kawasaki S, et al (2003) The sugary-type isoamylase gene from rice and Aegilops tauschii: characterization and composition with maize and Arabidopsis. Genome 46: 496–506 [DOI] [PubMed] [Google Scholar]
- Satoh H, Omura T (1979) Induction of mutation by the treatment of fertilized egg cell with N-methyl-N-nitrosourea in rice. J Fac Agr Kyushu U 24: 165–174 [Google Scholar]
- Smith AM, Denyer K, Martin C (1997) The synthesis of the starch granule. Annu Rev Plant Physiol Plant Mol Biol 48: 67–87 [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Wait R, Lu Z, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ (2004) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 16: 694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DB (2000) On the non-random nature of amylopectin branching. Carbohydr Polym 43: 223–239 [Google Scholar]
- Toki S (1997) Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol Biol Rep 15: 16–21 [Google Scholar]
- Turner M, Mukai Y, Leroy P, Charef B, Appels R, Rahman S (1999) The Ha locus of a polymorphic region for tracing grain hardness in crosses. Genome 42: 1242–1250 [PubMed] [Google Scholar]
- Van den Koornhuyse N, Libessart N, Delrue B, Zabawinski C, Decq A, Iglesias A, Carton A, Preiss J, Ball S (1996) Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. J Biol Chem 27: 16281–16287 [DOI] [PubMed] [Google Scholar]
- Wong KS, Kubo A, Jane JL, Harada K, Satoh H, Nakamura Y (2003) Structures and properties of amylopectin and phytoglycogen in the endosperm of sugary-1 mutant of rice. J Cereal Sci 37: 139–149 [Google Scholar]
- Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol 33: 985–991 [Google Scholar]
- Yu J, Hu S, Wang J, Wong GK-S, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, et al (2002) A draft sequence of the rice genome (Oryza sativa L. ssp Indica). Science 296: 79–92 [DOI] [PubMed] [Google Scholar]
- Zeeman SC, Umemoto T, Lue WL, Au-Yeung P, Martin C, Smith AM, Chen J (1998) A mutant of Arabidopsis lacking a chloroplastic isoamylase accumulates both starch and phytoglycogen. Plant Cell 10: 1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]