Abstract
Pulmonary artery intimal sarcoma is a highly aggressive disease, and is most often misdiagnosed as pulmonary thromboembolism (PTE) due to the similar clinical symptoms and its rarity, which leads to the use of inappropriate treatments such as prolonged anticoagulant therapy. We reported a case of pulmonary artery intimal sarcoma in a patient who was misdiagnosed as having PTE. Pathology after surgery confirmed malignant disease. We concluded that when a patient presents with mild clinical manifestations yet with strong imaging manifestations, pulmonary artery malignancy should be suspected.
Keywords: Intimal sarcoma, pulmonary artery, pulmonary embolism, pulmonary thromboembolism
Introduction
Pulmonary artery intimal sarcoma arises from the intimal layer of the right, left, or main pulmonary arteries 1. It is extremely rare, clinically unpredictable, and often misdiagnosed as pulmonary thromboembolism (PTE) because of the similar clinical presentation. Although the prognosis is poor, the misdiagnosis of pulmonary artery sarcoma may delay any possible treatment. In this study, we report a patient who was initially diagnosed with PTE but ultimately correctly diagnosed with pulmonary artery intimal sarcoma by pathology after surgical resection.
Case Report
A 63‐year‐old male presented to the emergency department of Zhongshan Hospital with complaints of right‐sided chest pain for the past 2 weeks. The patient reported that the pain had increased gradually. He had mild chest congestion with mild cough and no haemoptysis, breathlessness, or fever. He had no hypoxaemia (partial pressure of oxygen (PaO2) 84 mmHg, peripheral capillary oxygen saturation (SpO2) 95%). Electrocardiogram (EKG) showed a left anterior fascicular block. Computerized tomography (CT) showed suspicious embolus in the right pulmonary artery. Computerized tomographic pulmonary angiography (CTPA) showed pulmonary embolism (PE) in the right pulmonary artery and upper right pulmonary artery and possible pulmonary infarction focally (Fig. 1A–D). D‐dimer was 0.49 mg/L (normal range is 0.02–0.80 mg/L). Complete blood count (CBC), hepatic function, renal function, serum electrolytes, cardiac biomarkers, autoantibodies, and coagulation were normal. There was no internal bleeding. He was diagnosed with PE and was treated with low‐molecular weight heparin (LMWH) for 2 weeks.
Figure 1.
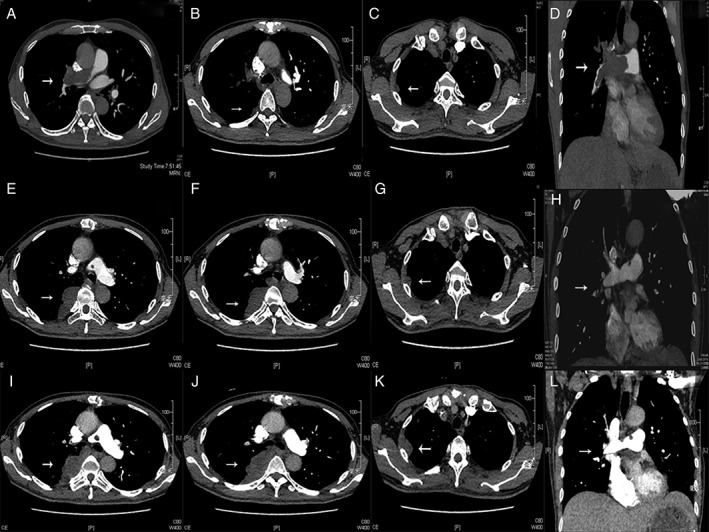
Series of images of computerized tomographic pulmonary angiography (CTPA) of the patient. (A–D) Images taken when the patient presented to hospital for the first time without any treatment. (E–H) Images taken 7 weeks after surgery and before chemotherapy. (I–L) Images taken after two cycles of chemotherapy.
The patient was then transferred to the respiratory department for further treatment. D‐dimer was monitored continuously and remained normal. Autoantibody, tumour markers, mycological examination, and T‐spot were all normal. Relative examinations showed no deep vein thrombosis (DVT) on colour Doppler ultrasound and the echocardiography (echo) was normal (left ventricular ejection fraction (LVEF) 66%; all chambers of the heart and pulmonary artery pressure were normal; no thrombus echo was observed in the main pulmonary artery and the right and left branches of pulmonary artery). Considering all the atypical clinical manifestations, we suggested that the patient undergo a (18)F‐positron emission tomography (PET)/CT, which revealed PE in the right pulmonary artery. The PET/CT also showed a mass originating from the endothelial cells in the right pulmonary artery (maximum standard uptake value (SUV) was 3.5). Meanwhile, there were multiple abnormal foci with high SUV in the right lung (maximum SUV was 7.9 and maximum diameter was 28.8 mm) (Fig. 2).
Figure 2.
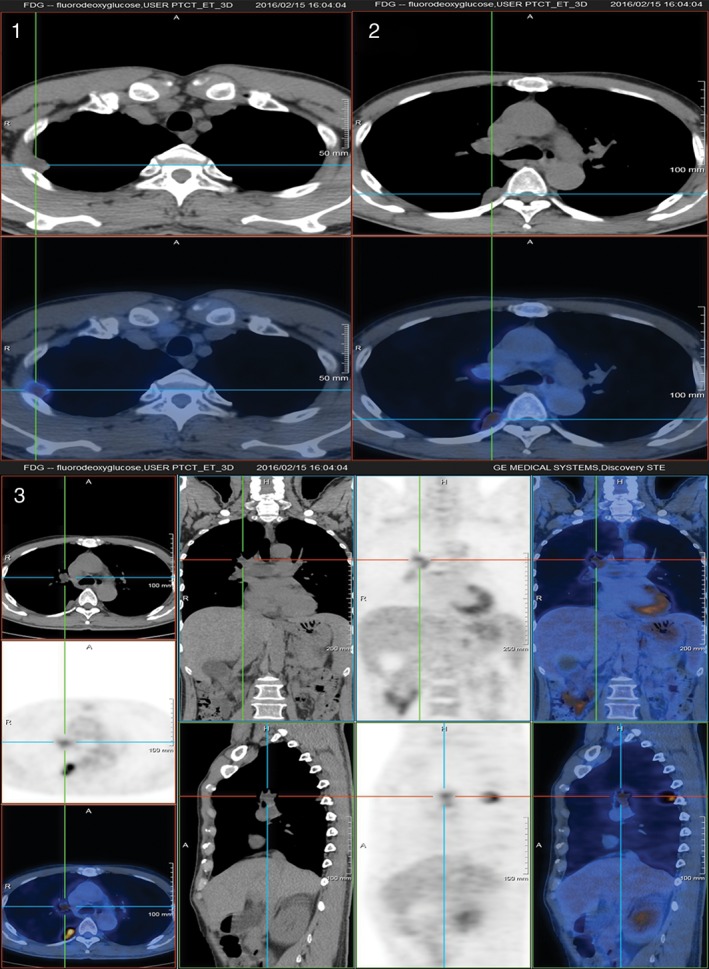
Images of (18)F‐positron emission tomography (PET)/computerized tomography (CT) of the patient.
After a cardiac surgery consultation, the tumour was resected. The patient received right pulmonary artery tumorectomy and pulmonary endarterectomy. During the surgery, we could see the right pulmonary artery was occupied by the tumour. Two arterial‐wall‐like tissues were sent for pathologic analysis. One mass, in which there was 4.5 × 2.5 × 2 cm large grey transparent sticky colloidal tissue, was 6.5 × 3.5 × 3 cm large and the wall was 0.3 cm thick. The other mass, with sticky colloidal tissue focally, was 3 × 2 × 1.5 cm large and the wall was 0.3 cm thick too. It was diagnosed as a spindle cell tumour that can be seen under an optical microscope. The immunological histological chemistry (IHC) results were S‐100 (small amount +), α‐smooth muscle actin (SMA; partial +), Desmin (Des) (−), CD34 (vascular +), CD31 (vascular +), Ki‐67 (30% positive), F8 (vascular +), epithelial membrane antigen (EMA) (−), CKpan: a kind of immunohistochemical mark (CKPAN) (−), vimentin (VIM; +) (Fig. 3). Both the gross investigation and the IHC suggested intimal sarcoma. Then we examined the relevant gene mutation. Next‐generation sequencing (NGS) showed gene mutations of CDK4, MDM2, FBXW7, FAT3, and FANCM.
Figure 3.
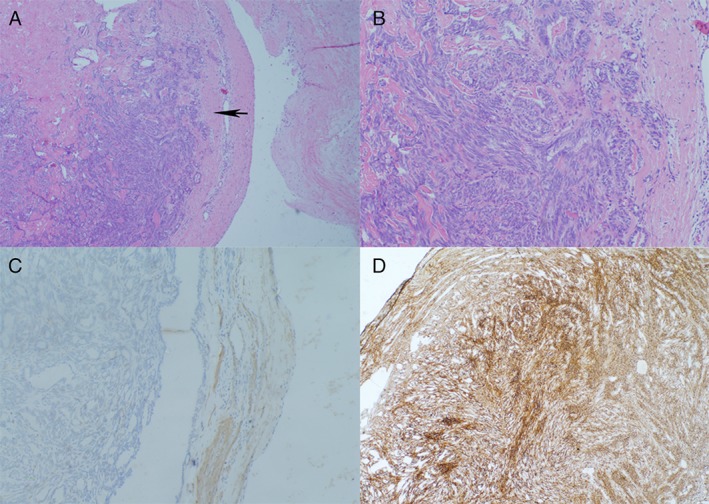
Pathology of the tumour. (A) Haematoxylin and eosin (HE) stain of the tumour shows that the tumour arose from the intimal of pulmonary artery (50×). The black arrow indicates the neoplasm from the artery wall. (B) Microscopically, the tumour was composed of pleomorphic spindle cells (HE stain, 200×). (C) Tumour cells were immunohistochemically positive for α‐smooth muscle actin (SMA). (D) Immunostaining for vimentin (VIM) highlights numerous neoplasm cells derived from mesenchymal tissue.
The patient was followed‐up closely after the surgery. Seven weeks after the surgery, the patient underwent CTPA examination again before he was started on chemotherapy. The second CTPA showed recurrence in the right pulmonary artery and the right upper pulmonary artery with nodular filling defect. Maximum metastasis seen was 6.3 cm in the right lung (Fig. 1E–H). The patient then agreed to receive chemotherapy the next day at our oncology department. The chemotherapy drug administered was epirubicin (90 mg/m2), for every 3 weeks. After two cycles of the chemotherapy, he underwent a third CTPA, which showed progress in the right pulmonary artery and the metastasis in the right lung (maximum diameter 9.7 cm) together with new lesions in the right lung and the right pleura (Fig. 1I–L). Afterwards, he was lost to follow‐up.
Discussion
Intimal sarcoma is a totipotent‐intimal‐cell originated tumour. Pulmonary artery intimal sarcoma arises from the intimal layer of the right, left, or main pulmonary arteries, where pulmonary metastases are common 2. In extremely rare cases, it may extend in a retrograde manner to the pulmonary valve and right ventricle 1. Most cases of this disease are diagnosed on autopsy or with surgical specimens; therefore, the true incidence of intimal sarcoma of the pulmonary artery is unknown.
The clinical presentation is often non‐specific and related to tumour emboli. The most common symptoms are dyspnoea, tachypnoea, followed by chest or back pain, cough, haemoptysis, and malaise, which is quite similar to PTE. With such non‐specific clinical features, the disease may mimic other diseases such as lung cancer, mediastinal masses, and especially acute or chronic PE 3, 4. Diagnosis in this patient was particularly difficult because of its long asymptomatic course and gradual development of symptoms of pulmonary hypertension and right heart failure 5. As for PTE, if the pulmonary trunk is occluded by a blood clot, the patient often presents rapid‐onset dyspnoea and chest tightness with right ventricular dysfunction such as pulmonary artery dilation or pulmonary hypertension 6. However, the patient's chest pain developed gradually and no obvious shortness of breath was observed. The echo showed relatively normal heart function as well. D‐dimer elevation is a sensitive indicator for thromboembolism either in lower limbs deep vein or pulmonary artery. D‐dimer was normal for this patient from onset of the disease and during follow‐up 7, 8.
From the viewpoint of imaging, pulmonary artery intimal sarcoma and PTE are difficult to distinguish in the differential diagnosis. The presence of extraluminal tumour extension as well as distant metastases are reliable signs in the differential diagnosis, but these are manifestations of advanced cancer of intimal sarcoma 9. The CTPA demonstrates a filling defect, raising the suspicion of thromboembolism. Although both show enhancement on the CTPA, intimal sarcoma tends to present as a unilateral, central, lobulated, pulmonary artery filling defect with an increase in the diameter of the pulmonary arteries 3, 9, 10, 11, 12 and acute angles with the vessel wall, in contrast to the PTE 13. And intimal sarcoma shows heterogeneous densities due to areas of necrosis, haemorrhage, and ossification within the mass 13. The PET/CT was used to confirm the suspicion of pulmonary artery intimal sarcoma and to distinguish with PTE on the basis of the increased radiopharmaceutical uptake of tumours 14, 15. The echo in these cases may reveal dilation of the right ventricle, with or without signs of obstruction of the right ventricular outflow tract or of the pulmonary trunk whereas PTE usually has right ventricular dysfunction such as right ventricular dilation, hypokinesis, or pressure overload 16, 17. The diagnosis of intimal sarcoma is usually made post‐operatively or at autopsy, but PET/CT can be of great help.
In our case, the patient had a subacute onset. His chief complaint was chest pain. The CTPA demonstrated an intraluminal filling defect occupying the entire lumen. The symptom and CTPA outcome indicated a type of vascular occlusion, such as PTE. But considering the mild clinical manifestation including the normal D‐dimer and echo results, and the following PET/CT examination, prompted a diagnosis of pulmonary artery malignancy. It was manifested by pathology after surgery. Finally, we diagnosed pulmonary artery intimal sarcoma that originated from the right pulmonary artery with metastasis in the right lung.
Knowledge about the molecular genetics and pathogenesis of intimal sarcoma is limited due to its rarity 18, 19. Intimal sarcomas are usually malignant mesenchymal tumours, which are poorly differentiated with fibroblastic or myofibroblastic differentiation 9. Pathologic diagnosis can be difficult because the intimal sarcoma does not express specific markers for immunohistochemistry 20.
There are no specific guidelines for the management of patients with pulmonary artery intimal sarcoma 21. The rarity of this diagnosis made it difficult to standardize the therapy. Surgery is the mainstay of pulmonary artery intimal sarcoma. Reported curative surgical procedures for treating the tumour include tumour endarterectomy, graft reconstruction of the pulmonary artery, and pneumonectomy. Aggressively repeated surgical interventions of the primary tumour, local recurrence, and metastatic lesions were helpful to increase patient survival.
The impact of chemotherapy and radiotherapy remains unclear, although some studies have demonstrated their effectiveness 2. The use of radiation therapy and chemotherapy after surgical treatment has been found to prolong survival time compared with surgery alone 3, 22, 23. Relative studies have shown that doxorubicin, and ifosfamide may be effective 24, 25, 26, 27. Clinical trials to better understand the role of surgery, chemotherapy, and radiation therapy are difficult to carry out due to the rarity of the disease.
The prognosis is also poor. In approximately 50% of the cases, pulmonary artery intimal sarcoma extends directly through the vessel wall to the adjacent lung, bronchial wall, lymph node, or heart. Distant metastases occur in 16–25% of the cases 28. It can metastasize to the brain, pancreas, adrenal glands, and lungs 21. The mean survival is 12–18 months 1, 29. Average survival at 5 years is less than 6% 30. Surgical resection has been reported to decrease clinical symptoms and lengthen survival 31, 32, 33, 34, 35, 36. The prognosis seems to depend on the location and vascular extension 24. Death is usually due to right heart failure because of obstruction of the pulmonary arteries 30.
In our case, the patient had mild chest pain with subacute onset, together with imaging that strongly suggested tumour involvement. His right pulmonary artery was totally blocked without any signs of heart failure or severe hypoxaemia and dyspnoea. Also, the D‐dimer tests were always normal. Furthermore, the patient had metastasis in his right lung. And finally, he had no risk factors for PTE. All of the above observations were quite different from PTE as we know. Therefore we opted for PET/CT and the following treatment. To summarize, attention should be paid when the patient presents with mild clinical manifestations and severe imaging manifestations. Especially when the D‐dimer is within the normal range and the presence of a low‐density lobulated filling defect with uneven distribution and contrast enhancement in the entire luminal diameter on CTPA, pulmonary artery malignancy should be strongly suspected. The high uptake on PET/CT scan can prompt suspicion of the disease.
In conclusion, untimely diagnosis leading to late stage presentations contributes to the poor prognosis of this disease. So early and proper diagnosis is necessary to establish appropriate therapy. Due to the high lethality of this tumour and the therapeutic and prognostic implications, it is of paramount importance for clinicians to be aware of the key features that differentiate this pathology from PTE. The PET/CT can be a proper way to help recognize intimal sarcomas when clinical manifestations are atypical. Given the dismal outcome and high risk of recurrence or metastatic spread of intimal sarcomas, the multimodal treatment, including a combinational treatment, appears justified. Difficulties in diagnosis and poor prognosis result in the lack of high volume studies and data registration, which means that further studies are necessary.
Disclosure Statement
No conflict of interest declared.
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81270078, 81470211 by Z. Chen), the Shanghai 3 year Plan of the Key Subjects Construction in Public Health‐Infectious Disease and Pathogenic Microorganism (15GWZK0102) and research fund from Shanghai Respiratory Research Institute. The authors would like to thank Yiqiu Zhang for providing the PET/CT figures.
Liu, X. , Hou, J. , Wang, X. and Chen, Z. (2017) An intimal sarcoma of pulmonary artery mimicking pulmonary embolism: a case report and literature review. Respirology Case Reports, 5 (5), e00248. doi: 10.1002/rcr2.248.
Associate Editor: Bei He
References
- 1. Ozbek C, Emrecan B, Calli AO, et al. 2007. Intimal sarcoma of the pulmonary artery with retrograde extension into the pulmonic valve and right ventricle. Tex. Heart Inst. J. 34(1):119–121. [PMC free article] [PubMed] [Google Scholar]
- 2. Tanaka A, Shirasaka T, Okada K, et al. 2015. Aggressive multiple surgical interventions to pulmonary artery sarcoma. Eur. J. Cardiothorac. Surg. 47(2):384–385. [DOI] [PubMed] [Google Scholar]
- 3. Cox JE, Chiles C, Aquino SL, et al. 1997. Pulmonary artery sarcomas: a review of clinical and radiologic features. J. Comput. Assist. Tomogr. 21(5):750–755. [DOI] [PubMed] [Google Scholar]
- 4. Simpson WL Jr, and Mendelson DS. 2000. Pulmonary artery and aortic sarcomas: cross‐sectional imaging. J. Thorac. Imaging 15(4):290–294. [DOI] [PubMed] [Google Scholar]
- 5. Scheidl S, Taghavi S, Reiter U, et al. 2010. Intimal sarcoma of the pulmonary valve. Ann. Thorac. Surg. 89(4):e25–e27. [DOI] [PubMed] [Google Scholar]
- 6. Miniati M, Cenci C, Monti S, et al. 2012. Clinical presentation of acute pulmonary embolism: survey of 800 cases. PLoS One 7(2):e30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miniati M, Prediletto R, Formichi B, et al. 1999. Accuracy of clinical assessment in the diagnosis of pulmonary embolism. Am. J. Respir. Crit. Care Med. 159(3):864–871. [DOI] [PubMed] [Google Scholar]
- 8. Stein PD, Beemath A, Matta F, et al. 2007. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am. J. Med. 120(10):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Attina D, Niro F, Tchouante P, et al. 2013. Pulmonary artery intimal sarcoma. Problems in the differential diagnosis. Radiol. Med. 118(8):1259–1268. [DOI] [PubMed] [Google Scholar]
- 10. Wittram C, Maher MM, Yoo AJ, et al. 2004. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics 24(5):1219–1238. [DOI] [PubMed] [Google Scholar]
- 11. Castaner E, Gallardo X, Rimola J, et al. 2006. Congenital and acquired pulmonary artery anomalies in the adult: radiologic overview. Radiographics 26(2):349–371. [DOI] [PubMed] [Google Scholar]
- 12. Kauczor HU, Schwickert HC, Mayer E, et al. 1994. Pulmonary artery sarcoma mimicking chronic thromboembolic disease: computed tomography and magnetic resonance imaging findings. Cardiovasc. Intervent. Radiol. 17(4):185–189. [DOI] [PubMed] [Google Scholar]
- 13. Parish JM, Rosenow EC III, Swensen SJ, et al. 1996. Pulmonary artery sarcoma. Clinical features. Chest 110(6):1480–1488. [DOI] [PubMed] [Google Scholar]
- 14. Ito K, Kubota K, Morooka M, et al. 2009. Diagnostic usefulness of 18F‐FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann. Nucl. Med. 23(7):671–676. [DOI] [PubMed] [Google Scholar]
- 15. Chong S, Kim TS, Kim BT, et al. 2007. Pulmonary artery sarcoma mimicking pulmonary thromboembolism: integrated FDG PET/CT. AJR Am. J. Roentgenol. 188(6):1691–1693. [DOI] [PubMed] [Google Scholar]
- 16. Austin BA, and Griffin BP. 2008. Pulmonary artery intimal sarcoma: a brief case series. J. Am. Soc. Echocardiogr. 21(8):978.e975–977. [DOI] [PubMed] [Google Scholar]
- 17. Cohen AT, Dobromirski M, and Gurwith MM. 2014. Managing pulmonary embolism from presentation to extended treatment. Thromb. Res. 133(2):139–148. [DOI] [PubMed] [Google Scholar]
- 18. Bode‐Lesniewska B, Zhao J, Speel EJ, et al. 2001. Gains of 12q13‐14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 438(1):57–65. [DOI] [PubMed] [Google Scholar]
- 19. Zhang H, Macdonald WD, Erickson‐Johnson M, et al. 2007. Cytogenetic and molecular cytogenetic findings of intimal sarcoma. Cancer Genet. Cytogenet. 179(2):146–149. [DOI] [PubMed] [Google Scholar]
- 20. Burke AP, and Virmani R. 1993. Sarcomas of the great vessels. A clinicopathologic study. Cancer 71(5):1761–1773. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto K, Nozue T, Tsuchida M, et al. 2012. Pulmonary embolism caused by intimal sarcoma of the pulmonary artery. Intern. Med. 51(21):3031–3034. [DOI] [PubMed] [Google Scholar]
- 22. Genoni M, Biraima AM, Bode B, et al. 2001. Combined resection and adjuvant therapy improves prognosis of sarcomas of the pulmonary trunk. J. Cardiovasc. Surg. (Torino) 42(6):829–833. [PubMed] [Google Scholar]
- 23. Hirose T, Ishikawa N, Hamada K, et al. 2009. A case of intimal sarcoma of the pulmonary artery treated with chemoradiotherapy. Intern. Med. 48(4):245–249. [DOI] [PubMed] [Google Scholar]
- 24. Hoiczyk M, Iliodromitis K, Bauer S, et al. 2012. Intimal sarcoma of the pulmonary artery with unusual findings: a case report. Clin. Res. Cardiol. 101(5):397–401. [DOI] [PubMed] [Google Scholar]
- 25. Penel N, Taieb S, Ceugnart L, et al. 2008. Report of eight recent cases of locally advanced primary pulmonary artery sarcomas: failure of Doxorubicin‐based chemotherapy. J. Thorac. Oncol. 3(8):907–911. [DOI] [PubMed] [Google Scholar]
- 26. Head HD, Flam MS, John MJ, et al. 1992. Long‐term palliation of pulmonary artery sarcoma by radical excision and adjuvant therapy. Ann. Thorac. Surg. 53(2):332–334. [DOI] [PubMed] [Google Scholar]
- 27. Uchida A, Tabata M, Kiura K, et al. 2005. Successful treatment of pulmonary artery sarcoma by a two‐drug combination chemotherapy consisting of ifosfamide and epirubicin. Jpn. J. Clin. Oncol. 35(7):417–419. [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Ren S, Li A, et al. 2014. A case report of chemo‐sensitive intimal pulmonary artery sarcoma. Cell Biochem. Biophys. 68(1):153–157. [DOI] [PubMed] [Google Scholar]
- 29. Huo L, Lai S, Gladish G, et al. 2005. Pulmonary artery angiosarcoma: a clinicopathologic and radiological correlation. Ann. Diagn. Pathol. 9(4):209–214. [DOI] [PubMed] [Google Scholar]
- 30. Kojima K, Okamoto I, Ushijima S, et al. 2003. Successful treatment of primary pulmonary angiosarcoma. Chest 124(6):2397–2400. [DOI] [PubMed] [Google Scholar]
- 31. Mattoo A, Fedullo PF, Kapelanski D, et al. 2002. Pulmonary artery sarcoma: a case report of surgical cure and 5‐year follow‐up. Chest 122(2):745–747. [DOI] [PubMed] [Google Scholar]
- 32. Blackmon SH, Rice DC, Correa AM, et al. 2009. Management of primary pulmonary artery sarcomas. Ann. Thorac. Surg. 87(3):977–984. [DOI] [PubMed] [Google Scholar]
- 33. Tucci M, Quatraro C, Calvani N, et al. 2005. Primary intimal sarcoma of the thoracic aorta. J. Exp. Clin. Cancer Res. 24(1):139–142. [PubMed] [Google Scholar]
- 34. Thalheimer A, Fein M, Geissinger E, et al. 2004. Intimal angiosarcoma of the aorta: report of a case and review of the literature. J. Vasc. Surg. 40(3):548–553. [DOI] [PubMed] [Google Scholar]
- 35. Osei‐Agyemang T, Geks J, Wagner HJ, et al. 2004. High‐grade intimal sarcoma in an aneurysm of the infrarenal aorta. Chirurg 75(8):823–827. [DOI] [PubMed] [Google Scholar]
- 36. Choi EY, Yoon YW, Kwon HM, et al. 2004. A case of pulmonary artery intimal sarcoma diagnosed with multislice CT scan with 3D reconstruction. Yonsei Med. J. 45(3):547–551. [DOI] [PubMed] [Google Scholar]


