Abstract
Currently, >35 Saudi Arabian medicinal plants are traditionally used for various liver disorders without a scientific rationale. This is the first experimental evaluation of the anti-hepatitis B virus (HBV) potential of the total ethanolic and sequential organic extracts of 60 candidate medicinal plants. The extracts were tested for toxicity on HepG2.2.15 cells and cytotoxicity concentration (CC50) values were determined. The extracts were further investigated on HepG2.2.15 cells for anti-HBV activities by analyzing the inhibition of HBsAg and HBeAg production in the culture supernatants, and their half maximal inhibitory concentration (IC50) and therapeutic index (TI) values were determined. Of the screened plants, Guiera senegalensis (dichloromethane extract, IC50=10.65), Pulicaria crispa (ethyl acetate extract, IC50=14.45), Coccinea grandis (total ethanol extract, IC50=31.57), Fumaria parviflora (hexane extract, IC50=35.44), Capparis decidua (aqueous extract, IC50=66.82), Corallocarpus epigeus (total ethanol extract, IC50=71.9), Indigofera caerulea (methanol extract, IC50=73.21), Abutilon figarianum (dichloromethane extract, IC50=99.76) and Acacia oerfota (total ethanol extract, IC50=101.46) demonstrated novel anti-HBV activities in a time- and dose-dependent manner. Further qualitative phytochemical analysis of the active extracts revealed the presence of alkaloids, tannins, flavonoids and saponins, which are attributed to antiviral efficacies. In conclusion, P. crispa, G. senegalensis and F. parviflora had the most promising anti-HBV potentials, including those of C. decidua, C. epigeus, A. figarianum, A. oerfota and I. caerulea with marked activities. However, a detailed phytochemical study of these extracts is essential to isolate the active principle(s) responsible for their novel anti-HBV potential.
Keywords: hepatitis B virus, anti-hepatitis B virus plants, Abutilon figarianum, Acacia oerfota, Capparis deciduas, Coccinea grandis, Corallocarpus epigeus, Fumaria parviflora, Guiera senegalensis, Indigofera coerulea, Pulicaria crispa
Introduction
Hepatitis B virus (HBV) is responsible for ~2 billion cases of liver infection worldwide, including ~40% of chronic carriers at the risk of developing fulminant hepatitis, cirrhosis and hepatocellular carcinoma (1,2). In total, >50% of the worldwide population lives in areas where HBV infection is highly endemic and >75% of this occurs in Asia, Africa and the Middle East, including the Kingdom of Saudi Arabia (1,3,4). Unfortunately, despite their high efficacies, all currently approved drugs against chronic hepatitis B have their own limitations. While long-term treatment with nucleot(s)ide analogues lead to the emergence of drug-resistance, chemotherapy with interferon-α is associated with a high incidence of adverse effects (3,4). Despite the global success of HBV vaccination programs, vaccine-escape mutants of the virus present another bottleneck in the preventive measures (1,2). In addition, the marketed anti-HBV agents are too expensive for the majority of developing countries. Therefore, there is an urgent need to search for novel anti-HBV agents with greater efficacy and safety.
Currently, there is an ongoing effort to identify anti-HBV products from a variety of plants and natural sources. Notably, it has been estimated that ~80% of Chinese patients with chronic hepatitis B (CHB) rely on traditional herbal remedies. Compared with the treatment of conventional drugs, such as interferons or lamivudine, a meta-analysis of clinical trials suggested that herbal preparations from Phyllanthus urinaria and Scutellaria baicalensis alone may have equivalent or better effects than lamivudine in the treatment of CHB (5). Additionally, many phytocompounds of different chemical classes have been reported to have promising anti-HBV activities (6–11).
Out of >1,000 species of medicinal plants documented in Saudi Arabia, at least 35 plants are used in Saudi folk medicine for the treatment of liver disorders (12,13). However, this wealth of herbal medicine has not been subjected to sustained scientific evaluations of their anti-HBV potentials to date. Therefore, the primary aim of the present study was to investigate the in vitro anti-HBV activities of 60 candidate plants and to perform a qualitative phytochemical analysis in order to identify the major secondary metabolites.
Materials and methods
Selection criteria
Candidate plants in the present study were selected on the basis of following one or more criteria: i) Claimed efficacies in treating various liver diseases in folk or traditional medicine; ii) reported in vitro or in vivo hepatoprotective potentials; iii) published antiviral activities against genetically-close human viruses, such as human immunodeficiency virus (HIV) and herpes simplex virus (HSV); and iv) taxonomically related to plants known for their antiviral activities.
Plant materials
A total of 60 medicinal plants were collected from different regions of the Kingdom of Saudi Arabia (n=57) as well as Sudan (n=3) (Table I). Plants were identified by an experienced plant taxonomist at the College of Pharmacy, King Saud University, (Riyadh, Saudi Arabia) and voucher specimens (Table I) were deposited at the college herbarium.
Table I.
List of medicinal plants (n=60) screened in the present study.
| No. | Plant name | Family | Part used | Voucher no. | Collection location |
|---|---|---|---|---|---|
| 1 | Abutilon figarianum | Malvaceae | L | 16,082 | Riyadh, KSA |
| 2 | Acacia hamulosa | Fabaceae | L + S | 16,221 | South, KSA |
| 3 | Acacia asak | Fabaceae | L | 16,387 | South, KSA |
| 4 | Acacia ehrenbergiana | Fabaceae | S | 16,385 | South, KSA |
| 5 | Acacia laeta | Fabaceae | S | 16,390 | South, KSA |
| 6 | Acacia oerfota | Fabaceae | S | 16,389 | South, KSA |
| 7 | Acacia salicina | Fabaceae | L | 15,007 | South, KSA |
| 8 | Acacia tortilis | Fabaceae | S | 14,977 | South, KSA |
| 9 | Achyranthes aspera | Amaranthaceae | Aerial parts (S, L, Fr) | 16,011 | Gabeel, KSA |
| 10 | Albizia procera | Fabaceae | L | 16,182 | Taif, KSA |
| 11 | Alternanthera pungens | Amaranthaceae | Aerial parts (S, L, Fr, Fl) | 16,391 | South, KSA |
| 12 | Amaranthus alba | Amaranthacea | Aerial parts (S, L, Fr, Fl) | 16,189 | Riyadh, KSA |
| 13 | Anagallis arvensis, var. caerulea | Primulaceae | Aerial parts (S, L, Fr, Fl) | 16,296 | South, KSA |
| 14 | Argemone ochroleuca | Papaveraceae | Aerial parts (S, L, Fr) | 16,185 | Taif, KSA |
| 15 | Atriplex suberecta | Chenopodiaceae | Aerial parts (S, L, Fr) | 16,195 | Taif, KSA |
| 16 | Aerva Javanica | Amaranthaceae | Aerial parts (S, L, Fl) | 16,196 | Taif, KSA |
| 17 | Bacopa monieri | Scrophulariaceae | L + S | 16,300 | South, KSA |
| 18 | Balanites aegyptiaca | Zygophyllaceae | B | 560 | Khartoum, Sudan |
| 19 | Boerhavia diffusa | Nyctaginaceae | L | 16,184 | Taif, KSA |
| 20 | Bougainvillea spectabilis | Nyctaginaceae | L | 16,177 | Taif, KSA |
| 21 | Capparis decidua | Capparaceae | S | 15,841 | Tabouk, KSA |
| 22 | Cassytha filiformis | Lauraceae | S | 15,716 | Taif, KSA |
| 23 | Chenopodium ambrosioides | Chenopodiaceae | Aerial parts (S, L, Fr) | 16,181 | Taif, KSA |
| 24 | Chenopodiumg laucum | Chenopodiaceae | L + S | 16,197 | Taif, KSA |
| 25 | Citrus maxima | Rutaceae | L | 16,173 | Riyadh, KSA |
| 26 | Cleome droserifolia | Crassulaceae | Aerial parts (S, L, Fl) | 15,830 | Taif, KSA |
| 27 | Clerodendrum inerme | Verbenaceae | L + S | 12,788 | Riyadh, KSA |
| 28 | Coccinia grandis | Cucurbitaceae | L + S | 16,275 | South, KSA |
| 29 | Combretum molle | Combretaceae | B | 15,496 | South, KSA |
| 30 | Corallocarpus epigeus | Cucurbitaceae | L | 16,393 | South, KSA |
| 31 | Daturai noxia | Solanaceae | L | 15,604 | Riyadh, KSA |
| 32 | Delonix elata | Fabaceae | L | 16,035 | South, KSA |
| 33 | Delonix regia | Fabaceae | L | 16,183 | Taif, KSA |
| 34 | Dodonea angustifolia | Sapindaceae | L | 15,787 | South, KSA |
| 35 | Eruca sativa | Brassicaceae | L + S | 16,318 | South, KSA |
| 36 | Euphorbia tirucalli | Euphorbiaceae | S | 16,172 | Riyadh, KSA |
| 37 | Euphorbia hirta | Euphorbiaceae | Aerial parts (S, L, Fr) | 16,084 | Omdurman, Sudan |
| 38 | Ficus benghalensis | Moraceae | L + B | 16,080 | Riyadh, KSA |
| 39 | Ficus palmata | Moraceae | L | 15,448 | Tanoma, KSA |
| 40 | Flaveria trineriva | Asreraceae | Arial parts (S, L, Fr) | 16,198 | Taif, KSA |
| 41 | Fumaria parviflora | Fumariaceae | L + S | 16,301 | South, KSA |
| 42 | Guiera senegalensis | Combretaceae | L | 798 | Kordofan, Sudan |
| 43 | Haplophyllum tuberculum | Rutaceae | Aerial parts (S, L, Fl) | 16,324 | South, KSA |
| 44 | Indigofera caerulea | Fabaceae | Aerial parts (S, L, Fl) | 16,392 | South, KSA |
| 45 | Ipomoea cairica (L.) sweet | Convolvulaceae | Aerial parts (S, L, Fr) | 16,075 | Riyadh, KSA |
| 46 | Indigofera tinctoria | Fabaceae | Aerial parts (S, L, Fr) | 16,390 | South, KSA |
| 47 | Jatropha curcas | Euphorbiaceae | Seeds | 15,189 | Riyadh, KSA |
| 48 | Juniperus phonicea | Cupressaceae | S + L | 16,179 | Taif, KSA, |
| 49 | Juniperus procera | Cupressaceae | S + L | 16,194 | Taif KSA, |
| 50 | Marrubium vulgare | Labiatae | Aerial parts (S, L, Fr) | 16,043 | Hadah, KSA |
| 51 | Momordica balsamina | Cucurbitaceae | L | 16,395 | South, KSA |
| 52 | Pergularia tomentosa | Asclepiadaceae | Aerial parts (S, L, Fr) | 16,075 | Riyadh, KSA |
| 53 | Psidiumg uajava | Myrtaceae | L | 16,085 | South, KSA |
| 54 | Pulicaria crispa | Asteraceae | Aerial parts (S, L, Fr) | 16,083 | Riyadh, KSA |
| 55 | Ricinus communis | Euphorbiaceae | L | 14,005 | Riyadh, KSA |
| 56 | Rumex dentatus | Polygonaceae | Aerial parts (S, L, Fr) | 16,186 | Taif, KSA |
| 57 | Senna obtusifolia | Fabaceae | Fr | 160,322 | South, KSA |
| 58 | Senna occidentalis | Fabaceae | Fr | 155,009 | South, KSA |
| 59 | Senna alexandrina | Fabaceae | L | 16,245 | South, KSA |
| 60 | Solanum surrattense | Solanaceae | L | 16,386 | South, KSA |
L, leaves; S, stems; B, bark; Fr, fruits; KSA, Kingdom of Saudi Arabia.
Preparation of the plants extracts
Dried plant materials were ground to a coarse powder using a mortar and pestle, extracted with 80% ethanol for 3 days followed by filtering with Whatman Grade 1 paper and were concentrated using a rotary evaporator (Buchi Labortechnik AG, Flawil, Switzerland) under reduced pressure at 4°C. Plants extracts showing anti-HBV activity were further extracted sequentially with different organic solvents of increasing polarity: Hexane, ethyl acetate, dichloromethane, methanol (all from Merck, Darmstadt, Germany), including the aqueous phase. Briefly, 100 g of each plant powder was soaked in a suitable volume of hexane with intermittent shaking for 24 h, and filtered using Whatman Grade 1 paper. Each of the residues were further extracted twice with fresh solvent, and the filtrates were pooled together. The residue was air-dried followed by sequential extractions with dichloromethane, ethyl acetate, methanol and double-distilled water similar to the procedure performed for hexane. Finally, solvents were removed under reduced pressure at 4°C using a rotary evaporator (Buchi Labortechnik AG). Following complete drying, the yield percentage of each extract was calculated (Table II). For biological screening, each extract was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Merck KGaA), and the stocks (100 mg/ml) were stored at −20°C until subsequent use.
Table II.
Determination of cytotoxicity concentration (CC50) and anti-hepatitis B virus activity (inhibition of HBsAg secretion via IC50) and the corresponding TI of the plant extracts.
| Plant name | Extraction solvent | Yield (%) | CC50 (µg/ml) | IC50 (µg/ml) | TI |
|---|---|---|---|---|---|
| Abutilon figarianum | EtOH | 8.71 | 366.67 | 106.46 | 3.44 |
| Hex | 1.10 | 1700.00 | NA | ND | |
| DCM | 0.64 | 1375.02 | 99.76 | 13.78 | |
| EtAc | 0.48 | 332.30 | NA | ND | |
| MeOH | 8.02 | 284.70 | NA | ND | |
| Aqua | 2.21 | NC | NA | ND | |
| Acacia oerfota | EtOH | 11.13 | 1375.00 | 101.46 | 13.55 |
| Hex | 2.64 | 1150.10 | NA | ND | |
| DCM | 0.19 | 960.00 | NA | ND | |
| EtAc | 0.79 | NC | NA | ND | |
| MeOH | 5.54 | 383.30 | 118.90 | 3.22 | |
| Aqua | 9.60 | 422.20 | 106.84 | 3.95 | |
| Capparis decidua | EtOH | 10.31 | 366.67 | 76.85 | 4.77 |
| Hex | 0.27 | 383.30 | NA | ND | |
| DCM | 0.51 | 400.00 | NA | ND | |
| EtAc | 0.18 | 833.10 | NA | ND | |
| MeOH | 3.86 | 1667.67 | NA | ND | |
| Aqua | 6.86 | 520.00 | 66.82 | 7.78 | |
| Coccinia grandis | EtOH | 8.71 | 219.44 | 31.57 | 6.95 |
| Hex | 1.33 | 800.00 | NA | ND | |
| DCM | 0.86 | 480.01 | 57.14 | 8.40 | |
| EtAc | 0.32 | NC | NA | ND | |
| MeOH | 7.28 | 800 | NA | ND | |
| Aqua | 5.61 | 557.14 | 87.21 | 6.38 | |
| Corallocarpus epigeus | EtOH | 7.35 | 1275.00 | 71.90 | 17.73 |
| Hex | 0.86 | 150.00 | NA | ND | |
| DCM | 0.50 | 112.90 | NA | ND | |
| EtAc | 0.31 | 153.37 | NA | ND | |
| MeOH | 0.72 | 2500.00 | NA | ND | |
| Aqua | 2.61 | 1094.00 | 70.91 | 15.42 | |
| Fumaria parviflora | EtOH | 9.35 | NC | 79.84 | ND |
| Hex | 0.88 | 425.00 | 35.44 | 11.99 | |
| DCM | 0.62 | 188.10 | NA | ND | |
| EtAc | 1.02 | 221.98 | NA | ND | |
| MeOH | 8.10 | 1950.00 | 79.19 | 24.62 | |
| Aqua | 2.61 | 766.67 | NA | ND | |
| Guiera senegalensis | EtOH | 9.76 | 1566.00 | 73.21 | 21.39 |
| Hex | 0.52 | 3330.00 | NA | ND | |
| DCM | 0.74 | 200.00 | 10.65 | 18.77 | |
| EtAc | 0.62 | 450.10 | NA | ND | |
| MeOH | 7.32 | 1000.06 | NA | ND | |
| Aqua | 2.08 | 370.69 | 76.67 | 4.83 | |
| Indigofera caerulea | EtOH | 8.61 | 1455.00 | 84.62 | 17.19 |
| Hex | 0.62 | 230.43 | NA | ND | |
| DCM | 0.66 | 208.00 | NA | ND | |
| EtAc | 0.42 | 642.80 | NA | ND | |
| MeOH | 2.47 | 1566 | 73.21 | 21.39 | |
| Aqua | 4.06 | 1250.00 | 125.10 | 9.99 | |
| Pulicaria crispa | EtOH | 12.17 | 686.71 | 23.10 | 29.72 |
| Hex | 1.24 | 160.10 | NA | ND | |
| Pulicaria crispa | DCM | 1.64 | 79.31 | NA | ND |
| EtAc | 0.48 | 603.00 | 14.45 | 41.04 | |
| MeOH | 7.80 | 258.33 | 141.72 | 1.82 | |
| Aqua | 4.41 | 733.30 | NA | ND |
CC50, 50% cytotoxicity concentration; IC50, half maximal inhibitory concentration; Hex, hexane; DCM, dichloromethane; EtOAc, ethyl acetate; MeOH, methanol; Aqua, water; NC, non-cytotoxic; NA, not active; TI, therapeutic index; ND, not determined.
Cell culture and drug
The HBV reporter cell line (HepG2.2.15) was a generous gift from Dr Shahid Jameel (International Center for Genetic Engineering & Biotechnology, New Delhi, India). HepG2.2.15 cells were grown in RPMI-1640 medium (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% heat-inactivated bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 1X penicillin-streptomycin, and 1X sodium pyruvate streptomycin (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified chamber (5% CO2). Lamivudine (Sigma-Aldrich; Merck KGaA), the approved anti-HBV nucleoside analog-based drug was used as a standard.
Cytotoxicity assessment of the plants extracts
Cytotoxicity of extracts was tested on HepG2.2.15 cells using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay kit (Tervigen, Gaithersburg, MD, USA) to determine the extract concentrations (doses) that did not affect cell viability, and were used in subsequent assays. MTT assay is based on the metabolic reduction of soluble MTT by mitochondrial enzyme activity of viable cells, into an insoluble colored formazan product which can be measured optically (14). Cells were seeded (0.5×105 cells/100 µl/well) in flat-bottom 96-well tissue culture plates (Corning Inc., Corning, NY, USA). Following 24 h of incubation, the cells were treated (in triplicate) with various concentrations of test samples (0, 6.25, 12.5, 25, 50 and 100 µg/ml) prepared in culture media, and incubated at 37°C for 48 h. The final concentration of DMSO never exceeded 0.1% in any of the assays and therefore, had no signs of toxicity. Blank (only media) and untreated/negative (0.1% DMSO in media) controls were also included. Cells were treated with MTT reagent (10 µl/well) and further incubated at 37°C for 3–4 h. Upon appearance of a purple color, the detergent solution (100 µl) from the kit was added to each well and further incubated at 37°C for 1 h. Optical density (OD) was recorded at 570 nm using a microplate reader (ELx800; BioTek Instruments, Inc., Winooski, VT, USA). Non-linear regression analysis was performed using Excel software (Microsoft Corp., Redmond, WA, USA) to determine the concentration that resulted in a 50% cytotoxicity concentration (CC50) using the following equation:
Where OD(s), OD(b) and OD(c) are the absorbances of the sample, blank and negative control, respectively.
Microscopy
At 24 and 48 h post-treatment, cells were visually monitored for morphological changes, such as lesions of the cell membrane and the compactness of cytoplasmic components under an inverted microscope (Bio Optical, Milan, Italy) at a magnification of ×200.
Dose-dependent analysis of HBsAg expression in treated cells
HepG2.2.15 cells were seeded in 96-well plates (0.5×105/well) and incubated at 37°C. The following day, the culture media was replaced with fresh media (150 µl, each in triplicate) containing various doses (0, 6.25, 12.5, 25 and 50 µg/ml) of the test samples and controls, and incubated at 37°C for 48 h. The culture supernatants of each sample were collected and stored at −20°C. The secreted HBsAg in the culture supernatants was analyzed using an ELISA kit (cat. no. 72348; Monolisa HBsAg ULTRA; Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the manufacturer's instructions. OD was recorded using an ELx800 microplate reader and analyzed according to the manufacturer's instructions. Non-linear regression analysis was performed using Excel software to determine the half maximal (50%) inhibitory concentration (IC50) of HBsAg secretion.
Time-course analysis of HBsAg inhibition
Based on the dose-dependent inhibition results, time-course (day 1, 3 and 5) analyses were performed to further investigate the antiviral potential of the most active extracts. The HBsAg expression study was performed by treating cells with the safest single-dose (50 µg/ml), as determined by the IC50 values.
Time-course analysis of HBeAg inhibition
Extracts exhibiting the most promising inhibitory effects on HBsAg secretions were further subjected to time-course (day 1, 3 and 5) analyses of HBeAg expression at a dose of 50 µg/ml. ELISA was performed on the culture media using an HBeAg/anti-HBe ELISA kit (cat. no. KAPG4BNE3; DIAsource ImmunoAssays; SA, Louvain-la-Neuve, Belgium) according to the manufacturer's instructions. OD was recorded using an ELx800 microplate reader, and analyzed following the DIASource manual.
Phytochemical constituent screening
Plants exhibiting the most promising anti-HBV activities were subjected to qualitative phytochemical screening for major secondary metabolites, including alkaloids, flavonoids, anthraquinones, tannins and saponins, following standard procedures (15–17) with minor modifications. Briefly, for alkaloids, the Mayer's test was performed. A total of 0.5 gm of the extract was dissolved in 2% hydrochloric acid (Sigma-Aldrich; Merck KGaA), and filtered. Mayer's reagent was freshly prepared by dissolving 0.68 g of mercuric chloride (Sigma-Aldrich; Merck KGaA) and 2.5 g of potassium iodide (Sigma-Aldrich; Merck KGaA) and made to 50 ml with distilled water. A few drops of the reagent were added to the 3 ml of extract solution in a test tube where formation of a yellowish precipitate indicated the presence of alkaloids. For flavonoids, the sodium hydroxide test was performed. A total of 5 ml of the extract solution was treated with few drops of 20% sodium hydroxide (Sigma-Aldrich; Merck KGaA) in a test tube. Formation of an intense yellow color, which becomes colorless on addition of diluted hydrochloric acid, indicated the presence of flavonoids. For tannins, the ferric chloride test was performed. A total of 0.25 gm of the extract was dissolved in 10 ml of distilled water in a test tube and few drops of 5% ferric chloride (Sigma-Aldrich; Merck KGaA) was added. Appearance of brownish green or blueish-black color indicated the presence of tannins. For saponins, the frothing test was performed. A total of 0.5 gm of the extract was dissolved in 10 ml of distilled water in a test tube and shaken vigorously. Formation of a thick persistent froth that persisted for at least 15 min indicated the presence of saponins. And lastly, for anthraquinone the Borntrager's test was performed. A total 0.5 gm of the extract residue was boiled with 5 ml of dilute hydrochloric acid and filtered while hot. The filtrate was combined with 5 ml of chloroform and shaken. The chloroform layer was transferred into a test tube and 2 ml of dilute ammonia solution (Sigma-Aldrich; Merck KGaA) was added. The appearance of a rose-pink to cherry-red color indicated the presence of anthraquinones.
Results
Effects of plants extracts on cell viability
CC50 values of different plants extracts were calculated (Table II), which allowed for the determination of the single optimal dose (50 µg/ml) with no sign of cytotoxicity. This observation was confirmed by microscopic observation of the cell morphology/growth at 24 and 48 h post-treatment with different concentrations of each extract (data not shown). Therefore, extracts at 50 µg/ml were used in the subsequent antiviral assays.
Dose-dependent inhibition of HBsAg by different extracts
Firstly, the total ethanolic-extracts of 60 medicinal plants were screened for anti-HBV activity by measuring the expression levels of viral HBsAg at 48 h. Of these, 9 plants showed inhibition of HBsAg production in a dose-dependent manner. These were Abutilon figarianum, Acacia oerfota, Capparis decidua, Coccinea grandis, Corallocarpus epigeus, Fumaria parviflora, Guiera senegalensis, Indigofera caerulea and Pulicaria crispa with IC50 values of 106.46, 101.46, 76.85, 31.57, 71.90, 79.84, 73.21, 84.62 and 23.10 µg/ml, respectively (Table II). Based on these results, 5 sequential extracts (hexane, dichloromethane, ethyl acetate, methanol and aqueous) of each of the 9 selected plants were prepared, and tested for cytotoxicity. The sequential extracts were further evaluated for dose-dependent HBsAg inhibition. The extraction yield percentage, IC50, CC50 and their corresponding TI values were calculated (Table II). Of these, 24 different extracts (from the 9 selected plants) that showed marked HBsAg inhibition were evaluated in a time-course study.
Time-course inhibition of HBsAg by selected extracts
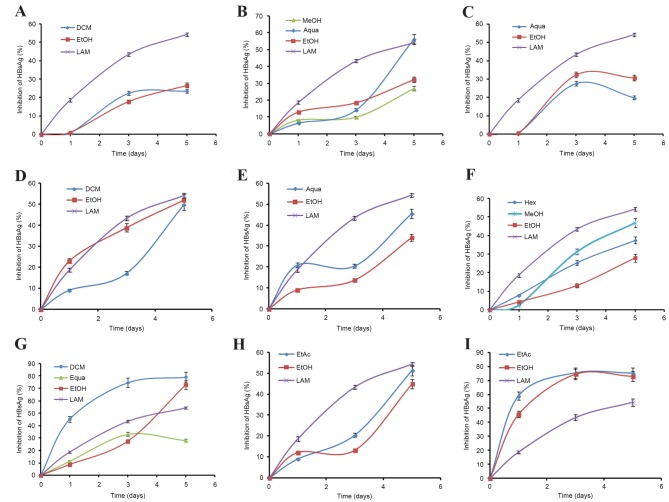
The selected extracts (of 9 plants) that showed marked HBsAg inhibition were evaluated in a time-course study, using 50 µg/ml doses for 5 days (Fig. 1). While prolonged treatment beyond day 5 did not show any notable inhibitory effect, further continuation of the culture resulted in cell overgrowth and death (data not shown). The optimal antiviral activities, in order, on day 5 post-treatment were: G. senegalensis (dichloromethane extract; IC50=10.65), P. crispa (ethyl acetate extract; IC50=14.45), C. gardis (total ethanol extract; IC50=31.57), F. parviflora (hexane extract; IC50=35.44), C. decidua (aqueous extract; IC50=66.82), C. epigeus (total ethanol extract; IC50=71.9), I. caerulea (methanol extract; IC50=73.21), A. figarianum (dichloromethane extract; IC50=99.76) and A. oerfota (total ethanol extract; IC50=101.46) (Table II).
Figure 1.
Time-course anti-HBV activity of selected plant extracts (50 µg/ml each). (A) Abutilon figarianum; (B) Acacia oerfota; (C) Capparis decidua; (D) Coccinia grandis; (E) Corallocarpus epigeus; (F) Fumaria parviflora; (G) Guiera senegalensis; (H) Indigofera caerulea; (I) Pulicaria crispa. ELISA showing inhibitions of HBsAg expression in HepG2.2.15 culture supernatants at days 1, 3 and 5 post-treatment. Lamivudine (2.0 µM) was used as a reference anti-HBV drug. Values (y-axis): means of three determinations. HBV, hepatitis B virus; Aqua, aqueous; DCM, dichloromethane; EtAc, ethyl acetate; EtOH, ethanol; Hex, hexane; MeOH, methanol; LAM, lamivudine.
Time-course downregulation of HBV replication by the active extracts
The HBeAg is a processed product of the ‘pre-Core’ gene that is co-translated with the ‘Core’ gene by bicistronic subgenomic-RNA. Therefore, in a natural infection, seropositivity of HBeAg is a hallmark of active viral DNA replication. Notably, this is analogous to the HIV ‘p24’ antigen where ELISA is a valid tool to monitor retroviral RNA replication. Therefore, the most promising active extracts that greatly suppressed HBsAg synthesis (Fig. 1), were analyzed for time-course effect on HBeAg production in the culture supernatants. While HBeAg secretion was inhibited maximally at day 3, there were no further improvements in antiviral activities at day 5 (Fig. 2). Limited by cell overgrowth and death, and unaffected virus replication (inhibition of HBeAg), the study was terminated at day 5. Furthermore, the antiviral activities on downregulating virus replication, as measured by HBeAg production, were: G. senegalensis (dichloromethane extract; 66%); I. caerulea (methanol extract; 58.60%); P. crispa (ethyl acetate extract; 55.30%); F. parviflora (hexane-extract; 54.68%); C. decidua (aqueous extract; 51.52%); C. epigeus (total ethanol extract; 43.31%); C. grandis (total ethanol extract; 35.51%); A. oerfota (total ethanol extract; 35.28%); and A. figarianum (dichloro methane extract; 27.56%) compared to the untreated control (Fig. 2). It is noteworthy that, with the exception of C. grandis and A. figarianum, all plant extracts (50 µg/ml) showed greater antiviral activity than lamivudine (2 µM).
Figure 2.
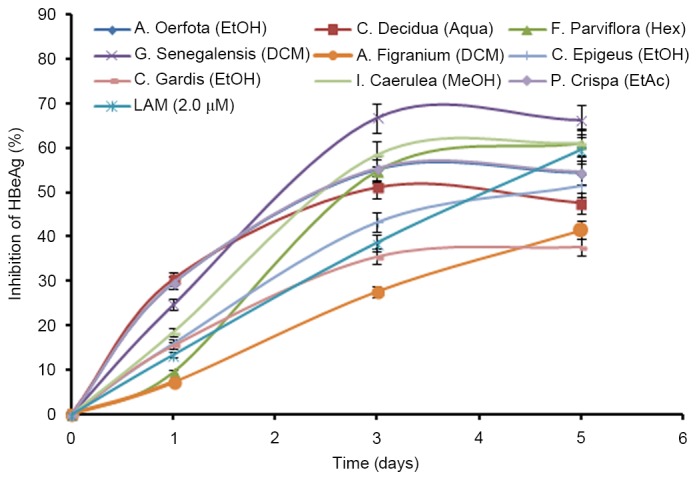
Time-course anti-HBV activity of the plants extracts with anti-HBV potential (50 µg/ml each). ELISA showing inhibitions of HBeAg expression in HepG2.2.15 culture supernatants at days 1, 3 and 5 post-treatment. Lamivudine (2.0 µM) was used as a reference anti-HBV drug. Values (y-axis): means of three determinations. HBV, hepatitis B virus; Aqua, aqueous; DCM, dichloromethane; EtAc, ethyl acetate; EtOH, ethanol; Hex, hexane; MeOH, methanol; LAM, lamivudine.
Phytochemical screening
Plants that exhibited anti-HBV activity showed the presence of alkaloids, flavonoids, tannins, saponins and anthraquinones (Table III), which have been previously reported for their antiviral activities (7,8).
Table III.
Qualitative phytochemical screening of plants with anti-hepatitis B virus activities.
| Phytochemicals | |||||
|---|---|---|---|---|---|
| Active plant extracts | Alkaloids | Flavonoids | Tannins | Saponins | Anthraquinones |
| Abutilon figarianum | + | − | − | − | − |
| Acacia oerfota | + | + | + | − | − |
| Capparis deciduas | + | + | + | + | − |
| Coccinea grandis | + | + | + | + | − |
| Corallocarpus epigeus | + | + | + | − | − |
| Fumaria parviflora | + | + | + | + | − |
| Guiera senegalensis | + | + | + | + | − |
| Indigofera caerulea | + | + | + | + | − |
| Pulicaria crispa | + | + | + | − | − |
+, detected; -, not detected.
Discussion
Development of anti-HBV therapies has been impeded until recently by the lack of suitable in vitro and in vivo experimental models that were able mimic natural chronic hepatitis B (18–21). Several lines of hepatoma cells stably transfected with HBV genome have been developed as an in vitro model to screen and identify potential antiviral therapeutic agents. Of these, in the present study, the widely used Hep G2.2.15 cells were used to evaluate the anti-HBV potential of candidate plants by measuring the expression of HBsAg (serological marker of viral infection) and HBeAg (serological marker of active DNA replication), respectively (22,23). A total of 60 medicinal plants were investigated for the first time, based on information on either their use in traditional medicine for curing liver diseases or experimental evidence of hepatoprotective or anti-retroviral potentials (12,13,24–37). The preliminary cell viability assay of the plants' total ethanolic extracts in the present study showed no cytotoxicity at concentrations up to 50 µg/ml. Further initial screening (dose-dependent HBsAg inhibition) identified 9 plants with notable anti-HBV activity that were therefore selected for sequential extractions (organic and aqueous phase) and subsequent screening.
The highest level of anti-HBV activity was observed in the dichloromethane extract of G. senegalensis, known as the ‘cure all’ medicine in African traditional medicine due to its wide applications (24). It is used to treat venereal, stomach, respiratory, dermatological and microbial diseases (25), including malaria (26). In agreement with its reported anti-HSV potential (27), G. senegalensis is likely to have exhibited anti-HBV activity because HSV and HBV are biologically and genetically similar. In addition to this, it has previously been reported that administration of G. senegalensis extract to Wistar rats for 6 months did not cause any significant hematological, biochemical or histological toxicity (28), confirming its in vivo safety. Assuming there was no synergy among the phytochemical constituents present in the dichloromethane-extract, it can be implied that the active compound(s) could be more potent than the lamivudine used as standard reference drugs.
F. parviflora has traditionally been used in Saudi folk medicine for the treatment of jaundice and hepatobiliary disorders (29). At day 5 post-treatment, hexane and methanol extracts of F. parviflora showed the best anti-HBV activities by ~37.45 and 46.86%, respectively. Besides its use in Sudanese traditional medicine to treat fever and jaundice, an aqueous-extract of C. decidua has been demonstrated to show hepatoprotective activity in rats (30). Notably, in line with its reported antiviral activity against HIV reverse-transcriptase (31), the aqueous-extract of C. decidua exhibited anti-HBV potential in the present study.
Acacia spp. constitute a large variety of medicinal plants worldwide, and of these, A. catechu has been previously reported for its anti-HIV activity (23). The present authors have previously demonstrated that A. mellifera ethyl acetate, n-butanol and aqueous extracts also have hepatoprotective and anti-HBV effects (32). In the present study, anti-HBV evaluation of A. oerfota extracts at a non-cytotoxic dose showed its association with the methanolic and aqueous extracts. Furthermore, the highest anti-HBV activity of C. grandis and C. epigeus was associated with the crude ethanolic-extract, indicating the possibility of synergy among the antiviral phytochemical constituents of the extract. Synergistic activity of antiviral components of plant extracts that act by different mechanisms has been reported previously (33). Traditionally, crude extracts of C. grandis are used to treat coughing, bronchitis, skin diseases, tongue sores and liver disorders (34). In previous studies, in vivo antioxidant and hepatoprotective efficacies of C. grandis (crude ethanolic-extract) and C. epigeus (ethanolic and aqueous extracts) have been demonstrated (35–37).
A variety of active phytochemicals (alkaloids, flavonoids, lignans, tannins, terpenoids, saponins and anthraquinones) of diverse geographic origins have already been reported to be effective against HBV in vitro and/or in vivo (7,8,38,39). Of these, promising anti-HBV phytoproducts such as picroliv (Picrorhiza kurroa), andrographolide (Andrographis paniculata), artemisinin (Artemisia annua) and silymarin have been reported for a long time (40). Notably, the most potent anti-HBV phytochemicals include isolated niranthin and hinokinin (lignans). From Phyllanthus spp. (41–43), helioxanthin from the Chinese Taiwania cryptomerioides (44), wogonin, another flavonoid from Scutellaria radix (45), the polyphenolic extract from Geranium carolinianum L. (46), protostane triterpenes from Alisma orientalis (47), dihydrochelerythrine alkaloids from Corydalis saxicola (48), Saikosaponin C from Bupleurum species (49), extracts from Rheum palmatum L. (50), and LPRP from Liriope platyphylla (51). Furthermore, the qualitative phytochemical analyses of the selected plant extracts in the present study that showed promising anti-HBV potential also revealed the presence of alkaloids, flavonoids, triterpenoids and tannin, which may have contributed to the antiviral activities observed. However, a detailed phytochemical investigation of these extracts is essential to elucidate the active principle(s) responsible for the anti-HBV potential.
In conclusion, antiviral screening in the present study discovered that extracts of G. senegalensis, F. parviflora and P. crispa had the most promising anti-HBV potential, followed by those of A. figarianum, A. oerfota, C. decidua, C. grandis, C. epigeus and I. caerulea with notable activities. From the results of the present study, it is possible to demonstrate the importance of the application of ethnobotanical information in the search and selection of traditionally used plants, which may provide new opportunities for the treatment of chronic hepatitis B. However, a detailed phytochemical study of these extracts is required in order to elucidate the active principle(s) responsible for their novel anti-HBV potential.
Acknowledgements
The project was supported by the National Plan for Science, Technology and Innovation (MARIFAAH) funded by King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia (grant no. MED11-1585-02).
References
- 1.Teo CG, Locarnini SA. Potential threat of drug-resistant and vaccine-escape HBV mutants to public health. Antivir Ther. 2010;15:445–449. doi: 10.3851/IMP1556. [DOI] [PubMed] [Google Scholar]
- 2.Torresi J. Hepatitis B antiviral resistance and vaccine escape: Two sides of the same coin. Antivir Ther. 2008;13:337–340. [PubMed] [Google Scholar]
- 3.Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C, Hepatitis B, Virus Drug Resistance Working Group Antiviral drug-resistant HBV: Standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254–265. doi: 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- 4.Locarnini S. Primary resistance, multidrug resistance, and cross-resistance pathways in HBV as a consequence of treatment failure. Hepatol Int. 2008;2:147–151. doi: 10.1007/s12072-008-9048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Zhu J. Anti-HBV effect of individual traditional chinese herbal medicine in vitro and in vivo: An analytic review. J Viral Hep. 2013;20:445–452. doi: 10.1111/jvh.12112. [DOI] [PubMed] [Google Scholar]
- 6.Qiu LP, Chen KP. Anti-HBV agents derived from botanical origin. Fitoterapia. 2013;84:140–157. doi: 10.1016/j.fitote.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Parvez MK, Arbab AH, Al-Dosari MS, Al-Rehaily AJ. Antiviral natural products against chronic hepatitis B: Recent developments. Curr Pharm Des. 2016;22:286–293. doi: 10.2174/1381612822666151112152733. [DOI] [PubMed] [Google Scholar]
- 8.Wu YH. Naturally derived anti-hepatitis B virus agents and their mechanism of action. World J Gastroenterol. 2016;22:188–204. doi: 10.3748/wjg.v22.i1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HC, Chou CK, Lee SD, Wang JC, Yeh SF. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1995;27:99–109. doi: 10.1016/0166-3542(94)00083-K. [DOI] [PubMed] [Google Scholar]
- 10.Chou SC, Huang TJ, Lin EH, Huang CH, Chou CH. Anti-hepatitis B virus constituents from Solanum erianthum. Nat Prod Commun. 2012;78:153–156. [PubMed] [Google Scholar]
- 11.Li J, Huang H, Zhou W, Feng M, Zhou P. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol Pharma Bull. 2008;31:743–747. doi: 10.1248/bpb.31.743. [DOI] [PubMed] [Google Scholar]
- 12.Rahman MA, Mossa JS, Al-Said MS, Al-Yahya MA. Medicinal plant diversity in the flora of Saudi Arabia 1: A report on seven plant families. Fitoterapia. 2004;75:149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Al-Asmari AK, Al-Elaiwi AM, Athar MT, Tariq M, Al Eid A, Al-Asmary SM. A review of hepatoprotective plants used in saudi traditional medicine. Evid Based Complement Alternat Med. 20142014:890842. doi: 10.1155/2014/890842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: The MTT assay. Methods Mol Biol. 2011;731:237–245. doi: 10.1007/978-1-61779-080-5_20. [DOI] [PubMed] [Google Scholar]
- 15.Satyajit D, Sarkar ZL, Gray AI. Natural Products Isolation. 2nd. Humana Press; Totowa, NJ, USA: 2006. [Google Scholar]
- 16.Kar A. Pharmacognosy and Pharmacobiotechnology. 2nd. New Age International; New Delhi, India: 2007. [Google Scholar]
- 17.Tiwari P, Mandeep BK, Kaur KG, Kaur H. Phytochemical screening and extraction: A review. Interl Pharma Sci. 2011;1:98–1062. [Google Scholar]
- 18.Hantz O, Zoulim F. Duck hepatitis B virus primary hepatocyte culture model. Methods Mol Med. 2004;96:189–197. doi: 10.1385/1-59259-670-3:189. [DOI] [PubMed] [Google Scholar]
- 19.Engler OB, Dai WJ, Sette A, Hunziker IP, Reichen J, Pichler WJ, Cerny A. Peptide vaccines against hepatitis b virus: From animal model to human studies. Mol Immunol. 2001;38:457–465. doi: 10.1016/S0161-5890(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 20.Thung SN, Gerber MA, Purcell RH, London WT, Michalik KB, Popper H. Animal model of human disease: Chimpanzee carriers of hepatitis B virus. Chimpanzee hepatitis B carriers. Am J Pathol. 1981;105:328–332. [PMC free article] [PubMed] [Google Scholar]
- 21.Aljofan M, Netter HJ, Aljarbou AN, Hadda TB, Orhan IE, Sener B, Mungall BA. Anti-hepatitis B activity of isoquinoline alkaloids of plant origin. Arch Virol. 2014;159:1119–1128. doi: 10.1007/s00705-013-1937-7. [DOI] [PubMed] [Google Scholar]
- 22.Bonino F, Hoyer B, Nelson J, Engle R, Verme G, Gerin J. Hepatitis B virus DNA in the sera of HBsAg carriers: A marker of active hepatitis B virus replication in the liver. Hepatology. 1981;1:386–391. doi: 10.1002/hep.1840010503. [DOI] [PubMed] [Google Scholar]
- 23.Arbab AH, Parvez MK, Al-Dosari MS, Al-Rehaily AJ, Al-Sohaibani M, Zaroug EE, AlSaid MS, Rafatullah S. Hepatoprotective and antiviral efficacy of Acacia mellifera leaves extracts against hepatitis B virus. Biomed Res Int. 2015;2015:929131. doi: 10.1155/2015/929131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akuodor GC, Essien AD, David-Oku E, Chilaka KC, Akpan JL, Ezeokpo B, Ezeonwumelu JO. Gastroprotective effect of the aqueous leaf extract of Guiera senegalensis in albino rats. Asian Pacific J Trop Med. 2013;6:771–775. doi: 10.1016/S1995-7645(13)60136-4. [DOI] [PubMed] [Google Scholar]
- 25.Silva O, Serrano R, Gomes ET. Botanical characterization of Guiera senegalensis leaves. Microsc Microanal. 2008;14:398–404. doi: 10.1017/S1431927608080860. [DOI] [PubMed] [Google Scholar]
- 26.Abubakar MS, Sule MI, Pateh UU, Abdurahman EM, Haruna AK, Jahun BM. In vitro snake venom detoxifying action of the leaf extract of Guiera senegalensis. J Ethnopharmacol. 2000;69:253–257. doi: 10.1016/S0378-8741(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 27.Silva O, Barbosa S, Diniz A, Valdeira ML, Gomes E. Plant extracts antiviral activity against herpes simplex virus type 1 and African swine fever virus. Interl J Pharmacog. 1997;35:12–16. doi: 10.1076/phbi.35.1.12.13264. [DOI] [Google Scholar]
- 28.Diouf A, Cisse A, Gueye SS, Mendes V, Siby T, Diop RM Diouf, Bassene E. Toxocological study of Guiera senegalensis Lam (Combretaceae) Dakar Med. 2000;45:89–94. (In French) [PubMed] [Google Scholar]
- 29.Najeeb-ur-Rehman Bashir S, Al-Rehaily AJ, Gilani AH. Mechanisms underlying the antidiarrheal, antispasmodic and bronchodilator activities of Fumaria parviflora and involvement of tissue and species specificity. J Ethnopharmacol. 2012;144:128–137. doi: 10.1016/j.jep.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 30.Ali H, König GM, Khalid SA, Wright AD, Kaminsky R. Evaluation of selected Sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-RT and tyrosine kinase inhibitory, and for cytotoxicity. J Ethnopharmacol. 2002;83:219–228. doi: 10.1016/S0378-8741(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 31.Ali SA, Gameel AA, Mohamed AH, Hassan T. Hepatoprotective activity of Capparis decidua aqueous and methanolic stems extracts against carbon tetrachloride induced liver histological damage in rats. J Pharmacol Toxicol. 2011;6:62–68. doi: 10.3923/jpt.2011.62.68. [DOI] [Google Scholar]
- 32.Nutan M, Modi C, Dezzutti CS, Kulshreshtha S, Rawat AK, Srivastava SK, Malhotra S, Verma A, Ranga U, Gupta SK. Extracts from Acacia catechu suppress HIV-1 replication by inhibiting the activities of the viral protease and tat. Virology J. 2013;10:309. doi: 10.1186/1743-422X-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhivya R, Enzo AP, Tiong CY, Lim SL Diana, Chu LT, Francois M, Lara G. Evidence of synergistic activity of medicinal plant extracts against neuraminidase inhibitor resistant strains of influenza viruses. Adv Microbiol. 2014;4:1260–1277. doi: 10.4236/aim.2014.416136. [DOI] [Google Scholar]
- 34.Niazi J, Singh P, Bansal Y, Goel RK. Anti-inflammatory, analgesic and antipyretic activity of aqueous extract of fresh leaves of Coccinia indica. Inflammopharmacology. 2009;17:239–244. doi: 10.1007/s10787-009-0010-3. [DOI] [PubMed] [Google Scholar]
- 35.Gopalakrishnan V, Rao KN, Devi M, Padmaha N, Lakshmi PM, Srividya T, Vadivukarasi G. Antihepatotoxic acticity of Coccinia indica. Anc Sci Life. 2001;21:12–15. [PMC free article] [PubMed] [Google Scholar]
- 36.Rangu MV, Suresh B, Sandeep VN, Narendra N, Ramesh M. Hepatoprotective activity of leaves of Corallocarpus epigaeus in carbon tetrachloride induced rats. Interl J Biol Pharma Res. 2012;3:567–570. [Google Scholar]
- 37.Madrigal-Santillán E, Madrigal-Bujaidar E, Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, Bautista M, Morales-González Á, García-Luna y González-Rubio M, Aguilar-Faisal JL, Morales-González JA. Review of natural products with hepatoprotective effects. World J Gastroenterol. 2014;20:14787–14804. doi: 10.3748/wjg.v20.i40.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wohlfarth C, Efferth T. Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharmacol Sin. 2009;30:25–30. doi: 10.1038/aps.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B: A systematic review. Liver. 2001;21:280–286. doi: 10.1034/j.1600-0676.2001.021004280.x. [DOI] [PubMed] [Google Scholar]
- 40.Mehrotra R, Rawat S, Kulshreshtha DK, Patnaik GK, Dhawan BN. In vitro studies on the effect of certain natural products against hepatitis B virus. Indian J Med Res. 1990;92:133–138. [PubMed] [Google Scholar]
- 41.Huang RL, Huang YL, Ou JC, Chen CC, Hsu FL, Chang C. Screening of 25 compounds isolated from Phyllanthus species for anti-human hepatitis B virus in vitro. Phytother Res. 2003;17:449–453. doi: 10.1002/ptr.1167. [DOI] [PubMed] [Google Scholar]
- 42.Ott M, Thyagarajan SP, Gupta S. Phyllanthus amarus suppresses hepatitis B virus by interrupting interactions between HBV enhancer I and cellular transcription factors. European J Clin Invest. 1997;27:908–915. doi: 10.1046/j.1365-2362.1997.2020749.x. [DOI] [PubMed] [Google Scholar]
- 43.Mehortra R, Rawat S, Kulshreshtha DK, Goyal P, Patnaik GK, Dhawan BN. In vitro effect of Phyllanthus amarus on hepatitis B virus. Indian J Med Res. 1991;93:71–73. [PubMed] [Google Scholar]
- 44.Cheng YC, Ying CX, Leung CH, Li Y. New targets and inhibitors of HBV replication to combat drug resistance. J Clin Virol. 2005;34(Suppl 1):S147–S150. doi: 10.1016/S1386-6532(05)80026-5. [DOI] [PubMed] [Google Scholar]
- 45.Guo Q, Zhao L, You Q, Yang Y, Gu H, Song G, Lu N, Xin J. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res. 2007;74:16–24. doi: 10.1016/j.antiviral.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Huang H, Feng M, Zhou W, Shi X, Zhou P. In vitro and in vivo anti-hepatitis B virus activities of a plant extract from Geranium carolinianum L. Antiviral Res. 2008;79:114–120. doi: 10.1016/j.antiviral.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Y, Zhang XM, Zhang FX, Liu N, Zhao F, Zhou J, Chen JJ. A new triterpene and anti-hepatitis B virus active compounds from Alisma orientalis. Planta Med. 2006;72:951–954. doi: 10.1055/s-2006-947178. [DOI] [PubMed] [Google Scholar]
- 48.Wu YR, Ma YB, Zhao YX, Yao SY, Zhou J, Zhou Y, Chen JJ. Two new quaternary alkaloids and anti-hepatitis b virus active constituents from Corydalis saxicola. Planta Medica. 2007;73:787–791. doi: 10.1055/s-2007-981549. [DOI] [PubMed] [Google Scholar]
- 49.Chiang LC, Ng LT, Liu LT, Shieh DE, Lin CC. Cytotoxicity and anti-hepatitis B virus activities of saikosaponins from Bupleurum species. Planta Med. 2003;69:705–709. doi: 10.1055/s-2003-42797. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Li LJ, Sun Y, Li J. Identification of natural compounds with anti-hepatitis B virus activity from Rheum palmatum L. ethanol extract. Chemotherapy. 2007;53:320–326. doi: 10.1159/000107690. [DOI] [PubMed] [Google Scholar]
- 51.Huang TJ, Tsai YC, Chiang SY, Wang GJ, Kuo YC, Chang YC, Wu YY, Wu YC. Anti-viral effect of a compound isolated from Liriope platyphylla against hepatitis B virus in vitro. Virus Res. 2014;192:16–24. doi: 10.1016/j.virusres.2014.07.015. [DOI] [PubMed] [Google Scholar]