Abstract
Large skin defects are commonly observed in the clinic and have attracted much attention recently. Therefore, finding an effective solution for large skin defects is a global problem. The objective of the present study was to assess the effectiveness of the EASApprox® skin-stretching system for closing large skin defects. Skin defects (5×5 cm) were created on the forearms of 9 Bama miniature pigs, which were randomly divided into the following three groups: Direct suture, the new EASApprox® skin-stretching device and Kirschner wires. Microcirculation was assessed before surgery and after wound closure. Following the different treatments, the defects were sutured, and wound healing was assessed based on a clinical score. Furthermore, microscopic and ultramicroscopic structures were evaluated, including collagen, elastic fibers and the microvessel density. Significant differences in the clinical score and microvessel density were observed among the groups. Additionally, the mean length obtained for elastic fibers was larger than that obtained for the other two groups. Finally, the new EASApprox® skin-stretching device resulted in successful wound management and with only minor side effects on skin histology and microcirculation. Therefore, this method has the potential to be used for healing large skin defects.
Keywords: wound closure, skin stretching, viscoelastic properties of skin
Introduction
Large skin defects, which are often observed in the clinic following trauma, burn, high-tension wounds, tumorectomy and large flap donor zones, have become a global problem that numerous surgeons encounter. It is often difficult to achieve primary closure on large skin defects on the trunk and limbs of patients due to a paucity of skin. Furthermore, a variety of methods have been used for closing large skin defects, including the use of skin grafts, local flaps, tissue stretching, skin expansion, free flaps and closure by secondary surgery. Limited by their considerable failure rate, risk of complications, complexity, delayed healing and poor appearance; these techniques have not been very widely used.
Biomechanical characteristics that define skin include creep, extensibility, viscoelasticity and stress relaxation (1–3). Creep describes the ability of the skin to extend under stretching at continuous tension (4). Viscoelasticity includes viscosity and elasticity, where elasticity describes the characteristic that the deformation due to stress loads can be temporary if the stress is rapidly relieved, whereas viscosity describes the permanent deformation that results when stress is maintained (3). As the corollary of creep, stress relaxation indicates that if skin is stretched over a constant distance, the amount of tension that is required to keep the skin stretched will slowly decrease over time (1,2,5).
Based on these principles, skin-stretching systems have been designed to exploit the potential afforded by these biomechanical characteristics to realize the primary closure of large skin defects within a short time. By applying a mechanical load to skin stretchers, one edge of the wound can be gradually pulled to the opposite side, achieving complete wound closure. This method is effective in theory. However, understanding how to achieve optimum efficiency in stretching skin in practice remains under investigation.
In 1987, Bashir (6) was the first to attempt a stretching technique to achieve skin expansion by traction with Kirschner and silver wires. Subsequently, several types of skin-stretching systems have emerged. In 1992, a specific piece of equipment termed a skin-stretching device was invented by Hirshowitz et al (7); this device attempted to close a wound by harnessing the viscoelastic property of the skin. This new device and improved versions accomplished the effective primary closure of large skin defects by markedly reducing the size of the defects in cases involving tumors, ulcers, scars, tattoos, burns, nevi and donor-site defects (6–12). Nevertheless, notable complications following skin stretching, including wound dehiscence, hypertrophic scar, infection, marginal necrosis and delayed healing arose, and this device was unable to prevent them (6–9).
Using a different form that grasped the skin edge without Kirschner wires, Wilhelm Fleischmann designed the EASApprox® skin-stretching system, which uses special hooked needles and a tension indicator (Fig. 1). This device was used to improve the potential of skin biomechanical properties. Three makers on the indicator included with the EASApprox® skin-stretching device represent 0.5 (5 N), 1.5 (15 N) and 3.0 kg (30 N) of stretching tension. This innovation has several advantages, including simple operation, increased healing and reduced complications. In order to evaluate the effectiveness of the new device at closing skin defects, the present study was performed using Bama miniature pigs; the new EASApprox® device was compared with methods including sutures and Kirschner wires in terms of cutaneous microcirculation, histology and the overall healing.
Figure 1.
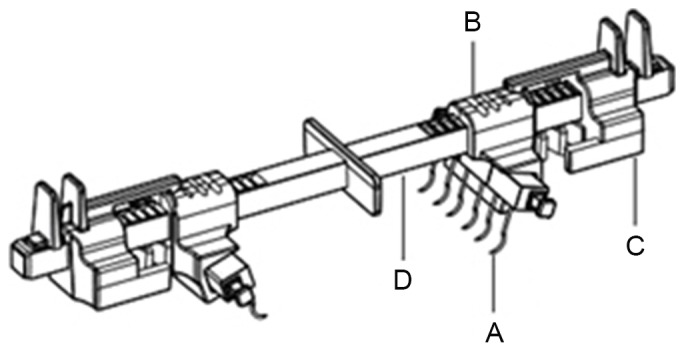
The EASApprox skin stretching system contains the following components: (A) Hook modules: A trio of hook needle units can be assembled by a connecting pin inserted into the top hole of the needles; (B) Needle holders: These can engage the needles by the concave slot; (C) The spring module: This indicator is designed to show the tension on the wound margin. There are three scales representing low, moderate and high tension. When tension was indicated to be on a low scale by the device, the wound could be safely closed and (D) Rod: The needles can be proximated together by pushing the spring modules and needle holders along the rod.
Materials and methods
Animals
The present study was approved by the Ethics Committee on Animal Care and Use of Dalian Medical University (Dalian, China). In total, 9 healthy, 10-month-old, purposely bred Bama miniature pigs (all females, weighing 20–25 kg) were purchased from Taizhou Taihe Biology Technology Co., Ltd., (Jiangsu, China). Furthermore, complete physical examinations and blood counts, serum biochemical analyses and urinalysis were performed on the miniature pigs before the study commenced. The animals were housed indoors, were allowed to move freely and had outdoor access twice daily. Commercial dry maintenance diets were administered twice daily, and water was available ad libitum. The pigs were then continuously fed as they participated in other experiments following the present study.
Study design
After the creation of skin defects on both forearms, the 18 forearms were randomly divided into 3 groups. In group A, sutures were used for direct closure; in group B, the new EASApprox® skin-stretching device [PFKZ-SL-02; BIOWIM (China), Ltd., Dalian, China] was used and in group C, Kirschner wires were inserted along each side of the wound and used to achieve closure.
Preoperative procedures
The pigs were premedicated with acepromazine (0.1 mg/kg; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), xylazine (2.2 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), ketamine (2 mg/kg; Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) and atropine (0.02 mg/kg; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Endotracheal general anesthesia via cannula was induced with 2.5% thiopental sodium (8 mg/kg; Shanghai New Asia Pharmaceutical Co., Ltd., Shanghai, China) and maintained with isoflurane (3–5%; Hebei Jiupai Pharmaceutical Co., Ltd., Shijiazhuang, China) in oxygen (1.5 l/min); and the body temperature, heart and respiration rates were monitored throughout. Both forearms of each pig were shaved from the middle of the humerus to the carpus and prepared for aseptic surgery. All drugs listed above were supported by the Central Research Laboratory of The First Affiliated Hospital of Dalian Medical University. Following the operation, one Panadol (Sino American Tianjin Smith Kline & French Laboratories, Ltd., Tianjin, China) was fed to each pig daily from postoperative day (POD) 1 to POD 10.
Surgical procedures
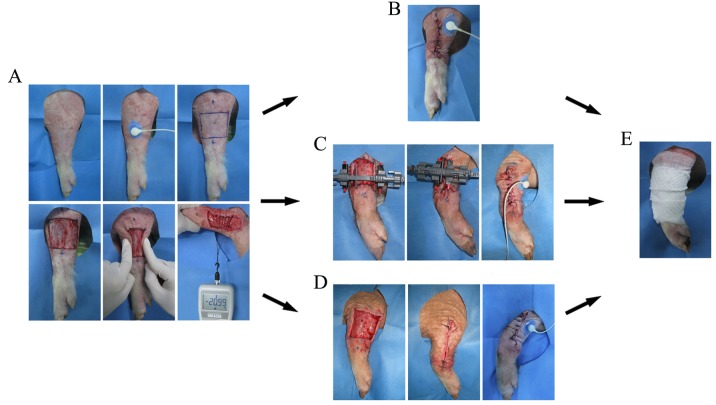
On the day of the operation, one skin defect (5×5 cm) was created by removing the intact skin and subcutaneous tissue using a No. 10 blade on the lateral surface of each mid-forearm. Furthermore, cutaneous microcirculation was measured based on the transcutaneous partial pressure of oxygen (TcpO2) using a PeriFlux System 5000 instrument (Perimed AB, Stockholm, Sweden) (Fig. 2A). Pinch tests were used to indicate high tension, and a push/pull gauge (Imada Co., Ltd., Toyohashi, Japan) measured the tension of each wound margin (19.52–21.05 N) to standardize the wounds. Defects without any undermining of the wound edges had lengths and widths of 5 cm.
Figure 2.
Wound creation and wound closure. (A) Wound creation, TcpO2 and tension measurement were accomplished. Different treatments for wounds included (B) direct suture, (C) stretching using the EASApprox® new device and (D) extension by Kirschner wires. (E) All the surgical areas were bandaged with the same sterile dressing. TcpO2, transcutaneous partial pressure of oxygen.
For group A (suture), a direct simple interrupted procedure was performed to cover the wound using 2/0 Mersilk sutures (Ethicon Inc., Somerville, NJ, USA). Side holes were 0.5 cm from the wound edges (Fig. 2B). For group B (Fig. 2C), the EASApprox® skin-stretching device was applied by installing hook needles ~0.5 cm from the wound edge. In total, three cycles of intermittent skin stretching (cycle loading) were subsequently loaded. Forces of 1.0–3.0 kg were loaded for 4 min, followed by 1-min periods of relaxation (13). After a second pinch test indicated low tension, simple interruption by 2/0 Mersilk sutures was performed between the two wound edges in group B. For group B (Fig. 2D), two 2.0-mm Kirschner wires were inserted along both wound sides ~0.5 cm from the wound edges (13,14). Subsequently, intermittent skin stretching was conducted similar to that applied in group B through the traction of Kirschner wires. A further pinch test was applied to estimate the actual tension of the wound. Subsequently, complete closure was achieved by simple interrupted suturing with 2/0 Mersilk sutures. Finally, all wounds in groups A-C were bandaged with sterile dressings and marked carefully on each pig (Fig. 2E).
Postoperative procedures
Following surgery, wound dressings were changed every 2 days, and wound care and healing recording were conducted at the same time. On postoperative day 10, all wound sutures were removed.
Microcirculation measurements
Cutaneous microcirculation was assessed based on TcpO2. Under general anesthesia, measurements of the lateral surface of the mid-forearm were performed to detect the baseline value for normal skin. Before suturing the wound in groups A-C, TcpO2 was measured using the same protocol as during wound closure. Variations of TcpO2 were recorded and compared.
Clinical scoring of wound healing
Healing was observed following primary wound closure in groups A, B and C; and the clinical score was recorded every two days until suture removal on POD 10. A modified clinical scale was used as follows: 1, no visible reaction; 2, minimal swelling or erythema; 3, suture line inflammatory reaction at least 1-cm thick with pain or redness; 4, seroma or abscess formation; and 5, dehiscence, skin necrosis or impossible primary closure (15). Clinical scores were assessed by a clinician who was blinded to the groupings of the present study.
Microscopic and ultramicroscopic structure evaluation
Samples for histological evaluation were taken at suture removal (POD 10). Specimens were obtained using a No. 10 blade while the pigs were under general anesthesia, as previously described. The area of resection included the healing area and the adjacent skin at a distance of 0.5 cm from the wound margin or suture line. Specimens were cut into two halves and pinned flat to a piece of wood. One half was fixed in 10% neutral-buffered formalin (Beijing Solarbio Science & Technology Co., Ltd.). Following routine processing, which included dehydration in an alcohol gradient series, clearing in xylene and paraffin embedding, the samples were cut into 4-µm sections, mounted on glass slides and stained with Verhoeff Van-Gieson (VVG) for elastin hematoxylin and eosin (HE) for microvessels, and Masson and Sirius Red (all Leagene Biotech Co., Ltd., Beijing, China) for collagen bundles. All histological results were observed and analyzed using Image Pro Plus 6.0 (Media Cybernetics, Inc., Silver Spring, MD, USA). The remaining half of the specimens was fixed in 2.5% glutaraldehyde (Beijing Solarbio Science & Technology Co., Ltd.) and treated by desiccation and spraying with gold. Scanning electron microscopy (SEM; SUPRA 55VP; Carl Zeiss AG, Oberkochen, Germany) was used to study the collagen microstructure.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Data were statistically analyzed based on the analysis of variance for repeated measures. Data were expressed as the mean ± standard deviation (as indicated), and the normality of the data distribution was assessed using the Shapiro-Wilk test. Cross-group differences were analyzed using the paired t-test. P≤0.05 was considered to indicate a statistically significantly difference.
Results
Microcirculation measurements
In different groups, cutaneous microcirculation was assessed twice (Fig. 3). Before creating the wound, basic TcpO2 was measured as 47.76±0.58, 55.33±1.53 and 57.67±1.53 mmHg, respectively, in groups A-C. When wounds were closed by different methods in groups A-C, TcpO2 decreased to 5.00±3.00, 38.33±12.74 and 34.33±10.02 mmHg, respectively. Finally, the differences in TcpO2 between the groups were calculated and compared using SPSS software. No statistically significant differences in TcpO2 were observed between groups A and C or between groups B and C. Significant differences were observed only between groups A and B (P=0.036), and the difference between preoperative and postoperative TcpO2 (skin microcirculation) was smaller for the EASApprox® skin-stretching device.
Figure 3.
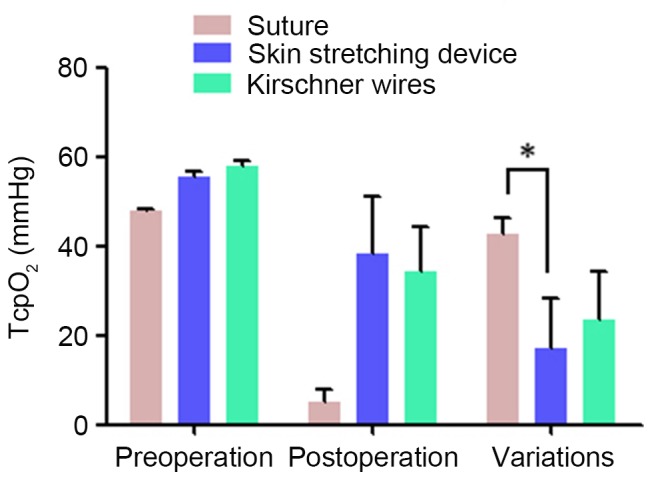
Comparison of TcpO2 measurement values among the different groups. The variation of TcpO2 in the EASApprox® device group was significantly lower than that in the direct group. No other significant differences were detected. Data are expressed as the mean + standard deviation from at least three experiments. *P<0.05. TcpO2, transcutaneous partial pressure of oxygen.
Clinical scoring of wound healing
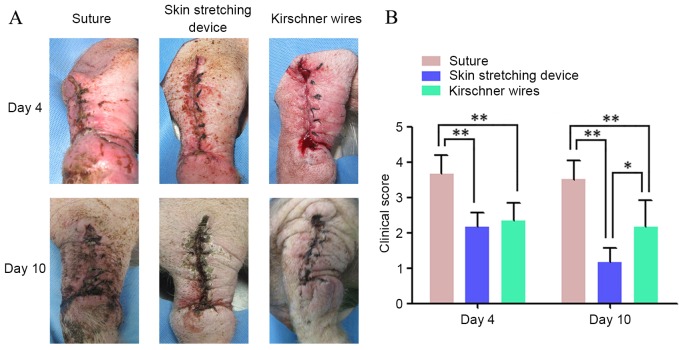
Clinical scores for wounds during the healing period (from wound closure to suture removal on POD 10) were recorded for all groups. Table I and Fig. 4 show the condition of wound healing on POD4 and 10. Wound healing in groups B and C was significantly better than that in group A on POD4 and POD10 (P<0.01). Although there were no significant differences between groups B and C in the clinical scores on POD4, at the final observation period, the outward appearances of wounds in group B significantly better than those in group C (P<0.05).
Table I.
Clinical scores of different wounds.
| Suture | Skin stretching device | Kirschner wires | ||||
|---|---|---|---|---|---|---|
| Serial number | Day 4 | Day 10 | Day 4 | Day 10 | Day 4 | Day 10 |
| 1 | 4 | 4 | 2 | 1 | 3 | 3 |
| 2 | 3 | 3 | 2 | 1 | 2 | 1 |
| 3 | 3 | 4 | 2 | 1 | 2 | 2 |
| 4 | 4 | 4 | 2 | 1 | 2 | 2 |
| 5 | 4 | 3 | 3 | 2 | 3 | 3 |
| 6 | 4 | 3 | 2 | 1 | 2 | 2 |
| Mean ± SD | 3.67±0.52 | 3.50±0.55 | 2.17±0.41 | 1.17±0.41 | 2.33±0.52 | 2.17±0.75 |
SD, standard deviation.
Figure 4.
Clinical scoring of wound healing. (A) The condition of different groups on postoperative days 4 and 10 are shown. (B) Comparison of the clinical score of different groups: Clinical scores of wounds in the EASApprox® skin-stretching device group were lower than the other two groups. Data are expressed as the mean + standard deviation from at least three experiments. *P<0.05 and **P<0.01.
Microscopic structure evaluation
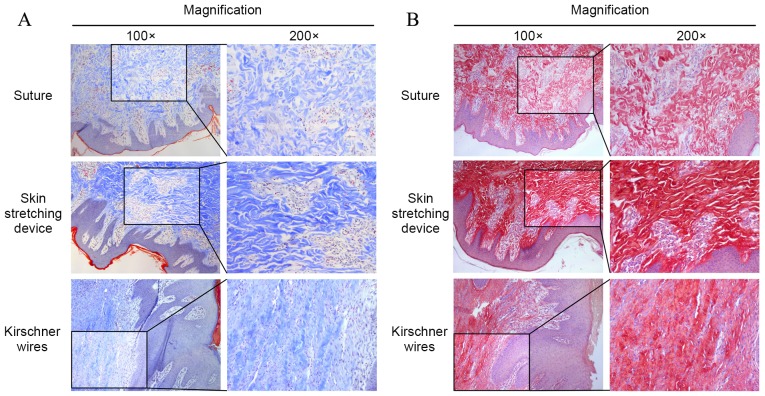
Histological staining of the three groups is shown in Fig. 5. VVG staining for elastin was performed (Fig. 5A). Overall, elastin stained as a deep color that ran perpendicularly to the epidermis. In group A, the overall length of elastin fiber was longer than that of groups B and C. Pathological sections of group C exhibited minimal long, straight elastin fibers; however, elastin fragments were apparent in groups B and C. The mean lengths of elastin in groups A-C are shown in Fig. 5B, with elastin length demonstrated to be the shortest in group B.
Figure 5.
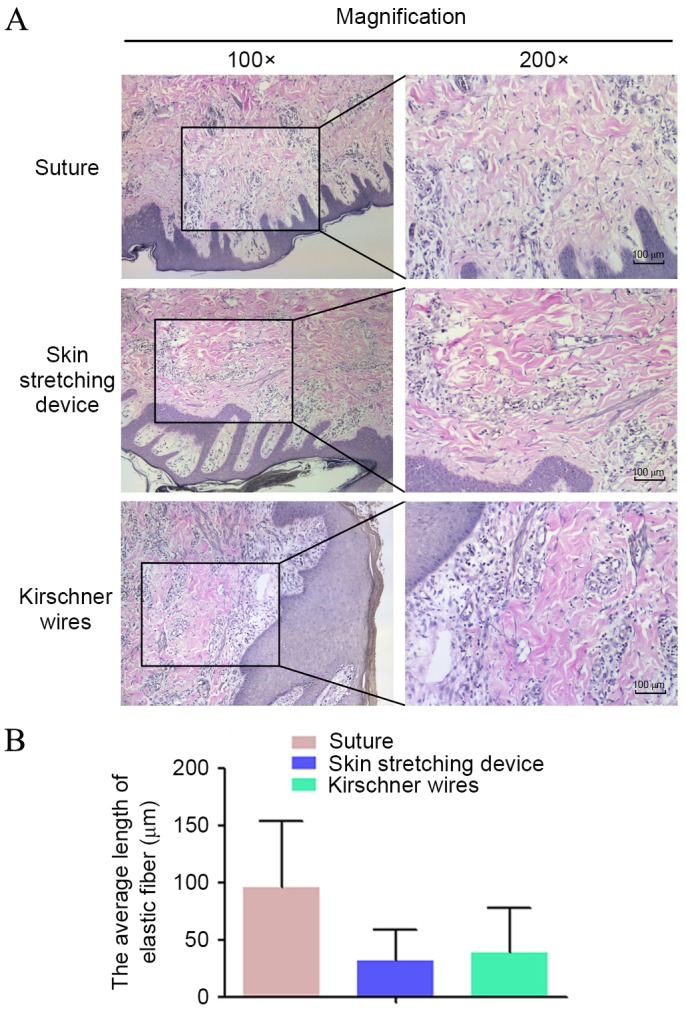
VVG staining. (A) Elastin deeply stained by VVG presented in the mid dermis. Elastin was straight, intact and ordered in the direct suture group, whereas elastin was fragmented and disordered in the EASApprox® skin-stretching device group. (B) The values for the average length of elastin are shown for each group. Data are expressed as the mean + standard deviation from at least three experiments. VVG, Verhoeff Van-Gieson.
Masson and Sirius Red staining were used to assess collagen bundles (Fig. 6A and B, respectively). Under the microscope, collagen bundles appeared blue after Masson staining and red after Sirius Red. In group A, collagen was predominantly oriented perpendicular to the skin surface. Most collagen bundles were disordered and exhibited crimping. Collagen bundles in groups B and C were stretched and oriented parallel to the epidermis, and the collagen bundles in group B were straighter and longer than those in groups A and C.
Figure 6.
Collagen bundle staining. (A) Masson staining showed that collagen bundles stained in blue. In the direct suture group, collagen bundles were disorderly arranged and presented crimp status. In the other groups, collagen bundles were stretched and paralleled to the epidermis. Additionally, collagen bundles were straighter and longer in the EASApprox® skin-stretching device group. (B) Sirius Red staining was used to indicate the shape of collagen bundles, and the results were similar to Masson staining.
Microvessel density was calculated after HE staining. Microscopically, microvessels comprised round vascular walls and intravascular red blood cells. Significant differences were observed among the three groups (P<0.05; Fig. 7). More microvessels were observed in group A than in the other groups. HE staining indicated that the pathological sections in group B had the lowest microvessel density.
Figure 7.
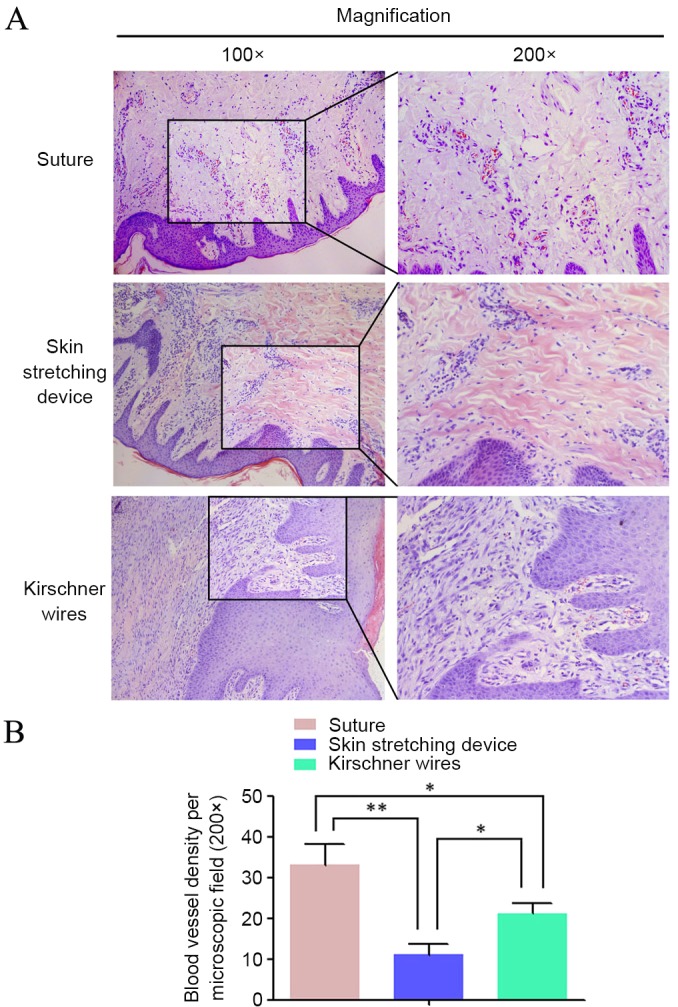
(A) Hematoxylin and eosin staining highlighted the distribution of microvessels, where the microvessel density in the direct suture group was higher than the others. (B) Microvessel density in different groups was compared with each other. Data are expressed as the mean + standard deviation from at least three experiments. *P<0.05 and **P<0.01.
The specimen surfaces of samples from different groups were scanned and observed on the SEM screen at magnifications of ×2,000 and ×5,000. Images of representative areas were captured (Fig. 8). The entire shape of the collagen bundle was easily observed under SEM. In group A, the collagen bundles presented with particularly small fibers and were seriously fractured. However, the collagen bundles in group B were bolder, straighter and more intact than those in the groups A and C. Randomly arranged, very small fibers were observed in specimens from group C.
Figure 8.
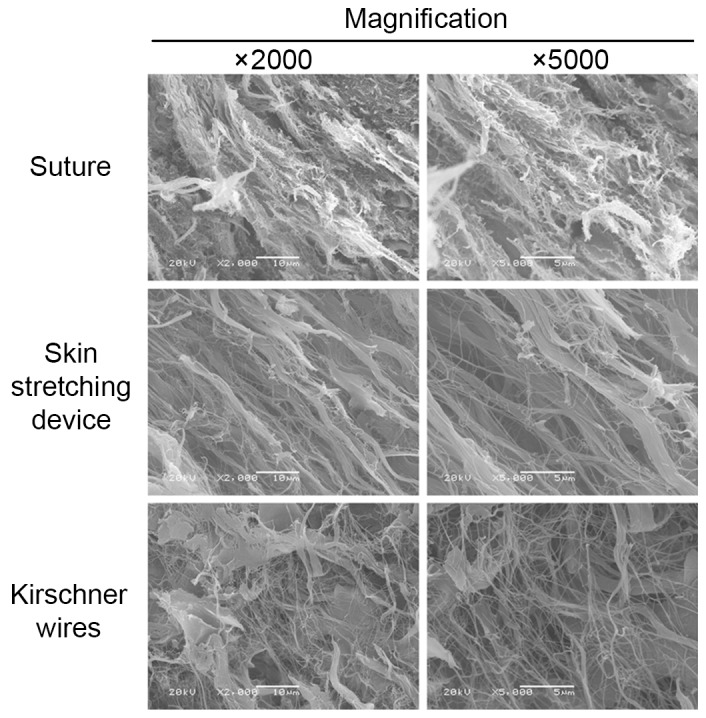
Scanning electron microscopy demonstrated the state of collagen bundles in the three groups: Collagen bundles in the EASApprox® skin-stretching device group were bolder, straighter and more parallel to each other than those in the other two groups.
Discussion
The closure of large skin defects is challenging for surgeons worldwide. Large local excisions or acute trauma can create defects that are usually not amenable to primary closure. In the past, the treatment of large skin defects required the application of a graft or flap. Due to advances in the research of biomechanical characteristics of skin, presuturing techniques and other mechanical means have been presented in the literature; these methods facilitate wound closure by manipulating skin that is in close proximity or adjacent to the margins of a wound or proposed operative area (16–20). Among several impressive wound closure techniques, the first skin-stretching device, which was designed by Hirshowitz et al (7), has been applied in many medical institutions with satisfactory efficiency. This device skillfully exploits the viscoelastic and stress relaxation properties of skin to stretch the tissue and close the defect. However, notable complications can occur after skin stretching, including wound dehiscence, hypertrophic scarring, infection, marginal necrosis and delayed healing; these complications limit the applications of this technique.
In order to facilitate the operating process and avoid complications that result from skin stretching, a new generation of skin-stretching devices has been designed. These devices are simple to insert at the wound edge, and an indicator measures wound tension. Compared with other devices, the new EASApprox® skin-stretching device investigated in the present study is able to mobilize large areas of skin that lie adjacent to and more distant from the operative area through cyclical stretching and relaxation. Consequently, skin can be stretched from more than one region and direction in relation to the surgical area.
Skin consists of the epidermis and dermis. The thickness of the horny layer in Bama miniature pigs is similar to that in humans and does not change with age. Liu et al (21) considered Bama miniature pigs a suitable animal model for studies of human skin; thus, these animals were used in the present study. Before the surgery commenced, the TcpO2 value of the forearms in the different pig groups ranged between 47.76±0.58 and 57.67±1.53 mmHg. Under the same resection of the entire skin, a pig model of a 5-cmx5-cm wound was achieved. The pinch test suggested that the wound tension was too high to achieve primary closure. Furthermore, the tension was ~20 N, representing unsafe conditions for acutely closing the wound.
The efficiency of the new EASApprox® skin stretching device was compared with two presuturing techniques. Direct suture is the method originally used to achieve wound closure, particularly for conditions without any tension. Skin stretching using Kirschner wires, a prototype of the skin-stretching device, is considered the most basic form of primary closure. Wounds in groups B and C were manipulated by cycle stretching. According to a safe threshold, the loaded force ranged between 0.5 and 4 kg (7). During the period of skin stretching, the tension adopted in group B ranged from 1 to 3 kg to ensure skin integrity. Greater forces stretch collagen bundles and small blood vessels, thereby damaging blood perfusion, resulting in subsequent necrosis of the skin margins (4,14). When used in combination with cycles of stretching and relaxation loading, this method contributed to skin extension, as previously described (6,22). Furthermore, when a pinch test suggested that the wound was under low tension following three sets of cycle stretching, primary closure was performed. The TcpO2 value of a second measurement indicated that the EASApprox® skin-stretching device protected skin microcirculation to a greater extent than direct suture. This protective function benefited from the ingenious design, rational structure and use of the cycle stretching method. Additionally, the sharply hooked needles were convenient to pin the wound margins and reduced side injuries caused by the surgery.
At the stage of wound healing (POD4 and POD10), the clinical scores of the wounds that were treated using the EASApprox® skin-stretching device were significantly lower than those achieved using the other methods. In general, the wounds showed light, visible reactions or minimal swelling without any inflammatory reaction. The treatment using the Kirschner wires group was the second most effective method. By contrast, direct suture had a long-term impact on wound healing, which was accompanied by evident inflammation or seroma. Therefore, treatment using the EASApprox® skin-stretching device promoted wound healing and prevented the occurrence of adverse reactions.
Collagen, elastin and ground substance are the most important structural components of the dermis (23). Among them, collagen constitutes the main structural component of the skin, accounting for >50% of its fat-free dry weight (3). Without any stress loading, collagen fibers are arranged haphazardly; however, when the skin is stretched, the fibers are aligned parallel to each other. Mechanically, collagen bundles have high tensile strength, are stiff and lack extensibility. These bundles are the main source of structural support for the skin but are not significant in its recoiling abilities (24,25).
According to observations made on skin that was stained with Masson and Sirius Red stain, the collagen bundles were longer and straighter in the skin-stretching device group than in the other groups. In addition, stretching by both the new EASApprox® device and Kirschner wires oriented the collagen bundles parallel to the skin surface. Observations made using microscopy were consistent with those obtained using SEM. Under SEM, when the skin was stretched using the new EASApprox® device the collagen bundles were bolder, straighter and more parallel to each other than those in the other groups. Furthermore, at the micro- and ultramicro-scales, the new EASApprox® skin-stretching device made full use of the skin viscoelasticity and achieved optimal stretching.
Different from collagen bundles, elastin comprises <5% of the fat-free dry weight of skin and is characterized by long-range elastic extensibility (3). Even after a maximal strain, elastin has the capacity to return to its original shape. Furthermore, it has a close association with collagen and promotes the return of collagen to its wavy posture when it is in a state of rest. Thus, elastin provides the ability of skin to recoil after the application of deforming stresses; however, if sufficient stretching load is applied, elastic fibers can fragment, resulting in a loss of recoil (24,25). Therefore, the shape of elastin could be treated as another stretch level.
Histological staining of sections by VVG demonstrated that elastic fibers were fragmented after a stretching load was applied. In the direct suture group, the mean length of the elastic fibers was greater than those in the other groups. Due to stretching by the new EASApprox® device, elastic fibers were thoroughly fragmented. By analyzing the observations of collagen bundles and elastic fibers, it was once more confirmed that there is a correlation between collagen and elastin; indicating that the EASApprox® skin-stretching device supported greater stretching efficiency.
Microvessel density among the three different methods was demonstrated to be in the order of: Direct suture> EASApprox® skin-stretching >Kirschner wires. Researchers consider high microvessel density beneficial for healing (26,27). The present study proposed that local inflammation following tissue damage can easily lead to vascularization. According to this hypothesis, the high microvessel density observed in the direct suture group indicated serious tissue damage. Conversely, use of the EASApprox® skin-stretching device resulted in the least tissue damage.
In conclusion, the EASApprox® skin-stretching system is a safe and effective method to treat large skin defects and has several potential advantages over other devices currently used for wound closure; thus, this device can eliminate the requirement for more costly flap, grafting or tissue-expansion techniques. We suggest that cycle stretching should be used in concert with the EASApprox® device during surgery. Tension applied to the skin during stretching (ranging between 1.0 and 3.0 kg) was found to be safe. Compared with direct suture and stretching using Kirschner wires, skin stretching by the device resulted in only minor side effects on skin histology and microcirculation. Additionally, the EASApprox® device may promote wound healing. In an era of increasing medical investment, this device is particularly attractive because it only requires a simple operation. However, more clinical and preclinical studies of the EASApprox® skin-stretching system are required.
Acknowledgements
The authors would like to thank Dr Wilhelm Fleischmann [BIOWIM (China), Ltd.] for his theoretical guidance during the execution of the present study, and BIOWIM (China), Ltd., for providing the EASApprox® skin-stretching system.
Glossary
Abbreviations
- TcpO2
transcutaneous partial pressure of oxygen
- POD
postoperative day
- VVG
Verhoeff Van-Gieson
- SEM
scanning electron microscopy
References
- 1.Armstrong DG, Sorensen JC, Bushman TR. Exploiting the viscoelastic properties of pedal skin with the sure closure skin stretching device. J Foot Ankle Surg. 1995;34:247–253. doi: 10.1016/S1067-2516(09)80055-0. [DOI] [PubMed] [Google Scholar]
- 2.Gibson T, Kenedi RM. Biochemical properties of skin. Surg Clin North Am. 1967;47:279–294. doi: 10.1016/S0039-6109(16)38180-4. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SH, Limthongkul B, Humphreys TR. The biomechanical properties of the skin. Dermatol Surg. 2013;39:193–203. doi: 10.1111/dsu.12095. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelmi BJ, Blackwell SJ, Mancoll JS, Phillips LG. Creep vs. stretch: A review of the viscoelastic properties of skin. Ann Plast Surg. 1998;41:215–219. doi: 10.1097/00000637-199808000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Liang MD, Briggs P, Heckler FR, Futrell JW. Presuturing-a new technique for closing large skin defects: Clinical and experimental studies. Plast Reconstr Surg. 1988;81:694–702. doi: 10.1097/00006534-198805000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Bashir AH. Wound closure by skin traction: An application of tissue expansion. Br J Plast Surg. 1987;40:582–587. doi: 10.1016/0007-1226(87)90151-2. [DOI] [PubMed] [Google Scholar]
- 7.Hirshowitz B, Lindenbaum E, Har-Shai Y. A skin-stretching device for the harnessing of the viscoelastic properties of skin. Plast Reconstr Surg. 1993;92:260–270. doi: 10.1097/00006534-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Marrero GM, Dufresne RG., Jr An intraoperative skin-stretching device to close wounds in mohs defects. Dermatol Surg. 1996;22:546–550. doi: 10.1111/j.1524-4725.1996.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 9.Signorini M, Blandini D, Rafanelli G, Colonna M, Candiani P. Experience with the skin stretching device. European J Plastic Surg. 1996;19:63–68. doi: 10.1007/BF00207916. [DOI] [Google Scholar]
- 10.Verhaegen PD, Bloemen MC, van der Wal MB, Vloemans AF, Tempelman FR, Beerthuizen GI, van Zuijlen PP. Skin stretching for primary closure of acute burn wounds. Burns. 2014;40:1727–1737. doi: 10.1016/j.burns.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Samis AJ, Davidson JS. Skin-stretching device for intraoperative primary closure of radial forearm flap donor site. Plast Reconstr Surg. 2000;105:698–702. doi: 10.1097/00006534-200002000-00034. [DOI] [PubMed] [Google Scholar]
- 12.Har-Shai Y, Hirshowitz B. The use of a modified skin stretching device (SSD) together with a lower cheek and neck flap for coverage of a large cheek skin defect. European J Plastic Surg. 1995;18:136–138. [Google Scholar]
- 13.Petro JA, Niazi ZBM. Immediate skin expansion: An old concept by a novel and inexpensive technique. Ann Plast Surg. 1996;36:479–484. doi: 10.1097/00000637-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Abramson DL, Gibstein LA, Pribaz JJ. An inexpensive method of intraoperative skin stretching for closure of large cutaneous wounds. Ann Plast Surg. 1997;38:540–542. doi: 10.1097/00000637-199705000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Freeman LJ, Pettit GD, Robinette JD, Lincoln JD, Person MW. Tissue reaction to suture material in the feline linea alba. A retrospective, prospective, and histologic study. Vet Surg. 1987;16:440–445. doi: 10.1111/j.1532-950X.1987.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 16.Topaz M, Carmel NN, Silberman A, Li MS, Li YZ. The TopClosure® 3S System, for skin stretching and a secure wound closure. Eur J Plast Surg. 2012;35:533–543. doi: 10.1007/s00238-011-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavletic MM. Use of an external skin-stretching device for wound closure in dogs and cats. J Am Vet Med Assoc. 2000;217:350–354. doi: 10.2460/javma.2000.217.350. [DOI] [PubMed] [Google Scholar]
- 18.Nordström RE, Devin JW. Scalp stretching with a tissue expander for closure of scalp defects. Plast Reconstr Surg. 1985;75:578–581. doi: 10.1097/00006534-198504000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Barnea Y, Gur E, Amir A, Leshem D, Zaretski A, Shafir R, Weiss J. Our experience with Wisebands: A new skin and soft-tissue stretch device. Plast Reconstr Surg. 2004;113:862–871. doi: 10.1097/01.PRS.0000105339.41838.D2. [DOI] [PubMed] [Google Scholar]
- 20.Macionis V. Noninvasive wound closure by stretching skin with hook needles enveloped in double-sided adhesive tape and stuck to the skin. Ann Plast Surg. 2001;47:345–346. doi: 10.1097/00000637-200109000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Chen JY, Shang HT, Liu CE, Wang Y, Niu R, Wu J, Wei H. Light microscopic, electron microscopic, and immunohistochemical comparison of Bama minipig (Sus scrofa domestica) and human skin. Comp Med. 2010;60:142–148. [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen BH, Cosmetto AJ. The suture tension adjustment reel. A new device for the management of skin closure. J Dermatol Surg Oncol. 1992;18:112–123. doi: 10.1111/j.1524-4725.1992.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 23.Uitto J. Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J Invest Dermatol. 1979;72:1–10. doi: 10.1111/1523-1747.ep12530093. [DOI] [PubMed] [Google Scholar]
- 24.Chu DH. Chapter 7. Development and Structure of Skin. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th. New York: McGraw-Hill; 2008. pp. 57–72. [Google Scholar]
- 25.Wilkes GL, Brown IA, Wildnauer RH. The biomechanical properties of skin. CRC Crit Rev Bioeng. 1973;1:453–495. [PubMed] [Google Scholar]
- 26.Guimarães GF, De Araújo VC, Nery JC, Peruzzo DC, Soares AB. Microvessel density evaluation of the effect of enamel matrix derivative on soft tissue after implant placement: A preliminary study. Int J Periodontics Restorative Dent. 2014;35:733–738. doi: 10.11607/prd.2044. [DOI] [PubMed] [Google Scholar]
- 27.Kant V, Gopal A, Kumar D, Pathak NN, Ram M, Jangir BL, Tandan SK, Kumar D. Curcumin-induced angiogenesis hastens wound healing in diabetic rats. J Surg Res. 2015;193:978–988. doi: 10.1016/j.jss.2014.10.019. [DOI] [PubMed] [Google Scholar]