Abstract
Health promotion strategies and lifestyle changes are important in disease prevention. Oral health has received a large amount of attention previously as it is a fundamental component of general health and it contributes to the quality of life. Therefore, the study of associations between diet, health and the presence of bioactive compounds in food is receiving a substantial amount of attention. In the present review the effects and targets of a natural polyohenolic stilbenoid compound; resveratrol (3,5,4′-trihydroxystilbene; RSV) is assessed, and the future prospects for RSV in promoting oral health are considered. RSV is a phytoalexin, synthesized by a wide range of plants and abundantly extracted in grape skin, it has been purported to exert a multiplicity of anti-inflammatory, anti-viral, anti-microbial, estrogenic, anticancer, cardioprotective, neuroprotective and immunomodulatory functions. In this review, following an introduction documenting the biochemistry of RSV and RSV glucosides, the bioavailability and pharmacokinetics of RSV are described. Considering its multiple properties, the present review has focused on the potential benefits of RSV as an antioxidant and chemopreventive agent.
Keywords: resveratrol, oral squamous cell carcinoma, chemoprevention, oral disease, oxidative stress
1. Introduction
The importance of preventing lifestyle-related diseases including heart disease, cerebrovascular disease, diabetes and cancer has been increasing. In particular, the Global Oral Health Programme of the World Health Organization (WHO) has prompted worldwide vigilance of oral health as it is a matter of considerable importance for general health and quality of life (1,2). Considerable attention has been given to the effective use of fluoride (3), controlling tobacco use (4), oral health at each age, from children to the elderly (5) and healthy diet and nutrition (6,7).
In order to prevent oral diseases, early detection and diagnosis is vital. As there is a potential link between poor oral health and chronic disease, it is important to integrate the prevention of oral diseases with general health promotion to enable the prevention of chronic diseases and improve oral health. Oral diseases affect a large proportion of individuals, for example oral cancer is the 8th most commonly diagnosed cancer in the world and its incidence ranges from one to 10 cases per 100,000 people globally (8). Therefore, a major priority is to establish innovative health promotion strategies by developing novel beneficial agents capable of improving the prevention of oral diseases, for example by integration of oral health into public health programmes or by supportive school policies. The physical environment and skills-based health education are essential in maintaining oral health and reducing risk factors (2).
The study of associations between diet, health and the presence of bioactive compounds in foods has previously received attention. A large number of natural substances, including curcumin (9), quercetin, genistein (10) and epigallocatechin-3-gallate (11) have been identified, which may provide safe, non-toxic alternative pharmacological treatments to prevent the onset of oral diseases. Nutraceutical substances may be used either as individual compounds in their natural form (isolated from matrixes of plant origin) or obtained from chemical synthesis and used alone, or in combination with alternative therapeutic agents, such as phytochemical compounds (10,12).
Traditional Chinese medicine has purported a wide range of remedies made from herbs rich in polyphenols, including Radix Curcumae formula to treat cardiovascular disease (13) and Yi Shen Juan Bi Tablets to treat rheumatoid arthritis (14), which are responsible not only for providing therapy for specific diseases but also for improving general health (15). The scientific evidence accumulated over the past 20 years has suggested that resveratrol (RSV), a natural polyphenolic compound, exhibits a number of pharmacological activities (16,17) with beneficial health effects. Evidence has indicated that RSV is involved in the modulation of numerous cell-signaling pathways (18,19), thus exerting a variety of antioxidant (20), anti-inflammatory (21), anti-viral, anti-microbial, estrogenic (22), anticancer (23,24), cardioprotective (25), neuroprotective (26) and immunomodulatory (27) functions. The potential beneficial effects of RSV have been linked with a wide variety of chronic diseases, including cancer and the analogues of RSV (28–30) are being investigated specifically as chemopreventive agents in humans (31). RSV has also been linked with cardiovascular diseases (32), skin disorders (33), diabetes (34), arthritis (35), neurological diseases (36) and the aging process (37).
The current review focused on the anticancer effects of RSV assessed in vivo and in vitro in addition to clinical trials, concentrating on the beneficial effects exerted on oral cancer, specifically the squamous cell carcinoma type that makes up ~90% of oral cancer diagnosed.
2. Biochemistry of RSV and RSV glucosides
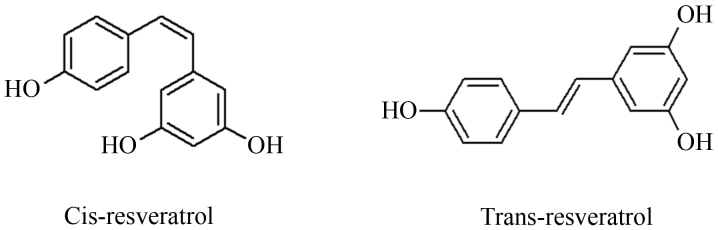
RSV is a polyphenolic compound that functionally acts as a phytoalexin and is identified in nature as both cis and trans isomers (Fig. 1). RSV is synthesized by plants as a defense mechanism in response to bacterial or fungal infection (17) and stress factors (such as UV irradiation or ozone exposure (38,39). RSV is synthesized through stilbene synthase by three different condensation reactions between three molecules of malonyl-coenzime A and one molecule of coumaroyl-coenzyme A (40). RSV is naturally occurring in >70 different plant species, such as Polygonum cuspidatum (41), eucalyptus (42) and Picea excelsa (43), and fruit species, including mulberries, raspberries, pines, peanuts, blueberries and grapes (44). Although these plants and their extracts have been used for various therapeutic purposes by traditional Chinese and Japanese medicine, RSV itself was first isolated in 1940 (45) from the roots of the medicinal herb hellebore (Veratrum grandiflorum) (46) and it was identified as a component of wine in 1992 (47,48) However, this compound exists in numerous foods and beverages consumed daily, including cocoa (49), grapes (50) and red wine (51). Red wine, the most notable dietary source of RSV (52), has received special attention and in 1992 an epidemiological study demonstrated a potential correlation between incidence of cardiovascular disease and the consumption of red wine, observed in Mediterranean populations (48).
Figure 1.
Resveratrol isomers.
RSV is composed of two phenol rings, linked by a styrene double bond to generate 3,4′,5-trihydroxystilbene. Different conjugate forms of this compound have been detected in plants and trans-resveratrol exists in glycosylated form; trans-isomer is sterically a more stable form and for this reason is considered to be the most abundant form (53). Isomerization is facilitated by UV, light and pH, therefore trans-resveratrol is rapidly converted into cis-resveratrol by visible light, high temperatures or low pH (54).
RSV has a high number of analogues and derivatives, differing in terms of type, number and position of substituents (hydroxyl, methoxyl, halogenated, glycosylated, esterified), the presence of stilbenic double bonds, modified steroisomery and oxidative dimerizations (to form oligomers) (16). Additionally, a number of studies indicate that certain resveratrol analogues and derivatives have pharmacological properties, such as apoptotic and antioxidant activities (55) and chemopreventive effects (56).
A number of RSV derivatives have been identified in the roots of Poligonum cuspidatum including piceid 2 (3-O-b-d-glucosylresveratrol), resveratroloside 3 (4′-O-b-d-glucosylresveratrol), piceatannol 4 (3,5,3′,4′-tetra-O-hydroxyestilbene) and its glucosylated derivative 5 (4′-O-b-dglucosyl-piceatannol) (57). Polyphenols are hydrophobic scaffolds exhibiting poor absorption and solubility, resulting in the very short half-life of RSV in the circulatory system (58). Presence of the glycosylated form of RSV is considered to improve physicochemical properties, such as solubility and partition coefficient in order to facilitate the entry of polyphenols into enterocytes (59) and improve bioavailability (60).
In grapes and wine, RSV has been identified as a free-species and in glycosylated form. In particular, piceid (resveratrol-3-O-beta-D-glucoside, also called polydatin) is a glucoside of RSV in which the hydroxyl group in the C-3 position is substituted by a glycoside group, a hydroxyl group substitute (61). The substitution of the glycosidic group leads to conformational changes occurring in the polydatin that are reflected in changes to the biological properties, greater bioavailability and a higher stability. The piceid maintains the antioxidant properties and the hydroxyl group at C-3, which in this compound is replaced by glycosidic group, is less reactive in regards to the activity of scavenging, undertaken by the hydroxyl group at C-4 that remains unchanged in polydatin (62). The piceid retains the biological activity of resveratrol but has a number of advantages over RSV in drug research: Polydatins are more resistant to enzymatic oxidation than RSV, penetrate the cell via an active carrier mechanism using glucose carrier and due to its solubility in water, it is absorbed from the intestine with greater efficiency (63,64).
3. Bioavailability and pharmacokinetics
The beneficial properties of RSV have been extensively investigated in the literature with studies in vitro (65) and in vivo (66); however, there is limited understanding regarding the pharmacokinetics of RSV. A number of studies in animals and humans (46) have demonstrated that unconjugated RSV has a poor in vivo bioavailability, due to its extremely rapid metabolism to glucuronide and sulphate derivatives in the liver and intestine (67). The plasma concentration of RSV and its metabolites depend on the dose administered. Marier et al (68) have shown that trans-resveratrol in in its aglycone and glucuronide forms exhibited increases in plasma concentrations 4–8 h after oral administration, with terminal elimination half-life of 1.48 and 1.58 h, respectively. Trans-resveratrol in its aglycone form has 38% bioavailability and its exposure was approximately 46-fold lower than that of the glucuronide form (The area under the curve extrapolated to infinity is 7.1 vs. 324.7 µmol·h/l). Due to its poor water solubility (69) RSV requires binding with serum proteins (70); however, it is able to passively diffuse through the plasma membrane (71). Although there is considerable inter-individual variability, five distinct metabolites may be detected in the urine following the moderate consumption of red wine: Resveratrol monosulfate, two isomeric forms of resveratrol monoglucuronide, dihydroresveratrol monosulfate and dihydroresveratrol (72). Further studies of the activity of its metabolites are required to understand the in vivo concentrations of different metabolites from ingested RSV that may be much higher than the concentration of RSV itself.
In vitro glycosylation of natural products is successfully employed to improve physicochemical properties such as solubility and partition coefficient (43,47). Glucosylated polyphenols, are initially deglycosylated at the intestinal wall prior to diffusing into the cells. Following absorption, polyphenol aglycones are conjugated with glucuronic acid or sulphate in the intestinal enterocytes and the liver (72). The presence of a mixture of glucosylated compounds may be of note in terms of the absorption of RSV at the intestinal wall when they are orally administered.
Biasutto et al (58) observed that glucosyl groups, added to RSV via a succinate linker, enhanced the bioavailability of RSV. This suggested that the administration of a combination of the aglycone (RSV) and its glycosylated derivative may produce a long-lasting increase in circulating levels of the polyphenols and its metabolites. RSV interacts with a high affinity with albumin and this binding may have a crucial role in improving the distribution of RSV in different body tissues and organs (70). The adsorption and distribution of RSV in organs such as the heart, liver and kidney following oral administration were described for the first time in 1996, by sub-ministration of a daily dose of 6.5 mg/l resveratrol to two different groups of rats (73), and have more recently been confirmed (in the liver, IC50 for the inhibition of resveratrol sulphation was 12±2 pM quercetin, 1.0±0.04 µM fisetin, 1.4±0.1 µM myricetin, 2.2±0.1 µM kaempferol and 2.8±0.2 µM apigenin) (74,75) and extended to the bile, stomach and duodenum (76). To the best of our knowledge, bioavailability of RSV and RSV derivatives is yet to be elucidated.
At present, attention is being paid to novel techniques and proposals to promote the bioavailability of these molecules, such as microparticles (77), nanoparticles (78), microsphere (79), microencapsulation (80) and nanoencapsulation (81). Evidence indicates that the reduction of particle size allows an increase in the contact surface, for example, trans-resveratrol is the most commonly used isomeric form due to its numerous health benefits, even though it has poor bioavailability due to low aqueous solubility and slow dissolution rate. However, these parameters increase following treatments to reduce the particle size (82).
With regards to toxicity it has been demonstrated that, unless extremely high doses are administered, there are no signs of toxicity following treatment with RSV. Animal models exhibited no adverse effects following 28 days of RSV administration at 1,000-fold the levels of RSV present in red wine (83).
4. Resveratrol as a potent antioxidant
Previous results have indicated that resveratrol is a free radical scavenger and potent antioxidant, counteracting the oxidative stress that is considered to be associated with the etiology and progression of multiple chronic and acute diseases (84). In clinical studies, oxidative stress has been associated with a number of degenerative diseases including atherosclerosis, cancer, asthma, hyperoxia, arthritis, dermatitis (85,86) and inflammatory conditions (87).
Reactive Oxygen Species (ROS), such as superoxide (O2−) and hydrogen peroxide (H2O2), are by-products of normal aerobic metabolism, which at low levels are fundamental in cell signaling processes (88), including cell proliferation, apoptosis or necrosis, induction or suppression of gene expression. Exogenous factors may cause ROS generation and an imbalance in their production may have negative effects on biomolecules, thus altering normal cell function (89). It has been indicated that ROS are able to induce proliferation, senescence, necrosis, apoptosis or cell death (89). At higher concentrations, ROS induces apoptosis, mediating the post-translational modifications of p53 and altering mitochondrial membrane permeability and apoptotic DNA fragmentation (90). RSV appears to provide protection against DNA damage caused by these ROS (84). Similar to the numerous plant polyphenols, RSV may also exhibit pro-oxidant properties, catalyzing cellular DNA degradation in the presence of transition metal ions (91) and mobilizing endogenous copper, such as chromatin bound copper (92).
The antioxidant system includes a number of antioxidant enzymes such as superoxide dismutase and catalase, non-enzymatic antioxidants such as reduced glutathione (GSH), protein-sulfhydryls and uric acid. It has been demonstrated that RSV significantly activates and prevents the oxidation of these endogenous antioxidant systems. RSV has been demonstrated to reduce the production of H2O2, and normalize the level of oxidized glutathione reductase and myeloperoxidase activities (93).
In a 2004 study by Cao and Li (94) investigating the protective role of RSV in various oxidative cardiovascular disorders, it was determined that RSV increased the level of endogenous antioxidants and phase 2 enzymes in cardiomyocytes. These modifications contribute to the increased resistance of oxidative and electrophilic cardiac cell injury (94). Yen et al (95) indicated that RSV, in combination with 4-hexylresorcinol, may exert considerable protection against oxidative DNA damage in human lymphocytes induced by H2O2 through modulation of antioxidant enzymes (glutathione peroxidase, glutathione reductase, glutathione-S-transferase) and increased level of GSH, accepted as the most important intracellular hydrophilic antioxidant (96). Another study by Ates et al (97) focused on the potential neuroprotective role of RSV and confirmed that an increase in glutathione levels is due to the free-radical scavenging properties of RSV. In a time dependent study of the capacity of RSV to prevent the oxidation of GSH in red blood cells, it was demonstrated that incubating human erythrocytes with resveratrol (10 µM) caused a significant activation of the plasma membrane redox system (41%) and ascorbate free radical reductase (30%) compared with the control (basal level). Furthermore, it was determined that the accumulation of resveratrol inside the erythrocyte is ~89% and does not significantly change after 30 min (86).
Lipophilic properties of RSV may improve its antioxidant activity (98,99). In order to increase the evidence demonstrating the involvement of free radicals in numerous disorders and diseases including cancer (100), cardio-vascular diseases and neurological disorders, ageing (16,101,102) and lipid peroxidation, RSV has received increased attention. RSV appears to prevent oxidation of low-density lipoprotein (LDL) by chelating copper and scavenging ROS. Furthermore, a RSV-rich diet has been demonstrated to produce a measurable increase in plasma antioxidant level and decreased lipid peroxidation (103). It has also been reported that RSV reduces intracellular ROS and prevents LDL oxidation in endothelial cells (104).
In 2008, Dani et al (105) indicated that RSV has the ability to prevent lipid peroxidation and intracellular oxidation in Saccharomyces cerevisiae. Under conditions of oxidative stress in skeletal muscle, it has been determined that RSV is able to alter protein catabolism and muscle function (106).
It is important to note that the protective effects of RSV against lipids and peroxidation occur over a very short time frame (16,107–109). RSV is rapidly absorbed and its peak plasma concentration is achieved within 15–60 min of its administration (73,110).
5. Resveratrol as a chemopreventive
RSV is comprised of a number of polypheinolic compounds, including curcumin (111), rottlerin (112), genistein (113) and quercetin (114,115). RSV has been purported to be of potential use in cancer treatment and evidence indicates that it may inhibit pathways contributing to cell proliferation (116). Yu et al (117) demonstrated the inhibitory effect exhibited by RSV on the proliferation of oral squamous cell carcinoma cells through the induction of apoptosis and G2/M phase cell cycle arrest.
Oral submucous fibrosis (OSF) is a precancerous condition that affects the oral mucosa, which currently cannot be treated by specific therapeutic drugs. Moderate-to-severe OSF is irreversible and current treatment strategies include injections or topical application of steroids, oral subministration of lycopene (16 mg daily) and pentoxyfilline (400 mg 3 times daily) (16,118,119). A previous study demonstrated that RSV epigenetically inhibits Zinc finger E-Box binding homeobox 1 expression to suppress the myofibroblast activity of fibrotic buccal mucosal fibroblasts, and may serve as a dietary supplement for OSF patients (120).
Mohan et al (121) developed a drug combination of RSV and doxorubicin loaded in liposomal nanoparticles (78). Their evaluation in vitro was conducted on a squamous cell carcinoma cell line, NT8e. Their data indicated that the drug-loaded nanoparticle exerted apoptosis inducing effects by controlling the cell cycle and downstream apoptosis by inducing proteins such as caspase-3 and poly (ADP-ribose) polymerase 1.
A study by ElAttar and Virji (122) aimed to evaluate the chemopreventive properties of RSV on oral squamous cell carcinoma cell (SCC-5). The study calculated the average incorporation of 3H-thymidine into nuclear DNA using a hemocytometer and demonstrated that RSV alone, or a combination of RSV and quercetin was able to effectively inhibit growth and proliferation.
Another study demonstrated that RSV inhibited matrix metalloproteinase-9 expression and metastasis in oral cancer cells (SCC-9) by downregulating the signaling pathways of c-Jun N-terminal kinase 1/2 and extra-cellular signal regulated kinase 1/2 signals, thus, exerting beneficial effects in chemoprevention (123).
6. Conclusions
Resveratrol is a promising nutraceutical for the treatment of cancer; however, the molecular mechanisms that explain the chemopreventive role of RSV remain unknown. Currently, there are numerous in vitro studies regarding the benefits of RSV as an anticancer agent (12,117,124,125). It is necessary to complete and confirm these effects using in vivo studies and clinical trials.
Finally, in order to resolve the primary problem associated with the poor bioavailability of RSV and its complicated pharmacokinetic profile, the development of specific nanotechnology and controlled and targeted-drug delivery systems are required.
References
- 1.Petersen PE. Oral cancer prevention and control-the approach of the World Health Organization. Oral Oncol. 2009;45:454–460. doi: 10.1016/j.oraloncology.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Petersen PE. World Health Organization global policy for improvement of oral health-World Health Assembly 2007. Int Dent J. 2008;58:115–121. doi: 10.1111/j.1875-595X.2008.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 3.Marthaler TM, Petersen PE. Salt fluoridation-an alternative in automatic prevention of dental caries. Int Dent J. 2005;55:351–358. doi: 10.1111/j.1875-595X.2005.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 4.Petersen PE. Tobacco and oral health-the role of the World Health Organization. Oral Health Prev Dent. 2003;1:309–315. [PubMed] [Google Scholar]
- 5.Petersen PE, Bourgeois D, Bratthall D, Ogawa H. Oral health information systems-towards measuring progress in oral health promotion and disease prevention. Bull World Health Organ. 2005;83:686–693. [PMC free article] [PubMed] [Google Scholar]
- 6.Moynihan P, Petersen PE. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004;7:201–226. doi: 10.1079/PHN2003589. [DOI] [PubMed] [Google Scholar]
- 7.Moynihan PJ. The role of diet and nutrition in the etiology and prevention of oral diseases. Bull World Health Organ. 2005;83:694–699. [PMC free article] [PubMed] [Google Scholar]
- 8.Oral Health Worldwide, corp-author. A report by FDI World Dental Federation. FDI World Dental Federation; Geneva: 2014. [Google Scholar]
- 9.Nagpal M, Sood S. Role of curcumin in systemic and oral health: An overview. J Nat Sci Biol Med. 2013;4:3–7. doi: 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elattar TM, Virji AS. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res. 2000;20:1733–1738. [PubMed] [Google Scholar]
- 11.Ramshankar V, Krishnamurthy A. Chemoprevention of oral cancer: Green tea experience. J Nat Sci Biol Med. 2014;5:3–7. doi: 10.4103/0976-9668.127272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan J, Yue W, E J, Malhotra J, Lu SE, Gu J, Xu F, Tan XL. In vitro comparative studies of resveratrol and triacetylresveratrol on cell proliferation, apoptosis, and STAT3 and NFκB signaling in pancreatic cancer cells. Sci Rep. 2016;6:31672. doi: 10.1038/srep31672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao W, Xu X, Wang X, Li B, Wang Y, Li Y, Yang L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J Ethnopharmacol. 2013;145:1–10. doi: 10.1016/j.jep.2012.09.051. [DOI] [PubMed] [Google Scholar]
- 14.Jiang M, Lu C, Chen G, Xiao C, Zha Q, Niu X, Chen S, Lu A. Understanding the molecular mechanism of interventions in treating rheumatoid arthritis patients with corresponding traditional Chinese medicine patterns based on bioinformatics approach. Evid Based Complement Alternat Med. 2012;2012:129452. doi: 10.1155/2012/129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S, Zhang B. Traditional Chinese medicine network pharmacology: Theory, methodology and application. Chin J Nat Med. 2013;11:110–120. doi: 10.3724/SP.J.1009.2013.00110. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 17.Pervaiz S, Holme AL. Resveratrol: Its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 18.Harikumar KB, Aggarwal BB. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 19.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerem Z, Chetrit D, Shoseyov O, Regev-Shoshani G. Protection of lipids from oxidation by epicatechin, trans-resveratrol, and gallic and caffeic acids in intestinal model systems. J Agric Food Chem. 2006;54:10288–10293. doi: 10.1021/jf0621828. [DOI] [PubMed] [Google Scholar]
- 21.Murias M, Handler N, Erker T, Pleban K, Ecker G, Saiko P, Szekeres T, Jäger W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: Synthesis and structure-activity relationship. Bioorg Med Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor; Proc Natl Acad Sci USA; 1997; pp. 14138–14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraft TE, Parisotto D, Schempp C, Efferth T. Fighting cancer with red wine? Molecular mechanisms of resveratrol. Crit Rev Food Sci Nutr. 2009;49:782–799. doi: 10.1080/10408390802248627. [DOI] [PubMed] [Google Scholar]
- 24.Benová B, Adam M, Onderková K, Královsky J, Krajicek M. Analysis of selected stilbenes in Polygonum cuspidatum by HPLC coupled with CoulArray detection. J Sep Sci. 2008;31:2404–2409. doi: 10.1002/jssc.200800119. [DOI] [PubMed] [Google Scholar]
- 25.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Jung JC, Lim E, Lee Y, Kang JM, Kim H, Jang S, Oh S, Jung M. Synthesis of novel trans-stilbene derivatives and evaluation of their potent antioxidant and neuroprotective effects. Eur J Med Chem. 2009;44:3166–3174. doi: 10.1016/j.ejmech.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 27.King RE, Kent KD, Bomser JA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact. 2005;151:143–149. doi: 10.1016/j.cbi.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Szekeres T, Fritzer-Szekeres M, Saiko P, Jäger W. Resveratrol and resveratrol analogues-structure-activity relationship. Pharm Res. 2010;27:1042–1048. doi: 10.1007/s11095-010-0090-1. [DOI] [PubMed] [Google Scholar]
- 30.Szekeres T, Saiko P, Fritzer-Szekeres M, Djavan B, Jäger W. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann N Y Acad Sci. 2011;1215:89–95. doi: 10.1111/j.1749-6632.2010.05864.x. [DOI] [PubMed] [Google Scholar]
- 31.Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat Res. 2012;750:60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- 33.Ndiaye M, Philippe C, Mukhtar H, Ahmad N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch Biochem Biophys. 2011;508:164–170. doi: 10.1016/j.abb.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635:1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 35.Wahba MG, Messiha BA, Abo-Saif AA. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm Biol. 2016;54:1705–1715. doi: 10.3109/13880209.2015.1125931. [DOI] [PubMed] [Google Scholar]
- 36.Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852:1195–1201. doi: 10.1016/j.bbadis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Markus MA, Morris BJ. Resveratrol in prevention and treatment of common clinical conditions of aging. Clin Interv Aging. 2008;3:331–339. [PMC free article] [PubMed] [Google Scholar]
- 38.Frémont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Tang K, Yang HR, Wen PF, Zhang P, Wang HL, Huang WD. Distribution of resveratrol and stilbene synthase in young grape plants (Vitis vinifera L. cv. Cabernet Sauvignon) and the effect of UV-C on its accumulation. Plant Physiol Biochem. 2010;48:142–152. doi: 10.1016/j.plaphy.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: A molecule whose time has come? And gone? Clin Biochem. 1997;30:91–113. doi: 10.1016/S0009-9120(96)00155-5. [DOI] [PubMed] [Google Scholar]
- 41.Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 2000;48:253–256. doi: 10.1021/jf9909196. [DOI] [PubMed] [Google Scholar]
- 42.Hathway DE, Seakins JW. Hydroxystilbenes of Eucalyptus wandoo. Biochem J. 1959;72:369–374. doi: 10.1042/bj0720369b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rolfs CH, Kindl H. Stilbene synthase and chalcone synthase: Two different constitutive enzymes in cultured cells of Picea excelsa. Plant Physiol. 1984;75:489–492. doi: 10.1104/pp.75.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King RE, Bomser JA, Min DB. Bioactivity of resveratrol. Comprehensive Reviews in Food Science and Food Safety. 2006;5:65–70. doi: 10.1111/j.1541-4337.2006.00001.x. [DOI] [Google Scholar]
- 45.Takaoka MJ. The phenolic substances of white hellebore (Veratrum grandiflorum Loes. fil.) J Faculty Sci. 1940;3:1–16. [Google Scholar]
- 46.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 47.Langcake P, Pryce RJ. A new class of phytoalexins from grapevines. Experientia. 1977;33:151–152. doi: 10.1007/BF02124034. [DOI] [PubMed] [Google Scholar]
- 48.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- 49.Counet C, Callemien D, Collin S. Chocolate and cocoa: New sources of trans-resveratrol and trans-piceid. Food Chemistry. 2006;98:649–657. doi: 10.1016/j.foodchem.2005.06.030. [DOI] [Google Scholar]
- 50.Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem. 2002;50:431–435. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- 51.Siemann EH, Creasy LL. Concentration of the Phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49–52. [Google Scholar]
- 52.Guerrero RF, Garcia-Parrilla MC, Puertas B, Cantos-Villar E. Wine, resveratrol and health: A review. Nat Prod Commun. 2009;4:635–658. [PubMed] [Google Scholar]
- 53.Stervbo U, Vang O, Bonnesen C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chemistry. 2007;101:449–457. doi: 10.1016/j.foodchem.2006.01.047. [DOI] [Google Scholar]
- 54.Figueiras TS, Neves-Petersen MT, Petersen SB. Activation energy of light induced isomerization of resveratrol. J Fluoresc. 2011;21:1897–1906. doi: 10.1007/s10895-011-0886-3. [DOI] [PubMed] [Google Scholar]
- 55.Cai YJ, Wei QY, Fang JG, Yang L, Liu ZL, Wyche JH, Han Z. The 3,4-dihydroxyl groups are important for trans-resveratrol analogs to exhibit enhanced antioxidant and apoptotic activities. Anticancer Res. 2004;24:999–1002. [PubMed] [Google Scholar]
- 56.Wieder T, Prokop A, Bagci B, Essmann F, Bernicke D, Schulze-Osthoff K, Dörken B, Schmalz HG, Daniel PT, Henze G. Piceatannol, a hydroxylated analog of the chemopreventive agent resveratrol, is a potent inducer of apoptosis in the lymphoma cell line BJAB and in primary, leukemic lymphoblasts. Leukemia. 2001;15:1735–1742. doi: 10.1038/sj.leu.2402284. [DOI] [PubMed] [Google Scholar]
- 57.Yang T, Wang L, Zhu M, Zhang L, Yan L. Properties and molecular mechanisms of resveratrol: A review. Pharmazie. 2015;70:501–506. [PubMed] [Google Scholar]
- 58.Biasutto L, Marotta E, Bradaschia A, Fallica M, Mattarei A, Garbisa S, Zoratti M, Paradisi C. Soluble polyphenols: Synthesis and bioavailability of 3,4′,5-tri(alpha-D-glucose-3-O-succinyl) resveratrol. Bioorg Med Chem Lett. 2009;19:6721–6724. doi: 10.1016/j.bmcl.2009.09.114. [DOI] [PubMed] [Google Scholar]
- 59.Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J Control Release. 2006;113:189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 60.Lin YL, Chang HC, Chen TL, Chang JH, Chiu WT, Lin JW, Chen RM. Resveratrol protects against oxidized LDL-induced breakage of the blood-brain barrier by lessening disruption of tight junctions and apoptotic insults to mouse cerebrovascular endothelial cells. J Nutr. 2010;140:2187–2192. doi: 10.3945/jn.110.123505. [DOI] [PubMed] [Google Scholar]
- 61.Du QH, Peng C, Zhang H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm Biol. 2013;51:1347–1354. doi: 10.3109/13880209.2013.792849. [DOI] [PubMed] [Google Scholar]
- 62.Plou FJ, Segura AGd, Ballesteros A. Application of Glycosidases and Transglycosidases in the synthesis of oligosaccharides. In: Polaina J, MacCabe AP, editors. Industrial enzymes: Structure, function and applications. Springer; Netherlands, Dordrecht: 2007. pp. 141–157. [DOI] [Google Scholar]
- 63.Bertrand A, Morel S, Lefoulon F, Rolland Y, Monsan P, Remaud-Simeon M. Leuconostoc mesenteroides glucansucrase synthesis of flavonoid glucosides by acceptor reactions in aqueous-organic solvents. Carbohydr Res. 2006;341:855–863. doi: 10.1016/j.carres.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Cichewicz RH, Kouzi SA. Biotransformation of resveratrol to piceid by Bacillus cereus. J Nat Prod. 1998;61:1313–1314. doi: 10.1021/np980139b. [DOI] [PubMed] [Google Scholar]
- 65.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–434. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 66.Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms (review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 67.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 68.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: Extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 69.Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol. 1997;53:1347–1355. doi: 10.1016/S0006-2952(96)00820-9. [DOI] [PubMed] [Google Scholar]
- 70.Jannin B, Menzel M, Berlot JP, Delmas D, Lancon A, Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: Plasmatic protein binding and cell uptake. Biochem Pharmacol. 2004;68:1113–1118. doi: 10.1016/j.bcp.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 71.Lançon A, Delmas D, Osman H, Thénot JP, Jannin B, Latruffe N. Human hepatic cell uptake of resveratrol: Involvement of both passive diffusion and carrier-mediated process. Biochem Biophys Res Commun. 2004;316:1132–1137. doi: 10.1016/j.bbrc.2004.02.164. [DOI] [PubMed] [Google Scholar]
- 72.Lucas R, Alcantara D, Morales JC. A concise synthesis of glucuronide metabolites of urolithin-B, resveratrol, and hydroxytyrosol. Carbohydr Res. 2009;344:1340–1346. doi: 10.1016/j.carres.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 73.Bertelli AA, Giovannini L, Stradi R, Bertelli A, Tillement JP. Plasma, urine and tissue levels of trans- and cis-resveratrol (3,4′,5-trihydroxystilbene) after short-term or prolonged administration of red wine to rats. Int J Tissue React. 1996;18:67–71. [PubMed] [Google Scholar]
- 74.El Mohsen MA, Marks J, Kuhnle G, Moore K, Debnam E, Srai S Kaila, Rice-Evans C, Spencer JP. Absorption, tissue distribution and excretion of pelargonidin and its metabolites following oral administration to rats. Br J Nutr. 2006;95:51–58. doi: 10.1079/BJN20051596. [DOI] [PubMed] [Google Scholar]
- 75.Vitrac X, Desmoulière A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Mérillon JM. Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci. 2003;72:2219–2233. doi: 10.1016/S0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 76.de Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Xenobiotica. 2000;30:609–617. doi: 10.1080/004982500406435. [DOI] [PubMed] [Google Scholar]
- 77.Koga CC, Andrade JE, Ferruzzi MG, Lee Y. Stability of trans-resveratrol encapsulated in a protein matrix produced using spray drying to UV light stress and simulated gastro-intestinal digestion. J Food Sci. 2016;81:C292–C300. doi: 10.1111/1750-3841.13176. [DOI] [PubMed] [Google Scholar]
- 78.Teskac K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2010;390:61–69. doi: 10.1016/j.ijpharm.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Nam JB, Ryu JH, Kim JW, Chang IS, Suh KD. Stabilization of resveratrol immobilized in monodisperse cyano-functionalized porous polymeric microspheres. Polymer. 2005;46:8956–8963. doi: 10.1016/j.polymer.2005.07.016. [DOI] [Google Scholar]
- 80.Shi G, Rao L, Yu H, Xiang H, Yang H, Ji R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int J Pharm. 2008;349:83–93. doi: 10.1016/j.ijpharm.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 81.Kesisoglou F, Panmai S, Wu Y. Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev. 2007;59:631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 82.Rasenack N, Müller BW. Crystal habit and tableting behavior. Int J Pharm. 2002;244:45–57. doi: 10.1016/S0378-5173(02)00296-X. [DOI] [PubMed] [Google Scholar]
- 83.Juan ME, Vinardell MP, Planas JM. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J Nutr. 2002;132:257–260. doi: 10.1093/jn/132.2.257. [DOI] [PubMed] [Google Scholar]
- 84.Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 85.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-B. [DOI] [PubMed] [Google Scholar]
- 86.Rizvi SI, Pandey KB. Activation of the erythrocyte plasma membrane redox system by resveratrol: A possible mechanism for antioxidant properties. Pharmacol Rep. 2010;62:726–732. doi: 10.1016/S1734-1140(10)70330-3. [DOI] [PubMed] [Google Scholar]
- 87.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 88.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 90.Kim CH, Jeon HM, Lee SY, Jeong EK, Ju MK, Park BJ, Park HG, Lim SC, Han SI, Kang HS. Role of reactive oxygen species-dependent protein aggregation in metabolic stress-induced necrosis. Int J Oncol. 2010;37:97–102. doi: 10.3892/ijo_00000657. [DOI] [PubMed] [Google Scholar]
- 91.la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem Soc Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 92.Azmi AS, Bhat SH, Hanif S, Hadi SM. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Lett. 2006;580:533–538. doi: 10.1016/j.febslet.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 93.Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res. 1999;25:65–77. [PubMed] [Google Scholar]
- 94.Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: Protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004;489:39–48. doi: 10.1016/j.ejphar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 95.Yen GC, Duh PD, Lin CW. Effects of resveratrol and 4-hexylresorcinol on hydrogen peroxide-induced oxidative DNA damage in human lymphocytes. Free Radic Res. 2003;37:509–514. doi: 10.1080/1071576031000083099. [DOI] [PubMed] [Google Scholar]
- 96.Melov S. Therapeutics against mitochondrial oxidative stress in animal models of aging. Ann N Y Acad Sci. 2002;959:330–340. doi: 10.1111/j.1749-6632.2002.tb02104.x. [DOI] [PubMed] [Google Scholar]
- 97.Ates O, Cayli SR, Yucel N, Altinoz E, Kocak A, Durak MA, Turkoz Y, Yologlu S. Central nervous system protection by resveratrol in streptozotocin-induced diabetic rats. J Clin Neurosci. 2007;14:256–260. doi: 10.1016/j.jocn.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 98.Belguendouz L, Frémont L, Gozzelino MT. Interaction of transresveratrol with plasma lipoproteins. Biochem Pharmacol. 1998;55:811–816. doi: 10.1016/S0006-2952(97)00544-3. [DOI] [PubMed] [Google Scholar]
- 99.Rotondo S, Rajtar G, Manarini S, Celardo A, Rotillo D, De Gaetano G, Evangelista V, Cerletti C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br J Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.West JD, Marnett LJ. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem Res Toxicol. 2006;19:173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 101.Lee HS, Kim HN, Jung MH, Choi JY. Electrically evoked potentials recorded in a patient with auditory neuropathy. Cochlear Implants Int. 2004;5(Suppl 1):S231. doi: 10.1179/cim.2004.5.Supplement-1.231. [DOI] [PubMed] [Google Scholar]
- 102.Rizvi SI, Maurya PK. Alterations in antioxidant enzymes during aging in humans. Mol Biotechnol. 2007;37:58–61. doi: 10.1007/s12033-007-0048-7. [DOI] [PubMed] [Google Scholar]
- 103.Wenzel U, Nickel A, Daniel H. Increased mitochondrial palmitoylcarnitine/carnitine countertransport by flavone causes oxidative stress and apoptosis in colon cancer cells. Cell Mol Life Sci. 2005;62:3100–3105. doi: 10.1007/s00018-005-5378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guo R, Su Y, Liu B, Li S, Zhou S, Xu Y. Resveratrol suppresses oxidised low-density lipoprotein-induced macrophage apoptosis through inhibition of intracellular reactive oxygen species generation, LOX-1, and the p38 MAPK pathway. Cell Physiol Biochem. 2014;34:603–616. doi: 10.1159/000363026. [DOI] [PubMed] [Google Scholar]
- 105.Dani C, Bonatto D, Salvador M, Pereira MD, Henriques JA, Eleutherio E. Antioxidant protection of resveratrol and catechin in Saccharomyces cerevisiae. J Agric Food Chem. 2008;56:4268–4272. doi: 10.1021/jf800752s. [DOI] [PubMed] [Google Scholar]
- 106.Naylor AJ Dirks. Cellular effects of resveratrol in skeletal muscle. Life Sci. 2009;84:637–640. doi: 10.1016/j.lfs.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 107.Pandey KB, Rizvi SI. Protective effect of resveratrol on formation of membrane protein carbonyls and lipid peroxidation in erythrocytes subjected to oxidative stress. Appl Physiol Nutr Metab. 2009;34:1093–1097. doi: 10.1139/H09-115. [DOI] [PubMed] [Google Scholar]
- 108.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizvi SI, Jha R, Pandey KB. Activation of erythrocyte plasma membrane redox system provides a useful method to evaluate antioxidant potential of plant polyphenols. Methods Mol Biol. 2010;594:341–348. doi: 10.1007/978-1-60761-411-1_24. [DOI] [PubMed] [Google Scholar]
- 110.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/S0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 111.Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, De Lillo A, Laino L, Lo Muzio L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med. 2015;10:1615–1623. doi: 10.3892/etm.2015.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maioli E, Torricelli C, Valacchi G. Rottlerin and cancer: Novel evidence and mechanisms. Scientific World Journal. 2012;2012:350826. doi: 10.1100/2012/350826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Russo M, Russo GL, Daglia M, Kasi PD, Ravi S, Nabavi SF, Nabavi SM. Understanding genistein in cancer: The ‘good’ and the ‘bad’ effects: A review. Food Chem. 2016;196:589–600. doi: 10.1016/j.foodchem.2015.09.085. [DOI] [PubMed] [Google Scholar]
- 114.Brito AF, Ribeiro M, Abrantes AM, Pires AS, Teixo RJ, Tralhão JG, Botelho MF. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem. 2015;22:3025–3039. doi: 10.2174/0929867322666150812145435. [DOI] [PubMed] [Google Scholar]
- 115.Zhao X, Zhang J. Mechanisms for quercetin in prevention of lung cancer cell growth and metastasis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2015;40:592–597. doi: 10.11817/j.issn.1672-7347.2015.06.004. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 116.Feitelson MA, Arzumanyan A, Kulathinal RJ, Blain SW, Holcombe RF, Mahajna J, Marino M, Martinez-Chantar ML, Nawroth R, Sanchez-Garcia I, et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin Cancer Biol. 2015;35(Suppl):S25–S54. doi: 10.1016/j.semcancer.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu XD, Yang JL, Zhang WL, Liu DX. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumour Biol. 2016;37:2871–2877. doi: 10.1007/s13277-015-3793-4. [DOI] [PubMed] [Google Scholar]
- 118.Ray JG, Ranganathan K, Chattopadhyay A. Malignant transformation of oral submucous fibrosis: Overview of histopathological aspects. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:200–209. doi: 10.1016/j.oooo.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 119.Sharma M, Radhakrishnan R. Limited mouth opening in oral submucous fibrosis: Reasons, ramifications, and remedies. J Oral Pathol Med. 2016 Oct 15; doi: 10.1111/jop.12513. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 120.Chang YC, Lin CW, Yu CC, Wang BY, Huang YH, Hsieh YC, Kuo YL, Chang WW. Resveratrol suppresses myofibroblast activity of human buccal mucosal fibroblasts through the epigenetic inhibition of ZEB1 expression. Oncotarget. 2016;7:12137–12149. doi: 10.18632/oncotarget.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mohan A, Narayanan S, Balasubramanian G, Sethuraman S, Krishnan UM. Dual drug loaded nanoliposomal chemotherapy: A promising strategy for treatment of head and neck squamous cell carcinoma. Eur J Pharm Biopharm. 2016;99:73–83. doi: 10.1016/j.ejpb.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 122.ElAttar TM, Virji AS. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anticancer Drugs. 1999;10:187–193. doi: 10.1097/00001813-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 123.Lin FY, Hsieh YH, Yang SF, Chen CT, Tang CH, Chou MY, Chuang YT, Lin CW, Chen MK. Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells. J Oral Pathol Med. 2015;44:699–706. doi: 10.1111/jop.12288. [DOI] [PubMed] [Google Scholar]
- 124.Masuelli L, Di Stefano E, Fantini M, Mattera R, Benvenuto M, Marzocchella L, Sacchetti P, Focaccetti C, Bernardini R, Tresoldi I, et al. Resveratrol potentiates the in vitro and in vivo anti-tumoral effects of curcumin in head and neck carcinomas. Oncotarget. 2014;5:10745–10762. doi: 10.18632/oncotarget.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shan Z, Yang G, Xiang W, Pei-Jun W, Bin Z. Effects of resveratrol on oral squamous cell carcinoma (OSCC) cells in vitro. J Cancer Res Clin Oncol. 2014;140:371–374. doi: 10.1007/s00432-013-1575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]