Abstract
The present study investigated the effect of rutin on high glucose-induced actin, α2, smooth muscle, aorta (ACTA2) and p38 protein expression in diabetic nephropathy (DN). Human mesangial cells were divided into a control group, high glucose-induced mesangial cell group, high glucose + captopril group, and high glucose + rutin group (low, middle and high doses of rutin). Cell viability, adenosine 5′-triphosphate (ATP) content, cell cycle, and ACTA2 and p38 protein expression were examined using MTT assay, ATP assay kit, flow cytometry and immunofluorescence staining in cultured human mesangial cells, respectively. Cell viability, ATP content, and ACTA2 and p38 expression increased significantly in high glucose-induced mesangial cells (P<0.05). However, at concentrations of 0.2, 0.4 and 0.8 µmol/l rutin was able to inhibit high glucose-induced human mesangial cell viability, ATP content, and ACTA2 and p38 expression and improve the cell cycle progression of mesangial cells. In conclusion, ACTA2 and p38 proteins may have important roles in DN. Rutin may inhibit the expression of ACTA2 and p38 and may be utilized in the prevention and treatment of DN.
Keywords: diabetic nephropathy, human mesangial cells, actin, α2, smooth muscle, aorta, p38, rutin
Introduction
Diabetic nephropathy (DN) is one of the most serious microvascular complications of diabetes and a relatively common disease, which is the leading cause of end-stage renal disease (1–4). According to reports, the prevalence rate of DN is 33–40% in type 1 diabetes (5). The prevalence rate of DN is 20–25% in type 2 diabetes (6). Increasing research has focused on the genetics of lesion formation in the coronary artery of patients with type 2 diabetes (7). Actin, α2, smooth muscle, aorta (ACTA2) is located on chromosome 10q(23.3) and is the most abundant α actin content of protein in vascular smooth muscle cells (8). ACTA2 accounts for 40% of the total protein in vascular smooth muscle cells, and 70% of total actin protein (9). Thus, ACTA2 is an important protein required for the contraction of vascular smooth muscle cells. p38 is a key component of mitogen-activated protein kinase-mediated signaling pathways, and is involved in a variety of physiological processes, including inflammation, proliferation and apoptosis (10). Rutin is a drug that has been used clinically for many years for the regulation of cardiovascular disease and for diabetes (11,12). Therefore, the present study aimed to investigate whether rutin is able to inhibit the expression of p38 and ACTA2 and improve the severity of diabetic nephropathy. The protective effects of rutin on diabetic kidney disease were investigated by examining mesangial cell viability, energy metabolism, cell cycle and protein expression with the aim of providing valuable data for the clinical prevention and treatment of DN.
Materials and methods
Materials
ACTA2 (BM1693) and p38 (MK2101) antibodies were purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan, China). G secondary antibodies (AP130P) were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). An MCO-5AC CO2 thermostat incubator was obtained from Sanyo Electric Biomedical Co., Ltd. (Tokyo, Japan).
Cell culture
Glomerular mesangial cells were provided by Jilin University School of Pharmaceutical Sciences (Changchun, China). Glomerular mesangial cells were divided into a control group (PBS washing), high glucose-induced group (incubation with DMEM high glucose to mimic an environment that would induce DN; Invitrogen; Thermo Fisher, Scientific, Inc., Waltham, MA, USA), high glucose + captopril group (0.4 µmol/l, 24 h), and high glucose + low, middle and high rutin groups (0.2, 0.4 and 0.8 µmol/l rutin, respectively). Cells were cultured in DMEM high glucose at 37°C in a humidified atmosphere containing 5% CO2 for 24 h. Cells in the high glucose + rutin groups were treated with rutin for 24 h. Subsequently, mesangial cells were prepared for assays of cell viability, adenosine 5′-triphosphate (ATP) content, cell cycle, and ACTA2 and p38 protein expression levels.
Cell viability assay
A cell viability assay was carried out with an MTT staining kit (Sigma-Aldrich; Merck KGaA), according to the manufacturer's protocol. Results were analyzed using a spectrophotometer (UV2550; Shimadzu Corporation, Kyoto, Japan) at 450 nm.
ATP content
Intracellular ATP was quantified using an ATP Assay Kit (S0026; Biyun Tian Biotechnology Research Institute). Cell suspension (100 µl) was transferred to the wells of a 96-well microplate and ATP Assay reagent (100 µl) was added. The plate was shaken using a plate shaker for 10 min and luminescence was measured using a spectrophotometer (UV2550, 450 nm).
Cell cycle analysis
Glomerular mesangial cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) in 50-cm2 plates (1×106 cells/well) for 24 h at 37°C then treated with 0.2, 0.4 and 0.8 µmol/l (low, medium and high) rutin. Following incubation for 24 h, the cells were digested with 0.25% trypsin. DNA profiles were evaluated using a flow cytometry station (MACSQuant Analyzer, Miltenyi Biotec, Inc., Cambridge, MA, USA). Histograms or density plots were generated using the MACSQuant digital software.
Immunofluorescence and DAPI staining
Cells were fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) for 15 min at room temperature, then washed three times with PBS for 10 min. Cells were incubated with ACTA2 (BM1693) and p38 (MK2101) antibodies (1:200) at 4°C overnight, washed three times for 5 min and subsequently incubated with anti-mouse immunoglobulin G secondary antibodies (1:500; AP130P; Sigma-Aldrich; Merck KGaA) at 4°C for 2 h. DAPI was then used for nuclear staining. Cells were mounted and images were taken using a Nikon eclipse 80i fluorescence microscope (Nikon Corporation, Tokyo, Japan). The protein expression levels of ACTA2 and p38 and apoptotic cell count (DAPI) were analyzed using Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockbille, MD, USA).
Statistical analysis
All data were obtained from at least three independent experiments and presented as the mean ± standard error of the mean. Statistical comparisons were made using Student's t-tests. Statistical analysis of the data was performed using SPSS v.11.0 for Windows (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Mesangial cell viability
Mesangial cells were treated with different doses of rutin. Results demonstrated that cell viability was significantly increased in the high glucose-induced group at 24 h compared with the control group (P<0.01; Fig. 1). However, cell viability was significantly reduced after treatment with rutin (low, middle and high doses) and captopril (P<0.05) compared with high glucose-induced cells. These results indicated that rutin was able to inhibit the viability of mesangial cells.
Figure 1.
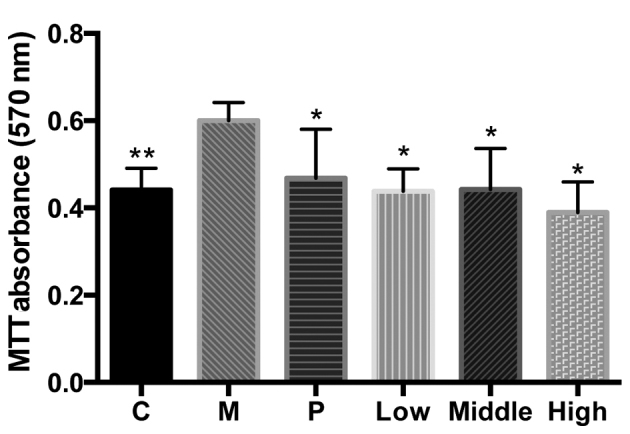
Mesangial cell viability. Data are presented as the mean ± standard error of the mean. **P<0.01 and *P<0.05 vs. M. C, control group; M, high glucose-induced group; P, captopril group; Low, group treated with 0.2 µmol/l rutin; Middle, group treated with 0.4 µmol/l rutin; High, group treated with and 0.8 µmol/l rutin.
ATP content analysis
The present results demonstrated that ATP content was significantly increased in all high glucose-induced mesangial cells compared with the control cells (P<0.05; Fig. 2).
Figure 2.
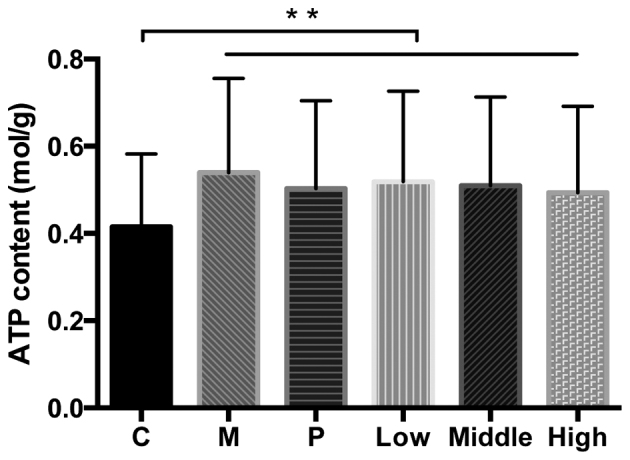
ATP content of mesangial cells. Data are presented as the mean ± standard error of the mean. **P<0.01. ATP, adenosine 5′-triphosphate; C, control group; M, high glucose-induced group; P, captopril group; Low, group treated with 0.2 µmol/l rutin; Middle, group treated with 0.4 µmol/l rutin; High, group treated with and 0.8 µmol/l rutin.
Cell cycle analysis
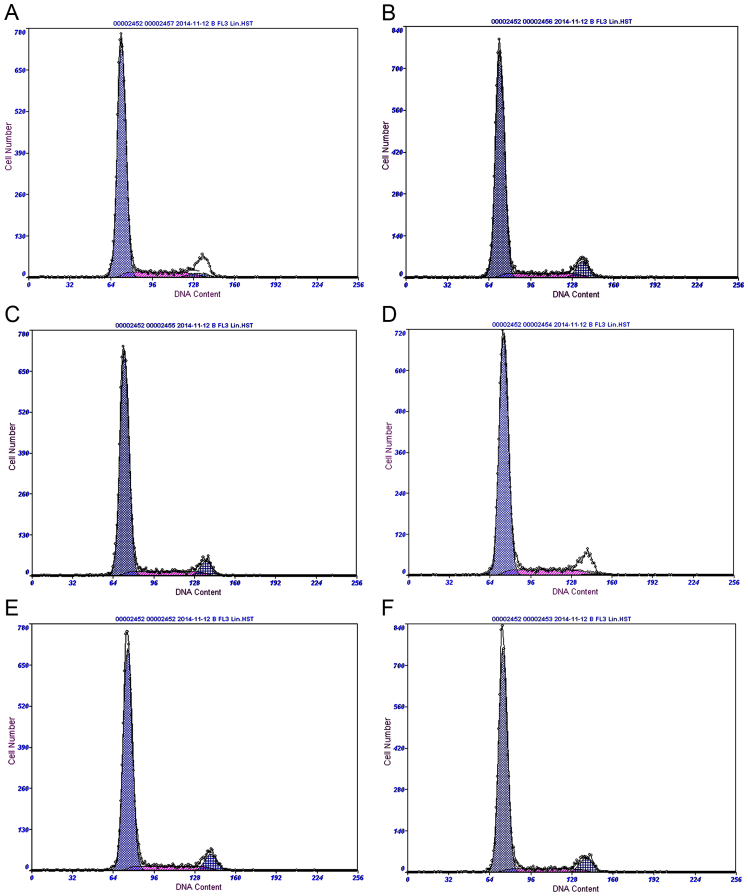
Flow cytometry was performed following treatment of mesangial cells with 0.2, 0.4 and 0.8 µmol/l rutin for 24 h. Results demonstrated that the cell cycle of mesangial cells changed markedly in the high glucose-induced mesangial cells group. However, the cell cycle progression of mesangial cells improved markedly in cells treated with rutin (Fig. 3; Table I). These results suggested that rutin may affect the regulation of the mesothelial cell cycle.
Figure 3.
Cell cycle analysis in each group of mesangial cells. Cell cycle analysis of the (A) control, (B) high glucose-induced, (C) captopril group and (D) 0.2 (low), (E) 0.4 (middle) and (F) 0.8 µmol/l (high) rutin treatment groups.
Table I.
Cell cycle distribution in each group of mesangial cells.
| Group | G1, % | G2, % | S, % |
|---|---|---|---|
| Control | 83.60 | 3.13 | 13.30 |
| High glucose-induced | 80.40 | 8.21 | 11.40 |
| Captopril | 81.70 | 6.84 | 11.50 |
| Rutin (0.2 µmol/l) | 85.00 | 1.08 | 13.90 |
| Rutin (0.4 µmol/l) | 82.70 | 7.94 | 9.29 |
| Rutin (0.8 µmol/l) | 80.80 | 7.79 | 11.50 |
Immunofluorescence staining
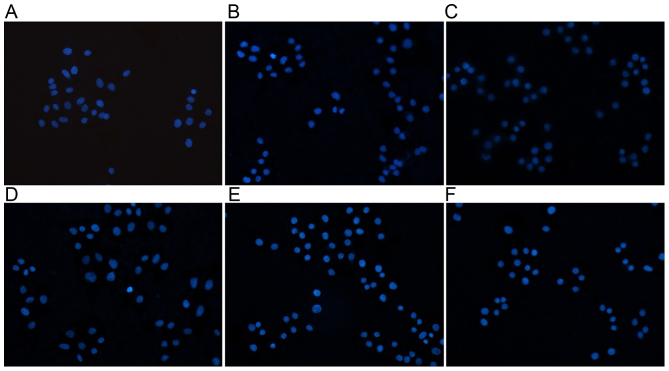
As demonstrated in Fig. 4 and Table II, the experimental results indicated apoptotic morphological changes of mesangial cells after 24 h rutin treatment was observed by fluorescence microscopy. Relative to the control group, cell groups treated with rutin exhibited higher numbers of detached cells with round and shrunken morphologies. DAPI nuclear staining also identified signs of mesangial cell apoptosis in the rutin treatment groups.
Figure 4.
Immunofluorescence staining with DAPI. Immunofluorescence images from the (A) control, (B) high glucose-induced, (C) captopril group and (D) 0.2, (E) 0.4 and (F) 0.8 µmol/l rutin treatment groups. Magnification, ×200.
Table II.
ACTA2 and p38 protein expression in each mesangial cell group (%).
| Group | Nucleus | ACTA2 | p38 |
|---|---|---|---|
| Control | 10.27±1.19 | 18.047±0.81 | 12.543±0.24 |
| High glucose-induced | 25.22±1.75a | 28.41±1.92a | 26.85±0.11a |
| Captopril | 12.26±0.97b | 22.57±1.36b | 17.05±0.60b |
| Rutin (0.2 µmol/l) | 17.92±1.79b | 25.61±1.66b | 22.63±0.72b |
| Rutin (0.4 µmol/l) | 17.84±2.35b | 35.60±2.53 | 19.26±0.65b |
| Rutin (0.8 µmol/l) | 14.63±1.09b | 20.77±1.40b | 19.93±0.73b |
Data are presented as the mean ± standard error of the mean.
P<0.05 vs. control group
P<0.05 vs. high glucose-induced group. ACTA2, actin, α2, smooth muscle, aorta.
ACTA2 protein expression
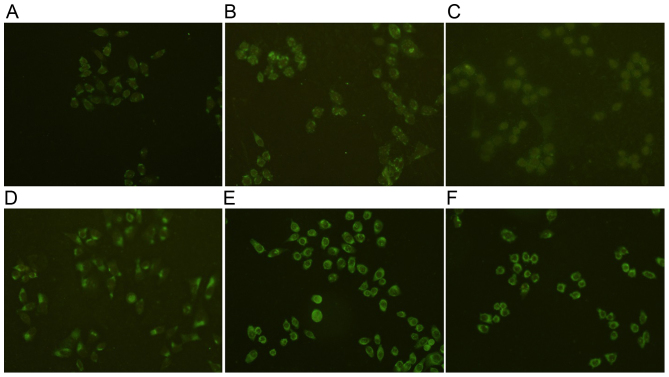
To validate the expression of ACTA2 in mesangial cells, ACTA2 specific fluorescent staining was performed on mesangial cells. The results demonstrated that cells in the control group had uniform chromatin, with a large nucleus and that they maintained integrity of the nuclear envelope. Expression of ACTA2 protein increased significantly in the high glucose group compared with the control (P<0.05; Fig. 5; Table II). The expression of ACTA2 protein decreased significantly in the rutin groups at 24 h compared with the high glucose group (P<0.05; Fig. 5; Table II), with the exception of the middle dose of rutin group.
Figure 5.
ACTA2 protein expression in the different mesangial cell groups. ACTA2 protein expression in the (A) control, (B) high glucose-induced, (C) captopril group and (D) 0.2, (E) 0.4 and (F) 0.8 µmol/l rutin treatment groups. ACTA2, actin, α2, smooth muscle, aorta. Magnification, ×200.
p38 protein expression
To validate the expression of p38 in mesangial cells, p38 specific fluorescent staining was performed on mesangial cells. The results demonstrated that cells in the control group had uniform chromatin, a large nucleus and maintained integrity of the nuclear envelope. The expression of p38 protein increased significantly in high glucose group compared with the control (P<0.05; Fig. 6; Table II). The expression of p38 protein decreased significantly in the rutin groups at 24 h compared with the high glucose group (P<0.05; Fig. 6; Table II).
Figure 6.
p38 protein expression in the different mesangial cell groups. p38 protein expression in the (A) control, (B) high glucose-induced, (C) captopril group and (D) 0.2, (E) 0.4 and (F) 0.8 µmol/l rutin treatment groups. Magnification, ×200.
Discussion
DN is one of the most serious microvascular complications of diabetes (13). Glucose metabolism and renal hemodynamics have an important role in the pathogenesis of DN (14). In the present experiment, high glucose induced the viability of mesangial cells. Rutin inhibited the viability of high glucose-induced mesangial cells. These results indicated that rutin had hypoglycemic effects. ATP is a versatile nucleotide and has an important role in cell biology as a coenzyme that is the ‘molecular unit of currency’ of intracellular energy transfer (15). The present experiment demonstrated that ATP content increased in high glucose-induced mesangial cells, indicating that high glucose may induce a change in ATP content. The content of ATP decreased after intervention with rutin. Therefore, rutin may have a hypoglycemic effect on cells and regulate energy metabolism. ACTA2 exists in birds and mammals. ACTA2 is predominantly distributed in vascular myofibroblastic cells, myoepithelial cells and smooth muscle cells under normal circumstances (16).
In the present experiment, the expression of ACTA2 increased in high glucose-induced mesangial cells. Expression of ACTA2 reduced after treatment with rutin. These results indicated that rutin may inhibit the expression of ACTA2 protein and protect against DN. p38 protein kinase is able to promote leukocyte aggregation and activation, regulate the activity of transcription factors and the synthesis of cell factors, and it has an important role in the regulation of inflammatory reactions (17). In the present experiment, the expression of p38 increased in high glucose-induced mesangial cells. p38 expression decreased after treatment with rutin. These results indicated that rutin may inhibit the expression of p38 protein, with protective effects against DN.
In conclusion, a diabetic model was successfully induced by high glucose, which promoted glomerular cell viability, increased ATP content, induced changes in the cell cycle, and increased the expression of ACTA2 and p38 proteins. Results suggested that rutin has hypoglycemic effects. Rutin may inhibit the expression of ACTA2 and p38 and may confer protective effects against DN. Rutin may also regulate the expression of p38.
Acknowledgments
The present study was supported by the Science and Technology Department of Jilin Province, China (grant nos. 20140312002ZG and YYZX201259).
References
- 1.Park CW. Diabetic kidney disease: From epidemiology to clinical Perspectives. Diabetes Metab J. 2014;38:252–260. doi: 10.4093/dmj.2014.38.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan E, McEvoy C, Sadlier D, Godson C, Martin F. The genetics of diabetic nephropathy. Genes (Basel) 2013;4:596–619. doi: 10.3390/genes4040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal D, Badhan Y, Gudala K, Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: A systematic review of randomized clinical trials. Diabetes Metab J. 2013;37:375–384. doi: 10.4093/dmj.2013.37.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol. 2015;224:R15–R30. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellastella G, Maiorino MI, Olita L, Volpe E Della, Giugliano D, Esposito K. Premature ejaculation is associated with glycemic control in Type 1 diabetes. J Sex Med. 2015;12:93–99. doi: 10.1111/jsm.12755. [DOI] [PubMed] [Google Scholar]
- 6.Ono K, Limbu YR, Rai SK, Kurokawa M, Yanagida J, Rai G, Gurung N, Sharma M, Rai CK. The prevalence of type 2 diabetes mellitus and impaired fasting glucose in semi-urban population of Nepal. Nepal Med Coll J. 2007;9:154–156. [PubMed] [Google Scholar]
- 7.Stolk RP, van Schooneveld MJ, Cruickshank JK, Hughes AD, Stanton A, Lu J, Patel A, Thom SA, Grobbee DE, Vingerling JR, AdRem Project Team and ADVANCE Management Committee Retinal vascular lesions in patients of Caucasian and Asian origin with type 2 diabetes: Baseline results from the ADVANCE Retinal Measurements (AdRem) study. Diabetes Care. 2008;31:708–713. doi: 10.2337/dc07-1657. [DOI] [PubMed] [Google Scholar]
- 8.Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 9.Whitesell TR, Kennedy RM, Carter AD, Rollins EL, Georgijevic S, Santoro MM, Childs SJ. An α-smooth muscle actin (acta2/αsma) zebrafish transgenic line marking vascular mural cells and visceral smooth muscle cells. PLoS One. 2014;9:e90590. doi: 10.1371/journal.pone.0090590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Kim SC, Yu T, Yi YS, Rhee MH, Sung GH, Yoo BC, Cho JY. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:352371. doi: 10.1155/2014/352371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YB, Ge ZM, Kang WQ, Lian ZX, Yao J, Zhou CY. Rutin alleviates diabetic cardiomyopathy in a rat model of type 2 diabetes. Exp Ther Med. 2015;9:451–455. doi: 10.3892/etm.2014.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panchal SK, Poudyal H, Arumugam TV, Brown L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J Nutr. 2011;141:1062–1069. doi: 10.3945/jn.111.137877. [DOI] [PubMed] [Google Scholar]
- 13.Soetikno V, Arozal W, Louisa M, Setiabudy R. New insight into the molecular drug target of diabetic nephropathy. Int J Endocrinol. 2014;2014:968681. doi: 10.1155/2014/968681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanjuliani AF, Fagundes VG, Francischetti EA. The effect of urapidil on blood pressure, renal hemodynamics, lipid and glucose metabolism. Arq Bras Cardiol. 1995;65:59–63. (In Portuguese) [PubMed] [Google Scholar]
- 15.Barata H, de Meis L. Uncoupled ATP hydrolysis and thermogenic activity of the sarcoplasmic reticulum Ca2+-ATPase: Coupling effects of dimethyl sulfoxide and low temperature. J Biol Chem. 2002;277:16868–16872. doi: 10.1074/jbc.M200648200. [DOI] [PubMed] [Google Scholar]
- 16.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1448–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 17.Lin YL, Liang YC, Lee SS, Chiang BL. Polysaccharide purified from Ganoderma lucidum induced activation and maturation of human monocyte-derived dendritic cells by the NF-kappaB and p38 mitogen-activated protein kinase pathways. J Leukoc Biol. 2005;78:533–543. doi: 10.1189/jlb.0804481. [DOI] [PubMed] [Google Scholar]