Abstract
Background and Objectives:
Subcentimetric (defined as <1 cm at short axis) lymph nodes are considered benign and there is limited literature on the results of fine needle aspiration (FNA) of these nodes.
Methods:
Endoscopic ultrasound (EUS) guided FNA was done on 189 lymph nodes in 166 patients with pyrexia of unknown origin (n = 113) or malignancy (n = 53). Subcentimetric lymph nodes (Group A) were compared to nodes with short axis diameter ≥1 cm (Group B). Data are shown as number, percentage, and median (25–75 interquartile range).
Results:
There was no significant difference between Group A and Group B regarding site of lymph nodes (mediastinal in 73.6 and 72.5%, abdominal in 26.3 vs. 27.4%), number of slides (median 14 vs. 15), needle passes (median 2), and needle used (22 G needle in 85.5% vs. 69.9%). Group A had significantly lesser long axis diameter (1.5 [1.2–2] vs. 2.1 [1.6–2.9] cm) and short axis diameter (0.7 [0.6–0.8) vs. 1.4 [1.1–1.6] cm). A diagnosis (pathologic or reactive) could not be made in 2 (2.6%) and 11 (9.7%) lymph nodes in Group A and Group B, respectively (P = 0.078), due to inadequate material. Respective diagnoses in Group A and Group B were reactive lymphadenopathy (51.3% vs. 18.5%, P = 0.000), granulomatous lymphadenopathy (34.2% vs. 53%, P = 0.011), and malignancy (11.8% vs. 18.5%, P = 0.231). The lymph nodes with granulomatous and malignant change were significantly larger and had higher chances of having sharply demarcated borders as compared to reactive nodes.
Conclusion:
EUS-guided FNA of subcentimetric lymph nodes have comparable results to larger nodes. Almost half of the subcentimetric lymph nodes are pathologic.
Keywords: Endoscopic ultrasound, fine needle aspiration, malignancy, pyrexia of unknown origin, subcentimetric lymph nodes
INTRODUCTION
Small lymph nodes are defined as short axis diameter <1 cm. Often, these lymph nodes are detected by computed tomography (CT), and several postmortem studies in patients with no chest infection or malignancy showed that these presence of nodes is common.[1,2,3] Small-sized mediastinal and abdominal lymph nodes are difficult to sample also. With introduction of endoscopic ultrasound (EUS) guided fine needle aspiration (FNA), even small nodes can be sampled under real-time vision, and EUS-FNA has been proved to be very safe from mediastinum where vascular structures are abundant.[4] There is lack of knowledge about small lymph nodes, particularly in the setting of pyrexia of unknown origin which is quite common in South Asia, and often these patients get empirical anti-tubercular therapy with a risk of hepatotoxicity. In the present study, we compare the results of EUS-guided FNA of subcentimetric mediastinal or abdominal lymph nodes to larger nodes (≥1 cm at short axis) in patients with pyrexia of unknown origin or malignancy.
MATERIALS AND METHODS
The study was conducted at a tertiary care center in North India. Our EUS facility serves as a referral center to nearby hospitals; in addition, we have patients from our hospital. The data were analyzed retrospectively from a prospectively collected database. The study was conducted from August 2014 to February 2015. Our center is a high volume EUS center (>1500 EUS examinations per year), and the main indication of lymph node FNA is pyrexia of unknown origin where patients are referred from internal medicine and pulmonary medicine followed by patients with malignancy. As EUS-FNA is a costly procedure (approximately 200 USD at our center), we do EUS-FNA only for those nodes which are not easily accessible by percutaneous ultrasound and when diagnosis could not be achieved by other simpler means. All the procedures were carried out in conscious sedation (midazolam). EUS-guided FNA was done using GF-UCT140 linear echo-endoscope (EUS scope, Olympus, Tokyo, Japan). Subcentimetric lymph nodes were defined as short axis <1 cm. The primary objective of the study was to compare the FNA outcome of subcentimetric lymph nodes to larger nodes. The following data were recorded: Age, sex, indication for procedure (pyrexia of unknown origin vs. malignancy), site of lymph node, echo-features of lymph node, size at short- and long-axis, type of needle used (19 G/22 G/25 G), number of needle passes, number of slides made, result of FNA, and procedural complications. In the presence of multiple lymph nodes, node with the following features was preferred for FNA: Larger size, sharply demarcated borders, and hypoechoic nature. A lymph node was considered reactive when FNA showed lymphoid cells in different stages of activation in the presence of adequate cellularity and absence of granulomas/necrosis/malignant cells. Blood products were given before EUS-guided FNA procedure if International Normalized Ratio was >1.5 or platelet count was <50,000/cmm.
Endoscopic ultrasound fine needle aspiration procedure
A lymph node was selected based on size and echo-features (larger, round, well-defined borders, hypoechoic lymph nodes were preferred) for FNA, if feasible (avoidance of vascular structures and vital organs). A linear array echo-endoscope was directly inserted into the esophagus; in cases of mediastinal lymphadenopathy, upper abdominal examination was also done to look for abdominal lymph nodes (porta, celiac axis), left adrenal, and spleen. We used EchoTip® (Cook Medical, Limerick, Ireland) and Expect™ Slimline (Boston Scientific, Spencer, USA) needles in a defined period. EUS-FNA needle with stylet was introduced in the working channel; Doppler was used to avoid any vascular structures in the needle path. The stylet was withdrawn slightly before puncture; it was reintroduced fully after puncture of lymph node to displace any material in needle (from gastrointestinal wall). After that stylet was completely withdrawn; 20–30 to and fro movements of needle were done within lymph node with application of fanning whenever possible. The material inside the needle was pushed on slides bit by bit with the help of stylet. One or 2 slides from each pass were immediately fixed in absolute alcohol and rest were air dried. The slides were stained with Papanicolaou, Giemsa stain, and Ziehl–Neelsen stain (wherever required). The type of needle, method of FNA (suction, no suction, or capillary), and number of needle passes (as we do not have on-site cytopathologist facility) were operator-dependent. A different needle was used for different lymph nodes to avoid cross contamination.
Statistical methods
The data are shown as number, percentage, and median (25–75 interquartile range). Two groups (pathological vs. reactive and subcentimetric vs. large lymph nodes) were compared with Mann–Whitney test (as it was nonparametric data) or Chi-square/Fisher's exact test (nominal data) and three groups (reactive, granulomatous, and malignant nodes) were compared with Kruskal–Wallis test. Area under receiver operating characteristic curve (AUROC) was calculated for long- and short-axis and pathological diagnosis (granulomatous etiology or malignant change). A two-tailed P < 0.05 was considered significant. All statistical analyses were performed with SPSS, version 16 (SPSS, SPSS Inc., Chicago, IL, USA).
RESULTS
The study group included 166 patients (112 males), a total of 189 lymph nodes were sampled. Indication for EUS-guided FNA was pyrexia of unknown origin (n = 113) and malignancy (n = 53). The patients were divided into Group A (lymph nodes with short axis diameter <1 cm) and Group B (lymph nodes with short axis diameter ≥1 cm) for the purpose of data analysis. Majority of the patients had pyrexia of unknown origin as indication of EUS-FNA, 52 and 61 in Groups A and B, respectively. Malignancy was the indication of procedure in 20 and 33 patients of Groups A and B, respectively. There was no significant difference between sex distribution and age between groups as shown in Table 1. Indication of procedure was pyrexia of unknown origin in the majority of patients in both groups, and mediastinal lymph nodes were sampled in 73.5% and 72.5% of the patients, respectively, in Group A and Group B. The main lymph node FNA sites were subcarinal (n = 87), porta hepatis (n = 31), aorto-pulmonary window (n = 11), and aortocaval region (n = 14). The patients in Group B had significantly higher long axis, short axis, and long-to-short axis ratio, P value being 0.000 for each of these parameters. Number of passes and number of slides were not significantly different between groups. There was significantly higher proportion of granulomatous (53% vs. 34.2%, P = 0.000) and lesser proportion of reactive lymph nodes (18.5% vs. 51.3%, P = 0.000) in Group B as compared with Group A; proportion of malignant lymph nodes (18.5% vs. 11.8%) could not reach statistical significance as shown in Table 2.
Table 1.
Comparison of subcentimetric (<1 cm at short axis) lymph nodes with larger (≥1 cm at short axis) lymph nodes
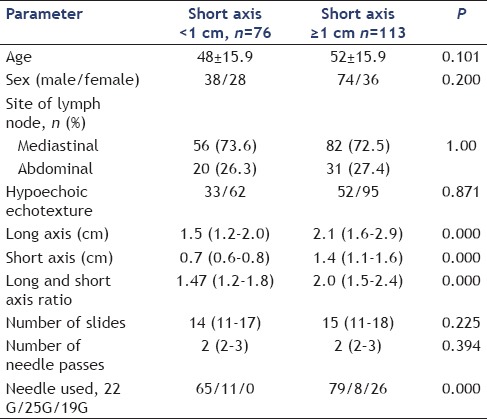
Table 2.
Fine needle aspiration diagnosis of 189 lymph nodes
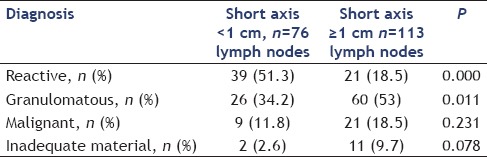
Diagnosis could not be made (inadequate material) in 2 (2.6%) and 11 (9.7%) lymph nodes in Group A and Group B, respectively (P = 0.000). An acid-fast bacillus staining was positive in 6 patients of Group A and 11 patients of Group B. The primary site of malignancy in cases of malignant lymphadenopathy (n = 30) was lung (n = 7 including 2 neuroendocrine cases), esophagus (n = 3), lymphoma (n = 5), gall bladder (n = 3), breast (n = 2), oropharyngeal (n = 3), and one each of rectum, prostate, pancreas, ureter, hepatocellular carcinoma, and cholangiocarcinoma. The primary site was unknown in one patient.
Two patients (out of 13 with inadequate samples) underwent EUS-guided reattempt and these two patients had reactive lymphadenopathy, rest 11 patients were not taken for re-FNA procedure as they were thought to have reactive lymph nodes based on EUS morphological criteria (heteroechoic lymph nodes with hyperechoic center). A total of 14 lymph nodes in 13 patients were <1 cm in size at both long and short axis (long axis 0.8 [0.7–0.9 cm] and short axis 0.6 [0.5–0.7 cm]). The FNA diagnosis in these 14 lymph nodes was malignancy in one, granulomatous etiology in six (including acid-fast Bacilli positivity in one) and reactive changes in seven.
Lymph nodes with granulomatous or malignant change were significantly larger at both long and short axis and had more chances of having sharply demarcated borders as compared to reactive lymph nodes [Tables 3 and 4], however long/short axis ratio (shape) was not significantly different. The subgroup analysis of subcentimetric lymph nodes for pathologic versus reactive diagnosis showed no significant difference at larger long axis (1.5 [1.2–1.9] vs. 1.5 [1.0–2.0], P = 0.425), a larger short axis (0.7 [0.6–0.9] vs. 0.7 [0.6–0.7], P = 0.662), and higher proportion of sharply defined borders (8/35 [22.8%] vs. 3/39 [7.6%], P = 0.101) that could not reach statistical significance. Both long axis and short axis measurements have relatively poor AUROC for the diagnosis of malignant or granulomatous change. The AUROC (95% confidence interval) was 0.727 (0.650–0.804, P = 0.00) and 0.730 (0.653–0.807, P = 0.00) for long axis and short axis for positive pathological diagnosis versus reactive lymphadenopathy, respectively. The long axis ≥2.75 cm and short axis ≥1.9 cm had 82% specificity for diagnosis of granulomatous or malignant change, but sensitivity at these values was 26% and 16% only. Representative EUS images of lymph nodes and cytopathology are shown in Figures 1 and 2.
Table 3.
Comparison of reactive, granulomatous, and malignant nodes
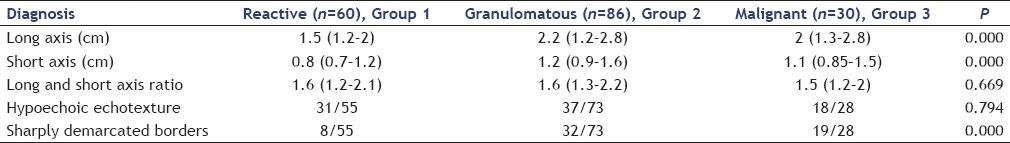
Table 4.
Comparison of reactive and pathologic (granulomatous and malignant) lymph nodes
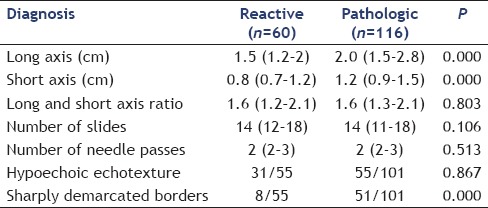
Figure 1.
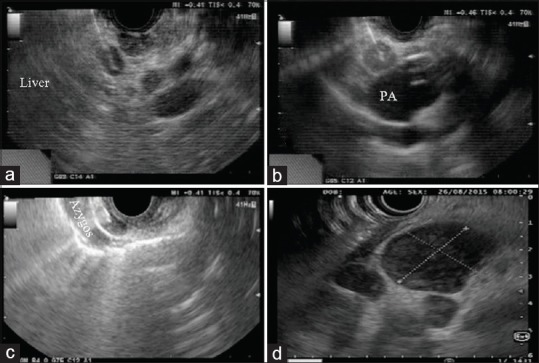
(a) Malignant node at porta (metastatic hepatocellular carcinoma); (b) tubercular node at aortopulmonary window (PA: pulmonary artery); (c) tubercular node in relation to azygos vein; (d) lymphoma lymph nodes
Figure 2.
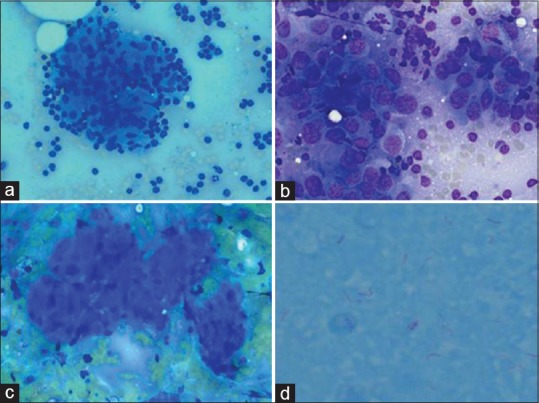
Representative cytopathological images; (a) tubercular granuloma, 40 times magnification, Giemsa stain); (b) metastatic hepatocellular carcinoma (60 times magnification, Giemsa stain), (c) metastatic gall bladder carcinoma (20 times magnification, Giemsa stain); (d) acid-fast Bacilli positivity (100 times magnification, ZN stain)
DISCUSSION
Presence of mediastinal lymph nodes in normal individuals without infection or malignancy is a common phenomenon as shown by postmortem studies and CT-based studies.[1,2,3] Kiyono et al. showed normal short axis diameter of 12 mm at subcarinal and 10 mm at region 4, 10R and 8 mm for regions 2, 5, 6, 8, 9, and 10 L in a postmortem study of forty cadavers without chest infection or malignancy. They showed greater variation in long axis size, thus proposed short axis as better criteria for normal mediastinal lymph nodes.[1] In another study of 64 cadavers who did not die due to infection and malignancy, Ziyade et al. suggested normal diameter of lymph nodes at the stations 4R and 7 as 1.5 cm and 2 cm and 1 cm at other stations.[2] In a CT-based study of 56 patients, Glazer et al. suggested 1 cm at short axis as normal mediastinal lymph nodes.[3] However, several studies have shown that not all lymph nodes with short axis diameter <1 cm are benign and some of these may show metastatic deposits in patients with lung carcinoma.[5,6,7,8] We have a major difference with other published studies; majority of our patients had pyrexia of unknown origin as indication of FNA as the profile of our patients is different from West. Granulomatous pathology was the predominant cause of pathological lymph node enlargement. Our findings are similar to two other studies from India in patients with mediastinal lymphadenopathy that suggested tuberculosis in 32 out of 60 patients and 22 out of 42 patients.[9,10] As expected, granulomatous and malignant nodes had significantly higher long and short axis, but AUROC was just near 0.73 for differentiation between reactive lymph node and pathologically enlarged node, thus supporting our strategy of sampling small lymph nodes in symptomatic patients. We did not encounter any complication during this study. EUS-guided FNA is a safe procedure. A meta-analysis of 32 studies of mediastinal lymph node FNA by Puli et al. showed complication rate of 0–2.3% across studies.[4] Our study has several limitations. It is retrospective in nature and we do not have follow-up data of these patients, thus we might have missed positive diagnosis in some cases with multiple lymph nodes that were reported as reactive. We do not have data on echo-features of some lymph nodes and method of FNA (capillary, suction vs. no suction); however, literature shows no effect of these methods on the outcome of FNA,[11,12] and poor predictive value of echo-features in differentiating between benign and pathological lymph nodes as shown by Jamil et al.[13] The authors compared 68 benign lymph nodes with 94 pathological lymph nodes and showed that various echo-features including echogenicity, margins, and shape were not significantly different between these groups, only size was significantly different.[13] The strengths of this study include a larger sample size and inclusion of pyrexia of unknown origin patients. The literature on EUS-FNA in pyrexia of unknown origin is limited to small series and case reports.[14,15,16] Pyrexia of unknown origin is quite common in South Asian region, and practice of empirical anti-tubercular therapy is common which may result in acute liver failure due to hepatotoxicity potential and should not be given.[17,18] In fact, the largest study regarding anti-tubercular therapy induced acute liver failure (n = 70) showed that 62.8% of the patients were given empirical treatment.[18] We do not want to suggest that all lymph nodes seen in mediastinum or abdomen should be sampled in asymptomatic patients (as they are present in normal individuals also). We suggest that small lymph nodes should be sampled when it is indicated like in our series (for pyrexia of unknown origin or to confirm malignant lymphadenopathy).
CONCLUSION
We show that lymph nodes with <1 cm short axis diameter are pathologically enlarged in almost half cases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors thank Mr. Yogesh Saini, Mr. Vipin Thomas (research coordinators), and Mr. Manish K. Singh (Biostatistician at Medanta) for their support.
REFERENCES
- 1.Kiyono K, Sone S, Sakai F, et al. The number and size of normal mediastinal lymph nodes: A postmortem study. AJR Am J Roentgenol. 1988;150:771–6. doi: 10.2214/ajr.150.4.771. [DOI] [PubMed] [Google Scholar]
- 2.Ziyade S, Pinarbasili NB, Ziyade N, et al. Determination of standard number, size and weight of mediastinal lymph nodes in postmortem examinations: Reflection on lung cancer surgery. J Cardiothorac Surg. 2013;8:94. doi: 10.1186/1749-8090-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glazer GM, Gross BH, Quint LE, et al. Normal mediastinal lymph nodes: Number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144:261–5. doi: 10.2214/ajr.144.2.261. [DOI] [PubMed] [Google Scholar]
- 4.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: It's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita T, Kuramitsu T, Kawamura M, et al. Bronchogenic carcinoma: Incidence of metastases to normal sized lymph nodes. Thorax. 1995;50:1267–9. doi: 10.1136/thx.50.12.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izbicki JR, Thetter O, Karg O, et al. Accuracy of computed tomographic scan and surgical assessment for staging of bronchial carcinoma. A prospective study. J Thorac Cardiovasc Surg. 1992;104:413–20. [PubMed] [Google Scholar]
- 7.Kondo D, Imaizumi M, Abe T, et al. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586–93. doi: 10.1378/chest.98.3.586. [DOI] [PubMed] [Google Scholar]
- 8.McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: Analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319–23. doi: 10.1148/radiology.182.2.1732943. [DOI] [PubMed] [Google Scholar]
- 9.Puri R, Vilmann P, Sud R, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462–7. doi: 10.1055/s-0029-1244133. [DOI] [PubMed] [Google Scholar]
- 10.Rana SS, Bhasin DK, Srinivasan R, et al. Endoscopic ultrasound (EUS) features of mediastinal tubercular lymphadenopathy. Hepatogastroenterology. 2011;58:819–23. [PubMed] [Google Scholar]
- 11.Storch IM, Sussman DA, Jorda M, et al. Evaluation of fine needle aspiration vs. fine needle capillary sampling on specimen quality and diagnostic accuracy in endoscopic ultrasound-guided biopsy. Acta Cytol. 2007;51:837–42. doi: 10.1159/000325857. [DOI] [PubMed] [Google Scholar]
- 12.Wani S, Early D, Kunkel J, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: A prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328–35. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 13.Jamil LH, Kashani A, Scimeca D, et al. Can endoscopic ultrasound distinguish between mediastinal benign lymph nodes and those involved by sarcoidosis, lymphoma, or metastasis? Dig Dis Sci. 2014;59:2191–8. doi: 10.1007/s10620-014-3164-9. [DOI] [PubMed] [Google Scholar]
- 14.Catalano MF, Rosenblatt ML, Chak A, et al. Endoscopic ultrasound-guided fine needle aspiration in the diagnosis of mediastinal masses of unknown origin. Am J Gastroenterol. 2002;97:2559–65. doi: 10.1111/j.1572-0241.2002.06023.x. [DOI] [PubMed] [Google Scholar]
- 15.Puri R, Thandassery RB, Choudhary NS, et al. Endoscopic ultrasound-guided fine-needle aspiration of the adrenal glands: Analysis of 21 patients. Clin Endosc. 2015;48:165–70. doi: 10.5946/ce.2015.48.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary NS, Puri R, Kotecha H, et al. An uncommon etiology of adrenal enlargement and fever in a patient of cirrhosis diagnosed with EUS guided FNAC. Trop Gastroenterol. 2014;35:258–60. doi: 10.7869/tg.228. [DOI] [PubMed] [Google Scholar]
- 17.Mir T, Nabi Dhobi G, Nabi Koul A, et al. Clinical profile of classical fever of unknown origin (FUO) Caspian J Intern Med. 2014;5:35–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar R, Shalimar, Bhatia V, et al. Antituberculosis therapy-induced acute liver failure: Magnitude, profile, prognosis, and predictors of outcome. Hepatology. 2010;51:1665–74. doi: 10.1002/hep.23534. [DOI] [PubMed] [Google Scholar]


