Abstract
A noninvasive, cell-autonomous reporter system was developed to monitor the generation and distribution of physiologically active pools of abscisic acid (ABA). ABA response (abi1-1) and biosynthesis (aba2-1) mutants of Arabidopsis (Arabidopsis thaliana) were used to validate the system in the presence and absence of water stress. In the absence of water stress, low levels of ABA-dependent reporter activation were observed in the columella cells and quiescent center of the root as well as in the vascular tissues and stomata of cotyledons, suggesting a nonstress-related role for ABA in these cell types. Exposure of seedlings to exogenous ABA resulted in a uniform pattern of reporter expression. In marked contrast, reporter expression in response to drought stress was predominantly confined to the vasculature and stomata. Surprisingly, water stress applied to the root system resulted in the generation of ABA pools in the shoot but not in the root. The analysis of the response dynamics revealed a spread of physiologically active ABA from the vascular tissue into the areoles of the cotyledons. Later, ABA preferentially activated gene expression in guard cells. The primary sites of ABA action identified by in planta imaging corresponded to the sites of ABA biosynthesis, i.e. guard cells and cells associated with vascular veins. Hence, water stress recognized by the root system predominantly results in shoot-localized ABA action that culminates in a focused response in guard cells.
Abscisic acid (ABA) plays a major role in plants' responses to biotic and abiotic stresses; increases in ABA levels have been reported during salt, cold, drought exposure, and wounding (Zeevaart and Creelman, 1988; Peña-Cortés et al., 1989; Shinozaki and Yamaguchi-Shinozaki, 2000). ABA affects plant resistance to heat exposure (Robertson et al., 1994) and plant-pathogen interaction (Mohr and Cahill, 2003). ABA action can be targeted to certain tissues (Casimiro et al., 2003) or to single cells such as the guard cells (Schroeder et al., 2001). In addition, ABA may generally enable plants to adjust to severe drought and cold stress (Shinozaki and Yamaguchi-Shinozaki, 1996). The ABA-induced responses to such abiotic stresses are complemented by ABA-independent regulatory pathways (Shinozaki and Yamaguchi-Shinozaki, 2000; Zhu, 2002).
Although ABA is considered to be a “stress hormone” (Zeevaart and Creelman, 1988), it is becoming clear that it also serves important regulatory functions in the absence of stress (Cheng et al., 2002; Sharp, 2002). ABA is recruited as an endogenous signal in many plant developmental processes including dormancy (Karssen et al., 1983; Koornneef et al., 2002), heterophylly in Marsilea (Hsu et al., 2001), sex determination in Cannabis (Mohan Ram and Juiswal, 1972), pollination (Kovaleva and Zakharova, 2003), and senescence (Hunter et al., 2004). One role for ABA in nonstressed plant development is assigned to the inhibition of ethylene generation (Sharp, 2002). The mode and sites of ABA action in the absence of stress are not yet elucidated.
During water stress, ABA is thought to be generated in the root and transported to the shoot to mediate stomatal closure as well as more general adaptive shoot responses. Our understanding of the effect of drought stress on the generation of ABA pools in intact plants has to date relied largely on whole leaf and xylem sap measurements (Jackson, 1993; Wilkinson and Davies, 2002). A limited number of studies have addressed ABA levels in specific groups of cells. While the latest mass-spectrometric tools offer the possibility of reliable quantification of ABA in minute amounts of plant samples (Müller et al., 2002), analysis of hormone pools in single cells or cell groups is usually restricted to specialized cell types such as guard cell pairs for which appropriate isolation procedures have been elaborated (Harris et al., 1988; Zhang et al., 2001).
For a better understanding of the regulatory role of ABA as an adaptive signal during drought stress, information about the dynamics of generation and distribution of physiologically active ABA pools is necessary. The comprehensive analysis of ABA action requires an evaluation of phytohormone action in a three-dimensional, cell-autonomous manner and, preferably, in vivo with noninvasive tools to reduce the impact of experimental manipulation. Several systems for in planta imaging were established, including luciferase (LUC)-based reporters (Millar et al., 1995; Ishitani et al., 1997; Chinnusamy et al., 2002) and green fluorescent protein (GFP)-using systems (Haseloff, 1999). Localization of phytohormone action at a histological level has been achieved predominantly by expression of the β-glucuronidase (GUS) reporter and subsequent in situ analysis, e.g. of an auxin-responsive promoter (Sabatini et al., 1999; Benkova et al., 2003) and recently in planta by a GFP-based sensor for auxin action (Ottenschläger et al., 2003). GFP-derived systems for in planta imaging require high intensities of near UV light for confocal analysis that are prone to photobleaching and heat generation of the specimen (Dixit and Cyr, 2003). A reporter system responsive to both drought and ABA has been very valuable in analyzing plant responses to abiotic stress (Ishitani et al., 1997; Xiong et al., 1999; Zhu, 2002). However, as the activity of the RD29A promoter used in this system is regulated by both ABA-dependent and ABA-independent signaling pathways (Narusaka et al., 2003), it is not solely indicative for the action of ABA.
In this report, we used two ABA-specific promoters to analyze ABA-indicative reporter responses at the whole plant level in a noninvasive system with single cell resolution. The study revealed insights into the spatial and temporal pattern of ABA action in the presence and absence of water stress.
RESULTS
Development and Validation of ABA-Specific, Sensitive Reporter Systems
Two ABA-specific promoters were used in this study, pLTI65/pRD29B (Nordin et al., 1993; Yamaguchi-Shinozaki and Shinozaki, 1994; Uno et al., 2000) and the AtHB6 promoter (pAtHB6), which shows over 1,000-fold ABA-specific induction (Himmelbach et al., 2002). Transgenic plants homozygous for the GUS and LUC reporter genes under control of these ABA-specific promoters were generated. The reporter system was tested at ABA concentrations ranging from 0.3 to 100 μm because micromolar concentrations of ABA accumulate in mesophyll and guard cells during water stress (Harris et al., 1988).
Reporter activation was seen above 0.3 μm and was saturated at approximately 30 μm in both the root and cotyledons (Fig. 1). The sensitivity and the dynamic range of pAtHB6::LUC at micromolar concentrations of ABA argue for the suitability of the reporter system to detect changes in endogenous ABA levels, at least during conditions of elevated phytohormone concentrations. To confirm this, we tested the ability of the pAtHB6::LUC and pRD29B::LUC reporter systems to respond to moderate water stress (Ψ = −0.8 MPa). Both systems respond to water stress with a strong induction of reporter expression whereby a 2- to 3-fold stronger signal was generated in the pAtHB6::LUC line (Fig. 2).
Figure 1.
Dependence of reporter response on exogenous ABA levels. LUC expression of 4-d-old pAtHB6::LUC seedlings was recorded after exposure to various ABA concentrations for 12 h. Light emission of cotyledons (A) and roots (B) in wild-type (black circles), ABA-deficient aba2-1 (white squares), and ABA-insensitive abi1-1 (white triangles) background. ABA restores the reporter response in the ABA-deficient aba2-1 mutant, whereas the response is abrogated in the abi1-1 background. Note the different saturation levels of wild-type cotyledons and roots. When related to the protein content of the samples, the saturation levels became similar for shoots and roots. Values are means of 3 independent measurements, each comprising 15 seedlings.
Figure 2.
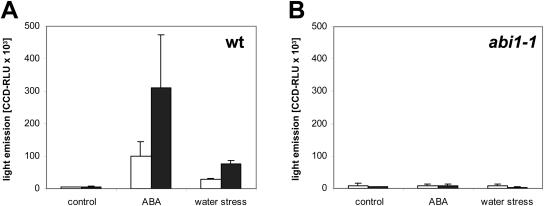
Activation of pRD29B::LUC and pATHB6::LUC by ABA and water stress. Seedlings expressing either the pRD29B::LUC or pAtHB6::LUC reporter gene were exposed to ABA (30 μm), water stressed (Ψ = −0.8 MPa), or not treated in the control for 24 h prior to measurement of reporter activity. The analysis was performed in ABI1 wild-type (wt; A) and abi1-1 background (B; n = 45). Activity is expressed as relative light units captured by the CCD camera within 10 min for pRD29B::LUC (white bars) and pAtHB6::LUC reporter (black bars).
In Arabidopsis (Arabidopsis thaliana), both ABA-deficient and ABA-insensitive mutants have been isolated (Koornneef et al., 1998) that are perfectly suited to test the ABA specificity of reporter responses. ABA deficiency is observed in aba2-1 (Léon-Kloosterziel et al., 1996), a mutant impaired in the conversion of xanthoxin to the immediate ABA precursor abscisic aldehyde (Schwartz et al., 1997; Gonzalez-Guzman et al., 2002). The mutation reduces ABA levels to approximately 20% of wild-type levels of nonstressed plants and almost abolishes the stress-induced increase in ABA (Cheng et al., 2002). Elevated levels of pAtHB6::LUC activity in the aba2-1 mutant background show that the reporter system is responding to exogenous as opposed to endogenous ABA pools (Fig. 1). abi1-1 is an ABA-insensitive mutant (Koornneef et al., 1984), defective for a protein phosphatase (Leube et al., 1998) that plays a central role in ABA signaling (Hoth et al., 2002; Himmelbach et al., 2003). The ABA-mediated activation of pAtHB6 requires ABI1 and is abolished in abi1 (Söderman et al., 1999; Himmelbach et al., 2002). Consistently, neither ABA exposure nor water stress significantly altered reporter expression in the abi1-1 mutant background (Figs. 1 and 2).
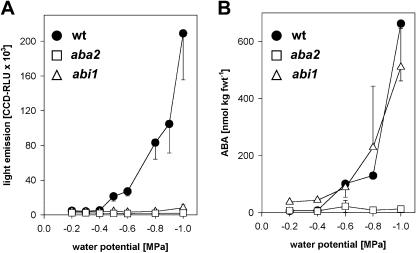
In the wild type, the intensity of reporter activation in the shoot depended on the water potential of the root medium (Fig. 3A). Within the water potential examined ranging from −0.2 to −1.0 MPa, a reporter response was detectable below −0.4 MPa, with maximal induction of up to 40-fold at −1.0 MPa. With roots exposed to Murashige and Skoog medium (approximately −0.2 MPa) and Murashige and Skoog containing 80 mm mannitol (−0.4 MPa), no induction of pAtHB6::LUC expression was observed in the wild type or in the mutants aba2-1 and abi1-1 (Fig. 3A). These water potentials were not sufficient to elevate detectably the ABA level either (Fig. 3B). The endogenous ABA level increased below −0.4 MPa and concomitant with reporter induction up to 100-fold at −1.0 MPa in wild type. ABA levels rose 14-fold in abi1-1 shoots at −1.0 MPa, whereas no increase in reporter expression occurred in the aba2-1. The results emphasize water-stress- and ABA-dependent activation of pAtHB6::LUC expression.
Figure 3.
Reporter response and ABA levels in dependence of water potential. LUC expression of 4-d-old pAtHB6::LUC seedlings was recorded 24 h after exposure to various water potentials calibrated by supplementing the solidified medium with mannitol. Light emission (A) and ABA (B) levels of shoots were recorded in wild-type (black circles), ABA-deficient aba2-1 (white squares), and ABA-insensitive abi1-1 (white triangles) background. Values are means of 3 independent measurements, each comprising 15 seedlings.
Distribution of ABA Pools in the Absence of Stress
Although ABA is thought to play important roles in the absence of stress, the mode and sites of ABA action under such conditions have not been elucidated. We therefore monitored ABA pools in well-watered plants using our ABA-specific promoters to drive the expression of both LUC and GUS reporter genes (Figs. 4 and 5). Well-watered pAtHB6::LUC and pRD29B::LUC seedlings revealed a faint but detectable LUC expression throughout the seedling (Fig. 4A) with no prominent recognizable areas of increased reporter activity in the absence of exogenous ABA. Low levels of GUS expression were detected in the shoot apical meristem, cotyledonary veins, guard cells, and in cells of the cotyledon hydathode (Fig. 5, A and F). In the root, the pRD29B::GUS plants showed clearly localized reporter expression in columella cells and the root quiescent center (Fig. 5P).
Figure 4.
Differential activation of ABA signaling induced by water stress. Seedlings of the pAtHB6::LUC line were exposed to control conditions (A), ABA (100 μm; B), and water stress (Ψ = −0.8 MPa; C) for 24 h prior to measurement of reporter activity. Luminescence intensity is depicted in false colors ranging from blue to red, indicating low and high intensity, respectively. In A, the scale of light emission is 5× enhanced compared to B and C. The nonenhanced image is shown as the inset. The seedling analyzed in B was deficient in ABA biosynthesis due to the aba2-1 mutation. The signals induced by water stress (C) are primarily confined to the stomata and vasculature of the cotyledons. The signal of the root and hypocotyl was hardly detectable. The pictures were taken by a CCD camera mounted to an inverted microscope with 2.5× magnification of the objective and an exposure time of 10 min with 4 × 4 pixel binning. The scale bars correspond to 1 mm.
Figure 5.
ABA signaling in the root tip: response specificity toward ABA. Seedlings containing the pRD29B::GUS expression cassette were either nontreated or exposed to 30 μm ABA (ABA) or their roots were water stressed (stress; Ψ = −1.0 MPa) for 24 h prior to histological staining for reporter activity. The analysis included seedlings with wild-type ABA response (wt), ABA deficiency, and ABA insensitivity provided by the aba2 and abi1 mutant background, respectively. The pictures arranged in a vertical row represent photographs of the same specimen with one specific treatment, while the horizontal rows represent photographs of the same organ or organ parts of seedlings treated differently. The first horizontal row (A–E) shows a close-up view of the cotyledon (scale bars represent 100 μm), the second the shoot (scale bars represent 1 mm), the third a section of the root (scale bars represent 100 μm), and the fourth the root tip (scale bars represent 100 μm). The specimens were stained for identical periods (1 h) to visualize reporter activity, with the exception of Figure 5, K, N, and O, which were stained for 3 h.
Visualization of GUS expression in cells of the root and hypocotyl stele required prolonged staining. Analysis of pRD29B::GUS reporter expression in the ABA-deficient aba2-1 or in the ABA-insensitive abi1-1 mutant backgrounds suggested that the pattern of reporter activity of pRD29B::GUS seedlings reflects gene regulation controlled indeed by endogenous ABA that escapes detection by the LUC system (Fig. 4A). In conclusion, our findings indicate that in the absence of stress, ABA signaling occurs in specific tissues and cell types.
Water Stress Applied to the Root System Results in the Generation of Active ABA Pools in the Shoot But Not Detectably in the Root
Water stress induces ABA biosynthesis and, as a consequence, elevated ABA levels are found in water-stressed plants (Harris et al., 1988; Zeevaart and Creelman, 1988). A prerequisite for a bona fide visualization of ABA changes in planta is the responsiveness of the reporter system throughout the plant. In response to exogenous ABA, LUC expression was seen throughout the seedling (Fig. 4B) and GUS expression was observed throughout the root (Fig. 5, L and Q). This supports a general capacity of the substrates to access plant cells that express the reporter genes in an ABA-responsive manner.
Water stress (Ψ = −0.8 MPa) applied to the root resulted in predominant activation of reporter activity in shoot rather than root tissues irrespective of the ABA-regulated promoter and the reporter enzyme used (Figs. 4C and 5, C, H, M, and R). In marked contrast to the uniform patterns of reporter expression observed in seedlings exposed to exogenous ABA, reporter expression in response to drought stress was confined to specific tissues and cell types (compare Fig. 4B with Fig. 4C, Fig. 5L with Fig. 5M, and Fig. 5Q with Fig. 5R). In the shoot, reporter expression was induced predominantly in the stomata and vasculature (Fig. 4C). In the root, low induction of reporter expression was restricted to the vascular tissues (Fig. 5M), while expression in the quiescent center and columella was not induced (compare Fig. 5R with Fig. 5P).
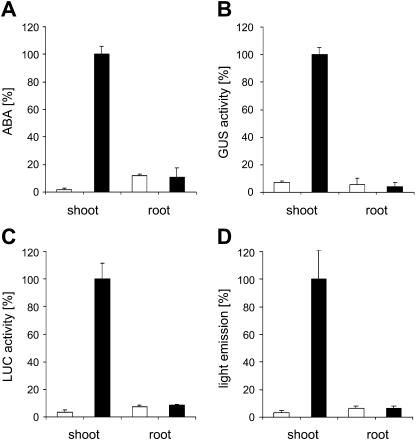
Current models postulate that, in response to water withdrawal, ABA pools are generated in the root and translocated to the shoot. It seemed surprising that our reporter systems failed to detect significant ABA pools in root tissue of water-stressed plants. We therefore reevaluated our findings by directly measuring ABA concentrations of shoot and root tissue 24 h after application of water stress (Ψ = −0.8 MPa) to the root system (Fig. 6). The analysis showed that ABA pools in the shoot increased approximately 50-fold in response to water stress. There was no detectable change in ABA pools in the root (Fig. 6A). We compared our direct measurement of ABA pools to induction levels indicated by reporter activity. Reporter activity was determined by ratiometric in vivo imaging as well as by enzymatic assay of cell-free extracts to rule out the possibility of limited substrate availability. In independent experiments (n = 3), induction rates of LUC reporter activity in shoots always closely matched the relative increase of ABA levels (compare Fig. 6A with Fig. 6, C and D). The specific reporter activity calculated from enzymatic assays in cell-free extracts (normalized to protein levels) in the shoot fraction was 14- and 30-fold enhanced by water stress over nonstressed controls in GUS and LUC reporter lines, respectively (Fig. 6, B and C). Similar induction rates of LUC were calculated from in vivo measurements (33-fold; Fig. 6D). However, no significant change in reporter activity was observed in extracts from roots or by ratiometric in vivo imaging of roots (Fig. 6, B–D). Seedlings and developed rosettes of Arabidopsis revealed the identical pattern of shoot versus root ABA action during water stress. Four-week-old rosette plants grown under sterile conditions were exposed to water stress (Ψ = −1.0 MPa) prior to recording light emission. Again, water stress induced light emission of the shoot but not of the root system (Fig. 7). In contrast, transgenic plants of the same age in which LUC expression was driven by the constitutively active 35S promoter showed reporter activity in both shoot and root (data not shown).
Figure 6.
Induction of ABA pools and ABA signaling in shoots by water-stressing roots. Seedlings of lines pRD29B::LUC and pRD29B::GUS were exposed to water stress (Ψ = −0.8 MPa) via the roots for 24 h prior to the analysis of shoots and roots. The analysis included determination of ABA levels (A), GUS reporter activity (B), and LUC activity (C), with both reporters assayed enzymatically in tissue extracts, and light emission analyzed ratiometrically by in vivo measurement (D). The values are shown for water-stressed seedlings (black bars) and controls (white bars) of either the pRD29B::LUC (A, C, and D) or pRD29B::GUS (B) line. The values are given relative to the highest value obtained within one type of analysis for better comparison with 100% marking in 148 fmol ABA μg protein−1 (A; this equals 164 nmol ABA kg−1 shoot fresh weight or 435 nmol ABA kg−1 root fresh weight), 8.12 RLU s−1 μg protein−1 (B), 1,890 RLU s−1 μg protein−1 (C), and 31 CCD-RLU s−1 μg protein−1 (D). The analysis was performed on 3 groups of 15 seedlings per data point (± sd).
Figure 7.
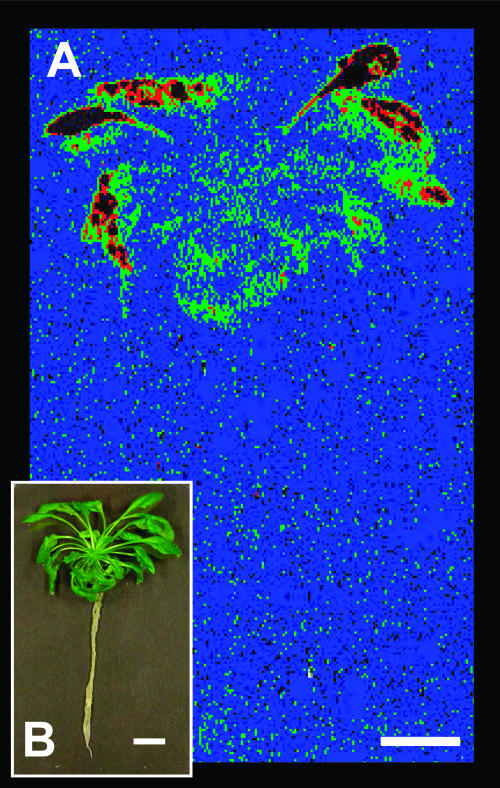
Reporter response in Arabidopsis rosette plant. The pAtHB6::LUC reporter plants cultivated for 4 weeks under sterile conditions were exposed to water stress (Ψ = −1.0 MPa) via their root system for 24 h prior to in vivo measurement of reporter activity shown in false colors (A) for a single plant and the corresponding brightfield image (B). Comparatively developed and nonstressed plants served as a control and revealed only background LUC activity, while p35S::LUC transgenic plants showed high reporter activity in root and shoot (not shown). Color code is as introduced in Figure 4. The scale bars correspond to 1 cm.
Dynamics of ABA Action in Response to Osmotic Stress
The above experiments document the sites of ABA action 1 d after the onset of water stress. The LUC reporter is well suited for monitoring changes of reporter expression within hours (Millar et al., 1992). This is a time frame during which drought stress induces massive alterations in gene expression (Seki et al., 2002). To record the dynamics in the generation of active ABA pools, a series of in vivo measurements were performed with pAtHB6::LUC transgenic seedlings from the beginning of root-applied water stress (Ψ = −1.0 MPa) until 14 h after.
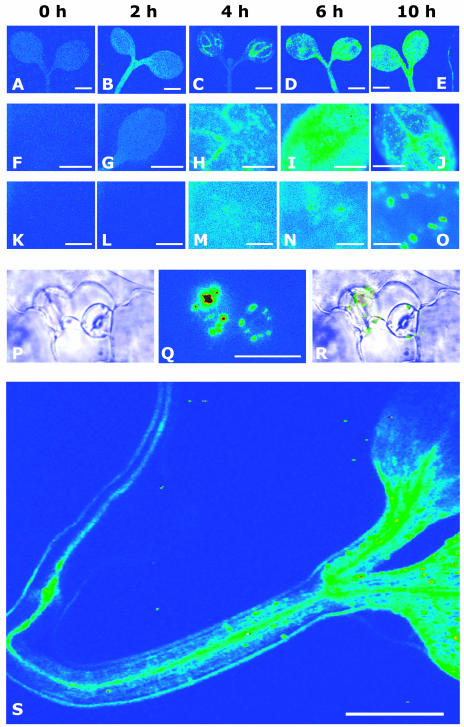
In the shoot, reporter expression was just detectable and evenly distributed at the onset of the experiment (Fig. 8, A, F, and K). The signal increased within 2 h of stress exposure in hypocotyl and cotyledons (Fig. 8B). Four hours after the application of drought stress, reporter activation was prominent around the vasculature of the cotyledons (Fig. 8, C and H) and subsequently dissipated throughout the cotyledon (6 h; Fig. 8, D and I). Thereafter, LUC expression within the cotyledons culminated more and more in stomata and clearly focused in guard cells at time point 14 h (compare Fig. 8, M, N, O, and Q). In roots, only background levels of reporter activity were detectable at time points 2 to 6 h (data not shown). However, a clear signal increase was observed in roots stressed for 10 and 14 h along the root vasculature that extended into the veins of hypocotyl and cotyledons (Fig. 8, E and S). Later on, the reporter response in the root decreased close to resting levels within 24 h after stress initiation. The dynamics of LUC activation were comparably observed with the pRD29B::LUC line, however, at a reduced sensitivity. In addition, a parallel analysis of pRD29B::LUC lines in the aba2-1 and abi1-1 backgrounds confirmed the ABA specificity of the signals recorded (data not shown).
Figure 8.
Dynamics of reporter response to root-sensed water stress. Seedlings of the reporter line pAtHB6::LUC were water stressed via their roots (Ψ = −1.0 MPa), and reporter activity was recorded noninvasively. The images depict light emission of cotyledons at different magnification and in dependence of time of water stress (0, 2, 4, 6, and 10 h). Background reporter activity (false color scale as before) is seen at the onset of the experiment (A, F, and K). The signal increases roughly 2-fold after 2 h, but no distinct pattern of reporter activation can be recognized (B, G, and L). Four hours after the beginning of stress exposure, the reporter is significantly induced in vascular tissues of the cotyledons (C and H), whereas after 2 additional h of treatment, a more generalized response is seen throughout the shoot (D, I, and N). After 10 h of water stress, reporter induction focused within vascular tissues (J) and guard cells (J, O–R). Reporter induction was also visible in the root (E; root positioned on the right) at that time point. However, no reporter induction was detected in the roots before. A similar pattern of ABA action is seen after 14 h of water stress (S). A to E, Pattern of light emission from representative shoots; objective 2.5×; bars correspond to 1 mm. F to J, Light emission from a representative cotyledon during water stress; objective 10×; bars correspond to 500 μm. K to O, Light emission from epidermal cells and guard cells during water stress; objective 40×; bars correspond to 100 μm. P to R, Light emission from guard cells after 10 h of water stress; objective 40×; bars correspond to 100 μm. P, Brightfield picture. Q, Pattern of light emission. R, Overlay picture of P and Q. S, Light emission from a seedling after 14 h of water stress treatment; objective 2.5×; exposure time 15 min; pixel binning 4 × 4; bar corresponds to 1 mm.
DISCUSSION
To study the distribution of active ABA pools in planta in the presence and absence of water stress, we made use of the noninvasive LUC reporter gene under the control of the ABA-specific promoters pAtHB6 and pRD29B. We used abi1-1 and aba2-1 mutant backgrounds to validate the system. ABA-specific in vivo imaging revealed the presence and relative abundance of physiologically active pools of ABA at the whole seedling level with single cell resolution. The pAtHB6 promoter was more ABA sensitive than the pRD29B, and the GUS reporter appears to be advantageous for monitoring very low levels of ABA-controlled gene expression not discerned by the LUC reporter. A difference in reporter stability (de Ruijter et al., 2003) may account for the more informative expression pattern observed with the GUS reporter system in the absence of stress.
Water Stress Applied to the Root System Results in the Generation of ABA Pools in the Shoot But Not in the Root
Current models postulate that water stress sensed by the root system generates ABA within the root, which subsequently is translocated to the shoot for regulation of transpiration (Wilkinson and Davies, 2002). In this study, three lines of evidence indicate elevation of ABA levels in the shoot rather than in the root exposed to water stress, namely by monitoring ABA-dependent gene expression in vivo, by enzymatic assays, and by directly measuring ABA concentrations in different organs. In these analyses, induction of ABA levels and the stimulation of ABA-dependent reporter expression was confined to the shoot with the single exception of a limited and localized ABA action along the root vasculature (Figs. 5M and 8, E and S). The root response was delayed in comparison to the shoot response by several hours.
Under the assumption that this model is correct, a potential explanation for our finding may be the rapid export of ABA from roots to shoots, such that ABA generated de novo in the root is efficiently depleted. Thus, changes in pool sizes could be too small to be detected. However, we are tempted to speculate that water stress sensed by the root induces a long distance-acting signal, which triggers ABA biosynthesis in the shoot. Such a concept is supported by grafting experiments, which revealed that ABA biosynthesis of tomato roots exposed to low-water potentials is not sufficient to induce proper stomatal closure in ABA-deficient shoots (Holbrook et al., 2002). The nature of the translocated signal is speculative, and there could be minute amounts of ABA not detectable by our system, a hydraulic signal, or pH changes within the xylem sap (Wilkinson and Davies, 2002; Sobeih et al., 2004).
The Distribution of ABA Pools in the Absence of Stress
In well-watered plants, ABA-specific gene expression was localized to the root columella, the cells of the quiescent center, and vascular tissues of root and shoot. Root tips are known as sites of ABA accumulation in the absence of stress (Rivier et al., 1977; Feldman et al., 1985), but the exact distribution of ABA within the root tip had not yet been elucidated. The finding of root cap-localized ABA-signaling may provide an explanation for the stress-independent expression of the ABA-responsive dehydrin genes LTI29 and ERD14 in the calyptra (Nylander et al., 2001). The role of the ABA signal in the root cap is not clear and could either reflect a response to mechanical stress or be linked to senescence or to osmotic adjustment. ABA-activated gene expression in the calyptra could also point to an important role of ABA in normal root development, which requires the root cap (Tsugeki and Fedoroff, 1999) for proper regulation of the ABA-dependent processes of cell division (Dewitte and Murray, 2003) and lateral root formation (De Smet et al., 2003). In line with this interpretation, the root system of the ABA-deficient mutant aba2-1 is shorter and has fewer lateral roots (Cheng et al., 2002).
The quiescent center of the root meristem comprises four cells, the so-called stem cells, which are mitotically inactive due to an arrest in the cell cycle (Dolan et al., 1993). ABA signaling in these cells (A. Christmann, unpublished data) was assigned by comparison with ABA biosynthesis and response mutants and by comparison with the auxin-responsive Arabidopsis DR5 promoter-GUS line that shows a maximum of auxin-specific signaling in the quiescent center, columella stem cells, and differentiated columella (Sabatini et al., 1999). Auxin, a cell-division-stimulating plant hormone, could be antagonized by ABA to keep the quiescent center in the mitotically dormant state (Dewitte and Murray, 2003). Interestingly, the root meristem was not visibly altered in aba2-1, indicating either that ABA is not required or that the reduced level of ABA present in the mutant is sufficient to exert its function.
Upon Drought Stress, ABA Accumulates in the Vasculature and in Stomata
Ten hours after the application of water stress to the root system, ABA pools were found almost exclusively in the stomata and vasculature of the shoot. The pattern of ABA action seems to coincide with sites of ABA biosynthesis. ABA aldehyde oxidase AAO3 catalyzes the final step in ABA biosynthesis (Seo et al., 2000; Gonzalez-Guzman et al., 2004). Its expression occurs predominantly along the vasculature throughout the plant and under water-stress protein levels increase in the epidermal tissues and guard cells (Koiwai et al., 2004). In addition, two other enzymes of ABA biosynthesis, the C15 xanthoxin-generating epoxycarotenoid dioxygenase (NCED; Tan et al., 2003) and the oxidase ABA2 (Cheng et al., 2002; Gonzalez-Guzman et al., 2002), are found in proximity to the vascular tissue. Promoter activity of AtNCED3, encoding the major stress-induced NCED of Arabidopsis (Iuchi et al., 2001), was observed in the root tip and along the root vasculature as well as in guard cells (Tan et al., 2003). Our findings clearly support a transmitter function of ABA in which primary sites of signal biosynthesis and signal action are located in tight proximity.
Despite the differential response of organs and cell types observed in planta during water stress, the whole plant has the capacity to respond to ABA as shown by exogenous ABA administration. This is conceivable because adaptation of a plant to low water potentials requires the concerted effort of the multicellular organism. Reduced water availability to root cells is counteracted by enhancement of the driving force of water uptake, the cellular osmotic potential. ABA provides a signal to mediate osmotic increase in root cells (Robertson et al., 1990). The increase in the osmotic potential of the root necessitates the orchestration of osmotic readjustments in the whole organism to avoid water loss of other organs and to retain turgescence in the shoot. During water stress, cells of the vasculature and stomata are foci of physiologically active ABA, as revealed by the noninvasive reporter system. A major function of the cells of the vasculature is loading and unloading xylem and phloem, which would be severely compromised by loss of cell turgescence. The other main target of ABA signaling, the guard cell, seems to differ in its ABA response from other cells. Rather than increasing the osmotic potential, guard cells reduce it in response to ABA to initiate stomatal closure (Schroeder et al., 2001). The long-lasting and strong ABA signaling observed in guard cells compared to mesophyll cells during drought stress could be required to keep the stomata in the closed state.
Perspective
The communication between root and shoot during water stress as well as the role of ABA in this process are still a conundrum (Sobeih et al., 2004). The noninvasive system to analyze ABA action in live plants without inflicting major physical stress provides a novel tool in phytohormone analysis. In appropriate specimens, the reporter system is able to visualize physiologically active ABA pools with cellular resolution. The integration of three-dimensional reconstructions in the analysis is an attractive perspective from which to dissect the spatiotemporal action of ABA in stress responses and development. Optical projection tomography allows the three-dimensional analysis of translucent samples of up to several millimeters in diameter (Sharpe et al., 2002). Phytohormones act specifically in time and space of multicellular organisms and mediate intercellular as well as interorgan communication. Addressing phytohormone action in vivo in a three-dimensional, noninvasive, and cell-autonomous manner is necessary for comprehensive analysis of these endogenous signals. Our understanding will surely benefit from the use of such noninvasive reporter systems.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized for 25 min with 80% ethanol containing 0.1% (v/v) Triton X-100 and for 3 min with 3% sodium hypochlorite solution. Sterilized seeds were then rinsed 5 times with sterile water and subsequently spread onto petri dishes containing Murashige and Skoog medium (Murashige and Skoog, 1962) supplied with 10 g/L Suc and 0.9% agar. Plates were kept for 2 d at 4°C and thereafter at 22°C under continuous illumination (60 μE m−2 s−1).
Plasmid Constructs
The RD29B promoter (−1,771 to −25) was PCR-amplified by using Arabidopsis (ecotype Columbia [Col-0]) genomic DNA as template and the primers 5′-TCCCCGCGGCTCAAGTTTACTTCATCC-3′ and 5′-GCGGATCCTTCAAGTGAATCAATCATCAAAC-3′. These primers introduced single BamHI and SacII sites into the promoter fragment, which was fused with the GUS gene derived from pBI121 (Jefferson et al., 1987) to form pRD29B::GUS or with the LUC gene from pGEM-Luc (Promega, Madison, WI) to form pRD29B::LUC. Construction of pAtHB6::LUC has been described previously (Himmelbach et al., 2002).
Transgenic Plants
Arabidopsis reporter lines were generated by genomic integration of reporter genes consisting of either a pAtHB6 or pRD29B promoter fragment fused to the LUC or GUS gene using Agrobacterium tumefaciens strain MP90 and Arabidopsis plants ecotype Col-0 and RLD as described (Meyer et al., 1994). Several independent pRD29B::LUC, pRD29B::GUS, and pAtHB6::LUC transgenic plants were recovered after Agrobacterium-mediated transformation and two lines of each version were randomly selected. Transgenic Col-0 lines homozygous for the pRD29B::LUC, pRD29B::GUS, or pAtHB6::LUC transgene were used to cross in the ABA-deficiency locus aba2-1. For analysis of the reporter lines in the abi1-1 background, RLD reporter lines were crossed with abi1-1 and as a control with ecotype Landsberg erecta. Lines homozygous for reporter constructs and for mutant alleles were used throughout the experiments.
ABA and Stress Treatments
For ABA exposure experiments, 4-d-old seedlings were transferred to Murashige and Skoog agar plates containing 5 g/L Suc and different concentrations of (+) cis-trans-ABA (Lomon Bio Technology, Deyang City, China). Seedlings were then sprayed with the same ABA concentration as supplied with the agar media in 10 mm MES buffer, pH 5.8. Exposure time to ABA was 24 h unless otherwise indicated. For water stress treatments, seedlings were transferred onto agar plates that contained different concentrations of mannitol (Figs. 2, 3, and 7) or that had been equilibrated with PEG6000 (Figs. 4, 5, 6, and 8; Fluka Chemie GmbH, Buchs, Switzerland) solutions of different concentrations to adjust water potentials according to van der Weele et al. (2000). From these plates, 8-mm wide horizontal strips of solidified medium had been removed prior to transfer of seedlings. Seedlings were positioned on the plates such that only roots but not shoots were in contact with the agar surface during incubation. Water potential of mannitol-agar and polyethylene glycol-equilibrated media was measured with a psychrometer (Wescor, Logan, UT). Incubation time on stress media was 24 h unless otherwise indicated. The relative humidity of the air was above 98% during incubation as measured by psychrometry. The petri dishes were sealed with a porous tape (SOEHNGEN Pore; SOEHNGEN, Taunusstein, Germany). In reconstruction experiments, 98% relative humidity was not sufficient to induce reporter expression. During the transfer process of seedlings to new plates, the plant material was exposed to 50% to 70% relative humidity for a short period, which also failed to activate the reporters.
Reporter and ABA Analysis
Transgenic seedlings carrying the GUS reporter gene were fixed in cold (4°C) 90% acetone and stained for GUS activity according to Jefferson et al. (1987). For quantification of GUS activity, plant tissues were homogenized in liquid nitrogen and GUS activity was measured in tissue extracts according to Breyne et al. (1993). LUC was extracted from plant tissues homogenized in liquid nitrogen and assayed in the presence of CoA according to Luehrsen et al. (1992). Light emission from extracts was measured with a Berthold “flash'n glow” luminometer (Berthold Technologies, Bad Wildbad, Germany) and is referred to as relative light units (RLU). Details are given in Himmelbach et al. (2002).
ABA was extracted from seedlings and quantified by gas chromatography-tandem mass spectrometry according to Müller et al. (2002).
In Vivo Imaging
For imaging of LUC activity, plants were sprayed with a 1-mm solution of luciferin (PJK, Kleinblittersdorf, Germany) in 10 mm MES, pH 7.0, 0.01% Tween 80. Light emission was detected by an intensified CCD camera (ORCAII ERG; Hamamatsu Photonics, Hamamatsu City, Japan) equipped with a Schneider Xenon 0.95/25 Objective (Schneider, Kreuznach, Germany) in a dark box. Collection of light started after 10 min preincubation in the dark for 10 min with 4 × 4 pixel binning. The intensity of light emission from plant organs of interest was measured after background subtraction using Simple PCI 5.2 software (Compix, Cranberry Township, PA). Gray levels of pixels within the measuring area corrected for background activity are referred to as CCD-RLU).
Detection of luminescence on a cellular level was performed with an inverted microscope (Axiovert 200; Carl Zeiss, Göttingen, Germany) equipped with Fluar objectives and the CCD camera mounted to the microscope base port. Seedlings were placed in a drop of luciferin solution in small (50 × 20 × 2 mm) self-constructed chambers that were sealed at the bottom with a coverslip. Seedlings were covered with black cheesecloth to reduce light reflection, and the whole chamber was loosely covered with a coverslip to reduce evaporation. Pixel binning was between 1 and 4, and exposure times varied between 10 and 30 min as indicated.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain material, please contact Dr. E. Grill, grill@botanik.biologie.tu-muenchen.de.
Acknowledgments
We thank the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie for their financial support and F. Assaad and U. Schuberth for help in preparing the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft, Fonds der Chemischen Industrie.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.053082.
References
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Breyne P, De Loose M, Dedonder A, Van Montagu M, Depicker A (1993) Quantitative analysis of β-glucuronidase activities using a computer-directed microtiter plate reader. Plant Mol Biol Rep 11: 21–31 [Google Scholar]
- Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang H, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8: 165–171 [DOI] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, et al (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Stevenson B, Lee BH, Zhu JK (2002) Screening for gene regulation mutants by bioluminescence imaging. Sci STKE 140: PL10. [DOI] [PubMed] [Google Scholar]
- de Ruijter NCA, Verhees J, van Leeuwen W, van der Krol AR (2003) Evaluation and comparison of the GUS, LUC and GFP reporter system for gene expression studies in plants. Plant Biol 5: 103–115 [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inze D, Foyer CH, Zhang H (2003) An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. Plant J 33: 543–555 [DOI] [PubMed] [Google Scholar]
- Dewitte W, Murray JA (2003) The plant cell cycle. Annu Rev Plant Biol 54: 235–264 [DOI] [PubMed] [Google Scholar]
- Dixit R, Cyr R (2003) Cell damage and reactive oxygen species production induced by fluorescence microscopy: effect on mitosis and guidelines for non-invasive fluorescence microscopy. Plant J 36: 280–290 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Feldman LJ, Arroyave NJ, Sun PS (1985) Abscisic acid, xanthoxin and violaxanthin in the caps of gravistimulated maize roots. Planta 166: 483–489 [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Abia D, Salinas J, Serrano R, Rodriguez PL (2004) Two new alleles of the abscisic aldehyde oxidase 3 gene reveal its role in abscisic acid biosynthesis in seeds. Plant Physiol 135: 325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Apostolova N, Belles JM, Barrero JM, Piqueras P, Ponce MR, Micol JL, Serrano R, Rodriguez PL (2002) The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 14: 1833–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MJ, Outlaw WH Jr, Mertens R, Weiler EW (1988) Water-stress-induced changes in the abscisic acid content of guard cells and other cells of Vicia faba L. leaves as determined by enzyme-amplified immunoassay. Proc Natl Acad Sci USA 85: 2584–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J (1999) GFP variants for multispectral imaging of living cells. Methods Cell Biol 58: 139–151 [DOI] [PubMed] [Google Scholar]
- Himmelbach A, Hoffmann T, Leube M, Hohener B, Grill E (2002) Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J 21: 3029–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach A, Yang Y, Grill E (2003) Relay and control of abscisic acid signaling. Curr Opin Plant Biol 6: 470–479 [DOI] [PubMed] [Google Scholar]
- Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: response of grafted plants to soil drying. J Exp Bot 53: 1503–1514 [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey SV, Chua NH (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci 115: 4891–4900 [DOI] [PubMed] [Google Scholar]
- Hsu TC, Liu HC, Wang JS, Chen RW, Wang YC, Lin BL (2001) Early genes responsive to abscisic acid during heterophyllous induction in Marsilea quadrifolia. Plant Mol Biol 47: 703–715 [DOI] [PubMed] [Google Scholar]
- Hunter DA, Ferrante A, Vernieri P, Reid MS (2004) Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus “Dutch Master”). Physiol Plant 121: 313–321 [DOI] [PubMed] [Google Scholar]
- Ishitani M, Xiong L, Stevenson B, Zhu JK (1997) Genetic analysis of osmotic and cold stress signal transduction in Arabidopsis: interactions and convergence of abscisic acid-dependent and abscisic acid-independent pathways. Plant Cell 9: 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Jackson MB (1993) Are plant hormones involved in root to shoot communication? Adv Bot Res 19: 103–187 [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karssen CM, Brinkhorst-van der Swan DLC, Breekland AE, Koornneef M (1983) Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157: 158–165 [DOI] [PubMed] [Google Scholar]
- Koiwai H, Nakaminami K, Seo M, Mitsuhashi W, Toyomasu T, Koshiba T (2004) Tissue-specific localization of an abscisic acid biosynthetic enzyme, AAO3, in Arabidopsis. Plant Physiol 134: 1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5: 33–36 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Léon-Kloosterziel KM, Schwartz SH, Zeevaart JAD (1998) The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem 36: 83–89 [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61: 377–383 [Google Scholar]
- Kovaleva LV, Zakharova EV (2003) Hormonal status of the pollen-pistil system at the progamic phase of fertilization after compatible and incompatible pollination in Petunia hybrida L. Sex Plant Reprod 16: 191–196 [Google Scholar]
- Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Leube MP, Grill E, Amrhein N (1998) ABI1 of Arabidopsis is a protein serine/threonine phosphatase highly regulated by the proton and magnesium ion concentration. FEBS Lett 424: 100–104 [DOI] [PubMed] [Google Scholar]
- Luehrsen KR, De Wet JR, Walbot V (1992) Transient expression analysis in plants using firefly luciferase reporter gene. Methods Enzymol 216: 397–414 [DOI] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264: 1452–1455 [DOI] [PubMed] [Google Scholar]
- Millar AJ, Carre IA, Strayer CA, Chua NH, Kay SA (1995) Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267: 1161–1163 [DOI] [PubMed] [Google Scholar]
- Millar AJ, Short SR, Chua NH, Kay SA (1992) A novel circadian phenotype based on firefly luciferase expression in transgenic plants. Plant Cell 4: 1075–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan Ram IIY, Juiswal VS (1972) Induction of male flowers on female plants of Cannabis sativa by gibberellins and its inhibition by abscisic acid. Planta 105: 263–266 [DOI] [PubMed] [Google Scholar]
- Mohr P, Cahill D (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30: 461–469 [DOI] [PubMed] [Google Scholar]
- Müller A, Düchting P, Weiler EW (2002) A multiplex GC-MS/MS technique for the sensitive and quantitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 216: 44–56 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, Abe H, Narusaka M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34: 137–148 [DOI] [PubMed] [Google Scholar]
- Nordin K, Vahala T, Palva ET (1993) Differential expression of two related, low-temperature-induced genes in Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 21: 641–653 [DOI] [PubMed] [Google Scholar]
- Nylander M, Svensson J, Palva ET, Welin BV (2001) Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol Biol 45: 263–279 [DOI] [PubMed] [Google Scholar]
- Ottenschläger I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100: 2987–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Cortés H, Sanchez-Serrano J, Mertens R, Willmitzer L, Prat S (1989) Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci USA 86: 9851–9855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier L, Milon H, Pilet P-E (1977) Gas chromatography-mass spectrometric determinations of abscisic acid levels in the cap and the apex of maize roots. Planta 134: 23–27 [DOI] [PubMed] [Google Scholar]
- Robertson AJ, Ishikawa M, Gusta LV, MacKenzie SL (1994) Abscisic acid-induced heat tolerance in Bromus inermis Leyss cell-suspension cultures. Heat-stable, abscisic acid-responsive polypeptides in combination with sucrose confer enhanced thermostability. Plant Physiol 105: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Hubick KT, Yeung EC, Reid DM (1990) Developmental responses to drought and abscisic acid in sunflower roots. I. Root growth, apical anatomy, and osmotic adjustment. J Exp Bot 41: 325–337 [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52: 627–658 [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Léon-Kloosterziel KM, Koornneef M, Zeevaart JA (1997) Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol 114: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, Nanjo T, Fujita M, Oono Y, Kamiya A, Nakajima M, Enju A, Sakurai T, et al (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31: 279–292 [DOI] [PubMed] [Google Scholar]
- Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, Koornneef M, Kamiya Y, Koshiba T (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Acad Sci USA 97: 12908–12913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE (2002) Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ 25: 211–222 [DOI] [PubMed] [Google Scholar]
- Sharpe J, Ahlgren U, Perry P, Hill B, Ross A, Hecksher-Sorensen J, Baldock R, Davidson D (2002) Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296: 541–545 [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (1996) Molecular responses to drought and cold stress. Curr Opin Biotechnol 7: 161–167 [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3: 217–223 [PubMed] [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ (2004) Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J Exp Bot 55: 2353–2363 [DOI] [PubMed] [Google Scholar]
- Söderman E, Hjellstrom M, Fahleson J, Engstrom P (1999) The HD-Zip gene ATHB6 in Arabidopsis is expressed in developing leaves, roots and carpels and up-regulated by water deficit conditions. Plant Mol Biol 40: 1073–1083 [DOI] [PubMed] [Google Scholar]
- Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty DR (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35: 44–56 [DOI] [PubMed] [Google Scholar]
- Tsugeki R, Fedoroff NV (1999) Genetic ablation of root cap cells in Arabidopsis. Proc Natl Acad Sci USA 96: 12941–12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc Natl Acad Sci USA 97: 11632–11637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J Exp Bot 51: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ (2002) ABA-based chemical signalling: the co-ordination of responses to stress in plants. Plant Cell Environ 25: 195–210 [DOI] [PubMed] [Google Scholar]
- Xiong L, Ishitani M, Lee H, Zhu JK (1999) HOS5-a negative regulator of osmotic stress-induced gene expression in Arabidopsis thaliana. Plant J 19: 569–578 [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart JAD, Creelman RA (1988) Metabolism and physiology of abscisic acid. Annu Rev Plant Physiol Plant Mol Biol 39: 439–473 [Google Scholar]
- Zhang SQ, Outlaw WH Jr, Aghoram K (2001) Relationship between changes in the guard cell abscisic acid content and other stress-related physiological parameters in intact plants. J Exp Bot 52: 301–308 [PubMed] [Google Scholar]
- Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]