Abstract
Background and Objectives:
The safety and utility of endoscopic ultrasound (EUS) for the evaluation and management of gastrointestinal (GI) tract disorders among adults has been established. The literature on safety and efficacy in a pediatric referral population (under 21 years of age) is limited. We hypothesized that EUS is safe and useful in the pediatric population. We reviewed the pediatric EUS experience at a single tertiary-care system. We describe the indications, findings, safety, technical success rate, and impact on clinical outcomes.
Patients and Methods:
All patients 21 years of age or younger referred for EUS between 5, 2007 and 11, 2012 were identified from our electronic medical record databases. Retrospective chart review was then conducted to document demographics, procedure indications, procedure type (diagnostic or therapeutic), type of anesthesia used, EUS findings, and the clinical impact of EUS on the subsequent management of the patients.
Results:
Seventy EUS procedures were attempted in 58 patients during the study. Of these, two EUS procedures were aborted due to inadequate moderate sedation and 68 were successfully completed. The median age at initial endoscopy was 18 years (range 6–21 years), 50% were male and 65% were Caucasian. Four patients underwent EUS-guided pseudocyst drainage. Among the remaining 54 patients, the indications for EUS were the evaluation of GI mucosal/submucosal lesions (n = 14), acute or recurrent pancreatitis (n = 10), localization of suspected insulinoma (n = 8), evaluation of pancreatic abnormalities seen on prior imaging (n = 6), surveillance of tumors or evaluation of luminal lesions in hereditary syndromes (n = 6), abdominal pain of suspected pancreatobiliary origin (n = 5), and other rare indications (n = 5). Fine-needle aspiration was performed in 13 (9 diagnostic, 4 therapeutic) and trans-gastric fine-needle contrast injection of the pancreatic duct was performed in one patient without any complications. Sedation (data available for 66 procedures) included general endotracheal anesthesia in 38 (57%), monitored anesthesia care (MAC) in 19 (29%), and moderate sedation in 9 (14%). There were 4 minor intra-procedural anesthesia-related complications (laryngospasm in 2 and hypoxemia from airway obstruction and secretions in 2) in MAC and general endotracheal anesthesia (GA) cases, and 1 postprocedural complication (fever after pseudocyst drainage). EUS can achieve the diagnostic or therapeutic goal and ruled out suspected pathology in 88% of cases precluding need for additional testing.
Conclusions:
(1) EUS in the pediatric population is technically successful and efficacious. (2) Therapeutic and diagnostic EUS impacted clinical care decisions. (3) There is a low risk of immediate significant complications. (4) The overall efficacy and safety support the performance of EUS in a pediatric population by experienced endoscopists.
Keywords: Clinical impact, endoscopic ultrasound, pancreas, pediatric
INTRODUCTION
Endoscopic ultrasound (EUS) is an established diagnostic and therapeutic modality with a well-defined safety profile in the adult population. It offers better diagnostic and therapeutic capability not offered by conventional trans-abdominal ultrasound. While this modality has been used among pediatric populations, the literature on safety, efficacy, and ability to make a clinical impact in this population is limited to only a few studies.[1,2,3,4,5] This is partly explained by the lower incidence of luminal and pancreaticobiliary malignancies among the pediatric population and also by a paucity of experienced endosonographers interacting with a pediatric patient population. This study was designed to analyze and report pediatric EUS experience at a single tertiary care system. We describe the indications, findings, safety, technical success rate, and its impact on clinical outcomes.
PATIENTS AND METHODS
The Institutional Review Boards at the University of Pennsylvania (HUP) and The Children's Hospital of Pennsylvania (CHOP) approved our study. All patients 21 years of age or younger referred for EUS between 5, 2007 and 11, 2012 were identified from our endoscopic electronic database. Most of these patients were referred to us from the CHOP. Retrospective chart review was conducted to document demographics, procedure indications, procedure type (diagnostic or therapeutic), type of anesthesia used, EUS findings, procedure-related complications, and impact of EUS findings on the patient's subsequent management. Procedural-related complication was defined as onset of hypotension, hypoxia, cardiac arrhythmia, bleeding or any unexpected event during or after the procedure. EUS was considered to have had meaningful clinical impact when the diagnostic or therapeutic goal was achieved or suspected pathology was ruled out while precluding the need for additional testing.
Equipment and procedure
All patients were admitted to CHOP before the procedures and were evaluated by pediatric and adult gastroenterology teams for safety and appropriateness of EUS. Written informed consent was then obtained from patients (for those older than 18 years) or parents or guardians (for patients <18-year-old). The procedures were performed under moderate sedation, monitored anesthesia care (MAC), or general endotracheal anesthesia (GA), using combinations of intravenous midazolam, fentanyl, or propofol with appropriate cardiorespiratory monitoring. Moderate sedation was performed under supervision of the endosonographer and MAC and general anesthesia (GA) were performed under the supervision of an anesthesiologist. The choice of moderate or deep sedation was made at the discretion of the endosonographers and anesthesiologists.
All EUS procedures were performed by one of 4 experienced endosonographers. Depending on the reason for examinations, the procedure was either started with a regular forward viewing endoscope (GIF-160, Olympus America, Inc.) or radial echoendoscope (GF-UC140P-AL5, GF-UM160, Olympus America, Inc.). When indicated, EUS with fine-needle aspiration (FNA) was performed using a linear echoendoscope (GF-UCT140, Olympus America, Inc.) with a 19 or 22-gauge EUSN-1, EUSN-2, EUSN-3, or Echotip Ultra Needle (Cook Medical Inc., Winston-Salem, NC, USA). Cytopathology service was on-site to assess for adequacy of sampling and for preliminary diagnosis.
RESULTS
Between 5, 2007 and 11, 2012, a total of seventy EUS procedures were attempted in 58 patients. At the time of last endoscopy, the median age of patients was 18 years (range 6–21 years), 50% were males, and 65% were Caucasian. Fifty-three (91%) patients were older than 10 years and five patients were 10 years or younger. Two of the seventy procedures, attempted with moderate sedation, had to be aborted due to inadequate sedation. Nine of the 58 patients underwent more than one EUS examination (ten total EUS in four patients for surveillance of known gastrointestinal (GI) tract tumors or history of familial cancer syndromes, five total EUS in two patients with pancreatic pseudocysts, four total EUS (two attempted and two completed) in two patients for prior incomplete procedures, and two separate procedures in one patient with a pancreatic cyst).
Four of the 58 patients underwent EUS guided pseudocyst drainage. Among the remaining 54 patients, the indications for EUS [Table 1] were evaluation of GI mucosal/submucosal lesions (n = 14), acute or recurrent pancreatitis (n = 10), localization of suspected insulinoma (n = 8), evaluation of pancreatic abnormalities seen on prior imaging (n = 6), screening for tumors in hereditary syndromes (n = 6), abdominal pain of suspected pancreatobiliary origin (n = 5), and other rare indications (n = 5). Of the six patients with hereditary syndromes who underwent EUS; one 20-year-old patient with von Hippel–Lindau syndrome underwent surveillance EUS for pancreatic lesions. Five other patients (three with FAP and two with Peutz–Jegher's syndrome) underwent evaluation of gastric and duodenal polyps.
Table 1.
Success and the indication for endoscopic ultrasound in 58 pediatric patients
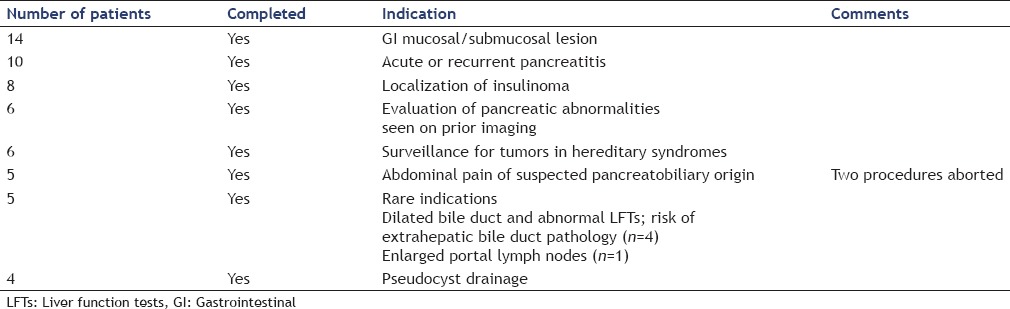
Among the patients <10 years of age, indications for EUS included evaluation for insulinoma (n = 2), pseudocyst drainage (n = 1), recurrent acute pancreatitis (n = 1), and evaluation of gastric polyps (n = 1). Although GI cancers are rare in the pediatric population, we staged and proved by cytology a pancreatic adenocarcinoma in an 18-year-old male and staged a 16-year-old male with biopsy proven adenocarcinoma of the esophagus.
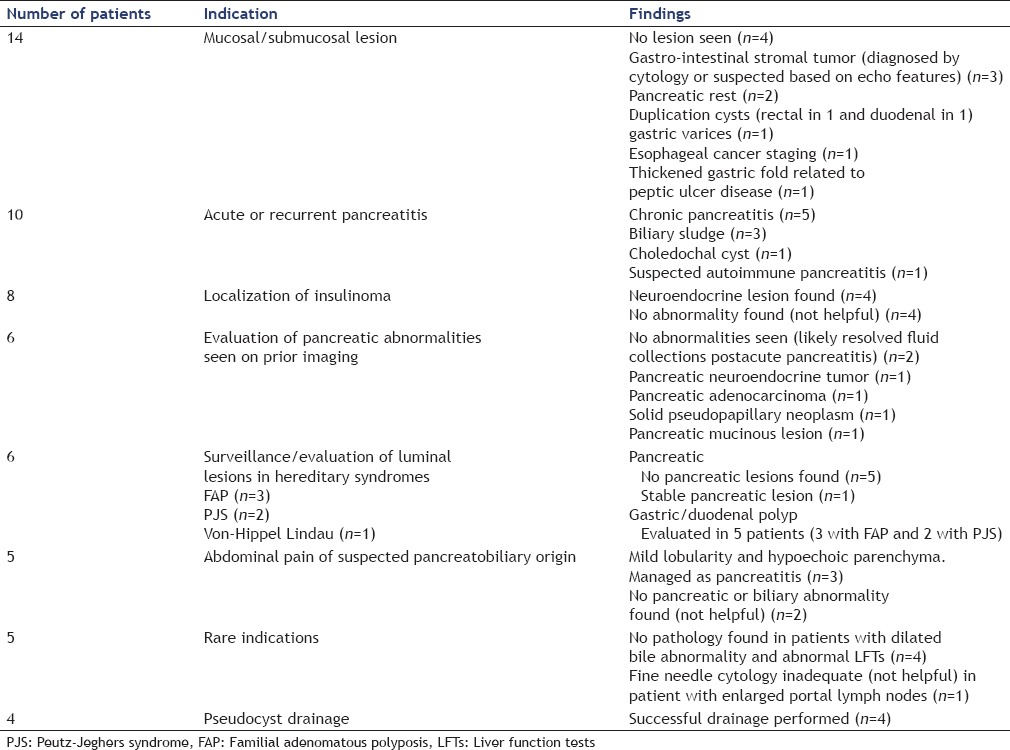
The findings of our study are listed in Table 2. Meaningful impact on clinical care was achieved in 51/58 (88%) cases. In seven patients, EUS did not achieve either a definitive diagnostic or therapeutic goal. In four of these patients, EUS was done for suspected insulinoma and did not reveal any mass. Two of these patients subsequently underwent surgical intervention and were found to have insulinomas. The two other patients continue to have symptomatic hypoglycemia and were undergoing further evaluation. In two patients with chronic abdominal pain, no explanatory abnormality was found. In one patient with abnormal abdominal lymphadenopathy, EUS with FNA was performed which was nondiagnostic; however, follow-up computed tomography scan demonstrated resolution of the lymphadenopathy.
Table 2.
Findings in 58 patients undergoing endoscopic ultrasound in 58 pediatric patients
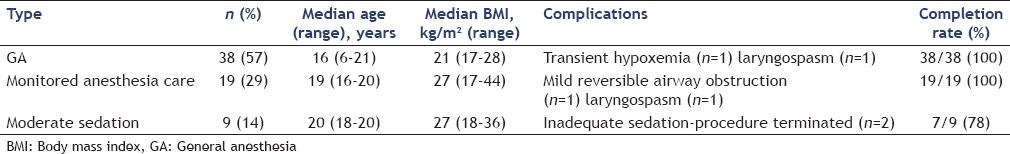
Sedation (complete data only available for 66 of 70 procedures) included GA in 38 (57%), monitored anesthesia case in 19 (29%), and moderate sedation in 9 (14%) [Table 3].
Table 3.
Mode of sedation, complications and completion rate in 66 endoscopic ultrasound cases performed on 58 pediatric patients
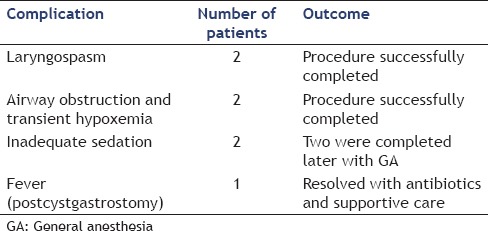
A few complications were encountered in the study population [Table 4]. There were two intra-procedural anesthesia-related complications (laryngospasm and hypoxemia from airway obstruction and secretions) in the patients who underwent GA. There were two intra-procedural anesthesia-related complications (laryngospasm and mild airway obstruction, relieved by nasal-trumpet) in the patients who underwent MAC. All four were managed successfully during the procedure and did not result in cancellation of the procedure or any postprocedure complications. Two of the nine procedures performed under moderate sedation had to be aborted due to inadequate sedation. Two of these procedures were repeated and completed on a subsequent day with GA. There was only one postprocedural complication (fever after pseudocyst drainage) in the cohort. This patient was managed with intravenous antibiotics with quick resolution of symptoms.
Table 4.
Complications during and after endoscopic ultrasound in 58 pediatric patients
FNA was performed during 13 of the 68 completed procedures. Nine of these were performed for diagnostic reasons and in four the FNA was performed as part of the pseudocyst drainage. In all patients undergoing diagnostic FNA, a 22-gauge FNA needle was used with a median of two passes (range 2–4) per procedure. In the four patients undergoing pseudocyst drainage, a 19-gauge needle was used with one pass made during each procedure. In one patient with a main pancreatic duct (PD) stricture, a trans-gastric fine-needle injection of the PD was performed using a 19-gauge needle without any complications.
DISCUSSION
The role of EUS has been firmly established in the adult population. However, data on its safety and ability to impact clinical care among pediatric patients is limited to a few case series [Table 3].[1,2,3,4,5] Most of these case series are based on a small cohort of patients, but they all have uniformly suggested that EUS is safe and makes a meaningful impact on the care of pediatric patients.[1,2,5] Here, we present our experience with pediatric EUS, and our study represents one of the largest case series addressing the feasibility and the safety of EUS in pediatric population. We demonstrated that EUS in pediatric population is safe and makes a meaningful impact on the care of patients. It, however, is important to note that our practice represents patients seen in a referral tertiary care setting, and all reported pediatric EUS were performed by experienced adult endosonographers. Our findings, therefore, may not be applicable to EUS in a community setting or with performance of EUS by endosonographers with limited experience. The accumulating feasibility and safety data does make a case for providing dedicated EUS training to selective pediatric gastroenterologists.
Similar to previous studies, evaluation of the pancreaticobiliary system and mucosal and submucosal lesions of the GI tract are the majority of our patients.[1,3,4,5,6] Some noticeable differences were that 14% (8/58) of our cases were for suspected insulinoma; six patients had hereditary cancer syndromes and were undergoing EUS for surveillance or for the evaluation of gastro-duodenal polyps. Although these differences are reflective of practice patterns and differences in the tertiary referral patterns, our study provides safety data on additional indications in the pediatric population.
Another important difference from prior studies is the age limit for inclusion. We chose to include patients aged 21 years or younger as most of these were referred to us from the CHOP. The American Academy of Pediatrics in its 1988 policy statement, “Age Limits of Pediatrics,” has suggested that the responsibility of pediatrics begins with the fetus and continue through 21 years. Many pediatric practices accept patients up to the age of 21 years.
The ASGE Technology Committee status evaluation report on equipment for pediatric endoscopy provides some guidance on feasibility of EUS in pediatric patients depending on their size: “Standard adult radial echoendoscopes have a tip diameter ranging from 12.7 to 14.2 mm; linear FNA echoendoscopes are slightly larger, measuring 12.1 to 14.6 mm in tip diameter. Use of the larger echoendoscopes should be limited to pediatric patients weighing at least 15 kg, and caution should be used, given their relatively rigid distal tip.” In patients weighing <15 kg it has been suggested that “through-the scope miniprobes with frequencies ranging from 12 to 30 MHz may be used through standard gastroscopes with a 2.8-mm working channel.”[7]
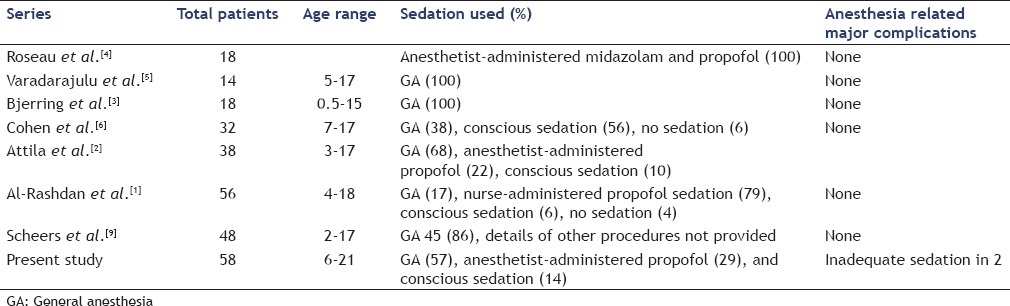
A multitude of factors have to be taken into consideration when deciding on the type of anesthesia to be used for endosonography. These include age, weight, other medical conditions, patient's condition, American Society of Anesthesiologists grade, patient and parent preference, anticipated cooperation of patient, preference of treating physicians, and the degree of complexity of the procedure.[8] The majority of EUS in our study were performed with GA (57%), which is similar to some of the previous studies [Table 5].[2,5,6] A notable difference, however, is that 29% of our patients (all more than 10 years-old) were successfully sedated with anesthesia administered propofol. In the initial studies on EUS in pediatric patients; significantly greater airflow resistance and increased episodes of dynamic and static airway occlusion among children were suggested as reasons for almost exclusively using inhalational GA among younger children.[3,5,10] However, Al-Rashdan et al. in a recent series of 56 patients demonstrated that 73% of cases (all more than 10 years of age) could be safely sedated with nurse-administered propofol.[1] While GA seems to be a modality suitable for younger children undergoing complex EUS procedures and for children undergoing lengthy procedures, using MAC selectively in otherwise healthy older children appears to be a safe alternative.
Table 5.
Published series regarding sedation given during endoscopic ultrasound in children
In this study, incorporation of EUS into the management algorithm had a significant impact on the clinical management in 88% of cases. These findings are similar to previous reports. Varadarajulu et al. found that EUS in evaluation of pancreaticobiliary disorders reported a significant impact on patient management in 13 of their 14 patients (93%).[5] Similarly Al-Rashdan et al. reported a new diagnosis made in 44 of their 56 patients (86%).[1] These data suggest that EUS when performed by expert endosonographers is safe, feasible, and makes a significant clinical impact among the pediatric patients.
Our study has certain limitations. The median age of children in our study was 18 years and most of our children were over 10 years of age. Our findings cannot be generalized to infants and very young children. Furthermore, this study was retrospective with limited long-term follow-up, although the follow-up was clinically meaningful.
CONCLUSIONS
We present one of the largest reported data on safety and utility of EUS in pediatric patients. Diagnostic and therapeutic capability of EUS has an established role in patient management in a less invasive fashion. In addition, accruing data from our and other studies confirms the safety profile of EUS among pediatric patients. Increase in EUS utilization by pediatric gastroenterologists is expected and offering dedicated EUS training to some select pediatric gastroenterologists might be indicated. Although there remains a need for the development of dedicated diagnostic and therapeutic probes in smaller children (<15 kg), studies are needed to assess the demand and potential cost-effectiveness of such endeavors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Al-Rashdan A, LeBlanc J, Sherman S, et al. Role of endoscopic ultrasound for evaluating gastrointestinal tract disorders in pediatrics: A tertiary care center experience. J Pediatr Gastroenterol Nutr. 2010;51:718–22. doi: 10.1097/MPG.0b013e3181dac094. [DOI] [PubMed] [Google Scholar]
- 2.Attila T, Adler DG, Hilden K, et al. EUS in pediatric patients. Gastrointest Endosc. 2009;70:892–8. doi: 10.1016/j.gie.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Bjerring OS, Durup J, Qvist N, et al. Impact of upper gastrointestinal endoscopic ultrasound in children. J Pediatr Gastroenterol Nutr. 2008;47:110–3. doi: 10.1097/MPG.0b013e31816c74af. [DOI] [PubMed] [Google Scholar]
- 4.Roseau G, Palazzo L, Dumontier I, et al. Endoscopic ultrasonography in the evaluation of pediatric digestive diseases: Preliminary results. Endoscopy. 1998;30:477–81. doi: 10.1055/s-2007-1001311. [DOI] [PubMed] [Google Scholar]
- 5.Varadarajulu S, Wilcox CM, Eloubeidi MA. Impact of EUS in the evaluation of pancreaticobiliary disorders in children. Gastrointest Endosc. 2005;62:239–44. doi: 10.1016/s0016-5107(05)00312-3. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Kalinin M, Yaron A, et al. Endoscopic ultrasonography in pediatric patients with gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2008;46:551–4. doi: 10.1097/MPG.0b013e31815ce571. [DOI] [PubMed] [Google Scholar]
- 7.Status Evaluation Report. Equipment for pediatric endoscopy. [Last accesed on 09 2016];Gastrointest Endosc. 2012 76:8–17. doi: 10.1016/j.gie.2012.02.023. Available from: http://www.asge.org/assets/0/71312/71314/c1dfe0de-4d9d-45f7-ac8b-be4e20f98d7b.pdf . [DOI] [PubMed] [Google Scholar]
- 8.ASGE Standards of Practice Guideline. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. 2008;67:1–9. doi: 10.1016/j.gie.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Scheers I, Ergun M, Aouattah T, et al. Diagnostic and therapeutic roles of endoscopic ultrasound in pediatric pancreaticobiliary disorders. J Pediatr Gastroenterol Nutr. 2015;61:238–47. doi: 10.1097/MPG.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 10.Krauss B, Brustowicz RM. Pediatric Procedural Sedation and Analgesia. Ch. 16. Philadelphia: Lippincott, Williams and Wilkins; 1999. Establishing procedural sedation guidelines and policies. [Google Scholar]


