A 58-year-old female with a gastric bulging in the greater curvature/posterior wall [Figure 1] was referred for endoscopic ultrasound (EUS) evaluation. Sectorial EUS (Olympus GF-UCT140-AL5, Olympus America Inc., New York, USA, coupled to an ultrasound unit Aloka Prosound alpha-5SX) detected a well-circumscribed, hypoechoic, and heterogeneous mass in the pancreatic body, with solid and cystic areas, measuring 5 cm × 5 cm [Figure 2]. Main pancreatic duct was not dilated. EUS-guided fine needle aspiration (EUS-FNA) was performed via a transgastric approach using a 19-gauge needle (EchoTip Ultra Echo-19, Cook Medical, Winston-Salem, USA) for three passes. A sample of 12 mL of dark-brown liquid was aspirated, as well as tissue cores. There was no on-site cytopathologist. Aspirate was acellular with amylase of 74 U/mL and carcinoembryonic antigen of 5.9 ng/mL. Tissue cores were processed as cell blocks for histologic examination and immunohistochemistry. Histopathology demonstrated a hypercellular tumor with branching papillary arrangements composed of fibrovascular cores and microadenoid structures with cuboidal cells [Figure 3a]. Nuclei were round or oval with granular chromatin and small nucleoli. Cytoplasm was granular or vacuolated. No mitotic activity was observed. Tumor cells were negative for periodic-acid Schiff alcian blue. Immunohistochemical stains were positive for beta-catenin (clone B-catenin 1) [Figure 3b] and CD10 (clone 56C6) [Figure 3c], whereas chromogranin (clone LK2H10) [Figure 3d] was negative. These findings confirmed a solid pseudopapillary tumor (SPT) of the pancreas. Tumor was successfully removed, and the patient remains very well so far.
Figure 1.
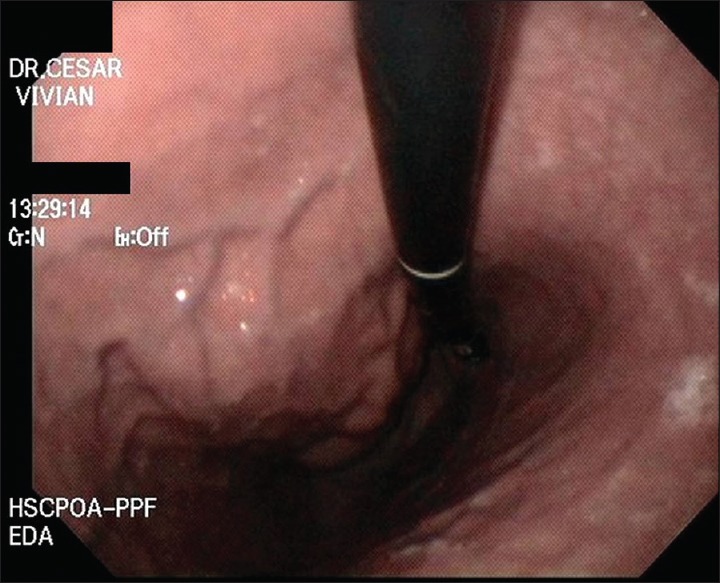
Retroflexed view of upper digestive endoscopy revealing a gastric bulging in the greater curvature/posterior wall of the gastric body
Figure 2.
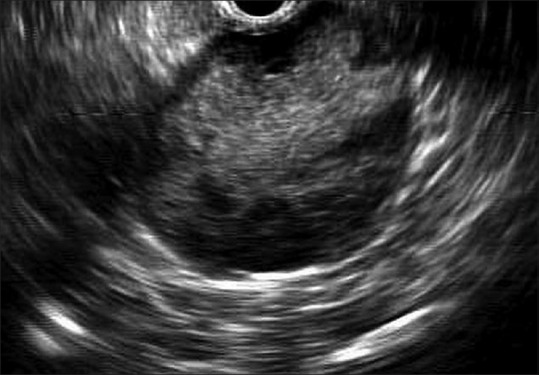
Sectorial endosonography revealing a solid-cystic lesion, with well-defined borders, hypoechoic and heterogeneous pattern, measuring 5 cm × 5 cm, in the pancreatic body
Figure 3.
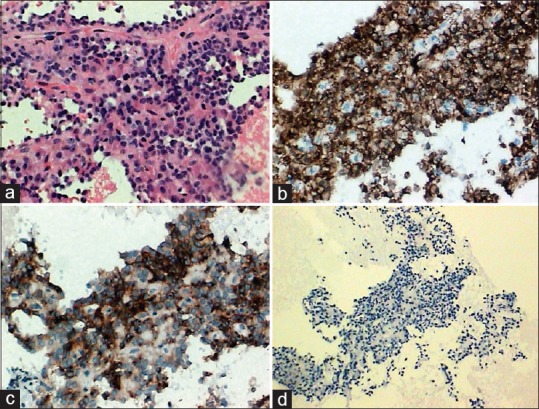
Histopathological findings on cell blocks. Pseudopapillae with prominent myxoid stroma and thin fibrovascular structures covered by cuboidal cells with no cellular atypia (H and E, ×200) (a). Immunohistochemically, tumor cells were positive for beta-catenin (nuclear and cytoplasmic pattern) (×200) (b), CD10 (×200) (c), and negative for chromogranin A (×100) (d)
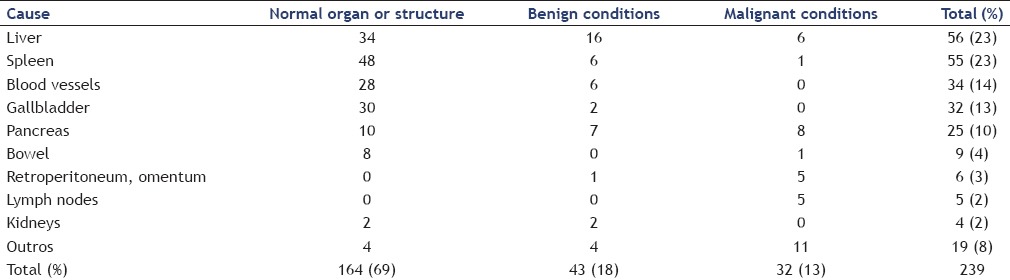
SPT constitutes 1%–2% of all exocrine tumors and 5% of cystic tumors of the pancreas.[1] In the English literature, less than 2800 cases have been reported.[2] It occurs in young women in the third decade of life with a frequency of up to 90%.[3,4] Besides, (pre) malignant pancreatic diseases accounted for 3% of the causes for gastrointestinal bulging [Table 1].[5] Symptoms may include abdominal pain and even the presence of a palpable mass. Tumors are usually large and approximately 70% arise in the body and tail of the pancreas.[2,4] Characteristic imaging findings include an encapsulated lesion with well-defined borders and variable central areas with cystic degeneration, necrosis, or hemorrhage.[2,4] Differential diagnosis includes a large number of lesions, such as neuroendocrine tumors, acinar cell carcinoma, intraductal papillary mucinous neoplasia, serous cystadenoma, pancreatoblastoma, mucinous cystadenoma, and pseudocysts. Local surgical excision is the treatment for SPT, even for those with local infiltration or distant metastasis.[2,4] Nadler et al.[6] first made a correct diagnosis of SPT using EUS-FNA in 2002. The diagnostic accuracy of EUS-FNA for SPTs ranges from 69.5% to 82.4%.[2,7,8] FNA samples, especially when evaluating cell blocks, demonstrate marked cellularity with pseudopapillae composed of fibrovascular stalks lined with one to several layers of neoplastic cells mixed with discohesive neoplastic cells.[1] Beta-catenin, CD10, and chromogranin are the most sensitive markers for the diagnosis of SPTs.[9] In conclusion, EUS-FNA provides an accurate preoperative diagnosis, differentiating SPT from other pancreatic neoplasms of similar radiologic appearance but with different biologic behavior and treatment.
Table 1.
Causes of extraluminal compression of the wall of the upper gastrointestinal tract
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understand that her name and initial will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: Histopathology. JOP. 2006;7:131–6. [PubMed] [Google Scholar]
- 2.Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: Are these rare lesions? Pancreas. 2014;43:331–7. doi: 10.1097/MPA.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: A review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–78. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 4.Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: Review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965–72. doi: 10.1016/j.jamcollsurg.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54, viii. doi: 10.1016/j.giec.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Nadler EP, Novikov A, Landzberg BR, et al. The use of endoscopic ultrasound in the diagnosis of solid pseudopapillary tumors of the pancreas in children. J Pediatr Surg. 2002;37:1370–3. doi: 10.1053/jpsu.2002.35028. [DOI] [PubMed] [Google Scholar]
- 7.Law JK, Stoita A, Wever W, et al. Endoscopic ultrasound-guided fine needle aspiration improves the pre-operative diagnostic yield of solid-pseudopapillary neoplasm of the pancreas: An international multicenter case series (with video) Surg Endosc. 2014;28:2592–8. doi: 10.1007/s00464-014-3508-8. [DOI] [PubMed] [Google Scholar]
- 8.Jani N, Dewitt J, Eloubeidi M, et al. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: A multicenter experience. Endoscopy. 2008;40:200–3. doi: 10.1055/s-2007-995364. [DOI] [PubMed] [Google Scholar]
- 9.Jahangir S, Loya A, Siddiqui MT, et al. Accuracy of diagnosis of solid pseudopapillary tumor of the pancreas on fine needle aspiration: A multi-institution experience of ten cases. Cytojournal. 2015;12:29. doi: 10.4103/1742-6413.171140. [DOI] [PMC free article] [PubMed] [Google Scholar]


