Abstract
Root apical aluminum (Al) exclusion via Al-activated root citrate exudation is widely accepted as the main Al-resistance mechanism operating in maize (Zea mays) roots. Nonetheless, the correlation between Al resistance and this Al-exclusion mechanism has not been tested beyond a very small number of Al-resistant and Al-sensitive maize lines. In this study, we conducted a comparative study of the physiology of Al resistance using six different maize genotypes that capture the range of maize Al resistance and differ significantly in their genetic background (three Brazilian and three North American genotypes). In these maize lines, we were able to establish a clear correlation between root tip Al exclusion (based on root Al content) and Al resistance. Both Al-resistant genotypes and three of the four Al-sensitive lines exhibited a significant Al-activated citrate exudation, with no evidence for Al activation of root malate or phosphate release. There was a lack of correlation between differential Al resistance and root citrate exudation for the six maize genotypes; in fact, one of the Al-sensitive lines, Mo17, had the largest Al-activated citrate exudation of all of the maize lines. Our results indicate that although root organic acid release may play a role in maize Al resistance, it is clearly not the only or the main resistance mechanism operating in these maize roots. A number of other potential Al-resistance mechanisms were investigated, including release of other Al-chelating ligands, Al-induced alkalinization of rhizosphere pH, changes in internal levels of Al-chelating compounds in the root, and Al translocation to the shoot. However, we were unsuccessful in identifying additional Al-resistance mechanisms in maize. It is likely that a purely physiological approach may not be sufficient to identify these novel Al-resistance mechanisms in maize and this will require an interdisciplinary approach integrating genetic, molecular, and physiological investigations.
Aluminum (Al) toxicity in acid soils negatively impacts the production of staple food crops, particularly grain crops. Given that approximately 30% of the world's total land area consists of acid soils, and as much as 50% of the world's potentially arable lands are acidic, Al toxicity represents one of the most important limitations to agricultural production worldwide (Von Uexkull and Mutert, 1995). As the soil pH drops below 5, toxic forms of Al become soluble into the soil solution, interfering with a wide range of physical and cellular processes, resulting in the inhibition of root growth and function and thus reducing crop yields. The mechanisms underlying Al toxicity have been extensively addressed in the literature. Fortuitously, crop plants have evolved resistance mechanisms that enable them to tolerate toxic levels of Al in acid soils (for recent reviews on Al resistance and Al toxicity, see Kochian, 1995; Matsumoto, 2000; Matsumoto et al., 2001; Barcelo and Poschenrieder, 2002; Kochian et al., 2004, 2005). Resistance to Al can be achieved via exclusion of Al from the root apex and/or via intracellular tolerance by sequestration of Al in the plant's symplasm (i.e. “true tolerance”). Although recent evidence for an Al-resistance mechanism involving internal detoxification and sequestration is starting to emerge, the most compelling evidence has focused on a resistance mechanism based on chelation and exclusion of extracellular Al via Al-activated root organic acid release (for review, see Ma, 2000; Ma et al., 2001; Ryan et al., 2001; Kochian et al., 2004, 2005). An early study in snapbean (Phaseolus vulgaris; Miyasaka et al., 1991), followed by a more extensive characterization of the same phenomena in wheat (Triticum aestivum; Delhaize et al., 1993a, 1993b), showed that Al-tolerant genotypes exhibit a strong, Al-activated exudation of Al-chelating organic acids (citrate in snapbean and malate in wheat), which is absent or much smaller in the Al-sensitive genotypes. Subsequent studies showed a close correlation between the degree of Al resistance and the magnitude of Al-activated root malate release in 36 different wheat genotypes differing in Al resistance, in addition to that shown for the Al tolerant near isogenic lines of wheat characterized originally (Ryan et al., 1995a, 1995b).
Although Al-tolerant genotypes from many plant species seem to share this general resistance mechanism, it is the identity of the organic acid released that varies in different plant species (see table I in Kochian et al., 2004). High levels of Al-activated citrate, oxalate, and malate release have been correlated with differential Al resistance in a large number of both monocot and dicot plant species. Al-activated malate exudation has been the most extensively characterized Al-exclusion mechanism, mainly due to the large number of studies published on wheat Al resistance. However, Al-tolerant genotypes for a number of other plant species (including maize [Zea mays]) exhibit an Al-activated root citrate exudation. Given its physicochemical properties, citrate3− (a tricarboxylic acid anion) chelates Al3+ more effectively than does the dicarboxylic malate2− anion, making it more effective at detoxifying Al. Consequently, several studies have characterized the Al-induced citrate exudation response in maize as a potential Al-exclusion mechanism (Pellet et al., 1995; Jorge and Arruda, 1997; Ishikawa et al., 2000; Kidd et al., 2001; Kollmeier et al., 2001; Piñeros et al., 2002; Mariano and Keltjens, 2003). However, it should be pointed out that the acceptance that Al resistance in maize is conferred by Al-activated citrate exudation is based, in part, on assumptions and/or extrapolations from the extensive body of work that has been carried out on wheat Al resistance. In each study on maize, the correlation between Al resistance and Al-activated citrate exudation has only been studied with a single Al-resistant genotype in comparison with one or two Al-sensitive lines. In general, other potential Al-resistance mechanisms in maize have been disregarded, and given the genetic complexity of maize Al resistance (Magnavaca et al., 1987; Ninamango et al., 2003; Kochian et al., 2005), are quite likely to exist. Since some recent findings on the physiology of Al resistance has suggested that alternative or additional mechanisms of Al resistance other than organic acid exudation may be operating in maize (Kidd et al., 2001; Piñeros et al., 2002), there is a need to investigate more rigorously the correlation between the degree of Al resistance and the magnitude of Al-activated root citrate release in maize.
Table I.
Root citrate exudation rates (pmol root−2 h−1) of six maize genotypes exposed to different Al3+ activities
Exudation collections were performed at two different 24-h period intervals (Day 1–2 and Day 3–4). Genotypes have been sorted from left to right in order of decreasing Al resistance. The values are the mean of six replicates ± the SEM.
| Time Interval
|
Al3+ Activity (μm)
|
Decreasing Al Resistance
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cateto-Colombia | → | Pioneer 3355 | → | Mo17 | → | B73 | → | L53 | → | 11×726 | ||
| Day 1–2 | 0 | 0 ± 0 | 18 ± 10 | 91 ± 22 | 0 ± 0 | 0 ± 0 | 11 ± 11 | |||||
| 5 | 33 ± 4 | 67 ± 40 | 173 ± 116 | 5 ± 5 | 0 ± 0 | 48 ± 26 | ||||||
| 10 | 41 ± 1 | 105 ± 23 | 675 ± 183 | 42 ± 12 | 28 ± 19 | 53 ± 15 | ||||||
| 20 | 203 ± 57 | 137 ± 44 | 511 ± 225 | 61 ± 40 | 301 ± 41 | 139 ± 9 | ||||||
| 40 | 368 ± 95 | 221 ± 82 | 829 ± 332 | 17 ± 10 | 309 ± 15 | 297 ± 99 | ||||||
| 80 | 324 ± 70 | 769 ± 168 | 911 ± 172 | 44 ±15 | 249 ± 59 | 284 ± 67 | ||||||
| Day 3–4 | 0 | 0 ± 0 | 9 ± 9 | 194 ± 88 | 8 ± 5 | 0 ± 0 | 4 ± 4 | |||||
| 5 | 33 ± 19 | 74 ± 63 | 38 ± 14 | 206 ± 70 | 12 ± 12 | 203 ± 55 | ||||||
| 10 | 120 ± 42 | 215 ± 74 | 523 ± 139 | 204 ± 78 | 22 ± 14 | 193 ± 17 | ||||||
| 20 | 236 ± 71 | 590 ± 114 | 298 ± 97 | 153 ± 24 | 107 ± 51 | 138 ± 16 | ||||||
| 40 | 441 ± 98 | 613 ± 153 | 572 ± 122 | 181 ± 65 | 449 ± 53 | 317 ± 44 | ||||||
| 80 | 484 ± 131 | 883 ± 446 | 1,481 ± 399 | 44 ± 17 | 671 ± 209 | 539 ± 183 | ||||||
Although Al-activated release of organic acids as an Al-resistance mechanism has gained wide acceptance for plants, alternative Al-resistance mechanisms have also been mentioned and have begun to receive attention in the literature. Examples of such mechanisms include internal detoxification of Al (Ma et al., 1997; Ma and Hiradate, 2000), root mediated changes in rhizosphere pH (Degenhardt et al., 1998), and root exudation of phenolic compounds (Kidd et al., 2001). Recent studies on the extremely Al-resistant forage grass, Bracchiaria decumbens, have suggested the existence of novel and undetermined mechanisms of Al resistance (Wenzl et al., 2001, 2002). Although this species is considerably more Al resistant than any of the most resistant genotypes of wheat, maize, rye (Secale cereale), and triticale reported in the literature, it does not seem to share any of the Al-resistance mechanisms described for these species. For example, although some degree of Al exclusion could be operating in Bracchiaria roots, no evidence for Al activation of root organic acid exudation or root-mediated alteration in rhizosphere pH was found. Likewise, changes in internal organic acid concentrations (i.e. internal detoxification and sequestration of Al) could not account for the much higher level of Al resistance in Bracchiaria.
The main objective of this study was to carry out a detailed comparative study of the physiology of maize Al resistance using six different maize genotypes that capture the range of maize Al resistance and differ significantly in their genetic background: three Brazilian genotypes and three North American genotypes. This comparison should help to confirm the validity of the role of root organic acid exudation in maize Al resistance, and should also indicate whether further research is needed to explore the existence of alternative Al-resistance mechanisms in maize.
RESULTS
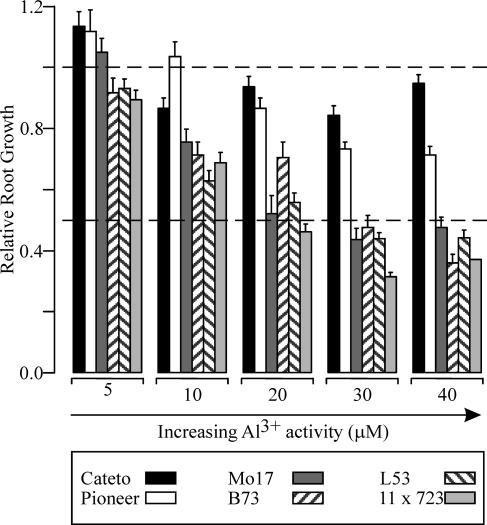
The degree of Al resistance of all six maize genotypes was correlated with physiological parameters associated with mechanisms that facilitate Al exclusion from the root apex and/or by mechanisms that confer the ability to tolerate Al in the plant symplasm (Al tolerance). Given that relative root growth (RRG) measurements have proved to be suitable phenotypic criteria to assess Al resistance in maize roots grown in full nutrient solution (Magnavaca et al., 1987; Cançado et al., 1999), we used this parameter to rank the degree of Al resistance among the six maize lines (Fig. 1). As expected, as the Al3+ activity in the growth solution was increased from 5 to 40 μm, an increasing degree of root growth inhibition in all six genotypes was observed. Although differential Al resistance was seen even at low Al3+ activities, higher Al levels (>20 μm Al3+) were used for most of the subsequent studies as these resulted in the greatest differences in Al resistance between the maize lines. For example, exposure of the most Al-tolerant genotype, Cateto-Colombia, to solutions containing Al3+ activities as high as 40 μm caused no more than a 20% inhibition of root growth (relative to the −Al-grown control), while inhibiting root growth by 45% to 70% in the other lines. Based on the root growth measurements we ranked the Al resistance of the genotypes into three main categories: highly Al tolerant (Cateto-Colombia), moderately Al tolerant (Pioneer 3355), and Al sensitive (Mo17, B73, L53, and 11 × 723). It is interesting to note that the genotype Cateto-Colombia was extremely Al tolerant, not only compared to the other five genotypes, but also to other maize genotypes and crop species reported in the literature (for comparisons, see Wenzl et al., 2001).
Figure 1.
Relative Al resistance of six different maize genotypes based on Al inhibition of root growth in response to increasing Al3+ activities in the growth solution. The RRG was calculated over a 3-d period as described in “Materials and Methods.” Vertical bars indicate the se of the mean (SEM).
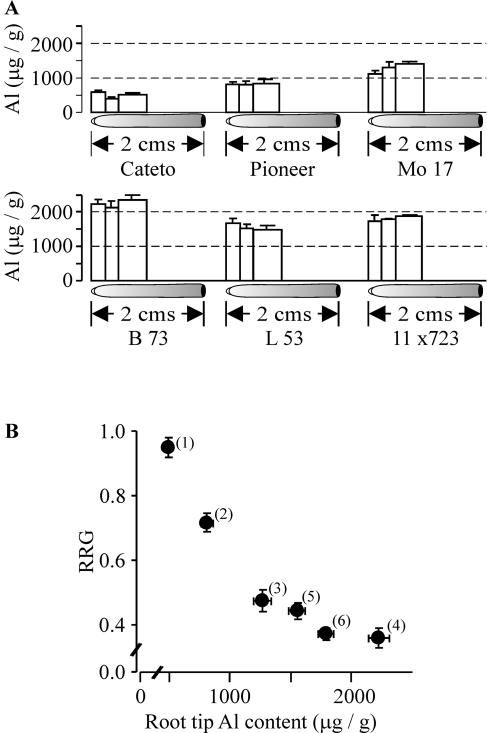
Possible Al-resistance mechanisms have been classified into two distinct classes: those operating to exclude Al from the root apex and those that allow the plant to tolerate Al accumulation in the root and shoot symplasm. Since Al resistance in maize has repeatedly been attributed to mechanisms aimed at excluding Al from the root tip, we proceeded to correlate the degree of Al resistance with the degree of Al exclusion (as measure by Al content in root tips) for each of the six genotypes. Figure 2A depicts the magnitude and spatial distribution of Al content over the first centimeter of the root apices for plants grown for 5 d in the presence of 40 μm Al3+ activity in the nutrient solution. The Al content over the root's first centimeter was relatively homogenous in every genotype, with no significant differences in the Al content among the three discrete root sections analyzed (0–0.25 cm, 0.25–0.5 cm, and 0.5–1 cm). However, the total Al content over the first centimeter was significantly different among the lines, ranging from as low as 495 ± 38 μg Al/g dry weight in Cateto-Colombia to as high as 2,226 ± 86 μg Al/g dry weight in the very Al-sensitive genotype, B73. Figure 2B illustrates the relationship between the degree of Al resistance and root tip Al content for the six lines. The very Al-resistant Cateto-Colombia exhibited the lowest Al content, followed by moderately Al-resistant Pioneer 3355. The remaining Al-sensitive genotypes had a 2- to 3-fold larger Al content in their root tips. The clear correlation between Al resistance and root Al content in Figure 2B strongly suggests that the differences in Al-resistance genotypes arise from differences in their potential to exclude Al from the root tip.
Figure 2.
Relationship between root Al content and Al resistance. A, Diagram illustrating the magnitude and spatial distribution of Al-tissue content along the first centimeter of the root apex of the six maize genotypes grown for 5 d in full nutrient solution plus 40 μm Al3+ activity. The bars indicate the magnitude of Al content at a given position, and the bar's width corresponds to the length of root segment along the root in which Al content was determined. Vertical bars indicate the SEM. B, Relationship between Al content and Al resistance, as measured by RRG in full nutrient solutions containing 40 μm Al3+ activity as shown in Figure 1. Al content values represent the average of Al content measured over the first centimeter of the root (data shown in A). The numbers refer to the different maize genotypes in order of decreasing Al resistance: highly Al-resistant Cateto-Colombia (no. 1), moderately Al-resistant Pioneer (no. 2), and Al-sensitive Mo17 (no. 3), B73 (no. 4), L53 (no. 5), and 11 × 723 (no. 6).
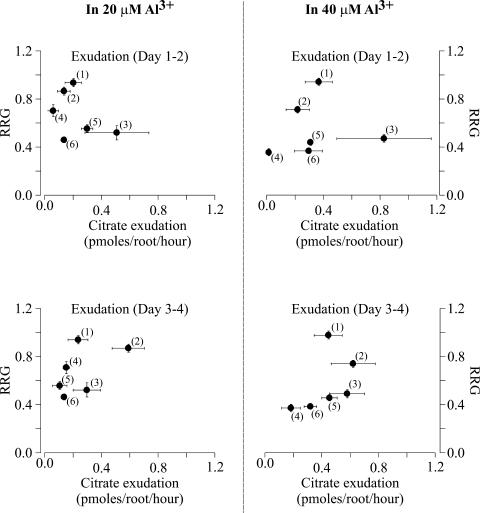
To determine whether these differences in root Al exclusion were due to root organic acid exudation, we proceeded to quantify and compare Al-activated root organic acid release in the six lines. Root exudates were collected over two different 24-h periods (between days 1 and 2 and days 3 and 4) for roots of intact seedlings exposed to different Al3+ activities. For all six maize genotypes, citrate was the only organic acid whose release was dependent on the presence of Al. Table I shows the complete set of data for time- and Al-activity-dependent rates of root citrate exudation obtained for the six maize genotypes. Five out of the six genotypes tested (B73 being the exception) showed an Al-concentration dependent increase in root citrate release. The Al-activated citrate exudation rates of these five genotypes tended to saturate as the Al3+ activities reached 40 μm, with the half-maximal rates of citrate release occurring at about 20 μm Al3+ (Table I). It is interesting to note that although the two most Al-resistant maize genotypes, Cateto-Colombia and Pioneer 3355, exhibit a significant rate of Al-activated root citrate release, the Al-sensitive genotype, Mo17, exhibited the highest citrate exudation rate of any of the six genotypes (from 570–830 pmol citrate root−2 h−1 at 40 μm Al3+). Rates of root Al-activated citrate exudation were correlated with the degree Al resistance (as determined by the RRG measurements) to evaluate the role of organic acid release in the Al-resistance response of the maize genotypes. Figure 3 shows the relationship between RRG and citrate exudation rates at two different Al activities. These activities were chosen as they represent the Al activities where the Al-induced citrate release rates were half-maximal and saturating, respectively, for all of the maize lines. Interestingly, as seen in Figure 3, there was no clear relationship between the magnitude of Al-activated citrate exudation and the Al resistance of a genotype. In general, the extremely Al-tolerant Cateto-Colombia genotype had citrate exudation rates that were not significantly different from those of several of the Al-sensitive genotypes. Furthermore, as mentioned above, the Al-sensitive genotype, Mo17, had a significantly larger citrate exudation rate than any of the other five maize lines for exudation measured during the first time period (day 1–2 in Al). Consequently, when these parameters were considered for all six genotype, we could not establish a significant correlation between root Al-activated citrate exudation and differential Al resistance.
Figure 3.
Relationship between the rate of citrate exudation and Al resistance, as measured by RRG. The two sections on the left show the citrate exudation rates [estimated between days 1 and 2 (top section) and days 3 and 4 (bottom section)] and RRG for the six maize genotypes exposed to 20 μm Al3+ activity. The two sections on the right show the citrate exudation rates [estimated between days 1 and 2 (top section) and days 3 and 4 (bottom section)] and RRG for the six maize genotypes exposed to 40 μm Al3+ activity. RRG values and exudation rates correspond to those shown in Figure 1 and Table I, respectively. Horizontal and vertical bars indicate the SEM. The numbers refer to the different maize genotypes in order of decreasing Al resistance: highly Al-resistant Cateto-Colombia (no. 1), moderately Al-resistant Pioneer (no. 2), and Al-sensitive Mo17 (no. 3), B73 (no. 4), L53 (no. 5), and 11 × 723 (no. 6).
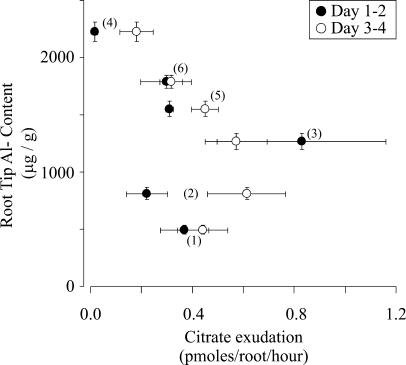
The lack of correlation between Al-induced citrate exudation and Al resistance contrasts with the clear correlation between Al exclusion (i.e. Al content) and Al resistance shown in Figure 2B. Therefore, we also examined the relationship between root tip Al content and Al-activated citrate exudation for the six genotypes (Fig. 4). These results again show the lack of a clear correlation between Al exclusion and citrate exudation rates at either of the two time periods tested. When one examines the three most Al-sensitive genotypes, B73, L53, and 11×723, the correlation between these two parameters holds up to some degree. However, although the Al-sensitive genotype, Mo17, had the highest citrate exudation rates, its root tip Al content was 1.5 and 2.5 times larger than that observed for the Al-tolerant genotypes, genotype Pioneer 3355 and Cateto-Colombia, respectively. Likewise, despite the 3-fold difference in root tip Al content between Cateto-Colombia, and the Al-sensitive lines, L53 and 11×723, the citrate exudation rates measured for these three genotypes were not significantly different. Thus, although root tip Al exclusion correlates nicely with the degree of Al resistance of a particular maize genotype, Al-activated organic acid exudation does not correlate strongly with either parameter. Consequently, since citrate exudation cannot entirely account for the Al exclusion observed in the most resistant genotype, the above results strongly suggest that although root citrate exudation may play a role in resistance (as all the tolerant maize genotypes studied thus far exhibit a substantial Al-activated root citrate release), other Al-resistance mechanisms most likely also contribute to the high degree of Al resistance seen in maize lines such as Cateto-Colombia.
Figure 4.
Relationship between root citrate exudation and root Al content. Al content values were estimated as in Figure 2A. Citrate exudation rates correspond to those shown in Table I. Horizontal and vertical bars indicate the SEM. The numbers refer to the different maize genotypes in order of decreasing Al resistance: highly Al-resistant Cateto-Colombia (no. 1), moderately Al-resistant Pioneer (no. 2), and Al-sensitive Mo17 (no. 3), B73 (no. 4), L53 (no. 5), and 11 × 723 (no. 6).
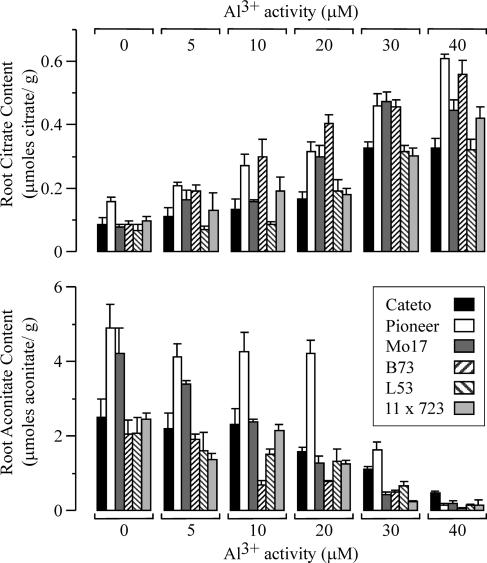
We also considered the possible role of the root exudation of some other potential Al chelators that could contribute to Al exclusion and consequently, Al resistance. Root malate and phosphate exudation was quantified and was found to not differ substantially between the six maize genotypes (see supplemental material, available at www.plantphysiol.org; Table I). Furthermore, in contrast to citrate exudation, malate and phosphate exudation were constitutive, not being dependent on the absence or presence of external Al. Consequently, we could not establish a correlation between malate and phosphate exudation rates and the degree of Al resistance or Al exclusion observed among the six genotypes. These results suggest that to account for the Al resistance observed across the six genotypes described above, mechanisms in addition to Al-activated organic release have to be operating in maize. Given that some recent evidence in the published literature suggests that Al resistance in some species can involve internal detoxification of Al by organic acid ligands, followed by translocation and sequestration of the Al-organic acid complexes in the shoot vacuole, we investigated this possibility. Table II shows the Al content of leaf tissue for the six maize genotypes when grown on 40 μm Al3+. The levels of Al in the shoot tissue of all six genotypes were significantly lower (20- to 100-fold) than those observed in the root tissue, indicating that there is no significant translocation of Al from the root to the shoot. Furthermore, as the shoot Al content was not significantly different among the six genotypes, it is not likely that this Al-resistance mechanism is operating in maize. Consequently, we then looked at the potential role of Al-chelating organic acids in the root in conferring an internal Al-tolerance mechanism. Figure 5 depicts the root tip concentrations of citrate and aconitate, the two organic acids whose root content respond significantly to Al exposure. In general, while exposure to increasing levels of Al elicited a strong (more than 3-fold) increase in root tip citrate content for all six maize lines, the aconitate content in the root tip showed an inverse relationship with increasing Al exposure, decreasing by 4- to 5-fold as the Al activities were increased. There was no clear correlation found between differential Al resistance and root tip citrate or aconitate content when all of the maize lines were examined. Furthermore, malate and phosphate root tip content were also not significantly correlated with Al resistance (see supplemental material; Table II). Overall, no significant relationship could be established between the Al resistance of a particular genotype and the absolute levels of changes in organic acids or phosphate in the root tip.
Table II.
Al content (μg Al/g FW) of shoot tissue for the six maize varieties grown on nutrient solution containing 40 μm Al3+ for a period of 5 d
| Maize Genotype | Al Content |
|---|---|
| μg Al/g dry weight | |
| Cateto-Colombia | 22.4 ± 1.2 |
| Pioneer 3355 | 31.7 ± 1.9 |
| Mo17 | 26.8 ± 1.6 |
| B73 | 31.8 ± 1.8 |
| L53 | 26.2 ± 1.7 |
| 11×723 | 30.5 ± 1.8 |
Figure 5.
Root citrate (top) and aconitate (bottom) content of six maize genotypes in response to increasing activities of Al3+ in the growth solution, as indicated in the top and bottom lines of the figure. The root citrate and aconitate content were measured in the apical 2 cm of the root tip after 5 d of the Al treatment indicated for each case.
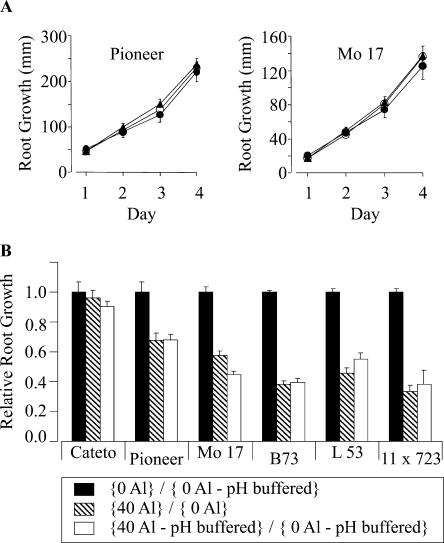
As an alternative Al-resistance mechanism we investigated the possibility of Al exclusion from the root apex being achieved via root-mediated alterations of rhizosphere pH. These efforts involved “pH clamping” of the rhizosphere solution by including the non-Al-chelating buffer, Homopipes, at a concentration (2.5 mm) that abolished pH gradients generated at the root surface due to root H+ influx or efflux (Degenhardt et al., 1998). Thus, we are able to control the pH of the rhizosphere solution at the root surface at predetermined pH values determined by the Homopipes buffer. Therefore, any Al-resistance mechanism based on root-mediated changes in rhizosphere pH should be abolished or significantly reduced by this treatment, and a decrease in overall Al resistance should be seen. We first established that 2.5 mm Homopipes had no effect on maize root growth in the absence of Al (Fig. 6A). Subsequently, we examined the relative Al resistance of the six maize genotypes by measuring RRG in the presence and absence of Al in buffered and unbuffered growth solutions. As seen in Figure 6B, there was no significant difference in relative Al resistance between the six genotypes whether the rhizosphere pH was clamped at pH 4 with Homopipes compared with unbuffered growth solution. These results suggest that the differential Al resistance in these six maize genotypes was probably not associated with differences in the ability of the more resistant genotypes to increase the rhizosphere pH in the presence of Al.
Figure 6.
Effect of pH clamping of rhizosphere pH on Al resistance. A, Examples of root growth of two maize varieties grown in unbuffered full nutrient solutions at pH 4.0 (white circles), pH 4.0 solutions buffered with 2.5 mm Homopipes (black circles), and unbuffered pH 6.0 full nutrient solutions (black triangles). Similar results were obtained for the remaining four cultivars examined in this study (data not shown). B, Comparison of RRG and Al resistance (based on Al inhibition of root growth) under conditions where the rhizosphere pH remained pH clamped at pH 4 with 2.5 mm Homopipes (white bars) or is unbuffered (striped bars). The RRG between control and Al-treated (39 μm Al3+) plants in buffered and unbuffered conditions was calculated over a 3-d period as described in “Materials and Methods.” The RRG between buffered and unbuffered control conditions is shown in black. Vertical bars indicate the SEM.
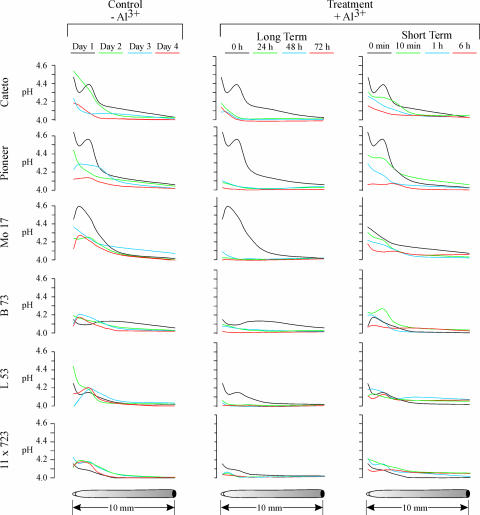
To examine this response in more detail, we then used pH microelectrodes to measure rhizosphere pH at a distance of 30 μm from the root surface at different locations along the first centimeter of the root tip of the six maize lines either for up to 4 d in the absence of Al or for short-term (up to 6 h) and long-term (up to 72 h) exposures to 40 μm Al3+. In the absence of Al, the pH profiles of all six maize varieties were quite similar (Fig. 7, left column), exhibiting a strong and persistent alkalinization (pH increase from 4 to between 4.2 and 4.6) over the first approximately 3 to 4 mm of the root apex. Although this pattern was maintained over a 4-d period in the absence of Al, the rhizosphere alkalinization was less pronounced on days 3 and 4, most likely due to developmentally related variations in rhizosphere pH.
Figure 7.
The diagram illustrates changes in rhizosphere pH measured with pH microelectrodes at 30 μm from the root surface along the terminal 10 mm of the maize root in the absence of Al (left column) and upon long (center column) and short (right column) Al-exposure periods. The six maize genotypes (rows) are labeled on the left side of the figure. The pH measurements were performed at the time periods that are color labeled at the top of each column. Each result is a representative of measurements done in for least three different roots of each maize genotype. The scale bars at the bottom of the figure indicate the position along the root.
Regardless of their degree of Al resistance all six varieties experienced a significant decrease in the rhizosphere pH at the root apex after being exposed to Al for 24 h (Fig. 7, middle column). Longer periods of Al exposure did not result in further reductions of the surface pH. Consequently, we examined the effect of a shorter-term exposure to Al (up to 6 h). Within 10 min after Al exposure, Al significantly reduced the surface pH in all genotypes tested. Further reduction in surface pH was observed after 1 h of Al exposure. After 6 h of exposure, the surface pH was reduced to values similar to those measured after the long-term Al exposures. In summary, we were unable to detect any significant differences in the Al-induced changes in surface pH among the six genotypes tested that could be associated with the differential Al resistance. Moreover, we were not able to observe any indication of an Al-induced alkalinization for any of the genotypes studied that could account for its Al-resistance profile. Consequently, the differences in Al exclusion and Al resistance among the genotypes examined cannot be attributed to Al-induced root-mediated alterations (i.e. alkalinization) of the rhizosphere pH.
DISCUSSION
It is widely accepted in the literature that a major plant Al-resistance mechanism involves Al-activated exudation of the organic acids, malate, citrate, or oxalate, depending on the plant species studied (see, for example, Ma, 2000; Ma et al., 2001; Ryan et al., 2001; Kochian et al., 2004). The extensive work in wheat has shown a clear and strong correlation between the degree of Al resistance in a large number of wheat genotypes and the magnitude of Al-activated root malate release (Delhaize et al., 1993a, 1993b; Ryan et al., 1995a, 1995b; Pellet et al., 1996; Papernik et al., 2001). These studies have laid the foundation for investigations into the cellular mechanism (i.e. membrane transporters) that mediates the release of malate from wheat root cells (Ryan et al. 1997; Zhang et al., 2001) that has culminated in the isolation of a candidate gene, ALMT1, for the Al-activated malate transporter in wheat (Sasaki et al., 2004). Al-activated organic acid (i.e. citrate) release (Pellet et al., 1995; Jorge and Arruda, 1997; Ishikawa et al., 2000; Kollmeier et al., 2001; Piñeros et al., 2002; Mariano and Keltjens, 2003) and putative membrane transporters capable of mediating the transport of these anions (Kollmeier et al., 2001; Piñeros and Kochian, 2001; Piñeros et al., 2002) have also been reported in roots from Al-resistant maize genotypes. However, in contrast to the overwhelming evidence presented for wheat, the work in maize has been limited to a few Al-resistant genotypes, and therefore evidence indicating that Al-activated citrate release is the main Al-resistance mechanism for maize is still lacking. Hence, in this study we addressed a number of possible physiological mechanisms that could account for the differential Al resistance observed in six different maize genotypes with diverse genetic backgrounds.
Are Organic Acids Involved in Maize Al Resistance?
The degree of Al resistance for all six genotypes in this study was clearly correlated with the root tip Al content (Fig. 2B), indicating that an Al-exclusion mechanism most likely underlies Al resistance in maize. With regards to the magnitude of Al-activated root organic acid exudation, five out of the six maize genotypes exhibited Al-activated citrate exudation, with the rates being similar in magnitude to those reported for other Al-resistant maize genotypes (SA3, Pellet et al., 1995; IAC-TAIUBA, Jorge and Arruda, 1997; ATP-Y, Kollmeier et al., 2001; CMS36, Mariano and Keltjens, 2003). The Al-activated citrate exudation rates were positively correlated with the level of Al in the nutrient solution. However, we could not establish a clear correlation between the magnitude of the Al-induced citrate exudation and the degree of Al resistance. For example, the most Al-resistant genotype, Cateto-Colombia, had similar or lower citrate exudation rates to the intermediately resistant and Al-sensitive genotypes (except for Al-sensitive B73, which exhibits a much lower Al-activated citrate release). The characteristics of the Al-induced citrate release observed in this study also differ from those reported for other maize genotypes. For example, other maize lines (e.g. SA3 and ATP-Y) exhibited a significant constitutive citrate release, and Al-activated exudation rates were significantly reduced at higher Al concentrations. Other studies have suggested that Al-activated citrate release in maize is an inducible process that increases as the length of the Al exposure increases (Pellet et al., 1995; Jorge and Arruda, 1997), which was not seen in this study.
The analytical techniques employed in this study allow us to identify a wide range of organic acids in the root exudates solution. The only organic acid that was released in an Al-activated manner was citrate (Table I). Furthermore, the only other potential Al-binding ligands we found to be released from the roots of the six maize genotypes were malate and phosphate, which were released from the roots in a constitutive (i.e. Al-independent) manner. The lack of correlation between root malate/phosphate exudation and Al resistance indicated that they do not play a role in the differences in Al resistance between the genotypes.
Changes in root organic acid content in Al-treated maize roots have been previously being reported (Pellet et al., 1995; Piñeros et al., 2002). Increases in root tip citrate content and citrate synthase activity have also been reported in Al-tolerant rye and soybean genotypes, where Al-stimulation of citrate release also takes place (Li et al., 2000; Silva et al., 2001). Given that the modulation of organic acid metabolism (via the TCA cycle) affects the accumulation and potentially the efflux of organic acids, we evaluated changes in internal organic acid content in root tips. The increases in Al-activated citrated exudation were accompanied by an up-regulation of root citrate levels, and a down-regulation of root aconitate content (Fig. 5). The increase in citrate content could result from a stimulation of citrate synthase and/or a reduction in the activity of aconitase, which could result in lower citrate turnover via the conversion to isocitrate. Although changes in the TCA cycle are likely a prerequisite to sustain the exudation of organic acids, it is unlikely that alterations in symplasmic organic acid metabolism drive the increases in organic acid release. First, although the Al-activated stimulation in citrate exudation saturates at around 20 μm Al3+, the Al-induced changes in organic acid content (i.e. increase in citrate content and decrease in aconitate) did not shown any saturation even at 40 μm Al3+. Furthermore, the changes in internal organic acid concentrations did not correlate with exudation rates. For instance, root organic acid content in B73 was not significantly different from that measured in the most Al-tolerant genotype (Cateto-Colombia) or the genotype with the highest citrate exudation rate (Mo17), even though B73 exhibited low Al-activated citrate exudation. This suggests that the capacity of the TCA cycle to maintain organic acid synthesis exceeds the demands of organic acid efflux. Our observations are consistent with the lack of correlation between internal organic acid concentrations and efflux rates reported for Al-tolerant and Al-sensitive maize and wheat genotypes (Delhaize et al., 1993b; Pellet et al., 1995). In addition to its potential role in root tip Al exclusion, organic acids can also play a role in internal Al detoxification. Studies in Al accumulator crop species such as buckwheat have shown that these species can tolerate Al by translocating it to the shoot tissue as an Al-organic acid complex, and subsequently storing the Al-organic acid complex in the vacuole of leaf cells (Ma et al., 1998; Ma and Hiradate, 2000). In this study, the Al concentrations found in the shoots were low and were not significantly different between the maize lines, suggesting that such a mechanism cannot explain the differences in Al resistance. In addition, given that the Al concentrations in the shoot were 20- to 100-fold lower than those found in the root tissue, it is unlikely that Al translocation to the shoot plays a significant role in Al resistance.
Are Other Al-Exclusion Mechanisms Operating in Maize Roots?
Given that the measurements of root Al content pointed to Al resistance being achieved by Al exclusion, we also evaluated the hypothesis of Al exclusion via root-mediated increases in rhizosphere pH (Taylor, 1991; Degenhardt et al., 1998). Alkalinization at the root surface would decrease the activity of the rhizotoxic Al3+ species in the rhizosphere and thus would confer an increase in Al resistance. Microelectrode techniques allowed us to directly characterize changes in the rhizosphere pH along the root tips of the maize genotypes in the presence and absence of Al. In the absence of Al, the rhizosphere pH profile along the root of all six maize genotypes was similar, showing a significant alkalinization along the root apical region, with the maximum gradient occurring acropetally from the meristematic region. The root cap and the more mature root regions maintained a slightly more acidic pH. The apical pH gradient rapidly collapsed (within minutes) upon exposure to Al. This weaker alkalinizing ability of the root apex in the presence of Al was observed in all the maize genotypes. These observations were in agreement with the root growth experiments, where exposure to Al cause a similar degree of toxicity (i.e. Al-induced root growth inhibition) in roots where the rhizosphere pH was either unbuffered or pH clamped with Homopipes buffer. Both approaches confirmed the lack of potential in maize roots of Al-resistant genotypes to mediate Al-stimulated alkalinization of the rhizosphere. Consequently, it is unlikely that changes in rhizosphere pH can account for the difference in Al resistance observed among the six genotypes.
What Are the Novel Al-Resistance Mechanisms Operating in Maize?
We had previously speculated that an Al-exclusion mechanism mediated via citrate exudation could be operating in conjunction with a second internal Al-tolerance mechanism based on increases in internal levels of citrate in the root to account for the maize Al resistance exhibited in very resistant genotypes such as Cateto-Colombia (Piñeros et al., 2002). That study and this one reinforce the idea that, in contrast to crop plants like wheat where Al resistance is relatively simple, in maize it is a genetically and physiologically complex trait (Magnavaca et al., 1987; Kochian et al., 2004). In the one major quantitative trait loci mapping study on maize Al resistance that has been published to date, five distinct genomic regions were identified as important for Al resistance (Ninamango et al., 2003), which could indicate that multiple mechanisms are pyramided in maize to result in the overall high level of resistance seen in Cateto-Colombia. Based on the findings presented here, we suggest that Al-activated root citrate exudation could provide a baseline level of Al resistance in many (but not all) maize genotypes. What is intriguing is that despite a fairly extensive investigation of several other possible mechanisms of Al exclusion and internal tolerance, including release of other Al-chelating ligands, alterations in rhizosphere pH, changes in internal levels of Al-chelating compounds in the root, and Al translocation to the shoot, we were unsuccessful in identifying additional Al-resistance mechanisms in maize. It seems likely that the additional Al-resistance mechanisms are associated with Al exclusion from the root tip. It is possible that in the very Al-resistant Cateto-Colombia, Al activates the release of a class of Al-chelating compounds we have not yet identified, such as phenolics. A less likely scenario that should not be dismissed, however, is that is that this Al exclusion could be due to Al efflux from the root-cell symplasm. It should be noted that our findings are similar to those recently presented for very Al-resistant Bracchiaria (signalgrass) species, where it was also shown that Al resistance did not correlate with organic acid release (Wenzl et al., 2001, 2002). It is likely that the discovery of these novel Al-resistance mechanisms in maize will require an interdisciplinary approach integrating genetic, molecular, and physiological investigations.
MATERIALS AND METHODS
Plant Material and Seedling Growth
The maize hybrids (SLP 181/71 × Cateto-Colombia 96/71 and 11×723) and line L53 were supplied by EMBRAPA Maize and Sorghum Research Center in Sete Lagoas, Brazil; the maize cultivars B73 and Mo17 were provided by Dr. Owen Hoekenga, Cornell University, NY; and Pioneer 3335 was obtained from Pioneer Hi-Bred International (Des Moines, IA). Seeds were germinated and grown as described previously (Piñeros et al., 2002). The full nutrient solution contained the following macronutrients (in mm): Ca, 3.53; K, 2.35; Mg, 0.85; NH4, 1.3; NO3, 10.86; PO4, 0.04; SO4, 0.59, and micronutrients (μm): BO3, 25; Cl, 596; Cu, 0.63; Fe-HEDTA, 77; MoO4, 0.83; Mn, 9.1; Zn, 2.3; and Na, 1.74. Seedlings were grown for 24 h in a growth chamber at 26°C/24°C (light:dark, 16:8 h) under a light intensity of 550 μmol photons m−2 s−1. Al treatments were initiated following a 24-h period of growth in nutrient solution by replacing the control nutrient solution with an identical solution that contained Al added as AlK(SO4)2 12H2O to the final concentrations. The desired Al3+ activities were estimated using GEOCHEM-PC speciation software (Parker et al., 1995). The pH of the control and treatment solutions was adjusted to 4.0 with HCl. For experiments evaluating the effects of pH on root growth and Al resistance, the full nutrient growing solution contained 2.5 mm Homopipes buffer, and the pH was adjusted to 4.0 with HCl. In all cases, seedlings were grown for an additional 1 to 5 d in the treatment solutions depending on the type of experiment performed. The time periods of the different treatments are given in the text.
Measurement of Root Growth Root Tip Al Content
Root growth measurements were performed as described previously (Piñeros et al., 2002), prior to Al treatments and then over the following 5 d in Al at 24-h intervals. Root measurements for at least 12 plants/treatment were averaged for each day. Root growth rates (RGR expressed in mm/d) for the control and the Al treatments were obtained from the regression coefficients (slopes) estimated from the linear regression of the root length values (mm) over the first 3 d as a function of time (4 points including initial length). RRG was calculated as: RRG = (RGR in Al solution/RGR in control solution). Root tip and leaf tissue Al content were measured by inductively coupled argon plasma emission spectrometry. The first centimeter of the root tips or the entire set of leaves from the shoot were collected and dried in an oven at 55°C overnight. Dry weights were determined using a microgram balance (MT2, Mettler, Greifensee, Switzerland). Dry samples were digested with 100 μL of 70% perchloric acid, resuspended in 2 mL of 0.5% nitric acid, and analyzed using an inductively coupled argon plasma model 51000 emission spectrometer (Perkin-Elmer/Sciex, Foster City, CA).
Whole Root Organic Acid Exudation and Root Tip Organic Acid Content
Following a 24-h Al treatment in full nutrient solution each seedling cup containing 4 seedlings was fitted on top of a plastic centrifuge tube containing 50 mL of aerated 4.3 mm CaCl2, plus or minus AlCl3 (to the desired activity) at pH 4.5 (adjusted with HCl). The choice of this particular simple salt solution and Ca2+ concentration for collection of exudates was previously determined to be optimal for measurement of root organic acid exudations (Piñeros et al., 2002). Root exudation was allowed to occur from roots of intact plants for 24 h (i.e. the second day of Al treatment). At this point, exudation samples were collected and analyzed as described below. The cups containing the seedlings were returned to their original treatment in full nutrient solution for an additional 24 h. To compare exudation rates at different time periods, a second set of root exudates were collected in the same simple salt solution over the subsequent 24 h (i.e. the fourth day of Al treatment). Preliminary assays indicated that root organic acid exudation determinations using capillary electrophoresis (CE) in simple salt solutions were similar to the values determined in full nutrient solutions using enzyme-based methods for organic acids (data not shown). Given that CE analysis of organic acids is much more sensitive than organic acid determinations based on using enzyme kits (Delhaize et al., 1993b; Jorge and Arruda, 1997), and also that it allows us to determine the identify and quantity of all organic acids released from the root, CE analysis was chosen as the preferred method for organic acid determination. Root exudate samples were passed through an OnGuard-Ag chromatography (DIONEX, Sunnyvale, CA) column to remove excess Cl− and analyzed by CE. Following the collection of the exudate samples, the root tips were excised and analyzed for organic acid content. Root tissue organic acids were extracted by homogenizing the first centimeter of the root tips in 18 MΩ water. Samples were centrifuged for 10 min in a microfuge and the supernatant was analyzed by CE with no further sample processing. Organic acids in root exudates and root homogenates were analyzed with a Beckman (Fullerton, CA) P/ACE 5510 CE system controlled by a Pentium II computer interfaced via PACE 1.2.1 software. The background electrolyte used for separation consisted of 0.5 mm dodecyltrimethylammonium bromide, 7.5 mm salicylic acid, and 15 mm Tris adjusted to pH 9.5 with NaOH. Organic acids were separated, detected, and identified as described previously (Piñeros et al., 2002). Given that malate and phosphate peaks had similar migration times, we used a combination of CE and colorimetric methods to quantify malate and phosphate separately in that study. Given that those results showed no Al-induced changes in the malate or phosphate exudation rates (in one of the same genotypes used in this study), in this study we rely solely on CE measurements and for practical purpose refer to this combined peak as malate + phosphate.
Microelectrode pH Measurements: H+-Selective Microelectrodes
Liquid-membrane ion-selective pH microelectrodes were constructed as previously described (Kochian et al., 1989). Briefly, borosilicate glass capillaries (1.5 mm in diameter, without filament, World Precision Instruments, Sarasota, FL) were cleaned in a mixture of 95% (v/v) concentrated H2SO4 and 5% (v/v) of 70% HClO4. Capillaries were pulled using a two-stage Flaming-Brown horizontal electrode puller (Model P-87; Sutter Instrument, Novato, CA), generating a microelectrode with a relatively short shank and a tip diameter of approximately 1 to 2 μm. Microelectrodes were heated (200°C, 3 h), silanized with tri(n-butyl) chlorosilane (200°C, 30 min), cooled, and then stored in an evacuated desiccator. Microelectrodes were initially completely backfilled with a 16-mm KH2PO4, 24 mm K2HPO4, and 15 mm NaCl, pH 7.0 solution. The microelectrode tip was then front filled with a short column (50 μm in length) of the H+ selective cocktail, which consisted of 6% (w/w) 4-nonadecylpyridine (Fluka, Ronkonkoma, NY), 1% (w/w) potassium tetrakis(4-chlorophenyl) borate (Fluka), and 93% (w/w) 2-nitrophenyl octyl ether (Fluka). Subsequently, the backfilling buffer was reduced to a column length of approximately 1.5 cm to minimize parasitic capacitance. Electrical contact between the microelectrode and the head stage was made through a 0.25-mm Ag/AgCl wire, and a single junction reference electrode (Model MI-409F, Microelectrodes, Londonderry, NH) was connected to the reference input of the head stage. Signals from the amplifier are fed into the computer by a DT-2801 analog-to-digital board from Data Translation (Marlboro, MA). Since the electrode was used in a stationary mode, then the output was followed off from the amplifier display. The stepper motors of the translation stages had sufficient resolution to position the electrode along the root, 30 μm from the root surface, as well as to quickly move the electrode into the background for solution reference measurements.
Supplementary Material
This work was supported by the U.S. Department of Agriculture National Research Initiative (grant no. 00–35100–9280 to M.A.P. and L.V.K.; grant no. 2001–35301–10647 to L.V.K.).
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.047357.
References
- Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48: 75–92 [Google Scholar]
- Cançado GMA, Loguercio LL, Martins PR, Perentoni SN, Paiva E, Borém A, Lopes MA (1999) Hematoxylin staining as a phenotypic index for aluminum tolerance selection in tropical maize (Zea mays L). Theor Appl Genet 99: 747–754 [Google Scholar]
- Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol 117: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ (1993. a) Aluminum tolerance in wheat (Triticum asetivum L.) I. Uptake and distribution of aluminum in root apices. Plant Physiol 103: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ (1993. b) Aluminum tolerance in wheat (Triticum aestivum L.) II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Wagatsuma T, Sasaki R, Ofei-Manu P (2000) Comparison of the amount of citric and malic acids in Al media of seven plant species and two cultivars each in five plant species. Soil Sci Plant Nutr 46: 751–758 [Google Scholar]
- Jorge RA, Arruda P (1997) Aluminum-induced organic acid exudation by roots of an aluminum-tolerant tropical maize. Phytochemistry 45: 675–681 [Google Scholar]
- Kidd PS, Llugany M, Poschenrieder C, Gunse B, Barcelo J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52: 1339–1352 [PubMed] [Google Scholar]
- Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46: 237–260 [Google Scholar]
- Kochian LV, Shaff JE, Lucas WJ (1989) High-affinity K+ uptake in maize roots: a lack of coupling with H+ efflux. Plant Physiol 91: 1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga AO, Piñeros MA (2004) How do plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Physiol Plant Mol Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- Kochian LV, Piñeros MA, Hoekenga AO (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. In H Lambers, ed, Root Physiology: From Gene to Function. Kluwer Publishers, Dordrecht, The Netherlands (in press)
- Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R (2001) Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex. A comparison between aluminum-sensitive and an aluminum-resistant cultivars. Plant Physiol 126: 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Ma JF, Matsumoto H (2000) Pattern of aluminum-induced secretion of organic acids differs between rye and wheat. Plant Physiol 123: 1537–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF (2000) Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol 41: 383–390 [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S (2000) Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench). Planta 211: 355–360 [DOI] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Matsumoto H (1998) High aluminum resistance in buckwheat. II. Oxalic acid detoxifies aluminum internally. Plant Physiol 117: 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H (1997) Internal detoxification mechanism in Hydrangea. Identification of the Al form in leaves. Plant Physiol 113: 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E (2001) Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6: 273–278 [DOI] [PubMed] [Google Scholar]
- Magnavaca R, Gardner CO, Clark RB (1987) Evaluation of inbred maize lines for aluminum tolerance in nutrient solution. In HW Gabelman, BC Loughman, eds, Genetic Aspects of Plant Mineral Nutrition. Martinus Nijhoff, Dordrecht, The Netherlands, pp 255–265
- Mariano ED, Keltjens WG (2003) Evaluating the role of citrate exudation as a mechanism of aluminum resistance in maize genotypes. Plant Soil 256: 469–479 [Google Scholar]
- Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200: 1–46 [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Yamamoto Y, Rama Devi S (2001) Aluminum toxicity in acid soils. Plant responses to aluminum. In M Prasad, ed, Metals in the Environment. Marcel Dekker, New York, pp 289–319
- Miyasaka SC, Buta JG, Howell RK, Foy CD (1991) Mechanisms of aluminum tolerance in snapbeans. Root exudation of citric acid. Plant Physiol 96: 737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninamango CFE, Guimaraes CT, Martins PR, Parentoni SN, Carneiro NP, Lopes MA, Moro SJ, Paiva E (2003) Mapping QTLs for aluminum tolerance in maize. Euphytica 130: 223–232 [Google Scholar]
- Papernik LA, Bethea AS, Singleton TE, Magalhaes JV, Garvin DF, Kochian LV (2001) Physiological basis of reduced Al tolerance in ditelosomic lines of Chinese Spring wheat. Planta 212: 829–834 [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL (1995) GEOCHEM-PC: a chemical speciation program for IBM and compatible computers. In RH Loeppert, AP Schwab, S Goldberg, eds, Chemical Equilibrium and Reaction Models. Soil Science Society of America, Madison, WI, pp 253–269
- Pellet DM, Grunes DL, Kochian LV (1995) Organic acid exudation as an aluminum-tolerance mechanism in maize (Zea mays L.). Planta 196: 788–795 [Google Scholar]
- Pellet DM, Papernik L, Kochian L (1996) Multiple aluminum-resistance mechanisms in wheat. Roles of root apical phosphate and malate exudation. Plant Physiol 112: 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros M, Kochian LV (2001) A patch clamp study on the physiology of aluminum toxicity and aluminum tolerance in maize. Identification and characterization of Al3+-induced anion channels. Plant Physiol 125: 292–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros M, Magalhaes J, Carvalho-Alves VM, Kochian LV (2002) The physiology and biophysics of an aluminum tolerance mechanism based on root citrate exudation in maize. Plant Physiol 29: 1194–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527–560 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ (1995. a) Characterisation of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta 196: 103–110 [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ (1995. b) Malate efflux from root apices: evidence for a general mechanism of Al-tolerance in wheat. Aust J Plant Physiol 22: 531–536 [Google Scholar]
- Ryan PR, Skerrett M, Findlay GP, Delhaize E, Tyerman SD (1997) Aluminum activates an anion channel in the apical cells of wheat roots. Proc Natl Acad Sci USA 94: 6547–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Silva IR, Smyth TJ, Raper CD, Carter TE, Rufty T (2001) Differential aluminum tolerance in soybean: an evaluation of the role of organic acids. Physiol Plant 112: 200–210 [DOI] [PubMed] [Google Scholar]
- Taylor GJ (1991) Current views of the aluminum stress response: the physiological basis of tolerance. Curr Top Plant Biochem Physiol 10: 57–93 [Google Scholar]
- Von Uexkull HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In RA Date, NJ Grundon, GE Raymet, ME Probert, eds, Plant-Soil Interactions at Low pH: Principles and Management. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 5–19
- Wenzl P, Chaves A, Patiño G, Mayer J, Rao I (2002) Aluminum stress stimulates the accumulation of organic acids in root apices of Brachiaria species. J Plant Nutr Soil Sci 165: 582–588 [Google Scholar]
- Wenzl P, Patiño GR, Chaves AL, Mayer JE, Rao IM (2001) The high levels of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol 122: 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ryan P, Tyerman SD (2001) Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol 125: 1459–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.