Abstract
Certain strains of lactobacilli have been reported to exert favorable effects on atopic dermatitis (AD). Jeotgal, a traditional Korean food, is a salted fermented seafood known to harbor many lactic acid bacteria. In the present study, two novel lactobacillus strains were isolated from Jeotgal, and their anti-AD effects were investigated. Lactobacilli isolated from Jeotgal were identified, according to conjugated linoleic acid-producing activity, as Lactobacillus plantarum (JBCC105645 and JBCC105683). AD-like skin lesions were induced in BALB/c mice using dinitrofluorobenzene (DNFB). Ear swelling, histological analysis and serum immunoglobulin E (IgE) levels in mice were evaluated to investigate the anti-AD effects of lactobacilli. Cytokine production of ex vivo cluster of differentiation (CD)4+ T cells, and interleukin (IL)-12 production of in vitro macrophages were also evaluated to establish a putative mechanism of the action of lactobacilli. Administration of JBCC105645 or JBCC105683 suppressed ear swelling and serum IgE levels in DNFB-treated mice (P<0.05). Notably, JBCC105645 was more effective than JBCC105683 (P<0.05). Treatment with the lactobacilli also induced a significant decrease in IL-4 production with concomitant increase in interferon (IFN)-γ production in DNFB-exposed CD4+ T cells, and an increase in IL-12 production in macrophages (P<0.05). Taken together, the lactobacilli isolated from Jeotgal may suppress the development of AD-like skin inflammation in mice by modulating IL-4 and IFN-γ production in CD4+ T cells, presumably via enhancing IL-12 production by macrophages.
Keywords: lactobacilli, atopic dermatitis, immunoglobulin E, interleukin-4, interferon-γ
Introduction
Adaptive immune responses are classified into two categories: T helper (Th) 1-type response (or cellular immune response) and Th2 response (or humoral immune response). These subsets of helper cluster of differentiation (CD)4+ T cells differ in their cytokine secretion patterns and effector functions, and counter-regulate one another (1). The majority of allergic diseases, including atopic dermatitis (AD), are considered to be due to the abnormal activation of Th2 cells, namely the polarization of allergen-specific T-cell memory towards the Th2 instead of the Th1 immune response (2). Interleukin (IL)-4, which is a representative cytokine of Th2 cells, induces immunoglobulin E (IgE) isotype switching, augments IgE production in B cells, and promotes Th2 differentiation (2). Interferon-γ (IFN-γ), a representative cytokine of Th1 cells, is known to suppress the development of Th2 cells and promote Th1 differentiation (3,4). Considering the evidence suggesting that the Th1 and Th2 types of immune responses are reciprocally regulated in vivo (5), modulation of the Th1/Th2 balance, namely shifting the balance from Th2 to Th1 dominance, may be an effective strategy for the treatment of allergic diseases associated with Th2 cells (6–9).
AD is a highly prevalent skin inflammatory disease characterized by allergic signs and symptoms of the skin, such as redness, edema, warmth, and pruritus (10–12). AD is a multifactorial disease with genetic and environmental components, and its pathogenesis has not yet been fully defined (13). AD is associated with elevated IgE and IL-4 production (13,14), indicating the predominance of the Th2 response over the Th1 response. The current therapeutic management of AD aims to relieve recurring skin inflammation and prevent flare-ups. Topical steroids and immunosuppressive agents have typically been the standard treatment for severe cases of AD (15,16). However, these agents are often associated with severe adverse effects and are not sufficiently effective in a substantial number of patients with AD (15,16). There is therefore a need to develop novel effective therapies for the treatment of AD. Notably, it has been reported that certain strains of lactobacilli may offer clinical benefits to patients with AD or other allergic diseases through the enhancement of Th1 immunity and suppression of Th2 immunity (17–19).
Lactobacilli are used widely in industrial and traditional fermentative food processes, and have a long history of safe use (20,21). It has been reported that Lactobacilli in a variety of food products may potentially provide health benefits (22–24). Certain Lactobacillus strains may potentially modulate the immune response via the production of interleukin (IL)-12, promoting the Th1/Th2 balance towards a Th1-dominant state in systemic immunity (25–34). This effect may be of use in the prevention and treatment of AD.
Jeotgal is a traditional Korean fermented food, which is made with various salted seafood, such as shrimp, oysters, shellfish, fish, and fish eggs, and is primarily used as a condiment in the production of other fermented foods (35–41). It has previously been reported that many Lactobacillus strains are involved in the fermentation of Jeotgal, suggesting that it is a good source of various Lactobacillus strains (35–41).
The aim of present study was to examine the in vivo anti-AD effects of two novel strains of Lactobacillus isolated from Jeotgal using a 2, 4-dinitrofluorobenzene (DNFB)-induced AD-like skin inflammation model. To explain a putative mechanism of action of the isolated Lactobacilli, the ex vivo effects on the secretion of cytokines by CD4+ T cells from lymph nodes isolated from mice that were sensitized and challenged with DNFB were examined, as well as the in vitro effects on IL-12 production in macrophages.
Materials and methods
Materials
MRS Broth was obtained from BD Biosciences (Franklin Lakes, NJ, USA). RPMI-1640, fetal bovine serum (FBS), penicillin/streptomycin, and DNFB were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). All other reagents were obtained from Sigma-Aldrich (Merck KGaA).
Lactobacillus strains
The authors of the present study searched for novel Lactobacillus strains that may be beneficial for AD treatment, and isolated novel Lactobacillus strains with conjugated linoleic acid (CLA)-producing ability, using a linoleic acid tolerance method, as previously described (42). The Lactobacillus strains used in the present study were isolated from five types of the Korean traditional fermented food Jeotgal obtained from Shinan-Suhyup Market (Shinan, Korea), of which the original sources were baby octopus (Octopus ocellatus), tripe of pollack (Pollachius pollachius), small octopus (Octopus minor), scallop (Patinopecten yessoensis) and oyster (Crassostrea gigas). The lactic acid bacteria that were able to produce high-level CLA were selected using the linoleic acid tolerance method (42) as shown in Table I and identified by 16S rRNA gene sequence analysis (43) as Lactobacillus plantarum (JBCC105645 and JBCC105683) with 99–100% similarity. JBCC105683 exhibited the highest CLA producing ability (748.8 µg/ml), and JBCC105645 produced 227.4 µg/ml (Table I). Following cultivation in MRS broth at 37°C for 24 h, the cells of both strains were harvested, washed with sterile distilled water and lyophilized in lyoprotectant consisting of 750 ml horse serum and 250 ml distilled water supplemented with 7.5% sucrose (both from Sigma-Aldrich; Merck KGaA), 0.63% nutrient broth (Sigma-Aldrich; Merck KGaA) and 1 mg/ml cysteine HCl. The cells were killed by heating at 105°C for 15 min, and the resultant heat-killed cells were lyophilized and then suspended in PBS to be used in the following experiments.
Table I.
Production of total CLA by lactobacilli isolated from Jeotgal.
| Strain | Linoleic acid (µg/ml) | Total CLA (µg/ml) |
|---|---|---|
| Lactobacillus plantarum | ||
| (JBCC105683) | 100 | 334.475±1.797 |
| 200 | 553.118±5.635 | |
| 600 | 748.807±1.919 | |
| 1,000 | 412.929±1.919 | |
| Lactobacillus plantarum | ||
| (JBCC105645) | 100 | 227.403±13.067 |
| 200 | 185.449±5.513 | |
| 600 | 192.061±0.000 | |
| 1,000 | 167.230±13.067 |
Data are presented as the mean ± standard deviation. CLA, conjugated linoleic acid.
Mice
A total of 100 female 5-week-old BALB/c mice weighing 15–20 g were purchased from the Da-Mul Experimental Animal Center (Daejeon, Korea) and were maintained conventionally in plastic cages at a temperature of ~22°C and humidity of ~50% under a 12-h light/dark cycle. The mice were provided with a standard diet and access to autoclaved water ad libitum throughout the experimental period. For experiments, mice were divided into 4 groups (n=6 in each group) as follows: Normal control group, unchallenged with DNFB and administrated with PBS; DNFB model, challenged with DNFB and administrated with PBS; JBCC195645-treated group, challenged with DNFB and administrated with JBCC195645; JBCC105683-treated group, challenged with DNFB and administrated with JBCC105683. The mice were anesthetized with 2% isoflurane (Sigma-Aldrich; Merck KGaA) in 98% oxygen before sacrifice by decapitation. The present study was performed according to the guidelines laid out by Ethics Committee for Animal Experiments of Wonkwang University (Iksan, Korea) and was approved by the Ethics Committee (WKU15-17).
Induction of AD-like skin inflammation
AD-like skin inflammation was induced by repeated treatment with DNFB solution on the surfaces of the right ear and shaved abdomen in BALB/c mice. After administration of anesthesia (1.2% isoflurane), shaved abdomen skin was coated with a total of 100 µl 0.5% DNFB in acetone/olive oil (4:1) once a day for 5 days. Following this, mouse ears were coated with 25 µl 0.2% DNFB (25 µl) for 2 days. Normal control mice were treated with acetone/olive oil (4:1) in the absence of DNFB. Ear thickness was measured with a spring-loaded micrometer (Oditest; Swiss Precision Instruments, Inc., Garden Grove, CA, USA) prior to DNFB and 24 and 48 h following the DNFB challenge.
Oral administration of lactobacilli
A total of 200 µl PBS containing heat-killed JBCC105645 or JBCC105683 strains [1×109 colony-forming units (CFU)/ml] was orally administrated once a day for 7 days before DNFB challenge. DNFB model mice were orally treated with 200 µl PBS in the absence of lactobacilli.
Histological study
Mice were anesthetized with 2% isoflurane (Sigma-Aldrich; Merck KGaA) in 98% oxygen and sacrificed by decapitation. Treated ears were harvested 48 h after the final application of DNFB or acetone/olive oil. The tissue was fixed for 10 min at room temperature in a 10% neutral formalin solution and embedded in paraffin wax by standard procedures (44). The sections (4–5 µm) were stained with hematoxylin and eosin and observed using a light microscope. Digital photomicrograph images were captured from five representative areas at a fixed magnification of ×100.
Analysis of total serum IgE production
Blood samples (1 ml) were collected from the heart following completion of treatment, and the sera were separated by centrifugation at 3,000 × g for 5 min at 4°C. Concentrations of total serum IgE were measured using a Mouse IgE ELISA MAX™ Deluxe kit (cat. no. 432406) purchased from BioLegend. Inc. (San Diego, CA, USA), according to the manufacturer's protocols.
Isolation of CD4+ T cells and analysis of IL-4 and IFN-γ production
On day 11 following the end of treatment, all mice were sacrificed and their lymph nodes were isolated as previously described (45). T cells were isolated from the lymph nodes and CD4+ T cells were purified using a CD4+ T cell isolation kit purchased from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany), according to the manufacturer's protocol. Isolated CD4+ T cells (1.5×105) were cultured for 2 h at 37°C in 96-well flat-bottom culture plates in RPMI-1640 medium with 10% FBS, 50 µM β-mercaptoethanol and 2 mM glutamine (both from Sigma-Aldrich; Merck KGaA), and then stimulated for 24 h at 37°C with immobilized anti-CD3 antibody (5 µg/ml; cat. no. 554829) and the soluble form of purified anti-CD28 antibody (2 µg/ml; cat. no. 553294) (both from BD Pharmingen, San Diego, CA, USA). Levels of IL-4 and IFN-γ were quantified using IL-4 ELISA (cat. no. 431103) and IFN-γ ELISA kits (cat. no. 430807) (both from BioLegend, Inc.), respectively, following T cell activation.
Macrophage culture and analysis of IL-12 production
RAW264.7 macrophages were obtained from American Type Culture Collection (cat. no. ATCC® TIB-71™; ATCC, Manassas, VA, USA). Cells were maintained for 18 h at 37°C in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Macrophages (1.5×105) were cultured for 2 h at 37°C in 96-well flat-bottom culture plates in RPMI-1640 medium with 10% heat-inactivated FBS, and then stimulated for 48 h at 37°C with 5×107 CFU/ml heat-killed JBCC105645 or JBCC105683. The production of IL-12 following stimulation was quantified using a mouse IL-12 ELISA kit (cat. no. 433606; BioLegend Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical differences between the means of treated and control groups were analyzed using one-way analysis of variance and Fisher's protected least significant difference and GraphPad Prism software version 3.03 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of JBCC105645 and JBCC105683 on DNFB-induced ear swelling response
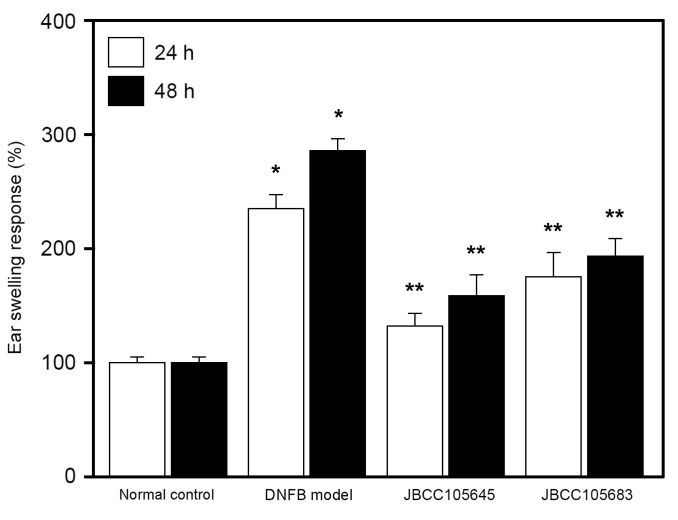
DNFB was used to induce a model of murine contact hypersensitivity. In the skin following exposure to DNFB, murine contact hypersensitivity is able to generate AD-like lesions and induce Th2-type immune responses, and is therefore often used to study the effects of drugs on AD pathogenesis (46,47). In the present study, repeated applications of DNFB were used to induce AD-like lesions in BALB/c mice. Following epidermal application of DNFB to the skin of mouse ears (DNFB challenge), significant ear swelling was induced in the DNFB model group compared with the normal control group (P<0.05; Fig. 1). This DNFB-induced ear swelling was significantly reduced in both the JBCC105645 and JBCC105683-treated groups at 24 and 48 h following treatment (P<0.05; Fig. 1); JBCC105645 was markedly more effective than JBCC105683 in reducing DNFB-induced edema.
Figure 1.
Effect of oral administration of lactobacilli on the ear swelling response of mice following exposure to DNFB. Mice were orally administrated with PBS (DNFB model), JBCC105645 or JBCC105683 (1×109 colony-forming units) daily for 7 days. The treated mice were contact-sensitized for 5 days with 0.2% DNFB and thereafter challenged or unchallenged (normal control). The thickness of the ear was measured 24 and 48 h following DNFB challenge. Data are presented as a percentage of the thickness of the ear measured prior to the DNFB challenge and are given as the mean ± standard deviation (n=6). *P<0.05 vs. normal control group. **P<0.05 vs. DNFB model group. DNFB, dinitrofluorobenzene.
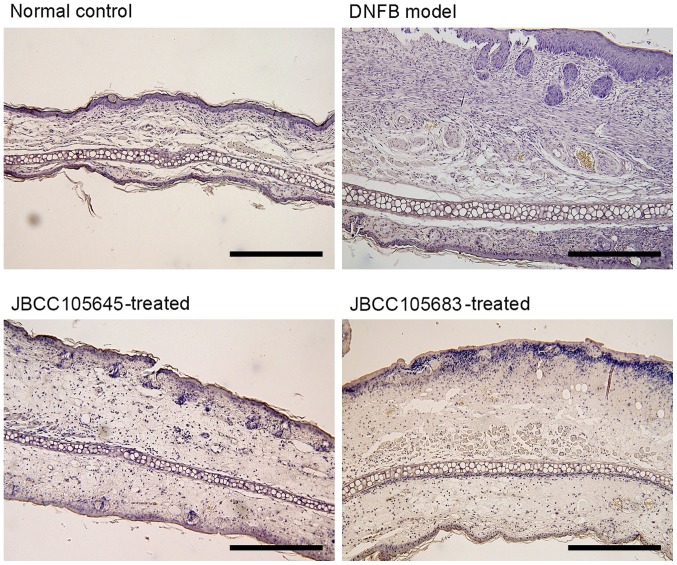
Effects of JBCC105645 and JBCC105683 on histopathological findings of DNFB-challenged ear skin
Histological analysis revealed spongiosis in the ear skin of mice in the DNFB model group (Fig. 2). Additionally, marked infiltration of inflammatory cells in the epidermis and dermis was also observed in DNFB model group (Fig. 2). DNFB-induced pathophysiological parameters, such as spongiosis and infiltration of inflammatory cells, were markedly reduced following oral treatment with JBCC105645 or JBCC105683 (Fig. 2).
Figure 2.
Histopathological changes of mice ear lesion. Mice were orally administrated with PBS (DNFB model), JBCC105645 or JBCC105683 (1×109 colony-forming units) daily for 7 days. The treated mice were contact-sensitized for 5 days with 0.2% DNFB and thereafter challenged or unchallenged (normal control). Each sample was obtained 48 h following the DNFB challenge. Sections were stained with hematoxylin and eosin (scale bar, 300 µm; magnification, ×100). Sections shown are representatives of >5 observations. DNFB, dinitrofluorobenzene.
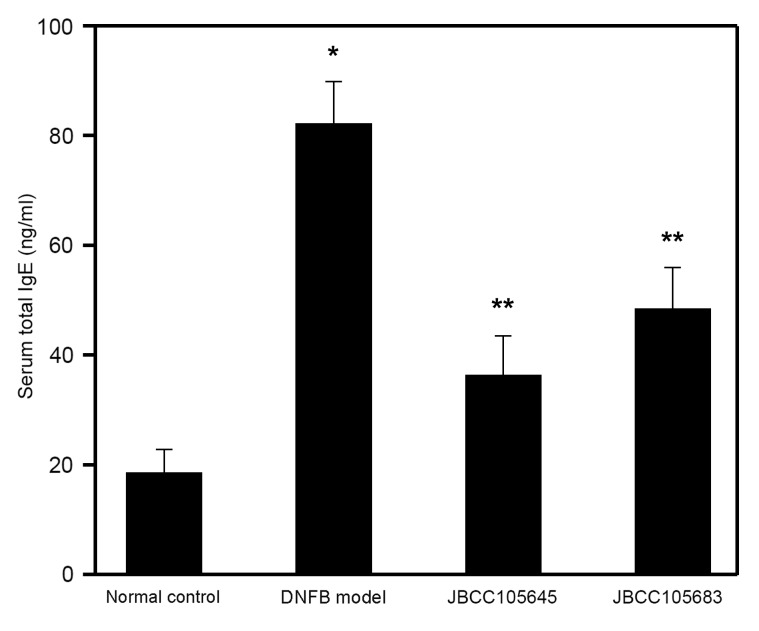
Effects of oral administration of JBCC105645 and JBCC105683 on total serum IgE levels
In addition to the clinical features, the levels of total serum IgE, which is one of the most common indicators of AD (13,14), were also assessed to characterize the immunological response during disease progression. Following the completion of DNFB treatment, serum samples were obtained, and total serum IgE levels were determined by ELISA. Total serum IgE levels were significantly increased by DNFB challenge compared with the normal control group (P<0.05; Fig. 3). The DNFB-induced elevation in serum IgE levels was significantly decreased by oral treatment with JBCC105645 or JBCC105683 (P<0.05; Fig. 3); JBCC105645 was markedly more effective than JBCC105683 in serum IgE inhibition.
Figure 3.
Effect of oral administration of lactobacilli on total serum IgE in DNFB-challenged mice. Mice were orally administered with PBS (DNFB model), JBCC105645 or JBCC105683 (1×109 colony-forming units) daily for 7 days. The treated mice were contact-sensitized for 5 days with 0.2% DNFB and thereafter challenged or unchallenged (normal control). The concentration of total serum IgE collected 48 h following DNFB challenge was determined using ELISA. Data are presented as the mean ± standard deviation (n=6). *P<0.05 vs. normal control group. **P<0.05 vs. DNFB model group. Ig, immunoglobulin; DNFB, dinitrofluorobenzene.
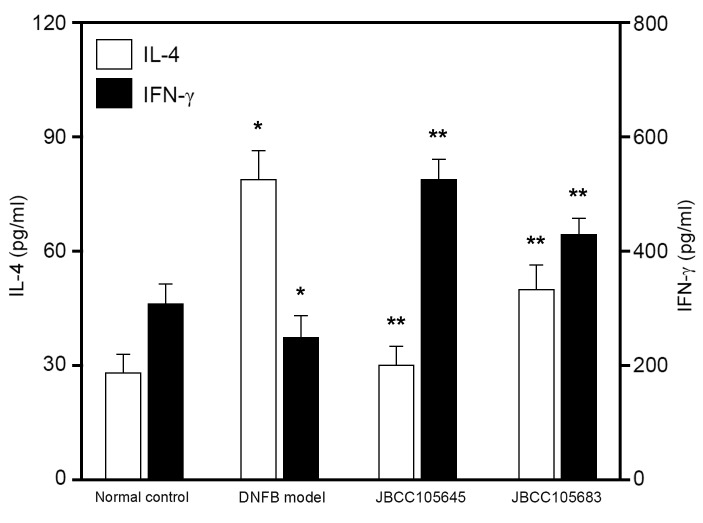
Effects of JBCC105645 and JBCC105683 on IL-4/IFN-γ production by ex vivo CD4+ T cells
To evaluate whether oral administration of lactobacilli would affect IL-4 and IFN-γ production, CD4+ T cells were isolated from the lymph nodes of mice following exposure to DNFB and re-stimulated for 48 h with anti-CD3 and anti-CD28 antibodies. IL-4 and IFN-γ concentrations in the culture supernatant were assessed using ELISA. Both IL-4 and IFN-γ production was measured in CD4+ T cells. IL-4 production in ex vivo re-stimulated CD4+ T cells was significantly increased in the DNFB model compared with the control, while IFN-γ production was significantly decreased (both P<0.05; Fig. 4). IL-4 production was significantly attenuated by JBCC105645 and JBCC105683 compared with the DNFB model (P<0.05; Fig. 4), while IFN-γ production was significantly augmented by these treatments (P<0.05; Fig. 4). JBCC105645 was markedly more effective in suppressing IL-4 production and enhancing IFN-γ production than JBCC105683.
Figure 4.
Effects of oral administration of lactobacilli on IL-4 and IFN-γ levels in CD4+ T cells isolated from the lymph nodes of DNFB-challenged mice. Mice were orally administered with PBS (DNFB model), JBCC105645 or JBCC105683 (1×109 colony-forming units) daily for 7 days. The treated mice were contact-sensitized for 5 days with 0.2% DNFB and thereafter challenged or unchallenged (normal control). CD4+ T cells were purified from the lymph nodes of DNFB-challenged mice. The cells (1.5×105 cells/ml) were stimulated for 48 h with immobilized anti-CD3 antibody (5 µg/ml) and soluble anti-CD28 antibody (2 µg/ml). IL-4 and IFN-γ levels were measured using ELISA. Data are presented as the mean ± standard deviation (n=6). *P<0.05 vs. normal control group. **P<0.05 vs. DNFB model group. IL, interleukin; IFN, interferon; CD, cluster of differentiation; DNFB, dinitrofluorobenzene.
Effects of JBCC105645 and JBCC105683 on IL-12 production by in vitro macrophages
In the present study, in vitro cell culture studies were performed by testing whether lactobacilli stimulated macrophages to produce IL-12, which is a Th1 differentiation-stimulatory cytokine (48). RAW264.7 macrophages were stimulated with heat-killed lactobacilli for 24 h, and levels of IL-12 in supernatants were subsequently measured using ELISA. Macrophages stimulated with JBCC105645 and JBCC105683 had significantly increased levels of IL-12 production compared with untreated cells (P<0.05; Fig. 5). At a given concentration (5×107 CFU/ml), JBCC105645 was markedly more effective in induction of IL-12 production than JBCC105683.
Figure 5.
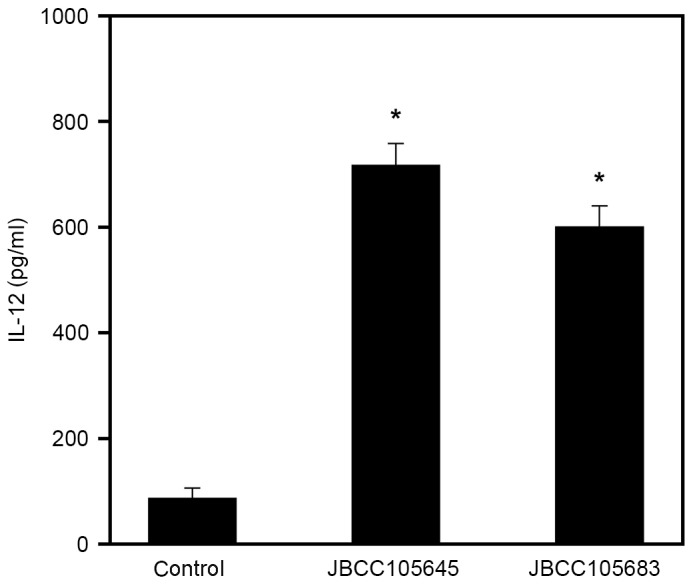
Effect of heat-killed lactobacilli on IL-12 production in macrophages. RAW264.7 macrophages were stimulated for 48 h with PBS (control) or 5×107 cells/ml of heat-killed JBCC105645 or JBCC105683. IL-12 levels in cultured supernatants were measured using ELISA. Data are presented as the mean ± standard deviation (n=9). *P<0.05 vs. control group. IL, interleukin.
Discussion
It has previously been reported that intestinal microflora serve an important role in Th1/Th2 balance (29,49), which is the primary mechanism associated with allergic diseases. Furthermore, human epidemiological data suggest that altered microbial exposure during childhood influences the outcome of allergic sensitization (50–52); therefore, the role of probiotics in allergic disease has been highlighted. There are a substantial number of reports that demonstrate the beneficial effects of probiotics in the management of AD and other allergic responses (53–55). A large number of organisms in probiotics have previously been studied, and certain strains of lactobacilli and bifidobacteria are now believed to have therapeutic effects on the extent and severity of allergic diseases, including AD (25–34,56). In the present study, it was addressed whether oral administration of lactobacilli (JBCC105645 and JBCC105683 strains) isolated from Jeotgal, a Korean traditional fermented food (35–41), was able to reduce the severity of AD-like skin lesions induced by DNFB in BALB/c mice. The results demonstrated that two lactobacillus strains significantly suppressed AD-like skin inflammation with varying effectiveness. JBCC105645 was more potent than JBCC105683, thus suggesting that the beneficial effects of the lactobacilli on the extent and severity of AD may be strain-specific.
The upregulation of total serum IgE is a hallmark of AD (13,14). Elevated serum IgE levels are known to cause both acute and chronic skin inflammation (13,14), and DNFB exposure increases serum IgE levels in several animal models (46,47). In line with this, a significant increase in total serum IgE levels was also observed in the present AD mouse model. However, oral treatment with the lactobacilli isolated from Jeotgal significantly reduced DNFB-induced IgE levels. Notably, the potency for IgE inhibition by the lactobacilli was similar to that for their suppression of AD-like skin inflammation.
The balance of the Th2 cytokine IL-4 and the Th1 cytokine IFN-γ has previously been reported to be critical for regulating IgE production (2–5). IL-4 produced by Th2 cells induces a switch to IgE production by differentiating B cells, resulting in an increase in IgE production, whereas IFN-γ produced by Th1 cells is able to inhibit that switch, consequently preventing IgE production (2–5). In this context, it is possible that the inhibition of serum IgE levels by JBCC105645 and JBCC105683 may result from downregulation of IL-4 production and/or upregulation of IFN-γ production. To test this possibility, it was investigated whether oral administration of lactobacillus was able to influence the balance of IL-4/IFN-γ production in ex vivo re-stimulated CD4+ T cells obtained from lymph nodes of DNFB-challenged mice. Oral administration of the lactobacilli induced a significant decrease in IL-4 production with concomitant increase in IFN-γ production. Interestingly, JBCC105645 was more effective in IL-4 inhibition and IFN-γ enhancement than JBCC105683, which was similar to their effects on IgE inhibition. These results suggest that oral administration of lactobacilli may reduce the levels of serum IgE, at least in part, via modulation of IL-4/IFN-γ production in DNFB-challenged mice. The results of the present study are also in agreement with previous reports that found that other lactobacillus strains are able to modulate IL-4/IFN-γ production in re-stimulated CD4+ T cells (28,57).
At present, the mechanism(s) by which the lactobacilli modulate IL-4/IFN-γ production remains unclear. It has been reported that IL-12, which is primarily produced by antigen-presenting cells such as macrophages and dendritic cells, typically induces differentiation of IFN-γ-producing Th1 cells, thereby preventing differentiation of IL-4-producing Th2 cells (25,28). Furthermore, it has been reported that various lactobacillus strains are able to induce IL-12 production in mouse macrophages and stimulate IFN-γ production in mouse splenocytes (25); which prompted the examination of whether JBCC105645 and JBCC105683 were also able to stimulate macrophages to produce IL-12 in the present study. The results demonstrated that the lactobacilli directly stimulated macrophages in vitro to induce IL-12 production, and JBCC105645 was more effective in IL-12 production than JBCC105683. This suggests that the enhancement of IFN-γ production by lactobacilli may be a result of the stimulating effects of JBCC105645 and JBCC105683 on IL-12 release from the antigen-presenting cell macrophages. The mechanism(s) by which the lactobacilli reduce IL-4 production in re-stimulated CD4+ T cells has yet to be elucidated. IFN-γ is able to prevent IL-4 production via the inhibition of Th2 differentiation (3–5), and so it may be assumed that inhibition of IL-4 production is due to enhanced IFN-γ production. Future studies should aim to produce a detailed study of the relevant mechanisms.
In conclusion, the results of the present study demonstrate that oral treatment with JBCC105645 and JBCC105683 strains isolated from Jeotgal inhibits AD-like skin lesions and reduces serum IgE levels in DNFB-treated mice. The cytokine production pattern of ex vivo CD4+ T cells demonstrated that administration of these lactobacillus strains induced a significant decrease in levels of the Th2-related cytokine IL-4 with a concomitant increase in the Th1-related cytokine IFN-γ. Additionally, lactobacilli stimulate macrophages to produce IL-12 in vitro. These results suggest that the lactobacillus strains isolated from Jeotgal are capable of reducing the severity of AD-like skin lesions, presumably by switching the immune response from Th2 type toward Th1 type via the activation of macrophages. Future studies should be directed towards identifying which components of the lactobacilli are responsible for their immunomodulatory effects.
Acknowledgements
The present study was supported by the High Value-Added Food Technology Development Program, (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, and the Ministry for Food, Agriculture, Forestry, and Fisheries of Republic of Korea; project no. 313037-3).
References
- 1.Yacoub MR, Colombo G, Marcucci F, Caminati M, Sensi L, Di Cara G, Frati F, Incorvaia C. Effects of sublingual immunotherapy on allergic inflammation: An update. Inflamm Allergy Drug Targets. 2012;11:285–291. doi: 10.2174/187152812800958988. [DOI] [PubMed] [Google Scholar]
- 2.Di Lernia V. Therapeutic strategies in extrinsic atopic dermatitis: Focus on inhibition of IL-4 as a new pharmacological approach. Expert Opin Ther Targets. 2015;19:87–96. doi: 10.1517/14728222.2014.965682. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–1166. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira LK, Fonseca BP, Barboza BA, Viola JP. The role of interferon-gamma on immune and allergic responses. Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):S137–S144. doi: 10.1590/S0074-02762005000900024. [DOI] [PubMed] [Google Scholar]
- 5.Mucida D, Cheroutre H. The many face-lifts of CD4 T helper cells. Adv Immunol. 2010;107:139–152. doi: 10.1016/B978-0-12-381300-8.00005-8. [DOI] [PubMed] [Google Scholar]
- 6.McFadden JP, Thyssen JP, Basketter DA, Puangpet P, Kimber I. T helper cell 2 immune skewing in pregnancy/early life: Chemical exposure and the development of atopic disease and allergy. Br J Dermatol. 2015;172:584–591. doi: 10.1111/bjd.13497. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang Y, Gu W, He L, Sun B. Th1/Th2 cell's function in immune system. Adv Exp Med Biol. 2014;841:45–65. doi: 10.1007/978-94-017-9487-9_3. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen TH, Casale TB. Immune modulation for treatment of allergic disease. Immunol Rev. 2011;242:258–271. doi: 10.1111/j.1600-065X.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumazawa Y, Takimoto H, Matsumoto T, Kawaguchi K. Potential use of dietary natural products, especially polyphenols, for improving type-1 allergic symptoms. Curr Pharm Des. 2014;20:857–863. doi: 10.2174/138161282006140220120344. [DOI] [PubMed] [Google Scholar]
- 10.Werfel T, Biedermann T. Current novel approaches in systemic therapy of atopic dermatitis: Specific inhibition of cutaneous Th2 polarized inflammation and itch. Curr Opin Allergy Clin Immunol. 2015;15:446–452. doi: 10.1097/ACI.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 11.Montes-Torres A, Llamas-Velasco M, Pérez-Plaza A, Solano-López G, Sánchez-Pérez J. Biological treatments in atopic dermatitis. J Clin Med. 2015;4:593–613. doi: 10.3390/jcm4040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015;7:221–229. doi: 10.4168/aair.2015.7.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu LC, Scheerens H. Targeting IgE production in mice and humans. Curr Opin Immunol. 2014;31:8–15. doi: 10.1016/j.coi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Yamanaka K, Mizutani H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr Probl Dermatol. 2011;41:80–92. doi: 10.1159/000323299. [DOI] [PubMed] [Google Scholar]
- 15.Svensson A, Chambers C, Gånemo A, Mitchell SA. A systematic review of tacrolimus ointment compared with corticosteroids in the treatment of atopic dermatitis. Curr Med Res Opin. 2011;27:1395–1406. doi: 10.1185/03007995.2011.582483. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg JI. Atopic dermatitis: An evidence-based treatment update. Am J Clin Dermatol. 2014;15:149–164. doi: 10.1007/s40257-014-0062-z. [DOI] [PubMed] [Google Scholar]
- 17.Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: A review of the evidence from studies conducted in humans. Curr Pharm Des. 2009;15:1428–11518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir O. Various effects of different probiotic strains in allergic disorders: An update from laboratory and clinical data. Clin Exp Immunol. 2010;160:295–304. doi: 10.1111/j.1365-2249.2010.04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitaliti G, Pavone P, Guglielmo F, Spataro G, Falsaperla R. The immunomodulatory effect of probiotics beyond atopy: An update. J Asthma. 2014;51:320–332. doi: 10.3109/02770903.2013.862259. [DOI] [PubMed] [Google Scholar]
- 20.Vogel RF, Ehrmann M. Genetics of lactobacilli in food fermentations. Biotechnol Annu Rev. 1996;2:123–150. doi: 10.1016/S1387-2656(08)70008-5. [DOI] [PubMed] [Google Scholar]
- 21.Derrien M, van Hylckama Vlieg JE. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015;23:354–366. doi: 10.1016/j.tim.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Pandey V, Berwal V, Solanki N, Malik NS. Probiotics: Healthy bugs and nourishing elements of diet. J Int Soc Prev Community Dent. 2015;5:81–87. doi: 10.4103/2231-0762.155726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuoka T. Development of functional foods. Biosci Microbiota Food Health. 2014;33:117–128. doi: 10.12938/bmfh.33.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemgang TS, Kapila S, Shanmugam VP, Kapila R. Cross-talk between probiotic lactobacilli and host immune system. J Appl Microbiol. 2014;117:303–319. doi: 10.1111/jam.12521. [DOI] [PubMed] [Google Scholar]
- 25.Won TJ, Kim B, Song DS, Lim YT, Oh ES, Lee do I, Park ES, Min H, Park SY, Hwang KW. Modulation of Th1/Th2 balance by lactobacillus strains isolated from Kimchi via stimulation of macrophage cell line J774A.1 in vitro. J Food Sci. 2011;76:H55–H61. doi: 10.1111/j.1750-3841.2010.02031.x. [DOI] [PubMed] [Google Scholar]
- 26.Hazebrouck S, Przybylski-Nicaise L, Ah-Leung S, Adel-Patient K, Corthier G, Langella P, Wal JM. Influence of the route of administration on immunomodulatory properties of bovine beta-lactoglobulin-producing Lactobacillus casei. Vaccine. 2009;27:5800–5805. doi: 10.1016/j.vaccine.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 27.Chuang L, Wu KG, Pai C, Hsieh PS, Tsai JJ, Yen JH, Lin MY. Heat-killed cells of lactobacilli skew the immune response toward T helper 1 polarization in mouse splenocytes and dendritic cell-treated T cells. J Agric Food Chem. 2007;55:11080–11086. doi: 10.1021/jf071786o. [DOI] [PubMed] [Google Scholar]
- 28.Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization; Proc Natl Acad Sci USA; 2005; pp. 2880–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross ML, Mortensen RR, Kudsk J, Gill HS. Dietary intake of Lactobacillus rhamnosus HNOO1 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med Microbiol Immunol. 2002;191:49–53. doi: 10.1007/s00430-002-0112-7. [DOI] [PubMed] [Google Scholar]
- 30.Drago L, Nicola L, Iemoli E, Banfi G, De Vecchi E. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res Notes. 2010;3:44. doi: 10.1186/1756-0500-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: Prediction for in vivo efficacy. PLoS One. 2009;4:e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18 and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JM, Kim YR, Kim JK, Jeong GT, Ha JC, Kong IS. Characterization of salt-tolerant β-glucosidase with increased thermostability under high salinity conditions from Bacillus sp. SJ-10 isolated from jeotgal, a traditional Korean fermented seafood; Bioprocess Biosyst Eng; 2015; pp. 1335–1346. [DOI] [PubMed] [Google Scholar]
- 36.Han KI, Kim YH, Hwang SG, Jung EG, Patnaik BB, Han YS, Nam KW, Kim WJ, Han MD. Bacterial community dynamics of salted and fermented shrimp based on denaturing gradient gel electrophoresis. J Food Sci. 2014;79:M2516–M2522. doi: 10.1111/1750-3841.12707. [DOI] [PubMed] [Google Scholar]
- 37.Jeong DW, Han S, Lee JH. Safety and technological characterization of Staphylococcus equorum isolates from jeotgal, a Korean high-salt-fermented seafood, for starter development. Int J Food Microbiol. 2014;188:108–115. doi: 10.1016/j.ijfoodmicro.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Lee KW, Park JY, Sa HD, Jeong JH, Jin DE, Heo HJ, Kim JH. Probiotic properties of Pediococcus strains isolated from jeotgals, salted and fermented Korean sea-food. Anaerobe. 2014;28:199–206. doi: 10.1016/j.anaerobe.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Jung J, Lee SH, Jin HM, Jeon CO, Park W. Pyrosequencing-based analysis of bacterial community and metabolites profiles in Korean traditional seafood fermentation: A flatfish-fermented seafood. Biosci Biotechnol Biochem. 2014;78:908–910. doi: 10.1080/09168451.2014.895659. [DOI] [PubMed] [Google Scholar]
- 40.Kim MS, Park EJ. Bacterial communities of traditional salted and fermented seafoods from Jeju Island of Korea using 16S rRNA gene clone library analysis. J Food Sci. 2014;79:M927–M934. doi: 10.1111/1750-3841.12431. [DOI] [PubMed] [Google Scholar]
- 41.Kim YR, Kim EY, Lee JM, Kim JK, Kong IS. Characterisation of a novel Bacillus sp. SJ-10 β-1,3-1,4-glucanase isolated from jeotgal, a traditional Korean fermented fish. Bioprocess Biosyst Eng. 2013;36:721–727. doi: 10.1007/s00449-013-0896-4. [DOI] [PubMed] [Google Scholar]
- 42.Romero-Pérez GA, Inoue R, Ushida K, Yajima T. A rapid method of screening lactic acid bacterial strains for conjugated linoleic acid production. Biosci Biotechnol Biochem. 2013;77:648–650. doi: 10.1271/bbb.120709. [DOI] [PubMed] [Google Scholar]
- 43.Luo W, Chen M, Chen A, Dong W, Hou X, Pu B. Isolation of lactic acid bacteria from pao cai, a Chinese traditional fermented vegetable, with inhibitory activity against Salmonella associated with fresh-cut apple, using a modelling study. J Appl Microbiol. 2015;118:998–1006. doi: 10.1111/jam.12741. [DOI] [PubMed] [Google Scholar]
- 44.Vinod KR, Jones D, Udupa V. A simple and effective heat induced antigen retrieval method. MethodsX. 2016;3:315–319. doi: 10.1016/j.mex.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broggi MA, Schmaler M, Lagarde N, Rossi SW. Isolation of murine lymph node stromal cells. J Vis Exp. 2014;90:e51803. doi: 10.3791/51803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita H, Ito T, Kato H, Asai S, Tanaka H, Nagai H, Inagaki N. Comparison of the efficacy of tacrolimus and cyclosporine A in a murine model of dinitrofluorobenzene-induced atopic dermatitis. Eur J Pharmacol. 2010;645:171–176. doi: 10.1016/j.ejphar.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 47.Yun Y, Kim K, Choi I, Ko SG. Topical herbal application in the management of atopic dermatitis: a review of animal studies. Mediators Inflamm. 2014;2014:752103. doi: 10.1155/2014/752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol. 2015;34:130–136. doi: 10.1016/j.coi.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross ML, Gill HS. Can immunoregulatory lactic acid bacteria be used as dietary supplements to limit allergies? Int Arch Allergy Immunol. 2001;125:112–119. doi: 10.1159/000053804. [DOI] [PubMed] [Google Scholar]
- 50.Vernocchi P, Del Chierico F, Fiocchi AG, El Hachem M, Dallapiccola B, Rossi P, Putignani L. Understanding probiotics' role in allergic children: The clue of gut microbiota profiling. Curr Opin Allergy Clin Immunol. 2015;15:495–503. doi: 10.1097/ACI.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 51.Panzer AR, Lynch SV. Influence and effect of the human microbiome in allergy and asthma. Curr Opin Rheumatol. 2015;27:373–380. doi: 10.1097/BOR.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 52.Putignani L, Del Chierico F, Petrucca A, Vernocchi P, Dallapiccola B. The human gut microbiota: A dynamic interplay with the host from birth to senescence settled during childhood. Pediatr Res. 2014;76:2–10. doi: 10.1038/pr.2014.49. [DOI] [PubMed] [Google Scholar]
- 53.Madonini ER. Probiotics and allergies: Myth or reality? Eur Ann Allergy Clin Immunol. 2014;46:196–200. [PubMed] [Google Scholar]
- 54.Foolad N, Armstrong AW. Prebiotics and probiotics: The prevention and reduction in severity of atopic dermatitis in children. Benef Microbes. 2014;5:151–160. doi: 10.3920/BM2013.0034. [DOI] [PubMed] [Google Scholar]
- 55.Żukiewicz-Sobczak W, Wróblewska P, Adamczuk P, Silny W. Probiotic lactic acid bacteria and their potential in the prevention and treatment of allergic diseases. Cent Eur J Immunol. 2014;39:104–108. doi: 10.5114/ceji.2014.42134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansfield JA, Bergin SW, Cooper JR, Olsen CH. Comparative probiotic strain efficacy in the prevention of eczema in infants and children: A systematic review and meta-analysis. Mil Med. 2014;179:580–592. doi: 10.7205/MILMED-D-13-00546. [DOI] [PubMed] [Google Scholar]
- 57.Won TJ, Kim B, Lim YT, Song DS, Park SY, Park ES, Lee DI, Hwang KW. Oral administration of Lactobacillus strains from Kimchi inhibits atopic dermatitis in NC/Nga mice. J Appl Microbiol. 2011;110:1195–1202. doi: 10.1111/j.1365-2672.2011.04981.x. [DOI] [PubMed] [Google Scholar]